Abstract
Purpose
Recently, we reported finding that circulating melatonin levels in age-related macular degeneration patients were significantly lower than those in age-matched controls. The purpose of this study was to investigate the hypothesis that melatonin deficiency may play a role in the oxidative damage of the retinal pigment epithelium (RPE) by testing the protective effect of melatonin and its receptor antagonist on RPE cells exposed to H2O2 damage.
Methods
Cultured human RPE cells were subjected to oxidative stress induced by 0.5 mM H2O2. Cell viability was measured using the microculture tetrazoline test (MTT) assay. Cells were pretreated with or without melatonin for 24 h. Luzindole (50 μM), a melatonin membrane-receptor antagonist, was added to the culture 1 h before melatonin to distinguish direct antioxidant effects from indirect receptor-dependent effects. All tests were performed in triplicate.
Results
H2O2 at 0.5 mM decreased cell viability to 20% of control levels. Melatonin showed dose-dependent protective effects on RPE cells against H2O2. Cell viability of RPE cells pretreated with 10−10, 10−8, 10−6, and 10−4 M melatonin for 24 h was 130%, 160%, 187%, and 230% of cells treated with H2O2 alone (all p<0.05). Using cells cultured without H2O2 as the control, cell viability of cells treated with H2O2 after pretreatment with 10−10-10−4 M melatonin was still significantly lower than that of the controls, suggesting that melatonin significantly decreased but did not completely abolish the in vitro cytotoxic effects of H2O2. Luzindole completely blocked melatonin’s protective effects at low concentrations of melatonin (10−10-10−8 M) but not at high concentrations (10−6-10−4 M).
Conclusions
Melatonin has a partial protective effect on RPE cells against H2O2 damage across a wide range of concentrations (10−10-10−4 M). This protective effect occurs through the activation of melatonin membrane receptors at low concentrations (10−10-10−8 M) and through both the direct antioxidant and indirect receptor activation effects at high concentrations (10−6-10−4 M).
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in aged people in developed countries. The prevalence of AMD in Americans 40 years of age or older is 1.5%, and it has been estimated that 1.75 million people suffer from this disease in the United States [1].
The retinal pigment epithelium is a single layer of pigmented cells that have multiple functions essential to the maintenance of the overlying photoreceptors and hence to visual function. Oxidative stress has been implicated in the pathogenesis of AMD, possibly due to the detrimental effects of reactive oxygen species (ROS) on the retinal pigment epithelial (RPE) cells [2-6]. In support of this hypothesis, supplementation with antioxidants and zinc has been demonstrated in several studies to slow the progression of disease and preserve vision [6,7].
Recently, we found that the amount of 6-sulphatoxymelatonin (aMT6s) in nocturnal urine (a well established and reliable parameter for estimating peak circulating melatonin levels) in AMD patients was significantly lower than that of age- and gender-matched controls [8]. Whether reduced melatonin levels in AMD patients play a role in the occurrence of the disease remains unknown. It is therefore important to study the protective effects of melatonin on RPE cells against oxidative stress to determine the relationship between melatonin deficiency and RPE cell damage. A demonstration of RPE protection by melatonin would support the hypothesis that melatonin supplementation may be helpful for the prevention and treatment of AMD.
Furthermore, Baba et al. [9] reported that MT1 receptor transcripts were localized in mouse photoreceptor cells and in some inner retinal neurons. A diurnal rhythm in the dark-adapted electroretinography (ERG) responses was observed in wild-type (WT) mice, but not in melatonin membrane receptor 1 (MT1) receptor-deficient mice [MT1(−/−) mice]. Injection of melatonin during the day influenced ERG in WT mice but not MT1(−/−) mice. MT1(−/−) mice showed a significant decrease of photoreceptor nuclei and ganglion cells, compared with WT mice. These results demonstrate the functional significance of melatonin and MT1 receptors in the mammalian retina and create the basis for future studies on the therapeutic use of melatonin in retinal degeneration.
The detrimental effects of H2O2 on various cell types could be reduced by melatonin in the lymphoma cells [10], astrocytes [11], breast cancer cells [12], cerebellar granular neurons [13], pituitary cells [14,15], brain astrocytes [16], neuroblastoma cells [17], astroglial cells [18], spermatozoa [19], hepatoma cells [20] and motoneurons [21]. The mechanism of melatonin mediated cytoprotection has been documented as a direct antioxidant effect [12], or an indirect effect via the activation of melatonin receptors and relevant signal pathways [10,14,21], or through both direct and indirect effects [16,17], therefore, the melatonin protective effects and its mechanisms are highly cell type-specific. Very little is known about melatonin’s ability to protect RPE cells from H2O2 damage [22]. In this study, we looked at the influence of the dose and timing of melatonin administration on melatonin’s ability to protect cultured human RPE cells against H2O2-induced damage. In addition, the cell cultures were challenged, with and without the addition of luzindole, to determine the direct antioxidant versus indirect receptor-mediated effects of melatonin across a wide spectrum of concentrations.
Methods
Cell culture
The human RPE cell line, ARPE-19 (an immortal cell line from a 19-year-old donor), was obtained from American Type Culture Collections (Manassas, VA). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Carlsbad, CA), supplemented with 10% fetal bovine serum (FBS; Gibco). Cells were incubated in a humidified 5% CO2 atmosphere at 37 °C. After reaching confluence, cells were detached by trypsin-EDTA solution (Gibco), diluted 1:3–1:4, plated for subculture, and passaged routinely at a dilution of 1:3–1:4 every 5–7 days.
A new separate culture of primary human RPE cells was isolated from a donor eye (56 years old) and cultured as previously described [23,24]. Briefly, the anterior segment of the eye, the vitreous and the retina were excised. The RPE layer was immersed with trypsin-EDTA solution at 37 °C for 1 h. Culture medium with 10% FBS was added, and the RPE cells were isolated and collected under direct observation using a dissecting microscope. Isolated cells were centrifuged, re-suspended and seeded to a culture flask. Cells were also cultured in DMEM with 10% FBS. After reaching confluence, cells were subcultured as described above. Phase-contrast microscopy revealed pigmentation of RPE cells during the primary culture and the first and second subcultures. Cells displayed characteristic epithelial morphology throughout the culture period. The purity of the cell lines was demonstrated by immunocytochemical methods. RPE cells display positive staining of cytokeratin, whereas fibroblasts and melanocytes do not [25].
Effects of H2O2 on the viability of retinal pigment epithelium cells
The effects of H2O2 on the viability of RPE cells were studied with the microculture tetrazoline test (MTT) test as described previously [24]. Briefly, RPE cells were plated in 96-well plates at a density of 5 × 103 cells per well. After incubation for 24 h, H2O2 (Sigma, St. Louis, MO) was added to the wells at various final concentrations (0.05 mM, 0.1 mM, 0.25 mM, 0.5 mM, 0.75 mM, and 1.0 mM) and cultured for 24 h. Next, 50 µl of tetrazolium bromide, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (1 mg/ml; Sigma), was added to each well and incubated for 4 h. The medium was withdrawn and 100 µl of DMSO (Sigma) was added to each well. The optical density was read at 540 nm using a microplate reader (Multiskan EX; Thermo, Vantaa, Finland). Cells cultured without H2O2 served as the controls. The effects of H2O2 on the viability of RPE cells were studied in the ARPE-19 cell line and the primary-culture RPE cell line separately. All groups were tested in triplicate.
Effect of melatonin on H2O2-induced damage in retinal pigment epithelium cells
RPE cells were seeded as detailed above. Twenty-four hours later, melatonin (Sigma) was added to the culture medium at different concentrations (10−10 M to 10−4 M). After 1 h, 24 h, and 48 h, H2O2 was added to the medium at a final concentration of 0.5 mM and cultured for 24 h. Then, cell viability was evaluated by the MTT test as described above. Cells cultured with H2O2 but without melatonin were used as positive controls. This study was performed in the ARPE-19 cell line and the primary-culture RPE cell line separately. All groups were tested in triplicate.
Influence of luzindole on the effects of melatonin on H2O2-induced damage in retinal pigment epithelium cells
RPE cells were seeded as previously detailed. After 24 h, luzindole (Sigma) was added to the culture medium at a final concentration of 50 μM. One hour later, melatonin was added to the culture medium at different concentrations (10−10 M to 10−4 M). After 24 h cultivation, H2O2 was added for a final concentration of 0.5 mM and cultured for 24 h. Then, cell viability was evaluated by the MTT test described above. This study was performed in the ARPE-19 cell line and the primary-culture RPE cell line separately. All groups were tested in triplicate.
Statistical analysis
Statistical significances of differences in means throughout this study were calculated by an ANOVA one-way test in comparing data from more than two groups, and by the Student’s t-test in comparing data between two groups. A difference at p<0.05 was considered statistically significant.
Results
Cytotoxic effects of hydrogen peroxide on cultured human retinal pigment epithelium
H2O2 showed dose-dependent cytotoxic effects on cultured human RPE cells (ARPE-19) at concentrations from 0.25 mM to 1.00 mM (Figure 1 and Figure 2). Cell viability in cells treated with 0.5 mM H2O2 decreased by 71%, compared to the controls (cells not treated with H2O2). Therefore, 0.5 mM H2O2 was selected as the concentration used for subsequent experiments. H2O2 had a similar cytotoxic effect on the primary-culture human RPE cells (Figure 2B).
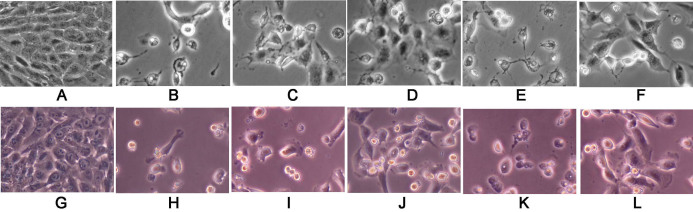
Figure 1.
Melatonin protected the retinal pigment epithelium (RPE) cells against H2O2 damage, especially in high concentrations (10−4 M) and luzindole decreased the protective effects of melatonin. Phase-contrast microscopic images of the effects of melatonin and its membrane-receptor antagonist (luzindole) on retinal pigment epithelial cells against H2O2 damage. A-F: The RPE cells are from the ARPE-19 cell line (an immortal RPE cell line from a 19-year-old donor). G-L: The RPE cells are from the primary culture (PC) from the donor eye. ARPE-19 and PC cells were cultured with or without H2O2, melatonin, and luzindole, as follows: without H2O2, melatonin, and luzindole(A and G); with H2O2 at 0.5 mM concentrations for 24 h (B and H); with H2O2 and a pretreatment of melatonin at 10−10 M (C and I) or at 10−4 M (D and J) for 24 h; or with luzindole (50 μM, 1 h), followed by melatonin at 10−10 M (E and K) or at 10−4 M for 24 h (F and L). H2O2 was then added and the ARPE-19 and PC cells cultured for 24 h.
Figure 2.
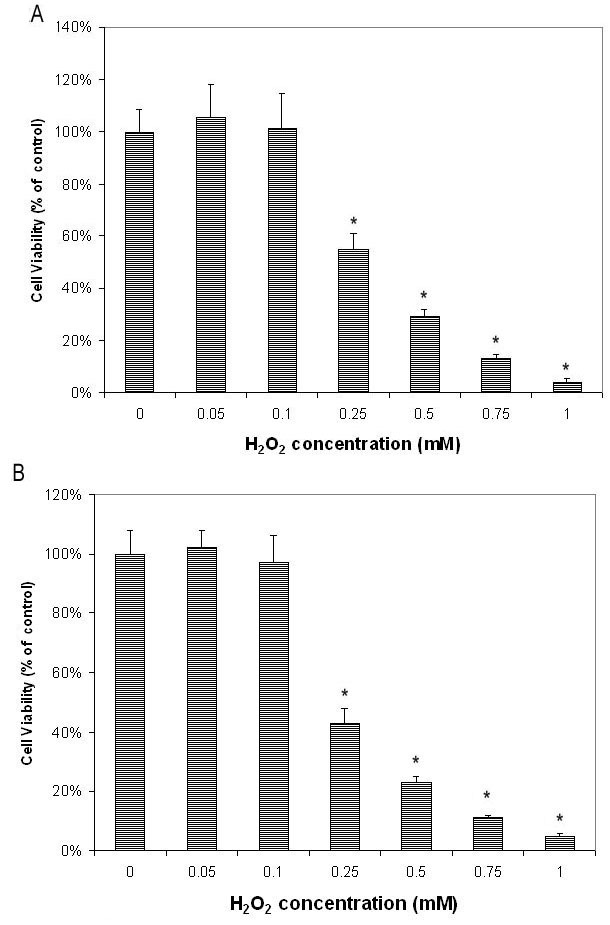
H2O2 dose-dependently decreased cell viability of retinal pigment epithelial cells as tested by microculture tetrazoline test. Retinal pigment epithelial (RPE) cells were plated in 96-well plates and treated with various concentrations of H2O2 for 24 h. Cell viability was evaluated by the microculture tetrazoline test (MTT) test and expressed as percentage of that in cells without H2O2 (mean±standard deviation [SD] in triplicate tests). A: H2O2 showed dose-dependent cytotoxic effects on ARPE-19 cells (cells from an immortal RPE cell line from a 19-year-old donor) at concentrations from 0.25 mM to 1.00 mM (p<0.05, compared to cells cultured without H2O2. B: Studies of primary cultures of human RPE cells isolated from donor eye showed similar results. *p<0.05, compared with the controls (cells cultured with H2O2 alone).
Ability of melatonin to protect retinal pigment epithelium cells against H2O2 damage
Melatonin at concentrations of 10−10 M to 10−4 M applied for different time periods did not influence the cell viability of cultured human RPE cells (ARPE-19 cell line). Pretreatment of RPE cells with melatonin at low (10−10 M) or high (10−6 M) concentrations for 1 h, 24 h, and 48 h significantly protected cells against H2O2 damage (Figure 3A,B). Cell viability for cultures pretreated with 10−10 M melatonin for 1 h, 24 h, and 48 h was, respectively, 125%, 133%, and 121% of that of cells treated with H2O2 alone. Differences between cells cultured with and without melatonin pretreatment were statistically significant (p<0.05 for all three different pretreatment periods; see Figure 3A). No significant difference (p>0.05) in cell viability could be detected between cells pretreated with melatonin at 1 h, 24 h, and 48 h before challenge (p>0.05). Pretreatment with 10−6 M melatonin for different periods also significantly protected cells (p<0.05 in all groups; Figure 3A). No difference in cell viability could be detected between cells pretreated with melatonin for different time periods (p>0.05). Therefore, pretreatment of melatonin for 24 h was selected for subsequent experiments. Studies of the primary-culture human RPE cells from the donors obtained similar results (data not shown).
Figure 3.
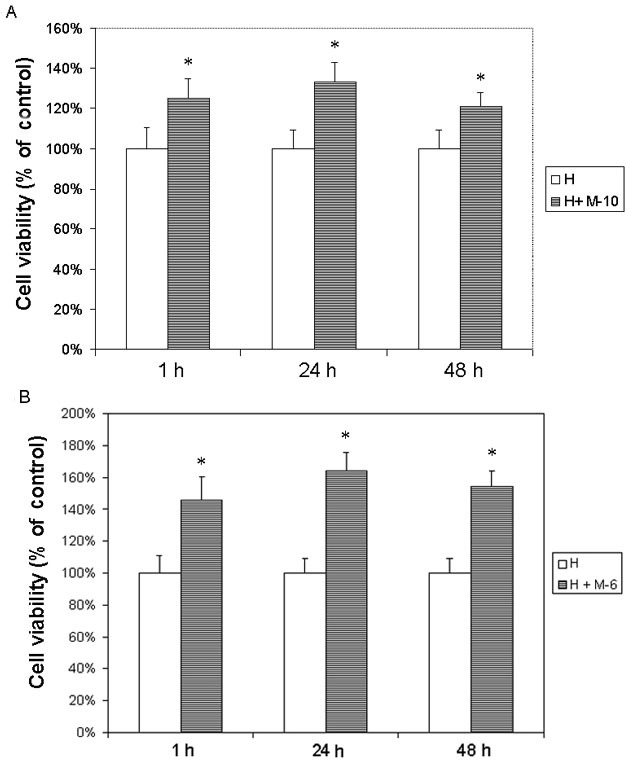
The ARPE19 cells (an immortal retinal pigment epithelial cell line from a 19-year-old donor) were treated with melatonin (M) at different concentrations. After 1 h, 24 h, and 48 h culture, 0.5 mM H2O2 (H) was added and cultured for 24 h. Cells cultured with H2O2 alone were used as the controls. Cell viability was evaluated by the microculture tetrazoline test and expressed as percentages of controls (mean±SD in triplicate tests). Error bars represent SD A: Pretreatment with low concentrations of melatonin at 10−10 M (M-10) for 1 h, 24 h, and 48 h significantly protected cells against H2O2. B: Pretreatment with high concentrations of melatonin at 10−6 M (M-6) obtained similar results. The difference between cells cultured with and without melatonin at both high and low concentrations was statistically significant at all three different pretreatment periods. *p<0.05, compared with the controls.
Pretreatment of melatonin at different concentrations for 24 h showed dose-dependent protective effects on RPE cells (ARPE-19) against H2O2 damage (Figure 1 and Figure 4A). Cell viability of RPE cells pretreated with 10−10 M, 10−8 M, 10−6 M, and 10−4 M melatonin was 130%, 160%, 187% and 230%, respectively, of cells treated with H2O2 alone. The differences in cell viability between cells treated with and without melatonin were significant (p<0.05) in all groups. Using cells cultured without H2O2 as the control, the cell viability of cells treated with H2O2 alone and that of cells treated with H2O2 after pretreatment with 0 M, 10−10 M, 10−8 M, 10−6 M, and 10−4 M melatonin was 20%, 26%, 31%, 37%, and 45% of the controls, respectively, which was significantly lower than the cell viability of cells cultured without H2O2 (one-way ANOVA, p>0.05; see Figure 5A). Studies of primary-culture human RPE isolated from the donor eye showed similar results (Figure 1, Figure 4B, and Figure 5B).
Figure 4.
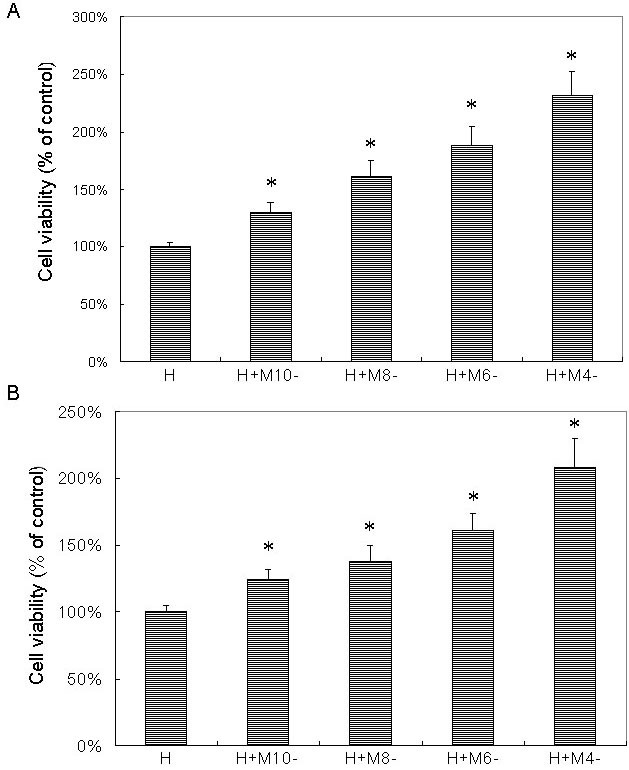
Melatonin dose-dependently protected retinal pigment epithelial cells against H2O2 damage as tested by microculture tetrazoline test. Retinal pigment epithelial (RPE) cells were pretreated with melatonin (M) at concentrations of 10−10 M (M-10), 10−8 M (M-8), 10−6 M (M-6), and 10−4 M (M-4). After 24 h, 0.5 mM H2O2 (H) was added and cultured for 24 h. Cells treated with H2O2 alone were used as the controls (H). Cell viability was evaluated by the microculture tetrazoline test and expressed as percentages of the controls (mean±standard deviation [SD] in triplicate tests). Error bars represent SD. Pretreatment with melatonin showed dose-dependent protective effects on ARPE-19 cells (an immortal RPE cell line from a 19-year-old donor) against H2O2 damage (A). Studies in primary culture of human RPE cells isolated from the donor eye showed similar results (B). *p<0.05, compared with the controls (cells treated with H2O2 alone).
Figure 5.
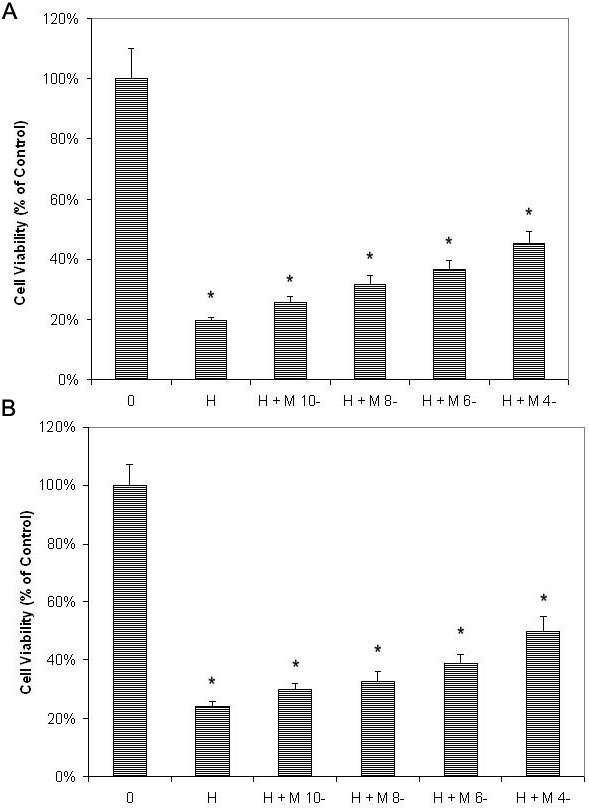
Melatonin dose-dependently protected retinal pigment epithelial cells against H2O2 damage as tested by microculture tetrazoline test, compared with cells not treated with H2O2. Retinal pigment epithelial (RPE) cells were pretreated with melatonin (M) at concentrations of 10−10 M (M-10), 10−8 M (M-8), 10−6 M (M-6), and 10−4 M (M-4). After 24 h, 0.5 mM H2O2 (H) was added and cultured for 24 h. Cells not treated with H2O2 were used as negative controls (0). Cell viability was evaluated by the microculture tetrazoline test and expressed as percentages of negative controls (mean±standard deviation [SD] in triplicate tests). Error bars represent SD A: Cell viability of cell treated with melatonin and H2O2 still significantly lower than that in cells cultured without H2O2 in the ARPE-19 cells (an immortal RPE cell line from a 19-year-old donor). B: The same was true in the primary-culture RPE cells. * p<0.05, compared with the negative controls (cells treated without H2O2).
Influence of luzindole on the protective effects of melatonin on retinal pigment epithelium cells
Cell viability of cultures treated with luzindole at 50 μM alone or luzindole with melatonin at various concentrations (10−10-10−4 M) did not show any effect in either of the RPE cell lines tested.
Cell viability of ARPE-19 cells (cultured with H2O2) with luzindole before melatonin was significantly decreased, compared with cells treated with melatonin at all concentrations (Figure 1 and Figure 6A). The difference in cell viability between cells treated with and without luzindole was statistically significant (p<0.05) in cells treated with 10−10 M to 10−4 M melatonin.
Figure 6.
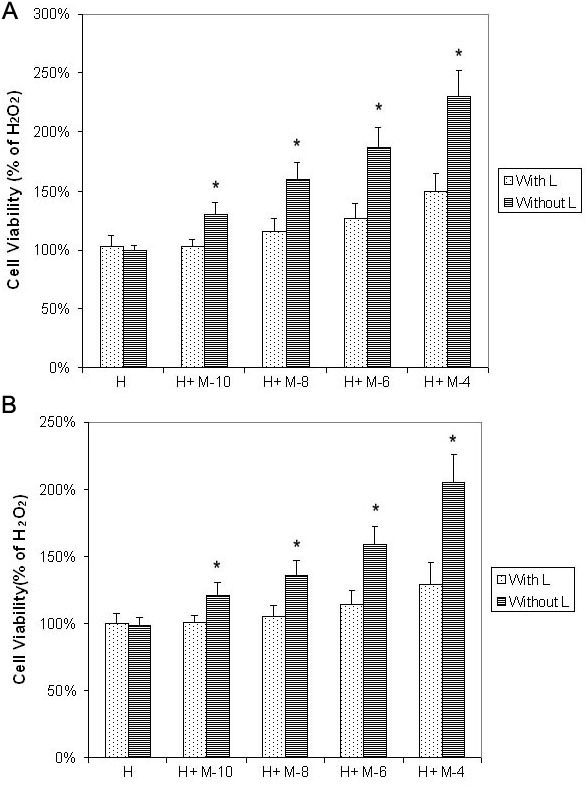
Luzindole decreased protective effects of melatonin on retinal pigment epithelial cells against H2O2 damage as tested by microculture tetrazoline test. Cultured retinal pigment epithelial (RPE) cells were treated with or without 50 μM luzindole (L). One hour later melatonin (M) was added to the culture medium at concentrations of 10−10 M (M-10), 10−8 M (M-8), 10−6 M (M-6), and 10−4 M (M-4). After 24 h, 0.5 mM H2O2 (H) was added and cultures were incubated for 24 h. Cell viability was evaluated by the microculture tetrazoline test and expressed as percentages of cells cultured with H2O2 alone (mean±standard deviation [SD] in triplicate tests). Error bars represent SD A: Luzindole significantly decreased melatonin-induced protective effects at 10−10 to 10−4 M in ARPE-19 cells (an immortal RPE cell line from a 19-year-old donor). B: The same was true in the primary-culture RPE cells. * p<0.05, comparison between cells treated with and without luzindole.
In cells pretreated with melatonin, the difference between cell cultures treated with H2O2 alone and cell cultures treated with H2O2 plus melatonin and luzindole was statistically nonsignificant (p>0.05) in melatonin at 10−10 M and 10−8 M (melatonin) and significant (p<0.05) in melatonin at 10−6 M and 10−4 M, by one-way ANOVA. Studies of primary-culture human RPE cells isolated from the donor eye showed similar results (Figure 1 and Figure 6B).
Discussion
H2O2 is a relatively weak oxidant, but in the presence of metal catalysts, it can convert to the reactive hydroxyl radical, which is cytotoxic. H2O2 is the most stable ROS; it presents in tissues with a relatively long half-life. H2O2 is soluble in both lipid and aqueous media, so it can easily diffuse in and out of the cell to reach targets. For these reasons, H2O2 exposure is one of the most common in vitro models used for evaluating oxidative-stress damage on cells [10-22,26].
Previous studies have demonstrated that melatonin protects various cells against H2O2 damage in vitro, and is cell type-specific [10-21]. Little is known about the protective effects of melatonin on RPE cells against H2O2 damage. Only one paper has been published on this subject [22], which tested the effects of melatonin at very high concentrations; and even the minimum concentration tested (10−7 M) is 100 to 500 fold the melatonin levels in the serum. The mechanism of the protective effects (whether a direct antioxidant effect or an indirect effect through the activation of melatonin receptors) has not been studied [22].
The present study tested the protective effects of melatonin on RPE cells against H2O2 across a wide range of concentrations. Melatonin showed dose-dependent protective effects from 10−10 M to 10−4 M. The protective effects present at both physiologic concentrations (10−10 M to 10−8 M, which are 1/10 to 10 fold the melatonin serum levels) and pharmacological concentrations (10−6 M to 10−4 M). Cell survival increased by 30%–130% in cells exposed to H2O2 following melatonin pretreatment, compared to those exposed to H2O2 alone, but the cell viability in cells treated with melatonin and H2O2 was still lower than that in cells cultured without H2O2. This suggests that melatonin can partially inhibit the cytotoxicity of H2O2 on cultured human RPE cells and is consistent with previous studies demonstrating the protective effects of melatonin on other cells (neurons, astrocytes, pituitary cells, neuroblastoma cells, etc.) exposed to H2O2 [10-21].
Normal melatonin levels in the serum during the night have been reported to vary from 2 × 10−10 M to 10−9 M [27-29], and the melatonin levels in ocular aqueous humors have been reported to range from 10−8-10−9 M [30,31]. Urinary aMT6s levels in AMD patients were 40% lower than in those of age- and gender-matched controls [8]. The present study suggests that melatonin at 10−8-10−10 M significantly protects human RPE cells against H2O2 in a dose-dependent manner. It corroborates the hypothesis that the deficiency of melatonin in AMD patients may play a role in the pathogenesis of AMD and that supplementation of melatonin may be helpful in the prevention and treatment of AMD.
Melatonin may protect cells against oxidative damage by at least two mechanisms. At high concentrations, melatonin may act as a scavenger of free radicals, ROS, and reactive nitrogen species. The redox properties of melatonin are similar to those of other tryptophan metabolites [32]: melatonin quenches free radicals (superoxide and hydroxyl radicals) efficiently but quenches singlet oxygen with only moderate efficiency [33]. On the other hand, it is able to activate membrane-bound melatonin receptors, which can stimulate the production of a variety of antioxidative enzymes through several signaling pathways [21,27,34-40].
To test whether the protective effects of melatonin against H2O2-induced oxidative stress demonstrated here were due to direct ROS quenching or due to indirect receptor-mediated effects, we employed luzindole, a melatonin receptor antagonist. Luzindole is a nonselective antagonist of melatonin membrane receptors MT1 and MT2, with a higher affinity for the MT2 subtype. It has been extensively used to distinguish the direct antioxidant effects from indirect receptor-mediated effects [35,41-47].
Very little is known about the effect of luzindole on melatonin-induced protection of cells against H2O2 damage. Only one report mentions that luzindole attenuated the effects of melatonin-induced protection of motoneurons against H2O2 damage, at a single melatonin concentration of 1.5×107 M [21].
Human RPE cells express MT2 but not MT1 melatonin membrane receptors [30,48]. Luzindole significantly decreased the melatonin-induced protective effects at all concentrations of melatonin, indicating that the receptor-dependent indirect effects are the main protective mechanism in RPE cells. This is consistent with studies performed on motoneurons at a single concentration [21]. Binding of melatonin with its membrane receptors appears to promote enzymes that metabolize oxidative stress. These enzymes include glutathione peroxidase, glutathione reductase, superoxide dismutases, glucose-6-phosphate dehydrogenase, and catalase [36]. Additionally, melatonin receptors can couple to multiple signaling pathways, which protects cells from oxidative stress damage [21].
In the present study, pretreatment of RPE cells with luzindole decreased but did not completely block the protective effects of melatonin at high concentrations (10−6-10−4 M), suggesting that at pharmacological concentrations, melatonin may have direct antioxidant effects. This indicates that luzindole, by blocking the activation of melatonin membrane receptors, can completely abolish the protective effect of melatonin at low concentrations. However, luzindole can reduce, but cannot completely abolish, the protective effect of high levels of melatonin against H2O2 damage.
Controversy exists as to whether melatonin can neutralize H2O2 directly [36,38]. However, H2O2 can convert to the hydroxyl radical, a highly reactive species, which is very toxic to cells and is the main cause of cell damage from H2O2. The hydroxyl radical is not enzymatically detoxified within cells and can only be neutralized by direct free-radical scavengers. Melatonin interacts with hydroxyl radicals at a rate-constant that is equivalent to that of other highly efficient hydroxyl radical scavengers [27,32,34,35,38]. Therefore, this could be the mechanism for receptor-independent melatonin protection.
In addition to the protective effects of melatonin on human RPE cells against oxidative stress, previous studies have also demonstrated that melatonin protects retinal homogenates against lipid peroxidation [49], and protects photoreceptors against various oxidative stressors [50-52]. These studies have also suggested that melatonin may be beneficial in the management of AMD.
Only one clinical study explored the treatment of AMD with melatonin supplementation: Yi et al. [53] reported encouraging results, but the study was nonrandomized, with only 6 months of follow-up and an attrition rate at follow-up of 45%. Still, these results are encouraging for organizing a randomized clinical trial evaluating the therapeutic value of melatonin supplementation for AMD management.
Supplementation of melatonin has been widely used in the treatment of various human disorders. The doses range from 1 mg to 5 mg per night (low dosage) for treating insomnia or jet lag [27,54] to 20 mg or more per day (high dosage) for managing malignant tumors [55]. Patients taking a low dosage at night usually have no significant side effects, whereas sleepiness, fatigue, seizures, or mild nausea may occur in patients taking a high dosage [55,56]. If melatonin protects cells against oxidative stress only at pharmacological concentrations (10−6-10−4 M), then a high dosage would be required (e.g., an orally administered dose of 80 mg melatonin raises melatonin serum levels from 10−7 M to 2×10−6 M) [57]. Our study suggests that melatonin effectively protects RPE cells at physiologic levels (10−8-10−10 M), which can be obtained from an orally administered, low dosage of melatonin (1–5 mg per night; e.g., an orally administered dose of 3 mg melatonin has been reported to raise the melatonin serum level to 2×10−8 M) [28]. This suggests that oral administration of 1–3 mg melatonin per night should be sufficient to test the clinical efficacy of melatonin therapy in AMD patients.
Acknowledgments
This work was supported by the Bendheim-Lowenstein Family Foundation and New York Eye and Ear Infirmary Pathology Research Fund, New York, NY.
References
- 1.Rein DB, Wittenborn JS, Zhang X, Honeycutt AA, Lesesne SB, Saaddine J, Vision Health Cost-Effectiveness Study Group Forecasting age-related macular degeneration through the year 2050: the potential impact of new treatments. Arch Ophthalmol. 2009;127:533–40. doi: 10.1001/archophthalmol.2009.58. [DOI] [PubMed] [Google Scholar]
- 2.Hogg R, Chakravarthy U. AMD and micronutrient antioxidants. Curr Eye Res. 2004;29:387–401. doi: 10.1080/02713680490517890. [DOI] [PubMed] [Google Scholar]
- 3.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 4.Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative Damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–21. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 5.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 6.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 7.van Leeuwen R, Boekhoorn S, Vingerling JR, Witteman JC, Klaver CC, Hofman A, de Jong PT. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005;294:3101. doi: 10.1001/jama.294.24.3101. [DOI] [PubMed] [Google Scholar]
- 8.Rosen R, Hu DN, Perez V, Tai K, Yu GP, Chen M, Tone P, McCormick SA, Walsh J. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Mol Vis. 2009;15:1673–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Baba K, Pozdeyev N, Mazzoni F, Contreras-Alcantara S, Liu C, Kasamatsu M. Martinez- Merlos T, Strettoi E, Iuvone PM, Tosini G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci USA. 2009;106:15043–8. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radogna F, Paternoster L, Albertini MC, Cerella C, Accorsi A, Bucchini A, Spadoni G, Diamantini G, Tarzia G, De Nicola M, D'Alessio M, Ghibelli L. Melatonin antagonizes apoptosis via receptor interaction in U937 monocytic cells. J Pineal Res. 2007;43:154–62. doi: 10.1111/j.1600-079X.2007.00455.x. [DOI] [PubMed] [Google Scholar]
- 11.Juknat AA, Méndez Mdel V, Quaglino A, Fameli CI, Mena M, Kotler ML. Melatonin prevents hydrogen peroxide-induced Bax expression in cultured rat astrocytes. J Pineal Res. 2005;38:84–92. doi: 10.1111/j.1600-079X.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin WS, Barrett JC. Melatonin attenuates hydrogen peroxide toxicity in MCF7 cells only at pharmacological concentrations. Biochem Biophys Res Commun. 1998;250:602–5. doi: 10.1006/bbrc.1998.9370. [DOI] [PubMed] [Google Scholar]
- 13.Lezoualc'h F, Sparapani M, Behl C. N-acetyl-serotonin (normelatonin) and melatonin protect neurons against oxidative challenges and suppress the activity of the transcription factor NF-kappaB. J Pineal Res. 1998;24:168–78. doi: 10.1111/j.1600-079x.1998.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoo YM, Jeung EB. Melatonin-induced calbindin-D9k expression reduces hydrogen peroxide-mediated cell death in rat pituitary GH3 cells. J Pineal Res. 2010;48:83–93. doi: 10.1111/j.1600-079X.2009.00730.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoo YM, Jeung EB. Melatonin-induced estrogen receptor alpha-mediated calbindin-D9k expression plays a role in H2O2-mediated cell death in rat pituitary GH3 cells. J Pineal Res. 2009;47:301–7. doi: 10.1111/j.1600-079X.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- 16.Martín V, Sainz RM, Antolín I, Mayo JC, Herrera F, Rodríguez C. Several antioxidant pathways are involved in astrocyte protection by melatonin. J Pineal Res. 2002;33:204–12. doi: 10.1034/j.1600-079x.2002.02113.x. [DOI] [PubMed] [Google Scholar]
- 17.Chetsawang B, Putthaprasart C, Phansuwan-Pujito P, Govitrapong P. Melatonin protects against hydrogen peroxide-induced cell death signaling in SH-SY5Y cultured cells: involvement of nuclear factor kappa B, Bax and Bcl-2. J Pineal Res. 2006;41:116–23. doi: 10.1111/j.1600-079X.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 18.Das A, Belagodu A, Reiter RJ, Ray SK, Banik NL. Cytoprotective effects of melatonin on C6 astroglial cells exposed to glutamate excitotoxicity and oxidative stress. J Pineal Res. 2008;45:117–24. doi: 10.1111/j.1600-079X.2008.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Espino J, Ortiz A, Bejarano I, Lozano GM, Monllor F, García JF, Rodríguez AB, Pariente JA. Melatonin protects human spermatozoa from apoptosis via melatonin receptor- and extracellular signal-regulated kinase-mediated pathways. Fertil Steril. 2011;95:2290–6. doi: 10.1016/j.fertnstert.2011.03.063. [DOI] [PubMed] [Google Scholar]
- 20.Kimball SR, Abbas A, Jefferson LS. Melatonin represses oxidative stress-induced activation of the MAP kinase and mTOR signaling pathways in H4IIE hepatoma cells through inhibition of Ras. J Pineal Res. 2008;44:379–86. doi: 10.1111/j.1600-079X.2007.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das A, McDowell M, Pava MJ, Smith JA, Reiter RJ, Woodward JJ, Varma AK, Ray SK, Banik NL. The inhibition of apoptosis by melatonin in VSC4.1 motoneurons exposed to oxidative stress, glutamate excitotoxicity, or TNF-alpha toxicity involves membrane melatonin receptors. J Pineal Res. 2010;48:157–69. doi: 10.1111/j.1600-079X.2009.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang FQ, Green L, Wang C, Alssadi R, Godley BF. Melatonin protects human retinal pigment epithelial (RPE) cells against oxidative stress. Exp Eye Res. 2004;78:1069–75. doi: 10.1016/j.exer.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Hu DN, Savage HE, Roberts JE. Uveal melanocytes, ocular pigment epithelium and Mueller cells in culture: in vitro toxicology. Int J Toxicol. 2002;21:465–72. doi: 10.1080/10915810290169891. [DOI] [PubMed] [Google Scholar]
- 24.Wu WC, Hu DN, Gao HX, Chen M, Wang D, Rosen R, McCormick SA. Subtoxic levels hydrogen peroxide-induced production of interleukin-6 by retinal pigment epithelial cells. Mol Vis. 2010;16:1864–73. [PMC free article] [PubMed] [Google Scholar]
- 25.Hu DN, McCormick SA, Ritch R, Pelton-Henrion K. Studies of human uveal melanocytes in vitro: Isolation, purification and cultivation of human uveal melanocytes. Invest Ophthalmol Vis Sci. 1993;34:2210–9. [PubMed] [Google Scholar]
- 26.Allegra M, Reiter RJ, Tan DX, Gentile C, Tesoriere L, Livrea MA. The chemistry of melatonin's interaction with reactive species. J Pineal Res. 2003;34:1–10. doi: 10.1034/j.1600-079x.2003.02112.x. [DOI] [PubMed] [Google Scholar]
- 27.Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R. Melatonin: Nature's most versatile biological signal? FEBS J. 2006;273:2813–38. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 28.Kovács J, Brodner W, Kirchlechner V, Arif T, Waldhauser F. Measurement of urinary melatonin: a useful tool for monitoring serum melatonin after its oral administration. J Clin Endocrinol Metab. 2000;85:666–70. doi: 10.1210/jcem.85.2.6349. [DOI] [PubMed] [Google Scholar]
- 29.Boutin JA, Dietzel M. Daily and annual rhythms in human melatonin secretion: role in puberty control. Ann N Y Acad Sci 1985; 453:205–14.29. Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–9. [Google Scholar]
- 30.Alarma-Estrany P, Pintor J. Melatonin receptors in the eye: location, second messengers and role in ocular physiology. Pharmacol Ther. 2007;113:507–22. doi: 10.1016/j.pharmthera.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Rohrbach JM, Wollmann H, Heinze J, Gupta D, Thanos S. The role of melatonin in growth of malignant choroid melanoma. Ophthalmologe. 1993;90:289–93. [PubMed] [Google Scholar]
- 32.Roberts JE, Hu DN, Wishart JF. Pulse radiolysis studies of melatonin and chloromelatonin. J Photochem Photobiol B. 1998;42:125. doi: 10.1016/s1011-1344(97)00132-2. [DOI] [PubMed] [Google Scholar]
- 33.Roberts JE, Hu DN, Martinez L, Chignell CF. Photophysical studies on melatonin and its receptor agonists. J Pineal Res. 2000;29:94–9. doi: 10.1034/j.1600-079x.2000.290205.x. [DOI] [PubMed] [Google Scholar]
- 34.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. J Biomed Sci. 2000;7:444–58. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 35.Boutin JA, Audinot V, Ferry G, Delagrange P. Molecular tools to study melatonin pathways and actions. Trends Pharmacol Sci. 2005;26:412–9. doi: 10.1016/j.tips.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50:1129–46. [PubMed] [Google Scholar]
- 37.Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S. Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv Med Sci. 2007;52:11–28. [PubMed] [Google Scholar]
- 38.Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Z. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol. 2007;54:1–9. [PubMed] [Google Scholar]
- 39.Martín M, Macías M, Escames G, León J, Acuña-Castroviejo D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J. 2000;14:1677. doi: 10.1096/fj.99-0865fje. [DOI] [PubMed] [Google Scholar]
- 40.Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Jr, Reed RL, Jones DP. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka D, Furusawa K, Kameyama K, Okamoto H, Doi M. Melatonin signaling regulates locomotion behavior and homeostatic states through distinct receptor pathways in Caenorhabditis elegans. Neuropharmacology. 2007;53:157–68. doi: 10.1016/j.neuropharm.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Tam CW, Mo CW, Yao KM, Shiu SY. Signaling mechanisms of melatonin in antiproliferation of hormone-refractory 22Rv1 human prostate cancer cells: implications for prostate cancer chemoprevention. J Pineal Res. 2007;42:191–202. doi: 10.1111/j.1600-079X.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 43.Shiu SY, Pang B, Tam CW, Yao KM. Signal transduction of receptor-mediated antiproliferative action of melatonin on human prostate epithelial cells involves dual activation of Gα(s) and Gα(q) proteins. J Pineal Res. 2010;49:301–11. doi: 10.1111/j.1600-079X.2010.00795.x. [DOI] [PubMed] [Google Scholar]
- 44.Scott FF, Belle MD, Delagrange P, Piggins HD. Electrophysiological effects of melatonin on mouse Per1 and non-Per1 suprachiasmatic nuclei neurones in vitro. J Neuroendocrinol. 2010;22:1148–56. doi: 10.1111/j.1365-2826.2010.02063.x. [DOI] [PubMed] [Google Scholar]
- 45.Sotthibundhu A, Phansuwan-Pujito P, Govitrapong P. Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J Pineal Res. 2010;49:291–300. doi: 10.1111/j.1600-079X.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- 46.Romero A, Egea J, García AG, López MG. Synergistic neuroprotective effect of combined low concentrations of galantamine and melatonin against oxidative stress in SH-SY5Y neuroblastoma cells. J Pineal Res. 2010;49:141–8. doi: 10.1111/j.1600-079X.2010.00778.x. [DOI] [PubMed] [Google Scholar]
- 47.Cabrera J, Negrín G, Estévez F, Loro J, Reiter RJ, Quintana J. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J Pineal Res. 2010;49:45–54. doi: 10.1111/j.1600-079X.2010.00765.x. [DOI] [PubMed] [Google Scholar]
- 48.Zmijewski MA, Sweatman TW, Slominski AT. The melatonin-producing system is fully functional in retinal pigment epithelium (ARPE-19). Mol Cell Endocrinol. 2009;307:211–6. doi: 10.1016/j.mce.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siu AW, Reiter RJ, To CH. The efficacy of vitamin E and melatonin as antioxidants against lipid peroxidation in rat retinal homogenates. J Pineal Res. 1998;24:239–44. doi: 10.1111/j.1600-079x.1998.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 50.Marchiafava PL, Longoni B. Melatonin as an antioxidant in retinal photoreceptors. J Pineal Res. 1999;26:184–9. doi: 10.1111/j.1600-079x.1999.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 51.Guajardo MH, Terrasa AM, Catalá A. Protective effect of indoleamines on in vitro ascorbate- Fe2+ dependent lipid peroxidation of rod outer segment membranes of bovine retina. J Pineal Res. 2003;35:276–82. doi: 10.1034/j.1600-079x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 52.Guajardo MH, Terrasa AM, Catalá A. Lipid-protein modifications during ascorbate-Fe2+ peroxidation peroxidation of photoreceptor membranes: protective effect of melatonin. J Pineal Res. 2006;41:201–10. doi: 10.1111/j.1600-079X.2006.00352.x. [DOI] [PubMed] [Google Scholar]
- 53.Yi C, Pan X, Yan H, Guo M, Pierpaoli W. Effects of melatonin in age-related macular degeneration. Ann N Y Acad Sci. 2005;1057:384–92. doi: 10.1196/annals.1356.029. [DOI] [PubMed] [Google Scholar]
- 54.Arendt J, Van Someren EJ, Appleton R, Skene DJ, Akerstedt T. Clinical update: melatonin and sleep disorders. Clin Med. 2008;8:381–3. doi: 10.7861/clinmedicine.8-4-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mills E, Wu P, Seely D, Guyatt G. Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J Pineal Res. 2005;39:360–6. doi: 10.1111/j.1600-079X.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez R, Sanchez A, Ferguson JA, Balmer C, Daniel C, Cohn A, Robinson WA. Melatonin therapy of advanced human malignant melanoma. Melanoma Res. 1991;1:237–43. doi: 10.1097/00008390-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Waldhauser F, Waldhauser M, Lieberman HR, Deng MH, Lynch HJ, Wurtman RJ. Bioavailability of oral melatonin in humans. Neuroendocrinology. 1984;39:307–13. doi: 10.1159/000123997. [DOI] [PubMed] [Google Scholar]