Abstract
Background
The criteria by Camitta for diagnosis in severe aplastic anemia (SAA) has been used since 1976. However, there has been no attempt to verify the Camitta's criteria, that the survival in patients with SAA may differ by absolute neutrophil count (ANC), platelet count (PLT), and corrected reticulocyte count (CRC), which are components of the Camitta's criteria.
Methods
117 SAA patients diagnosed by the Camitta's criteria were analyzed, retrospectively. Univariate and multivariate analyses were used to evaluate the factors affecting overall survival (OS).
Results
Response by immunosuppressive therapy (IST) or stem cell transplantation (SCT) significantly affected OS (P=0.001). Therefore, we excluded treatment responders for analysis. Finally, 92 SAA patients including treatment non-responders by IST or SCT and conservative care group were analyzed by using univariate and multivariate analyses. The median age of analyzed patients was 54.5 years. Male to female ratio was 1:1. The median follow-up duration was 74.23 months (range, 54.71-93.74 months). The median ANC, PLT, and CRC were 394/µL, 12,000/µL, and 0.39%, respectively. In multivariate analyses, ANC <500/µL or ≥500/µL (P=0.015, HR 2.694, 95% CI: 1.20-6.01) and age (P=0.015, HR 1.022, 95% CI: 1.00-1.04) were the significant factors for OS.
Conclusion
ANC could be an essential, not an optional criterion for diagnosing SAA. This study suggests the possibility that the Camitta's criteria be modified. Studies in large cohorts are needed to transform the Camitta's criteria.
Keywords: Camitta's criteria, Severe aplastic anemia, Absolute neutrophil count
INTRODUCTION
Aplastic anemia (AA) is a disorder characterized by pancytopenia (a reduction in the number of red blood cells, WBC, and platelets), hypoplastic bone marrow, and the absence of underlying malignancy [1].
The severity of AA varies widely from mild, chronic pancytopenia to total hematopoietic failure. The clinical outcome and choice of initial management depend on the severity of AA [2]. Stem cell transplantation (SCT) or immunosuppressive therapy (IST) is recommended for patients with severe AA (SAA) to improve their long-term survival with hematologic recovery [3]. Without treatment, the disease is known to be fatal. Severe cytopenia may cause many complications such as anemia, bleeding, and life-threatening infections. Bacterial sepsis and fungal infections are the most common causes of death as the disease progresses [4].
SAA is diagnosed when patients meet at least 2 of the following criteria: an absolute neutrophil count (ANC) of less than 0.5×109/L, a platelet count (PLT) of less than 20×109/L, or a corrected reticulocyte count (CRC) of less than 1%. These criteria have been almost unchanged since they were proposed by Camitta in 1976 [5, 6]. However, should we still use Camitta's criteria for the diagnosis of SAA? Since severe neutropenia leads to life-threatening infections, which is the most common cause of death in SAA patients, we wonder whether we should put the same weight on each of the 3 components of Camitta's criteria. There has been no attempt to verify Camitta's criteria, even though survival in SAA may differ based on ANC, PLT, and CRC, which are the components of Camitta's criteria. Therefore, this study was performed to determine survival in SAA according to ANC, PLT, and CRC to verify the relevance of Camitta's criteria in the diagnosis of SAA.
MATERIALS AND METHODS
We conducted a retrospective analysis of 117 patients diagnosed with SAA at Dong-A University Hospital in Busan, South Korea from January 1995 to July 2011. Diagnosis and assessment of the disease were on the basis of Camitta's criteria, which defines SAA as bone marrow cellularity of less than 30% and severe pancytopenia with at least 2 of the following peripheral blood count criteria: (1) ANC <0.5×109/L; (2) PLT <20×109/L; and (3) CRC <1%.
Bone marrow biopsy and aspiration for morphology and cytogenetics were performed before diagnosis. All patients were tested for paroxysmal nocturnal hemoglobinuria clones using the Ham test until 2000, at which point the test was replaced by a flow cytometric assay [7].
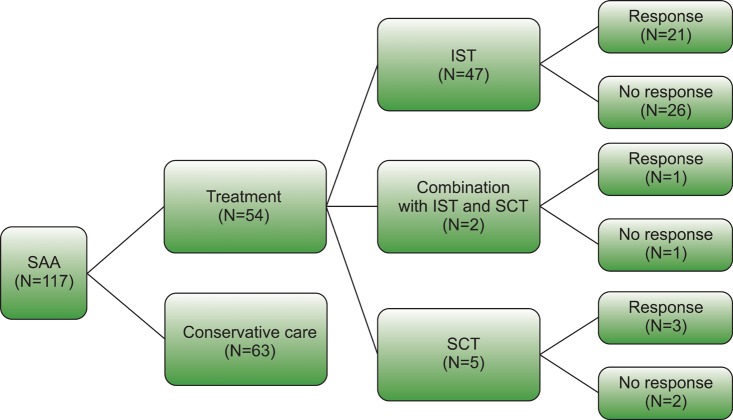
In our study, all 117 patients were treated with SCT, IST, or conservative care. Patients who were less than 40 years of age, medically fit, and had a histocompatible donor underwent SCT. The others received IST, including doses of antithymocyte globulin alone or in combination with cyclosporine and prednisone. As shown in Fig. 1, 54 patients received IST and/or underwent SCT, of which 47 received IST, 5 underwent SCT, and 2 received both treatments. Response to treatment was defined as no longer meeting the criteria for SAA and was evaluated 3 months after SCT or IST [8-10]. The number of treatment responders and non-responders in each treatment method is shown in Fig. 1. Sixty-three patients received conservative care, including proper empirical antibiotic therapy and intermittent red cell and/or platelet transfusions. We analyzed patients who received conservative care and treatment non-responders in the final analysis.
Fig. 1.
Diagram of patients included in this study. Abbreviations: SAA, severe aplastic anemia; IST, immunosuppressive therapy; SCT, stem cell transplantation.
The variables included in the analysis were age, sex, WBC, hemoglobin, ANC, PLT, CRC, hepatitis B surface antigen, and hepatitis C virus antibody (anti-HCV) at diagnosis. Descriptive statistics were used for covariables. Univariate and multivariate analyses were used to evaluate the factors affecting overall survival (OS). For the purpose of presenting a simple picture of the effect of these covariates on survival, variables were categorized according to Camitta's criteria.
OS obtained using the Kaplan-Meyer method was compared by the log-rank test. Cox proportional hazard regression analysis was used for univariate and multivariate analyses of factors affecting OS. P values less than 0.05 were considered statistically significant.
RESULTS
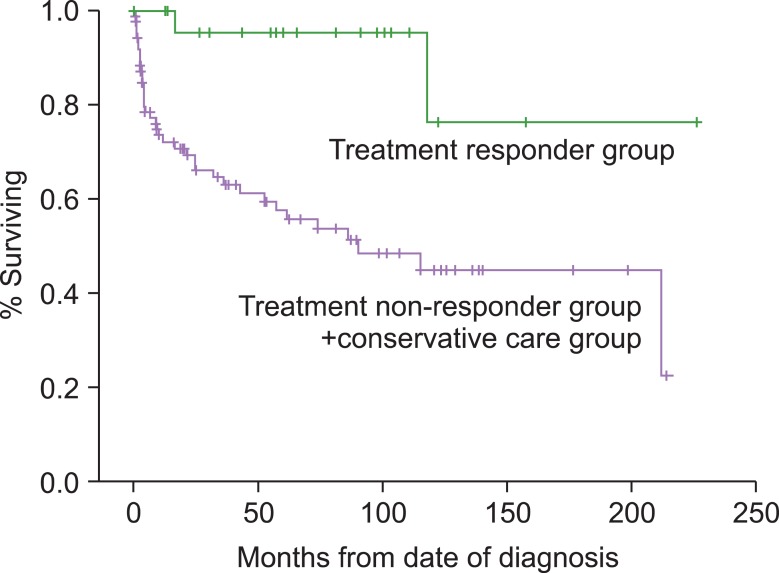
Among the 117 patients with SAA, the median OS of the 54 patients in the treatment group and the 63 patients in the conservative care group were 152 months (95% CI, 119.58-184.83) and 90 months (95% CI, 16.17-163.82), respectively. OS in the treatment group was significantly higher than in the conservative care group (P=0.03). Among the 54 patients in the treatment group, 25 patients responded to IST or SCT (Fig. 1). The median OS of the 25 patients in the treatment responder group and the 92 patients in the treatment non-responder and conservative care group was 195 months (95% CI, 156.06-235.59) and 90 months (95% CI, 26.29-153.70), respectively. The treatment responder group had a significantly higher survival rate than the treatment non-responder and conservative care group (P=0.001) (Fig. 2). There was no significant difference between the OS of the treatment non-responder and conservative care groups. Based on this analysis, response to treatment with IST or SCT significantly affected OS. Since these findings could obscure an analysis of the natural course of SAA, we included the 92 patients only in the final analysis who either did not respond to treatment or only received conservative care to validate Camitta's criteria for predicting survival in SAA patients.
Fig. 2.
Survival curves according to the treatment response. The median survival was 195 months in the treatment responder group vs. not yet reached in the treatment non-responder and conservative care group (P=0.0014).
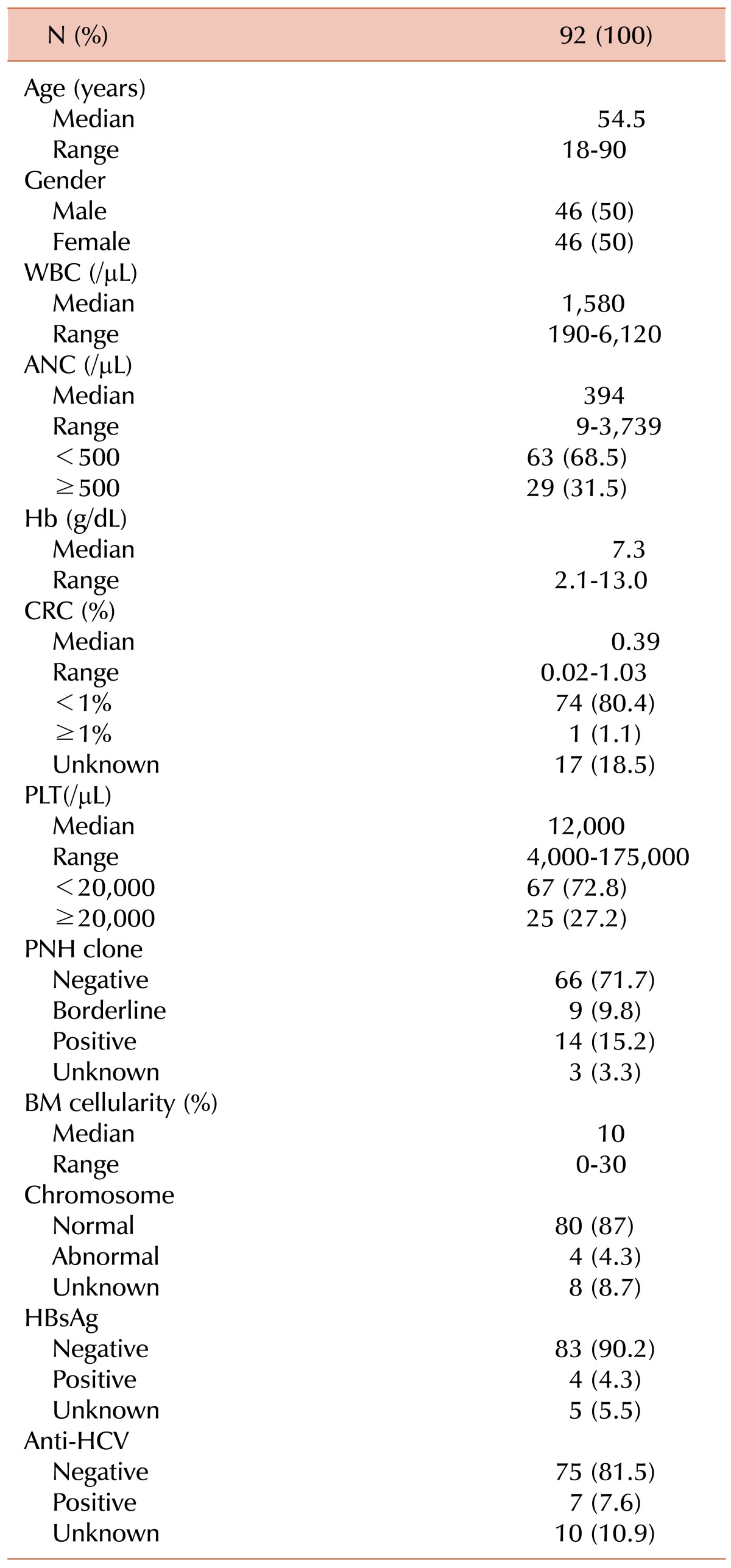
The baseline characteristics of the 92 patients are listed in Table 1. The median follow-up duration was 74.23 months (range, 54.71-93.74 months). The numbers of patients who satisfied Camitta's criteria for ANC and CRC, PLT and CRC, ANC and PLT were 45 (48.9%), 49 (53.3%), and 38 (41.3%), respectively, and 20 patients (21.7%) met all 3 components of Camitta's criteria.
Table 1.
Baseline characteristics of the study population.
Abbreviations: ANC, absolute neutrophil count; CRC, corrected reticulocyte count; PLT, platelet count; PNH, paroxysmal nocturnal hemoglobinuria; BM, bone marrow; HBsAg, hepatitis B surface antigen; Anti-HCV, hepatitis C virus antibody.
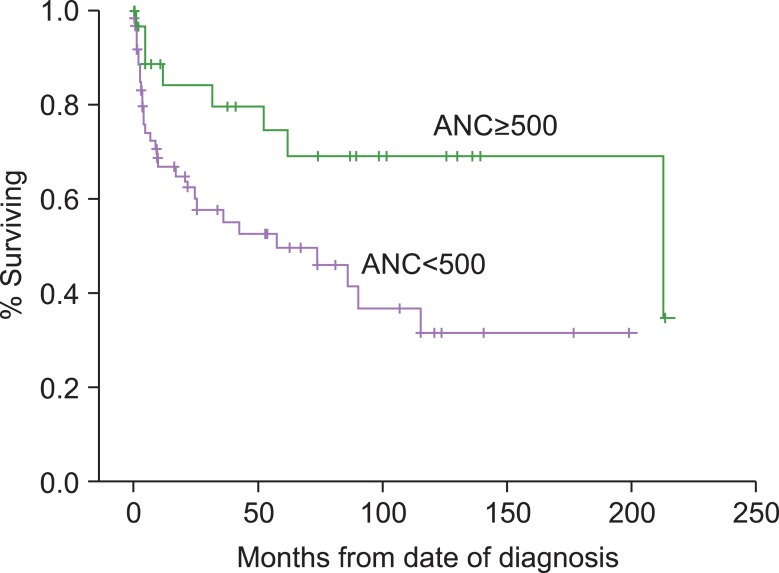
The median OS of the 92 patients was 90 months (95% CI, 26.29-153.70). The median OS of patients with ANC <0.5×109/L was 57.53 months (95% CI, 4.97-110.08) and the median OS of patients with ANC ≥0.5×109/L was 212.23 months (95% CI, 0-425.03). OS was significantly longer in patients with ANC ≥0.5×109/L (P=0.014) (Fig. 3). However, the OS of patients with CRC <1% or ≥1% did not differ significantly (P=0.352), nor did the OS of patients with PLT <20×109/L or ≥20×109/L (P=0.063).
Fig. 3.
Survival curves according to absolute neutrophil count (ANC). The median survival was 195 months in ANC <500/µL vs. not yet reached in ANC ≥500/µL (P=0.014). Abbreviation: ANC, absolute neutrophil count.
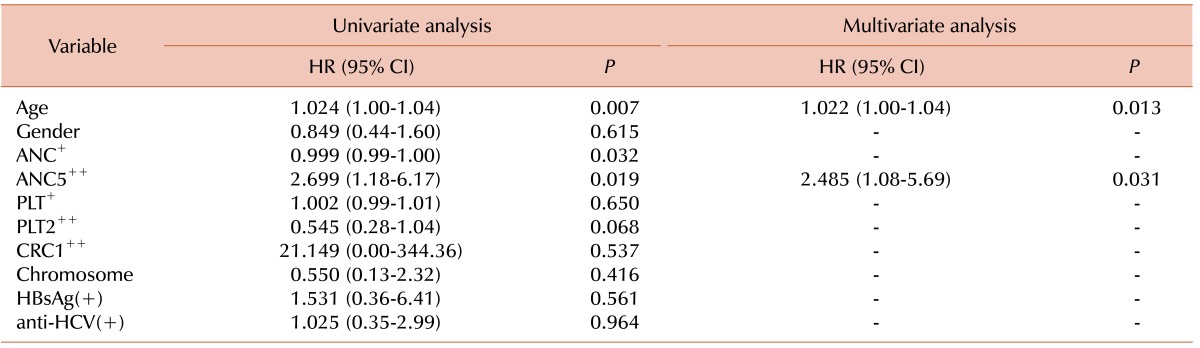
Analysis of the effect of ANC <0.5×109/L or ≥0.5×109/L; PLT <20×109/L or ≥20×109/L; and CRC <1% or ≥1% on survival by Cox proportional hazard regression analysis showed that ANC <0.5×109/L or ≥0.5×109/L was the only significant factor affecting OS in both univariate analysis (P=0.01; HR=2.699; 95% CI, 1.18-6.17) and multivariate analysis (P=0.006; HR=3.213; 95% CI, 1.39-7.43). PLT <20×109/L or ≥20×109/L, and CRC <1% or ≥1% did not affect OS.
Univariate analysis of covariables, including age (continuous), sex, chromosome, HBsAg, anti-HCV, ANC (continuous and categorical by 0.5×109/L), PLT (continuous and categorical by 20×109/L), and CRC (categorical by 1%), showed that age, ANC, and PLT significantly affected OS. In multivariate analyses, OS was significantly affected by age and ANC (Table 2).
Table 2.
Univariate and multivariate analyses of the factors that influence survival.
Abbreviations: ANC, absolute neutrophil count; ANC5, ANC <500/µL; PLT, platelet count; PLT2, PLT <20,000/µL; CRC1, corrected reticulocyte count <1%; HBsAg, hepatitis B surface antigen; anti-HCV, hepatitis C virus antibody; +, continuous variable; ++, categorical variable.
DISCUSSION
The symptoms of SAA are caused by pancytopenia. Patients with severe anemia may have life-threatening conditions due to failure of the cardiac or circulatory system, especially if they have not received transfusions [11, 12]. Although bleeding due to thrombocytopenia was a major cause of death in the past, it is gradually declining due to the use of transfusions. Currently, infection leads to the highest percentage of death [13]. Recovery from anemia and thrombocytopenia by immediate transfusions is temporary, but patients can achieve tolerable states. Moreover, transfusions allow patients for whom IST or SCT are not indicated to maintain life. In contrast, despite empirical antibiotic therapy, uncontrolled infections caused by neutropenia end in septic shock and deprive patients of an opportunity to receive treatment for SAA (SCT or IST). Therefore, neutropenia seems to have the worst survival compared with the other 2 components of Camitta's criteria.
In the past, several predictive models have been proposed to predict survival in SAA patients. However, none gained wide acceptance because they were impractical (some models included complex formulas) or relied on subjective indices (such as bone marrow morphologies and differential counts) [14-16]. In 1976, Camitta et al. proposed more accurate criteria for SAA in a prospective randomized trial [17]. Patients who met the SAA criteria had better survival because they received earlier allo-SCT. After this study, the SAA criteria by Camitta et al. became the most widely accepted standard for the diagnosis of AA [18, 19]. However, it is difficult to understand the process or reason why Camitta chose ANC, PLT, and CRC for the diagnosis of SAA, and why he set the standard values as ANC <0.5×109/L, PLT <20×109/L, and CRC <1%, and stated that 2 out of these 3 components must be satisfied. Until now, no studies have reviewed or validated Camitta's criteria.
The importance of ANC for predicting the prognosis of AA has been emphasized in other studies. Bacigalupo et al. subclassified SAA by ANC: very SAA (<0.2×109/L neutrophils) and moderately SAA (0.2-0.5×109/L neutrophils) [20], and then patients were randomly assigned to receive IST or SCT. Patients with more severe AA showed better survival when treated with SCT than with IST. Another retrospective analysis of SAA patients treated with horse anti-thymocyte globulin (ATG)-based IST showed that baseline ANC was the most significant contributor to short-term survival [21]. Most patients with low ANC counts present to the clinic with septic conditions; therefore, they are not able to undergo aggressive treatment or receive SCT or IST. Since patients with low ANC tend to have shorter survival times or poor responses to therapy, these patients have not been included in prospective studies.
In our study, patients with low ANC also had significantly poor OS. ANC <0.5×109/L and age significantly affected OS in multivariate analysis. A patient with low ANC might have a high rate of infection and short OS. ANC was the only statistically significant component of Camitta's 3 criteria to have an effect on OS. Based on our analysis, we believe that ANC <0.5×109/L should be an essential component of SAA diagnosis. Age was another significant prognostic factor for OS. Older patients tolerate severe infections less well than younger patients, and this might have influenced their poor OS. As this study was retrospective in nature, there was little information on the performance status of the patients; therefore, we could not analyze survival by performance status.
Few studies have verified the prognostic impact of CRC or PLT on SAA. Furthermore, in our study, these 2 components did not have a significant impact on OS. However, there was only 1 patient with a CRC ≥1% among the analyzed patients. Of the 92 analyzed patients, 17 (18.5%) who satisfied Camitta's criteria for ANC and PLT were not tested for CRC. Therefore, we excluded CRC as a continuous variable in the univariate and multivariate analyses.
Our study is limited by its retrospective nature. However, it is difficult to perform a prospective study that randomizes patients into supportive care only or the appropriate treatment groups, because SAA is fatal if not treated. Since this study population only included those who were not able to receive more intensive treatments such as allogeneic SCT or IST, it is possible that this analysis is only valid for patients who are excluded from intensive treatment. Therefore, this analysis may be biased due to the inequality of the study population. If the conclusion is the same for all severe AA patients regardless of treatment type, then the conclusion that ANC is an essential component for the diagnosis of SAA will be more solid. However, to ensure that the response to IST or SCT significantly influenced OS, and to more properly determine the natural course of SAA in patients, we excluded patients who responded to treatment. Few studies have analyzed the optimal diagnostic criteria for SAA or reported the survival rate of untreated SAA. This study is the first report arguing for a change in the diagnostic criteria of SAA.
In conclusion, ANC should be an essential and not an optional criterion for diagnosing SAA. This study suggests the need for a modified Camitta's criteria. Further studies in large populations are required to verify whether each of the 3 components of Camitta's criteria has the same statistical power in SAA diagnosis.
References
- 1.Camitta BM. Pathogenesis and treatment of aplastic anemia. Rinsho Ketsueki. 1984;25:459–469. [PubMed] [Google Scholar]
- 2.Pitcher LA, Hann IM, Evans JP, Veys P, Chessells JM, Webb DK. Improved prognosis for acquired aplastic anaemia. Arch Dis Child. 1999;80:158–162. doi: 10.1136/adc.80.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camitta BM, Thomas ED, Nathan DG, et al. A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood. 1979;53:504–514. [PubMed] [Google Scholar]
- 4.Bacigalupo A, Passweg J. Diagnosis and treatment of acquired aplastic anemia. Hematol Oncol Clin North Am. 2009;23:159–170. doi: 10.1016/j.hoc.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Camitta BM, Storb R, Thomas ED. Aplastic anemia (first of two parts): pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645–652. doi: 10.1056/NEJM198203183061105. [DOI] [PubMed] [Google Scholar]
- 6.Camitta BM, Storb R, Thomas ED. Aplastic anemia (second of two parts): pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:712–718. doi: 10.1056/NEJM198203253061204. [DOI] [PubMed] [Google Scholar]
- 7.Dunn DE, Tanawattanacharoen P, Boccuni P, et al. Paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure syndromes. Ann Intern Med. 1999;131:401–408. doi: 10.7326/0003-4819-131-6-199909210-00002. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995;85:3058–3065. [PubMed] [Google Scholar]
- 9.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 10.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sebastiani G, Pantopoulos K. Disorders associated with systemic or local iron overload: from pathophysiology to clinical practice. Metallomics. 2011;3:971–986. doi: 10.1039/c1mt00082a. [DOI] [PubMed] [Google Scholar]
- 12.Quarta A, Melpignano A, Quarta G. Oral iron chelator deferasirox in the treatment of secondary hemochromatosis following bone marrow transplantation in a patient with severe aplastic anemia. Acta Haematol. 2011;125:219–221. doi: 10.1159/000322802. [DOI] [PubMed] [Google Scholar]
- 13.Wali R, Fadoo Z, Adil S, Naqvi MA. Aplastic anemia: clinicohaematological features, treatment and outcome analysis. J Coll Physicians Surg Pak. 2011;21:219–222. [PubMed] [Google Scholar]
- 14.Najean Y, Pecking A. Prognostic factors in acquired aplastic anemia. A study of 352 cases. Am J Med. 1979;67:564–571. doi: 10.1016/0002-9343(79)90226-2. [DOI] [PubMed] [Google Scholar]
- 15.Williams DM, Lynch RE, Cartwright GE. Prognostic factors in aplastic anaemia. Clin Haematol. 1978;7:467–474. [PubMed] [Google Scholar]
- 16.Rozman C, Marin P, Grañena A, et al. Prognosis in acquired aplastic anaemia. A multivariate statistical analysis of 80 cases. Scand J Haematol. 1981;26:321–329. doi: 10.1111/j.1600-0609.1981.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 17.Camitta BM, Thomas ED, Nathan DG, et al. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976;48:63–70. [PubMed] [Google Scholar]
- 18.Hormann A, Berchthold W, Rhyner K, Wiedemann-Sigg C, Gmür J. Prognosis in acquired aplastic anemia. Acta Haematol. 1984;71:81–89. doi: 10.1159/000206562. [DOI] [PubMed] [Google Scholar]
- 19.Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009;144:206–216. doi: 10.1111/j.1365-2141.2008.07450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177–182. doi: 10.1111/j.1365-2141.1988.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 21.Sleijfer DT, Mulder NH, Nieweg HO. The value of prognostic indices in aplastic anaemia. Blut. 1981;42:69–78. doi: 10.1007/BF01030028. [DOI] [PubMed] [Google Scholar]