Abstract
UBP43 (also known as USP18) plays a role in the negative regulation of interferon-α/β signaling, and bone marrow cells in Ubp43-deficient mice exhibited hypersensitivity to interferon-α/β-mediated apoptosis. Here, we show that the mitochondrial apoptotic pathway and reactive oxygen species are major contributors to the elevated interferon-α/β-mediated apoptosis in Ubp43-deficient mouse bone marrow cells and in UBP43-knockdown THP-1 cells. Furthermore, TRAIL and FASL, which were proposed as apoptosis inducers upon interferon-α/β treatment in UBP43-knockdown adherent cancer cells, did not cause apoptosis in these hematopoietic cells. Therefore, although UBP43 depletion can cause hypersensitivity to interferon-α/β-mediated apoptosis in a broad range of cell types, the downstream pathway may vary depending on the cell type.
Keywords: UBP43, USP18, ubiquitin-like protein, ISG15, interferon-α/β, apoptosis, mitochondrial pathway, reactive oxygen species (ROS)
Type I interferons (IFNs), represented by IFN-α/β, are pleiotropic cytokines that play a variety of biological roles in anti-viral, immunomodulatory, anti-proliferatory, anti-tumor, and apoptotic pathways [1–4]. IFN-α/β induces the expression of several hundred genes called interferon-stimulated genes (ISGs) via the JAK-STAT signaling pathway [5, 6]. Among the ISGs, UBP43 was identified as a deconjugating protease for a ubiquitin-like protein ISG15 [7, 8], and the expression of UBP43 was specifically induced by IFN- α/β [9]. A knockout mouse model for the Ubp43 gene revealed a negative regulatory role for UBP43 in IFN-α/β-mediated JAK-STAT signaling [10]. Ubp43-deficient cells were found to be hypersensitive to IFN-α/β through the increased and prolonged activation of the JAK-STAT signaling pathway, leading to a much higher expression of ISGs compared to the normal cells. Along with the hypersensitivity to IFN-α/β, Ubp43-deficient mice are more resistant to viral and bacterial infections [9, 11]. Furthermore, Ubp43 deficiency increased the resistance to oncogenic transformation by BCR-ABL, the causative agent of chronic myeloid leukemia [12]. Detailed analyses for the cause of the hypersensitivity to IFN-α/β in Ubp43-deficient mice and cells have revealed that UBP43 negatively regulates JAK-STAT signaling independent of its deISGylating enzyme activity [13]. Regardless of its enzymatic activity, UBP43 directly interacts with the IFNAR2 subunit of the IFN-α/β receptor such that UBP43 inhibits the activation of receptor-associated JAK1 by blocking the interaction between JAK1 and IFNAR2 [13].
It has been shown that IFN-α/β induces apoptosis in many types of malignant cells [14] and in hematopoietic cancer cells [15–17]. IFN-α/β induces the extrinsic apoptotic pathway through FADD/caspase-8 signaling and the mitochondrial pathway [3]. One interesting phenotype of the Ubp43-deficient mice that is in agreement with the hypersensitivity to IFN-α/β is increased apoptosis in hematopoietic cells [10]. The administration of polyI:C or LPS, which in turn induces IFN-α/β production, is more lethal to Ubp43-deficient mice than their wild-type counterparts owing to the extensive apoptosis especially in hematopoietic cells [9, 10]. Another group also reported elevated apoptosis in UBP43-knockdown cells upon IFN-α/β administration. The depletion of UBP43 from adherent types of cells, such as E1A-transformed IMR90 fibroblasts (IMR90-E1A) and MCF7, promoted the activation of the extrinsic apoptotic pathway by IFN-α, in accordance with an increased TRAIL production and upregulated expression of transcription factors IRF-1, IRF-7, and IRF-9 [18]. In spite of the obvious apoptotic phenotype in Ubp43-deficient hematopoietic cells, the exact downstream mechanism that causes the increased apoptotic cell death was not clearly defined.
Here we show that, as in Ubp43-deficient mouse bone marrow cells, UBP43 depletion dramatically increases IFN-α/β sensitivity in UBP43-knockdown THP-1 cells, as exemplified by enhanced and prolonged STAT1 phosphorylation and several-fold increases in apoptosis. A detailed analysis of the apoptotic pathway revealed that the mitochondrial pathway rather than the extrinsic pathway plays the major role in the IFN-α/β-mediated apoptotic cell death in both Ubp43-deficient mouse bone marrow cells and UBP43-knockdown THP-1 cells. Furthermore, the elevated generation of ROS upon IFN-α treatment and the reduction of IFN-α-mediated apoptosis by the elimination of ROS in the UBP43-knockdown THP-1 cells indicated that ROS is also a major contributor to the elevated IFN-α/β-mediated apoptosis in the UBP43-depledted hematopoietic cells.
Materials and methods
Plasmid construction and transfection
The shRNA targeting the human UBP43 gene, pLKO.1-shUBP43 (TRCN0000004194), and control shRNA, pLKO.1-TRcontrol, were purchased from Open Biosystems (USA). pLKO.1-shUBP43 and pLKO.1-TRcontrol were transfected into THP-1 cells using an Amaxa nucleofector (Amaxa, USA). The transfected cells were selected in the presence of puromycin (0.5 3μg/ml) for 2 weeks.
Cell culture and treatment
The mouse bone marrow cells were cultured in RPMI 1640 medium (Invitrogen, USA) containing 10% FBS (Invitrogen, USA), 10 ng/ml IL-3, 10 ng/ml IL-6, and 100 ng/ml stem cell factor (PeproTech, USA), and the THP-1 cells were cultured in RIPM 1640 medium containing 10% FBS and 2 mM L-glutamine (Invitrogen, USA). Recombinant human IFN-α and mouse IFN-β (PBL Interferon Source, USA) were used at 1,000 units/ml and 500 units/ml, respectively. Recombinant human or mouse FASL (R&D System, USA) were used at two concentrations, 100 or 300 ng/ml. Recombinant human TRAIL (R&D System, USA) or recombinant mouse TRAIL (PeproTech, USA) were used at 300 or 500 ng/ml. An ROS antagonist, N-acetylcysteine (NAC; Sigma-Aldrich, USA), was used at 10 mM.
Antibodies
The anti-Bid, anti-caspase-3, anti-cleaved caspase-3, anti-cytochrome c, anti-STAT1, and anti-phosphoSTAT1Tyr701 antibodies were purchased from Cell Signaling (USA). The anti-Bax and anti-caspase-8 antibodies were purchased from Santa Cruz (USA). The antibody generated against human UBP43 was described previously [13].
Immunocytochemistry
THP-1 cells (2 x 105 cells/slide) were attached onto slides using Shandon Cytospin 4 (Thermo Scientific, USA). After fixing with 3.7% paraformaldehyde, the cells were permeabilized with 0.5% Triton X-100 in PBS and blocked with PBST containing 10% FBS and 1% BSA. The cells were incubated with primary antibodies diluted in 3% BSA at room temperature (RT) and then incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Zhongshan Goldenbridge Biotech, China) in the dark. After being washed four times with PBST, the cells were mounted using a Vectorshield mounting medium containing DAPI (Vector Labs, USA) and observed with a fluorescence microscope (Olympus, Japan).
Measurement of apoptotic cell death
The apoptotic cell death was analyzed by fluorescence activated cell sorting (FACS) after staining using the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences, USA). Briefly, the THP-1 cells were harvested and washed twice with cold PBS. The cells (1 x 106 cells) were resuspended in 100 μl of the kit’s binding buffer and incubated with Annexin V-FITC for 15 min at RT. Flow cytometry was performed using the FACSCanto II (BD Bioscience, USA).
Measurement of ROS
Intracellular ROS production was determined by incubating the cells (1 x 106 cells) with 10 μM H2DCFDA (Sigma-Aldrich, USA) for 30 min at 37 °C in the dark. The cells were washed twice in PBS and analyzed by flow cytometry or observed using a fluorescence microscope (Olympus, Japan).
Measurement of the mitochondrial membrane potential
The mitochondrial membrane potential was evaluated by staining the cells (1 x 106 cells) using the JC-1 Mitochondrial Membrane Potential Detection Kit (Biotium, USA). The cells were then analyzed by flow cytometry.
Caspase activity assay
Cells (5 x 106 cells) were harvested and resuspended in extraction buffer (40 mM HEPES [pH 7.5], 0.1% Triton X-l00, 20% glycerol, and 4 mM DTT) on ice for 10 min. The extracts were then incubated with 50 μM Ac-LEHD-AFC (Alexis Corp., USA) at 37 °C for 2 hrs. The caspase activity was measured (excitation 400 nm/emission 505 nm) using the SpectraMax M5e (Molecular Devices, USA).
Results
Increased IFN-α/β-mediated apoptosis in Ubp43-deficient mouse bone marrow cells and UBP43-knockdown THP-1 cells
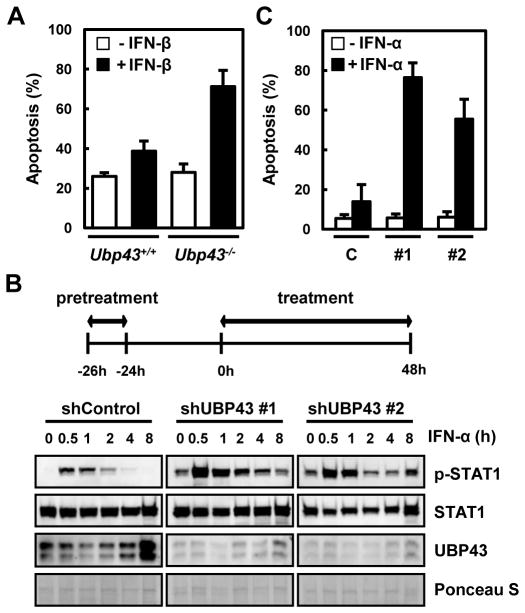
Previously we had shown that the loss of Ubp43 in mice resulted in an enhanced and prolonged STAT1 phosphorylation upon IFN-α/β stimulation and increased apoptosis of hematopoietic cells upon polyI:C or LPS administration [10]. We confirmed whether the elevated death of the Ubp43-deficient hematopoietic cells by polyI:C or LPS in vivo is in fact mediated by IFN-α/β. Bone marrow cells from Ubp43+/+ and Ubp43−/− mice were cultured in vitro and treated with IFN-β. Both cells showed similar basal levels of spontaneous cell death in the absence of IFN-β. In contrast, the addition of IFN-β increased cell death by approximately 40% in the Ubp43-deficient cells, whereas the wild-type cells showed only approximately 10% increases in cell death (Fig. 1A). Thus, the Ubp43-deficient mouse bone marrow cells are more susceptible to IFN-α/β-mediated apoptosis.
Fig. 1. Increased IFN-α/β-mediated apoptosis in UBP43-depleted cells.
(A) Bone marrow cells from Ubp43+/+ and Ubp43−/− mice were cultured in the absence or presence of IFN-β (500 units/ml) for 24 hrs, and apoptotic cell death was analyzed by FACS after staining with Annexin V-FITC. (B) THP-1 cells stably expressing control shRNA (shControl) or UBP43- specific shRNAs (#1 and #2) were treated with IFN-α, as indicated on the top of the panel. The cells were pretreated with 1,000 units/ml of IFN-α for 2 hrs, washed with regular medium without IFN-α, and cultured another 24 hrs. The cells were again treated with 1,000 units/ml of IFN-α and harvested at various time points. The total proteins were analyzed by immunoblotting with anti-STAT1, anti-phosphoSTAT1Tyr701 (p-STAT1), and anti-UBP43 antibodies. Relative protein loading was shown by Ponceau S staining. (C) Cells were treated with IFN-α as indicated in (B) and analyzed for apoptosis after 48 hrs of treatment using FACS. C, #1, and #2 represent the THP-1 cells stably expressing control shRNA, UBP43- specific shRNA#1, or UBP43-specific shRNA#2, respectively. All data in bar graphs throughout the figures represent the mean value for three independent experiments.
To test whether the elevated susceptibility to apoptosis is also applied to human cells, we generated stable UBP43 knockdown cells using human monocytic leukemia cell line, THP-1. We first examined whether UBP43-knockdown THP-1 cells showed similar hypersensitivity to IFN-α/β with UBP43-deficient bone marrow cells. Since UBP43 functions as a negative feedback regulator against JAK/STAT signaling after its expression by IFN-α/β, we challenged the cells with IFN-α twice. As shown in Fig. 1B, the cells were pre-treated with IFN-α for 2 hrs and cultured in the same medium but without IFN-α for 24 hrs. The cells were then treated with IFN-α again and analyzed for the phosphorylation of STAT1 as an indication of signal activation at various time points. Two independent lines of UBP43- knockdown THP-1 cells showed a significant elevation and extended duration of STAT1 phosphorylation when compared to the control cells (Fig. 1B). With the elevated STAT activation, the UBP43-knockdown THP-1 cells were several-fold more susceptible to IFN-α/β-mediated apoptosis. When the cells were treated with IFN-α as in Fig. 1B and examined for apoptosis 48 hrs later, the UBP43-knockdown THP-1 cells exhibited 4–5-fold higher apoptotic cell death compared to the control cells (Fig. 1C), suggesting that the UBP43- knockdown THP-1 cells have a similar hypersensitivity to IFN-α/β as the UBP43-deficient mouse bone marrow cells.
Increased caspase-3 activation by IFN-α/β in UBP43-depleted hematopoietic cells does not occur through the extrinsic apoptotic pathway
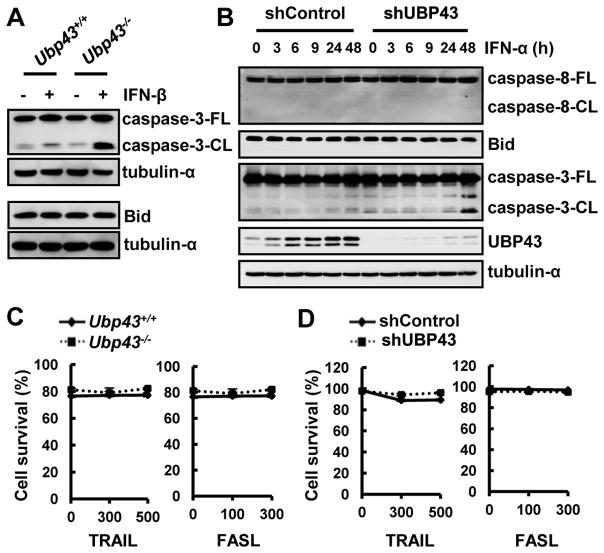
We investigated which apoptotic pathway mainly contributes to the observed IFN-α/β-mediated apoptosis in UBP43-depleted hematopoietic cells. Cultured bone marrow cells from Ubp43+/+ and Ubp43−/− mice were treated with IFN-β for 24 hrs and assessed for the activation of downstream caspase, caspase-3, and Bid cleavage. Consistent with the elevated apoptosis, a much higher level of cleaved caspase-3, representing the active form, was detected in the Ubp43-deficient cells compared to the wild-type cells (Fig. 2A, top). However, Bid, a well-known substrate of the initiator caspase, caspase-8, was not cleaved upon IFN-β treatment in either the wild-type or Ubp43-deficient cells (Fig. 2A, bottom), suggesting that the extrinsic pathway might not be responsible for the mediation of apoptosis in this case. These observations in mouse the bone marrow cells led us to examine the UBP43-knokdown THP-1 cells. Because the two UBP43-knockdown THP-1 cells showed similar hypersensitivities to IFN-α/β, we used cell line #1 for the following experiments. We applied the same IFN-α pretreatment condition as described in Fig. 1B for the detection of apoptosis. The cells were harvested at various time points after the 2nd treatment with IFN-α and the activation of caspase-8 and -3 and the cleavage of Bid were visualized by immunoblotting (Fig. 2B). We did not detect the activation of caspase-8 or the cleavage of Bid at any time point in either the control or UBP43-knockdown THP-1 cells, whereas we clearly detected a strong and elevated activation of caspase-3 in the UBP43-knockdown THP-1 cells at 48 hrs of IFN-α treatment (Fig. 2B). These results confirm that caspase-8 activation might not be involved in the initiation of the IFN-α/β-mediated apoptosis in these cell types.
Fig. 2. Resistance to the extrinsic apoptotic pathway in Ubp43-deficient mouse bone marrow cells and UBP43 knockdown THP-1 cells upon IFN-α/β treatment.
(A) Bone marrow cells from Ubp43+/+ and Ubp43−/− mice were cultured in the absence or presence of IFN-β (500 units/ml) for 24 hrs (top panel for caspase-3 detection) or for 6 hrs (bottom panel for Bid detection). caspase-3-FL, full-length form of caspase-3; caspase-3-CL, activated cleaved form of caspase-3. (B) THP-1 cells stably expressing shControl or UBP43-specific shRNA#1 were treated with IFN-α as in Fig. 1B. The cells were harvested after the 2nd treatment of IFN-α at various time points and analyzed by immunoblotting. (C and D) Cultured bone marrow cells from Ubp43+/+ and Ubp43−/− mice (C) or THP-1 cells stably expressing shControl or UBP43-specific shRNA#1 (D) were treated with TRAIL (0, 300, or 500 ng/ml) or FASL (0, 100, or 300 ng/ml) for 24 hrs, and analyzed for apoptosis.
In a previous report, the extrinsic pathway through TRAIL was suggested as a major route to induce IFN-α/β-mediated apoptosis in UBP43-knockdown adherent cells [18]. Because we did not detect the critical activation of the extrinsic apoptotic pathway in either the Ubp43-deficient mouse bone marrow cells or UBP43-knockdown THP-1 cells upon IFN-α/β treatment, we examined whether TRAIL and FASL, major inducers of the extrinsic pathway, could cause apoptosis in these hematopoietic cell types. Bone marrow cells from Ubp43+/+ and Ubp43−/− mice or control THP-1 and UBP43-knockdown THP-1 cells were treated with increasing concentrations of either TRAIL or FASL for 24 hrs and the cell death was measured. Interestingly, neither TRAIL nor FASL induced cell death in any of the cells even in the absence of UBP43 (Fig. 2C and 2D). Thus, although IFN-α/β induces high levels of caspase-3 activation in UBP43-depleted hematopoietic cells, the downstream apoptotic pathway may differ in these cell types from the previously reported extrinsic pathway.
The mitochondrial pathway as a major contributor to IFN-α/β-mediated apoptosis in Ubp43-deficient hematopoietic cells
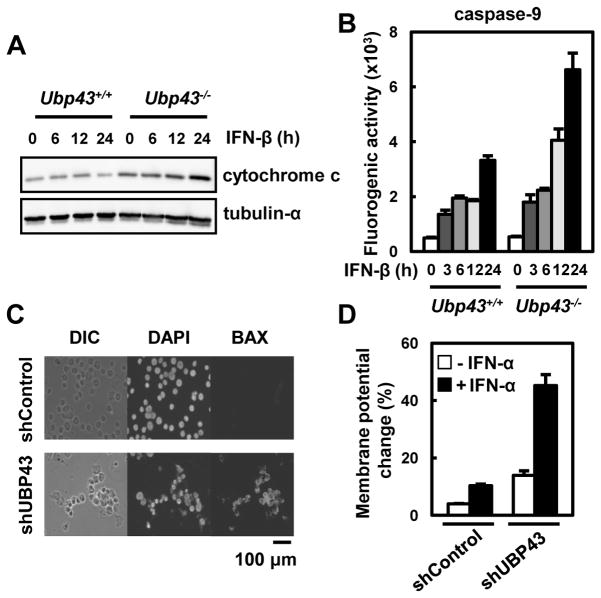
Because the extrinsic pathway is not responsible for the observed IFN-α/β-mediated apoptosis in hematopoietic cells, we then examined the activation of the mitochondrial apoptotic pathway upon IFN-α/β treatment. In addition to the lack of extrinsic pathway activation, the mitochondrial pathway showed significantly increased activation in both the Ubp43-deficient bone marrow cells and UBP43-knockdown THP-1 cells compared to their control cells. The cytoplasmic level of cytochrome c that is released from the mitochondria was much higher in the Ubp43-deficient bone marrow cells compared to the control cells upon IFN-β treatment (Fig. 3A). In addition, the activity of caspase-9 was also increased by approximately two fold in the Ubp43-deficient cells (Fig. 3B). In accordance with these observations, two other characteristics of an activated mitochondrial pathway were significantly increased in the UBP43-knockdown THP-1 cells. Upon IFN treatment, the amount of activated Bax that is visualized by immunostaining with activated Bax-specific antibody and the change in the mitochondrial membrane potential were significantly increased in the UBP43-knockdown THP-1 cells compared to the control cells (Fig. 3C and 3D). Taken together, these results suggest that the mitochondrial pathway is responsible for the hypersensitivity to IFN-α/β-mediated apoptosis in UBP43-depleted hematopoietic cells.
Fig. 3. Increased activation of the mitochondrial apoptotic pathway in Ubp43-deficient mouse bone marrow cells and UBP43 knockdown THP-1 cells upon IFN-α/β treatment.
(A and B) Cultured bone marrow cells from Ubp43+/+ and Ubp43−/− mice were treated with IFN-β (500 units/ml) for various time periods. The cytoplasmic release of cytochrome c was measured by immunoblotting (A), and the caspase-9 activity was measured using a fluorogenic substrate (B). (C and D) THP-1 cells stably expressing shControl or UBP43-specific shRNA#1 were treated with IFN-α as in Fig. 1B. After 48 hrs of the 2nd treatment with IFN-α, the cells were analyzed by immunostaining with the anti-Bax antibody (C), or the fluorescence intensity was detected by FACS after JC-1 staining (D).
ROS as a mediator of IFN-α/β induced apoptosis in UBP43-knockdown THP-1 cells
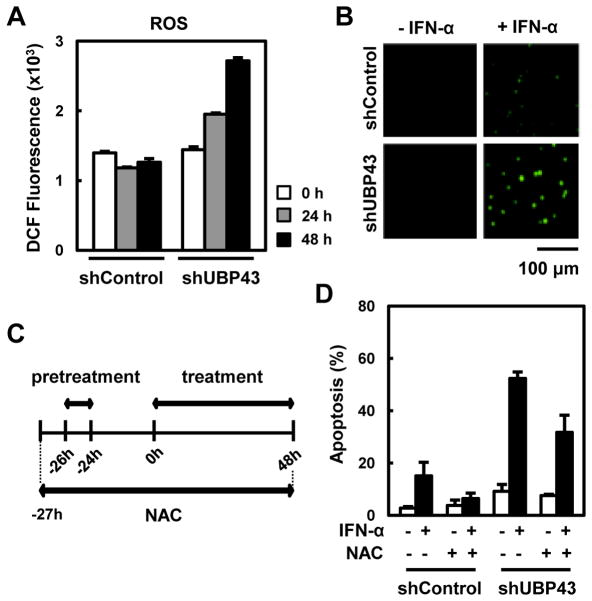
ROS has been known to play a role in the induction of apoptosis and to cooperate with the mitochondrial pathway [19]. We investigated whether the hypersensitivity to IFN-α/β-mediated apoptosis in the UBP43-deficient cells is related to ROS. First, we detected the accumulation of ROS in the cells upon IFN treatment. Both control and UBP43-knockdown THP-1 cells were treated with IFN-α for 0, 24, and 48 hrs and the amount of ROS was measured using H2DCFDA and analyzed by flow cytometry. Compared to the control cells, the UBP43-knockdown THP-1 cells showed approximately 2.5-fold increased accumulation of ROS at 48 hrs in response to IFN-α (Fig. 4A). The accumulation of ROS in the UBP43-knockdown THP-1 cells was confirmed once again by the increased intensity of H2DCFDA using fluorescence microscopy (Fig. 4B).
Fig. 4. Involvement of ROS in IFN-α/β-mediated apoptosis in UBP43-knockdown THP-1 cells.
(A) THP-1 cells stably expressing shControl or UBP43-specific shRNA#1 were treated with IFN-α as in Fig. 1B. The cells were harvested after the 2nd treatment of IFN-α at various time points, incubated with 10 μM H2DCFDA, and then analyzed by FACS. (B) The cells in (A) were harvested on a slide at 48 hrs after IFN-α treatment. An increase in ROS (green dots) was observed using a fluorescence microscope. (C and D) DMSO or NAC (10 mM) was added to the culture of THP-1 cells stably expressing shControl or UBP43-specific shRNA#1 one hour prior to IFN-α pretreatment as shown schematically in (C). The cells were harvested at 48 hrs after the IFN-α treatment and analyzed for apoptosis (D).
To investigate further whether the enhanced ROS accumulation in the UBP43-knockdown THP-1 cells is directly associated with the elevated IFN-α/β-mediated apoptosis, we blocked ROS accumulation by treating the cells with the ROS scavenger NAC and measured the intensity of the IFN-α/β-mediated apoptosis. As shown in Fig. 4C, cells were exposed to NAC one hour prior to the IFN-α treatment. IFN-α induced apoptotic cell death in approximately 60% of the UBP43-knockdown THP-1 cells in the absence of NAC, whereas the cell death was decreased by approximately 20% in the presence of NAC (Fig. 4D). In addition, the pre-treatment of NAC also reduced apoptosis in the control THP-1 cells. Taken together, ROS may play a role in IFN-α/β-mediated apoptosis, at least in hematopoietic cells and the elevated accumulation of ROS in UBP43-depleted cells contributes, at least in part, to increased IFN-α/β-mediated apoptosis.
Discussion
In accordance with the proapoptotic function of IFN-α/β and within the context of negative regulatory role of the UBP43 in IFN-α/β signaling, the deficiency of Ubp43 in mice caused elevated apoptosis in hematopoietic cells upon the administration of IFN-α/β-producing reagents, polyI:C or LPS [9, 10]. One characteristic of IFN-α/β-mediated apoptosis is the late-onset timing of apoptosis, suggesting the indirect effect of IFN-α/β on the initiation of apoptosis [20]. In our studies, the deficiency of UBP43 in mouse bone marrow cells or human promonocytic THP-1 cells caused greatly elevated levels of apoptosis. However, the timing of the onset of apoptosis was not changed dramatically. Therefore, the deficiency of UBP43 is likely to increase only the intensity of proapoptotic signals without turning on other cell death signals.
Several interferon-inducible genes have been suggested as candidate mediators of IFN-α/β-induced apoptosis including TRAIL and FAS, which can induce apoptosis via the extrinsic pathway through FADD/caspase-8 signaling [3]. Indeed, TRAIL and FAS have been shown to play roles in IFN-α/β-induced apoptosis in several cell types such as melanoma, multiple myeloma, and chronic myelogenous leukemia cells [20–23]. In addition, the promoted proapoptotic effects of IFN-α/β on UBP43-knockdown cells such as IMR90-E1A and MCF7 were also mediated by the extrinsic pathway and could be abrogated by FLIP overexpression or TRAIL silencing [18].
In contrast, our data using Ubp43-deficient mouse bone marrow cells and UBP43-knockdown THP-1 cells exhibited somewhat different patterns from the previous report in terms of the major contribution of the mitochondrial pathway rather than the extrinsic pathway in IFN-α/β-mediated apoptosis. TRAIL and FAS have been identified as IFN-α/β-inducible proteins [24, 25] and, in our study, also exhibited elevated expression levels in both the Ubp43-deficient mouse bone marrow cells and UBP43-knockdown THP-1 cells upon IFN-α/β treatment (data not shown). However, the in vitro treatment with TRAIL or FASL did not induce apoptosis in either cell type; the resistance of THP-1 cells to TRAIL-induced apoptosis has also been reported by others [26]. Although the Ubp43-deficient mouse bone marrow cells and UBP43-knockdown THP-1 cells were not sensitive to TRAIL or FASL, IFN-α/β still triggered severe and elevated apoptosis process in these cells compared to their control counterparts, suggesting that neither TRAIL nor FASL is a major mediator of IFN-α/β-induced apoptosis at least in these cell types. In contrast to the extrinsic pathway, the activation of the mitochondrial pathway was notably enhanced in both the Ubp43-deficient mouse bone marrow cells and UBP43-knockdown THP-1 cells upon IFN-α/β administration. In addition, we found that ROS production was greatly increased by IFN-α/β treatment in the UBP43-knockdown THP-1 cells in comparison to its control cells. Furthermore, the removal of ROS by the treatment with ROS scavenger NAC reduced the IFN-α/β-mediated apoptosis in the same cells. ROS has been known to cooperate with the mitochondrial apoptotic pathway by changing the permeability of the mitochondrial outer membrane, resulting in the translocation of apoptosis transducers, including cytochrome c [27]. Thus, the elevated activation of the mitochondrial apoptotic pathway in the UBP43-depleted hematopoietic cells might be the result of the cooperation between the intrinsic activation of mitochondrial signaling and the ROS-mediated enhancement of the same pathway. In summary, the results presented in this report may represent the divergence of downstream mediator signaling in IFN-α/β-induced apoptosis, which can vary depending on the specific cell type.
Highlights.
Ubp43-deficient mouse bone marrow cells and UBP43-knockdown THP-1 cells are hypersensitive to interferon-α/β induced apoptosis.
Increased caspase-3 activation by interferon-α/β in UBP43-depleted hematopoietic cells is not mediated through the extrinsic apoptotic pathway.
The mitochondrial pathway is a major contributor to interferon-α/β-mediated apoptosis in Ubp43-deficient hematopoietic cells.
ROS is also a mediator of interferon-α/β induced apoptosis in UBP43-knockdown THP-1 cells.
Acknowledgments
This work was supported by the Ubiquitome Research Program (2011-0002136) and the Science Research Center (SRC) Program (No. 2011-0006469) to K.I.K. through the National Research Foundation (NRF) grant funded by the Korean government (MEST), and NIH HL091549 to D-E.Z.
Abbreviations
- ROS
reactive oxygen species
- IFN
interferon
- ISGs
interferon stimulated genes
- NAC
N-acetylcysteine
- FACS
fluorescence activated cell sorting
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Biron CA. Interferons alpha and beta as immune regulators: a new look. Immunity. 2001;14:661–4. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 2.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–49. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 4.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–90. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 6.Jeon YJ, Yoo HM, Chung CH. ISG15 and immune diseases. Biochim Biophys Acta. 2010;1802:485–96. doi: 10.1016/j.bbadis.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem. 2002;277:9976–81. doi: 10.1074/jbc.M109078200. [DOI] [PubMed] [Google Scholar]
- 8.Kim KI, Zhang DE. UBP43, an ISG15-specific deconjugating enzyme: expression, purification, and enzymatic assays. Methods Enzymol. 2005;398:491–499. doi: 10.1016/S0076-6879(05)98040-3. [DOI] [PubMed] [Google Scholar]
- 9.Kim KI, Malakhova OA, Hoebe K, Yan M, Beutler B, Zhang DE. Enhanced antibacterial potential in UBP43-deficient mice against Salmonella typhimurium infection by up-regulating type I IFN signaling. J Immunol. 2005;175:847–54. doi: 10.4049/jimmunol.175.2.847. [DOI] [PubMed] [Google Scholar]
- 10.Malakhova OA, Yan M, Malakhov MP, Yuan Y, Ritchie KJ, Kim KI, Peterson LF, Shuai K, Zhang DE. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev. 2003;17:455–60. doi: 10.1101/gad.1056303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie KJ, Hahn CS, Kim KI, Yan M, Rosario D, Li L, de la Torre JC, Zhang DE. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat Med. 2004;10:1374–8. doi: 10.1038/nm1133. [DOI] [PubMed] [Google Scholar]
- 12.Yan M, Luo JK, Ritchie KJ, Sakai I, Takeuchi K, Ren R, Zhang DE. Ubp43 regulates BCR-ABL leukemogenesis via the type 1 interferon receptor signaling. Blood. 2007;110:305–12. doi: 10.1182/blood-2006-07-033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malakhova OA, Kim KI, Luo JK, Zou W, Kumar KG, Fuchs SY, Shuai K, Zhang DE. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J. 2006;25:2358–67. doi: 10.1038/sj.emboj.7601149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thyrell L, Erickson S, Zhivotovsky B, Pokrovskaja K, Sangfelt O, Castro J, Einhorn S, Grander D. Mechanisms of Interferon-αlpha induced apoptosis in malignant cells. Oncogene. 2002;21:1251–62. doi: 10.1038/sj.onc.1205179. [DOI] [PubMed] [Google Scholar]
- 15.Sangfelt O, Erickson S, Castro J, Heiden T, Einhorn S, Grander D. Induction of apoptosis and inhibition of cell growth are independent responses to interferon-α in hematopoietic cell lines. Cell Growth Differ. 1997;8:343–52. [PubMed] [Google Scholar]
- 16.Otsuki T, Yamada O, Sakaguchi H, Tomokuni A, Wada H, Yawata Y, Ueki A. Human myeloma cell apoptosis induced by interferon-α. Br J Haematol. 1998;103:518–29. doi: 10.1046/j.1365-2141.1998.01000.x. [DOI] [PubMed] [Google Scholar]
- 17.Panaretakis T, Pokrovskaja K, Shoshan MC, Grander D. Interferon-α-induced apoptosis in U266 cells is associated with activation of the proapoptotic Bcl-2 family members Bak and Bax. Oncogene. 2003;22:4543–56. doi: 10.1038/sj.onc.1206503. [DOI] [PubMed] [Google Scholar]
- 18.Potu H, Sgorbissa A, Brancolini C. Identification of USP18 as an important regulator of the susceptibility to IFN-α and drug-induced apoptosis. Cancer Res. 2010;70:655–65. doi: 10.1158/0008-5472.CAN-09-1942. [DOI] [PubMed] [Google Scholar]
- 19.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–62. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chawla-Sarkar M, Leaman DW, Borden EC. Preferential induction of apoptosis by interferon (IFN)-β compared with IFN-α2: correlation with TRAIL/Apo2L induction in melanoma cell lines. Clin Cancer Res. 2001;7:1821–31. [PubMed] [Google Scholar]
- 21.Selleri C, Sato T, Del Vecchio L, Luciano L, Barrett AJ, Rotoli B, Young NS, Maciejewski JP. Involvement of Fas-mediated apoptosis in the inhibitory effects of interferon-α in chronic myelogenous leukemia. Blood. 1997;89:957–64. [PubMed] [Google Scholar]
- 22.Spets H, Georgii-Hemming P, Siljason J, Nilsson K, Jernberg-Wiklund H. Fas/APO-1 (CD95)-mediated apoptosis is activated by interferon-γ and interferon-α in interleukin-6 (IL-6)-dependent and IL-6-independent multiple myeloma cell lines. Blood. 1998;92:2914–23. [PubMed] [Google Scholar]
- 23.Chen W, O'Sullivan MG, Hudson W, Kersey J. Modeling human infant MLL leukemia in mice: leukemia from fetal liver differs from that originating in postnatal marrow. Blood. 2011;117:3474–5. doi: 10.1182/blood-2010-11-317529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Der SD, Zhou A, Williams BR, Silverman RH. Identification of genes differentially regulated by interferon a, β, or γ using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;95:15623–8. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, Silverman RH, Williams BR. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–20. [PubMed] [Google Scholar]
- 26.Wei W, Wang D, Shi J, Xiang Y, Zhang Y, Liu S, Liu Y, Zheng D. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induces chemotactic migration of monocytes via a death receptor 4-mediated RhoGTPase pathway. Mol Immunol. 2010;47:2475–84. doi: 10.1016/j.molimm.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]