Abstract
The Terebridae are a diverse family of tropical and subtropical marine gastropods that use a complex and modular venom apparatus to produce toxins that capture polychaete and enteropneust preys. The complexity of the terebrid venom apparatus suggests that venom apparatus development in the Terebridae could be linked to the diversification of the group and can be analyzed within a molecular phylogenetic scaffold to better understand terebrid evolution. Presented here is a molecular phylogeny of 89 terebrid species belonging to 12 of the 15 currently accepted genera, based on Bayesian inference and Maximum Likelihood analyses of amplicons of 3 mitochondrial (COI, 16S and 12S) and one nuclear (28S) genes. The evolution of the anatomy of the terebrid venom apparatus was assessed by mapping traits of six related characters: proboscis, venom gland, odontophore, accessory proboscis structure, radula, and salivary glands. A novel result concerning terebrid phylogeny was the discovery of a previously unrecognized lineage, which includes species of Euterebra and Duplicaria. The non- monophyly of most terebrid genera analyzed indicates that the current genus-level classification of the group is plagued with homoplasy and requires further taxonomic investigations. Foregut anatomy in the family Terebridae reveals an inordinate diversity of features that covers the range of variability within the entire superfamily Conoidea, and that hypodermic radulae have likely evolved independently on at least three occasions. These findings illustrate that terebrid venom apparatus evolution is not perfunctory, and involves independent and numerous changes of central features in the foregut anatomy. The multiple emergence of hypodermic marginal radular teeth in terebrids are presumably associated with variable functionalities, suggesting that terebrids have adapted to dietary changes that may have resulted from predator-prey relationships. The anatomical and phylogenetic results presented serve as a starting point to advance investigations about the role of predator-prey interactions in the diversification of the Terebridae and the impact on their peptide toxins, which are promising bioactive compounds for biomedical research and therapeutic drug development.
Keywords: character evolution, key innovations, predator-prey system, radula, teretoxins, toxins
1. Introduction
At the macroevolutionary level, it is hypothesized that the tempo of evolution can be viewed through the lens of key innovations (Sanderson and Donoghue, 1994). Key innovations are biological traits that promote lineage diversification (Heard and Hauser, 1995; Hodges and Arnold, 1995). The development of a venom apparatus in the marine gastropod superfamily Conoidea is a key innovation that can be used as an organizational framework to decipher the evolutionary history of this megadiverse group. Here the evolution of the venom apparatus in auger snails (Neogastropoda; Conoidea; Terebridae) is investigated using a molecular phylogenetic scaffold.
The Terebridae are a diverse family of medium to large-sized (mostly 15-150 mm) marine gastropods distributed throughout most tropical and subtropical oceans. Terebrids use their venom apparatus to capture prey, and perhaps also to defeat competitors or predators (Olivera, 1997). Similar to the peptide toxins produced by cone snails (Neogastropoda; Conoidea; Conidae), the peptide toxins produced by terebrids, teretoxins, are promising bioactive compounds for biomedical research and therapeutic drug development (Puillandre and Holford, 2010). Peptide toxins from a venom source are of increasing interest in the pharmacological industry (Chin et al., 2006; Newman and Cragg, 2007; Butler, 2008; Casewell et al., 2009; Hong, 2011). As recently demonstrated (Fry et al., 2003; Modica and Holford, 2010; Puillandre et al., 2010; Saslis-Lagoudakis et al., 2011), understanding how the organisms that produce these toxins have emerged and evolved over time, may become central in the process of drug discovery. Specifically, in the case of the Terebridae, not all species have a venom apparatus, therefore identifying the lineages that have a venom apparatus is an effective route to peptide toxin characterization. Currently, the extent of species diversification of the Terebridae is largely underestimated and the evolutionary pathways explored by the terebrid groups, especially regarding the peptide toxins they produce, remains largely unknown.
Whether used for defense or attack, the diversity of toxins developed by venomous organisms is often attributed to the process of co-evolution in predator-prey relationships (Kordis and Gubensek, 2000; Lynch, 2007; Duda, 2008; Kozminsky-Atias et al., 2008; Barlow et al., 2009). Co-evolutionary predator-prey interactions may lead to the development of specialized adaptations in the predator that are followed by counter-adaptations in the prey, which in turn can lead to further adaptations in the predator, and so on, as dictated by biotic, “Red Queen” (Van Valen, 1973) or abiotic, “Court Jester” (Barnosky, 2001) pressures. For example, numerous plants produce toxic secondary compounds that influence the behavior, growth, or survival of insects and other herbivores. In addition, herbivores have developed ways to detoxify, sequester, or render ineffective specific plant poisons (Laycock, 1978; Fowler, 1983; Zangerl et al., 2008). In snakes, it has been demonstrated that venom diversity may result by adaptation toward specific diets (Daltry et al., 1996; Wüster et al., 1999; Barlow et al., 2009). In parallel, some snake prey have developed the ability to inhibit specific venom toxins (Heatwole and Poran, 1995; Biardi et al., 2005). By its indirect effect on fitness, the predator-prey arms race can represent a driving force of speciation and species diversification in both predators and preys populations. This is referred to as the “escalation/diversification hypothesis” (Ehrlich and Raven, 1964; but see also Berenbaum and Feeny, 1981; Berenbaum, 1983; Vermeij, 1993). Phylogenetic analyses can provide seminal evidence on rates and patterns of predation-traits evolution and species diversification (Farrell et al., 1991). However, the correlation between adaptative changes of predation- traits and species-diversification in predator-prey systems is difficult to study. Such a study requires a good understanding of the biology and the ecology of the species involved and necessitates a thorough taxonomic sampling of both predator and prey taxa. A good alternative, as attempted here with the Terebridae, is to obtain an exhaustive taxonomic sampling of one of the two taxa (predator or prey) and to study the traits or innovations that affect the ability to accomplish or avoid predation. Mapping these innovations on a phylogenetic tree then reveals patterns that may impact species diversification.
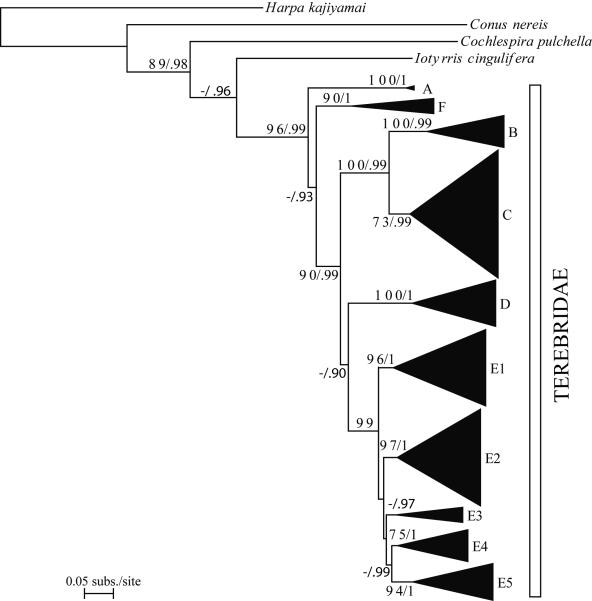
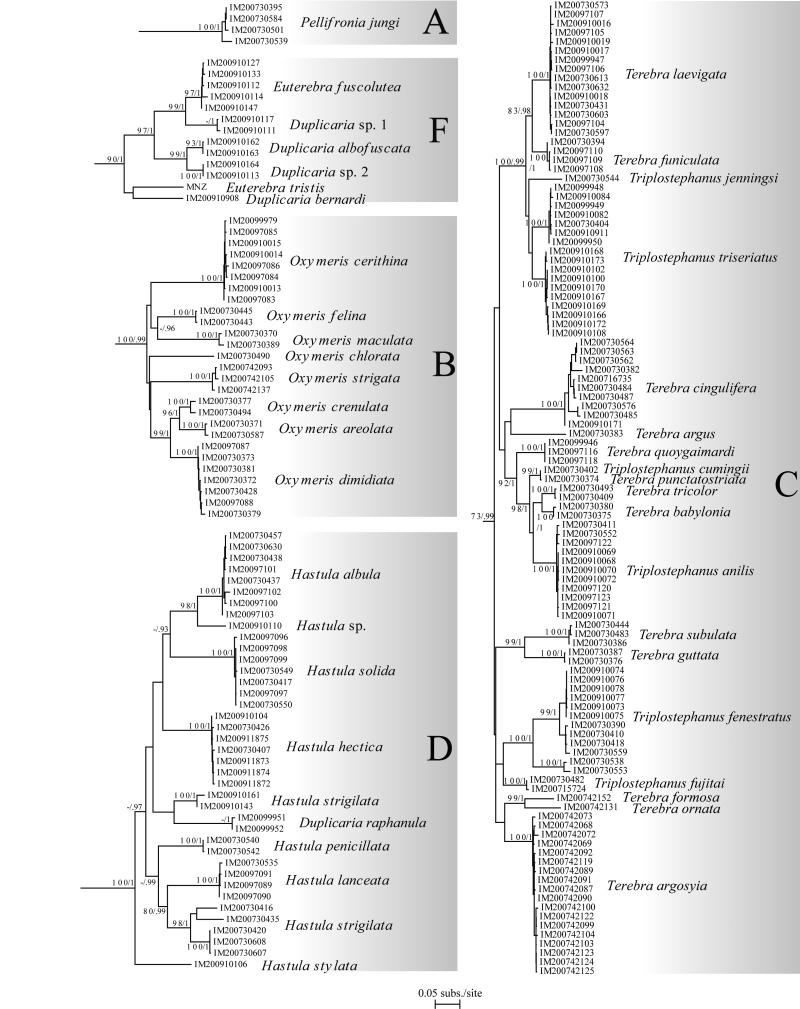
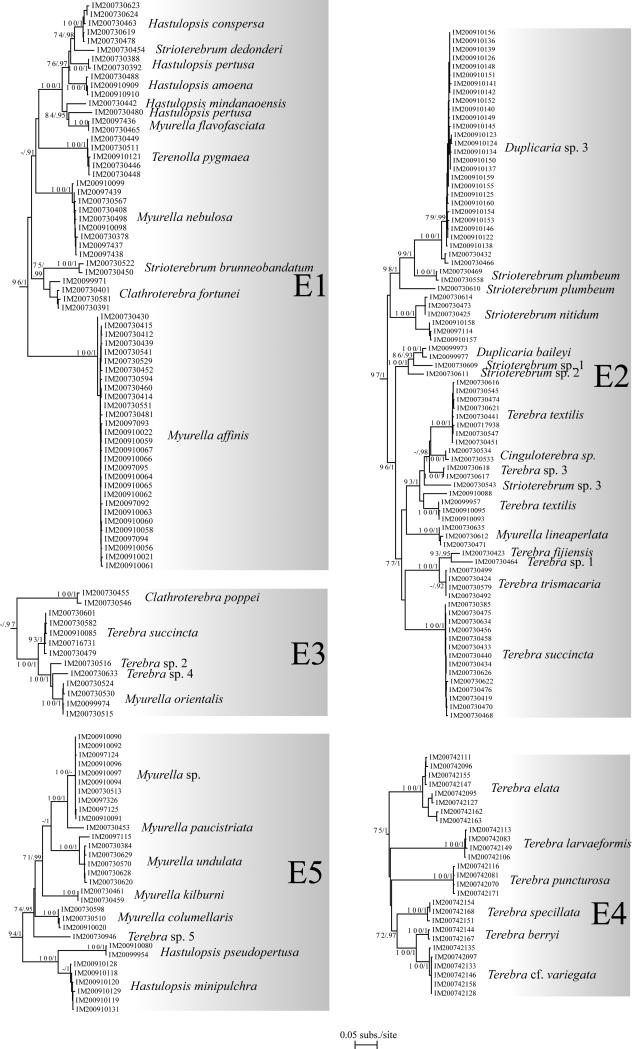
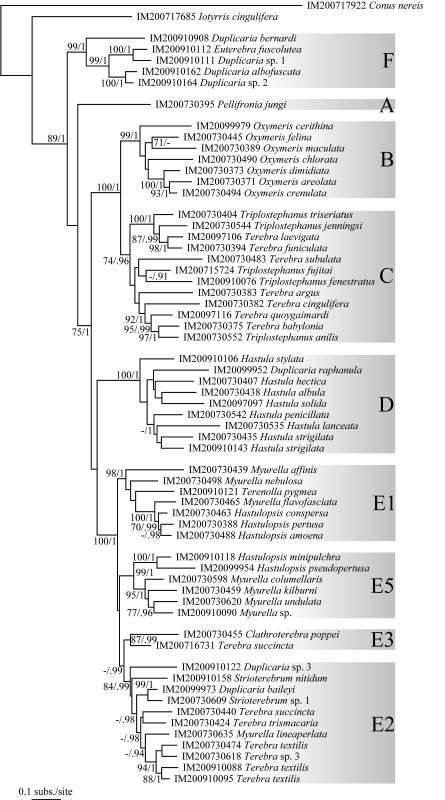
Understanding the evolutionary patterns of venom apparatus evolution in the Terebridae would significantly advance clarifying the phylogeny and systematics of the group, in addition to advancing the characterization of terebrid peptide toxins for biomedical applications. Recent molecular phylogenies (Holford et al., 2009a, 2009b; Puillandre et al., 2011) of the family Terebridae based on samples from Western and Eastern Pacific demonstrated the monophyly of terebrids relative to the other families of conoideans. Also illustrated in these phylogenetic studies is the existence of five distinctive clades, Pellifronia, Oxymeris [= Acus], Terebra, Hastula, and Myurella, numbered clades A to E, respectively, with clade A, containing the recently revised Pellifronia jungi (Terryn and Holford, 2008), as sister species of all the other terebrids. Previous molecular analyses combined with mapping of venom apparatus morphology also indicated that the Terebridae have lost the venom apparatus at least twice during their evolution (in clades B and E). However, these phylogenies were based on a limited number of species (~ 50 for the most complete, vs the ~ 400 currently described species), and sampling was limited to the Pacific Ocean. Additionally, only the presence and absence of the venom glands were studied, overlooking other morphological and anatomical innovations potentially linked to the evolution of terebrid predatory skills and toxin diversity. In contrast, the present expanded study of the molecular phylogeny of the family Terebridae almost doubles the number of species from 50 to 89, including 12 out of the 15 accepted genera, almost triples the number of specimens, and increases the geographical area sampled by including the western Indian Ocean. The molecular phylogeny in this study is based on the three mitochondrial genes, COI, 12S, 16S, previously used in conoidean phylogenies, with the addition of one nuclear gene, 28S, shown to be useful in resolving relationships at the genus level in Conoidea and other gastropods (Williams and Ozawa, 2006; Puillandre et al., 2008). The analysis of the venom apparatus, previously reduced to the presence or absence of the venom gland, and thus underestimating the diversity of the evolutionary pathways the terebrids may have explored, is here extended to other anatomical features linked to the venom apparatus. The morphology of the radula, in particular, has been linked to prey capture, and consequently different radula types may correlate to innovations in predatory behavior, including venom evolution.
2. Material and methods
2.1. Taxon sampling
All the material studied herein was collected during several expeditions conducted by the Museum National d’Histoire Naturelle of Paris (MNHN), in partnership with Pro-Natura International (PNI), Instituto Español de Oceanografia (IOE), and Institut de Recherche pour le Développement (IRD), the Natural History Museum of London (NHM), and the Smithonian Tropical Research Institute (STRI) (See Table 1 and acknowledgements). Samples include 406 specimens assigned to 89 species collected off New Caledonia (4 specimens), Philippine Islands (49), Vanuatu (115), Solomon Islands (12), Australia (4), the Coral Sea (4), Panama (50), Madagascar (87), Mozambique (75), Tahiti (4), New- Zealand (1) and Fiji (1) (Fig. 1). These samples originate from depths ranging from 0 m to ~ 800 m (Table 1). In the field, all specimens were specifically fixed for molecular analysis. Living specimens were anesthetized using magnesium chloride (MgCl2), a piece of tissue was cut from the head-foot, and fixed in 95% ethanol. Shells were kept intact for identification. Vouchers are deposited in MNHN. Taxonomy follows Terryn (2007), with updates in Terryn (2011) (Cinguloterebra synonymized with Triplostephanus, Impages with Hastula, and Acus and Perirhoe with Oxymeris). Three specimens of the family Turridae (putative sister-group of the Terebridae – Puillandre et al., 2011), Cochlespiridae (Conoidea) and Conidae (Conoidea) were used as closely related outgroups. Harpa kajiyamai, belonging to another neogastropod family (Harpidae), was used as a distant outgroup to root the tree.
Table 1.
List of specimens analysed
| MNHN Ids | Genus | species | Country | Coordinates; depth (m) | Clade | BOLD ID | Genbank COI | GenBank 12S | GenBank 16S | GenBank 28S |
|---|---|---|---|---|---|---|---|---|---|---|
| IM_2007_30391 | Clathroterebra | fortunei | Solomon Islands | 7°59′ S, 157°33′ E; 260 | E1 | CONO381-08 | EU685535 | EU685384 | EU685675 | |
| IM_2007_30401 | Clathroterebra | fortunei | Philippines | 9°39′N, 123°48′E; 255-268 | E1 | CONO284-08 | EU685526 | EU685371 | EU685663 | |
| IM_2007_30581 | Clathroterebra | fortunei | Philippines | 9°39′N, 123°48′E; 255-268 | E1 | XXX | XXX | XXX | ||
| IM_2009_9971 | Clathroterebra | fortunei | Mozambique | 25°33′S, 33°13′E; 253-262 | E1 | XXX | XXX | XXX | XXX | |
| IM_2007_30455 | Clathroterebra | poppei | Philippines | 9°36.4′N, 123°53.8′E; 60-62 | E3 | CONO266-08 | EU685523 | EU685368 | EU685660 | XXX |
| IM_2007_30546 | Clathroterebra | poppei | Vanuatu | 15°36′S, 167°03′E; 86-118 | E3 | CONO482-08 | EU685596 | EU685455 | EU685748 | |
| IM_2009_10162 | Duplicaria | albofuscata | South Madagascar | 25°03.7-8′S, 46°57.7′E; 3-4 | F | XXX | XXX | XXX | XXX | |
| IM_2009_10163 | Duplicaria | albofuscata | South Madagascar | 25°03.7-8′S, 46°57.7′E; 3-4 | F | XXX | XXX | XXX | ||
| IM_2009_9973 | Duplicaria | baileyi | South New-Caledonia | 22°06′S, 167°03′E; 190-200 | E2 | XXX | XXX | XXX | XXX | XXX |
| IM_2009_9977 | Duplicaria | baileyi | South New-Caledonia | 22°06′S, 167°03′E; 190-200 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10908 | Duplicaria | bernardi | Australia | 26°56′607"S, 153°23′813"E; 40 | F | XXX | XXX | XXX | XXX | |
| IM_2009_9951 | Duplicaria | raphanula | North Madagascar | 14°31′S, 47°25′E; 50-107 | D | XXX | XXX | XXX | ||
| IM_2009_9952 | Duplicaria | raphanula | North Madagascar | 14°31′S, 47°25′E; 50-107 | D | XXX | XXX | XXX | XXX | XXX |
| IM_2009_10111 | Duplicaria | sp. 1 | South Madagascar | 25°04.4-7′S, 46°55.3-56.3′E; 19- 26 |
F | XXX | XXX | XXX | XXX | |
| IM_2009_10117 | Duplicaria | sp. 1 | South Madagascar | 25°04.4-7′S, 46°55.3-56.3′E; 19- 26 |
F | XXX | XXX | XXX | ||
| IM_2009_10113 | Duplicaria | sp. 2 | South Madagascar | 25°03.7-8′S, 46°57.6-7′E; 2-7 | F | XXX | XXX | XXX | ||
| IM_2009_10164 | Duplicaria | sp. 2 | South Madagascar | 25°03.7-8′S, 46°57.6-7′E; 2-7 | F | XXX | XXX | XXX | XXX | |
| IM_2007_30432 | Duplicaria | sp. 3 | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | E2 | XXX | XXX | XXX | XXX | |
| IM_2007_30466 | Duplicaria | sp. 3 | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | E2 | XXX | XXX | XXX | ||
| IM_2009_10122 | Duplicaria | sp. 3 | South Madagascar | 25°26.1-4′S, 44°55.2-6′E; 17-20 | E2 | XXX | XXX | XXX | XXX | XXX |
| IM_2009_10123 | Duplicaria | sp. 3 | South Madagascar | 25°25.80-8′S, 44°55.7-8′E; 11-13 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10124 | Duplicaria | sp. 3 | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10125 | Duplicaria | sp. 3 | South Madagascar | 25°26.1-4′S, 44°55.2-6′E; 17-20 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10126 | Duplicaria | sp. 3 | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10134 | Duplicaria | sp. 3 | South Madagascar | 25°25.9′S, 44°55.1-2′E; 18-20 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10136 | Duplicaria | sp. 3 | South Madagascar | 25°26.1-4′S, 44°55.2-6′E; 17-20 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10137 | Duplicaria | sp. 3 | South Madagascar | 25°25.80-8′S, 44°55.7-8′E; 11-13 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10138 | Duplicaria | sp. 3 | South Madagascar | 25°23.1-2′S, 44°51.4-6′E; 20-23 | E2 | XXX | XXX | XXX | XXXe | |
| IM_2009_10139 | Duplicaria | sp. 3 | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10140 | Duplicaria | sp. 3 | South Madagascar | 25°23.6-7′S, 44°53.3-5′E; 10-12 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10141 | Duplicaria | sp. 3 | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10142 | Duplicaria | sp. 3 | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10145 | Duplicaria | sp. 3 | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10146 | Duplicaria | sp. 3 | South Madagascar | 25°26.1-4′S, 44°55.2-6′E; 17-20 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10148 | Duplicaria | sp. 3 | South Madagascar | 25°26.7′S, 44°55.8′E; 15 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10149 | Duplicaria | sp. 3 | South Madagascar | 25°26.7′S, 44°55.8′E; 15 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10150 | Duplicaria | sp. 3 | South Madagascar | 25°26.7′S, 44°55.8′E; 15 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10151 | Duplicaria | sp. 3 | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10152 | Duplicaria | sp. 3 | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10153 | Duplicaria | sp. 3 | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10154 | Duplicaria | sp. 3 | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10155 | Duplicaria | sp. 3 | South Madagascar | 25°25.80-8′S, 44°55.7-8′E; 11-13 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10156 | Duplicaria | sp. 3 | South Madagascar | 25°25.80-8′S, 44°55.7-8′E; 11-13 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10159 | Duplicaria | sp. 3 | South Madagascar | 25°25.80-8′S, 44°55.7-8′E; 11-13 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10160 | Duplicaria | sp. 3 | South Madagascar | 25°26.1-4′S, 44°55.2-6′E; 17-20 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10112 | Euterebra | fuscolutea | South Madagascar | 25°04.4-7′S, 46°55.3-56.3′E; 19- 26 |
F | XXX | XXX | XXX | XXX | |
| IM_2009_10114 | Euterebra | fuscolutea | South Madagascar | 25°03.7′S, 46°57.8′E; 7 | F | XXX | XXX | XXX | ||
| IM_2009_10127 | Euterebra | fuscolutea | South Madagascar | 25°26.0-1′S, 44°54.2-9′E; 21-24 | F | XXX | XXX | XXX | ||
| IM_2009_10133 | Euterebra | fuscolutea | South Madagascar | 25°26.8′S, 44°54.9′E; 27 | F | XXX | XXX | XXX | ||
| IM_2009_10147 | Euterebra | fuscolutea | South Madagascar | 25°25.9′S, 44°55.1-2′E; 18-20 | F | XXX | XXX | XXX | ||
| Museum of New Zealand |
Euterebra | tristis | New-Zealand | 35°13.20′S, 174°14,30′E, 2-8 | F | HQ401611 | HQ401677 | |||
| IM_2007_30437 | Hastula | albula | Vanuatu | 15°26.6′S, 167°15.2′E; | D | CONO477-08 | EU685592 | EU685743 | ||
| IM_2007_30438 | Hastula | albula | Vanuatu | 15°26.6′S, 167°15.2′E; | D | CONO478-08 | EU685593 | EU685451 | EU685744 | XXX |
| IM_2007_30457 | Hastula | albula | Vanuatu | 15°22.6′S, 167°11.6′E; | D | CONO501-08 | EU685612 | EU685471 | EU685764 | |
| IM_2007_30630 | Hastula | albula | Vanuatu | 15°35.7′S, 166°59.3′E; 12 | D | CONO511-08 | EU685620 | EU685480 | EU685773 | |
| IM_2009_7100 | Hastula | albula | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | XXX | |
| IM_2009_7101 | Hastula | albula | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | XXX | |
| IM_2009_7102 | Hastula | albula | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | ||
| IM_2009_7103 | Hastula | albula | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | ||
| IM_2007_30407 | Hastula | hectica | Philippines | 07°38,5′N, 008°25,1′W; 883 | D | CONO260-08 | EU685518 | EU685363 | EU685655 | XXX |
| IM_2007_30426 | Hastula | hectica | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | D | CONO498-08 | EU685610 | EU685469 | EU685762 | |
| IM_2009_10104 | Hastula | hectica | South Madagascar | 25°08.9′S, 46°45.4′E; 0-1 | D | XXX | XXX | XXX | XXX | |
| IM_2009_11872 | Hastula | hectica | Tahiti | 17°30′28.28"S, 149°27′0.14"W; 0 | D | XXX | XXX | XXX | ||
| IM_2009_11873 | Hastula | hectica | Tahiti | 17°30′28.28"S, 149°27′0.14"W; 0 | D | XXX | XXX | XXX | ||
| IM_2009_11874 | Hastula | hectica | Tahiti | 17°30′28.28"S, 149°27′0.14"W; 0 | D | XXX | XXX | XXX | ||
| IM_2009_11875 | Hastula | hectica | Tahiti | 17°30′28.28"S, 149°27′0.14"W; 0 | D | XXX | XXX | XXX | ||
| IM_2007_30535 | Hastula | lanceata | Philippines | 9°33.0′N, 123°46.5′E; 8-14 | D | CONO203-08 | EU685495 | EU685631 | XXX | |
| IM_2009_7089 | Hastula | lanceata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | XXX | |
| IM_2009_7090 | Hastula | lanceata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | XXX | |
| IM_2009_7091 | Hastula | lanceata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | XXX | |
| IM_2007_30540 | Hastula | penicillata | Vanuatu | 15°22.6′S, 167°11.6′E; | D | CONO503-08 | EU685614 | EU685473 | EU685766 | |
| IM_2007_30542 | Hastula | penicillata | Vanuatu | 15°22.6′S, 167°11.6′E; | D | CONO502-08 | EU685613 | EU685472 | EU685765 | XXX |
| IM_2007_30417 | Hastula | solida | Vanuatu | 15°26.6′S, 167°15.2′E; | D | XXX | XXX | EU685450 | EU685742 | |
| IM_2007_30549 | Hastula | solida | Vanuatu | 15°26.6′S, 167°15.2′E; | D | XXX | EU685449 | EU685741 | ||
| IM_2007_30550 | Hastula | solida | Vanuatu | 15°26.6′S, 167°15.2′E; | D | CONO476-08 | EU685591 | EU685448 | EU685740 | |
| IM_2009_7096 | Hastula | solida | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | XXX | |
| IM_2009_7097 | Hastula | solida | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | XXX | XXX |
| IM_2009_7098 | Hastula | solida | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | ||
| IM_2009_7099 | Hastula | solida | Mozambique | 25°59.0′S, 32°54.5′E; 0 | D | XXX | XXX | XXX | ||
| IM_2009_10110 | Hastula | sp. | South Madagascar | 25°03.7′S, 46°57.8′E; 7 | D | XXX | XXX | XXX | ||
| IM_2007_30416 | Hastula | strigilata | Vanuatu | 15°35.2′S, 167°59.4′E; | D | XXX | XXX | EU685435 | EU685727 | |
| IM_2007_30420 | Hastula | strigilata | Vanuatu | 15°35.2′S, 167°59.4′E; | D | CONO466-08 | EU685581 | EU685434 | EU685726 | |
| IM_2007_30435 | Hastula | strigilata | Vanuatu | 15°33.4′S, 167°12.4′E; 2-6 | D | XXX | XXX | XXX | XXX | XXX |
| IM_2007_30607 | Hastula | strigilata | Vanuatu | 15°35.2′S, 167°59.4′E; | D | XXX | XXX | EU685433 | EU685725 | |
| IM_2007_30608 | Hastula | strigilata | Vanuatu | 15°35.2′S, 167°59.4′E; | D | CONO465-08 | EU685580 | EU685724 | ||
| IM_2009_10143 | Hastula | strigilata | South Madagascar | 25°23.6-7′S, 44°53.3-5′E; 10-12 | D | XXX | XXX | XXX | XXX | |
| IM_2009_10161 | Hastula | strigilata | South Madagascar | 25°08.9′S, 46°45.4′E; 0-1 | D | XXX | XXX | XXX | ||
| IM_2009_10106 | Hastula | stylata | South Madagascar | 24°47.1′S, 47°11.9′E; 0-1 | D | XXX | XXX | XXX | XXX | XXX |
| IM_2007_30488 | Hastulopsis | amoena | Vanuatu | 15°31.7′S, 167°09.4′E; 9-13 | E1 | XXX | XXX | XXX | XXX | XXX |
| IM_2009_10909 | Hastulopsis | amoena | Australia | 26°56′607"S, 153°23′813"E; 40 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10910 | Hastulopsis | amoena | Australia | 26°56′607"S, 153°23′813"E; 40 | E1 | XXX | XXX | XXX | ||
| IM_2007_30463 | Hastulopsis | conspersa | Vanuatu | E1 | CONO445-08 | EU685560 | EU685411 | EU685702 | XXX | |
| IM_2007_30478 | Hastulopsis | conspersa | Vanuatu | E1 | CONO446-08 | EU685561 | EU685412 | EU685703 | ||
| IM_2007_30619 | Hastulopsis | conspersa | Vanuatu | 15°33.1′S, 167°12.2′E; 3-40 | E1 | CONO437-08 | EU685552 | EU685403 | EU685694 | |
| IM_2007_30623 | Hastulopsis | conspersa | Vanuatu | E1 | CONO443-08 | EU685558 | EU685409 | EU685700 | ||
| IM_2007_30624 | Hastulopsis | conspersa | Vanuatu | 15°33.4′S, 167°12.4′E; 2-6 | E1 | CONO518-08 | EU685623 | EU685483 | EU685776 | |
| IM_2007_30442 | Hastulopsis | mindanaoensis | Philippines | E1 | CONO207-08 | EU685499 | EU685344 | EU685635 | ||
| IM_2009_10118 | Hastulopsis | minipulchra | South Madagascar | 25°30.2′S, 45°46.3′E; 41-42 | E5 | XXX | XXX | XXX | XXX | XXX |
| IM_2009_10119 | Hastulopsis | minipulchra | South Madagascar | 25°28.6′S, 44°56.8′E; 12 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_10120 | Hastulopsis | minipulchra | South Madagascar | 25°22.8-23.7′S, 44°51.1′E; 18-21 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_10128 | Hastulopsis | minipulchra | South Madagascar | 25°22.8-23.7′S, 44°51.1′E; 18-21 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_10129 | Hastulopsis | minipulchra | South Madagascar | 25°28.6′S, 44°56.8′E; 12 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_10131 | Hastulopsis | minipulchra | South Madagascar | 25°22.8-23.7′S, 44°51.1′E; 18-21 | E5 | XXX | XXX | XXX | XXX | |
| IM_2007_30388 | Hastulopsis | pertusa | Vanuatu | E1 | CONO447-08 | EU685562 | EU685413 | EU685704 | XXX | |
| IM_2007_30392 | Hastulopsis | pertusa | Vanuatu | E1 | CONO448-08 | EU685563 | EU685414 | EU685705 | ||
| IM_2007_30480 | Hastulopsis | pertusa | Vanuatu | E1 | CONO444-08 | EU685559 | EU685410 | EU685701 | ||
| IM_2009_10080 | Hastulopsis | pseudopertusa | North Madagascar | 13°25′S, 47°57′E; 71-158 | E5 | XXX | XXX | XXX | ||
| IM_2009_9954 | Hastulopsis | pseudopertusa | North Madagascar | 13°25′S, 47°57′E; 71-158 | E5 | XXX | XXX | XXX | XXX | XXX |
| IM_2007_30412 | Myurella | affinis | Vanuatu | 15°26.6′S, 167°15.2′E; | E1 | CONO468-08 | EU685583 | EU685437 | EU685729 | |
| IM_2007_30414 | Myurella | affinis | Vanuatu | 15°26.6′S, 167°15.2′E; | E1 | CONO467-08 | EU685582 | EU685436 | EU685728 | |
| IM_2007_30415 | Myurella | affinis | Vanuatu | 9°32.8′N, 123°45.9′E; 2 | E1 | XXX | XXX | XXX | XXX | |
| IM_2007_30430 | Myurella | affinis | Philippines | 9°37.4′N, 123°54.5′E; 6-8 | E1 | CONO214-08 | EU685506 | EU685351 | EU685642 | |
| IM_2007_30439 | Myurella | affinis | Philippines | 08°36.7′N, 079°00′W; 28 | E1 | CONO218-08 | EU685508 | EU685353 | EU685644 | XXX |
| IM_2007_30452 | Myurella | affinis | Philippines | 9°37.4′N, 123°54.5′E; 6-8 | E1 | CONO215-08 | EU685507 | EU685352 | EU685643 | |
| IM_2007_30460 | Myurella | affinis | Philippines | 9°35.7′N, 123°44.4′E; 0-2 | E1 | CONO239-08 | EU685512 | EU685356 | EU685648 | |
| IM_2007_30481 | Myurella | affinis | Philippines | 9°35.7′N, 123°44.4′E; 0-2 | E1 | CONO283-08 | EU685525 | EU685370 | EU685662 | |
| IM_2007_30529 | Myurella | affinis | Philippines | 08°36.7′N, 079°00′W; 28 | E1 | XXX | XXX | XXX | ||
| IM_2007_30541 | Myurella | affinis | Vanuatu | 15°36.8′S, 167°08.5′E; 1-42 | E1 | CONO485-08 | EU685599 | EU685458 | EU685751 | |
| IM_2007_30551 | Myurella | affinis | Vanuatu | 9°32.8′N, 123°45.9′E; 2 | E1 | XXX | XXX | XXX | XXX | |
| IM_2007_30594 | Myurella | affinis | Vanuatu | 9°32.8′N, 123°45.9′E; 2 | E1 | CONO475-08 | EU685590 | EU685447 | EU685739 | |
| IM_2009_10021 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10022 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10056 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10058 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10059 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10060 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10061 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10062 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10063 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10064 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10065 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10066 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | ||
| IM_2009_10067 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_7092 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_7093 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_7094 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_7095 | Myurella | affinis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | XXX | |
| IM_2007_30510 | Myurella | columellaris | Philippines | 9°35.7′N, 123°44.4′E; 0-2 | E5 | CONO237-08 | EU685510 | EU685646 | ||
| IM_2007_30598 | Myurella | columellaris | Vanuatu | 15°26.6′S, 167°15.2′E; | E5 | CONO469-08 | EU685584 | EU685438 | EU685730 | XXX |
| IM_2009_10020 | Myurella | columellaris | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E5 | XXX | XXX | XXX | XXX | |
| IM_2007_30465 | Myurella | flavofasciata | Philippines | 9°29.4′N, 123°56.0′E; 15-20 | E1 | CONO247-08 | EU685515 | EU685360 | EU685652 | XXX |
| IM_2009_7436 | Myurella | flavofasciata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E1 | XXX | XXX | XXX | ||
| IM_2007_30459 | Myurella | kilburni | Philippines | 9°35.7′N, 123°44.4′E; 0-2 | E5 | CONO238-08 | EU685511 | EU685355 | EU685647 | XXX |
| IM_2007_30461 | Myurella | kilburni | Vanuatu | 15°42.7′S, 167°15.1′E; 2-3 | E5 | CONO491-08 | EU685604 | EU685463 | EU685756 | |
| IM_2007_30471 | Myurella | lineaperlata | Vanuatu | 15°29′S, 167°14.9′E; 2-4 | E2 | CONO461-08 | EU685576 | EU685429 | EU685720 | |
| IM_2007_30612 | Myurella | lineaperlata | Vanuatu | 15°29′S, 167°14.9′E; 2-4 | E2 | CONO460-08 | EU685575 | EU685428 | EU685719 | |
| IM_2007_30635 | Myurella | lineaperlata | Vanuatu | 15°33.4′S, 167°12.4′E; 2-6 | E2 | CONO519-08 | EU685624 | EU685484 | EU685777 | XXX |
| IM_2007_30378 | Myurella | nebulosa | Vanuatu | 15°33.1′S, 167°12.2′E; 3-40 | E1 | CONO407-08 | EU685392 | EU685683 | ||
| IM_2007_30408 | Myurella | nebulosa | Philippines | 9°29.4′N, 123°56.0′E; 15-20 | E1 | CONO248-08 | EU685516 | EU685361 | EU685653 | |
| IM_2007_30498 | Myurella | nebulosa | Vanuatu | 15°27.6′S, 167°14.3′E; 6-35 | E1 | CONO479-08 | EU685594 | EU685453 | EU685746 | XXX |
| IM_2007_30567 | Myurella | nebulosa | Vanuatu | 15°34.7′S, 167°13.8′E; 14-25 | E1 | CONO459-08 | EU685574 | EU685426 | EU685717 | |
| IM_2009_10098 | Myurella | nebulosa | Mozambique | 26°12′S, 35°03′E; 87-90 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_10099 | Myurella | nebulosa | Mozambique | 26°12′S, 35°03′E; 87-90 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_7437 | Myurella | nebulosa | Mozambique | 26°12′S, 35°03′E; 87-90 | E1 | XXX | XXX | XXX | ||
| IM_2009_7438 | Myurella | nebulosa | Mozambique | 26°12′S, 35°03′E; 87-90 | E1 | XXX | XXX | XXX | XXX | |
| IM_2009_7439 | Myurella | nebulosa | Mozambique | 26°12′S, 35°03′E; 87-90 | E1 | XXX | XXX | XXX | XXX | |
| IM_2007_30515 | Myurella | orientalis | Chesterfield Islands | 20°06′S, 160°23′E; 280-304 | E3 | CONO202-08 | EU685494 | EU685340 | EU685630 | |
| IM_2007_30524 | Myurella | orientalis | Solomon Islands | 9°07′ S, 158°21′ E; 267-329 | E3 | XXX | XXX | XXX | XXX | |
| IM_2007_30530 | Myurella | orientalis | Chesterfield Islands | 20°29′S, 158°42′E; 197-230 | E3 | CONO201-08 | EU685493 | EU685339 | EU685629 | |
| IM_2009_9974 | Myurella | orientalis | North New-Caledonia | 18°02′S, 163°04′E; 320-337 | E3 | XXX | XXX | XXX | XXX | |
| IM_2007_30453 | Myurella | paucistriata | Vanuatu | 15°29.6′S, 167°14.9′E; 2-5 | E5 | CONO480-08 | EU685595 | EU685454 | EU685747 | |
| IM_2007_30513 | Myurella | sp. | Philippines | 9°36.4′N, 123°53.8′E; 60-62 | E5 | CONO265-08 | EU685522 | EU685367 | EU685659 | |
| IM_2009_10090 | Myurella | sp. | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | E5 | XXX | XXX | XXX | XXX | XXX |
| IM_2009_10091 | Myurella | sp. | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_10092 | Myurella | sp. | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_10094 | Myurella | sp. | North Madagascar | 15° 30,15′ S, 46° 4,3′ E; 29-36 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_10096 | Myurella | sp. | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_10097 | Myurella | sp. | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_7124 | Myurella | sp. | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_7125 | Myurella | sp. | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E5 | XXX | XXX | XXX | XXX | |
| IM_2009_7326 | Myurella | sp. | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E5 | XXX | XXX | XXX | ||
| IM_2007_30384 | Myurella | undulata | Vanuatu | 15°26.6′S, 167°15.2′E; | E5 | CONO472-08 | EU685587 | EU685441 | EU685733 | |
| IM_2007_30570 | Myurella | undulata | Vanuatu | 15°38.1′S, 167°05.9′E; | E5 | CONO494-08 | EU685606 | EU685465 | EU685758 | |
| IM_2007_30620 | Myurella | undulata | Vanuatu | 15°31.3′S, 167°10.4′E; 3-18 | E5 | CONO440-08 | EU685555 | EU685406 | EU685697 | XXX |
| IM_2007_30628 | Myurella | undulata | Vanuatu | 15°33.1′S, 167°12.2′E; 3-40 | E5 | CONO409-08 | EU685543 | EU685394 | EU685685 | |
| IM_2007_30629 | Myurella | undulata | Vanuatu | 15°33.1′S, 167°12.2′E; 3-40 | E5 | CONO408-08 | EU685542 | EU685393 | EU685684 | |
| IM_2009_7115 | Myurella | undulata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E5 | XXX | XXX | XXX | XXX | |
| IM_2007_30371 | Oxymeris | areolata | Vanuatu | 15°28.7′S, 167°15.2′E; 19 | B | CONO406-08 | JN589001 | HQ401637 | HQ401700 | XXX |
| IM_2007_30587 | Oxymeris | areolata | Philippines | 9°37.4′N, 123°46.9′E; 3-20 | B | CONO241-08 | EU685513 | EU685357 | EU685649 | |
| IM_2009_10013 | Oxymeris | cerithina | Mozambique | 25°59.0′S, 32°54.5′E; 0 | B | XXX | XXX | XXX | XXX | |
| IM_2009_10014 | Oxymeris | cerithina | Mozambique | 25°59.0′S, 32°54.5′E; 0 | B | XXX | XXX | XXX | XXX | |
| IM_2009_10015 | Oxymeris | cerithina | Mozambique | 25°59.0′S, 32°54.5′E; 0 | B | XXX | XXX | XXX | XXX | |
| IM_2009_7083 | Oxymeris | cerithina | Mozambique | 25°59.0′S, 32°54.5′E; 0 | B | XXX | XXX | XXX | XXX | |
| IM_2009_7084 | Oxymeris | cerithina | Mozambique | 25°59.0′S, 32°54.5′E; 0 | B | XXX | XXX | XXX | XXX | |
| IM_2009_7085 | Oxymeris | cerithina | Mozambique | 25°59.0′S, 32°54.5′E; 0 | B | XXX | XXX | XXX | ||
| IM_2009_7086 | Oxymeris | cerithina | Mozambique | 25°59.0′S, 32°54.5′E; 0 | B | XXX | XXX | XXX | XXX | |
| IM_2009_9979 | Oxymeris | cerithina | Mozambique | 25°59.0′S, 32°54.5′E; 0 | B | XXX | XXX | XXX | XXX | XXX |
| IM_2007_30490 | Oxymeris | chlorata | Vanuatu | 15°22.6′S, 167°11.6′E; | B | CONO504-08 | EU685615 | EU685474 | EU685767 | XXX |
| IM_2007_30377 | Oxymeris | crenulata | Vanuatu | 15°34.4′S, 167°13.1′E; 9 | B | CONO442-08 | EU685557 | EU685408 | EU685699 | |
| IM_2007_30494 | Oxymeris | crenulata | Vanuatu | 15°34.4′S, 167°13.1′E; 9 | B | CONO441-08 | EU685556 | EU685407 | EU685698 | XXX |
| IM_2007_30372 | Oxymeris | dimidiata | Vanuatu | 15°32.5′S, 167°10.5′E; 5-10 | B | CONO487-08 | EU685601 | EU685460 | EU685753 | |
| IM_2007_30373 | Oxymeris | dimidiata | Vanuatu | B | CONO449-08 | EU685564 | EU685415 | EU685706 | XXX | |
| IM_2007_30379 | Oxymeris | dimidiata | Vanuatu | 15°32.5′S, 167°10.5′E; 5-10 | B | CONO486-08 | EU685600 | EU685459 | EU685752 | |
| IM_2007_30381 | Oxymeris | dimidiata | Vanuatu | 15°35.4′S, 166°59.7′E; 3-37 | B | CONO510-08 | EU685619 | EU685479 | EU685772 | |
| IM_2007_30428 | Oxymeris | dimidiata | Vanuatu | 15°38.1′S, 167°05.9′E; | B | CONO495-08 | EU685607 | EU685466 | EU685759 | |
| IM_2009_7087 | Oxymeris | dimidiata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | B | XXX | XXX | XXX | XXX | |
| IM_2009_7088 | Oxymeris | dimidiata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | B | XXX | XXX | XXX | ||
| IM_2007_30443 | Oxymeris | felina | Philippines | 9°37.4′N, 123°54.5′E; 6-8 | B | CONO208-08 | EU685500 | EU685345 | EU685636 | |
| IM_2007_30445 | Oxymeris | felina | Philippines | 9°37.4′N, 123°54.5′E; 6-8 | B | CONO210-08 | EU685502 | EU685347 | EU685638 | XXX |
| IM_2007_30370 | Oxymeris | maculata | Philippines | 9°37.4′N, 123°46.9′E; 3-20 | B | CONO204-08 | EU685496 | EU685341 | EU685632 | |
| IM_2007_30389 | Oxymeris | maculata | Vanuatu | 15°28.7′S, 167°15.2′E; 19 | B | CONO405-08 | EU685541 | EU685391 | EU685682 | XXX |
| IM_2007_42093 | Oxymeris | strigata | Panama | 08°11.8′N, 078°57.1′W; 24 | B | CONO974-09 | FJ707455.1 | FJ707388.1 | FJ707422.1 | |
| IM_2007_42105 | Oxymeris | strigata | Panama | 08°11.8′N, 078°57.5′W; 22 | B | CONO979-09 | FJ707460.1 | FJ707393.1 | FJ707428.1 | |
| IM_2007_42137 | Oxymeris | strigata | Panama | 08°14.7′N, 079°05.6′W; 18 | B | CONO990-09 | FJ707471.1 | FJ707404.1 | FJ707439.1 | |
| IM_2007_30395 | Pellifronia | jungi | Philippines | 9°38′N, 123°40′E; 606-631 | A | CONO292-08 | EU685530 | EU685375 | EU685666 | XXX |
| IM_2007_30501 | Pellifronia | jungi | Solomon Islands | 8°26′ S, 159°26′ E; 543-593 | A | XXX | EU685385 | EU685676 | ||
| IM_2007_30539 | Pellifronia | jungi | Vanuatu | 15°44′S, 167°03′E; 618-722 | A | XXX | XXX | XXX | XXX | |
| IM_2007_30584 | Pellifronia | jungi | Philippines | 9°34′N, 123°38′E; 729-733 | A | CONO347-08 | EU685532 | EU685380 | EU685671 | |
| IM_2007_30450 | Strioterebrum | brunneobandatum | Philippines | 9°43′N, 123°49′E; 123-135 | E1 | CONO256-08 | EU685517 | EU685362 | EU685654 | |
| IM_2007_30522 | Strioterebrum | brunneobandatum | Solomon Islands | 8°38′ S, 157°22′ E; 195-197 | E1 | XXX | XXX | XXX | ||
| IM_2007_30454 | Strioterebrum | dedonderi | Philippines | 9°36.4′N, 123°53.8′E; 60-62 | E1 | CONO263-08 | EU685521 | EU685366 | EU685658 | |
| IM_2007_30425 | Strioterebrum | nitidum | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | E2 | CONO506-08 | EU685616 | EU685475 | EU685768 | |
| IM_2007_30473 | Strioterebrum | nitidum | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | E2 | CONO507-08 | EU685617 | EU685476 | EU685769 | |
| IM_2007_30614 | Strioterebrum | nitidum | Vanuatu | 15°31.7′S, 167°09.4′E; 9-13 | E2 | XXX | EU685424 | EU685715 | ||
| IM_2009_10157 | Strioterebrum | nitidum | South Madagascar | 25°26.1-4′S, 44°55.2-6′E; 17-20 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10158 | Strioterebrum | nitidum | South Madagascar | 25°24.1-2′S, 44°51.1-7′E; 24-26 | E2 | XXX | XXX | XXX | XXX | XXX |
| IM_2009_7114 | Strioterebrum | nitidum | Mozambique | 25°59.0′S, 32°54.5′E; 0 | E2 | XXX | XXX | XXX | ||
| IM_2007_30610 | Strioterebrum | plumbeum | Vanuatu | 15°35.2′S, 167°59.4′E; | E2 | CONO463-08 | EU685578 | EU685431 | EU685722 | |
| IM_2007_30469 | Strioterebrum | plumbeum | Vanuatu | 15°31.7′S, 167°09.4′E; 9-13 | E2 | CONO456-08 | EU685571 | EU685422 | EU685713 | |
| IM_2007_30558 | Strioterebrum | plumbeum | Vanuatu | 15°31.7′S, 167°09.4′E; 9-13 | E2 | CONO455-08 | EU685570 | EU685421 | EU685712 | |
| IM_2007_30609 | Strioterebrum | sp. 1 | Vanuatu | 15°35.2′S, 167°59.4′E; | E2 | CONO464-08 | EU685579 | EU685432 | EU685723 | XXX |
| IM_2007_30611 | Strioterebrum | sp. 2 | Vanuatu | 15°35.2′S, 167°59.4′E; | E2 | CONO462-08 | EU685577 | EU685430 | EU685721 | |
| IM_2007_30543 | Strioterebrum | sp. 3 | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | E2 | CONO499-08 | EU685611 | EU685470 | EU685763 | |
| IM_2007_42068 | Terebra | argosyia | Panama | 08°37.2′N, 079°01.1′W; 25 | C | CONO962-09 | FJ707443.1 | FJ707376.1 | FJ707408.1 | |
| IM_2007_42069 | Terebra | argosyia | Panama | 08°37.2′N, 079°01.1′W; 25 | C | CONO963-09 | FJ707444.1 | FJ707377.1 | FJ707409.1 | |
| IM_2007_42072 | Terebra | argosyia | Panama | 08°15.6′N, 078°51.6′W; 24 | C | CONO964-09 | FJ707445.1 | FJ707378.1 | FJ707411.1 | |
| IM_2007_42073 | Terebra | argosyia | Panama | 08°15.6′N, 078°51.6′W; 24 | C | CONO965-09 | FJ707446.1 | FJ707379.1 | FJ707412.1 | |
| IM_2007_42087 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.1′W; 21 | C | CONO969-09 | FJ707450.1 | FJ707383.1 | FJ707417.1 | |
| IM_2007_42089 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.1′W; 21 | C | CONO970-09 | FJ707451.1 | FJ707384.1 | FJ707418.1 | |
| IM_2007_42090 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.1′W; 21 | C | CONO971-09 | FJ707452.1 | FJ707385.1 | FJ707419.1 | |
| IM_2007_42091 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.1′W; 21 | C | CONO972-09 | FJ707453.1 | FJ707386.1 | FJ707420.1 | |
| IM_2007_42092 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.1′W; 21 | C | CONO973-09 | FJ707454.1 | FJ707387.1 | FJ707421.1 | |
| IM_2007_42099 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.5′W; 24 | C | CONO975-09 | FJ707456.1 | FJ707389.1 | FJ707423.1 | |
| IM_2007_42100 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.5′W; 24 | C | CONO976-09 | FJ707457.1 | FJ707390.1 | FJ707424.1 | |
| IM_2007_42103 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.5′W; 22 | C | CONO977-09 | FJ707458.1 | FJ707391.1 | FJ707426.1 | |
| IM_2007_42104 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.5′W; 22 | C | CONO978-09 | FJ707459.1 | FJ707392.1 | FJ707427.1 | |
| IM_2007_42119 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.5′W; 22 | C | CONO981-09 | FJ707462.1 | FJ707395.1 | FJ707430.1 | |
| IM_2007_42122 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.5′W; 22 | C | CONO984-09 | FJ707465.1 | FJ707398.1 | FJ707433.1 | |
| IM_2007_42123 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.5′W; 22 | C | CONO985-09 | FJ707466.1 | FJ707399.1 | FJ707434.1 | |
| IM_2007_42124 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.5′W; 22 | C | CONO986-09 | FJ707467.1 | FJ707400.1 | FJ707435.1 | |
| IM_2007_42125 | Terebra | argosyia | Panama | 08°11.8′N, 078°57.5′W; 22 | C | CONO987-09 | FJ707468.1 | FJ707401.1 | FJ707436.1 | |
| IM_2007_30383 | Terebra | argus | Vanuatu | 15°26.6′S, 167°15.2′E; | C | XXX | XXX | EU685442 | EU685734 | XXX |
| IM_2007_30375 | Terebra | babylonia | Vanuatu | 9°32.8′N, 123°45.9′E; 2 | C | XXX | XXX | EU685445 | EU685737 | XXX |
| IM_2007_30380 | Terebra | babylonia | Vanuatu | 9°32.8′N, 123°45.9′E; 2 | C | CONO474-08 | EU685589 | EU685446 | EU685738 | |
| IM_2007_42144 | Terebra | berryi | Panama | 08°14.7′N, 079°05.6′W; 18 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42167 | Terebra | berryi | Panama | 08°33′N, 079°04′W; 19 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42097 | Terebra | cf. variegata | Panama | 08°11.8′N, 078°57.1′W; 26 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42128 | Terebra | cf. variegata | Panama | 08°14.9′N, 079°05.7′W; 14 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42133 | Terebra | cf. variegata | Panama | 08°14.8′N, 079°05.9′W; 13 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42135 | Terebra | cf. variegata | Panama | 08°14.8′N, 079°05.9′W; 13 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42146 | Terebra | cf. variegata | Panama | 08°14.7′N, 079°05.6′W; 18 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42158 | Terebra | cf. variegata | Panama | 08°24.5′N, 079°04.7′W; 18 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_16735 | Terebra | cingulifera | Philippines | 9°36′N, 123°44′E; 382-434 | C | CONO340-08 | EU015735 | EU685379 | EU685670 | EU015620 |
| IM_2007_30382 | Terebra | cingulifera | Vanuatu | 15°26.6′S, 167°15.2′E; | C | XXX | XXX | EU685443 | EU685735 | XXX |
| IM_2007_30484 | Terebra | cingulifera | Solomon Islands | 8°38′ S, 157°22′ E; 195-197 | C | XXX | XXX | XXX | ||
| IM_2007_30485 | Terebra | cingulifera | Vanuatu | 15°32.5′S, 167°10.5′E; 5-10 | C | CONO490-08 | EU685603 | EU685462 | EU685755 | |
| IM_2007_30487 | Terebra | cingulifera | Solomon Islands | 8°40′ S, 157°23′ E; 214-243 | C | CONO382-08 | EU685536 | EU685386 | EU685677 | |
| IM_2007_30562 | Terebra | cingulifera | Philippines | 16°04′N, 121°57′E; 98-107 | C | XXX | XXX | XXX | ||
| IM_2007_30563 | Terebra | cingulifera | Philippines | 16°05,85′N, 121°58,85′E; 83 | C | XXX | XXX | XXX | ||
| IM_2007_30564 | Terebra | cingulifera | Philippines | 15°54′N, 121°42′E; 125-198 | C | XXX | XXX | XXX | ||
| IM_2007_30576 | Terebra | cingulifera | Vanuatu | 15°36.8′S, 167°08.7′E; 3-36 | C | XXX | XXX | XXX | ||
| IM_2009_10171 | Terebra | cingulifera | South Madagascar | 25°04.7′S, 47°03.4′E; 64-65 | C | XXX | XXX | XXX | ||
| IM_2007_42095 | Terebra | elata | Panama | 08°11.8′N, 078°57.1′W; 26 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42096 | Terebra | elata | Panama | 08°11.8′N, 078°57.1′W; 26 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42111 | Terebra | elata | Panama | 08°11.8′N, 078°57.5′W; 22 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42127 | Terebra | elata | Panama | 08°11.8′N, 078°57.5′W; 22 | E4 | XXX | XXX | XXX | ||
| IM_2007_42147 | Terebra | elata | Panama | 08°14.7′N, 079°05.4′W; 18 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42155 | Terebra | elata | Panama | 08°24.5′N, 079°04.7′W; 18 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42162 | Terebra | elata | Panama | 08°31.2′N, 079°06.8′W; 32 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42163 | Terebra | elata | Panama | 08°31.2′N, 079°06.8′W; 32 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_30423 | Terebra | fijiensis | Vanuatu | 15°33′S, 167°16.7′E; 92 | E2 | CONO520-08 | EU685625 | EU685485 | EU685778 | |
| IM_2007_42152 | Terebra | formosa | Panama | 08°16.9′N, 079°02.7′W; 39 | C | CONO991-09 | FJ707472.1 | FJ707405.1 | FJ707440.1 | |
| IM_2007_30394 | Terebra | funiculata | Vanuatu | C | CONO450-08 | EU685565 | EU685416 | EU685707 | XXX | |
| IM_2009_7108 | Terebra | funiculata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_7109 | Terebra | funiculata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_7110 | Terebra | funiculata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2007_30376 | Terebra | guttata | Vanuatu | 15°33.1′S, 167°12.2′E; 3-40 | C | CONO439-08 | EU685554 | EU685405 | EU685696 | |
| IM_2007_30387 | Terebra | guttata | Vanuatu | 15°33.1′S, 167°12.2′E; 3-40 | C | CONO438-08 | EU685553 | EU685404 | EU685695 | |
| IM_2007_30431 | Terebra | laevigata | Philippines | 9°36.8′N, 123°52.2′E; | C | CONO262-08 | EU685520 | EU685365 | EU685657 | |
| IM_2007_30573 | Terebra | laevigata | Vanuatu | 15°29.6′S, 167°14.9′E; 2-5 | C | XXX | XXX | XXX | XXX | |
| IM_2007_30597 | Terebra | laevigata | Vanuatu | 15°26.6′S, 167°15.2′E; | C | CONO471-08 | EU685586 | EU685440 | EU685732 | |
| IM_2007_30603 | Terebra | laevigata | Vanuatu | 15°43.4′S, 167°15.0′E; 6 | C | CONO484-08 | EU685598 | EU685457 | EU685750 | |
| IM_2007_30613 | Terebra | laevigata | Vanuatu | 15°31.7′S, 167°09.4′E; 9-13 | C | CONO458-08 | EU685573 | EU685425 | EU685716 | |
| IM_2007_30632 | Terebra | laevigata | Vanuatu | 15°31.7′S, 167°09.4′E; 9-13 | C | CONO457-08 | EU685572 | EU685423 | EU685714 | |
| IM_2009_10016 | Terebra | laevigata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | ||
| IM_2009_10017 | Terebra | laevigata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10018 | Terebra | laevigata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10019 | Terebra | laevigata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_7104 | Terebra | laevigata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_7105 | Terebra | laevigata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_7106 | Terebra | laevigata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | XXX |
| IM_2009_7107 | Terebra | laevigata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | ||
| IM_2009_9947 | Terebra | laevigata | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2007_42083 | Terebra | larvaeformis | Panama | 08°11.8′N, 078°57.1′W; 21 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42106 | Terebra | larvaeformis | Panama | 08°11.8′N, 078°57.5′W; 22 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42113 | Terebra | larvaeformis | Panama | 08°11.8′N, 078°57.5′W; 22 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42149 | Terebra | larvaeformis | Panama | 08°14.7′N, 079°05.4′W; 18 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42131 | Terebra | ornata | Panama | 08°16.9′N, 079°02.7′W; 39 | C | CONO988-09 | FJ707469.1 | FJ707402.1 | FJ707437.1 | |
| IM_2007_30374 | Terebra | punctatostriata | Vanuatu | 15°31.4′S, 167°09.7′E; 4-18 | C | XXX | EU685427 | EU685718 | ||
| IM_2007_42070 | Terebra | puncturosa | Panama | 08°15.6′N, 078°51.6′W; 24 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42081 | Terebra | puncturosa | Panama | 08°11.8′N, 078°57.1′W; 21 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42116 | Terebra | puncturosa | Panama | 08°11.8′N, 078°57.5′W; 22 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42171 | Terebra | puncturosa | Panama | 08°33′N, 079°04′W; 19 | E4 | XXX | XXX | XXX | XXX | |
| IM_2009_7116 | Terebra | quoygaimardi | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | XXX |
| IM_2009_7118 | Terebra | quoygaimardi | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | ||
| IM_2009_9946 | Terebra | quoygaimardi | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2007_30464 | Terebra | sp. 1 | Philippines | 9°35.3′N, 123°52.2′E; 84-87 | E2 | CONO206-08 | EU685498 | EU685343 | EU685634 | |
| IM_2007_30516 | Terebra | sp. 2 | Chesterfield Islands | 24°46′S, 159°43′E; 400-418 | E3 | XXX | XXX | XXX | XXX | |
| IM_2007_30617 | Terebra | sp. 3 | Vanuatu | 15°31.7′S, 167°09.7′E; 18-21 | E2 | CONO430-08 | EU685549 | EU685400 | EU685691 | |
| IM_2007_30618 | Terebra | sp. 3 | Vanuatu | 15°31.7′S, 167°09.7′E; 18-21 | E2 | CONO431-08 | EU685550 | EU685401 | EU685692 | XXX |
| IM_2007_30633 | Terebra | sp. 4 | Solomon Islands | 9°07′ S, 158°21′ E; 267-329 | E3 | XXX | XXX | XXX | XXX | |
| IM_2007_30946 | Terebra | sp. 5 | Chesterfield Islands | 20°21′S, 158°46′E; 345-351 | E5 | XXX | XXX | XXX | ||
| IM_2007_42151 | Terebra | specillata | Panama | 08°16.9′N, 079°02.7′W; 39 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42154 | Terebra | specillata | Panama | 08°24.5′N, 079°04.7′W; 18 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_42168 | Terebra | specillata | Panama | 08°33′N, 079°04′W; 19 | E4 | XXX | XXX | XXX | XXX | |
| IM_2007_30386 | Terebra | subulata | Vanuatu | 15°36.6′S, 167°10.1′E; 8-20 | C | CONO436-08 | EU685551 | EU685402 | EU685693 | |
| IM_2007_30444 | Terebra | subulata | Philippines | 9°37.4′N, 123°54.5′E; 6-8 | C | CONO209-08 | EU685501 | EU685346 | EU685637 | |
| IM_2007_30483 | Terebra | subulata | Philippines | C | CONO277-08 | EU685524 | EU685369 | EU685661 | XXX | |
| IM_2007_16731 | Terebra | succincta | Philippines | 9°30′N, 123°42′E; 356-396 | E3 | CONO331-08 | EU015732 | EU685378 | EU685669 | EU015617 |
| IM_2007_30385 | Terebra | succincta | Vanuatu | 15°26.6′S, 167°15.2′E; | E2 | CONO470-08 | EU685585 | EU685439 | EU685731 | |
| IM_2007_30419 | Terebra | succincta | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | E2 | XXX | XXX | XXX | XXX | |
| IM_2007_30433 | Terebra | succincta | Vanuatu | 15°33.4′S, 167°12.4′E; 2-6 | E2 | CONO516-08 | EU685621 | EU685481 | EU685774 | |
| IM_2007_30434 | Terebra | succincta | Vanuatu | 15°33.4′S, 167°12.4′E; 2-6 | E2 | CONO517-08 | EU685622 | EU685482 | EU685775 | |
| IM_2007_30440 | Terebra | succincta | Vanuatu | 15°31.7′S, 167°09.7′E; 18-21 | E2 | CONO426-08 | EU685545 | EU685396 | EU685687 | XXX |
| IM_2007_30456 | Terebra | succincta | Vanuatu | 15°31.7′S, 167°09.7′E; 18-21 | E2 | CONO427-08 | EU685546 | EU685397 | EU685688 | |
| IM_2007_30458 | Terebra | succincta | Vanuatu | 15°31.7′S, 167°09.7′E; 18-21 | E2 | CONO428-08 | EU685547 | EU685398 | EU685689 | |
| IM_2007_30468 | Terebra | succincta | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | E2 | XXX | XXX | XXX | XXX | |
| IM_2007_30470 | Terebra | succincta | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | E2 | XXX | XXX | XXX | XXX | |
| IM_2007_30475 | Terebra | succincta | Vanuatu | E2 | CONO451-08 | EU685566 | EU685417 | EU685708 | ||
| IM_2007_30476 | Terebra | succincta | Vanuatu | E2 | CONO452-08 | EU685567 | EU685418 | EU685709 | ||
| IM_2007_30479 | Terebra | succincta | Solomon Islands | 7°14′ S, 158°29′ E; 286-423 | E3 | CONO379-08 | EU685534 | EU685381 | EU685672 | |
| IM_2007_30582 | Terebra | succincta | Philippines | 9°39′N, 123°48′E; 255-268 | E3 | CONO285-08 | EU685527 | EU685372 | ||
| IM_2007_30601 | Terebra | succincta | Vanuatu | 15°41′S, 167°00′E; 517-614 | E3 | CONO492-08 | EU685605 | EU685464 | EU685757 | |
| IM_2007_30622 | Terebra | succincta | Vanuatu | E2 | XXX | XXX | XXX | XXX | ||
| IM_2007_30626 | Terebra | succincta | Vanuatu | 15°31.7′S, 167°09.7′E; 18-21 | E2 | CONO425-08 | EU685544 | EU685395 | EU685686 | |
| IM_2007_30634 | Terebra | succincta | Vanuatu | 15°31.7′S, 167°09.7′E; 18-21 | E2 | CONO429-08 | EU685548 | EU685399 | EU685690 | |
| IM_2009_10085 | Terebra | succincta | North New-Caledonia | 20°17′S, 163°50′E; 590-809 | E3 | XXX | XXX | XXX | XXX | |
| IM_2007_17938 | Terebra | textilis | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | E2 | CONO509-08 | EU015750 | EU685478 | EU685771 | EU015635 |
| IM_2007_30441 | Terebra | textilis | Vanuatu | 15°33.4′S, 167°12.4′E; 2-6 | E2 | XXX | XXX | XXX | ||
| IM_2007_30451 | Terebra | textilis | Philippines | 9°36.8′N, 123°52.2′E; | E2 | CONO261-08 | EU685519 | EU685364 | EU685656 | |
| IM_2007_30474 | Terebra | textilis | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | E2 | CONO508-08 | EU685618 | EU685477 | EU685770 | XXX |
| IM_2007_30545 | Terebra | textilis | Vanuatu | 15°31.3′S, 167°09.9′E; 1-6 | E2 | CONO497-08 | EU685609 | EU685468 | EU685761 | |
| IM_2007_30547 | Terebra | textilis | Vanuatu | 15°31.3′S, 167°09.9′E; 1-6 | E2 | CONO496-08 | EU685608 | EU685467 | EU685760 | |
| IM_2007_30616 | Terebra | textilis | Vanuatu | E2 | CONO454-08 | EU685569 | EU685420 | EU685711 | ||
| IM_2007_30621 | Terebra | textilis | Vanuatu | E2 | CONO453-08 | EU685568 | EU685419 | EU685710 | ||
| IM_2009_10088 | Terebra | textilis | North Madagascar | 14° 31,9′ S, 47° 26,54′ E; 46-54 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_10093 | Terebra | textilis | North Madagascar | 15° 30,15′ S, 46° 4,3′ E; 29-36 | E2 | XXX | XXX | XXX | ||
| IM_2009_10095 | Terebra | textilis | North Madagascar | 15° 30,15′ S, 46° 4,3′ E; 29-36 | E2 | XXX | XXX | XXX | XXX | |
| IM_2009_9957 | Terebra | textilis | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | E2 | XXX | XXX | XXX | ||
| IM_2007_30409 | Terebra | tricolor | Vanuatu | 15°33.1′S, 167°17.8′E; 15-25 | C | CONO404-08 | EU685540 | EU685390 | EU685681 | |
| IM_2007_30493 | Terebra | tricolor | Vanuatu | 15°38.5′S, 167°15.1′E; 13 | C | CONO488-08 | EU685602 | EU685461 | EU685754 | |
| IM_2007_30424 | Terebra | trismacaria | Solomon Islands | 8°37′ S, 157°21′ E; 150-160 | E2 | CONO380-08 | EU685383 | EU685674 | XXX | |
| IM_2007_30492 | Terebra | trismacaria | Solomon Islands | 8°40′ S, 157°23′ E; 214-243 | E2 | CONO384-08 | EU685538 | EU685388 | EU685679 | |
| IM_2007_30499 | Terebra | trismacaria | Solomon Islands | 8°40′ S, 157°23′ E; 214-243 | E2 | CONO385-08 | EU685539 | EU685389 | EU685680 | |
| IM_2007_30579 | Terebra | trismacaria | Solomon Islands | 8°40′ S, 157°23′ E; 214-243 | E2 | CONO383-08 | EU685537 | EU685387 | EU685678 | |
| IM_2007_30446 | Terenolla | pygmaea | Philippines | 9°37.4′N, 123°54.5′E; 4-5 | E1 | CONO211-08 | EU685503 | EU685348 | EU685639 | |
| IM_2007_30448 | Terenolla | pygmaea | Philippines | 9°37.4′N, 123°54.5′E; 4-5 | E1 | CONO212-08 | EU685504 | EU685349 | EU685640 | |
| IM_2007_30449 | Terenolla | pygmaea | Philippines | 9°37.4′N, 123°54.5′E; 4-5 | E1 | CONO213-08 | EU685505 | EU685350 | EU685641 | |
| IM_2007_30511 | Terenolla | pygmaea | Philippines | 9°35.7′N, 123°44.4′E; 0-2 | E1 | CONO236-08 | EU685509 | EU685354 | EU685645 | |
| IM_2009_10121 | Terenolla | pygmaea | South Madagascar | E1 | XXX | XXX | XXX | XXX | XXX | |
| IM_2007_30411 | Triplostephanus | anilis | Vanuatu | 15°35.4′S, 166°58.7′E; 3-8 | C | CONO493-08 | XXX | XXX | XXX | |
| IM_2007_30552 | Triplostephanus | anilis | Vanuatu | 15°35.2′S, 167°59.4′E; | C | CONO473-08 | EU685588 | EU685444 | EU685736 | XXX |
| IM_2009_10068 | Triplostephanus | anilis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10069 | Triplostephanus | anilis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10070 | Triplostephanus | anilis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10071 | Triplostephanus | anilis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10072 | Triplostephanus | anilis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_7120 | Triplostephanus | anilis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_7121 | Triplostephanus | anilis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_7122 | Triplostephanus | anilis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2009_7123 | Triplostephanus | anilis | Mozambique | 25°59.0′S, 32°54.5′E; 0 | C | XXX | XXX | XXX | XXX | |
| IM_2007_30402 | Triplostephanus | cumingii | Fiji | 18°26.4′S, 178°02.4′E; 50-51 | C | XXX | EU685487 | EU685779 | ||
| IM_2007_30390 | Triplostephanus | fenestratus | Philippines | 9°29′N, 123°44′E; 271-318 | C | CONO305-08 | EU685531 | EU685376 | EU685667 | |
| IM_2007_30410 | Triplostephanus | fenestratus | Philippines | 9°39′N, 123°48′E; 255-268 | C | CONO287-08 | EU685529 | EU685374 | EU685665 | |
| IM_2007_30418 | Triplostephanus | fenestratus | Philippines | 9°39′N, 123°48′E; 255-268 | C | CONO286-08 | EU685528 | EU685373 | EU685664 | |
| IM_2007_30538 | Triplostephanus | fenestratus | Philippines | 9°39′N, 123°48′E; 255-268 | C | XXX | XXX | XXX | ||
| IM_2007_30553 | Triplostephanus | fenestratus | Vanuatu | 15°42′S, 167°02′E; 268-445 | C | XXX | XXX | XXX | XXX | |
| IM_2007_30559 | Triplostephanus | fenestratus | Philippines | 9°39′N, 123°48′E; 255-268 | C | XXX | XXX | XXX | ||
| IM_2009_10073 | Triplostephanus | fenestratus | North Madagascar | 14°30′S, 47°27′E; 274-325 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10074 | Triplostephanus | fenestratus | North Madagascar | 14°30′S, 47°27′E; 274-325 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10075 | Triplostephanus | fenestratus | North Madagascar | 14°30′S, 47°27′E; 274-325 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10076 | Triplostephanus | fenestratus | North Madagascar | 14°30′S, 47°27′E; 274-325 | C | XXX | XXX | XXX | XXX | XXX |
| IM_2009_10077 | Triplostephanus | fenestratus | North Madagascar | 14°30′S, 47°27′E; 274-325 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10078 | Triplostephanus | fenestratus | North Madagascar | 14°30′S, 47°27′E; 274-325 | C | XXX | XXX | XXX | XXX | |
| IM_2007_15724 | Triplostephanus | fujitai | Philippines | 9°27′N, 123°49′E; 273-356 | C | CONO306-08 | EU015725 | EU685377 | EU685668 | EU015610 |
| IM_2007_30482 | Triplostephanus | fujitai | Vanuatu | 15°42′S, 167°02′E; 268-445 | C | CONO181-08 | EU685492 | EU685628 | ||
| IM_2007_30544 | Triplostephanus | jenningsi | Vanuatu | 15°28.6′S, 167°15.1′E; 3-31 | C | CONO483-08 | EU685597 | EU685456 | EU685749 | XXX |
| IM_2007_30533 | Triplostephanus | sp. | Philippines | 9°42.1′N, 123°51.4′E; 3-4 | E2 | XXX | XXX | XXX | ||
| IM_2007_30534 | Triplostephanus | sp. | Philippines | 9°42.1′N, 123°51.4′E; 3-4 | E2 | CONO243-08 | EU685514 | EU685359 | EU685651 | |
| IM_2007_30404 | Triplostephanus | triseriatus | Philippines | 9°35.3′N, 123°52.2′E; 84-87 | C | CONO205-08 | EU685497 | EU685342 | EU685633 | XXX |
| IM_2009_10082 | Triplostephanus | triseriatus | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10084 | Triplostephanus | triseriatus | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10100 | Triplostephanus | triseriatus | South Madagascar | 25°22.4′S, 47°02.8′E; 89-95 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10102 | Triplostephanus | triseriatus | South Madagascar | 25°02.4-5′S, 47°03.2-6′E; 54-56 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10108 | Triplostephanus | triseriatus | South Madagascar | 25°22.4′S, 47°02.8′E; 89-95 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10166 | Triplostephanus | triseriatus | South Madagascar | 25°04.7′S, 47°03.4′E; 64-65 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10167 | Triplostephanus | triseriatus | South Madagascar | 25°04.7′S, 47°03.4′E; 64-65 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10168 | Triplostephanus | triseriatus | South Madagascar | 25°04.7′S, 47°03.4′E; 64-65 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10169 | Triplostephanus | triseriatus | South Madagascar | 25°04.7′S, 47°03.4′E; 64-65 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10170 | Triplostephanus | triseriatus | South Madagascar | 25°04.7′S, 47°03.4′E; 64-65 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10172 | Triplostephanus | triseriatus | South Madagascar | 25°04.7′S, 47°03.4′E; 64-65 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10173 | Triplostephanus | triseriatus | South Madagascar | 25°04.7′S, 47°03.4′E; 64-65 | C | XXX | XXX | XXX | XXX | |
| IM_2009_10911 | Triplostephanus | triseriatus | Australia | 27°02′069"S, 153°19′00"E; 3,5- 7,8 |
C | XXX | XXX | XXX | ||
| IM_2009_9948 | Triplostephanus | triseriatus | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | C | XXX | XXX | XXX | XXX | |
| IM_2009_9949 | Triplostephanus | triseriatus | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | C | XXX | XXX | XXX | XXX | |
| IM_2009_9950 | Triplostephanus | triseriatus | North Madagascar | 12° 35,92′ S, 48° 35,22′ E; 50-52 | C | XXX | XXX | XXX | XXX | |
|
| ||||||||||
| IM_2007_40568 | Cochlespira | pulchella | Outgroup | FRANZ207-08 | EU685627 | EU685488 | EU685781 | |||
| IM_2007_17922 | Conus | nereis | Outgroup | CONO339-08 | EU015734 | EU685489 | EU685782 | EU015619 | ||
| IM_2007_40569 | Harpa | kajiyamai | Outgroup | EU685626 | EU685491 | EU685783 | ||||
| IM_2007_17685 | Iotyrris | cingulifera | Outgroup | CONO515-08 | EU127881 | EU685490 | EU685780 | EU127890 | ||
Figure 1.
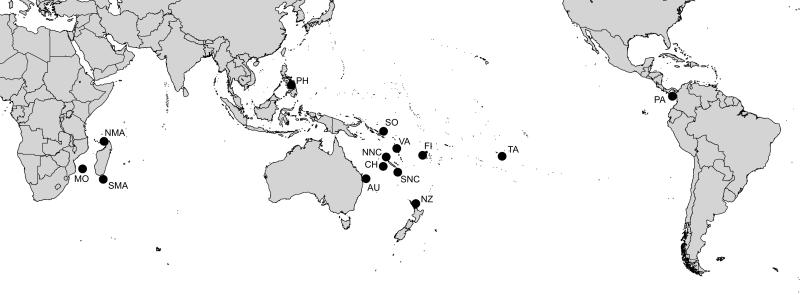
Map showing localities sampled for Terebridae study. AU, Queensland, Australia; CH, Coral Sea; FI, Fiji; SMA, South Madagascar; MO, Mozambique; NMA, North Madagascar; NNC, North New Caledonia; PA, Pacific Panama; PH, Philippines; SNC, South New Caledonia; SO, Solomon Islands; TA, Tahiti; VA, Vanuatu.
2.2. PCR amplification and DNA sequencing
Total genomic DNA was extracted from muscle tissue using NucleoSpinR 96 Tissues (Macherey- Nagel) and following the manufacturer’s instructions. Fragments of the mitochondrial genes Cytochrome Oxidase I (COI), 16S rRNA and 12S rRNA as well as the nuclear 28S rRNA were amplified (Table 2). PCR reactions were performed in 25 μL final volume, containing approximately 3 ng template DNA, 1.5 mM MgCl2, 0.26 mM of each nucleotide, 0.3 μM of each primer, 5% DMSO and 0.75 U of Taq Polymerase (Qbiogene). Amplification products were generated by an initial denaturation step of 4 min at 94 °C followed by 35 cycles at 94 °C for 40 s, annealing at 50°C for COI, 52°C for 28S, 51°C for 12S rRNA and 16S rRNA for 40 s and by an extension at 72°C for 1 min. PCR products were purified using ExonucleaseI and Phosphatase and sequenced using BigDye Terminator V3.1 kit (Applied biosystem) and the AB3730XL sequencer. All genes were sequenced for both directions to confirm accuracy of each sequence. Chromatograms were edited using CodonCode Aligner version 3.7.1.1. All the sequences were deposited in GenBank and BOLD (Table 1).
Table 2.
Primers used for gene amplification and sequencing. PCG = Protein Coding Gene
| Gene | Primer name | Primer Sequences (5′-3′) | Sens | Tm | References | Length of Amplification | Gene type |
|---|---|---|---|---|---|---|---|
| COI | LCOI1490 | GGT CAA CAA ATC ATA AAG ATA TTG G | F | 48/50 | Folmer et al., 1994 | 660 | |
| COI | HCOI2198 | TAA ACT TCA GGG TGA CCA AAA AAT CA | R | 48/50 | Folmer et al. 1994 | mtDNA PCG | |
| 16S | 16Sa-L | CGC CTG TTT ATC AAA AAC AT | F | 51 | Palumbi, 1996 | 460 | |
| 16S | 16Sb-H2 | CTC CGG TTT GAA CTC AGA TCA | R | 51 | Palumbi 1996 | mtDNA rRNA | |
| 12S | 12SA | AAA CTG GGA TTA GAT ACC CCA CTA T | F | 51 | Palumbi 1996 | 370 | |
| 12S | 12SB | GAG GGT GAC GGG CGG TGT GT | R | 51 | Palumbi 1996 | mtDNA rRNA | |
| 28S | C1′ | ACC CGC TGA ATT TAA GCA T | F | 56 | Jovelin and Justine, 2001 | 830 | |
| 28S | D2 | TCC GTG TTT CAA GAC GGG | R | 56 | Jovelin and Justine, 2001 | nDNA rRNA |
2.3. Datasets
Six datasets were analyzed. The first three datasets were analyzed for all taxa listed in Table 1 and consisted of three independent gene analyses performed from COI, 16S and 12S genes. The fourth dataset consisted of a combined data set of COI, 16S, and 12S and is referred to as CD1. To evaluate the robustness of the mitochondrial phylogeny, a fifth dataset corresponding to the nuclear 28S gene set was built, with one representative for most of the species. This reduced dataset was then combined with the three mitochondrial genes and is referred to as CD2.
2.4. Phylogenetic analyses
Sequences were aligned for each gene independently using MUSCLE (Edgar, 2004). The accuracy of automatic alignments was confirmed by eye using BioEdit version 7.0.0.0 (Hall, 1999). Hyper-variable regions of 12S and 16S rRNA genes were excluded from further analyses to avoid ambiguities in the homology hypotheses. Best-fit substitution models were identified for each gene separately and for each combined dataset using Modelgenerator V.85 (Keane et al., 2006). Best-scoring Maximum Likelihood (ML) trees were estimated using RaxML (Stamatakis, 2006) from 100 independant searches each starting from distinct random trees. Robustness of the nodes were assessed using the thorough bootstrapping algorithm (Felsenstein, 1985a) with 1000 replicates. Bayesian Analyses (BA) were performed running two parallel analyses in MrBayes (Huelsenbeck and Ronquist, 2001), consisting each of eight Markov chains of 100,000,000 generations with a sampling frequency of one tree each ten thousand generations. The number of swaps chains was set to 5, and the chain temperature at 0.02. Convergence of each analysis was evaluated using Tracer 1.4.1 (Rambaut and Drummond, 2007) to check that ESS values were all greater than 200. A consensus tree was then calculated after omitting the first 25% trees as burn-in. For the treatment of combined data using ML and BA, the data were separated into six unlinked partitions: 16S, 12S, 28S and the three codon positions of the COI gene. Analyses were performed on the Cipres Science Gateway (http://www.phylo.org/portal2), using the RAxML-HPC2 on TG tool for ML and the MrBayes on TG tool for BA.
2.5. Overview of Terebridae anatomy and foregut characters
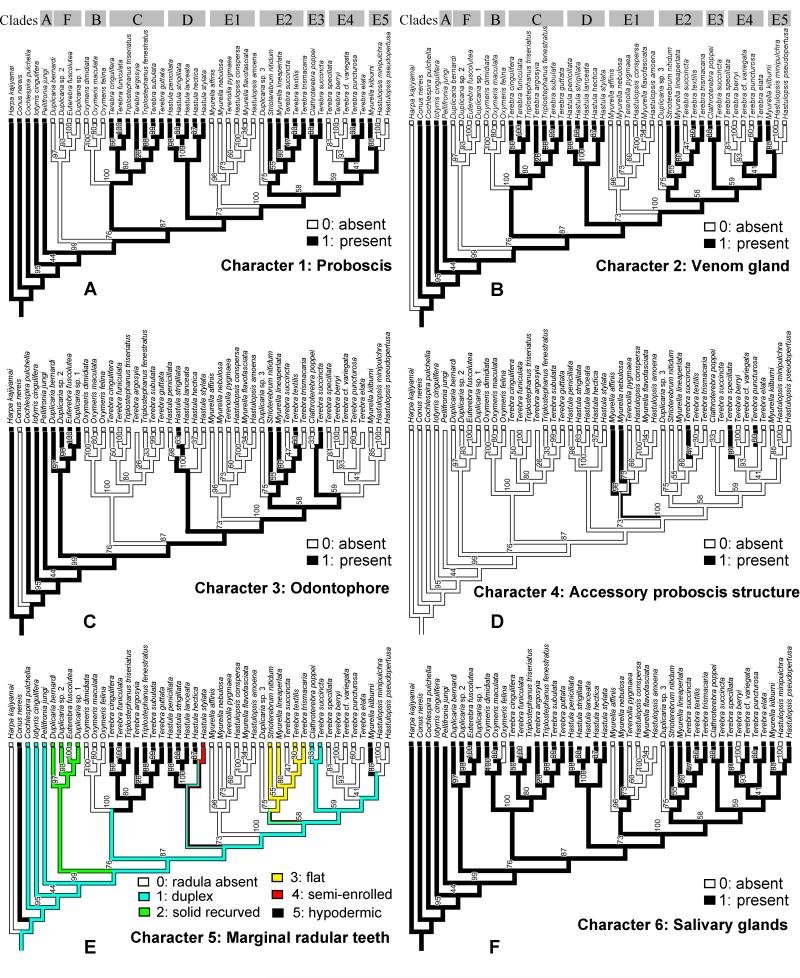
Foregut anatomy was examined by dissecting sequenced specimens. The radulae were cleaned with diluted bleach (1 part of commercially available bleach to 3-4 parts of water), rinsed several times in distilled water, mounted on clear glass cover-slips and air-dried. The cover-slips were glued to stubs, coated with gold and examined by scanning electron microscopy. Terminology previously used for description of the foregut structures in Terebridae is rather inconsistent and confusing (Miller, 1970, 1975, 1979). Here the terminology of Taylor et al. (1993), which reflects the supposed homologies within the entire Conoidea was followed. Six characters of the foregut were examined and used for tracing evolutionary pathways on the molecular tree (Table 3):
Table 3.
Matrix of the anatomical characters used for the character mapping. Numbers in parentheses in the column “MNHN vouchers” correspond to specimens used to reconstruct the phylogenetic tree when the dissected specimen was not available or when its sequencing failed. JDT and YK: species dissected by John D. Taylor and Yuri Kantor.
1. Proboscis (PR): 0 – absent, 1 – present
2. Venom gland (VG): 0 – absent, 1 – present
3. Odontophore (OD): 0 – absent, 1 – present
4. Accessory proboscis structure (APS): 0 – absent, 1 – present
5. Marginal radular teeth (RadT): 0 – radula absent, 1 – duplex, 2 – solid recurved, 3 – flat, 4 – semienrolled, 5 – hypodermic
6. Salivary gland(s) (SG): 0 – absent, 1 – present
| Species | MNHN vouchers | clade | PR | VG | OD | APS | RadT | SG |
|---|---|---|---|---|---|---|---|---|
| Clathroterebra poppei | IM_2007_30546 | E3 | 1 | 1 | 0 | ? | 1 | 1 |
| Duplicaria bernardi | IM_2009_10908 | F | 0 | 0 | 1 | 0 | 2 | 1 |
| Duplicaria sp. 1 | IM_2009_10111 | F | 0 | 0 | 1 | 0 | 2 | 1 |
| Duplicaria sp. 2 | IM_2009_10164 | F | 0 | 0 | 1 | 0 | 2 | 1 |
| Duplicaria sp. 3 | IM_2009_10134 | E2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Euterebra fuscolutea | IM_2009_10127 | F | 0 | 0 | 1 | 0 | 2 | 1 |
| Hastula hectica | YK (IM_2009_10104) | D | 1 | 1 | 0 | 0 | 5 | 1 |
| Hastula lanceata | IM_2007_30535 | D | 1 | 1 | 0 | 0 | 5 | ? |
| Hastula penicillata | IM_2007_30540 | D | 1 | 1 | 0 | 0 | 5 | 1 |
| Hastula strigillata | IM_2007_30607 | D | ? | 1 | 1 | 0 | 5 | ? |
| Hastula stylata | IM_2009_10106 | D | 1 | 1 | 0 | 0 | 4 | 1 |
| Hastulopsis amoena | IM_2009_10909 | E1 | 0 | 0 | 0 | ? | 0 | ? |
| Hastulopsis conspersa | IM_2007_30619 | E1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hastulopsis minipulchra | IM_2009_10129 | E5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hastulopsis pseudopertusa | IM_2009_9953 (9954) | E5 | 0 | 0 | 0 | 0 | 0 | 1 |
| Myurella affinis | IM_2007_30439 | E1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Myurella flavofasciata | IM_2007_30465 | E1 | 0 | 0 | 0 | ? | 0 | ? |
| Myurella kilburni | IM_2007_30461 | E5 | 1 | 1 | 0 | 0 | 5 | 1 |
| Myurella lineaperlata | IM_2007_30635 | E2 | 1 | 1 | 1 | 0 | 3 | 1 |
| Myurella nebulosa | IM_2007_30408 | E1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Oxymeris dimidiata | JDT (IM_2007_30373) | B | 0 | 0 | 0 | 0 | 0 | 1 |
| Oxymeris felina | IM_2007_30443 | B | 0 | 0 | 0 | 0 | 0 | 0 |
| Oxymeris maculata | JDT (IM_2007_30389) | B | 0 | 0 | 0 | 0 | 0 | 1 |
| Pellifronia jungi | IM_2007_30591 (30395) |
A | 1 | 1 | 1 | ? | 1 | ? |
| Strioterebrum nitidum | IM_2009_7114 | E2 | 1 | 1 | 1 | 0 | 3 | 1 |
| Terebra argosyia | IM_2007_42087 | C | ? | 1 | ? | ? | ? | 1 |
| Terebra berryi | IM_2007_42167 | E4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Terebra cf. variegata | IM_2007_42128 | E4 | 0 | 0 | 0 | 0 | 0 | 1 |
| Terebra cingulifera | IM_2007_30382 | C | 1 | 1 | 0 | ? | 5 | 1 |
| Terebra elata | IM_2007_42095 | E4 | 1 | 1 | ? | 0 | ? | 1 |
| Terebra funiculata | IM_2007_30394 | C | ? | 1 | 0 | ? | 5 | ? |
| Terebra guttata | IM_2007_30376 | C | 1 | 1 | 0 | 0 | 5 | 1 |
| Terebra puncturosa | IM_2007_42171 | E4 | 1 | 0 | 0 | 1 | 0 | ? |
| Terebra specillata | IM_2007_42168 | E4 | 0 | 0 | 0 | 0 | 0 | 1 |
| Terebra subulata | JDT (IM_2007_30444) | C | 1 | 1 | 0 | 0 | 5 | 1 |
| Terebra succincta | IM_2007_30385 | E2 | 1 | 0 | 0 | 1 | 0 | 1 |
| Terebra succincta | IM_2007_30582 | E3 | 1 | 1 | 1 | ? | 1 | 1 |
| Terebra textilis | IM_2007_30547 | E2 | 1 | 1 | 1 | 0 | 3 | 1 |
| Terebra trismacaria | IM_2007_30579 | E2 | 1 | 1 | 0 | 0 | 3 | 1 |
| Terenolla pygmaea | IM_2007_30449 | E1 | 0 | 0 | 0 | 0 | 0 | 0 |
|
Triplostephanus
fenestratus |
IM_2007_30418 | C | 1 | 1 | 0 | 0 | 5 | 1 |
| Triplostephanus triseriatus | IM_2007_30404 | C | 1 | 1 | 0 | ? | 5 | ? |
|
| ||||||||
| Cochlespira pulchella | IM_2007_40568 | Out | 1 | 1 | 1 | 0 | 1 | 1 |
| Conus nereis | IM_2007_17922 | Out | 1 | 1 | 0 | 0 | 5 | 1 |
| Harpa kajiyamai | IM_2007_40569 | Out | 1 | 0 | 1 | 0 | 0 | 1 |
| Iotyrris cingulifera | IM_2007_17685 | Out | 1 | 1 | 1 | 0 | 1 | 1 |
Character 1
Proboscis (PR): 0 – absent, 1 – present. PR is very variable in length, from extremely short to very long. In long proboscises, walls often form telescopic folds, while the proboscis can be coiled within the rhynchodaeum. The proboscis contains the buccal tube, i.e., the portion of the alimentrary canal extending between the buccal cavity and the true mouth, which is situated at the distal end of the proboscis (Taylor et al., 1993). The buccal tube is absent only in those species where the proboscis is lost. All examined terebrid species possess a more or less long rhynchodeal introvert (also known as labial tube – Miller, 1970). The length of the introvert correlates with the presence of the proboscis: in species without proboscis, the rhynchodeal introvert is much longer than in species with proboscis.
Character 2
Venom gland (VG): 0 – absent, 1 – present. VG, sometimes called venom duct, is an autapomorphy of Conoidea (Taylor et al., 1993); when present it always has a muscular bulb, also referred to as the venom bulb. The venom gland in Terebridae opens just posterior to the radular sac.
Character 3
Odontophore (OD): 0 – absent, 1 – present. OD, consisting of subradular cartilages and muscles, usually present in species having a radula with a strong subradular membrane. In Terebridae it can vary from being massive (e.g., Duplicaria bernardii) to being vestigial and hardly recognizable (e.g., Terebra succincta, clade E3).
Character 4
Accessory proboscis structure (APS): 0 – absent, 1 – present. APS is an extensible muscular structure that arises from the wall of the rhynchodaeum. It can be branching or club-shaped, distally papillated, or simple, stalk-shaped. A somewhat similar structure, named rhynchodeal outgrowth, is found in other Conoidea – Horaiclavidae and Zemacies (Borsoniidae) (Fedosov and Kantor, 2008).
Character 5
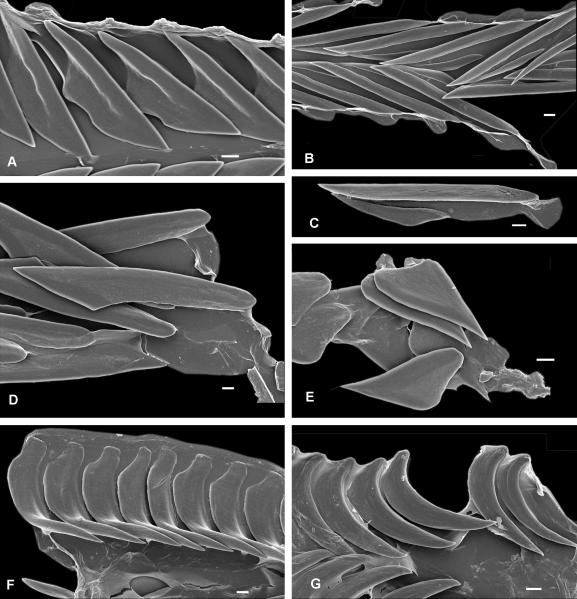
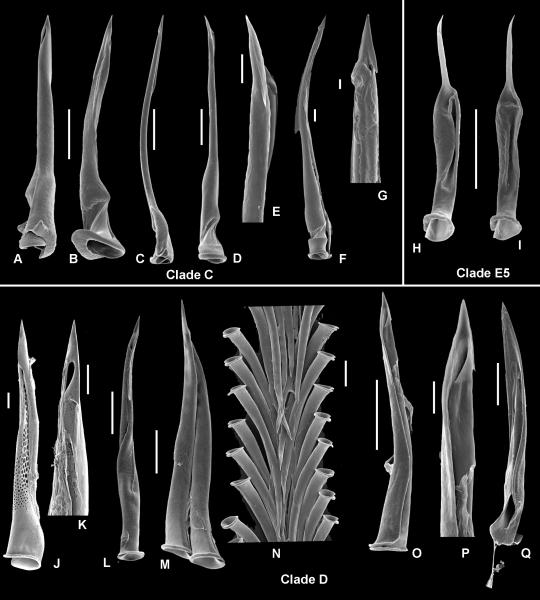
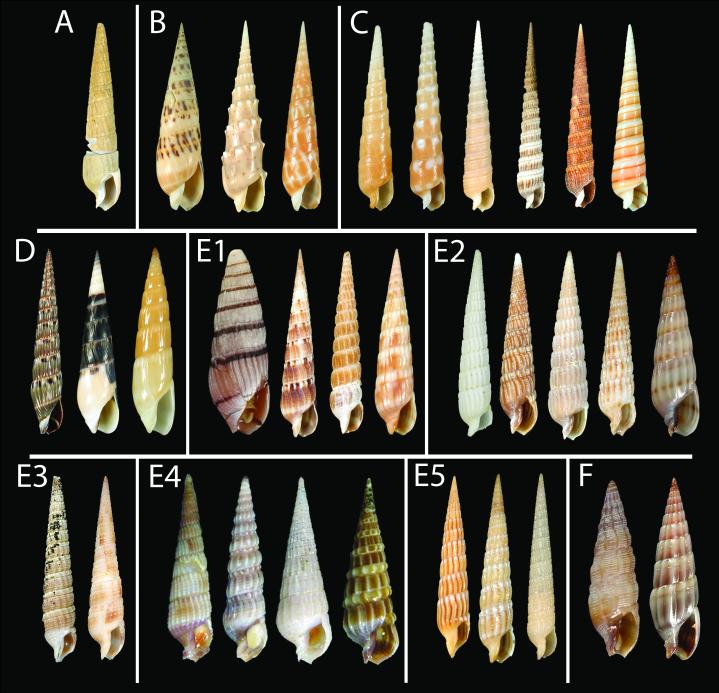
Radula (RadT): 0 – absent, 1 – consists of duplex marginal teeth, 2 – consists of solid recurved marginal teeth, 3 – consists of flat marginal teeth, 4 – consists of semi-enrolled marginal teeth, 5 – consists of hypodermic marginal teeth. Radula in Terebridae consists only of a pair of marginal teeth per transverse row. The radula was completely lost in several lineages, but when present the marginal teeth exhibit a range of morphological types, and five major types are here recognized: (1) Duplex teeth (Fig. 2 A-C), consisting of a major element (limb), attached to the subradular membrane along most of its length, and an accessory limb, which is the thickened edge of the major element, usually somewhat elevated above the membrane. Here, the radula has about 20-25 rows of teeth; (2) Solid recurved teeth (Fig. 2 F-G) with a broad flatened base, which is attached to the relatively strong subradular membrane. In species with this type of teeth, the radula is short, with only 15-20 rows; (3) Flat and simple teeth (Fig. 2 D-E), attached by a narrow base to the subradular membrane. Two, not clearly delimitated, variants - broad triangular (Fig. 2E) and long irregular (Fig. 2D) - are coded as the same radular type in the analysis. The subradular membrane is usually very thin and fragile, and easily tears apart. Radulae with this type of teeth consist of 20 or more rows; (4) Semi-enrolled teeth with tooth edges overlaping at the base, forming a loosely enrolled tube, while closer to the tip the tooth is trough shape in section. Radulae with this type of teeth are very short, with only about 10 rows; (5) Hypodermic hollow teeth (Fig. 3 A-P), rather similar to the hypodermic teeth present in other Conoidea. Such teeth have a very broad basal opening of the tooth canal, with usually a reflected outward edge of the tooth, forming a collar-like structure; the apical opening can be unarmed or it can have small barb(s) or blade(s). The subradular membrane is usually very thin and vestigial. The number of rows of teeth varies from about 10 (Terebra jenningsi) to about 30 (Hastula hectica and H. penicillata).
Figure 2.
Flat (A-E) and solid recurved (F-G) teeth of Terebridae. A – Pellifronia jungi (IM_2007_30591), ventral view of radular membrane, only half shown; B – Clathroterebra poppei (IM_2007_30546), ventral view of radular membrane; C – Terebra succincta (IM_2007_30582), separate marginal tooth; D – Terebra trismacaria (IM_2007_30579), ventral vies of radular membrane; E – Myurella lineaperlata (IM_2007_30635), group of teeth attached to the subradular membrane; F – Euterebra fuscolutea (IM_2009_10133), ventral view of radular membrane, only half shown; G – Duplicaria sp. 2 (IM_2009_10164), ventral view of radular membrane, only half shown. Scale bars – 10 μm.
Figure 3.
Hypodermic (A-O) and semienrolled (Q) teeth in Terebridae. Clade C (A-G): A, B – Terebra cingulifera (IM_2007_30382); C – Triplostephanus fenestratus (IM_2007_30418); D-E – Triplostephanus triseriatus (IM_2007_30404); F-G – Terebra guttata (IM_2007_30376);. Clade E5 (H-I) – Myurella kilburni (IM_2007_30461); Clade D (J-P): J- K – Hastula hectica, Philippines, Panglao Island; L – Hastula lanceata (IM_2007_30535); M-N – Hastula penicillata (IM_2007_30540), N – central part of the radular membrane; O-P – Hastula strigilata (IM_2007_30607); Q – Hastula stylata (IM_2009_10106). Scale bars: 50 μm (except E, G, P – 10 μm).
Character 6
Salivary glands (SG): 0 – absent, 1 – present. SG can be paired, but are more often fused, bipartite with paired ducts. In some species, a single gland is present.
Accessory salivary gland(s) are present in different species of Terebridae, as well as in some other conoideans. They usually are very small and difficult to find by dissection, therefore not used in the analysis.
2.6. Evolution of the anatomy
A reduced dataset was built for the 46 species (including the four outgroups) for which anatomical data were available. To minimize the risk of undetected cryptic species, the dissected and sequenced specimens were the same in most cases. However, for Pellifronia jungi and Hastulopsis pseudopertusa (Table 3), sequences were not obtained from the dissected specimens, and a conspecific specimen was used. Four species, Oxymeris dimidiata, O. maculata, Terebra subulata and Hastula hectica, were dissected by YK and John D. Taylor using non-sequenced material, and conspecific specimens were used for sequencing. ML analyses were performed using the method described above. The evolution of the six characters listed in Table 3, and described in the anatomy overview above, was assessed with Mesquite V2.74 (Maddison and Maddison, 2009), using the option “tracing character history” and the parsimony ancestral reconstruction method. The characters PR (proboscis), VG (venom gland), OD (odontophore), and RadT (marginal radular teeth anatomy) were treated as ordered characters (using a stepmatrix), prohibiting some of the transformation sequences, in our case from absent to present, as reapparition of these features is highly unlikely. Other characters were treated as unordered. Additionally, Bayestraits (Pagel and Meade, 2006) was used to test if the evolution of foregut characters were correlated. As Bayestraits cannot compare characters with more than two states, the character 5 (RadT) were recoded in two different characters, RadT1 and RadT2, with the states 0 “radula absent” and 1 “radula present” for RadT1, and states 0 “radula solid” and 1 “radula hypodermic” for RadT2. In the latter case, an absence of radula was coded as missing data. Independent and dependent models of Bayesdiscrete were compared. MCMC were run with default parameters, except for the number of generations, which were set to 2050000.
3. Results
3.1. Genetic diversity
Of the total of 406 samples of Terebridae used to reconstruct the molecular phylogeny of the family, 389 were sequenced for the COI gene, 400 for the 16S gene, 369 for the 12S gene and 63 for the 28S gene. For COI, 658 bp were sequenced and no indels were found. After the alignments and the removal of ambiguously aligned sites, fragments of 591, 654 and 761 bp in length were obtained for the 16S, 12S and 28S genes, respectively. For the COI gene, 218 different haplotypes were found, displaying 121 polymorphic sites and 278 parsimony informative sites. For the 16S gene, 162 different haplotypes were found, displaying 277 polymorphic sites and 235 parsimony informative sites. For the 12S gene, 164 different haplotypes were found, displaying 412 polymorphic sites and 369 parsimony informative sites. Representatives of the mitochondrial diversity were also sequenced for the 28S gene (62 specimens, including 2 outrgoups). Overall, the variability for the 28S gene was less important than for the mitochondrial genes, with 127 polymorphic sites and 94 parsimony informative sites.
3.2. Phylogenetic analyses: single-gene data sets
Modelgenerator results indicated that GTR + I + G model was the best-fit model of evolution for the four genes analyzed (COI: I = 0.47, α = 0.55; 16S: I = 0.56, α = 0.6; 12S: I = 0.3, α = 0.6 and 28S: I = 0.63, α = 0.4). Parameters of the models were estimated during the maximum likelihood and bayesian analyses for both single-gene and concatenated datasets (see below). For each gene analyzed, no supported conflict was found between the different analyses. In each of the four single gene analyses, the consensus tree showed the Terebridae to be monophyletic however, the relationships within terebrids were generally poorly resolved, with few well-supported clades (Supplementary data 1-4). Therefore only the results obtained for the combined datasets CD1 and CD2 are presented.
3.3. Phylogenetic analyses: combined data set 1 (CD1)
The best-fit model of evolution was GTR + I + G (I = 0.45, α = 0.59). Topologies derived from ML analyses of the combined data set 1 (CD1) were congruent with the topology derived from BA analyses. From these combined analyses, the Terebridae were found monophyletic, CD1, Posterior Probabilities PP = 0.99, Bootstraps B = 96% (Fig. 4). Within the Terebridae, the five major clades, Pellifronia, Oxymeris [= Acus], Terebra, Hastula and Myurella (clades A-E, respectively) previously identified in Holford et al. (2009a) were recovered. Each were still strongly supported (PP > 0.90, B > 70%), and the topological relationships among the clades were similar, e.g., clades B-E were grouped together (PP = 0.99, B = 90%) (Fig. 4, and see Fig. 2 in Holford et al. (2009a). A sixth clade, hereafter designated as clade F, is novel in the molecular analysis and presented here for the first time. Intra-clade relationships for clades A-F are detailed in Figures 5 and 6, and some shells are illustrated for each clade in Figure 7. Clade F appeared to be the sister group to clades B-E, although the corresponding node is not supported (PP = 0.93, B = 46%). It is comprised of six newly-sampled species, four from South Madagascar, one from Australia and one from New-Zealand. The species composition of clade A remained unchanged compared to Holford et al., 2009a and 2009b, still including a single species, and appearing to be the sister group to all the other clades (althgough without statistical support). A newly-sampled species from South Mozambique was added to clade B, now totalling eight species (PP = 0.99, B = 100%). Three newly- sequenced species, one from South Madagascar, one from South Mozambique, and one from Philippines and the Solomon Islands, were added to clade C, now comprising nineteen species (PP = 0.99, B = 73%). Clade D included eleven species, of which one species, sampled in Madagascar, was new to the taxon set (PP = 1, B = 100%). Clade E contained five well-supported subclades (E1-E5), but the relationships among these were in general poorly resolved. Clade E1 (PP = 1, B = 96%) included eleven species of which one, from Vanuatu and Australia, was new to the taxon set. Two newly-sequenced species, one from New Caledonia and one from Vanuatu and South Madagascar, were added to the thirteen species previously included in clade E2 (PP = 1, B = 97%). Clade E3 (PP = 0.97, B = 66%) included five species of which two, from the Coral Sea and Solomon Islands respectively, were new to the taxon set. Clade E4 (PP = 1, B = 75%) was new to the taxon set, with six species from Pacific Panama. Two newly-sampled species from Madagascar were added to clade E5, now comprising eight species (PP = 1, B = 94%).
Figure 4.
Likelihood phylogenetic tree obtained with 410 specimen sequences for the COI, 12S and 16S genes. Boostraps and Posterior Probabilities are indicated for each node (when > B = 70% and > PP = 0.90 respectively). The ten collapsed clades of Terebridae (A, B, C, D, E1, E2, E3, E4, E5 and F) are detailed on Figures 2-5.
Figure 5.
Likelihood phylogenetic tree for clades A, B, C, D, F. Boostraps and Posterior Probabilities are indicated for each node (when > 70 and > 0.90 respectively). For clarity purposes, intraspecific support values are not shown.
Figure 6.
Likelihood phylogenetic tree for the clades E1-E5. Boostraps and Posterior Probabilities are indicated for each node (when B > 70% and PP > 0.90 respectively). For clarity purposes, intraspecific support values are not shown.
Figure 7.
Illustration of some specimens in each clade. From left to right: Clade A: Pellifronia jungi IM_2007_30539; Clade B: Oxymeris maculata IM_2007_30370, Oxymeris crenulata IM_2007_30377, Oxymeris dimidiata IM_2007_30379; Clade C: Terebra argus IM_2007_30383, Terebra guttata IM_2007_30387, Terebra funiculata IM_2007_30394, Triplostephanus fujitai IM_2007_30482, Terebra cingulifera IM_2007_30485, Terebra tricolor IM_2007_30493; Clade D: Hastula strigilata IM_2007_30416, Hastula hectica IM_2007_30426, Hastula albula IM_2007_30437; Clade E1: Terenolla pygmaea IM_2009_10121, Hastulopsis pertusa IM_2007_30388, Clathroterebra fortunei IM_2007_30391, Myurella affinis IM_2007_30415; Clade E2: Terebra fijiensis IM_2007_30423, Terebra succincta IM_2007_30433, Terebra textilis IM_2007_30451, Myurella lineaperlata IM_2007_30471, Duplicaria sp. 3 IM_2009_10151; Clade E3: Terebra succincta IM_2007_16731, Myurella orientalis IM_2007_30515; Clade E4: Terebra elata IM_2007_42111, Terebra larvaeformis IM_2007_42113, Terebra puncturosa IM_2007_42116, Terebra berryi IM_2007_42144; Clade E5: Myurella undulata IM_2007_30384, Myurella paucistriata IM_2007_30453, Terebra sp. 5 IM_2007_30946; Clade F: Euterebra fuscolutea IM_2009_10112, Duplicaria albofuscata IM_2009_10162
Molecular analyses highlighted several incongruencies at the genus and species levels. With the exception of three genera (Oxymeris – clade B, Pellifronia – clade A and Terenolla – clade E1, the last two represented each by a single species), all the analyzed genera were found to be non-monophyletic. Clade B comprises eight species of the genus Oxymeris. As previously found (Holford et al., 2009a), clade C consists of 6 species of Triplostephanus and 13 of Terebra (s.s.), including Terebra subulata, the type species of Terebra. Clade D comprises eight species of Hastula and one Duplicaria. Clade E, the largest clade in terms of number of species, comprises primarily species of the genera Myurella, Clathroterebra, Terenolla, Hastulopsis, Strioterebrum, and the “Terebra” textilis-group (Terryn, 2007). However, as shown in Holford et al. (2009a), all these genera (except Terenolla) are polyphyletic, with species of each genus placed in several of the five clades E1-E5. Specifically, Myurella species were found in E1, E2, E3 and E5, Clathroterebra in E1 and E3, Hastulopsis in E1 and E5, Strioterebrum in E1 and E2, and species of Terebra (s.s.) are distributed in clades C, E2, E3, E4 and E5. Also, the addition of newly sampled species impacted the generic composition of clade E. For example, clade E2 now includes two species that were attributed to Duplicaria, D. baileyi and a new species D. sp3, and one species currently attributed to Triplostephanus. A newly sampled species, currently attributed to Hastulopsis (H. pseudopertusa), was included in clade E5. The new lineage, clade F, includes both Duplicaria and Euterebra species.
At species level, plumbeum, pertusa, strigilata, succincta and textilis each end up in two distinct clades, revealing cryptic species. The COI pairwise genetic distances (K2P) between the two clades were 9.6% for plumbeum, 9.9% for pertusa, 6.4% for strigilata, 12.47% for succincta and 7.73% for textilis. Fourteen different lineages (five in the genus Terebra, three in Strioterebrum, three in Duplicaria, and one each in Myurella, Triplostephanus and Hastula) were not identified to species level and may represent new species. Conversely, two specimens identified as Triplostephanus cumingii and Terebra punctatostriata (Clade C, Fig. 5) share almost identical sequences (no difference in the 16S gene and only four mutations in the 12S gene); revealing initial misidentification and/or synonymy of a species in the T. anilis complex.
3.4. Phylogenetic analyses: combined-gene data set 2 (CD2)
The best-fit model of evolution was GTR + I + G (I = 0.58, α = 0.55). The combined data set 2 (CD2) included 62 specimens for which at least two mitochondrial genes and the nuclear 28S gene were available. Topologies derived from both ML and BA analyses using CD2 were similar and consistent with the topology derived from analyses of the CD1 data set (Fig. 8). The family Terebridae was confirmed monophyletic (PP = 1, B = 89%). The nine clades (A-D, E1, E2, E3, E5 and F) represented in this dataset were also strongly supported, for some of them with PP and/or B superior to the supports obtained in CD1 analysis. Relationships between and within the main clades are generally similar, except for some non-supported nodes. For example, clade A is sister-group to all the other terebrids in CD1, but in CD2 its position is inverted with clade F.
Figure 8.
Likelihood phylogenetic tree obtained with 63 specimens sequences for the COI, 12S, 16S and 28S genes.
3.5. Evolution of foregut characters
Reconstruction of the evolution of the proboscis (character 1) clearly demonstrates that it was lost six times in Terebridae: in clades F, B, E1 (all species), and partially in clades E2, E4, and E5 (Fig. 9A). The venom gland (character 2) was lost eight times – in clades F, B, and E1 (all species), and partially in clades E2 (twice), E4 (twice), and E5 (Fig. 9B). In many lineages the odontophore (character 3) is completely absent (including all species having hypodermic marginal radular teeth) (Fig. 9C). Reconstruction of the presence of the odontophore showed that it was lost in most of the clades independently. It is present in clades A and F, and in some species of clades D, E3 and E2. It is vestigial, and hardly discernable in Hastula strigilata, to the extent that its presence was revealed only on serial histological sections (J.D.Taylor, personal communication). It is possible that a rudiment of the odontophore may be present in some other species of Hastula as well. Reconstruction of the presence of accessory proboscis structure (character 4) showed that it appeared independently in clades E1, E2, and E4 (Fig. 9D).
Figure 9.
Character mapping of the six characters presented in the Table 2. Bootstraps are shown for each node.
Reconstruction of the presence of the radula and of the morphology of marginal radular teeth (character 5) revealed a complicated evolutionary history of radular transformations (Fig. 9E). The radula was lost several times: in the entire clades B and E1, and in some species of clades E2 and E5. The most parsimonious ancestral state for the Terebridae radular teeth is the duplex type. Duplex teeth are variable in shape: in some species (Terebra succincta, clade E3, and Clathroterebra poppei – Figs. 2 B-C) the limb also has a thickened edge, while in Pellifronia jungi (Fig. 2A) the limb edge is not thickened. Analysis suggests that duplex teeth are the most parsimonious ancestral state for the entire clade E and that flat teeth originated from duplex ones in clade E2. Analysis was not able to resolve a single most parsimonious state for clade D, with duplex and semi-enrolled teeth being equally parsimonious. Solid recurved teeth appeared in the single clade F. Semi-enrolled teeth were found so far in a single of the species examined here, Hastula stylata (Fig. 3Q). Teeth of rather similar shape were recorded in Hastula bacillus (Taylor and Miller, 1990). Finally, hypodermic teeth appeared independently three times – in clade C, in clade D and in the single species, Myurella kilburni, from clade E5. However, the structure of the hypodermic teeth is slightly different in these three lineages. In the species belonging to clade C (Fig. 3A-G), the teeth are slender, have a constriction at the base, and usually a basal spur, i.e. an anterior projection on the base of the tooth. Another important character for the hypodermic radula of clade C is that the teeth are attached to the subradular membrane at their bases. In species of clade D (Hastula spp.), the hypodermic teeth are conical, without constriction at the base and without spur. Contrary to the species of clade C, the teeth are attached along most of their length to the subradular membrane. Species in clade D can have a barb or blade at the tip of the tooth. In Hastula hectica the walls of the tooth are penetrated by numerous holes as previously described (Imperial et al., 2007) (Fig. 3J). The only species in clade E5 with hypodermic teeth (Myurella kilburni) has teeth with a peculiar syringe-like shape, with very narrow, attenuated distal end (slightly less than half of tooth length) and broad and probably rather flacid basal part of the tooth. As the specimen examined was badly damaged, it was not possible to examine the radula of the single species of clade E4, Terebra elata, that possesses a venom gland, although the presence of a venom duct was noticed (Holford, personal observation) and the presence of a radula is highly probable.
Although found in several species, such as Triplostephanus fenestratus and Hastula hectica, the presence or absence of the accessory salivary glands cannot be confirmed without histological sections and therefore the character was excluded from the analysis. Reconstruction of the presence and absence of salivary glands (character 6) suggested independent loss in one species of clade B (Oxymeris felina), in most species of clade E1, in one species of clade E5 (Hastulopsis minipulchra) and one species of clade E2 (Duplicaria sp. 3) (Fig. 9F).
Bayestraits analyses revealed that the evolution of several characters is strongly correlated. As shown in Table 4, the results from Bayestraits analyses indicate that the evolution of the proboscis and the venom gland, of the proboscis and the radula (presence/absence), of the venom gland and the radula (presence/absence) and of the odontophore and the radula (solid/hypodermic) are all strongly correlated with bayes factors > 10. Additionally, the evolution of the proboscis and salivary glands, of the venom gland and the salivary glands, and of the radula (presence/absence) and the salivary gland are weakly correlated with bayes factors between 5 and 10 (Table 4).
Table 4.
Bayesfactor obtained with bayestraits from comparing the posterior probabilities of the independent and dependent models for seven discrete characters (PR = Proboscis, VG = venom gland, OD = Odontophore, APS = Accessory proboscis Structure, RadT1 and 2 = Marginal radular Teeth – see text for details, SG = Salivary glands).
| PR | VG | OD | APS | RadT1 | RadT2 | SG | |
|---|---|---|---|---|---|---|---|
| PR | |||||||
| VG | 35.16 | ||||||
| OD | −4 | −0.82 | |||||
| APS | −2.48 | 1.16 | −7.6 | ||||
| RadT1 | 14.9 | 27.48 | 4.14 | 4.3 | |||
| RadT2 | 1.58 | 0.04 | 11.56 | −2.72 | −0.38 | ||
| SG | 8.38 | 6.68 | −0.12 | −1.38 | 7.54 | −2.64 |
4. Discussion
A robust phylogenetic context was used to both clarify the phylogenetic relationships of the Terebridae and to provide a framework to trace the evolution of several anatomical features linked to the venom apparatus, a key innovation of the Conoidea. The molecular phylogeny of the Terebridae presented here was based on an extended dataset compared to the previous large-scale phylogeny of the group (Holford et al., 2009a, 2009b; Puillandre et al., 2011), tripling the number of specimens, doubling the number of species to include twelve out of the fifteen accepted genera, extending the sampled diversity to the West-Indian Ocean, and including an additional nuclear gene that strengthened the initial phylogeny exclusively based on mitochondrial genes. Analysis of terebrid foregut anatomy for the characters related to the presence of a venom apparatus, namely proboscis, venom gland and radula, and other characters, such as odontophore, accessory proboscis structure and salivary glands, identified unexpected evolutionary traits within the Terebridae, with implications for the whole superfamily Conoidea. Summarized below are our findings on the taxonomy, venom apparatus evolution, and predator-prey and toxin relationships in the Terebridae.
4.1. Taxonomy
The phylogenetic trees in this analysis confirmed the monophyly of the family Terebridae (Holford et al., 2009a, 2009b) and the existence of five major clades previously identified as Pellifronia, Acus [now Oxymeris], Terebra, Hastula, and Myurella, clades A-E, respectively (Holford et al., 2009a). A novel result for terebrid molecular analysis is the discovery of a new lineage, Clade F, which includes Euterebra and Duplicaria species, and appears to be the sister group to Clades B-E.
Our results suggest that taxonomic diversity of the family Terebridae is still inadequately understood. In several cases molecular data suggest the existence of at least two distinct species within what has been identified as a single morphospecies. In three cases (S. plumbeum, H. pertusa and T. succincta), the two cryptic species identified morphologically as one, were collected sympatrically, i.e. co-occuring in the same region, and sometimes syntopically, i.e. co-occuring at the same sampling station. This is the case for H. pertusa with includes two molecular species sampled at the same station in Santo, Vanuatu. The detection of several new cryptic lineages emphasizes that species diversity in the family Terebridae may be underestimated. Additonally, among the ca. hundred species analyzed in this study, about twenty could not be attributed to a species name according to the taxonomic literature, suggesting that they could represent new species or nominal species currently treated as synonyms.
Increasing the geographic and species diversity of Terebridae analysed in the molecular tree demonstrates that the current genus-level classification of the group is not tenable. Most of the genera recognized in the last working identification guide of the family are non-monophyletic (10 out of the 12 genera analyzed). For example, the genus Duplicaria, sampled for the first time in this study, represented by six species in our sampling, was found in three distinct clades (D, E2 and F). This was an unanticipated finding since Duplicaria, which is characterized by a shell axially ribbed, and a well- marked suture doubled on the whorls by an axial sculpture on the subsutural band (Terryn, 2007), is widely accepted in the taxonomy community and was one of the unambiguous genera recognized by Bratcher and Cernhorsky (1987). Similar problems were observed for Terebra and Myurella, where species were found in five (C, E2-5) and three (E3-5) distinct clades, respectively (see also Clathroterebra, Hastulopsis, Strioterebrum, Triplostephanus – Figs. 5-6). These examples imply that shell morphology, used to describe the diversity of terebrids, can be misleading at both genus and species levels, and can lead to an incorrect classification of the family.
Despite the extensive sampling efforts deployed to complete the taxonomic coverage, our dataset is still not exhaustive. It covers less than one quarter of the species diversity of the family, with 100 analyzed species out of the ~ 400 currently accepted species (WORMS – www.marinespecies.org), representing 12 out of the 15 currently accepted genera. Further sampling is needed to obtain the missing genera Granuliterebra, Microtrypetes and Pristiterebra. In addition, among the genera analyzed, numerous type-species are not represented. Considering that recent studies have shown that most terebrid genera are non-monophyletic, it will also be essential to include the numerous synonymised genera. Although further taxonomic investigations are needed to stabilize the classification of the family, the phylogeny presented here provides a robust framework to analyze the evolution of several characters linked to the venom apparatus in the Terebridae.
4.2. Venom apparatus evolution
The formation of the venom gland and the appearance of the feeding mechanism of Conoidea was the initial key apomorphy of the group (Kantor and Puillandre, in press). The unique mechanism of prey envenomation is the most outstanding character of Conoidea and includes use of individual marginal radular teeth (detached from the subradular membrane) at the proboscis tip for stabbing and injecting neurotoxins into prey (Taylor et al., 1993). Teeth of very different morphologies, i.e. not only hypodermic, are used in a similar manner. This was observed directly (e.g., Kohn, 1956) and inferred from serial sectioning of different conoideans (Kantor and Taylor, 1991). Until recently, the Terebridae remained relatively poorly studied anatomically and existing data confirmed a great disparity of anatomy of the foregut, with loss of major organs, including proboscis, venom gland and radula in many species. Nevertheless, due to the absence of a robust phylogeny, the evolution of the foregut remained largely uncertain, and loss and apparition of novel features were considered ancedotal. The results from this study indicate that the evolution of the venom apparatus is not straightforward, as key features, together with the loss of various structures of the foregut anatomy, have arisen independently on at least three occasions within terebrids. These anatomical modifications appear to be the rule rather than the exception.
Terebridae were always treated as a major independent lineage of Conoidea until the recent molecular phylogeny of the Conoidea superfamily was published (Puillandre et al., 2011). The Conoidea molecular phylogeny suggests that Terebridae is a sister group of the family Turridae (s.s.), the component species of which can possess a venom gland, a radula with strong subradular membrane, and have duplex marginal teeth. The discovery of true duplex teeth, and flat teeth, their derivatives in Terebridae was thus quite unexpected. Prior to this study only two types of radula were known in Terebridae, solid recurved teeth and hypodermic teeth. Duplex teeth appeared to be the ancestral state for the entire family Terebridae and this is consistent with the Turridae and Terebridae being sister- groups. Clade A, represented at the moment only by Pellifronia jungi and likely the sister clade to all other terebrids, has similar radula to that of Turridae.
As suggested by the Bayestraits analyses, the reduction and losses of foregut characters in many lineages of the Terebridae are not casual and have a functional explanation. All species possessing a venom gland have a corresponding radula and proboscis, as the bayes factors >10 for these characters indicate (Table 4). This is explained by the peculiarities of conoidean feeding mechanism, where envenomation of the prey requires the aid of the tooth gripped at the proboscis tip and used for stabbing the prey, or channelling the toxins through the internal lumen of hypodermic teeth. Currently, feeding of radulate terebrids was observed only in different Hastula and Terebra species with hypodermic radular teeth (Marcus and Marcus, 1960; Miller, 1970, 1979; Taylor, 1990; Taylor and Miller, 1990). The observations established that these species fed in a similar manner to other conoideans, with the use of marginal teeth at the proboscis tip. The prey reported were various sedentary polychaetes, mostly spionids. A characteristic feature of terebrid feeding is the well-developed rhynchostomal introvert, which is playing an active role in capturing and engulfing the prey.
Analysis of the anatomical characters revealed that hypodermic teeth originated three times independently in Terebridae, in clades C, D, and in a single species from clade E5, Myurella kilburni. As detailed in the results section, the hypodermic teeth of these three groups appear to be rather different (Fig. 3). Independent apparitions of hypodermic teeth suggest increasing the effectiveness of prey envenomation. A very interesting peculiarity was found in Hastula cinerea and H. hectica, both in clade D, where in most of the specimens examined, a tooth was held at the proboscis tip even when the species was not feeding, concealed within the proboscis with its base resting on the large sphincter (Marcus and Marcus, 1960; Imperial et al., 2007). This can be explained by the presence of a relatively strong subradular membrane and tough attachment of the teeth to the membrane. In Hastula, because the teeth in the radular cecum are still attached to the membrane, they cannot be immediately used for stabbing prey when required. In the process of radular growth, the oldest part of the membrane, situated in the radular cecum, is permanently destroyed and the teeth are dislodged. When the tooth is separated from the membrane, it is transferred to the proboscis tip, where it is presumably held until it is used. This is also assumed for members of the other families of “turrids” that have a strong subradular membrane. In most turrid specimens examined, there was a tooth at the proboscis tip held by the sphincter(s) (Kantor and Taylor, 1991).
Although nothing is known on the feeding of species with duplex/flat teeth, it is reasonable to suppose that they are used on the proboscis tip in a manner similar to other conoideans with non- hypodermic teeth. In this respect it was interesting to find in Terebra textilis at the proboscis tip flat teeth very similar to those of Terebra trismacaria (Fig. 2D). A group of four teeth attached to the subradular membrane was found in the buccal tube somewhat posterior to the proboscis tip. It is obvious that in this case the teeth cannot be used separately for stabbing the prey, but the mechanism of transport of the teeth from radular sac to the proboscis tip persists in this species. A probable explanation in this case represents an intermediate stage of reduction of radulae and transition to feeding without use of marginal teeth at the proboscis tip.
An odontophore is present in species that have a more or less strong subradular membrane and non-hypodermic radular teeth (bayes factor >10, Table 4). It is large and powerful in species of clade F, Duplicaria and Euterebra, which lack proboscis and venom gland and therefore do not utilize teeth for stabbing and envenomation of the prey. A well-developed odontophore suggests that the radula is functioning as a whole organ only, probably for transferring the prey from rhynchodaeum to oesophagus. There is no observation on feeding of species of this clade and diet is known for only one species with similar anatomy, Terebra nassoides, feeding on capitellid polychaetes (Taylor, 1990). Similarly to species with hypodermic radulae, an active role of the introvert in prey capture was also shown in Terebra gouldi, a species lacking venom apparatus, radula and proboscis. and that preys on the enteropneust Ptychodera flava, which is swallowed alive.
While reduction of the venom gland provides economy of energy that is otherwise used for producing toxins and constant formation of the radula, the rhynchostomal introvert, which is present and well-developed in all terebrids, may explain the numerous independent losses of the venom gland and associated organs. With the rhynchostomal introvert present, feeding becomes possible without stabbing and envenomation of the prey. In addition, the proboscis also becomes unnecessary, as its primary function, gripping the tooth, does not exist any more. The muscular buccal lip, which is well developed in radular-less species, serves for transferring the swallowed prey further into oesophagus. Although very little is known about diet of terebrids with such foregut anatomy, Miller (1975) suggested that they feed on different hemichordates. The family Raphitomidae is the only other taxon of Conoidea that possesses a developed rhynchostomal introvert. In that family numerous independent reductions and losses of the venom gland and radula were hypothesized (Kantor and Taylor, 2002). It was also suggested that these reductions were connected with the role of introvert in prey capture.
Bayestraits analysis revealed only weak correlations between presence of the salivary glands and proboscis, and of venom gland and presence/absence of radula (bayes factors between 6.68 and 8.38, Table 4). The low bayes factors suggest that salivary glands are not directly involved in process of envenomation of the prey. It should be noted however, that the salivary glands of cone snail species Conus pulicarius contained peptide toxins when analysed by transcriptome data (Biggs et al., 2008). The functions of the accessory proboscis structure remain unclear as its presence is not correlated with other foregut structures. It was suggested that it has chemosensory functions (Taylor, 1990; Taylor and Miller, 1990). The present data supports the idea that the accessory proboscis structure is not used directly in feeding processes, but may be related to detection of the prey.
4.3. Predator-prey and toxins
Numerous terebrid lineages have lost the venom apparatus, and by contrast the lineages that kept it each developed novel anatomical features, such as hypodermic marginal radular teeth. The components of the venom apparatus, radular, venom duct, venom bulb, and proboscis, were thought to be so complicated that they certainly evolved once or twice. However, the Terebridae acquired or lost similar structure several times, resulting in an anatomy sometimes convergent with that of other conoideans. In the Terebridae alone, a remarkable finding is that the hypodermic teeth, in association with reduction of the odontophore, have likely evolved on multiple and independent occasions. Additionally, the detailed anatomy demonstrates not only different origins of the teeth but also suggests differences in functional use. Analysis of radular evolution in the entire Conoidea indicate that besides terebrids, hypodermic teeth appeared only once in a major clade that unites the families Conidae, Conorbidae, Borsoniidae, Clathurellidae, Mitromorphidae, Mangeliidae and Raphitomidae (Kantor and Puillandre, in press).
The diversity of foregut anatomy in the single family Terebridae is as large as in the whole superfamily Conoidea, which includes 14 other families. For example, all major types of conoidean radular marginal teeth were recorded in the Terebridae. From prototypic duplex teeth they evolved: solid recurved teeth, which appeared independently in some Pseudomelatomidae; flat teeth, which appeared from duplex in some Drilliidae; and hypodermic teeth, which appeared independently in common ancestor of a major clade of Conoidea (Bouchet et al., 2011; Kantor and Puillandre, in press). Moreover, the flat triangular teeth of some Terebridae are unique among Conoidea. The overview of the foregut anatomy presented in this study revealed an inordinate diversity of features in the family Terebridae. These results suggest that predator-prey relationships have played an important role in the evolutionary history of Terebridae. Indeed, repeated innovations in the foregut anatomy of terebrids suggest that they adapted to different diets (e.g., deposit-feeding or carnivorous polychaetes). To date, this hypothesis remains untested as the prey of most of the analyzed terebrid species are unknown. This could be analysed by direct observation, or by indirect approaches, such as DNA-barcoding of the gut contents (Garros et al., 2008; Oliverio et al., 2009) or analysis of stable isotopes composition (Fujikura et al., 2009).
Based on the hypothesis that the diversity of foregut structures in the Terebridae is linked to the diversity of feeding types and preys, it could also be argued that the species diversity of the Terebridae could be linked to the prey diversity, and thus to foregut anatomy. However, the results also illustrate that several species may share an apparently identical foregut structure, suggesting that the diversity of the foregut and the prey are not the only factor at the origin of the species diversity in the Terebridae and other features of the prey-capture system should be investigated e.g., reduced dispersion abilities and geographical isolation (Bouchet, 1981; Duda and Palumbi, 1999; Cunha et al., 2005; Meyer et al., 2005; Cunha et al., 2008; Castelin et al., 2010), or differential selection by abiotic factors such as depth (Chase et al., 1998; Quattro et al., 2001; Zardus et al., 2006). Given the rate of evolution of conopeptides in cone snails, it can be argued that various Terebridae species evolved different toxins as an answer, or a consequence, to prey adapation. Integrative approaches will be employed to complete the phylogeny of the Terebridae, identify their respective preys, and compare their foregut anatomy and the peptide toxins they produce. An integrated approach is not only a promising way to identify the factors that led to the diversification of the Terebridae and potentially the (co-)evolution of their prey, but is also a step forward in the characterization of novel terebrid toxins with novel function and potentially new therapeutic applications. Terebrids have clearly evolved different responses to the costs and benefits of having a venom apparatus under varying conditions. Using, for example, phylogenetic independent contrasts (Felsenstein, 1985b), the large-scale phylogeny presented here could assist in analysing the potential correlation between the anatomical innovations developed by the Terebridae and various biotic and abiotic parameters.
Supplementary Material
Supplementary Figure 1. Likelihood phylogenetic tree obtained with 389 specimen sequences for the COI gene.
Supplementary Figure 2. Likelihood phylogenetic tree obtained with 400 specimen sequences for the16S gene.
Supplementary Figure 3. Likelihood phylogenetic tree obtained with 369 specimen sequences for the 12S gene.
Supplementary Figure 4. Likelihood phylogenetic tree obtained with 63 specimen sequences for the 28S gene.
Highlights.
An expanded molecular phylogeny of venomous marine snails Terebridae is presented. > Six characters associated with the venom apparatus are used to map terebrid evolution. > Hypodermic teeth and other innovations have likely evolved on multiple occasions. > Multiple radular origins may reflect variable functionalities associated to feeding. > Terebrids may have adapted to dietary changes following predator-prey relationships.
ACKNOWLEDGMENTS
In addition to the sources enumerated in Holford et al. (2009b), a large part of the material used in the present paper was collected in Mozambique and Madagascar in 2009-2010 during expeditions Mainbaza, Miriky and Atimo Vatae, a cluster of expeditions funded by the Total Foundation, Prince Albert II of Monaco Foundation, and Stavros Niarchos Foundation, and conducted by MNHN and Pro- Natura International (PNI) as part of their "Our Planet Reviewed" programme. The authors also thank Felix Rodriquez, Edwin Diaz, Trinidad Pardo, Moises Bernal, and the crew of the RV-Urraca with collection efforts in Panama. This work was supported by a grant from The Alfred P. Sloan Foundation (B2010-37), NSF (0940108), and NIH-NIGMS (GM088096) to MH; by the “Consortium National de Recherche en Génomique” and the “Service de Systématique Moléculaire” (UMS 2700 CNRS-MNHN) as part of agreement 2005/67 between Genoscope and MNHN for the project “Macrophylogeny of life” directed by Guillaume Lecointre; and by grant RFBR 11-04-01284 “Evolution of digestive system of carnivorous gastropods: testing of morphologically-based hypotheses by molecular data” (PI Yu. Kantor). NP was partly funded by CONCO, the cone snail genome project for health, funded by the European Commission: LIFESCIHEALTH-6 Integrated Project LSHB-CT-2007-037592. The phylogenetic analyses were performed on the Cipres Science Gateway. The authors want to thank Dr. John Taylor for sharing unpublished information on terebrid anatomy and providing material from Australia, and Barbara Buge and José Utge for processing and curation of the molecular collection. The authors thank Dr. Thomas F Duda, Jr. and Dr. Suzanne Williams for very constructive comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Barlow A, Pook CE, Harrison RA, Wüster W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. R. Soc. Biol. Sci. Ser. B. 2009;276:2443–2449. doi: 10.1098/rspb.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnosky AD. Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the northern Rocky Mountains. J. Vertebr. Paleontol. 2001;21:172–185. [Google Scholar]
- Berenbaum M. Coumarins and caterpillars: a case for coevolution. Evolution. 1983;37:163–179. doi: 10.1111/j.1558-5646.1983.tb05524.x. [DOI] [PubMed] [Google Scholar]
- Berenbaum M, Feeny P. Toxicity of angular furanocoumarins to swallowtail butterflies: escalation in a coevolutionary arms race? Science. 1981;212:927–929. doi: 10.1126/science.212.4497.927. [DOI] [PubMed] [Google Scholar]
- Biardi JE, Chien DC, Coss RG. California ground squirrel (Spermophilus beecheyi) defenses against rattlesnake venom digestive and hemostatic toxins. J. Chem. Ecol. 2005;31:2501–2518. doi: 10.1007/s10886-005-7610-1. [DOI] [PubMed] [Google Scholar]
- Biggs JS, Olivera BM, Kantor YI. [alpha]-Conopeptides specifically expressed in the salivary gland of Conus pulicarius. Toxicon. 2008;52:101–105. doi: 10.1016/j.toxicon.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet P. Evolution of larval development in eastern Atlantic Terebridae (Gastropoda), Neogene to Recent. Malacologia. 1981;21:363–369. [Google Scholar]
- Bouchet P, Kantor YI, Sysoev A, Puillandre N. A new operational classification of the Conoidea (Gastropoda) J. Molluscan Stud. 2011;77:273–308. [Google Scholar]
- Bratcher T, Cernohorsky WO. Living terebras of the world: a monograph of the recent Terebridae of the world. American Malacologists; Melbourne: 1987. [Google Scholar]
- Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- Casewell N, Harrison R, Wüster W, Wagstaff S. Comparative venom gland transcriptome surveys of the saw-scaled vipers (Viperidae: Echis) reveal substantial intra-family gene diversity and novel venom transcripts. BMC Genomics. 2009;10:564–576. doi: 10.1186/1471-2164-10-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelin M, Lambourdiere J, Boisselier C, Lozouet P, Couloux A, Cruaud C, Samadi S. Hidden diversity and endemism on seamounts: focus on poorly dispersive neogastropods. Biol. J. Linn. Soc. 2010;100:420–438. [Google Scholar]
- Chase MR, Etter RJ, Rex MA, Quattro JM. Bathymetric patterns of genetic variation in a deep-sea protobranch bivalve, Deminucula atacellana. Mar. Biol. 1998;131:301–308. [Google Scholar]
- Chin YW, Balunas MJ, Chai HB, Kinghorn AD. Drug discovery from natural sources. The AAPS Journal. 2006;8:239–253. doi: 10.1007/BF02854894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RL, Castilho R, R¸ber L, Zardoya R. Patterns of cladogenesis in the venomous marine gastropod genus Conus from the Cape Verde Islands. Syst. Biol. 2005;54:634–650. doi: 10.1080/106351591007471. [DOI] [PubMed] [Google Scholar]
- Cunha RL, Tenorio MJ, Afonso C, Castilho R, Zardoya R. Replaying the tape: recurring biogeographical patterns in Cape Verde Conus after 12 million years. Mol. Ecol. 2008;17:885–901. doi: 10.1111/j.1365-294X.2007.03618.x. [DOI] [PubMed] [Google Scholar]
- Daltry JC, Wüster W, Thorpe RS. Diet and snake venom evolution. Nature. 1996;379:537–540. doi: 10.1038/379537a0. [DOI] [PubMed] [Google Scholar]
- Duda TF. Differentiation of venoms of predatory marine gastropods: divergence of orthologous toxin genes of closely related Conus species with different dietary specializations. J. Mol. Evol. 2008;67:315–321. doi: 10.1007/s00239-008-9155-8. [DOI] [PubMed] [Google Scholar]
- Duda TF, Jr., Palumbi SR. Molecular genetics of ecological diversification: duplication and rapid evolution of toxin genes of the venomous gastropod Conus. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6820–6823. doi: 10.1073/pnas.96.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich PR, Raven PH. Butterflies and plants: a study in coevolution. Evolution. 1964;18:586–608. [Google Scholar]
- Farrell BD, Dussourd DE, Mitter C. Escalation of plant defense: do latex and resin canals spur plant diversification? Am. Nat. 1991;138:881–900. [Google Scholar]
- Fedosov A, Kantor Y. Toxoglossan gastropods of the subfamily Crassispirinae (Turridae) lacking a radula, and a discussion of the status of the subfamily Zemaciinae. J. Molluscan Stud. 2008;74:27. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985a;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985b;125:1–15. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- Fowler M. Plant poisoning in free-living wild animals: a review. J. Wildl. Dis. 1983;19:34–43. doi: 10.7589/0090-3558-19.1.34. [DOI] [PubMed] [Google Scholar]
- Fry BG, Wüster W, Kini RM, Brusic V, Khan A, Venkataraman D, Rooney AP. Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J. Mol. Evol. 2003;57:110–129. doi: 10.1007/s00239-003-2461-2. [DOI] [PubMed] [Google Scholar]
- Fujikura K, Sasaki T, Yamanaka T, Yoshida T. Turrids whelk, Phymorhynchus buccinoides feeds on Bathymodiolus mussels at a seep site in Sagami Bay, Japan. Plank. Benth. Res. 2009;4:23–30. [Google Scholar]
- Garros C, Ngugi N, Githeko AE, Tuno N, Yan G. Gut content identification of larvae of the Anopheles gambiae complex in western Kenya using a barcoding approach. Mol. Ecol. Res. 2008;8:512–518. doi: 10.1111/j.1471-8286.2007.02013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acid. S. 1999;41:95–98. [Google Scholar]
- Heard SB, Hauser DL. Key evolutionary innovations and their ecological mechanisms. Hist. Biol. 1995;10:151–173. [Google Scholar]
- Heatwole H, Poran NS. Resistances of sympatric and allopatric eels to sea snake venoms. Copeia. 1995;1:136–147. [Google Scholar]
- Hodges SA, Arnold ML. Spurring plant diversification: are floral nectar spurs a key innovation? Proc. Biol. Sci. 1995:343–348. [Google Scholar]
- Holford M, Puillandre N, Modica M, Watkins M, Collin R, Bermingham E, Olivera B. Correlating molecular phylogeny with venom apparatus occurrence in Panamic auger snails (Terebridae) PLoS ONE. 2009a;4:e7667. doi: 10.1371/journal.pone.0007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holford M, Puillandre N, Terryn Y, Cruaud C, Olivera B, Bouchet P. Evolution of the Toxoglossa venom apparatus as inferred by molecular phylogeny of the Terebridae. Mol. Biol. Evol. 2009b;26:15–25. doi: 10.1093/molbev/msn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. Role of natural product diversity in chemical biology. Curr. Opin. Chem. Biol. 2011;15:350–354. doi: 10.1016/j.cbpa.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MrBayes: a program for the Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Imperial JS, Kantor Y, Watkins M, Heralde FM, Iii, Stevenson B, Chen P, Hansson K, Stenflo J, Ownby JP, Bouchet P. Venomous auger snail Hastula (Impages) hectica (Linnaeus, 1758): molecular phylogeny, foregut anatomy and comparative toxinology. J. Exp. Zool. B Mol. Dev. Evol. 2007;308:744–756. doi: 10.1002/jez.b.21195. [DOI] [PubMed] [Google Scholar]
- Jovelin R, Justine J-L. Phylogenetic relationships within the Polyopisthocotylean monogeneans (Plathyhelminthes) inferred from partial 28S rDNA sequences. Int. J. Parasitol. 2001;31:393–401. doi: 10.1016/s0020-7519(01)00114-x. [DOI] [PubMed] [Google Scholar]
- Kantor Y, Puillandre N. Evolution of the radular apparatus in Conoidea (Gastropoda: Neogastropoda) as inferred from a molecular phylogeny. Malacologia. in press. [Google Scholar]
- Kantor YI, Taylor JD. Evolution of the toxoglossan feeding mechanism: new information on the use of the radula. J. Molluscan Stud. 1991;57:129. [Google Scholar]
- Kantor YI, Taylor JD. Foregut anatomy and relationships of raphitomine gastropods (Gastropoda: Conoidea: Raphitominae) In: Oliverio M, Chemello R, editors. Systematics, phylogeny and biology of the Neogastropoda. Bollettino Malacologico; Roma: 2002. pp. 161–174. [Google Scholar]
- Keane T, Creevey C, Pentony M, Naughton T, Mclnerney J. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol. Biol. 2006;6:29. doi: 10.1186/1471-2148-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AJ. Piscivorous gastropods of the genus Conus. Proc. Natl. Acad. Sci. U. S. A. 1956;42:168–171. doi: 10.1073/pnas.42.3.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordis D, Gubensek F. Adaptive evolution of animal toxin multigene families. Gene. 2000;261:43–52. doi: 10.1016/s0378-1119(00)00490-x. [DOI] [PubMed] [Google Scholar]
- Kozminsky-Atias A, Bar-Shalom A, Mishmar D, Zilberberg N. Assembling an arsenal, the scorpion way. BMC Evol. Biol. 2008;8 doi: 10.1186/1471-2148-8-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock W. Coevolution of poisonous plants and large herbivores on rangelands. J. Range Manag. 1978;31:335–342. [Google Scholar]
- Lynch V. Inventing an arsenal: adaptive evolution and neofunctionalization of snake venom phospholipase A2 genes. BMC Evol. Biol. 2007;7:2. doi: 10.1186/1471-2148-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison D. Mesquite: a modular system for evolutionary analysis. 2009.
- Marcus E, Marcus E. On Hastula cinerea. Boletim da Faculdade de Filosofía Ciencias e Letras. Universidade de Sao Paulo (Zoologia); 1960. pp. 25–66. [Google Scholar]
- Meyer CP, Geller JB, Paulay G. Fine scale endemism on coral reefs: archipelagic differentiation in Turbinid gastropods. Evolution. 2005;59:113–125. [PubMed] [Google Scholar]
- Miller B. Studies on the biology of Indo-Pacific Terebra (Ph. D. dissertation) University of New Hampshire; Durham: 1970. [Google Scholar]
- Miller BA. The biology of Terebra gouldi Deshayes, 1859, and a discussion of life history similarities among other terebrids of similar proboscis type. Pac. Sci. 1975;29:227–241. [Google Scholar]
- Miller BA. The biology of Hastula inconstans (Hinds, 1844) and a discussion of life history similarities among other Hastulas of similar proboscis type. Pac. Sci. 1979;33:289–306. [Google Scholar]
- Modica MV, Holford M. The Neogastropoda: evolutionary innovations of predatory marine snails with remarkable pharmacological potential. In: Pontarotti P, editor. Evolutionary biology - concepts, molecular and morphological evolution. Springer; Heidelberg: 2010. pp. 249–270. [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Olivera BM. Conus venom peptides, receptor and ion channel targets and drug design: 50 million years of neuropharmacology (EE Just Lecture, 1996) Mol. Biol. Cell. 1997;8:2101–2109. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliverio M, Barco A, Modica M, Richter A, Mariottini P. Ecological barcoding of corallivory by second internal transcribed spacer sequences: hosts of coralliophiline gastropods detected by the cnidarian DNA in their stomach. Mol. Ecol. Res. 2009;9:94–103. doi: 10.1111/j.1755-0998.2008.02388.x. [DOI] [PubMed] [Google Scholar]
- Pagel M, Meade A. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. Am. Nat. 2006;167:808–825. doi: 10.1086/503444. [DOI] [PubMed] [Google Scholar]
- Palumbi SR. Nucleic acids II: the polymerase chain reaction. In: Hillis DM, Mable BK, Moritz C, editors. Molecular systematics. Sinauer Associates; Sunderland: 1996. pp. 205–247. [Google Scholar]
- Puillandre N, Holford M. The Terebridae and teretoxins: combining phylogeny and anatomy for concerted discovery of bioactive compounds. BMC Chem. Biol. 2010;10:7. doi: 10.1186/1472-6769-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puillandre N, Kantor YI, Sysoev A, Couloux A, Meyer C, Rawlings T, Todd J, Bouchet P. The dragon tamed? A molecular phylogeny of the Conoidea (Gastropoda) J. Molluscan Stud. 2011;77:259–272. [Google Scholar]
- Puillandre N, Samadi S, Boisselier MC, Sysoev AV, Kantor YI, Cruaud C, Couloux A, Bouchet P. Starting to unravel the toxoglossan knot: molecular phylogeny of the “turrids”(Neogastropoda: Conoidea) Mol. Phylogenet. Evol. 2008;47:1122–1134. doi: 10.1016/j.ympev.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Puillandre N, Sysoev A, Olivera B, Couloux A, Bouchet P. Loss of planktotrophy, fragmentation and speciation: the deep-water gastropod genus Bathytoma (Gastropoda, Conoidea) in the western Pacific. Syst. Biodivers. 2010;8:371–394. [Google Scholar]
- Quattro, Chase, Rex, Greig, Etter Extreme mitochondrial DNA divergence within populations of the deep-sea gastropod Frigidoalvania brychia. Mar. Biol. 2001;139:1107–1113. [Google Scholar]
- Rambaut A, Drummond AJ. Tracer. 2007.
- Sanderson MJ, Donoghue MJ. Shifts in diversification rate with the origin of angiosperms. Science. 1994;264:1590–1593. doi: 10.1126/science.264.5165.1590. [DOI] [PubMed] [Google Scholar]
- Saslis-Lagoudakis CH, Klitgaard BB, Forest F, Francis L, Savolainen V, Williamson EM, Hawkins JA. The use of phylogeny to interpret cross-cultural patterns in plant use and guide medicinal plant discovery: an example from Pterocarpus (Leguminosae) PloS One. 2011;6:e22275. doi: 10.1371/journal.pone.0022275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Taylor J. The anatomy of the foregut and relationships in the Terebridae. Malacologia. 1990;32:19–34. [Google Scholar]
- Taylor J, Miller J. A new type of gastropod proboscis: The foregut of Hastula bacillus (Gastropoda: Terebridae) J. Zool. 1990;220:603–617. [Google Scholar]
- Taylor JD, Kantor YI, Sysoev AV. Foregut anatomy, feeding mechanisms, relationships and classification of the Conoidea (= Toxoglossa)(Gastropoda) Bull. Natl. Hist. Mus. Zool. Ser. 1993;59:125–170. [Google Scholar]
- Terryn Y. A collectors guide to recent Terebridae (Mollusca: Neogastropoda) ConchBooks/Natural Art; Hackenheim: 2007. [Google Scholar]
- Terryn Y. Family Terebridae Mörch, 1852. In: Severns M, editor. Shells of the Hawaiian Islands. The sea shells. Conchbooks; Hackenheim: 2011. pp. 370–381. [Google Scholar]
- Terryn Y, Holford M. The Terebridae of the Vanuatu archipelago with a revision of the genus Granuliterebra Oyama 1961. Visaya Supplement. 2008;3:6–118. [Google Scholar]
- Van Valen L. A new evolutionary law. Evol. Theor. 1973;1:1–30. [Google Scholar]
- Vermeij GJ. Evolution and escalation: an ecological history of life. Princeton University Press; Princeton: 1993. [Google Scholar]
- Williams ST, Ozawa T. Molecular phylogeny suggests polyphyly of both the turban shells (family Turbinidae) and the superfamily Trochoidea (Mollusca: Vetigastropoda) Mol. Phylogenet. Evol. 2006;39:33–51. doi: 10.1016/j.ympev.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Wüster W, Daltry JC, Thorpe RS. Can diet explain intraspecific venom variation? Reply to Sasa. Toxicon. 1999;37:253–258. doi: 10.1016/s0041-0101(98)00121-4. [DOI] [PubMed] [Google Scholar]
- Zangerl A, Stanley M, Berenbaum M. Selection for chemical trait remixing in an invasive weed after reassociation with a coevolved specialist. Proc. Natl. Acad. Sci. 2008;105:4547–4552. doi: 10.1073/pnas.0710280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardus JD, Etter RJ, Chase MR, Rex MA, Boyle EE. Bathymetric and geographic population structure in the pan Atlantic deep sea bivalve Deminucula atacellana (Schenck, 1939) Mol. Ecol. 2006;15:639–651. doi: 10.1111/j.1365-294X.2005.02832.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Likelihood phylogenetic tree obtained with 389 specimen sequences for the COI gene.
Supplementary Figure 2. Likelihood phylogenetic tree obtained with 400 specimen sequences for the16S gene.
Supplementary Figure 3. Likelihood phylogenetic tree obtained with 369 specimen sequences for the 12S gene.
Supplementary Figure 4. Likelihood phylogenetic tree obtained with 63 specimen sequences for the 28S gene.