Abstract
To identify potential human-safe insecticides against the malaria mosquito we undertook an investigation of the structure activity relationship of aryl methylcarbamates inhibitors of acetylcholinesterase (AChE). Compounds bearing a β-branched 2-alkoxy or 2-thioalkyl group were found to possess good selectivity for inhibition of Anopheles gambiae AChE over human AChE; up to 530-fold selectivity was achieved with carbamate 11d. A 3D QSAR model is presented that is reasonably consistent with log inhibition selectivity of 34 carbamates. Toxicity of these compounds to live Anopheles gambiae was demonstrated using both tarsal contact (filter paper) and topical application protocols.
Keywords: acetylcholinesterase, species-selectivity, malaria, mosquito, mechanism-based inactivator
Malaria exacts an enormous toll in the developing world, killing nearly 700,000 in 2010, most of whom were children under 5 years old.1 Malaria therapeutic drugs directed at the parasite Plasmodium sp. play a critical role in reducing mortality,2 but an important complementary approach involves controlling the vector of the malaria parasite, the mosquito Anopheles gambiae. Among vector control measures, the use of insecticide-treated nets (ITNs) has been particularly successful in reducing malaria mortality.3 To date only two biological targets have been successfully exploited in the design of insecticides against adult vector mosquitoes: the voltage-gated Na+ ion channel, and acetylcholinesterase (AChE).4 Current generation ITNs rely on pyrethoids, which modulate the former target. However, widespread use of these nets has led to the emergence of pyrethroid-resistant mosquitoes, jeopardizing this important disease control measure.5
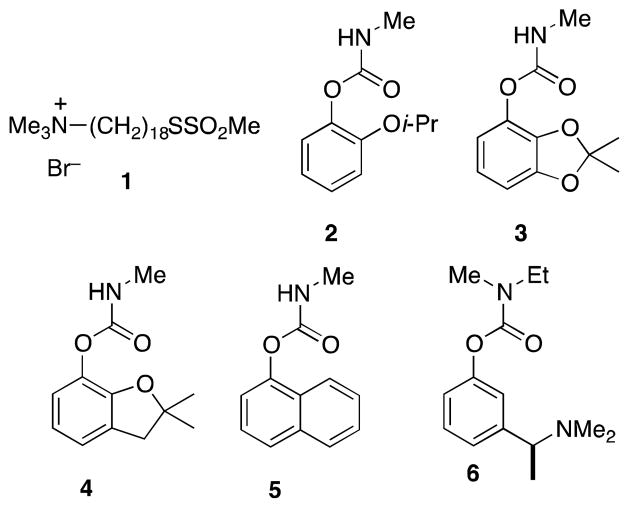
To confront this growing threat we sought to identify a class of insecticidal AChE inhibitors that would only weakly inhibit human AChE (hAChE). One intriguing strategy to achieve such selective inhibition relies upon the presence of a free cysteine in the active site gorge of Anopheles gambiae AChE (AgAChE); human (hAChE) has no such free cysteine.6 Recently, Pang and co-workers reported that the thiol-reactive compound 1 inhibited >95% of AgAChE activity after a 1 h exposure at 6 μM; under these conditions hAChE activity was unchanged.6d
We chose to pursue a different strategy: namely, redesign of the classic insecticidal aryl methylcarbamate pharmacophore7 (e.g. 2–5, Figure 1) to achieve species-selectivity. This goal appeared feasible since hAChE is only 49% identical to AgAChE.6c In addition, precedent for high species-selectivity for a carbamate is serendipitously provided by the Alzheimer’s therapeutic drug rivastigmine 6, which is 1,500-fold more potent towards hAChE than Torpedo californica AChE.8 In previous work6c we assessed the inhibitory selectivity of several carbamates (including 2) by measuring IC50 values following a 10 minute incubation. However since carbamates inhibit AChE by covalent modification of the catalytic serine, in this study we used the Ellman Assay9 to monitor time-dependent inhibition of the enzyme, by measuring enzyme velocities as a function of incubation time at fixed inhibitor concentrations. These velocities (v/v0) were used to calculate pseudo first-order rate constants kobs (min−1) for inactivation by plotting ln(v/v0) vs incubation time t. For each inhibitor kobs values were determined at three or more inhibitor concentrations ([I]). Plots of kobs vs [I] were constructed and the slope of the linear fit provided the apparent second-order rate constants ki (mM−1 min−1) for inactivation (See Supplementary Material for more detail).8,10 Inhibition of both recombinant AgAChE and hAChE by commercial carbamate insecticides 2–5 is described in Table 1.
Figure 1.
Thiol-reactive AgAChE-selective inhibitor 1, insecticidal aryl methylcarbamates 2–5, and hAChE-selective carbamate rivastigmine 6.
Table 1.
Ag AChE and hAChE inactivation rate constants ki of commercial agricultural carbamates
| Compound | AgAChEa ki (mM−1 min−1) | hAChEb ki (mM−1 min−1) | Ag/h selectivityc |
|---|---|---|---|
| 2 (propoxur) | 266 ± 9 | 17.0 ± 0.4 | 16 ± 1 |
| 3 (bendiocarb) | 839 ± 22 | 111 ± 5 | 7.6 ± 0.4 |
| 4 (carbofuran) | 2,620 ± 150 | 428 ± 12 | 6.1 ± 0.4 |
| 5 (carbaryl) | 386 ± 10 | 15.4 ± 0.4 | 25 ± 1 |
Recombinant AgAChE purified to 2,500 ± 100 U/mg (Supporting Information).
Commercial recombinant hAChE (Sigma C1682).
Defined as ki(Ag)/ki (h); error in ratio calculated by standard propagation of error.
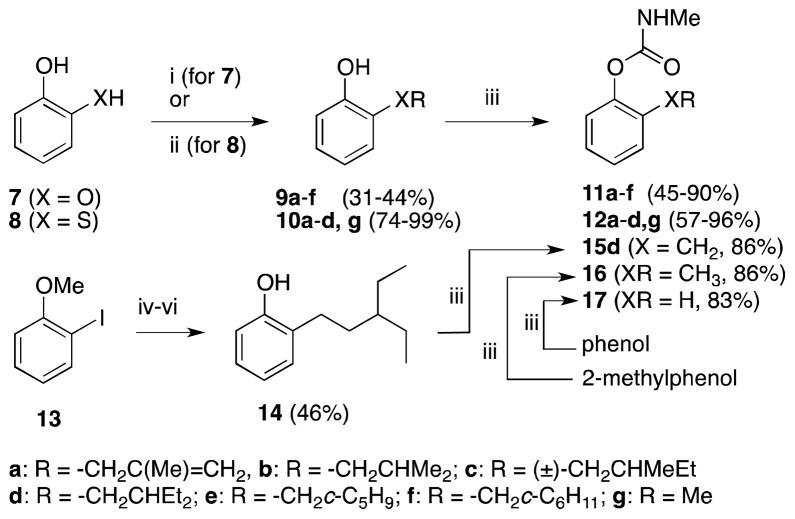
As assessed by ki ratio, 2 (propoxur) is 16-fold selective for inhibition of AgAChE, greater than the 2-fold selectivity we reported previously based on IC50 values.6c However none of these carbamates 2–5 exhibits high (i.e. >100-fold) Ag/h selectivity, and thus it is not surprising that to date the World Health Organization has not authorized the use of any carbamate on ITNs.11 We thus undertook the synthesis of a wide range of aryl carbamates to look for structural determinants of Ag/h selectivity. In this effort we focused on the use of methylcarbamates since they are known to have favorable insecticidal properties.7a In addition basic or quaternary nitrogen functionality were avoided, since these are known to reduce insecticidal potency.7a,7b One promising series to emerge featured β-branched 2-alkoxy and 2-thioalkyl substituents.12 These compounds were trivially synthesized by alkylation of catechol (7) or 2-thiophenol (8), followed by carbamoylation (Scheme 1). O-Alkylated products 9a–f were obtained in low to moderate yield due to competing dialkylation; S-alkylation to 10a–d,g occurred in higher yield. Carbamoylation was achieved in moderate to excellent yield by deprotonation of the phenols 9 and 10 with KOt-Bu and trapping with MeNHC(O)Cl. New compounds 11a–f and 12d were recently described by the authors in a patent.12 Inactivation rate constants for these compounds are given in Table 2.
Scheme 1.
Synthesis of 2-substituted aryl methylcarbamates and unsubstituted control 17
i) Cs2CO3, R-Br (or Cl, I, OTs), DMF, 80 °C, 16 h. ii) NaHCO3, R-Br (or Cl, I, OTs), DMF, 50 °C, 16 h. iii) KOt-Bu, THF; MeNHC(O)Cl. iv) i-PrMgCl, THF, −78 °C; MeO(Me)NC(O)CH2CH(Et)2. v) BBr3, CH2Cl2, 0°C. vi) NaBH4, CH3OH; Pd/C, H2, CH3OH.
Table 2.
Ag AChE and hAChE inactivation rate constants ki of 2-substituted aryl methylcarbamates 11a–f, 12a–d, g 15d, 16 and unsubstituted control 17.
| Cpd | X | R | AgAChEa ki (mM−1 min−1) | hAChEb ki (mM−1 min−1) | Ag/h selectivityc |
|---|---|---|---|---|---|
| 11a | O | -CH2CMe=CH2 | 62.2 ± 2.0 | 0.74 ± 0.05 | 84 ± 7 |
| 11b | O | -CH2CHMe2 | 52.8 ± 2.7 | 0.65 ± 0.20 | 81 ± 5 |
| 11c | O | (±)-CH2CHMeEt | 125 ± 2.8 | 0.58 ± 0.05 | 216 ± 18 |
| 11d | O | -CH2CHEt2 | 255 ± 12 | 0.48 ± 0.12 | 530 ± 130 |
| 11e | O | -CH2c-C5H9 | 9.8 ± 0.3 | 0.28 ± 0.02 | 36 ± 3 |
| 11f | O | -CH2c-C6H11 | 0.48 ± 0.02 | 0.21 ± 0.04 | 2.3 ± 0.4 |
| 12a | S | -CH2CMe=CH2 | 414 ± 18 | 7.5 ± 0.3 | 55 ± 3 |
| 12b | S | -CH2CHMe2 | 526 ± 11 | 6.5 ± 0.2 | 81 ± 3 |
| 12c | S | (±)-CH2CHMeEt | 806 ± 15 | 16.8 ± 0.4 | 48 ± 1 |
| 12d | S | -CH2CHEt2 | 1,850 ± 100 | 14.5 ± 1.5 | 130 ± 15 |
| 12g | S | Me | 68.9 ± 1.6 | 5.1 ± 0.3 | 14 ± 1 |
| 15d | CH2 | -CH2CHEt2 | 75.3 ± 2.7 | 0.75 ± 0.03 | 100 ± 5 |
| 16 | none | -CH3 | 1.31 ± 0.07 | 0.24 ± 0.07 | 5.4 ± 1.5 |
| 17 | none | -H | 2.75 ± 0.26 | 0.20 ± 0.06 | 13 ± 4.4 |
Recombinant AgAChE purified to 2,500 ± 100 U/mg (Supporting Information).
Commercial recombinant hAChE (Sigma C1682).
Defined as ki(Ag)/ki (h); error in ratio calculated by standard propagation of error.
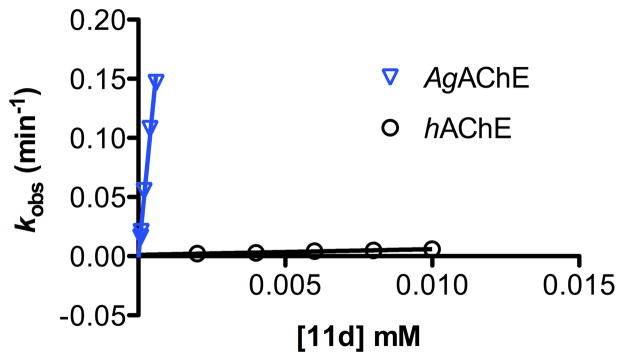
As can be seen in Table 2, β-branched 2-alkoxyphenyl methylcarbamates 11a–d are all more selective than 2, which is an α-branched 2-alkoxyphenyl methylcarbamate. The highest selectivity, 530-fold, is observed for 11d, which bears a 2-(2-ethylbutoxy) group. To illustrate the high selectivity of this compound, Figure 2 provides plots of kobs vs [11d] at both AgAChE and hAChE.
Figure 2.
Plot of kobs vs [11d] at both AgAChE and hAChE. Second-order rate constants for inactivation ki (mM−1 min−1) derive from the slope of each line.
Compounds 11e and 11f represent conformationally constrained analogs of 11d; these modifications dramatically decreased Ag/h selectivity, by reducing AgAChE ki, without significantly affecting the hAChE ki values. Thus on binding to AgAChE, the ethyl groups in 11d appear to be splayed apart, rather than adopting the syn-pentane-like conformation required by 11e–f. 2-Thioalkylphenyl methylcarbamates 12a–d also bear unconstrained β-branched alkyl groups, and offer 48- to 130-fold selectivity for inhibition of AgAChE over hAChE. The highest selectivity in this series is seen for 12d, which like 11d bears a 2-ethylbutyl substituent on the heteroatom. To further assess the scope of isosteric substitution, novel carbamate 15d was prepared, which features an X = CH2 spacer unit (Scheme 1). As can be seen in Table 2, carbamate 15d offers 100-fold selectivity for inhibition of AgAChE over hAChE. Reviewing the performance of 11d and 12d, it is clear that the replacement of O by S renders 12d significantly more inhibitory at both AgAChE and hAChE. In contrast, for 11d and 15d, the replacement of O by CH2 reduced the AgAChE ki value 3-fold, but left the hAChE ki value relatively unchanged. But regardless of the spacer unit (O vs. S vs. CH2) the 2-ethylbutyl group confers ≥ 100-fold selectivity for AgAChE. Finally, to assess the effect of minimal 2-substitution on Ag/h inhibition potency and selectivity, compounds 12g, 16 and 17 were prepared, that featured –SMe, -Me and –H in the ortho-position. As can be seen, AgAChE ki values of these compounds decrease dramatically relative to 11a–d, 12a–d, and 15d, but hAChE ki values remain largely unchanged.
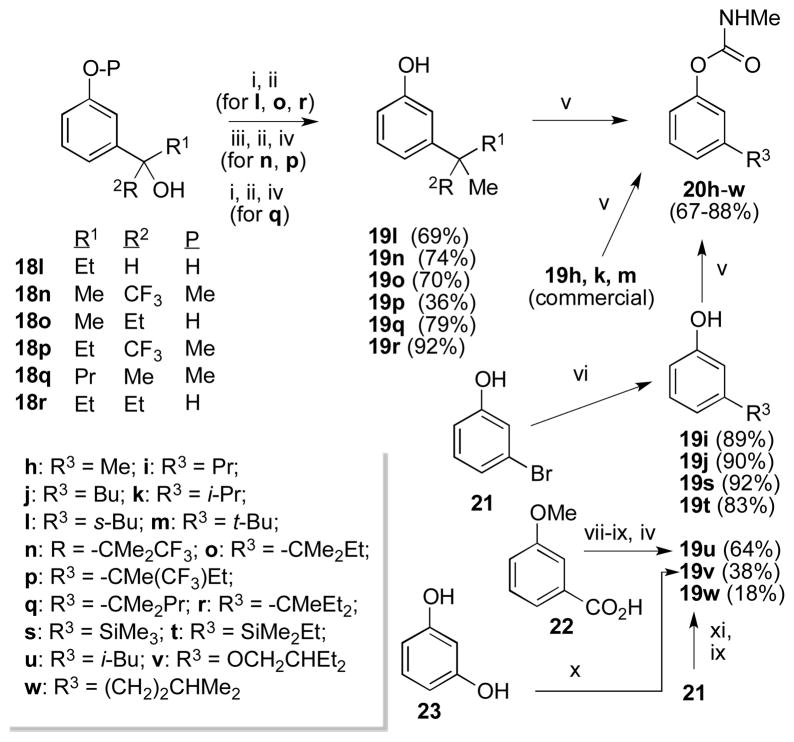
In our 2008 report6c we also discussed the inhibition selectivity of 3-substituted aryl methylcarbamates, particularly those bearing t-Bu (20m) and trialkylsilyl substituents (20s,t). To ascertain whether the 3-position had potential to confer high selectivity for AgAChE, a variety of 3-substituted aryl methylcarbamates were prepared, as shown in Scheme 2. 3-Alkylphenols 19l, n–r were prepared by methylation of the corresponding 2° or 3° benzylic alcohols, using the Shishido protocol (19n, p)13 or our recent modification of this method (19l,o,q,r).14 These benzylic alcohols 18l,n–r were in turn prepared from commercially available acetophenones or benzoate esters, by addition of the corresponding Grignard reagents or CF3SiMe3.15 Phenols 19i,j,s,t were prepared by Li/Br exchange on 21 and trapping with the appropriate electrophile. Phenol 19u was prepared from 22 by a standard Weinreb amide/Wolff-Kishner approach. 3-Alkoxyphenol 19v was prepared by alkylation of resorcinol 23. Phenol 19w was prepared from 21 by Li/Br exchange, trapping with the required Weinreb amide, and Wolff-Kishner reduction.
Scheme 2.
Synthesis of 3-substituted aryl methylcarbamates 20h–w.
NHMe i) SOCl2, 0°C. ii) AlMe3, CH2Cl2, 0 °C. iii) NaH, THF, MsCl. iv) BBr3, CH2Cl2.v) KOt-Bu, THF; CH3NHC(O)Cl. vi) 2.1 equiv n-BuLi, THF, −78 to 0 °C; 2 equiv R3-Br or R3-Cl (for 19s, t); aq. HCl. vii) DMAP, EDCI, HN(OMe)Me, CH2Cl2. viii) i-PrMgCl, Et2O, reflux. ix) NH2NH2, EtOH; KOH, ethylene glycol, 160 °C. x) NaOMe, BrCH2CHEt2, MeOH. xi) 2.1 equiv n-BuLi, THF, −78 to 0 °C; Me(MeO)NC(O)CH2CHMe2.
As can be seen in Table 3, most of the 3-substituted aryl methylcarbamates do not offer appreciable selectivity for inhibition of AgAChE over hAChE. In particular 20s and 20t are only about 10-fold selective, as opposed to the previously reported 130-fold selectivity.6c Similarly 20m is only 12-fold selective rather than the 38-fold figure reported earlier. We trace these variations, and that of 2 mentioned above, to an unanticipated but reproducible effect of our earlier inhibitor dilution protocol, which in this work has been replaced with a protocol delivering a constant final assay concentration of 0.1% (v/v) DMSO.16 Compounds 20n, p, r, t, v, and w have not been described previously. AgAChE inactivation rate constants ki are sensitive to the identity of the 3-substituent, varying from 17 (20h) to 10,000 mM−1 min−1 (20l).17 In particular, α-branching of the substituent plays an important role: AgAChE ki increases significantly as the substituent R3 is varied from methyl/1° alkyl (20h,i,j,u,w) to 2° alkyl (20k,l) or 3° alkyl (20m–r). It appears that 2° alkyl groups confer slightly faster inactivation than related 3° alkyl groups (cf. 20k vs 20m,n; 20l vs 20o,p,r). However, in general a similar structural dependence is seen for hAChE ki values, resulting in lower Ag/h inhibition selectivities than those seen for select 2-substituted aryl methylcarbamates (e.g. 11c, 11d, 12d, 15d, Table 2). The highest Ag/h inhibition selectivity in this class is seen for 3-isopentylphenyl methylcarbamate 20w (53-fold). We note that the γ-branched isopentyl group of 20u bears a resemblance to the β-branched alkoxy/thioalkyl and γ-branched 2-ethylbutyl groups that confer selectivity in the 2-substituted aryl methylcarbamate class (Table 2, 11a–d, 12a–d, 15d). Yet interestingly, 20v, which bears a 2-ethylbutoxy group at the C3 position, is poorly (6-fold) selective.
Table 3.
Apparent bimolecular rate constants ki for inactivation of AgAChE and hAChE by 3-substituted aryl methylcarbamates
| Cpd | R3 | AgAChEa ki (mM−1 min−1) | hAChEb ki (mM−1 min−1) | Ag/h selectivityc |
|---|---|---|---|---|
| 20h | -CH3 | 17.2 ± 0.3 | 0.52 ± 0.08 | 33 ± 5 |
| 20i | -Pr | 881 ± 17 | 63.7 ± 1.9 | 14.0 ± 0.5 |
| 20j | -Bu | 741 ± 5 | 32.1 ± 0.6 | 23.0 ± 0.5 |
| 20k | -i-Pr | 3,270 ± 70 | 383 ± 19 | 8.5 ± 0.5 |
| 20l | -s-Bu (±) | 10,000 ± 100 | 1,910 ± 110 | 5.3 ± 0.4 |
| 20m | -t-Bu | 1,510 ± 110 | 126 ± 3 | 12 ± 1 |
| 20n | -CMe2CF3 | 1,580 ± 40 | 139 ± 6 | 11 ± 0.6 |
| 20o | -CMe2Et | 7,600 ± 300 | 784 ± 28 | 9.7 ± 0.5 |
| 20p | -CMe(CF3)Et (±) | 3,270 ± 70 | 375 ± 6 | 8.7 ± 0.2 |
| 20q | -CMe2Pr | 6,690 ± 240 | 1,360 ± 40 | 4.9 ± 0.2 |
| 20r | -CMeEt2 | 5,280 ± 170 | 553 ± 13 | 9.5 ± 0.4 |
| 20s | -SiMe3 | 648 ± 29 | 67.8 ± 2.6 | 9.6 ± 0.6 |
| 20t | -SiMe2Et | 1,510 ± 70 | 110 ± 6 | 14 ± 1 |
| 20u | -CH2CHMe2 | 155 ± 6 | 5.5 ± 0.30 | 28 ± 2 |
| 20v | -OCH2CHEt2 | 28.8 ± 0.4 | 5.0 ± 0.6 | 5.8 ± 0.7 |
| 20w | -(CH2)2CHMe2 | 272 ± 10 | 5.2 ± 2 | 53 ± 3 |
Recombinant AgAChE purified to 2,500 ± 100 U/mg (Supporting Information).
Commercial recombinant hAChE (Sigma C1682).
Defined as ki(Ag)/ki (h); error in ratio calculated by standard propagation of error.
The high Ag/h inhibition selectivities demonstrated by 11c, 11d, 12d, and 15d (Table 2) must find their origin in differences in the primary sequences of AgAChE and hAChE, and the possible role of particular amino acid substitutions is actively under study.
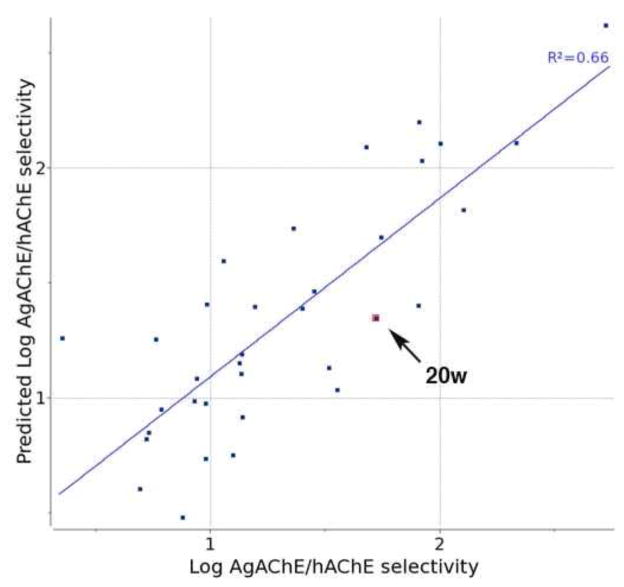
But at present we have constructed 3D QSAR models for Log (AgAChE ki), Log (hAChE ki), and Log AgAChE/hAChE selectivity. Multiple conformations of all compounds described in Tables 1–3 (except for 20w) were generated and aligned in 3D using the Atomic Property Fields (APF) method18 as implemented in ICM.19 APF molecular alignment accuracy was favorably compared to other methods in a recent benchmarking study.20 Following simplified APF 3D QSAR methodology, the pair-wise ligand APF scores were used to build a partial least square (PLS) models and derive property fields predictive of Log ki values at both enzymes, as well as Log selectivity. Both enantiomers of chiral compounds 11c, 12c, 20l, and 20p were included in the initial modeling, and the worst fitting enantiomer in each pair was discarded. Thus the final 3D QSAR model presented below is chiral, and was selected arbitrarily from one of two equally valid enantiomeric solutions. The leave-one-out cross-validated R2 values of PLS models of Log (AgAChE ki), Log (hAChE ki), and Log AgAChE/hAChE selectivity were 0.75, 0.81, and 0.66, respectively. The predicted value of 20w was then used as an external test in the plot of predicted vs observed Log AgAChE/hAChE selectivity in Figure 3. As can be seen, the prediction for 20w falls fairly close to the regression line (predicted and actual Log selectivities are 1.35 and 1.72, respectively). Because the compounds in Tables 1–3 vary primarily in the structure of the lipophilic 2- or 3- substituents, the lipophilic atomic property fields of the 3D QSAR models are useful to illustrate regions of 3D space which, when occupied by a lipophilic moiety, enhance or reduce inhibition potency or selectivity. The 3D QSAR lipophilic property field visualization for Log selectivity is presented in Figure 4.
Figure 3.
Predicted vs observed Log AgAChE/hAChE selectivity of inhibitors in Tables 1–3 except 20w (33 points); an R2 value of 0.66 is calculated. Note that prediction for each compound was made with a PLS model trained on a dataset excluding that particular compound, i.e. this is a leave-one-out prediction validation. Subsequently predicted vs. actual Log selectivity was determined for 20w (indicated by arrow).
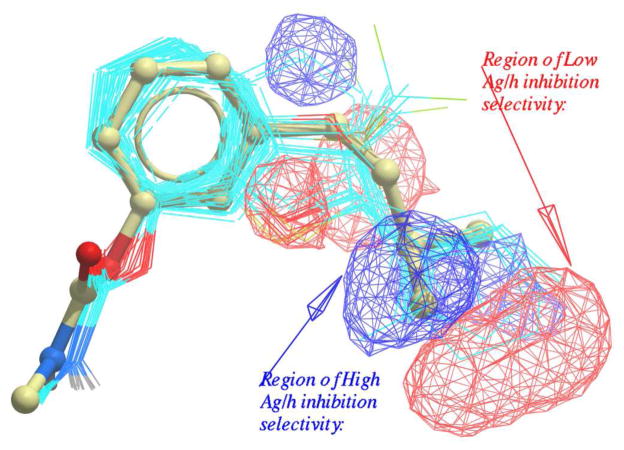
Figure 4.
3D QSAR lipophilic field visualizations for the Ag/h inhibition selectivity of the compounds in Tables 1–3. Lipophilic occupancy of blue regions improves Ag/h inhibition selectivity, and lipophilic occupancy of red regions decreases selectivity. 20w is shown in stick; other compounds are shown in CPK-colored wire (carbon is cyan).
Overall, the 3D QSAR lipophilic field indicates that in the subsite where the aromatic ring docks, AgAChE has a slightly larger ligand pocket than hAChE, so that steric bulk approximately two bonds away from the phenyl core is tolerated by AgAChE but not hAChE, conferring selectivity. Regions in which lipophilic occupancy improves Ag/h inhibition selectivity are colored blue; regions in which lipophilic occupancy decreases inhibition selectivity are colored red. The aligned structure of 20w is depicted in stick, and the placement of the terminal methyl groups in the selectivity-conferring distal blue region of the 3D QSAR lipophilicity field is evident; note that 20w is moderately (53-fold) selective. Highly selective (100–500 fold) inhibitors bearing β-branched alkoxy/thioalkyl or γ-branched alkyl groups at the 2-position (e.g. 11c–d, 12d, 15d) were also examined in this way, and in each case the terminal methyl groups fell principally in the distal blue region and did not impinge the distal red region significantly (see Supplementary Material for Figures). In contrast, the cycloalkyl moieties of constrained analogs 11e and 11f were found to extend into the distal red region, consistent with the reduced selectivities (36- and 2-fold, respectively; see Supplementary Material). Finally, the low (6-fold) selectivity of 20v, which bears a β-branched alkoxy substituent at the 3-position, is not well explained by the 3D QSAR lipophilicity field. The predicted selectivity of 20v is relatively close to the regression line (predicted and actual log selectivies are 1.26 and 0.76, respectively) and the accuracy of this prediction must be due to contribution from other property fields, likely penalizing the presence of an oxygen atom at C3.
Finally, toxicity of select carbamates to adult Anopheles gambiae was assessed using two standard protocols: tarsal contact of live mosquitoes with carbamate-treated filter papers,21 and topical application22 of ethanolic solutions of carbamates to the dorsal thorax of anesthetized mosquitoes (Table 4). With regard to the tarsal contact assay, commercial carbamates 2–5 gave LC50 values in the range of 16 – 42 ug/mL, and several of the low-selectivity aryl carbamates prepared in this work offered similar toxicities (20i,l,m,n,p). Compounds 20l,m also proved comparable to 2–5 in the topical application assay. However, the highly species-selective carbamates (11c, 11d, 12d) were less toxic than commercial carbamates 2–5 in both tarsal contact and topical application assays.
Table 4.
Toxicity of select carbamates to Anopheles gambiae using tarsal contact (filter paper) and topical application protocols.
| Compound | Tarsal Contact to treated filter paper LC50 ug/mL (95% CI) |
Topical application LD50 ng/mosquito (95% CI) |
|---|---|---|
| 2 (propoxur) | 39 (32–45) | 3.2 (2.4–4.2) |
| 3 (bendiocarb) | 16 (14–17) | 0.74 (0.52–0.97) |
| 4 (carbofuran) | 16 (11–25) | 0.85 (0.7–1.1) |
| 5 (carbaryl) | 42 (32–55) | 4.1 (3–5) |
| 11b | 290 (271–309) | 19 (12–27) |
| 11c | 445 (396–503) | 33 (24–43) |
| 11d | 27% @ 1,000 ug/mL | 81 (64–94) |
| 12a | 317 (256–389) | 12 (7–19) |
| 12b | 212 (145–279) | 10 (7–14) |
| 12d | 27% @ 1,000 ug/mL | 10 (8–12) |
| 20h | 712 (539–887) | nd |
| 20i | 41 (35–46) | nd |
| 20j | 237 (217–257) | nd |
| 20l | 31 (29–34) | 1.6 (1.4–1.8) |
| 20m | 37 (14–60) | 4.5 (3.6–5.4) |
| 20n | 61 (43–124) | nd |
| 20o | 115 (95–147) | nd |
| 20p | 68 (64–72) | nd |
| 20q | 236 (210–259) | nd |
| 20r | 72% @ 250 ug/mL | nd |
| 20t | 169 (162–176) | 8 (6–10) |
| 20w | 342 (267–472) | nd |
Mosquitoes were exposed (1 h) to dried filter papers previously treated with ethanolic solutions of carbamates; mortality was recorded after 24 h. LC50 values derive from the concentrations of inhibitor used to treat the papers.
Ethanolic solutions of the carbamate (0.2 uL) were applied to the dorsal thorax of anesthetized mosquitoes; mortality was recorded after 24 h.
Although there is no simple relationship between the measured AgAChE ki value and toxicity to live Anopheles gambiae, the expected negative correlations between LC50/LD50 and ki were seen (see Supplementary Material). In the 2-alkoxyphenyl series (2, 4, 11b–d), 4 (carbofuran) is the most rapid inactivator and has the lowest LC50/LD50 values in the tarsal contact and topical application assays. Yet, 2 (propoxur) is 25-fold more toxic than 11d in the topical application assay, despite their similar AgAChE ki values (266 ± 9 and 255 ± 12 mM−1 min−1, respectively). Similarly, in the 3-alkylphenyl series (20h–r,w), 20l is the most rapid AgAChE inactivator (AgAChE ki = 10,000 ± 100 mM−1 min−1), and in the tarsal contact assay it is among the most toxic inhibitors. However 20i, which is 11-fold slower than 20l for inactivation of AgAChE, has very similar toxicity to 20l in the tarsal contact assay. Thus AgAChE inhibitory potency is not the only determinant of mosquito toxicity. Ability to penetrate the mosquito exoskeleton, as well as traditional ADME issues are likely at work. Work to address possible metabolic and transport liabilities of highly selective carbamates continues and will be reported in due course.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (AI082581), the Foundation for the National Institutes of Health (GCGH-1497) through the Grand Challenges in Global Health Initiative, and Virginia Tech (the College of Science, the Department of Chemistry, and Fralin Life Science Institute) for financial support of this work.
Footnotes
Synthetic protocols and analytical characterization data for inhibitors; enzyme expression, assay, and mosquito toxicity protocols; and overlays of select inhibitors on the 3D QSAR lipophilic field visualization for Ag/h inhibition selectivity may be found in the online version, at doi.xxx.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.World Malaria Report 2011. The World Health Organization; http://www.who.int/malaria/world_malaria_report_2011/9789241564403_eng.pdf. [Google Scholar]
- 2.(a) Garner P, Graves PM. Plos Medicine. 2005;2:287. doi: 10.1371/journal.pmed.0020105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Burrows JN, Chibale K, Wells TNC. Curr Top Med Chem. 2011;11:1226. doi: 10.2174/156802611795429194. [DOI] [PubMed] [Google Scholar]; (c) Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GAT. Plos Medicine. 2005;2:338. doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wells TNC, Alonso PL, Gutteridge WE. Nature Rev Drug Disc. 2009;8:879. doi: 10.1038/nrd2972. [DOI] [PubMed] [Google Scholar]
- 3.(a) Lindblade KA, Eisele TP, Gimnig JE, Alaii JA, Odhiambo F, ter Kuile FO, Hawley WA, Wannemuehler KA, Phillips-Howard PA, Rosen DH, Nahlen BL, Terlouw DJ, Adazu K, Vulule JM, Slutsker L. JAMA. 2004;291:2571. doi: 10.1001/jama.291.21.2571. [DOI] [PubMed] [Google Scholar]; (b) Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, Vulule JM, Hawley WA, Hamel MJ, Walker ED. Malaria J. 2010:9. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rietveld A, Kurdova-Mintcheva R. Eliminating Malaria: Learning from the Past, Looking Ahead. World Health Organization; 2011. [Google Scholar]; (d) Lim SS, Fullman N, Stokes A, Ravishankar N, Masiye F, Murray CJL, Gakidou E. Plos Medicine. 2011:8. doi: 10.1371/journal.pmed.1001091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nauen R. Public Health - Bayer Environ Sci J. 2006;18:8. [Google Scholar]
- 5.N’Guessan R, Boko P, Odjo A, Knols B, Akogbeto M, Rowland M. Trop Med Int Health. 2009;14:389. doi: 10.1111/j.1365-3156.2009.02245.x. [DOI] [PubMed] [Google Scholar]
- 6.(a) Radić Z, Taylor P. In: Toxicology of Organophosphate and Carbamate Compounds. Gupta RC, editor. Elsevier; Amsterdam: 2006. p. 161. [Google Scholar]; (b) Pang YP. Bioorg Med Chem Lett. 2007;17:197. doi: 10.1016/j.bmcl.2006.09.073. [DOI] [PubMed] [Google Scholar]; (c) Carlier PR, Anderson TD, Wong DM, Hsu DC, Hartsel J, Ma M, Wong EA, Choudhury R, Lam PCH, Totrov MM, Bloomquist JR. Chemico-Biol Interact. 2008;175:368. doi: 10.1016/j.cbi.2008.04.037. [DOI] [PubMed] [Google Scholar]; (d) Pang YP, Ekstrom F, Polsinelli GA, Gao Y, Rana S, Hua DH, Andersson B, Andersson PO, Peng L, Singh SK, Mishra RK, Zhu KY, Fallon AM, Ragsdale DW, Brimijoin S. PLOS One. 2009;4:e6851. doi: 10.1371/journal.pone.0006851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Kolbezen MJ, Metcalf RL, Fukuto TR. J Agric Food Chem. 1954;2:864. [Google Scholar]; (b) Metcalf RL, Fukuto TR. J Agric Food Chem. 1967;15:1022. [Google Scholar]; (c) Metcalf RL. Bull Wld Hlth Org. 1971;44:43. [PMC free article] [PubMed] [Google Scholar]
- 8.Bar-On P, Millard CB, Harel M, Dvir H, Enz A, Sussman JL, Silman I. Biochemistry. 2002;41:3555. doi: 10.1021/bi020016x. [DOI] [PubMed] [Google Scholar]
- 9.Ellman GL, Courtney KD, Andres VJ, Featherstone RM. Biochem Pharm. 1961;7:88. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, Liu S, Zhao P, Pope C. Insect Biochem Mol Biol. 2009;39:646. doi: 10.1016/j.ibmb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The World Health Organization Pesticide Evaluation Scheme (WHOPES) has approved 2 (propoxur) and 3 (bendiocarb) for spraying on walls and other vertical surfaces inside dwellings, but not for deployment on ITNs: http://www.who.int/whopes/en/
- 12.Carlier Paul R, Bloomquist Jeffrey R, Paulson Sally L, Wong Eric A. U.S. Patent 8,129,428. Species-Selective Insecticidal Carbamates for Mosquito Control. issued March 6, 2012.
- 13.Tanaka H, Shishido Y. Bioorg Med Chem Lett. 2007;17:6079. doi: 10.1016/j.bmcl.2007.09.053. [DOI] [PubMed] [Google Scholar]
- 14.Hartsel JA, Craft DT, Chen Q-H, Ma M, Carlier PR. J Org Chem. 2012;77:3127. doi: 10.1021/jo202371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakash GKS, Panja C, Vaghoo H, Surampudi V, Kultyshev R, Mandal M, Rasul G, Mathew T, Olah GA. J Org Chem. 2006;71:6806. doi: 10.1021/jo060835d. [DOI] [PubMed] [Google Scholar]
- 16.Previously, inhibitor stock solutions in DMSO were diluted consecutively with buffer, causing variable final assay DMSO concentrations that were proportionate to the final inhibitor concentration. How the previous dilution protocol led to different results is under study and will be reported in due course; it is noteworthy that the selectivities of some highly selective inhibitors (e.g. 11c, 11d) were relatively insensitive to DMSO concentration.
- 17.The inactivation rate of AgAChE by 20l is very high, faster than the rate of inactivation of AgAChE by chlorpyrifos oxon (Alout H, Djogbenou L, Berticat C, Chandre F, Weill M. Comp Biochem Physiol B-Biochem Mol Biol. 2008;150:271. doi: 10.1016/j.cbpb.2008.03.008.) and similar to the rate of inactivation of hAChE by nerve agents (Bartling A, Worek F, Szinicz L, Thiermann H. Toxicology. 2007;233:166. doi: 10.1016/j.tox.2006.07.003.).
- 18.(a) Totrov M. Chem Biol Drug Des. 2008;71:15. doi: 10.1111/j.1747-0285.2007.00605.x. [DOI] [PubMed] [Google Scholar]; (b) Grigoryan A, Kufareva I, Totrov M, Abagyan R. Journal of Computer-Aided Molecular Design. 2010;24:173. doi: 10.1007/s10822-009-9316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abagyan RA. ICM software manual. Molsoft LLC; San Diego, CA: 2010. http://www.molsoft.com/man/index.html. [Google Scholar]
- 20.Giganti D, Guillemain Hln, Spadoni J-L, Nilges M, Zagury J-Fo, Montes M. J Chem Inf Model. 2010;50:992. doi: 10.1021/ci900507g. [DOI] [PubMed] [Google Scholar]
- 21.Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets. World Health Organization; Geneva: 2006. WHO/CDS/NTD/WHOPES/GCDPP/2006.3. [Google Scholar]
- 22.Pridgeon JW, Pereira RM, Becnel JJ, Allan SA, Clark GC, Linthicum KJ. J Med Entomol. 2008;45:82. doi: 10.1603/0022-2585(2008)45[82:soaacq]2.0.co;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.