Abstract
Biomedical evidence in the last 20 years has shown that the consumption of partially hydrolyzed guar gum may influence lipid and/or carbohydrate metabolism at many levels. Since intestine represents the first interface to interact with dietary partially hydrolyzed guar gum in vivo, we evaluated gene expression profiles in small intestinal mucosa of db/db mice fed with partially hydrolyzed guar gum in an effort to delineate its effect on the small intestine. DNA microarray and real-time PCR analyses were performed to evaluate the gene expression profiles in mice small intestinal mucosa. Among the 28,853 transcripts represented on the GeneChip® microarray, no more than 20 genes exhibited up- or down-regulation by 1.5-fold or more after four weeks following partially hydrolyzed guar gum consumption. No adverse effects were apparent. We detected up- or down-regulation of some genes known to be involved in host defense functions and cholesterol absorption.
Keywords: partially hydrolyzed guar gum, DNA microarray, db/db mice, host defense function, cholesterol
Introduction
Metabolic syndrome represents a group of physiological and biochemical abnormalities characterized by diabetes or high fasting glucose, central obesity, abnormal cholesterol and triglyceride levels, and hypertension.(1,2) The number of patients diagnosed with metabolic syndrome is rapidly increasing, and this disease is a major problem in the field of epidemics worldwide since it increases the risk of cardiovascular and cerebrovascular disease.(3)
Partially hydrolyzed guar gum (PHGG) is a water-soluble dietary fiber produced by the controlled partial enzymatic hydrolysis of guar gum seeds,(4) and its average molecular weight is 20 k (range, 1–100 k). Partially hydrolyzed guar gum, which is readily soluble in water, has a molecular weight that is almost 10 times lower and a viscosity that is 200 to 300 times lower than that of guar gum.(5) Partially hydrolyzed guar gum is used in a variety of ways in various beverages and foods such as a bulking agent, an alternative to wheat flour, a food stabilizer and a source of dietary fiber.(6)
Generally, dietary fibers are indigestible food ingredients that reach the colon and are then fermented by colonic bacteria, resulting mainly in the formation of short-chain fatty acids (SCFA) such as acetate, propionate and butyrate.(7) An in vitro study showed that PHGG moderately enhanced the growth of several bacterial strains,(8) and fresh feces inoculated with PHGG resulted in the production of SCFA.(9)
Considerable attention over the last 20 years has focused on the possible beneficial effect of dietary PHGG on treating diarrhea,(10) cholera,(11) irritable bowel syndrome,(12) and metabolic syndrome-related functions such as elevated fat and cholesterol levels,(13) and increases in postprandial blood glucose levels.(14–16) Taken together, it is clear that PHGG is capable of regulating, either directly or indirectly, a number of processes in the intestine, the first interactive interface with dietary PHGG in vivo, and other tissues. Many of these effects are likely to be mediated at the level of the transcriptome, although the molecular mechanism of action of PHGG on intestinal cellular processes, including cholesterol and glucose absorption, remains unknown. Elucidation of the molecular mechanism of action of PHGG may contribute towards the development of effective treatments of metabolic syndrome.
The availability of microarray technology, which allows for the investigation of the effect of substances such as PHGG on gut function by measuring the simultaneous expression of thousands of genes, offers the potential to gain valuable insights into the possible mechanism of action of PHGG. Therefore, in an effort to better understand the molecular mechanism by which PHGG affects intestinal cells, we compared the effect of PHGG on global gene expression in mouse intestinal mucosa using microarray technology. In addition to investigating the effect on global gene expression, particular emphasis was given to investigating the effect of PHGG on the expression of genes pertaining to processes involved in host defense functions and cholesterol absorption.
Materials and Methods
Partially hydrolyzed guar gum used
A commercial preparation of PHGG, ”Sunfiber” (Taiyo Kagaku Co., Ltd., Yokkaichi, Japan), was used in this study. The PHGG was prepared by treatment of guar gum with β-endogalacto-mannase from a strain of Aspergillus niger, and the average molecular mass of the PHGG as measured by HPLC was approximately 20 kDa. The total dietary fiber content of the PHGG was 80% as determined by the method of the Association of Official Agricultural Chemists.
Animals and diet
Maintenance of the animals and experimental procedures were carried out in accordance with the guidelines of the US National Institutes of Health for the Use of Experimental Animals. The procedures were approved by the Animal Care Committee of the Taiyo Kagaku Co., Ltd.
Four-week-old male db/db mice, a rodent model of type 2 diabetes, were purchased from Charles River Japan (Yokohama, Japan). Mice were housed individually in cages in a room kept at 18–24°C and 40–70% relative humidity with a 12-h light/dark cycle. Animals were allowed free access to their food and drinking water. First, mice were fed with a diet recommended by the American Institute of Nutrition, AIN-93G, prepared by Oriental Yeast Co., Ltd. (Tokyo, Japan) for 1 week during their acclimatization period. They were then divided into three groups of 10 animals each, comprising: (1) 0% PHGG group; diet without PHGG, (2) 2.5% PHGG group; diet with 2.5% of PHGG and 2.5% of cellulose, (3) 5.0% PHGG group; diet with 5.0% of PHGG. The composition of each diet is described in Table 1.
Table 1.
Composition of AIN-93G diet, modified by an increase in the quantity of cellulose or substitution of cellulose with PHGG (control diet)
| Ingredients (g/kg) | 0% PHGG | 2.5% PHGG | 5.0% PHGG |
|---|---|---|---|
| Cellulose | 50 | 25 | 0 |
| PHGG | 0 | 25 | 50 |
| Casein | 200 | ||
| L-cystine | 3 | ||
| Corn starch | 397.486 | ||
| Gelatinized corn starch | 132 | ||
| Sucrose | 100 | ||
| Soybean oil | 70 | ||
| AIN-93 mineral mixture | 35 | ||
| AIN-93G vitamin mixture | 10 | ||
| Choline bitartrate | 2.5 | ||
| t-Butylhydroquinone | 0.014 | ||
Blood and tissue collection
After 4 weeks of feeding, mice from each group were deprived of food overnight. The following morning, mice were lightly anesthetized with ether and blood samples were obtained from the retro-orbital plexus of mice into a syringe containing heparin. Mice were then killed by cervical dislocation. The intestinal mucosa (lower 1/3 part of the small intestine, from the pylorus to vermiform appendix) was recovered by scraping with glass slides.
Determination of blood lipid parameters and glucose levels
Plasma was obtained from blood isolated by low speed centrifugation at 1,700 × g for 10 min at 4°C. Plasma triglyceride (TG), total cholesterol (T-CHO), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and glucose (GLU) levels were analyzed by the Oriental Yeast Co. Ltd.
DNA microarray analysis
RNA was recovered using ISOGEN (Nippon Gene, Tokyo, Japan) according to the manufacturer’s instructions, and subjected to microarray analysis using Mouse Gene 1.0 ST Array (Affymetrix, Santa Clara, CA) according to the manufacturer’s instructions. Details of sample preparation and processing for microarray analysis are available from Affymetrix.
RT and quantitative PCR analysis
Reverse transcription (RT) was performed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster, CA), Quantitative real-time PCR was performed using a Power SYBR® Green PCR Master Mix and 7300 Real time PCR system (Applied Biosystems). The β-actin gene was used as an internal control. PCR reactions for each target and control gene were performed in triplicate. Primer sequences used were as follows: Oas3 based on NM_145226: forward, 5'-CCAACCCAAGTGCCAATAAAA-3', and reverse, 5'-AACATGACTTGAGATACTATCCAATGCT-3', Oas1g based on NM_011852: forward, 5'-GCTGTGGTACCC ATGTTTTATGAA-3', and reverse, 5'-AACCACCGTCGGCA CATC-3', Duox2 based on NM_177610: forward, 5'-GCAACCC AACGTTTTCTTCTG-3', and reverse, 5'-GCAAGCCGTCCG TTGTG-3', Nlrc5 based on FJ889356: forward, 5'-AAGGGA CCTCGAGTGACAGTACTACT-3', and reverse, 5'-TCCAGCG GAGCCGATATG-3', Soat1 based on NM_009230: forward, 5'-GAATATCAAACAGGAGCCCTTCA-3', and reverse, 5'-AAG ACACCTGGCAAGATGGAGTT-3', and β-actin based on NM_007393: forward, 5'-TATCCACCTTCCAGCAGATGT-3', and reverse, 5'-AGCTCAGTAACAGTCCGCCTA-3'.
Statistical analyses
All results are expressed as mean ± standard deviation (SD). The microarray data were analyzed statistically using one-way analysis of variance (ANOVA) between the 0% PHGG, 2.5% PHGG and 5.0% PHGG groups (Partek Genomics Suite; Partek Inc., St. Louis, MO). Quantitative real-time PCR, weight gain, food intake, blood lipid parameters and glucose data were analyzed statistically by a student t test using the Microsoft Excel data analysis program. p values less than 0.05 and 0.10 were considered to indicate significant difference and a tendency to be different, respectively.
Results
Effect of PHGG on weight gain and food intake
When db/db mice were fed diets containing PHGG for 4 weeks, there was no significant difference among the groups; either in terms of food intake or weight gain (Table 2).
Table 2.
Effect of dietary PHGG on weight gain and food intake of mice
| 0% PHGG | 2.5% PHGG | 5.0% PHGG | |
|---|---|---|---|
| Initial body weight (g) | 17.8 ± 1.4 | 18.1 ± 1.2 | 17.7 ± 1.2 |
| Final body weight (g) | 32.3 ± 1.7 | 31.1 ± 2.4 | 31.9 ± 2.0 |
| Weight gain (g) | 16.3 ± 5.6 | 13.0 ± 2.2 | 14.2 ± 2.1 |
| Food intake (g/day) | 5.8 ± 0.9 | 5.4 ± 0.4 | 4.4 ± 0.4 |
Effect of PHGG on blood lipid parameters and glucose levels
Dietary PHGG did not significantly affect plasma lipid parameters or glucose levels (Table 3). However, lower levels of LDL-C were found in the 2.5% PHGG group compared to the 0% PHGG group (p = 0.067), and lower levels of T-CHO (p = 0.095) and GLU (p = 0.080) were found in the 5.0% PHGG group compared to the 0% PHGG group.
Table 3.
Effect of dietary PHGG on blood lipid parameters and glucose levels in mice
| 0% PHGG | 2.5% PHGG | 5.0% PHGG | |
|---|---|---|---|
| TG (mg/dl) | 78 ± 23 | 89 ± 31 | 58 ± 30 |
| T-CHO (mg/dl) | 161 ± 19 | 141 ± 31 | 138 ± 19* |
| HDL-C (mg/dl) | 91 ± 11 | 81 ± 18 | 78 ± 12 |
| LDL-C (mg/dl) | 6 ± 2 | 5 ± 1* | 5 ± 2 |
| GLU (mg/dl) | 621 ± 105 | 533 ± 78 | 429 ± 187* |
Data are shown as mean ± SD. *p<0.1 vs 0% PHGG group. TG indicates triglycerides; T-CHO, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; GLU, glucose.
Gene expression analysis
We used a high-density genome-wide microarray technique for the mRNA expression profile of small intestinal mucosa in order to investigate the effect of chronic treatment with PHGG on gene expression changes in the levels of mRNA. We used the GeneChip® Mouse Gene 1.0 ST Array (Affymetrix), which interrogates 28,853 well-annotated genes with 770,317 distinct probes. Comparison of the expression profiles in PHGG-treated db/db mice enabled the identification of differentially regulated genes associated with the feeding of PHGG.
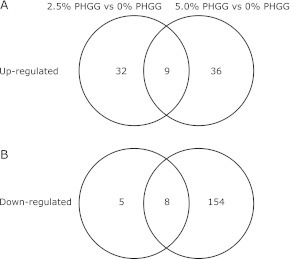
The present study showed that of the 28,853 transcripts examined, 54 were up-regulated (41 probes) or down-regulated (13 probes) by ⩾1.5-fold in 2.5% PHGG-treated mice, and 207 were up-regulated (45 probes) or down-regulated (162 probes) by ⩾1.5-fold in 5.0% PHGG-treated mice in comparison with 0.0% PHGG-treated mice (Fig. 1). A Venn diagram revealed 9 common transcripts that were up-regulated and 8 common transcripts that were down-regulated by PHGG (Fig. 1). Common up-regulated and down-regulated transcripts are listed in Tables 4 and 5, respectively.
Fig. 1.
Number of transcripts putatively associated with chronic treatment of PHGG. A Venn diagram showing the number of transcripts differing significantly between differing composition of PHGG (A) Transcripts of genes significantly up-regulated between 0% PHGG diet group and 2.5% PHGG diet group, and between 0% PHGG diet group and 5.0% PHGG diet group. Combination of these two comparisons by Venn diagram illustrates the number of genes up-regulated by chronic treatment of PHGG. (B) Transcripts of genes significantly down-regulated between 0% PHGG diet group and 2.5% PHGG diet group, and between 0% PHGG diet group and 5.0% PHGG diet group. Combination of these two comparisons by Venn diagram illustrates the number of genes down-regulated by chronic treatment of PHGG. Genes that satisfied the criteria of fold change ≥1.5 and p value ≥0.05 were considered significantly regulated.
Table 4.
The common up-regulated transcripts by PHGG
| Probe ID | Gene assignment | Description | Fold-change |
|
|---|---|---|---|---|
| 2.5% PHGG vs 0% PHGG | 5.0% PHGG vs 0% PHGG | |||
| 10424670 | NM_010416 | hematopoietic cell transcript 1 | 1.90 | 4.16 |
| 10533213 | NM_145226 | 2'-5' oligoadenylate synthetase 3 | 3.49 | 2.92 |
| 10486956 | NM_177610 | dual oxidase 2 | 3.34 | 2.87 |
| 10533246 | NM_011852 | 2'-5' oligoadenylate synthetase 1g | 2.88 | 2.16 |
| 10439321 | NM_021301 | solute carrier family 15 | 1.89 | 1.93 |
| 10533256 | NM_145211 | 2'-5' oligoadenylate synthetase 1a | 2.65 | 1.91 |
| 10574166 | NM_153507 | copine II | 1.53 | 1.72 |
| 10524631 | NM_145209 | 2'-5' oligoadenylate synthetase-like 1 | 1.95 | 1.70 |
| 10574145 | FJ889356 | NLR family, CARD domain containing 5 | 1.57 | 1.58 |
Table 5.
The common down-regulated transcripts by PHGG
| Probe ID | Gene assignment | Description | Fold-change |
|
|---|---|---|---|---|
| 2.5% PHGG vs 0% PHGG | 5.0% PHGG vs 0% PHGG | |||
| 10498797 | XR_035682 | predicted gene 5277 | 0.65 | 0.66 |
| 10430679 | — | — | 0.61 | 0.64 |
| 10359161 | NM_009230 | sterol O-acyltransferase 1 | 0.65 | 0.60 |
| 10421970 | — | — | 0.64 | 0.57 |
| 10582658 | NM_007428 | angiotensinogen | 0.64 | 0.53 |
| 10571567 | NM_172752 | sorbin and SH3 domain containing 2 | 0.64 | 0.52 |
| 10538706 | BC137623 | multimerin 1 | 0.66 | 0.49 |
| 10372503 | NM_010195 | leucine rich repeat containing G protein coupled receptor 5 | 0.54 | 0.48 |
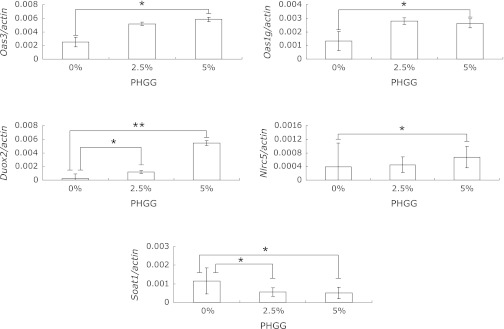
As described in the Discussion, the expression of 2'-5' oligoadenylate synthetases (Oas), dual oxidase 2 (Duox2), and NLR family, CARD domain containing 5 (Nlrc5) are considered to involve host defense functions, while the expression of Sterol O-acyltransferase 1 (Soat1) is considered to involve cholesterol absorption in intestine. Hence, we further analyzed the gene expression of the seven molecules Oas3, Oas1G, Oas1A, Oasl1, Duox2, Nlrc5 and Soat1 by quantitative real-time PCR to confirm the results obtained from the microarray analysis. PHGG treatment markedly increased the gene expression of Oas3, Oas1G, Duox2 and Nlrc5, and decreased the gene expression of SOAT1 compared with vehicle administration (Fig. 2). With regard to the gene expression of Oas3, Duox2, Oas1G, Nlrc5 and Soat1, the results obtained from the quantitative real-time PCR analysis paralleled those obtained from the microarray analysis.
Fig. 2.
Dietary effects of PHGG on gene expression in db/db mouse intestinal mucosa as assessed by real-time PCR. RNA was harvested from mouse intestinal mucosa and analyzed for Oas3, Oas1g, Duox2, Nlrc5 and Soat1, as well as actin by real-time PCR, as described in Materials and Methods. Data presented represent mean ± SD (n = 7). One and two asterisks indicate p values of <0.05 and <0.01, respectively, as determined by student t tests.
Discussion
The objective of this study was to determine whether PHGG affects gene expression in diabetic mouse small intestine at the mRNA level. Using microarray technology, we showed that 9 genes were up-regulated and 8 genes were down-regulated by chronic treatment with PHGG (Tables 4 and 5).
Dual oxidases; DUOX1 and DUOX2 localize to the apical plasma membrane of epithelial cells in major airways, salivary glands and the gastrointestinal tract, and provide extracellular hydrogen peroxide for lactoperoxidase to produce antimicrobial hypothiocyanite ions.(17) 2'-5' Oligoadenylate synthetases (OAS) are enzymes induced by and mediate the antiviral action of interferon, and synthesize 2',5'-linked phosphodiester bonds to polymerize ATP into oligomers of adenosine.(18,19) These unique 2',5'-oligomers specifically activate the latent form of RNaseL, leading to viral RNA degradation. Nucleotide-binding oligomerization domain-like receptor NLRC5 is a cytosolic protein which functions in innate immune responses.(20) Since DUOX2, OAS3, OAS1G and NLRC5 participate in host defense functions as mentioned above, dietary PHGG may increase immune functions. We previously reported on the preventive effect of PHGG on the colonization of Salmonella enteritidis in young and laying hens.(21) Our present results derived from the gene expression assay shed light on the mechanism of this preventive effect.
Sterol O-acyltransferase (SOAT; Acyl-coenzyme A:cholesterol acyltransferase/ACAT) is an intracellular enzyme that catalyzes the formation of cholesteryl esters from cholesterol and fatty acyl-coenzyme A.(22) In mammals, two isoenzymes, SOAT1 and SOAT2, encoded by two different genes, are present. SOAT1 is expressed in many tissues;(23) in mice, the highest expression levels are in macrophages, adrenal glands, dermal sebaceous glands and preputial glands. SOAT1 is also expressed in atherosclerotic lesions. SOAT2, however, is expressed only in the liver and intestine. Although SOAT2 is the major cholesterol-esterifying enzyme in mouse enterocytes as shown by studies of SOAT2-deficient mice,(24,25) we showed the possibility that PHGG decreased blood cholesterol through down-regulating Soat1 mRNA and decreasing cholesterol esterification in mouse small intestine.
Dietary fiber has been reported to exert hypocholesterolemic effects.(26) Several mechanisms of this cholesterol-lowering effect of dietary fiber have been suggested, such as direct binding of cholesterol within the intestinal lumen, interference with the diffusion of luminal cholesterol toward the epithelial cell surface, and antagonization of the cholesterol emulsification process.(26) Information concerning the effect of dietary fiber consumption on the expression of genes that regulate intestinal cholesterol absorption and hepatic sterol balance is limited. For the most part, published reports on the molecular mechanisms associated with the hypocholesterolemic effects of dietary fibers have pertained to the hepatic mRNA expression of HMG-CoAR and CYP7A1.(27–29) Additionally, GG consumption has been shown to reduce hepatic free-cholesterol concentrations and increase SREBP2-dependent hepatic LDLR mRNA and protein expression.(26) GG consumption was also shown to increase hepatic ABCG5/G8 mRNA and protein expression and biliary cholesterol concentrations in comparison to control-fed pigs.(26)
In summary, dietary fiber has been reported to modulate the expression of several hepatic genes.(26) In the present paper, we reported for the first time that PHGG up-regulated the expression of nine genes, including Oas3, Oas1g, Duox2 and Nlrc5, potentially related to host defense functions, and down-regulated the expression of 8 genes, including Soat1, which is involved in cholesterol esterification and absorption, in small intestine. We have yet to determine whether this effect is specific to PHGG (galactomannan; galactose:mannose 1:2, average molecular weight 20,000), or is common to dietary fiber. This question should be discussed in more detail, and is currently being investigated.
Acknowledgments
This research was supported by Adaptable and Seamless Technology Transfer Program through Target-driven R&D (A-STEP) of the Japan Science and Technology Agency (JST).
Abbreviations
- ABCG5
ATP-binding cassette transporter G5
- ABCG8
ATP-binding cassette transporter G8
- AIN-93G
American Institute of Nutrition
- CYP7A1
cholesterol 7α-hydroxylase
- DUOX
dual oxidase
- GG
guar gum
- GLU
glucose
- HMG-CoAR
3-hydroxy-3-methylglutaryl coenzyme A receptor
- NLRC5
NLR family CARD domain containing 5
- OAS
2'-5' oligoadenylate synthetases
- PHGG
partially hydrolyzed guar gum
- SOAT
Sterol O-acyltransferase
- SREBP2
sterol regulatory element binding protein 2
- T-CHO
total cholesterol
References
- 1.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 2.Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–375. doi: 10.1016/j.ecl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 4.Kuo DC, Hsu SP, Chien CT. Partially hydrolyzed guar gum supplement reduces high-fat diet increased blood lipids and oxidative stress and ameliorates FeCl3-induced acute arterial injury in hamsters. J Biomed Sci. 2009;16:15. doi: 10.1186/1423-0127-16-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon SJ, Chu DC, Raj Juneja L. Chemical and physical properties, safety and application of partially hydrolized guar gum as dietary fiber. J Clin Biochem Nutr. 2008;42:1–7. doi: 10.3164/jcbn.2008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapoor MP, Juneja LR. Fiber Ingredients. Food Applications and Health Benefits. CRC Press; 2009. pp. 79–121. [Google Scholar]
- 7.Scharlau D, Borowicki A, Habermann N, et al. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat Res. 2009;682:39–53. doi: 10.1016/j.mrrev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Okubo T, Ishihara N, Takahashi H, et al. Effects of partially hydrolyzed guar gum intake on human intestinal microflora and its metabolism. Biosci Biotechnol Biochem. 1994;58:1364–1369. [Google Scholar]
- 9.Velázquez M, Davies C, Marett R, Slavin JL, Feirtag JM. Effect of oligosaccharides and fibre substitutes on short-chain fatty acid production by human faecal microflora. Anaerobe. 2000;6:87–92. [Google Scholar]
- 10.Nakamura S, Hongo R, Moji K, Oku T. Suppressive effect of partially hydrolyzed guar gum on transitory diarrhea induced by ingestion of maltitol and lactitol in healthy humans. Eur J Clin Nutr. 2007;61:1086–1093. doi: 10.1038/sj.ejcn.1602623. [DOI] [PubMed] [Google Scholar]
- 11.Alam NH, Ashraf H, Sarker SA, et al. Efficacy of partially hydrolyzed guar gum-added oral rehydration solution in the treatment of severe cholera in adults. Digestion. 2008;78:24–29. doi: 10.1159/000152844. [DOI] [PubMed] [Google Scholar]
- 12.Giannini EG, Mansi C, Dulbecco P, Savarino V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition. 2006;22:334–342. doi: 10.1016/j.nut.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Minekus M, Jelier M, Xiao JZ, et al. Effect of partially hydrolyzed guar gum (PHGG) on the bioaccessibility of fat and cholesterol. Biosci Biotechnol Biochem. 2005;69:932–938. doi: 10.1271/bbb.69.932. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K, Tokunaga Y, Ikeda A, et al. Effect of dietary fiber on the lipid metabolism and immune function of aged Sprague-Dawley rats. Biosci Biotechnol Biochem. 2003;67:429–433. doi: 10.1271/bbb.67.429. [DOI] [PubMed] [Google Scholar]
- 15.Maenaka T, Yokawa T, Ishihara N, Gu Y, Juneja LR. Effects of partially hydrolyzed guar gum on postprandial blood glucoselevel and disaccharidase. J Jpn Soc Med Use Func Foods. 2007;4:195–201. [Google Scholar]
- 16.Gu Y, Yamashita T, Suzuki I, Juneja LR, Yokawa T. Effect of enzyme hydrolyzed guar gum on the elevation of blood glucose levels after meal. Med Biol. 2003;142:19–24. [Google Scholar]
- 17.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverman RH. Viral encounters with 2',5'-oligoadenylate synthetase and RNase L during the interferon antiviral response. J Virol. 2007;81:12720–12729. doi: 10.1128/JVI.01471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neerincx A, Lautz K, Menning M, et al. A role for the human nucleotide-binding domain, leucine-rich repeat-containing family member NLRC5 in antiviral responses. J Biol Chem. 2010;285:26223–26232. doi: 10.1074/jbc.M110.109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishihara N, Chu DC, Akachi S, Juneja LR. Preventive effect of partially hydrolyzed guar gum on infection of Salmonella enteritidis in young and laying hens. Poult Sci. 2000;79:689–697. doi: 10.1093/ps/79.5.689. [DOI] [PubMed] [Google Scholar]
- 22.Chang TY, Li BL, Chang CC, Urano Y. Acyl-coenzyme A: cholesterol acyltransferases. Am J Physiol Endocrinol Metab. 2009;297:E1–E9. doi: 10.1152/ajpendo.90926.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Accad M, Smith SJ, Newland DL, et al. Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA: cholesterol acyltransferase 1. J Clin Invest. 2000;105:711–719. doi: 10.1172/JCI9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buhman KK, Accad M, Novak S, et al. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 25.Repa JJ, Buhman KK, Farese RV, Jr., Dietschy JM, Turley SD. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 2004;40:1088–1097. doi: 10.1002/hep.20439. [DOI] [PubMed] [Google Scholar]
- 26.Rideout TC, Harding SV, Jones PJ, Fan MZ. Guar gum and similar soluble fibers in the regulation of cholesterol metabolism: current understandings and future research priorities. Vasc Health Risk Manag. 2008;4:1023–1033. doi: 10.2147/vhrm.s3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horton JD, Cuthbert JA, Spady DK. Regulation of hepatic 7 alpha-hydroxylase expression by dietary psyllium in the hamster. J Clin Invest. 1994;93:2084–2092. doi: 10.1172/JCI117203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kishida T, Nogami H, Ogawa H, Ebihara K. The hypocholesterolemic effect of high amylose cornstarch in rats is mediated by an enlarged bile acid pool and increased fecal bile acid excretion, not by cecal fermented products. J Nutr. 2002;132:2519–2524. doi: 10.1093/jn/132.9.2519. [DOI] [PubMed] [Google Scholar]
- 29.Han KH, Sekikawa M, Shimada K, Sasaki K, Ohba K, Fukushima M. Resistant starch fraction prepared from kintoki bean affects gene expression of genes associated with cholesterol metabolism in rats. Exp Biol Med (Maywood) 2004;229:787–792. doi: 10.1177/153537020422900811. [DOI] [PubMed] [Google Scholar]