Abstract
Purpose
The aim of this study was to investigate the prevalence of acute low back pain (ALBP) and associated factors in high school students from a Southern Brazilian city.
Methods
The study was cross-sectional and interviewed 1,233 students 13- to 19-year-olds, attending high schools. A total of 25 schools were included in the sample (15 state institutions, 7 private, 2 federal and 1 municipal). The ALBP was evaluated using two questions. The outcome was LBP in the previous 30 days.
Results
The prevalence of ALBP was 13.7%. Non-white students, who commuted to school walking, showed a higher prevalence of ALBP. The prevalence of ALBP is relatively high.
Conclusions
Further studies with follow-ups to adulthood are needed to investigate whether physical cumulative loads on the lumbar spine (for example, duration/transport, school bags and inadequate school furniture) during adolescence, may influence the development of ALBP later in life.
Keywords: Low back pain, Adolescent’s health, Cross-sectional studies, Epidemiology
Introduction
Low back pain (LBP) is a condition that affects 70–80% of adult population at least once in life [1], it usually is not presented as an isolated single event. Genetics and environment influence LBP and its consequences throughout adult life. Hestbaeck et al. [2] suggest that both kind of exposures, shared and non-shared aspects, contribute to LBP occurrence.
Recently, LBP among youth was considered common among adults. LBP during adolescence has been associated with persistent pain up to adulthood because LBP sufferers at the age of 14 are more likely to have pain later in life compared those without pain earlier [3]. Epidemiologic studies present a wide range of rates among adolescents (12–74%), mainly due to the different methods of assessment [4] and cut-off points.
During the last two decades, many aspects as anthropometry, psychosocial, age, gender, smoking, screen time, computer use, backpacks and school furniture, physical activity, working, genetics and socioeconomic status have been associated with LBP in adolescents [5, 6]. Age is especially relevant as associated factors and occurrence change across age groups [7]. Therefore, it would be interesting to evaluate if factors associated with LBP in adults are the same as in adolescents.
Although LBP is a physical and psychological disorder, mostly related to occupational exposures [8], it is common among school and graduate students before the work life begins. It may change the understanding about the importance of physical factors alone and their role in LBP occurrence in adolescents.
Thus, the goal of this study is to assess the prevalence of LBP in the previous 30 days (LBP30) in a high-school-based sample of adolescents from Pelotas (Southern Brazil) and to measure its association with sociodemographics, behavior, ergonomics and nutritional information.
Materials and methods
The study was cross-sectional and interviewed students 13- to 19-year-olds, attending high schools from Pelotas. Fieldwork was carried out between June and September (2009), and was mostly based on a questionnaire about LBP, socioeconomic and demographic characteristics, nutrition and lifestyle. A pilot-study was carried out in a school that was not sampled for the main study.
At first, all schools were visited to establish the number of students, shifts and grades available at each school. Then, we randomly selected the schools using a strategy that considered the proportionality between municipal, state, federal and private institutions.
Before data collection, all schools agreed to participate in the research and, within each class, students attended a brief lecture about the research and were handed a consent form. Students younger than 18 were asked to take the consent form to their parents or legal tutor/guardian.
A total of 25 schools were included in the sample accounting for 9,233 students (15 state institutions, 7 private, 2 federal and 1 municipal). Sample size was estimated using EpiInfo. Because this research was included in a broader project, the final sample size was established by the research demanding the highest number of subjects. For the prevalence calculation, the following parameters were considered: LBP expected frequency of 20%, worst acceptable result of 3.0 percentage points, resulting in a sample size of 682 students; considering losses and refuses and to make up for the design effect (1.3), the final sample size was estimated in 750 individuals. A higher number would be necessary for the association analysis based on the following parameters: confidence level at 95%, statistical power of 80%, exposed:unexposed ratio = 1:9 (socioeconomic level), relative risk = 2 and expected frequency in unexposed = 15%, resulting in a sample size of 778 students. To make up for losses and refuses and the design effect (1.3), the final sample size was estimated in 1,280 individuals. However, as another research within the project demanded a larger sample, the overall sample size was established at 1,350 students.
The questionnaire was administered in the classrooms by the researchers. After questionnaire completion, students were taken to another room to measure height, weight and backpack weight (SOEHNLE digital scale).
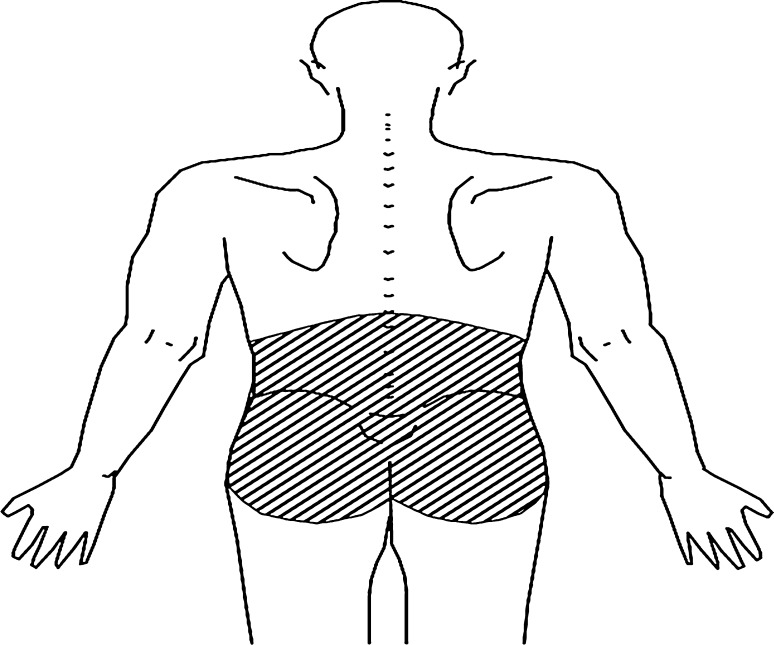
The outcome—LBP in the last 30 days (LBP30)—was assessed by two questions. First, have you ever had low back pain in the site shown in this figure? The figure showed the posterior (dorsal) view of a man standing and with the lumbar region shaded (Fig. 1). We only considered as presenting the outcome students reporting lumbar pain. Second, when did you have this pain? According to a LBP standardization consensus (Delphi), there is a large variation with respect to cultural aspects, language and methodologies to assess LBP, hindering the measurement of LBP frequency [9]. We based our assessments on this consensus; hence the outcome definition was based on a 30-day recall, in an attempt to rule out recall bias.
Fig. 1.
Anatomical depiction of low back pain
The following exposures were included in the analysis: sex, age, skin color; economic level, school type (public or private); body mass index; physical activity, smoking; and ergonomic information such as transportation to school, backpack use, backpack weight perception, backpack actual weight, school chair ergonomics (height and comfort) daily hours of computer and/or TV watching.
The economic level classification was based on the Brazilian Economic Criterion [10], where “A” is the wealthiest status. Physical activity engagement was assessed by an instrument proposed by Bastos et al. [11] and active subjects were those performing at least 300 min of weekly physical activity (moderate to vigorous) [12]. As for smoking, our choice was to evaluate subjects as having previous experience with tobacco (yes/no), regular smoker and heavy smoker, besides the age of regular smoking onset [13]. For analysis purposes, smoking was categorized as current smoker or never smoker/former smoker (not smoking in the previous month. The project obtained approval from the Ethics Research Committee of the Federal University of Pelotas and information was only collected after the completion of the consent forms (students or responsible for those younger than 18).
Data were entered twice into EpiInfo 6.04. Statistic analyses were done with STATA 9.0. Pearson’s Chi-square test was used to assess bivariate associations (p < 0.05) or linear trend when appropriate. Multivariable analysis was carried out by Poisson regression to control simultaneously risk factors for acute LBP, considering a hierarchical model [14].
A four-level hierarchical model was used to control for potential confounders. The first level included sociodemographic information (gender, age and skin color), the second level was economic status, the third level included ergonomic characteristics (commute to school, seating position, daily screen time—computer and TV), and the fourth level included backpack weight and usage. This statistical model controls for variables of the same level and levels above. All variables presenting a p value lower than 0.2 during crude analysis were included in the model to control for confounding. All variables presenting p < 0.05 were kept in the Poisson regression [14].
Results
The mean age of subjects was 15.9 years (SD 1.2), 54% were girls. As for skin color, 79% were whites and 70.3% were high school freshmen and sophomores. In terms of economic level, 89.9% belonged to classes B/C and 63.9% were classified as physically inactive. Most students (87%) were attending public schools (Table 1).
Table 1.
Sample characteristics. High school students from Pelotas to Brazil (n = 1,233)
| Variable | Boys | Girls | Total | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Economic level (n = 1,036) | ||||||
| A | 44 | 9.1 | 38 | 6.9 | 82 | 7.9 |
| B | 273 | 56.6 | 290 | 52.3 | 563 | 54.4 |
| C | 158 | 32.8 | 210 | 37.9 | 368 | 35.5 |
| D | 7 | 1.5 | 16 | 2.9 | 23 | 2.2 |
| Schooling (n = 1,233) | ||||||
| First year | 225 | 39.7 | 245 | 36.8 | 470 | 38.1 |
| Second year | 174 | 30.7 | 223 | 33.5 | 397 | 32.2 |
| Third year | 168 | 29.6 | 198 | 29.7 | 366 | 29.7 |
| Age (n = 1,233) | ||||||
| 13/14 | 73 | 12.9 | 83 | 12.4 | 156 | 12.7 |
| 15 | 126 | 22.2 | 151 | 22.7 | 277 | 22.5 |
| 16 | 176 | 31.1 | 225 | 33.8 | 401 | 32.5 |
| 17 | 143 | 25.2 | 148 | 22.2 | 291 | 23.6 |
| 18/19 | 49 | 8.6 | 59 | 8.9 | 108 | 8.8 |
| Skin color (n = 1,201) | ||||||
| White | 428 | 78.0 | 521 | 79.9 | 949 | 79.0 |
| Non-white | 121 | 22.0 | 131 | 20.1 | 252 | 21.0 |
| Physical activity (n = 1,233) | ||||||
| Inactive | 284 | 50.1 | 504 | 75.7 | 788 | 63.9 |
| Active | 283 | 49.9 | 162 | 24.3 | 445 | 36.1 |
| School type (n = 1,233) | ||||||
| Public | 484 | 85.4 | 587 | 88.1 | 1,071 | 86.9 |
| Private | 83 | 14.6 | 79 | 11.9 | 162 | 13.1 |
| BMI (kg/m2) (n = 1,191) | ||||||
| Normal | 399 | 72.3 | 490 | 76.7 | 889 | 74.7 |
| Overweight | 111 | 20.1 | 124 | 19.4 | 235 | 19.7 |
| Obese | 42 | 7.6 | 25 | 3.9 | 67 | 5.6 |
| Smoking (n = 1,221) | ||||||
| Former smoker/never smoked | 525 | 93.4 | 625 | 94.8 | 1,150 | 94.2 |
| Smoker | 37 | 6.6 | 34 | 5.2 | 71 | 5.8 |
| Low back pain last 30 days (n = 1,233) | ||||||
| No | 498 | 87.8 | 566 | 85.0 | 1,064 | 86.3 |
| Yes | 69 | 12.2 | 100 | 15.0 | 169 | 13.7 |
According to BMI classification, 74.7% were within normal range; 5.6% were obese and 19.7% overweight. With respect to smoking, 94.2% of students were former smokers or reported never smoking. The LBP prevalence during the previous 30 days was 13.7% (Table 1).
Table 2 presents the crude analysis of LBP30 and independent variables. Student’s age presented a U-shaped association (p = 0.01), compared to 13- to 14-year-olds the 15–17 age group presented lower frequencies of LBP, but in the 18–19 age group, the prevalence was higher than in the reference category. A 40% risk increase was observed among non-white skin color students compared to whites (p = 0.05). After control for confounders (Table 4), age was no longer significantly associated to the outcome and only skin color remained associated presenting the same risk magnitude (40% increase).
Table 2.
Low back pain in the last 30 days among high school students from Pelotas (Brazil) and its distribution according to demographic, socioeconomic and behavioral variables, 2009
| Variables | Acute low back pain prevalence | Crude analysis prevalence ratios (95%CI) | p | |
|---|---|---|---|---|
| N | % | |||
| Gender (n = 1,233) | 0.1* | |||
| Boys | 69 | 12.2 | 1.0 | |
| Girls | 100 | 15.2 | 1.2 (0.9–1.6) | |
| Schooling (n = 1,233) | ||||
| First year | 69 | 14.7 | 1.0 | 0.7* |
| Second year | 54 | 13.6 | 1.0 (0.7–1.3) | |
| Third year | 46 | 12.6 | 0.9 (0.6–1.2) | |
| Economic level (n = 1,036) | ||||
| A | 19 | 23.2 | 0.7 (0.3–1.9) | 0.2** |
| B | 69 | 12.3 | 0.7 (0.3–1.8) | |
| C | 47 | 12.8 | 0.7 (0.6–0.9) | |
| D | 4 | 17.4 | 1.0 | |
| Age (n = 1,233) | 0.01* | |||
| 13/14 | 27 | 17.3 | 1.0 | |
| 15 | 25 | 9.0 | 0.5 (0.3–0.9) | |
| 16 | 60 | 15.0 | 0.9 (0.6–1.3) | |
| 17 | 35 | 12.0 | 0.7 (0.4–1.1) | |
| 18/19 | 22 | 20.0 | 1.2 (0.7–2.0) | |
| Skin color (n = 1,201) | 0.05* | |||
| White | 120 | 12.6 | 1.00 | |
| Non-white | 44 | 17.5 | 1.4 (1.0–1.9) | |
| Physical activity (n = 1,233) | 0.3* | |||
| Inactive | 114 | 14.5 | 1.0 | |
| Active | 55 | 12.4 | 0.9 (0.6–1.2) | |
| BMI (kg/m2) (n = 1,191) | 0.6** | |||
| Normal | 122 | 13.7 | 1.0 | |
| Overweight | 32 | 13.6 | 1.0 (0.7–1.4) | |
| Obese | 7 | 10.5 | 0.8 (0.4–1.6) | |
| Smoking (n = 1,221) | 0.4* | |||
| Former smoker/never smoked | 156 | 13.6 | 1.0 | |
| Smoker | 12 | 16.9 | 1.2 (0.7–2.1) | |
* Chi-square test for heterogeneity
** Wald’s test for linear trend
Table 4.
Adjusted analysis of factors associated to low back pain in the last 30 days among high school students from Pelotas, Brazil, 2009
| Variables | Adjusted analysis | |
|---|---|---|
| PR (95%CI) | p | |
| First level | 0.04* | |
| Skin color | ||
| White | 1.0 | |
| Non-white | 1.4 (1.0–1.9) | |
| Second level | ||
| Economic level | 0.09** | |
| A | 1.7 (0.6–4.9) | |
| B | 0.9 (0.3–2.3) | |
| C | 0.8 (0.3–2.2) | |
| D | 1.0 | |
| Third level | ||
| Transportation to school | 0.009* | |
| Walking | 1.0 | |
| Motorized | 0.6 (0.5–0.9) | |
PR prevalence ratios, 95%CI 95% confidence intervals, First level adjusted for skin color, age and gender, Second level adjusted for economic level and first-level variables, Third level adjusted for means of transportation to school, school chair comfort, daily hours of TV watching and computer use, and levels above
* Chi-square test for heterogeneity
** Wald’s test for linear trend
Crude analysis of behavioral and ergonomic variables with LBP30 prevalence (Table 3) showed an association between the outcome and means of transportation to school (p = 0.02), inactive commuting (i.e., by car) decreased the LBP30 risk in up to 30%. The variable represented by school chair characteristics (height and comfort level of chair) was associated with LBP (p = 0.01) those reporting comfortable chairs presented a 30% lower risk for LBP30. After controlling for confounders (Table 4), only means of transportation to school remained associated to the outcome (students going to school by car were less likely to present LBP). The economic level (Table 4) was entered into the model (p = 0.2), however, it was not associated with LBP after confounding control (p > 0.05).
Table 3.
Low back pain in the last 30 days among high school students from Pelotas (Brazil) and its distribution according to ergonomic and behavioral variables, 2009
| Variables | Acute low back pain prevalence | Crude analysis prevalence ratios (95%CI) | p | |
|---|---|---|---|---|
| N | % | |||
| Commuting to school (n = 1,180) | 0.02* | |||
| Active | 96 | 16.2 | 1.0 | |
| Inactive | 68 | 11.6 | 0.7 (0.5–1.0) | |
| Backpack user (n = 1,233) | 0.1* | |||
| Yes | 140 | 13.2 | 1.4 (0.9–2.0) | |
| No | 28 | 17.7 | 1.0 | |
| Backpack weight (kg) (n = 1,233) | 0.3* | |||
| Lighter than 2.00 | 17 | 9.9 | 1.0 | |
| 2.00–3.99 | 108 | 14.8 | 1.5 (0.8–2.2) | |
| 4.00–5.99 | 36 | 12.9 | 1.3 (0.8–2.2) | |
| Heavier than 6.00 | 5 | 20.0 | 2.0 (0.8–5.0) | |
| Comfortable chair/adequate height (n = 1,232) | 0.01* | |||
| No | 96 | 18.4 | 1.0 | |
| Yes | 73 | 11.3 | 0.7 (0.5–0.9) | |
| Daily hours watching TV (n = 1,152) | 0.2** | |||
| Less than 2.00 | 72 | 14.7 | 1.0 | |
| 2.00–4.59 | 65 | 13.10 | 0.9 (0.7–1.2) | |
| >5.00 | 18 | 10.8 | 0.7 (0.5–1.2) | |
| Daily hours using computer (n = 1,063) | ||||
| Less than 2.00 | 47 | 11.8 | 1.0 | 0.3** |
| 2.00–4.59 | 65 | 14.6 | 1.2 (0.9–1.8) | |
| >5.00 | 31 | 14.2 | 1.2 (0.8–1.8) | |
* Chi-square test for heterogeneity
** Wald’s test for linear trend
Discussion
One aspect of our study to be highlighted is that our analyses were performed on a representative sample of high school students from Pelotas (Brazil)—1,233 students (13- to 19-year-olds)––the sampling strategy and the low refuse rate (8.7%) assure that our final sample represents the reality of the city. Another point to be highlighted is the adoption of previously established LBP definitions (DELPHI) for prevalence studies [9].
Some limitations must be considered, as the cross-sectional nature of the study, which does not allow causality speculations. Besides the subjective nature of LBP studies, we can never totally rule out the recall bias and the exclusion of the rural area schools. Young people living in rural areas usually work harder and longer than their urban counterparts [15]. In addition, we must consider that the combination of informed symptoms through questionnaires may represent a measurement error since the classification of pain and sensations that may indicate a health condition is sometimes hard to distinguish, even by physicians.
The LBP30 prevalence was 13.7% and is similar to Pellisé et al. [16], Quinnette et al. [17] and Erne and Elfering [18] studies. During the last two decades, a growing number of studies have showed that non-specific LBP in adolescents is higher than previously reported. Among adolescents, studies must consider developmental, biologic, psychosocial, educational and cultural aspects using a multidisciplinary approach. The adolescence is a learning period, when even the report of the pain may be affected by self report peculiarities. Thus, LBP among 11- to 15-year olds in Europe and Canada ranges from 1 to 22% among girls and 1–12% among boys, probably a result of cultural differences with respect to the report of the pain [19]. The results show how subjective and complex the evaluation of back pain is among adolescents considering their physical and psychosocial conditions.
After controlling for confounders, the LBP30 was associated to skin color; non-white presented a 40% higher risk. According to a review paper by Jeffries et al. [4], only the study by Olsen et al. [20] considered ethnics in the report of pain, and found a significant difference only among black adolescents (higher prevalence of back pain when compared to whites of the same age group—15-year-olds). However, in Brazil, the results may biased by socioeconomic conditions. According to IBGE [15], Black/mixed skin color people represent nearly 74% among the poorer, but only around 11% among the richer.
In the present study, an effect of regional socioeconomics was evidenced: 20% of the students were non-white and belonging to higher economic classes (A, B and C); 79% were white. Poverty may lead to physical workloads among adolescents, which is a risk factor for LBP according to Sjolie [21]. Data from IBGE [15] show that in 2005, 5.4 million children and adolescents were in the workforce—53.9% younger than 16. Domestic work is probably on similar levels, but is not measured properly. A comparison of these data with richer countries shows that working is associated to LBP in school-age Europeans. We must consider that exposure to work among students in Europe is part time (contrary to most adults who work full time) and muscular fatigue may have influenced pain reports [22].
In our study, going to school by car provided a protective effect of nearly 40%. Our results agree with those reported by Siambanes et al. [23] and Viry et al. [24]—in their study active commuting to school was associated with higher levels of LBP. On the other hand, our results are distinct from Skoffer et al. [25] and Sato et al. [26], which showed that commuting by car and some activities between classes were positively associated with LBP; and contrasting to the study by Szpalski et al. [27], reporting more LBP episodes among students who did not walk to school. This result is contrary to the hypothesis that being physically active is a risk factor for low back pain. However, studies on this subject carried out among adolescents are inconclusive, especially because most findings are based on cross-sectional studies.
Based on our results, we conclude that acute LBP prevalence among adolescents from the urban zone of Pelotas is high and consistent with previously reported, especially among non-white people who walk to school.
Further studies are needed to establish if cumulative workloads on lumbar spine (i.e. heavy backpacks or poor postures in school chairs) contribute to LBP during adulthood. Care must be taken when dealing with pain among young people since the definitions of this condition may be affected by their perceptions, and to use the same definitions of LBP among adults and adolescents does not seem to be the right approach [28]. Changes in the context of low back pain in adolescents are structural-dependent and comprehend better housing and familiar schooling level but also may be influenced by teachers’ training and community and school-level actions.
Conflict of interest
None.
References
- 1.Deyo RA, Mirza SK, Martin BI (2006) Back pain prevalence and visit rates: estimates from US national surveys, 2002. Spine (Phila Pa 1976) 31:2724–2727 [DOI] [PubMed]
- 2.Hestbaek L, Iachine IA, Leboeuf-Yde C, Kyvik KO, Manniche C. Heredity of low back pain in a young population: a classical twin study. Twin Res. 2004;7:16–26. doi: 10.1375/13690520460741408. [DOI] [PubMed] [Google Scholar]
- 3.Brattberg G. Do pain problems in young school children persist into early adulthood? A 13-year follow-up. Eur J Pain. 2004;8:187–199. doi: 10.1016/j.ejpain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Jeffries LJ, Milanese SF, Grimmer-Somers KA. Epidemiology of adolescent spinal pain: a systematic overview of the research literature. Spine (Phila Pa 1976) 2007;32:2630–2637. doi: 10.1097/BRS.0b013e318158d70b. [DOI] [PubMed] [Google Scholar]
- 5.Duggleby T, Kumar S. Epidemiology of juvenile low back pain: a review. Disabil Rehabil. 1997;19:505–512. doi: 10.3109/09638289709166043. [DOI] [PubMed] [Google Scholar]
- 6.Hestbaek L, Korsholm L, Leboeuf-Yde C, Kyvik KO. Does socioeconomic status in adolescence predict low back pain in adulthood? A repeated cross-sectional study of 4, 771 Danish adolescentes. Eur Spine J. 2008;17:1727–1734. doi: 10.1007/s00586-008-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trevelyan FC, Legg SJ. Back pain in school children—where to from here? Appl Ergon. 2006;37:45–54. doi: 10.1016/j.apergo.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Hoogendoorn WE, Bongers PM, Vet HC, Ariens GA, Mechelen W, Bouter LM. High physical work load and low job satisfaction increase the risk of sickness absence due to low back pain: results of a prospective cohort study. Occup Environ Med. 2002;59:323–328. doi: 10.1136/oem.59.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dionne CE, Dunn KM, Croft PR, Nachemson AL, Buchbinder R, Walker BF, et al. A consensus approach toward the standardization of back pain definitions for use in prevalence studies. Spine (Phila Pa 1976) 2008;33:95–103. doi: 10.1097/BRS.0b013e31815e7f94. [DOI] [PubMed] [Google Scholar]
- 10.ABEP (2009) Critério de Classificação Econômica Brasil. http://www.abep.org/codigosguias/CCEB2008-Base2006e2007.pdf. Accessed 14 April 2011
- 11.Bastos JP, Araujo CL, Hallal PC. Prevalence of insufficient physical activity and associated factors in Brazilian adolescents. J Phys Act Health. 2008;5:777–794. [PubMed] [Google Scholar]
- 12.Biddle SJH, Whitehead SH, O’Donovan TM, Nevill ME. Correlates of participation in physical activity for adolescent girls: a systematic review of recent literature. J Phys Act Health. 2005;2:421–432. [Google Scholar]
- 13.Horta BL, Calheiros P, Pinheiro RT, Tomasi E, Costa do Amaral K. Smoking among teenagers in an urban area in Southern Brazil. Rev Saude Publica. 2001;35:159–164. doi: 10.1590/S0034-89102001000200009. [DOI] [PubMed] [Google Scholar]
- 14.Victora CG, Huttly SR, Fuchs SC, Olinto MT. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epidemiol. 1997;26:224–227. doi: 10.1093/ije/26.1.224. [DOI] [PubMed] [Google Scholar]
- 15.IBGE (2006) Suplemento Trabalho Infantil–PNAD. http://www.ibge.gov.br/home/presidencia/noticias/noticia_impressao.php?id_noticia=1117, editor. Accessed 30 March 2010
- 16.Pellisé F, Balagué F, Rajmil L, Cedraschi C, Aguirre M, Fontecha CG, Pasarin M, Ferrer M. Prevalence of low back pain and its effect on health-related quality of life in adolescents. Arch Pediatr Adolesc Med. 2009;163:65–71. doi: 10.1001/archpediatrics.2008.512. [DOI] [PubMed] [Google Scholar]
- 17.Quinnette LA, Morris LD, Grimmer-Somers K. The prevalence of low back pain in Africa: a systematic review. BMC Musculoskelet Disord. 2007;8:105. doi: 10.1186/1471-2474-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erne C, Elfering A (2011) Low back pain at school: unique risk deriving from insatisfactory grade in maths and school-type recommendation. Eur Spine J [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 19.Cardon G, Balagué F. Low back pain prevention’s effects in schoolchildren. What is the evidence? Eur Spine J. 2004;13:663–679. doi: 10.1007/s00586-004-0749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen TL, Anderson RL, Dearwater SR, Kriska AM, Cauley JA, Aaron DJ, LaPort RE. The epidemiology of low back pain in an adolescent population. Am J Public Health. 1992;82:606–608. doi: 10.2105/AJPH.82.4.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sjolie AN, Thuen F. School journeys and leisure activities in rural and urban adolescents in Norway. Health Promot Int. 2002;17:21–30. doi: 10.1093/heapro/17.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Burton AK, Balagué F, Cardon G, Eriksen HR, Henrotin Y, Lahad A, Leclerc A, Müller G, Beek AJ. Chapter 2. European guidelines for prevention in low back pain: November 2004. Eur Spine J Suppl. 2006;2:S136–S168. doi: 10.1007/s00586-006-1070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siambanes D, Martinez JW, Butler EW, Haider T. Influence of school backpacks on adolescent back pain. J Pediatr Orthop. 2004;24:211–217. doi: 10.1097/01241398-200403000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Viry P, Creveuil C, Marcelli C. Nonspecific back pain in children. A search for associated factors in 14-year-old schoolchildren. Rev Rhum Engl Ed. 1999;66:381–388. [PubMed] [Google Scholar]
- 25.Skoffer B, Foldspang A. Physical activity and low-back pain in schoolchildren. Eur Spine J. 2008;17:373–379. doi: 10.1007/s00586-007-0583-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato T, Ito T, Hirano T, Morita O, Kikuchi R, Endo N, Tanabe N. Low back pain in childhood and adolescence: assessment of sports activities. Eur Spine J. 2011;20:94–99. doi: 10.1007/s00586-010-1485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szpalski M, Gunzburg R, Balagué F, Nordin M, Melot C. A 2-year prospective longitudinal study on low back pain in primary school children. Eur Spine J. 2002;11:459–464. doi: 10.1007/s00586-002-0385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balagué F, Dudler J, Nordin M. Low-back pain in children. Lancet. 2003;361:1403–1404. doi: 10.1016/S0140-6736(03)13148-0. [DOI] [PubMed] [Google Scholar]