Abstract
Introduction
Various studies have shown that spine stabilisation exercise therapy elicits improvements in symptoms/disability in patients with chronic non-specific low back pain (cLBP). However, few have corroborated the intended mechanism of action by examining whether clinical improvements (1) are greater in patients with functional deficits of the targeted muscles and (2) correlate with post-treatment improvements in abdominal muscle function.
Methods
Pre and directly after 9 weeks’ therapy, 32 cLBP patients (44.0 ± 12.3 years) rated their LBP intensity (0–10) and disability (0–24, Roland–Morris; RM) and completed psychological questionnaires. At the same timepoints, the voluntary activation of transversus abdominis (TrA), obliquus internus and obliquus externus during “abdominal-hollowing” and the anticipatory (“feedforward”) activation of these muscles during rapid arm movements were measured using M-mode ultrasound with tissue Doppler imaging.
Results
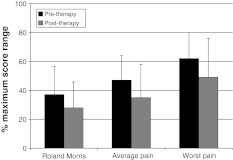
Pre-therapy to post-therapy, RM decreased from 8.9 ± 4.7 to 6.7 ± 4.3, and average pain, from 4.7 ± 1.7 to 3.5 ± 2.3 (each P < 0.01). The ability to voluntarily activate TrA increased by 4.5% (P = 0.045) whilst the anticipatory activation of the lateral abdominal muscles showed no significant change (P > 0.05). There was no significant correlation between the change in RM scores after therapy and either baseline values for voluntary (r = 0.24, P = 0.20) or anticipatory activation (r = 0.04, P = 0.84), or their changes after therapy (voluntary, r = 0.08, P = 0.66; anticipatory, r = 0.16, P = 0.40). In multiple regression, only a reduction in catastrophising (P = 0.0003) and in fingertip–floor distance (P = 0.0006) made unique contributions to explaining the variance in the reduction in RM scores.
Conclusion
Neither baseline lateral abdominal muscle function nor its improvement after a programme of stabilisation exercises was a statistical predictor of a good clinical outcome. It is hence difficult to attribute the therapeutic result to any specific effects of the exercises on these trunk muscles. The association between changes in catastrophising and outcome serves to encourage further investigation on larger groups of patients to clarify whether stabilisation exercises have some sort of “central” effect, unrelated to abdominal muscle function per se.
Keywords: Spine stabilisation exercise therapy, Outcome, Chronic low back pain
Introduction
Over the last decade, a number of studies have reported dysfunction in both the feedforward and the voluntary activation of the deep-lying trunk muscles in connection with recurrent or chronic low back pain (LBP) [7, 22, 25, 27, 47]. Compared with healthy controls, in patients with LBP the onset of activation of, in particular, the transversus abdominis (TrA) during rapid movements of the arm or leg was shown to be delayed, i.e., the involuntary “anticipatory” function of the TrA was compromised [22, 25, 27]. Not only the “anticipatory” function of the TrA but also the ability to voluntarily activate it during standardised exercises, as determined by changes in TrA thickness, has been shown to be deficient in patients with chronic LBP (cLBP) [7, 47]. Based on the hypothesis that these dysfunctions may pose a threat to spine segmental stability and perhaps predispose to continuing/future episodes of pain [24], specific spine stabilisation exercises, aimed at restoring these various aspects of deep trunk muscle function [23, 49], have become a popular concept in physiotherapy.
Since their introduction some 10 years ago, a number of randomised controlled trials have been carried out to examine the effectiveness of these exercises for LBP, and the pooled results have been summarised in two systematic reviews [12, 48]. These conclude that stabilisation exercises are superior to usual medical care and education (or “general practitioner treatment”), but not other forms of physical therapy/exercise, and there is limited evidence for any additional effect when stabilisation exercises are added to conventional physiotherapy programmes. These findings have been further substantiated in two subsequent trials [10, 30]. Although segmental stabilisation exercises enjoy wide popularity in the treatment of LBP patients worldwide, their application and the underlying rationale for their use is not endorsed unreservedly by all [4, 29, 39]. Some aspects of its plausibility have been challenged in the more recent literature [2, 3, 5, 16, 36, 37, 40].
Another reservation concerning the rationale for the treatment is that although some cross-sectional studies [7, 11, 47] (though by no means not all [44, 45]) show statistically significant differences in deep trunk muscle recruitment/activity levels between groups of cLBP patients and controls, few have quantified either the prevalence or the ‘diagnostic accuracy’ of such dysfunctions in large samples of individuals. Recent work acknowledges the small, but statistically significant group differences but suggests that the discriminatory power may be rather low and clinically non-relevant [47]. The final concern about the treatment relates to the paucity of data in the literature indicating that a positive outcome after a programme of segmental stabilisation exercises is contingent upon improvements in deep trunk muscle function. One small study showed a low (r = −0.35) but significant correlation between improved TrA recruitment during leg flexion/extension tasks and reduced disability, but not between TrA recruitment and reduced pain, improved patient-specific functional scores, or perceived recovery [13]. Another found no correlation between improved TrA recruitment and changes in sports restriction [28]. Interpretation of these findings is not straightforward, and in none of the studies were other aspects of physical function or psychological attributes assessed that might otherwise have helped to explain the changes in clinical outcome after treatment. As suggested for other types of exercise therapy [55], it is conceivable that the mechanism of action for this type of treatment does not concern trunk muscle function or segmental stabilisation, per se, and instead resides in some other positive influence of the treatment programme such as improvements in self-efficacy, coping strategies, catastrophising, or fear-avoidance [33, 52], changes in cortical organisation [14, 66] or simply a positive therapist–patient interaction/relationship [19].
The present study sought to test the following hypotheses: (1) that there is a significant relationship between changes in self-rated disability and changes in deep trunk muscle dysfunction function after a 3-month therapy programme of spine stabilisation exercises for patients with cLBP and (2) that this relationship is stronger than the relationships between changes in disability and changes in either psychological status or a general functional test (trunk flexion).
Methods
Patients
37 patients with cLBP participated in the study (see later for further details). The methods of recruitment and the inclusion/exclusion criteria have been described in detail elsewhere [32]. Briefly, they were recruited from the departments of rheumatology, orthopaedics and neurology of local participating hospitals, had non-specific LBP (as defined in European Guidelines [1]) with or without referred pain of a non-radicular nature for at least 3 months, for which they had just received a prescription to undergo physiotherapy.
All participants gave their signed informed consent to participate after receiving verbal and written information about the study. The study was approved by the local medical ethics committee.
Therapy
Patients underwent a 9-week programme of therapeutic spinal segmental stabilisation exercises, carried out once/week and directed by a physiotherapist trained in this therapy concept [32]. The treatment approach has been described in detail in the literature (summarised in [49]).
Patients were asked to perform home exercises comprising a sequence of 10 repetitions, 10 times a day. They were given illustrated information brochures describing the exercises and their purpose, and giving various tips and advice on how to perform them properly.
Questionnaires
Before and after therapy, the patients completed a questionnaire booklet containing the following instruments: the primary clinical outcome measure, the Roland and Morris Disability Questionnaire (RM), which measures 24 activity limitations due to back pain (score 0–24: higher score, increased disability) [9, 50]; a Pain Graphic Rating Scale (PGRS) [31], in which the average and worst back pain intensity during the last week was indicated on a 0–10 graphic rating scale; the Fear Avoidance Beliefs Questionnaire [54, 65]; the Pain Catastrophising Questionnaire [41, 56]; and the Modified Somatic Perception Questionnaire (heightened somatic awareness) and modified Zung self-rating depression questionnaire, the combined scores of which yielded a measure of Psychological Disturbance [17, 34].
Overview of functional tests
Tests were carried out to assess general trunk flexibility (fingertip-to-floor distance when standing with straight legs) and two aspects of lateral abdominal muscle function: the voluntary activation during abdominal hollowing exercises and the anticipatory (feedforward) activation during rapid movements of the contralateral arm. Voluntary activation was assessed by measuring the relative changes in TrA muscle thickness over time, based on M (motion)-mode ultrasound images recorded during the abdominal hollowing manoeuvre [35, 38, 47]. Anticipatory activation was measured using tissue velocity information from tissue Doppler imaging and was given by the first onset of activity of any of the lateral abdominal muscles (TrA, OI, OE), expressed in relation to the onset of activity of medial deltoid measured with surface electromyography (EMG) [18, 37].
Ultrasound equipment
For both functional tests, ultrasound images were recorded using a Philips HDI-5000 (Philips Medical Systems, Bothell, WA, USA) with a linear-array transducer (5–12 MHz); the images were superimposed with tissue Doppler image (TDI) data. The methods have been described in detail before [18, 35, 37, 38, 47]. Briefly, using firstly B (brightness)-mode ultrasound, the appropriate recording site was identified as a position approximately 2.5 cm anteromedial to the mid-point between the iliac crest and the costal margin on the mid-axillary line [42]. The ultrasound grey-scale data, superimposed with TDI, were sampled in M-mode at 333 Hz, and these plus the event-marker data [indicating the instruction to start the hollowing manoeuvre (voluntary function test) or the arm movement (anticipatory function test)] were exported in digital form using the ResearchLink option of the HDI-5000 system, and stored on computer.
Voluntary activation of the lateral abdominal muscles during abdominal hollowing
Abdominal hollowing exercises were performed in the supine hook-lying position, by slowly contracting the abdominals to draw in the abdomen, and holding for 5 s. The patients received a practice session (5–15 min), using ultrasound as a biofeedback tool [20]. Ten repeated abdominal hollowing exercises were then performed (5 with the transducer over the right abdominals and 5 with it over the left), with a 1–2 min rest period between each. During the measurement the subjects were not able to see the ultrasound imagines and they received no verbal feedback on their performance. They were instructed to breathe normally during the task.
Anticipatory activation of the lateral abdominal muscles during rapid arm movements
The methodology for examining the feedforward activation of the lateral abdominal muscles using tissue velocity information from TDI has been described in detail before [18, 37]. The test set-up was similar to that described by Hodges and Richardson [26], in which trunk muscle activity was assessed during rapid movements of the contralateral arm. Briefly, in response to a computerised visual stimulus, the participant performed rapid shoulder flexion (up to 60°), abduction (up to 60°) or extension (up to 40°) in randomised order, moving the extended arm as quickly as possible in the direction displayed on the computer screen. 10 arm movements were performed in each of the three directions, on each of the right and left body sides. A contact switch, with one part attached to the wrist and its counter-piece attached to the outer thigh, was used to indicate the start of the arm movement and to time-synchronise the TDI signals from the abdominal muscles and surface EMG (sEMG) signals from the medial deltoid (MD). The latter were recorded with pairs of disposable Ag/AgCl bipolar sEMG electrodes (Electrodes ECG Universelles; Contrôle-Graphique S.A., Brie Compte Robert Cedex, France) placed over the muscle after appropriate skin preparation with an inter-electrode distance of 2 cm [21]. A reference electrode was placed over the C7 spinous process. The raw sEMG signals were band-pass filtered (50–500 Hz), amplified, analogue-to-digital converted at a sampling rate of 5,000 Hz (Dantec, Medtronic Functional Diagnostics A/S, Skovlunde, Denmark) and stored on the hard disc of the computer.
Data processing: voluntary activation of the lateral abdominal muscles
Full details of the data processing methods for determination of the voluntary activation of the lateral abdominal muscles have been reported previously [35, 47]. Briefly, for the assessment of muscle thicknesses, the leading edge points (i.e. the upper border) of the fascia of the muscle of interest were marked as manually selected control points at regular intervals throughout the M-mode image, and a custom-written plug-in of the HDI-Lab software (version 1.9 ATL/Philips Medical Systems, Bothell, WA, USA) was then used to automatically track the borders between adjacent control points. This allowed subsequent calculation of the vertical distance between the top and bottom lines, i.e., the thickness of the given muscle over time. The data were exported as text data into a custom-written LabView (National Instruments Corporation, Austin, TX, USA) software programme to determine the resting thicknesses of TrA, given by the 1 s value during quiet rest, just before the contraction began, and the maximal thickness of TrA over any given 3 s period during the contraction. The TrA contraction ratio (Tr-CR) was then determined as the TrA thickness contracted/TrA thickness at rest. This index has been shown to give the most relevant and reliable (test–retest) estimation of the activation of TrA [38, 47, 57].
Data processing: anticipatory activation of the lateral abdominal muscles
Full details of the data processing methods for determination of the anticipatory activation of the lateral abdominal muscles have been reported before [37]. Briefly, the ultrasound data were firstly exported into a customised program written in MATLAB (Student Version 7.1 R14, The Math Works Inc., Natick, MA, USA), the area of interest of each abdominal muscle was identified, and the corresponding tissue velocity over time was calculated. This and the corresponding raw EMG data were imported into a second customised MATLAB program for the manual identification of the muscle activity onsets. The investigator was blind to the subject, the specific muscle, the type of movement, and the time-point of assessment (before or after therapy). For both TDI-velocity and surface EMG data, the onsets were given by the earliest rise above baseline levels [26].
For each individual arm movement trial, the onset time for the earliest muscle activity (in either TrA, OI or OE muscles) was expressed in relation to the onset of MD; a mean value for the “earliest onset of activity” was then calculated for each movement direction on each body side for a given person (i.e., from their 10 trials in each direction, on each body side) [18, 37].
Statistical analysis
The main aim of the study was to examine the association between changes in lateral abdominal muscle function and the clinical outcome (self-rated disability) after therapy. The final sample size allowed for the determination of a correlation coefficient of a moderate size, r = 0.5 (i.e., 25% shared variance) with a probability of 85% (power) against the null hypothesis, at a two-sided significance level of 5%, allowing for up to 20% therapy drop-outs.
Descriptive data are presented as mean and standard deviations (SD), unless otherwise indicated. Changes in lateral abdominal muscle function with therapy were examined using repeated measures ANOVA [repeated (“within”) factors: body side, movement direction, pre/post-therapy]. The interrelationships between the different measures of deep trunk muscle function and outcome were quantified using Pearson product–moment correlation coefficients or Spearman rank correlation coefficients, as appropriate [depending on the normality and nature (interval or ordinal) of the data]. Stepwise multiple regression was used to identify unique predictors of outcome, entering only the most significant variable (on bivariate testing) from the three domains of interest: lateral abdominal function (voluntary Tr-CR and anticipatory “earliest onset”), general function (fingertip-to-floor) and psychological attributes (fear avoidance beliefs, catastrophising, psychological disturbance).
Where missing data for one body side or movement direction would have resulted in the entire loss of data for an individual (e.g. in the repeated measures analyses), the missing mean value was estimated from the other body side or movement directions and adjusted for any differences in sides (or movement directions) for the group mean values. Note was made in the text when this was done.
Following the reasoning of Perneger et al. [43], no corrections were made for multiple testing. Significance was accepted at the 5% level.
The analyses were carried out using Statview 5.0 (SAS Institute Inc., San Francisco, USA) and SPSS 16.0 for Macintosh (SPSS Inc., Chicago, IL, USA).
Results
Changes in pain, disability and psychological factors after therapy
Details concerning the patients’ adherence to and completion of the physiotherapy programme, and the accompanying changes in pain and disability, have already been reported [32]. Briefly, 32/37 (86%) patients completed the treatment and returned pre- and post-therapy questionnaires. There were 11 men and 21 women, and their mean age, height, weight and BMI were 44.0 ± 12.3 years, 1.70 ± 0.07 m, 73.4 ± 11.5 kg and 25.5 ± 4.8 kg m−2, respectively. There were significant improvements in pain and disability after therapy (P < 0.01) (Fig. 1). There were no differences in the mean values for fear avoidance beliefs, pain catastrophising or psychological disturbance from pre-therapy to post-therapy (each P > 0.24).
Fig. 1.
Self-rated pain and disability before and after therapy (all scores normalised such that 100% = maximum possible score for the scale)
Changes in lateral abdominal muscle function following therapy
Table 1 shows the baseline and post-therapy values for the TrA-CR during the voluntary activation of TrA (i.e., during abdominal hollowing) for left and right body sides. The main effect from the ANOVA of “time of assessment” (pre- vs. post-therapy) indicated a significant 4.5% increase in TrA-CR post-therapy (P = 0.045). There was no significant main effect of body side, and no significant interaction (i.e., no difference in the extent of improvement after therapy dependent on body side).
Table 1.
TrA contraction ratios during abdominal hollowing before and after the 9-week programme of therapeutic spinal segmental stabilisation exercises
aData from one patient missing from left side; value estimated from right side after adjustment for group mean differences in right and left sides
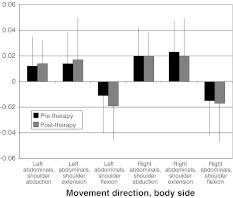
Figure 2 shows the baseline and post-therapy values for the anticipatory activation (“earliest onset”) of the lateral abdominal muscles during rapid arm movements. There was a significant main effect for movement direction (P < 0.0001), with the mean onsets in flexion (−0.016 ms) being approximately 30 ms earlier than those in abduction or extension (0.016 and 0.019 ms, respectively). There was no significant main effect for body side (P = 0.13), time of assessment (pre- vs. post-therapy) (P = 0.67), or for any interactions (P > 0.15).
Fig. 2.
Onset of the earliest activation of the lateral abdominal muscles before and after therapy
Relationships between LBP-related disability and lateral abdominal muscle function and psychological factors
At baseline, RM scores (LBP-related disability) showed non-significant correlations with TrA-CR (r = −0.26, P = 0.15) and with the anticipatory onset of the lateral abdominal muscles (r = 0.01, P = 0.95).
There was no significant correlation between the change in RM scores after therapy and baseline values of either TrA-CR (r = 0.24, P = 0.20) or the anticipatory onset of the lateral abdominal muscles (r = 0.04, P = 0.84). In other words, the degree of improvement in disability after the programme of spine stabilisation exercises was not dependent on the baseline (in)ability to activate the muscles.
The individual changes in RM scores from pre-therapy to post-therapy did not correlate significantly with the changes in either TrA-CR (r = 0.08, P = 0.66) or the anticipatory onset of the lateral abdominal muscles (r = 0.16, P = 0.40), i.e., improvements in RM scores did not go hand in hand with improvements in lateral abdominal muscle activity.
In contrast, there was a significant positive relationship between the improvements in RM scores and improvements in fingertip–floor distance (r = 0.45, P = 0.009). Improvements in RM scores were also significantly associated with reductions in fear avoidance beliefs about work (r = 0.48, P = 0.006), in pain catastrophising (r = 0.58, P = 0.0004) and in psychological disturbance (r = 0.44, P = 0.01).
In multiple regression, the reduction from pre-therapy to post-therapy in catastrophising (P = 0.0003) and fingertip–floor distance (P = 0.0006) each made a unique contribution to explaining the variance in the reduction in RM scores, together explaining 58.3% variance in the reduction in LBP-related disability. Neither of the lateral abdominal muscle function test scores made a significant contribution in the model.
When either the average pain or highest pain in the last week was used as the dependent variable in examining the correlations with lateral abdominal muscle function, the above findings were in each case similar to those reported using RM as the dependent variable (in terms of the strength of the correlations and their statistical significance), with the exception that the correlation between baseline values of highest pain and TrA-CR achieved statistical significance [r = −0.40, P = 0.03, compared with r = −0.26, P = 0.15 for RM (see above)].
Discussion
Main findings
The main findings of the present study were that there was a significant increase in the ability to voluntarily activate TrA after therapy, but neither the TrA-CR recorded prior to treatment nor its improvement following treatment bore any significant relationship to the change in the primary clinical outcome (self-rated RM disability score). In other words, the effect of spine stabilisation exercises on clinical outcome did not depend on the patient’s ability (or lack of ability) to recruit TrA during abdominal hollowing in the first place or their improved ability to recruit TrA during the same task after the programme. For the anticipatory activity of the lateral abdominal muscles, there was no significant group change after therapy and also no relationship between either baseline or change scores and the improvement in RM scores on an individual basis. The finding that the improvement in RM scores showed a moderately high, significant correlation with the improvement in trunk maximal flexion (fingertip-to-floor distance), considered a somewhat more “objective” measure of function, added credibility to the self-ratings of disability.
TrA voluntary activation during abdominal hollowing
The significant improvement in the ability to contract the TrA after a programme of spine stabilisation exercises concurs with the findings of Ferreira et al. [13]. In their study, 11 patients undertook stabilisation exercise therapy, and a further 23 patients either performed general exercise or received spinal manipulation. Compared with the latter two groups, the stabilisation group showed significantly greater changes after therapy in the ability to recruit TrA, though this was mainly due to a worsening in the other groups, rather than a significant improvement per se in the stabilisation group. In another small study (N = 15 per group), Franca et al. [15] showed a greater increase in the “TrA muscle activation capacity” (indirectly measured using a pressure biofeedback unit) in patients who trained with segmental stabilisation exercises than in those who carried out abdominal/trunk strengthening exercises, and again a reduced ability to activate TrA in the strength-training group was observed. In contrast to these two studies, Vasseljen et al. [61] found no significant improvements in group mean TrA contraction ratios during abdominal hollowing after a programme of either stabilisation exercises or other exercises. Franca et al. [15] did not evaluate the correlation between improved TrA activation and reduced pain/disability, whilst Ferreira et al. [13] found (for all three groups together) a low but significant correlation with reduced disability, but interestingly not with reduced pain, improved patient-specific functional scores, or perceived recovery. The significant correlation found with disability appears, at first sight, to contrast with our results, although closer examination of the strength of the correlation reported by Ferreira et al. (r = −0.35) and its wide 95% confidence intervals (r = 0.02–0.62) suggests that there is a large uncertainty about the true extent of the correlation. The group performing stabilisation exercises was always more likely to improve their TrA activation (in accordance with the training specificity principle) and this group also coincidentally had 25% higher disability scores at baseline (rendering them, mathematically, more likely to show a greater absolute reduction in their scores); these facts may have served to spuriously elevate the correlation between the change scores for the two variables in the group data. Interestingly, the correlation coefficient for the same relationship within the stabilisation group alone was not reported, which might otherwise have shed some light on the reliability of the finding. Also somewhat puzzling was the lack of any significant relationship between the improvement in TrA activation and the other closely related clinical measures examined (pain, patient-specific function and perceived recovery). Although Vasseljen et al. [61] reported that increases in TrA and decreases in OI contraction ratios were significantly related to changes in pain after exercise therapy (in the pooled data from all exercise types), together these muscle activation variables explained just 18% of the variance. Again, the relationship within the segmental stabilisation group alone was not reported.
Combining the results of all these studies, we would likely have to conclude that there is, at best, only a weak relationship between the improvement in TrA voluntary activation and the improvement in (some aspects of) the clinical status after various programmes of exercise therapy.
TrA anticipatory activation during rapid arm movements
To our knowledge, only one previous study [58] has examined the association between clinical outcome after a programme of spine stabilisation exercises and improvements in the anticipatory activation of the lateral abdominal muscles. The authors reported non-significant relationships between the change in TrA onsets (during shoulder flexion and extension) and changes in either pain (flexion r = −0.10, P = 0.80, extension r = 0.10, P = 0.80) or function (flexion r = −0.10, P = 0.68; extension r = 0.18, P = 0.64). The present study similarly found no evidence to suggest that these two attributes improve hand in hand. Confirming previous studies using the same methodology as that used here, and recording abdominal muscle activity with intramuscular wire EMG [37] and/or TDI [18, 37], shoulder flexion movements elicited significantly earlier abdominal muscle onsets than did abduction or extension movements. However, these timing patterns remained unchanged after therapy.
The active ingredient in segmental stabilisation exercises
Overall, our findings and those of others make it difficult to attribute with any confidence the therapeutic results of spine stabilisation exercises to specific effects on the trunk muscles. Spine stabilisation exercises are a popular treatment for cLBP and are currently subject to much investigation in clinical trials; we strongly recommend that the interrelationships between outcome and lateral abdominal muscle function be further examined in larger groups, to clarify the specificity of the treatment effect and determine whether these exercises might also have some kind of “general” effect, unrelated to abdominal muscle function per se.
Some may argue that if a treatment is effective, then establishment of its active ingredient is immaterial; however, such arguments are not tenable when dealing with a common, costly, and complicated problem such as cLBP, for which the currently available plethora of treatment modalities [51, 59] is serving to make management of the problem even more difficult [8, 64]. We must continuously strive to identify less expensive but similarly effective treatments, in order to make best use of the limited resources available, and this relies on us knowing why a given treatment works.
In the present study, the changes in a number of psychological factors correlated with the change in self-rated disability, a phenomenon reported before for exercise therapy [66]. Even in multiple regression, changes in one of these psychological variables (pain catastrophising) remained a significant unique variable explaining the change in disability after therapy. It is impossible to say whether the psychological changes followed the improvement in symptoms and function after therapy, or vice versa, i.e., which was the cause and which was the effect. Studies have emerged showing that the effects of physical treatment, with no specific cognitive-behavioural component, may be mediated by a decrease in pain catastrophising [53]. Possibly, this is the result of “enforced” exposure to activities that challenge the notion of movement representing an impending threat, allowing the patient to enjoy the positive experience of completing the given exercises without undue harm. As previously suggested [63] and supporting the contention that cognitions are best altered by treatment techniques that produce changes in motor behaviour [6, 53], changes in pain catastrophising may have occurred as a consequence of the improved physical function (itself brought about by yet-to-be identified mechanisms). Alternatively, other aspects of the therapy that were not formally monitored here—for example, the educative nature of the therapy, in terms of therapist feedback and information conveyed, or simply the positive patient-therapist interaction itself [19]—may have influenced outcome. Accepting that persistent back pain may be a problem of cortical reorganisation and degeneration, it is also possible that the treatment may have served to normalise abnormal cortical representation, whereby simply mastering a skill that the patient finds difficult may be all that is required [66]. And finally, it is not possible to rule out a placebo effect of the specific exercises [46], although our previous finding of a significant “dose–effect” relationship between adherence to the home exercises and outcome [32] does not necessarily support such an explanation. Either way, it is clear that these central factors have to be examined as potentially important attributes to target, or at least monitor, in designing appropriate therapies for cLBP. Certainly, more large-scale studies examining the mediating factors that explain outcome after therapy for cLBP are required.
Limitations of the study
Certain limitations of the present study are worthy of mention. First, TDI ultrasound tissue velocity measures do not measure muscle contractile activity per se but indicate tissue movement in association with contraction. Whilst ultrasound recordings have been shown to be reliable and valid for assessing the “earliest onset of activity” within deep-lying the lateral abdominal muscle group [37, 62, 67] they cannot be used to identify with any certainty which of the lateral abdominal muscles (TrA, OI or OE) is activated first. Possible reasons for this have been discussed in detail before [37, 60, 62, 67]. Nonetheless, the latter authors maintain that the “earliest muscle active” gives an adequate measure for clinical studies in this area, delivering a valid representation of the phenomenon under investigation. Indeed, due to the wide intraindividual and interindividual variabilities in the onset responses of the three muscles, some authors consider that examination of the “earliest onset of activity” may even convey certain advantages, by taking account of individual activation strategies that can otherwise be obscured by averaging group data for any given muscle [37, 62, 67]. This, coupled with the obvious benefit of using a non-invasive procedure in the assessment of patients, appears to make it a feasible approach for studies of deep muscle activation in connection with cLBP. Another limitation of the present study is that no control or comparator (treatment) group was examined that would have allowed evaluation of the relationship between changes in clinical variables (pain/disability) and changes in lateral abdominal muscle activation in those receiving no specific segmental stabilisation treatment. The sample size was also relatively small, and the findings should be interpreted with caution until such times as they can be confirmed in larger groups of patients. And finally, our assessments were only made immediately post-treatment. Although this might be the best stage at which to examine the direct association between respective changes in the variables of interest (since if changes are not seen here they might be unlikely to emerge later) it would still be of interest to re-examine the relationships at a longer-term follow-up.
Acknowledgments
This work was supported by a grant from the Swiss National Research Program NRP 53 “Musculoskeletal Health-Chronic Pain” of the Swiss National Science Foundation (Project 405340-104787/2) and the Schulthess Klinik Research Funds. We would like to express our thanks to: Prof Beat A. Michel for providing the infrastructure to carry out this work within the Department of Rheumatology and Institute of Physical Medicine, University Hospital Zürich, Switzerland; Deborah Gubler and Valeriu Toma for their assistance with the data collection; the physiotherapists Martin Litschi, Tamar Bon, Konstanze Wagner, Elfi Raffainer, Luca Scascighini, Raymond Denzler, Wiebke Schubien, Manuela Meier, Melanie Knecht, Selina Bühler, Christina Gruber and Diana Brun-Walser for treating the patients and completing the necessary documentation; Doctors Bischoff, Camenzind, Distler, Haltinner, Klipstein, Rörig, Schmidt, Stärkle-Bär, Tamborrini, Thoma, Zimmermann (USZ), Bartanusz, Kramers-de Quervain, Marx, Pihan (Schulthess Klinik), Brunner (Balgrist), Kern, Kurmann, Schuler, Stössel and Zoller (GP practices) for referring patients into the study; all the patients who took part in the study.
Conflict of interest
None.
References
- 1.Airaksinen O, Brox JI, Cedraschi C, Hildebrandt J, Klaber-Moffett J, Kovacs F, Mannion AF, Reis S, Staal JB, Ursin H, Zanoli G, et al. Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J. 2006;15(Suppl 2):S192–S300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison GT. Trunk muscle onset detection technique for EMG signals with ECG artefact. J Electromyogr Kinesiol. 2003;13:209–216. doi: 10.1016/S1050-6411(03)00019-1. [DOI] [PubMed] [Google Scholar]
- 3.Allison GT, Henry SM. The influence of fatigue on trunk muscle responses to sudden arm movements, a pilot study. Clin Biomech (Bristol, Avon) 2002;17:414–417. doi: 10.1016/S0268-0033(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 4.Allison GT, Morris SL. Transversus abdominis and core stability—has the pendulum swung? Br J Sports Med. 2008;42:930–931. doi: 10.1136/bjsm.2008.048637. [DOI] [PubMed] [Google Scholar]
- 5.Allison GT, Morris SL, Lay B. Feedforward responses of transversus abdominis are directionally specific and act asymmetrically: implications for core stability theories. J Orthop Sports Phys Ther. 2008;38:228–237. doi: 10.2519/jospt.2008.2703. [DOI] [PubMed] [Google Scholar]
- 6.Bandura A (1977) Self-efficacy: towards a unifying theory of behaviour change. Psychol Rev 84 [DOI] [PubMed]
- 7.Critchley DJ, Coutts FJ. Abdominal muscle function in chronic low back pain patients. Physiotherapy. 2002;88:322–332. doi: 10.1016/S0031-9406(05)60745-6. [DOI] [Google Scholar]
- 8.Deyo RA (1998) Low-back pain. Scientific Am August 279:48–53 [DOI] [PubMed]
- 9.Exner V, Keel P. Erfassung der Behinderung bei Patienten mit chronischen Rückenschmerzen. Schmerz. 2000;14:392–400. doi: 10.1007/s004820070004. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira ML, Ferreira PH, Latimer J, Herbert RD, Hodges PW, Jennings MD, Maher CG, Refshauge KM. Comparison of general exercise, motor control exercise and spinal manipulative therapy for chronic low back pain: a randomized trial. Pain. 2007;131:31–37. doi: 10.1016/j.pain.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira PH, Ferreira ML, Hodges PW. Changes in recruitment of the abdominal muscles in people with low back pain: ultrasound measurement of muscle activity. Spine. 2004;29:2560–2566. doi: 10.1097/01.brs.0000144410.89182.f9. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira PH, Ferreira ML, Maher CG, Herbert RD, Refshauge K. Specific stabilisation exercise for spinal and pelvic pain: a systematic review. Aust J Physiother. 2006;52:79–88. doi: 10.1016/S0004-9514(06)70043-5. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira PH, Ferreira ML, Maher CG, Refshauge K, Herbert RD, Hodges PW. Changes in recruitment of transversus abdominis correlate with disability in people with chronic low back pain. Br J Sports Med. 2010;44:1166–1172. doi: 10.1136/bjsm.2009.061515. [DOI] [PubMed] [Google Scholar]
- 14.Flor H. Cortical reorganisation and chronic pain: implications for rehabilitation. J Rehabil Med. 2003;41:66–72. doi: 10.1080/16501960310010179. [DOI] [PubMed] [Google Scholar]
- 15.Franca FR, Burke TN, Hanada ES, Marques AP. Segmental stabilization and muscular strengthening in chronic low back pain: a comparative study. Clinics (Sao Paulo) 2010;65:1013–1017. doi: 10.1590/S1807-59322010001000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorbet N, Selkow NM, Hart JM, Saliba S (2010) No Difference in transverse abdominis activation ratio between healthy and asymptomatic low back pain patients during therapeutic exercise. Rehabil Res Practice 459738 [DOI] [PMC free article] [PubMed]
- 17.Greenough CG, Fraser RD. Comparison of eight psychometric instruments in unselected patients with back pain. Spine. 1991;16:1068–1074. doi: 10.1097/00007632-199109000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Gubler D, Mannion AF, Schenk P, Gorelick M, Helbling D, Gerber H, Toma V, Sprott H. Ultrasound tissue Doppler imaging reveals no delay in abdominal muscle feed-forward activity during rapid arm movements in patients with chronic low back pain. Spine (Phila Pa 1976) 2010;35:1506–1513. doi: 10.1097/BRS.0b013e3181c3ed41. [DOI] [PubMed] [Google Scholar]
- 19.Hall AM, Ferreira PH, Maher CG, Latimer J, Ferreira ML. The influence of the therapist-patient relationship on treatment outcome in physical rehabilitation: a systematic review. Phys Ther. 2011;90:1099–1110. doi: 10.2522/ptj.20090245. [DOI] [PubMed] [Google Scholar]
- 20.Henry SM, Teyhen DS. Ultrasound imaging as a feedback tool in the rehabilitation of trunk muscle dysfunction for people with low back pain. J Orthop Sports Phys Ther. 2007;37:627–634. doi: 10.2519/jospt.2007.2555. [DOI] [PubMed] [Google Scholar]
- 21.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–374. doi: 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 22.Hodges PW. Changes in motor planning of feedforward postural responses of the trunk muscles in low back pain. Exp Brain Res. 2001;141:261–266. doi: 10.1007/s002210100873. [DOI] [PubMed] [Google Scholar]
- 23.Hodges PW. Core stability exercise in chronic low back pain. Orthop Clin North Am. 2003;34:245–254. doi: 10.1016/S0030-5898(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 24.Hodges PW, Moseley G. Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J Electromyogr Kinesiol. 2003;13:361–370. doi: 10.1016/S1050-6411(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 25.Hodges PW, Richardson C. Delayed postural contraction of transversus abdominis in low back pain associated with movement of the lower limb. J Spin Disord. 1998;11:46–56. [PubMed] [Google Scholar]
- 26.Hodges PW, Richardson CA. Inefficient muscular stabilisation of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine. 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- 27.Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil. 1999;80:1005–1012. doi: 10.1016/S0003-9993(99)90052-7. [DOI] [PubMed] [Google Scholar]
- 28.Jansen JA, Mens JM, Backx FJ, Stam HJ. Changes in abdominal muscle thickness measured by ultrasound are not associated with recovery in athletes with longstanding groin pain associated with resisted hip adduction. J Orthop Sports Phys Ther. 2009;39:724–732. doi: 10.2519/jospt.2009.3068. [DOI] [PubMed] [Google Scholar]
- 29.Kavcic N, Grenier S, McGill SM. Determining the stabilizing role of individual torso muscles during rehabilitation exercises. Spine. 2004;29:1254–1265. doi: 10.1097/00007632-200406010-00016. [DOI] [PubMed] [Google Scholar]
- 30.Macedo L, Latimer J, Maher CG, Hodges P, Nicholas M, Tonkin L, McAuley J, Stafford R. The effect of motor control exercise versus graded activity in patients with chronic nonspecific low back pain: immediate aftertreatment results from a randomised controlled trial (ACTRN12607000432415) Sweden: International Society for the Study of the Lumbar Spine; 2011. [Google Scholar]
- 31.Mannion AF, Balague F, Pellise F, Cedraschi C. Pain measurement in patients with low back pain. Nat Clin Pract Rheumatol. 2007;3:610–618. doi: 10.1038/ncprheum0646. [DOI] [PubMed] [Google Scholar]
- 32.Mannion AF, Helbling D, Pulkovski N, Sprott H. Spinal segmental stabilisation exercises for chronic low back pain: programme adherence and its influence on clinical outcome. Eur Spine J. 2009;18:1881–1891. doi: 10.1007/s00586-009-1093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannion AF, Junge A, Taimela S, Muntener M, Lorenzo K, Dvorak J. Active therapy for chronic low back pain: part 3. Factors influencing self-rated disability and its change following therapy. Spine. 2001;26:920–929. doi: 10.1097/00007632-200104150-00015. [DOI] [PubMed] [Google Scholar]
- 34.Mannion AF, Müntener M, Taimela S, Dvorak J. A randomised clinical trial of three active therapies for chronic low back pain. Spine. 1999;24:2435–2448. doi: 10.1097/00007632-199912010-00004. [DOI] [PubMed] [Google Scholar]
- 35.Mannion AF, Pulkovski N, Gubler D, Gorelick M, O’Riordan D, Loupas T, Schenk P, Gerber H, Sprott H. Muscle thickness changes during abdominal hollowing: an assessment of between-day measurement error in controls and patients with chronic low back pain. Eur Spine J. 2008;17:494–501. doi: 10.1007/s00586-008-0589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mannion AF, Pulkovski N, Schenk P, Hodges PW, Gerber H, Gorelick M, Sprott H (2008) “Direction-dependent” feed-forward activation of transversus abdominis during rapid arm movements challenges its unique role in spine stabilisation. International Society for the Study of the Lumbar Spine (Spine Week). Geneva
- 37.Mannion AF, Pulkovski N, Schenk P, Hodges PW, Gerber H, Loupas T, Gorelick M, Sprott H. A new method for the noninvasive determination of abdominal muscle feedforward activity based on tissue velocity information from tissue Doppler imaging. J Appl Physiol. 2008;104:1192–1201. doi: 10.1152/japplphysiol.00794.2007. [DOI] [PubMed] [Google Scholar]
- 38.Mannion AF, Pulkovski N, Toma V, Sprott H. Abdominal muscle size and symmetry at rest and during abdominal hollowing exercises in healthy control subjects. J Anat. 2008;213:173–182. doi: 10.1111/j.1469-7580.2008.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGill SM (2004) Appropriate back exercise: from rehabilitation to high performance. 5th Interdisciplinary World Congress on low back and pelvic pain, pp 229–235
- 40.McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13:353–359. doi: 10.1016/S1050-6411(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 41.Meyer K, Sprott H, Mannion AF. Cross-cultural adaptation, reliability, and validity of the German version of the Pain Catastrophizing Scale. J Psychosom Res. 2008;64:469–478. doi: 10.1016/j.jpsychores.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Misuri G, Colagrande S, Gorini M, Iandelli I, Mancini M, Duranti R, Scano G. In vivo ultrasound assessment of respiratory function of abdominal muscles in normal subjects. Eur Respir J. 1997;10:2861–2867. doi: 10.1183/09031936.97.10122861. [DOI] [PubMed] [Google Scholar]
- 43.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinto RZ, Ferreira PH, Franco MR, Ferreira MC, Ferreira ML, Teixeira-Salmela LF, Oliveira VC, Maher C (2011) The effect of lumbar posture on abdominal muscle thickness during an isometric leg task in people with and without non-specific low back pain. Man Ther 16(6):578–584 [DOI] [PubMed]
- 45.Pinto RZ, Ferreira PH, Franco MR, Ferreira ML, Ferreira MC, Teixeira-Salmela LF, Maher CG. Effect of 2 lumbar spine postures on transversus abdominis muscle thickness during a voluntary contraction in people with and without low back pain. J Manipulative Physiol Ther. 2011;34:164–172. doi: 10.1016/j.jmpt.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Puhl AA, Reinhart CJ, Rok ER, Injeyan HS. An examination of the observed placebo effect associated with the treatment of low back pain—a systematic review. Pain Res Manag. 2011;16:45–52. doi: 10.1155/2011/625315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pulkovski N, Mannion AF, Caporaso F, Toma V, Gubler D, Helbling D, Sprott H (2011) Ultrasound assessment of transversus abdominis muscle contraction ratio during abdominal hollowing: a useful tool to distinguish between patients with chronic low back pain and healthy controls? Eur Spine J. doi: 10.1007/s00586-011-1707-8 [DOI] [PMC free article] [PubMed]
- 48.Rackwitz B, Bie R, Limm H, Garnier K, Ewert T, Stucki G. Segmental stabilizing exercises and low back pain. What is the evidence? A systematic review of randomized controlled trials. Clin Rehabil. 2006;20:553–567. doi: 10.1191/0269215506cr977oa. [DOI] [PubMed] [Google Scholar]
- 49.Richardson C, Jull G, Hodges P, Hides J. Therapeutic exercise for spinal segmental stabilization in low back pain: scientific basis and clinical approach. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- 50.Roland M, Fairbank J. The Roland–Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25:3115–3124. doi: 10.1097/00007632-200012150-00006. [DOI] [PubMed] [Google Scholar]
- 51.Rubinstein SM, Middelkoop M, Kuijpers T, Ostelo R, Verhagen AP, Boer MR, Koes BW, Tulder MW. A systematic review on the effectiveness of complementary and alternative medicine for chronic non-specific low-back pain. Eur Spine J. 2010;19:1213–1228. doi: 10.1007/s00586-010-1356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smeets R. Active rehabilitation for chronic low back pain: cognitive-behavioural, physical, or both? Maastricht: University of Maastricht; 2006. [Google Scholar]
- 53.Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Staerkle R, Mannion AF, Elfering A, Junge A, Semmer NK, Jacobshagen N, Grob D, Dvorak J, Boos N. Longitudinal validation of the fear-avoidance beliefs questionnaire (FABQ) in a Swiss-German sample of low back pain patients. Eur Spine J. 2004;13:332–340. doi: 10.1007/s00586-003-0663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steiger F, Wirth B, de Bruin ED, Mannion AF (2011) Is a positive clinical outcome after exercise therapy for chronic non-specific low back pain contingent upon a corresponding improvement in the targeted aspect(s) of performance? A systematic review. Eur Spine J. doi:10.1007/s00586-011-2045-6 [DOI] [PMC free article] [PubMed]
- 56.Sullivan M, Bishop S, Pivik J. The pain catastrophising scale. Development and validation. Psychol Assess. 1995;7:524–532. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- 57.Teyhen DS, Miltenberger CE, Deiters HM, Toro YM, Pulliam JN, Childs JD, Boyles RE, Flynn TW. The use of ultrasound imaging of the abdominal drawing-in maneuver in subjects with low back pain. J Orthop Sports Phys Ther. 2005;35:346–355. doi: 10.2519/jospt.2005.35.6.346. [DOI] [PubMed] [Google Scholar]
- 58.Tsao H, Hodges PW. Persistence of improvements in postural strategies following motor control training in people with recurrent low back pain. J Electromyogr Kinesiol. 2008;18:559–567. doi: 10.1016/j.jelekin.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Middelkoop M, Rubinstein SM, Kuijpers T, Verhagen AP, Ostelo R, Koes BW, Tulder MW. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur Spine J. 2011;20:19–39. doi: 10.1007/s00586-010-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vasseljen O, Dahl HH, Mork PJ, Torp HG. Muscle activity onset in the lumbar multifidus muscle recorded simultaneously by ultrasound imaging and intramuscular electromyography. Clin Biomech (Bristol, Avon) 2006;21:905–913. doi: 10.1016/j.clinbiomech.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Vasseljen O, Fladmark AM. Abdominal muscle contraction thickness and function after specific and general exercises: a randomized controlled trial in chronic low back pain patients. Man Ther. 2010;15:482–489. doi: 10.1016/j.math.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Vasseljen O, Fladmark AM, Westad C, Torp HG. Onset in abdominal muscles recorded simultaneously by ultrasound imaging and intramuscular electromyography. J Electromyogr Kinesiol. 2009;19:e23–e31. doi: 10.1016/j.jelekin.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Vlaeyen JWS, Kole-Snijders AMJ, Rotteveel AM, Ruesink R, Heuts PHTG. The role of fear of movement/(re)injury in pain disability. J Occ Rehab. 1995;5:235–252. doi: 10.1007/BF02109988. [DOI] [PubMed] [Google Scholar]
- 64.Waddell G. Low back pain: twentieth century health care enigma. Spine. 1996;21:2820–2825. doi: 10.1097/00007632-199612150-00002. [DOI] [PubMed] [Google Scholar]
- 65.Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52:157–168. doi: 10.1016/0304-3959(93)90127-B. [DOI] [PubMed] [Google Scholar]
- 66.Wand BM, O’Connell NE. Chronic non-specific low back pain—sub-groups or a single mechanism? BMC Musculoskelet Disord. 2008;9:11. doi: 10.1186/1471-2474-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westad C, Mork PJ, Vasseljen O. Location and sequence of muscle onset in deep abdominal muscles measured by different modes of ultrasound imaging. J Electromyogr Kinesiol. 2010;20:994–999. doi: 10.1016/j.jelekin.2010.01.005. [DOI] [PubMed] [Google Scholar]