Abstract
Introduction
Atlantoaxial instability (AAI) is an uncommon disease in children. Surgical treatment of pediatric patients with AAI poses a challenge to spine surgeons because of the patients’ immature bone quality, extensive anatomical variability, and smaller osseous structures. In this study, the authors report complications and outcomes after posterior fusion in children with AAI.
Methods
The authors reviewed medical records of patients 13 years old and younger with AAI who underwent posterior fusion in the Nagoya Spine Group hospitals, a multicenter cooperative study group, from January 1995 to December 2007. We identified 11 patients who underwent posterior fusion, and analyzed their clinical outcomes and complications. To determine if vertical growth within the construct continued after posterior fusion, in three patients at 5 or more years following occipito-cervical (O-C) fusion, intervertebral disc heights and vertebral heights between the fused and non-fused levels were compared on the final follow-up.
Results
The initial surgeries were C1–C2 fusions in six patients and O-C fusion in five patients. Successful fusion ultimately occurred in all patients, however, the complication rate related to the operations was high (64%). Complications included neurologic deterioration, pedicle fracture with pedicle screw insertion, C1 posterior arch fracture with lateral mass screw insertion, perforation of the skull with a head pin placement, and fusion extension to adjacent vertebrae. Two patients required reoperation. The mean fixed and non-fixed intervertebral disc heights on the final follow-up were 2.6 and 5.3 mm, respectively, showing that the disc height of the fixed level was less than the non-fused level. Each vertebra lengthened similarly between fused and non-fused levels except for C2 which had a lower growth rate than the other vertebrae.
Conclusions
A high complication rate should be anticipated after posterior fusion in children with AAI. Careful consideration should be paid to pediatric patients with AAI treated by screw and/or rod systems. After posterior fusion in pediatric patients, each vertebra continued to grow, in contrast the disc height decreased between fused levels.
Keywords: Atlantoaxial instability, Posterior fusion, Os odontoideum, Down syndrome, Complications, Children
Introduction
Atlantoaxial instability (AAI) is an uncommon disease in children. Surgical treatment of pediatric patients with AAI poses a challenge to spine surgeons because of children’s immature bone quality, the extensive anatomical variability, and the smaller osseous structures. Also, the incidence of complications increases and surgery is more difficult in children with Down syndrome. Moreover, a high incidence of non-union after posterior fusion with the wiring technique has been reported [1, 2]. Magerl et al. [3] first proposed the use of C1–C2 transarticular screws in adults, and an alternative technique, namely C1 lateral mass screws and C2 pedicle screws [4, 5] or C2 translaminar screws [6] has been reported. More recently, these techniques are being used in pediatric patients [7–17]; however, there are no detailed reports on the risks with these techniques when used in children. Furthermore, the medical community does not fully understand the long-term effects of posterior cervical spine fusion in the skeletally immature spine, and there are only a few published articles on these effects [18, 19]. In this study, we investigated the complications and outcomes after posterior fusion in children with AAI. In addition, in three patients at five or more years following occipito-cervical fusion, intervertebral disc heights and vertebral heights between the fused and non-fused levels were compared at the final follow-up.
Methods
The research protocol for this study was approved by our Institutional Review Board. Of 19,364 patients registered for spinal surgery in a multicenter cooperative study group on spinal diseases, the Nagoya Spine Group (NSG), between January 1995 and December 2007, 11 pediatric patients, five boys and six girls, with AAI underwent posterior fusion. Pre-operative imaging included a combination of lateral cervical radiography, CT scanning, and MRI imaging. All patients had documented instability on pre-operative flexion-extension radiographs. CT scanning consisted of thin-cut axial images of the cervical spine with coronal and sagittal reconstructions to evaluate anatomic suitability for the planned screws. Surgeons placed all screws under the guidance of an image intensifier or CT navigation system. All patients underwent internal fixation, and the constructs included C1 lateral mass screw, pedicle screw, sublaminar wiring with rod or transarticular screws. Autogenous iliac crest bone grafts were used in all patients. For postoperative immobilization, we applied a hard cervical collar after C1–C2 fusion (average 2.4 months), and a halo vest after occipito-cervical (O-C) fusion (average 2 months). We investigated the surgical procedures, union rate, and complications. The clinical features of the patients in this study are summarized in Table 1. To determine if vertical growth within the construct continued after posterior fusion, we also measured and compared intervertebral disc heights and vertebral heights between the fused and non-fused levels on the final follow-up in three patients who had been followed for five or more years after their O-C fusions. We calculated the percentage of vertical growth of the cervical spine that occurred within the fused and non-fused levels as follows: height of construct (final follow-up − postoperative)/height of construct (postoperative) × 100.
Table 1.
Clinical information of pediatric patients with atlantoaxial instability
| Case no | Age at ope (yrs) | Diagnosis | Fusion construct | FU (mos) | Complications |
|---|---|---|---|---|---|
| 1 | 10 | Chronic AARF | C1–C2 transarticular screws | 109 | Fusion extension C2 to C3 |
| 2 | 13 | Os odontoideum | C1–C3 transarticular screws | 64 | – |
| 3 | 8 | Chronic AARF | C1 lat mass + C2 PS | 41 | Perforation of the skull with a head pin placement |
| 4 | 11 | Klippel–Feil syndrome, basilar impression | O-C3 fusion (rod + sublaminar wiring) | 68 | Loss of reduction, reoperation |
| 5 | 12 | AAS | C1 lat mass + C2 PS | 20 | – |
| 6 | 4 | AAS, Down syndrome | C1 lat mass + C2 PS | 20 | C1 posterior arch fracture with lateral mass screw insertion Loss of reduction, reoperation |
| 7 | 5 | Os odontoideum, congenital kyphosis | O-C7 fusion (rod + PS) | 16 | Pedicle fracture with pedicle screw insertion fusion extension C7 to Th1 |
| 8 | 8 | Chronic AARF | C1 lat mass + C2 PS | 12 | – |
| 9 | 10 | Os odontoideum | O-C3 fusion (rod + sublaminar wiring) | 90 | – |
| 10 | 7 | AAS, Down syndrome | O-C3 fusion (rod + sublaminar wiring) | 93 | Loss of reduction |
| 11 | 6 | Chronic AARF | O-C3 fusion (rod + sublaminar wiring) | 95 | Fusion extension C3 to C4 |
Ope operation, yrs years, FU follow-up, mos months, AARF atlantoaxial rotatory fixation, AAS atlantoaxial subluxation, lat mass lateral mass screw, PS pedicle screw
Results
Eleven pediatric patients with AAI underwent posterior fusion during the study period. The average age at surgery was 8.5 years (4–13 years), and the mean follow-up period was 57.1 months (12–109 months). The indications for surgery were chronic AARF in four patients, os odontoideum in two patients, AAS associated with Down syndrome in two patients, AAS in one patient, os odontoideum and congenital kyphosis in one patient, and Klippel–Feil syndrome and basilar impression in one patient. Five patients were myelopathic before surgery with upper motor neuron abnormalities and gait disturbance. CT angiography revealed a high-riding vertebral artery in two patients.
Surgical procedure
The initial surgical procedure was C1–C2 fusion in six patients and O-C fusion in five patients. For the C1–C2 fusions, surgeons performed the C1 lateral mass screw + C2 pedicle screw method (Goel–Harms constructs) in four patients, and the C1–C2 transarticular screw method (Magerl technique) in two patients. For the five patients who required O-C fusion, we used a rod and wiring technique in three of them for O-C3 fusions, a rod and sublaminar wiring technique in one patient for an O-C4 fusion, and a rod and sublaminar wiring/pedicle screw method in one patient for an O-C7 fusion. In one case (case 6), we converted C1–C2 fusion to O-C2 fusion finally, because of loss of reduction and neurological deterioration. Monocortical structural autografts and morselized cancellous autografts were harvested from the posterior iliac crest in all patients. The structural autograft was wired between the two rods or around the C2 spina to span the decorticated surfaces of the occiput to the cervical spine or C1–C2. The morselized cancellous autograft was packed around the remaining laminar surface and decorticated facet joints.
Radiographic fusion
Nine patients achieved solid osseous fusion demonstrated on plain radiographs or CT scanning. Non-union occurred in two patients (cases 4 and 6). Non-union with loss of reduction and neurologic deterioration necessitated reoperation in these patients. We performed an O-C2 fusion because of non-union and neurologic deterioration after the previous C1–C2 fusion for AAS associated with Down syndrome (Case 6). We performed transoral odontoidectomy and O-C2 fusion because of non-union and neurologic deterioration after O-C3 fusion in the patient with Klippel–Feil syndrome and basilar impression (case 4). After reoperation, these patients proceeded to solid fusions and neurologic improvement.
Complications
Complications related to the operation occurred in seven patients (64%) (Table 1). They included neurologic deterioration (three patients), non-union in two patients, fusion extension to adjacent vertebrae in three patients, pedicle fracture with pedicle screw insertion in one patient, C1 posterior arch fracture with lateral mass screw insertion in one patient, and perforation of the skull with a head pin placement in one patient. Cervical spine X-ray and CT demonstrated fusion extension from C1–2 to C3 (case 2), from O-C7 to Th1 (case 7), and from O-C3 to C4 (case 11).
There were no wound infections and no instances of iatrogenic vertebral artery injury. No cases of acute neurologic deterioration occurred intra-operatively or in the immediate postoperative period. Furthermore, during the follow-up period, no patients developed abnormal motion adjacent to the fused segments.
The rates of increase in vertebral body height and cervical spine alignment
In three patients whom we followed five or more years after their O-C3 fusions, the rates of increase in vertebral body heights were 20% for C2, 64% for C3, 73% for C4, 48% for C5, 45% for C6, and 35% for C7. Each vertebra grew taller during the follow-up period whether fused or not. The mean fixed and non-fixed intervertebral disc heights on the final follow-up were 2.6 and 5.3 mm, respectively, showing that the disc height of a fixed vertebral level was less than a non-fused level (Fig. 1). In this group, the cervical alignment was lordosis in one, straight in one, and kyphosis in one at the time of the final observation.
Fig. 1.
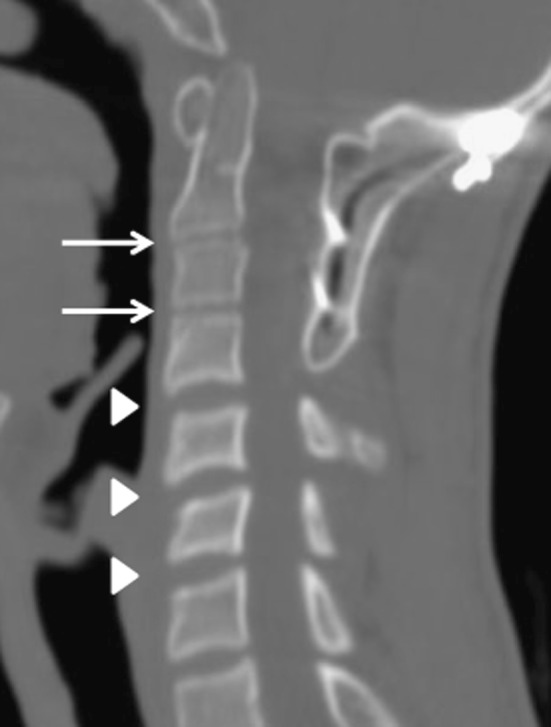
Sagittal CT reconstruction demonstrates the fixed and non-fixed intervertebral disc heights at the final follow-up. Arrow indicates the fixed intervertebral disc height, and arrow head indicates the non-fused disc height
Illustrative cases
Case 6
This 7-year-old girl presented with a gait disturbance due to AAS associated with Down syndrome. The CT sagittal image indicated AAS, and the MRI sagittal image indicated high intensity change of the spinal cord and stenosis at the C1 level. At her first operation, we performed C1–2 fusion using a C1 lateral mass screw and C2 pedicle screw. C1 posterior arch fracture occurred when the C1 lateral mass screw was inserted. Four months after the first operation, she developed recurrent stenosis between the occipital bone and the dens and became non-ambulatory again. We performed an O-C2 fusion for salvage, and a halo vest was applied after reoperation for 3 months. Bone union occurred, and she was able to walk (Fig. 2).
Fig. 2.
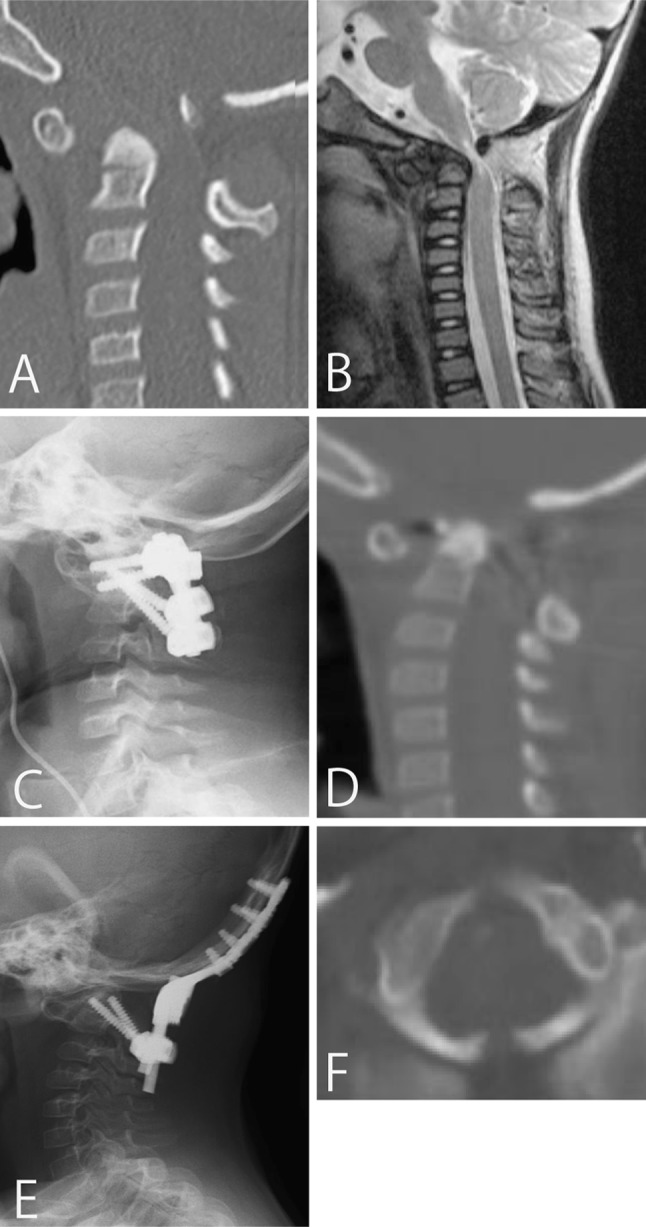
Case 6. A pre-operative CT scan reconstruction of the cervical spine (a) suggests an atlantoaxial subluxation, and a pre-operative sagittal MR image (b) demonstrates spinal canal stenosis and cord signal change. Postoperative radiograph (c) of the lateral cervical spine shows correction of atlantoaxial alignment using C1 lateral mass screw and C2 pedicle screws. 4 months after primary surgery, sagittal CT reconstruction of the cervical spine (d) shows stenosis between occipito and dens. After reoperation, radiograph of the lateral cervical spine (e) shows successful O-C2 fusion. Pre-operative axial CT scan of the atlas (f) shows spina bifida of the posterior arch
Case 11
This 6-year-old boy presented with torticollis and neck pain, which occurred after an upper respiratory infection. At our hospital, we diagnosed AARF (Fielding type 3) by X-ray and CT scanning, and he was treated conservatively: neck collar, Glisson neck traction, and halo vest treatment for 5 months. However, the condition did not improve, and 5 months after the onset of symptoms, we performed open reduction and O-C3 posterior fusion using a rod and wiring. Postoperatively, the patient was placed in a halo vest for 1 month and in a neck collar for 2 months. On follow-up, the patient had a solid fusion construct on CT scanning, however, cervical spine x-ray and CT demonstrated fusion extension from O-C3 to C4 (Fig. 3).
Fig. 3.
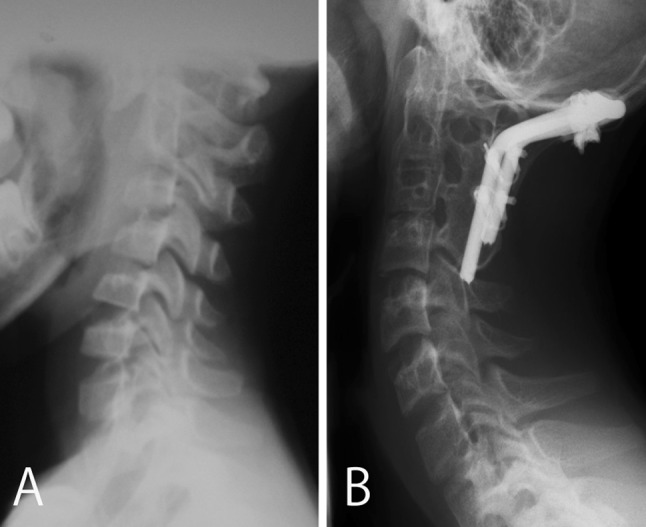
Case 11. Pre-operative (a) and postoperative (b) radiographs of the lateral cervical spine show realignment of the occipito-cervical and atlantoaxial complexes, however, at final follow-up the radiograph (b) demonstrates fusion extension from O-C3 to C4
Discussion
The cause of AAI in children includes congenital abnormalities, trauma, inflammation and infection. Congenital causes of AAI include the ligamentous laxity associated with Down syndrome and anomalies of the odontoid process [20–23]. Surgical treatment of pediatric patients with AAI poses a challenge to spine surgeons because of the patients’ immature bone quality, extensive anatomical variability, and their smaller osseous structures. In the past, a C1–C2 fusion using autogenous iliac crest and sublaminar wiring such as Brooks-Jenkins, Gallie, and Sonntag have been reported [24–26], but this technique resulted in non-union in most cases, especially for patients with Down syndrome [1, 2].
C1–2 transarticular screw fixation (Magerl technique [3]) and its effectiveness in pediatric patients have been described [8, 16, 17]. Similarly, some studies reported that the C1 lateral screw and C2 pedicle screw technique (Goel–Harms constructs [4, 5]) provide stabilization and promotes fusion in pediatric patients with AAI [9–13]. Also, unlike wiring techniques, C1–2 transarticular screw and Goel–Harms constructs do not require that the patient be placed in a halo vest postoperatively [13]. Likewise, Leonard et al. [15] reported successful results with rigid fixation in three children in whom bilateral crossing C2 translaminar screws were placed to avoid vertebral artery injury.
Although these techniques for upper cervical surgery have been extensively reported in adult series [27–29], there have been reports on its use in only a small series of pediatric populations. We performed the C1 lateral mass screw and C2 pedicle screw technique and C1–C2 transarticular screw technique for C1–C2 fusion in six patients and achieved successful outcomes in five of the six cases. We thought that this technique could be useful for C1–C2 fusion in children, as it has been in adults; however, in one patient (AAS associated with Down syndrome, case 6), we subsequently carried out an O-C2 fusion because of non-union and neurologic deterioration after a C1–C2 fusion. As we described in “Methods” on O-C fusion in our series, four patients were treated with rod and wiring technique and one patient by O-C7 fusion with rod and sublaminar wiring/pedicle screw. Of the four patients receiving rod and wiring, two patients deteriorated further as a result of loss of reduction. Thus, the rod and wiring was weak making it difficult to obtain solid fusion.
Recently, some small case series on O-C fusions with rod and screw fixation in pediatric patients were reported [11, 15, 16]. Jea et al. [14] recently reported on a series of pediatric patients in whom they incorporated lateral mass screws and pedicle screws within occipito-cervical or atlantoaxial constructs. One patient sustained injury to the vertebral artery. Similarly, Haque et al. [11] reported on four O-C fusion cases with a C1 lateral mass screw, C2 pars screw, and laminar screw. These patients did not have any significant morbidity associated with the procedure and all achieved solid fusion.
Although screw and rod fixation techniques increased the fusion rate, complications associated with screw fixation can and did occur in pediatric patients. For example, in case 6, when the C1 lateral mass screw was inserted, a bilateral C1 posterior arch fracture occurred, and we could not prepare the appropriate graft beds and transplant a sufficient bone graft. In this case, CT scanning revealed a C1 anomaly, spina bifida, before the operation. Since the patient’s AAS was reducible in the extension radiograph film, we should have performed a C1–C2 transarticular technique anticipating the possibility of posterior arch fracture with a C1 lateral mass screw insertion. In addition, in case 7, a pedicle fracture occurred with pedicle screw insertion to the C2 pedicle. To avoid these complications, we must take note of the pedicle size and any vertebral anomaly, and if we determine that screw insertion in the pedicle or lateral mass is difficult or risky, we should change the strategy.
Cervical X-ray and CT scanning demonstrated fusion extension to adjacent vertebrae in three patients. After C1–C2 or O-C fusion, neck motion is compensated by the subaxial segments. To maintain optimum range of motion in these patients, we must try to preserve more vertebrae after cervical fixation. Thus, we should expose the minimum surgical field needed so as to not damage the periosteum of adjacent vertebrae, especially in pediatric patients.
We measured the growth of cervical vertebrae after O-C fusion in three of our cases. Occipito-cervical fusion has been documented previously to lead to height loss of the fused vertebrae, particularly C2 [18, 19]. In contrast, Anderson et al. [30] demonstrated that, on average, 34% of the overall vertical growth of the cervical spine occurred within the fusion construct (occiput-C2 or C1–2). However, all cases in those studies were O-C2 or C1–2 fusion, yet they discussed C2 vertebral growth. To the authors’ knowledge, there has been no study of vertebral growth after cervical fusion cases longer than O-C2. On long-term follow-up in our three cases receiving longer fusions, the C2 vertebral body height increased at a lower rate than the other vertebrae, but the other vertebrae lengthened almost equally regardless of the type of posterior fusion was performed.
On the other hand, we could not find any previous studies accounting for the growth of disc spaces after O-C fusion. In our study, we investigated the fused and non-fused intervertebral disc heights in three patients five or more years after surgery, and the disc height of the fused level was less than the non-fused levels, suggesting that the continued growth of the anterior spine would result in decreasing disc height of the fused level in the skeletally immature patient. Cervical alignment after O-C or C1–2 fusion also could be affected. Nakagawa et al. [18] found an increase in cervical lordosis after O-C fusion from 12.9 to 23.6° at 5.9 years of follow-up. Similarly, Rodgers et al. [31] found that O-C lordosis increased a mean of 1° per level fused per year until skeletal maturity after O-C2 fusion using wire. However, our three cases indicated different cervical alignment after O-C fusion at final follow-up.
This study has some limitations. First, since it was a multicenter-study, there were various causes of atlantoaxial instability, and thus, the surgical approaches used at the different institutions were not uniform. Second, because of the small number of cases, this study lacks powerful statistical analysis of the variation in growth of vertebrae and intervertebral disc heights between the fused and non-fused levels.
Conclusions
In our study, 11 children with AAI underwent posterior fusion. The complication rate related to surgery was high (64%) and varied. In three patients whom we followed five or more years after their O-C3 fusions, each vertebra had similar growth; however, the disc height of a fixed vertebral level was less than a non-fused level. Thus, parents with a child suffering from AAI needing posterior fusion should be thoroughly informed of the various complications associated with surgery, and surgical treatment of the lesions associated with AAI in children should be extensively evaluated to avoid these various complications.
Acknowledgments
The authors are grateful to all the staff of Nagoya Spine Group for allowing them to study their patients and for their assistance with data collection.
Conflict of interest
None.
Glossary
- AAI
Atlantoaxial instability
- AARF
Atlantoaxial rotatory fixation
- AAS
Atlantoaxial subluxation
References
- 1.Doyle JS, Lauerman WC, Wood KB, Krause DR. Complications and long-term outcome of upper cervical spine arthrodesis in patients with Down syndrome. Spine. 1996;21:1223–1231. doi: 10.1097/00007632-199605150-00016. [DOI] [PubMed] [Google Scholar]
- 2.Segal LS, Drummond DS, Zanotti RM, Ecker ML, Mubarak SJ. Complications of posterior arthrodesis of the cervical spine in patients who have Down syndrome. J Bone Joint Surg Am. 1991;73:1547–1554. [PubMed] [Google Scholar]
- 3.Jeanneret B, Magerl F. Primary posterior fusion C1/2 in odontoid fractures: indications, technique, and results of transarticular screw fixation. J Spinal Disord. 1992;5:464–475. doi: 10.1097/00002517-199212000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Goel A, Laheri V. Plate and screw fixation for atlanto-axial subluxation. Acta Neurochir (Wien) 1994;129:47–53. doi: 10.1007/BF01400872. [DOI] [PubMed] [Google Scholar]
- 5.Harms J, Melcher RP. Posterior C1-C2 fusion with polyaxial screw and rod fixation. Spine. 2001;26::2467–2471. doi: 10.1097/00007632-200111150-00014. [DOI] [PubMed] [Google Scholar]
- 6.Wright NM. Posterior C2 fixation using bilateral, crossing C2 laminar screws: case series and technical note. J Spinal Disord Tech. 2004;17:158–162. doi: 10.1097/00024720-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Belen D, Simsek S, Yigitkanli K, Bavbek M. Internal reduction established by occiput-C2 pedicle polyaxial screw stabilization in pediatric atlantoaxial rotatory fixation. Pediatr Neurosurg. 2006;42:328–332. doi: 10.1159/000094073. [DOI] [PubMed] [Google Scholar]
- 8.Brockmeyer DL, York JE, Apfelbaum RI. Anatomical suitability of C1–2 transarticular screw placement in pediatric patients. J Neurosurg. 2000;92:7–11. doi: 10.3171/jns.2000.92.1.0007. [DOI] [PubMed] [Google Scholar]
- 9.Chamoun RB, Whitehead WE, Curry DJ, Luerssen TG, Jea A. Computed tomography morphometric analysis for C-1 lateral mass screw placement in children. Clinical article. J Neurosurg Pediatr. 2009;3:20–23. doi: 10.3171/2008.10.PEDS08224. [DOI] [PubMed] [Google Scholar]
- 10.Desai R, Stevenson CB, Crawford AH, Durrani AA, Mangano FT. C-1 lateral mass screw fixation in children with atlantoaxial instability: case series and technical report. J Spinal Disord Tech. 2010;23:474–479. doi: 10.1097/BSD.0b013e3181bf9f24. [DOI] [PubMed] [Google Scholar]
- 11.Haque A, Price AV, Sklar FH, Swift DM, Weprin BE, Sacco DJ. Screw fixation of the upper cervical spine in the pediatric population. Clinical article. J Neurosurg Pediatr. 2009;3:529–533. doi: 10.3171/2009.2.PEDS08149. [DOI] [PubMed] [Google Scholar]
- 12.Hedequist D, Proctor M. Screw fixation to C2 in children: a case series and technical report. J Pediatr Orthop. 2009;29:21–25. doi: 10.1097/BPO.0b013e3181924367. [DOI] [PubMed] [Google Scholar]
- 13.Heuer GG, Hardesty DA, Bhowmick DA, Bailey R, Magge SN, Storm PB. Treatment of pediatric atlantoaxial instability with traditional and modified Goel-Harms fusion constructs. Eur Spine J. 2009;18:884–892. doi: 10.1007/s00586-009-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jea A, Taylor MD, Dirks PB, Kulkarni AV, Rutka JT, Drake JM. Incorporation of C-1 lateral mass screws in occipitocervical and atlantoaxial fusions for children 8 years of age or younger. Technical note. J Neurosurg. 2007;107:178–183. doi: 10.3171/PED-07/08/178. [DOI] [PubMed] [Google Scholar]
- 15.Leonard JR, Wright NM. Pediatric atlantoaxial fixation with bilateral, crossing C-2 translaminar screws. Technical note. J Neurosurg. 2006;104:59–63. doi: 10.3171/ped.2006.104.1.59. [DOI] [PubMed] [Google Scholar]
- 16.Reilly CW, Choit RL. Transarticular screws in the management of C1–C2 instability in children. J Pediatr Orthop. 2006;26:582–588. doi: 10.1097/01.bpo.0000230337.26652.55. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Vokshoor A, Kim S, Elton S, Kosnik E, Bartkowski H. Pediatric atlantoaxial instability: management with screw fixation. Pediatr Neurosurg. 1999;30:70–78. doi: 10.1159/000028766. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa T, Yone K, Sakou T, Yanase M. Occipitocervical fusion with C1 laminectomy in children. Spine. 1997;22:1209–1214. doi: 10.1097/00007632-199706010-00006. [DOI] [PubMed] [Google Scholar]
- 19.Parisini P, Di Silvestre M, Greggi T, Bianchi G. C1-C2 posterior fusion in growing patients: long-term follow-up. Spine. 2003;28:566–572. doi: 10.1097/01.BRS.0000049961.22749.49. [DOI] [PubMed] [Google Scholar]
- 20.Burke SW, French HG, Roberts JM, Johnston CE, 2nd, Whitecloud TS, 3rd, Edmunds JO., Jr Chronic atlanto-axial instability in Down syndrome. J Bone Joint Surg Am. 1985;67:1356–1360. [PubMed] [Google Scholar]
- 21.Juhl M, Seerup KK. Os odontoideum. A cause of atlanto-axial instability. Acta Orthop Scand. 1983;54:113–118. doi: 10.3109/17453678308992879. [DOI] [PubMed] [Google Scholar]
- 22.Tredwell SJ, Newman DE, Lockitch G. Instability of the upper cervical spine in Down syndrome. J Pediatr Orthop. 1990;10:602–606. doi: 10.1097/01241398-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Uno K, Kataoka O, Shiba R. Occipitoatlantal and occipitoaxial hypermobility in Down syndrome. Spine. 1996;21:1430–1434. doi: 10.1097/00007632-199606150-00006. [DOI] [PubMed] [Google Scholar]
- 24.Brooks AL, Jenkins EB. Atlanto-axial arthrodesis by the wedge compression method. J Bone Joint Surg Am. 1978;60:279–284. [PubMed] [Google Scholar]
- 25.Dickman CA, Sonntag VK, Papadopoulos SM, Hadley MN. The interspinous method of posterior atlantoaxial arthrodesis. J Neurosurg. 1991;74:190–198. doi: 10.3171/jns.1991.74.2.0190. [DOI] [PubMed] [Google Scholar]
- 26.Gallie WE. Skeletal traction in the treatment of fractures and dislocations of the cervical spine. Ann Surg. 1937;106:770–776. doi: 10.1097/00000658-193710000-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aryan HE, Newman CB, Nottmeier EW, Acosta FL, Jr, Wang VY, Ames CP. Stabilization of the atlantoaxial complex via C-1 lateral mass and C-2 pedicle screw fixation in a multicenter clinical experience in 102 patients: modification of the Harms and Goel techniques. J Neurosurg Spine. 2008;8:222–229. doi: 10.3171/SPI/2008/8/3/222. [DOI] [PubMed] [Google Scholar]
- 28.Hott JS, Lynch JJ, Chamberlain RH, Sonntag VK, Crawford NR. Biomechanical comparison of C1–2 posterior fixation techniques. J Neurosurg Spine. 2005;2:175–181. doi: 10.3171/spi.2005.2.2.0175. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida G, Kamiya M, Yoshihara H, Kanemura T, Kato F, Yukawa Y, Ito K, Matsuyama Y, Sakai Y. Subaxial sagittal alignment and adjacent-segment degeneration after atlantoaxial fixation performed using C-1 lateral mass and C-2 pedicle screws or transarticular screws. J Neurosurg Spine. 2010;13:443–450. doi: 10.3171/2010.4.SPINE09662. [DOI] [PubMed] [Google Scholar]
- 30.Anderson RC, Kan P, Gluf WM, Brockmeyer DL. Long-term maintenance of cervical alignment after occipitocervical and atlantoaxial screw fixation in young children. J Neurosurg. 2006;105:55–61. doi: 10.3171/ped.2006.105.1.55. [DOI] [PubMed] [Google Scholar]
- 31.Rodgers WB, Coran DL, Kharrazi FD, Hall JE, Emans JB. Increasing lordosis of the occipitocervical junction after arthrodesis in young children: the occipitocervical crankshaft phenomenon. J Pediatr Orthop. 1997;17:762–765. doi: 10.1097/00004694-199711000-00011. [DOI] [PubMed] [Google Scholar]


