Abstract
CD36, a class B scavenger receptor, has been implicated in the pathogenesis of a host of vascular inflammatory diseases. Through a high-throughput screening (HTS) assay for CD36 antagonist, we previously identified salvianolic acid B (SAB), a hydrophilic component derived from the herb Danshen, as a potential candidate. Danshen, the dried roots of Salvia miltiorrhiza, has been widely used in China for the prevention and treatment of atherosclerosis-related disorders. Previous studies showed that SAB acted as an anti-oxidant by preventing lipid peroxidation and oxidized LDL (oxLDL) formation. The present study was to investigate the specificity and efficacy of SAB in the inhibition of CD36-mediated lipid uptake. SAB reduced modified LDL (mLDL) uptake in a dose-dependent manner in phorbol-12-myristate-13-acetate (PMA)-stimulated THP-1 and RAW 264.7 cells. In the CD36 silenced THP-1 cells, SAB had no effect in reducing mLDL uptake, whereas its over-expression in CHO cells reinstates the effect, indicating a specific involvement of SAB in antagonizing the CD36's function. Surface plasmon resonance (SPR) analysis revealed a direct binding of SAB to CD36 with a high affinity (KD =3.74 μM), confirming physical interactions of SAB with the receptor. Additionally, SAB reduced oxLDL-induced CD36 gene expression in the cultured cell lines and primary macrophages. In ApoE KO mice fed a high fat diet, SAB reduced CD36 gene expression and lipid uptake in macrophages, showing its ability to antagonize CD36 pathways in vivo. These results demonstrate that SAB is an effective CD36 antagonist and suggest SAB as a potential anti-atherosclerotic agent.
Keywords: scavenger receptor class B, CD36, modified LDL, antagonist, salvianolic acid B, atherosclerosis
1. Introduction
CD36, a member of the scavenger receptor class B family, has been thought to play a critical role in foam cell formation and inflammatory vascular disease [1–6]. Although a role of CD36 in atherosclerosis has been controversial [4,5,7,8], studies using CD36 knock-out (KO) mice and pharmacological inhibitors suggest that CD36 is a potential therapeutic target to treat inflammatory diseases such as atherosclerosis and stroke [9–12]. CD36 is expressed on many types of cells and tissues, including monocytes/macrophages [13,14], adipocytes [15], platelets [16], endothelial cells [17], cardiac and skeletal muscle [18], and retinal pigment epithelial cells [19]. The receptor shows high affinity for lipid-based ligands including oxidized/modified low density lipoprotein (oxLDL/mLDL), long chain fatty acids (LCFA) and structurally distinct non-lipid based ligands such as thrombospondins, fibrillar β-amyloid, and apoptotic cells [20,21]. It is involved in regulating an array of functions in different physiological and pathological processes such as atherosclerosis, innate immunity, inflammation, angiogenesis, and lipid metabolism [21,22].
During the last decade, several pharmacological agents have been identified in antagonizing CD36 activity. Hexarelin, a hexapeptide of the growth hormone releasing peptide (GHRP) family, was reported to be able to act as a CD36 ligand [23]. Treatment of mice with hexarelin or EP 80317, a structurally related analog devoid of any growth hormone releasing activity, resulted in a marked decrease in atherosclerotic lesions which was CD36 dependent [11,24]. Further studies by Demers et al. [23] showed that the binding domain of hexarelin on CD36 overlaps that of oxLDL, suggesting that hexarelin exerts its protective effect by blocking CD36-mediated uptake of oxLDL. Besides targeting oxLDL binding sites, other approaches have been used to modulate CD36 expression or its downstream effect. For instance, SS31, one of a new class of antioxidant SS peptides [25,26], has been shown to attenuate oxLDL-induced CD36 expression and foam cell formation in mouse peritoneal macrophages and CD36-mediated ischemic injury in vivo [27]. Some other antioxidants, such as α-tocopherol or polyphenolic compounds from plant, were reported not only to protect LDL from oxidation but also to reduce the expression of CD36 and uptake of oxLDL into macrophages [28–33].
Salvianolic acid B (SAB) is a water-soluble polyphenolic antioxidant isolated from the root of red-rooted salvia (Salvia miltiorrhiza Bunge), the Chinese herb Danshen. Danshen is widely used for the prevention and treatment of vascular diseases including atherosclerosis and stroke in China and other Asian countries. Early research focused mainly on its lipophilic components such as tanshinone IIA and cryptotanshinone, while recent studies have given more attention to its hydrophilic components, especially SAB [34]. SAB, also known as lithospermic acid B or tanshinoate B, can prevent LDL from oxidation and inhibit lipid peroxidation [35–37]. However, this study revealed that SAB can also antagonize the binding of oxLDL to CD36 and prevent the following foam cell formation when the LDL has already turned to oxLDL. In addition, SAB attenuates multiple inflammatory factors or inhibits the associated pathways including the expression of cyclooxygenase-2 [38], MMP-2 and MMP-9 [39] and TGF-β/Smad [40] or NF-κB signaling pathways [41], which may be involved in CD36 related pathway and trigger the atherosclerotic and other vascular disease.
Through a high-throughput screening (HTS) for CD36 antagonists based on the competition of soluble CD36-oxLDL binding assay [42], SAB was identified as a potential candidate molecule that antagonized the CD36-oxLDL binding. The present study investigates the specificity and efficacy of SAB in blocking CD36 pathways. Here we report that SAB inhibits CD36 function and expression in culture and in hyperlipidemic ApoE KO mice. The study provides evidence that SAB is a specific CD36 antagonist both in vitro and in vivo.
2. Materials and methods
2.1. Chemicals and materials
Salvianolic acid B (SAB, tested 98% purity) was purchased from National Institute for the Control of Pharmaceutical and Biological Products (Lot No. 111562-200807, Beijing, China). Oxidized LDL (oxLDL), acetylated LDL (acLDL) and 1,1'-Dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate-labeled acLDL (DiI-acLDL) were purchased from Biomedical Technologies (Stoughton, MA). Polypropylene microplates with 96-well, 24-well and 6-well plates were purchased from Corning (Acton, MA). Cell culture media and fetal bovine serum (FBS) for RAW 264.7 and THP-1 cells were purchased from Hyclone (Logan, UT). For primary peritoneal macrophage cultures, the media from Invitrogen (Carlsbad, CA) and FBS from Mediatech (Manassas, VA) were used.
2.2. Cell culture
Human acute monocytic leukemia cell line THP-1 cells were grown in suspension in RPMI1640 medium containing 10% FBS (v/v). For differentiation to macrophages, THP-1 cells were cultured in 24-well plates at a density of 200,000 cells in each well and stimulated with 160 nM PMA (Sigma-Aldrich, St. Louis, MO) for 24 h. Both RAW 264.7 murine macrophage cells and Chinese hamster ovary (CHO) cells were cultured in Dulbecco's modified Eagle's medium (DMEM, high glucose) containing 10% FBS (v/v). CD36-transfected CHO (CHO/CD36) cells [43] were cultured in DMEM containing 10% FBS (v/v) and 0.5 mg/ml G418 (Invitrogen, Carlsbad, CA). Resident macrophages were collected from mouse peritoneal cavities by lavage with 15 ml of PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4 [pH 7.3]) as previously described [9]. Harvested macrophages were washed with PBS and centrifuged at 1,000 rpm, at 4°C for 10 min, and resuspended in DMEM containing 10% FBS. The cells were cultured at 37°C in a humidified 5% CO2 incubator.
2.3. Flow cytometry analysis and photograph for cellular uptake of DiI-labeled acLDL
RAW 264.7 cells, PMA-induced THP-1 cells or primary macrophages were plated in 6 or 24-well culture dishes at respective 500,000 or 200,000 cells/well, followed by incubation with 2 μg/mL DiI-acLDL in the presence of vehicle (PBS) or an indicated concentration of SAB for 4 h at 37°C. For flow cytometry analysis, cells were collected and washed by PBS. The cell suspension was centrifuged (800 rpm, 3 min, 4°C) and the obtained pellet was resuspended in PBS buffer. Total 10,000 cells were counted and DiI fluorescence was analyzed using an Epics XL flow cytometer (Coulter Corporation, Miami, FL). For the visualization, cells were photographed by IN Cell Analyzer 1000 (GE Healthcare, Piscataway, NJ) after washing with PBS twice.
2.4. Surface plasmon resonance analysis
All experiments were performed in PBS buffer (pH 7.4) on a Biacore T100 System (GE Healthcare). The recombinant human CD36 (R&D, Minneapolis, MN) was diluted to 50 μg/mL in 10 mM sodium acetate (pH 4.5) and immobilized on a CM5 sensor chip by amino coupling at a total signal of 8,000 response units (RU). Different concentrations of SAB and hexareli were prepared in PBS and injected consecutively across the surface of the chip at a flow rate of 10 μl/min for 3 min at 25°C. Several concentration replications were applied to evaluate the parallelism of data. The chip surface was regenerated with a 20 s pulse of 10 mM NaOH between each run. A blank flow cell was included as a reference surface on the chip for bulk shift and nonspecific binding changes in resonance units (RU) and was subtracted from the experimental data. The resulting binding and dissociation curves were analyzed by 1:1 fitting model. The association rate constant (ka) and the dissociation rate constant (kd) were calculated according to the BiaEvaluation software version 2.1 provided by the manufacturer. The affinity constant was calculated from the equation KD=kd/ka.
2.5. siRNA knockdown assay
For suppression of endogenous CD36 expression in THP-1 cells, 3 siRNAs (HSS101567, HSS190373, HSS190374) that specifically target human CD36 mRNA and scrambled siRNA (Low GC Duplex 36%) were purchased from Invitrogen (Carlsbad, CA). THP-1 cells were transfected with the CD36 siRNAs or scrambled siRNA as negative control using the Lipofectaminetm RNAiMAX reagent (Invitrogen), according to the instructions of the manufacturer. After 24 hours, cells were treated with 100 μM SAB or vehicle. Cells were harvested 24 h later and real time RT-PCR or Flow cytometry analysis was performed.
2.6. Real time RT-PCR
RNA isolation and real time reverse transcriptase (RT)-PCR were performed as described previously [9,44]. For RAW 264.7 or THP-1 cells, FastStart Universal SYBR Green PCR Master (Roche, Basel, Schweiz) was used on a CFX96TM Real-Time PCR Detection System (Bio-Rad, California, USA). The sequences of the forward and reverse primers used for amplification were 5'-CCA AGC TAT TGC GAC ATG ATT-3' and 5'-TCT CAA TGT CCG AGA CTT TTC A-3' for murine CD36 (the size of the amplicon is 78 bp), 5'-AGC TTG TCA TCA ACG GGA AG-3' and 5'- TTT GAT GTT AGT GGG GTC TCG-3' for murine GAPDH (the size of the amplicon is 62 bp), 5'- TGG AAC AGA GGC TGA CAA CTT -3' and 5'-TTG ATT TTG ATA GAT ATG GGA TGC -3' for human CD36 (the size of the amplicon is 73 bp), 5'-AGC CAC ATC GCT CAG ACA C-3' and 5'-GCC CAA TAC GAC CAA ATC C-3' for human GAPDH (the size of the amplicon is 66 bp). The reactions were subjected to a heat dissociation protocol after the final cycle of PCR to indicate the proper temperature for fluorescence detection. Data were analyzed with CFX Manager version 1.5.534.0511 software (Bio-Rad). For primary macrophage and in vivo experiments, fluorescent TaqMan technology (Applied Biosystems, Foster City, CA) was used. PCR primers specific for CD36 and internal control β-actin were obtained from Applied Biosystems. The PCR reactions were performed using Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems).
2.7. Animals and SAB administration
The use of animals and procedures performed were approved by the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University. Experiments were performed in C57BL/6 (Jackson Laboratory, Bar Harbor, Maine) and ApoE knock-out (KO) mice. The ApoE KO mice were backcrossed seven times into the C57BL/6 strain and are housed at the Burke Medical Research Institute. The procedures for breeding and genotyping were described previously [4,45]. Six week old ApoE KO mice were fed a high fat diet (HFD; Harlan Teklad Madison, WI. TD88137) for 8 weeks. During the diet intervention, the mice were treated with either vehicle (saline) or SAB (100 mg/kg, prepared in saline) by intraperitoneal injection every other day.
2.8. Oil red O staining and quantification
Peritoneal macrophages were cultured in 4 chamber slide with indicated concentration of SAB and/or oxLDL. After 48 h of incubation, cells were washed by PBS and stained with oil red O according to the methods previously described [9,42]. Data were presented in two ways: mean intensity (integrated optical density, IOD) and mean area of oil red O staining using Image-Pro Plus 5.1 software.
2.9. Lipid content on aortic vessels
Thoracic aorta in the posterior mediastinal cavity spanning from the fourth to twelfth thoracic vertebra (T4-T12), including the aortic arch and ascending aorta, were dissected out. Wet weights of the vessels were obtained prior to drying them for lipid content assessment. Lipid was extracted according to the procedure described previously [9] using a mixture of chloroform/methanol (2:1) and centrifuged at 13,000 rpm for 5 min at room temperature to collect the lower lipid-containing phase. The procedure was repeated three times, and total lipid content was weighed after removal of solvent.
2.10. Data analysis
Data were expressed as mean ± SEM. Statistical significance of the data was evaluated by Student t-test. Multiple comparisons were made using ANOVA followed by a post hoc Newman-Keuls test. Values of p<0.05 were considered statistically significant.
3. Results
3.1. SAB reduces DiI-acLDL uptake in RAW 264.7 and THP-1 cells
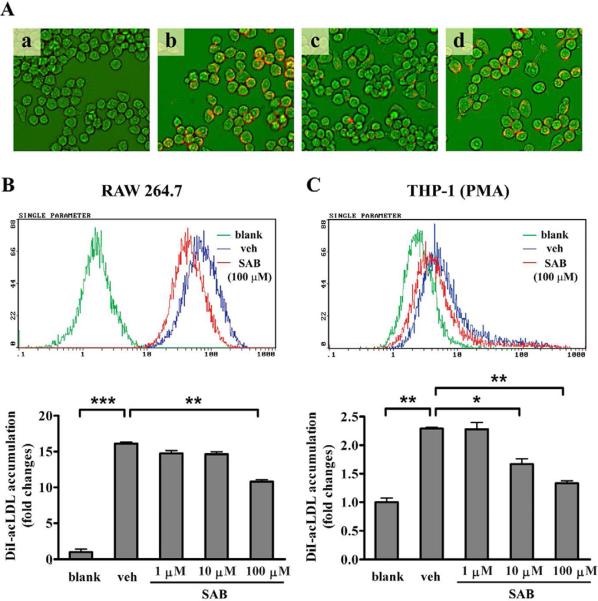
CD36, one of the main scavenger receptors expressed in macrophage, was reported to be responsible for the binding and internalizing of modified LDL (mLDL) in the process of foam cell formation [21]. This led us to investigate whether the lipid accumulation in macrophage was decreased by SAB using fluorescence-labeled acLDL. Fluorescence visualization by IN Cell Analyzer 1000 (GE Healthcare) showed that lipid accumulation was readily detected in murine macrophage cell line RAW 264.7 cells after incubation of 2 μg/mL DiI-acLDL for 4 h (Fig. 1Ab vs. 1Aa). Compared with vehicle-treated cultures (Fig. 1Ab), fluorescence was dramatically decreased in SAB-treated cells (Fig. 1Ac) as well as the cultures that contained excess (80 μg/mL) amount of unlabeled acLDL (Fig. 1Ad). The incubation of 100 μM SAB for 4 hours significantly reduced the uptake of DiI-acLDL in RAW 264.7 cells by 33.0% (Fig. 1B) detected by flow cytometry analysis. Similarly, PMA stimulated human acute monocytic leukemia THP-1 cells showed 27.1% and 41.9% reduction of DiI-acLDL uptake by 10 and 100 μM SAB (Fig. 1B). The inhibitory rate for SAB was similar at 1 h, 2 h, 4 h, 12 h or 24 h incubation of SAB (data not shown). These results indicated that SAB antagonized the mLDL uptake by cultured macrophage cells.
Fig. 1.
The effect of SAB on DiI-acLDL uptake by PMA-induced THP-1 cells and RAW 264.7 cells. (A) RAW 264.7 cells incubated with DiI-acLDL, in the presence or absence of SAB, were visualized and photographed using IN Cell Analyzer 1000 with a 20×objective. a, blank cells; b, 2 μg/mL DiI-acLDL treated cells; c, 2 μg/mL DiI-acLDL+100 μM SAB treated cells; d, 2 μg/mL DiI-acLDL+80 μg/mL acLDL treated cells. (B, C) Flow cytometry analyses for the SAB effect on lipid uptake in RAW 264.7 cells (B) and PMA-induced THP-1 cells (C). The macrophages were incubated with 2 μg/mL DiI-acLDL for 4 h in the presence of indicated concentrations of SAB or vehicle (veh). Blank cells were neither incubated with DiI-acLDL nor treated by SAB. Relative fluorescence intensities of each group of cells are demonstrated by integrogram. The experiments were repeated for at least 4 times. *, p<0.05 and **, p<0.01 compared with vehicle (veh).
3.2. SAB attenuates lipid uptake in a CD36-dependent manner by directly binding to CD36
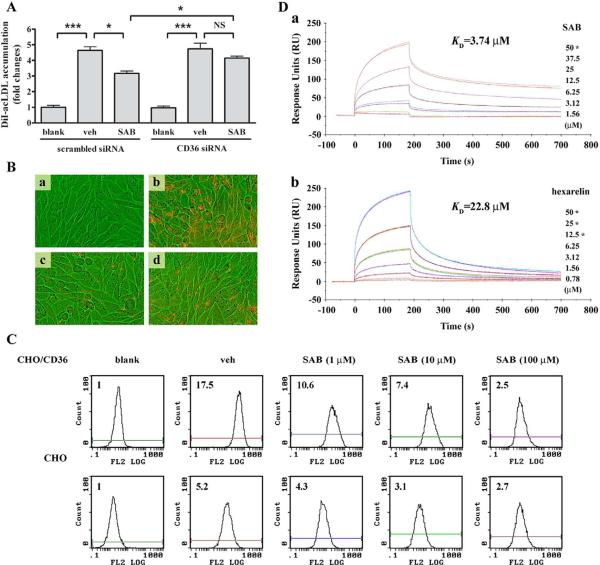
To address the specific effect of SAB on CD36, we determined the extent of DiI-acLDL uptake in CD36 silenced THP-1 cells. Transfection with CD36-specific siRNA resulted in a significant decrease in CD36 mRNA level (the inhibitory efficiency equaled to 91%) compared to scrambled siRNA treatment. As expected, SAB significantly reduced DiI-acLDL uptake in cultures transfected with scrambled siRNA. However , the inhibitory effect of SAB was impaired in the cultures transfected with CD36 siRNA. Furthermore, the difference of DiI-acLDL uptake by SAB treatment between scrambled siRNA and CD36 siRNA was significant, suggesting that CD36 is essential for SAB-mediated inhibition (Fig. 2A). On the other hand, the inhibitory effect of SAB on DiI-acLDL uptake was similar in the cultures transfected with LDLR-specific siRNA and scrambled siRNA in RAW 264.7 cells (data not shown), indicating that LDLR is not responsible for SAB-mediated antagonism of mLDL uptake. In addition, visualization of DiI-acLDL fluorescence in CD36-overexpressed CHO (CHO/CD36) cells revealed that the DiI-acLDL-induced fluorescence accumulation was markedly attenuated in the presence of 100 μM SAB or in the culture that contains an excess amount (80 μg/mL) of unlabeled acLDL (Fig. 2B). These changes were absent in CHO cells treated identically (data not shown). Further investigation by flow cytometry analysis showed that, although there was a background increase in DiI-acLDL uptake in CHO cells, CHO/CD36 cells profoundly increased DiI-acLDL accumulation. This increased lipid uptake was significantly reduced by SAB in a dose-dependent manner (Fig. 2C). The inhibition of lipid uptake by SAB was also confirmed when CHO/CD36 cultures were incubated with DiI-oxLDL (data not shown). Collectively, the data showed that CD36 was specifically involved in SAB mediated inhibition of lipid uptake. The direct binding ability of SAB to CD36 was determined by surface plasmon resonance (SPR) using CM5 chips immobilized with recombinant human CD36. Analysis of binding activity between SAB and immobilized CD36 showed that the binding occurred in a dose-dependent manner similar to that of hexarelin, a known CD36 ligand [23] (Fig. 2D). In addition, determination of the kinetics by association (ka), dissociation constant (kd) and affinity (KD) between the receptor and ligands revealed that SAB had higher binding affinity and indicated that SAB was a more potent CD36 antagonist than hexarelin (ka, 264.3 M−1s−1 vs. 240.1 M−s−1; kd, 9.89×10−4 s−1 vs. 5.48×10−3 s−1; KD, 3.74 μM vs. 22.8 μM).
Fig. 2.
SAB-induced inhibition of DiI-acLDL uptake was CD36-dependent. (A) The effect of SAB on DiI-acLDL uptake by PMA-induced THP-1 cells with CD36 siRNA using flow cytometry. The experiment was repeated for 4 times. *, p<0.05, ***, p<0.001 and NS, not significant. (B) The uptake of DiI-acLDL by CHO/CD36 cells. CHO/CD36 cells incubated with DiI-acLDL in the presence of SAB, vehicle or 80 μg/mL acLDL were visualized and photographed using IN Cell Analyzer 1000 with a 20×objective. a, blank cells; b, 2 μg/mL DiI-acLDL treated cells; c, 2 μg/mL DiI-acLDL+100 μM SAB treated cells; d, 2 μg/mL DiI-acLDL+80 μg/mL acLDL treated cells. (C) Flow cytometry analysis for uptake of DiI-acLDL by CHO cells and CHO/CD36 cells. Indicated concentrations of SAB were applied on both cell lines. The uptake of DiI-acLDL was detected in 10,000 cells from the whole portion of each group by FACS. Experiment was repeated 3 times and the representative result was presented. The bar graph reflected the quantified abundance of fluorescence. blank, cells only control; veh, 2 μg/ml DiI-acLDL treated cells; SAB, 2 μg/ml DiI-acLDL and 100 μM SAB treated cells. (D) Surface plasmon resonance (SPR) analysis of the binding of SAB (a) or hexarelin (b) to immobilized CD36. Recombinant human CD36 was immobilized on the surface of a CM5 sensor chip, and indicated concentrations of SAB or hexarelin were injected across the surface. Injections were aligned at t=0. The star symbol (*) means that the duplicate concentration was applied to evaluate the parallelism of data.
3.3. SAB reduces oxLDL-induced CD36 expression in macrophages
The effect of SAB on oxLDL-induced CD36 gene expression was investigated. Interaction of oxLDL with CD36 was shown to trigger downstream signal pathways, which led to an increased presence of CD36 on the surface of macrophages [46]. Accordingly, the inhibitory effect of SAB on CD36 expression in oxLDL-induced RAW 264.7 cells, THP-1 cells and primary macrophages was determined. The treatment of 25 μg/ml oxLDL in cultures significantly increased CD36 mRNA levels in all 3 different cell lines (Fig. 3). In RAW 264.7 cells, SAB reduced the CD36 mRNA level by 11.2%, 48.9% and 78.3% at respective 1 μM, 10 μM and 100 μM in the presence of oxLDL (Fig. 3A). Similar dose-dependent inhibition of CD36 gene expression by SAB was also observed in THP-1 cells (Fig. 3B) and peritoneal macrophages obtained from C57BL/6 mice (Fig. 3C).
Fig. 3.
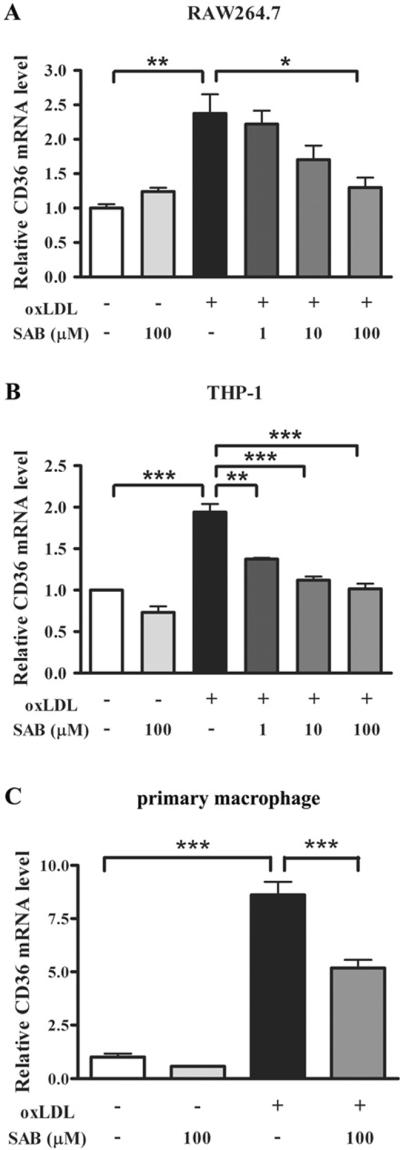
SAB inhibits oxLDL-induced upregulation of CD36 mRNA in RAW 264.7 cells (A), THP-1 cells (B) and primary macrophages (C). Cells were exposed to the indicated concentration of SAB in the presence or absence of 25 μg/mL oxLDL, and the CD36 mRNA level was detected using real time RT-PCR analysis. Values represent the mean ± SEM of three independent experiments. The asterisks denote a significant difference (*, p<0.05, **, p<0.01 and ***, p<0.001).
3.4. SAB suppresses lipid uptake and CD36 expression in hyperlipidemic ApoE KO mice
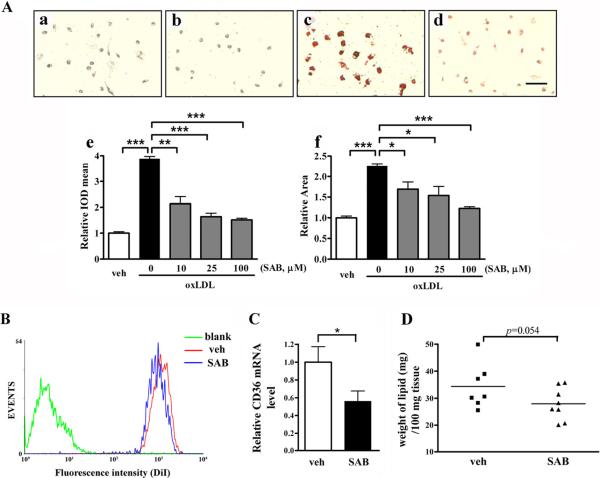
The effect of SAB on lipid uptake and CD36 expression was investigated in an in vivo hyperlipidemic condition. Previously, ApoE KO mice fed a high fat diet for 8 weeks showed significantly increased plasma cholesterol levels when compared to wild type mice fed a normal chow (900 vs. 120 mg/dl) [9]. Peritoneal macrophages from the hyperlipidemic ApoE KO mice showed intense oil red O staining, an indication of intracellular lipid accumulation, in the presence of oxLDL (Fig. 4Ac vs. 4Aa). The staining was significantly reduced in co-treatment with SAB (Fig. 4Ad vs. 4Ac). Quantification of the extent of lipid accumulation revealed that SAB attenuated the intensity as well as the area of the staining area in a dose-dependent manner (Fig. 4Ae–f). Further investigation on DiI-acLDL uptake was performed by flow cytometry analysis. Compared to vehicle-treated cells, SAB treatment reduced DiI-acLDL accumulation in peritoneal macrophage from the hyperlipidemic ApoE KO mice (Fig. 4B). Inhibition rate for SAB treatment was 17.65%. In addition, CD36 mRNA levels in peritoneal macrophages from SAB treated mice were significantly reduced (Fig. 4C). To address whether SAB had effects on lipid uptake in vivo, lipid contents of aortic vessels were determined in ApoE KO mice. The result showed that SAB treatment reduced lipid accumulation on the vessels compared to that of vehicle-treated group (Fig. 4D). Taken together, these findings suggest that SAB is an effective CD36 antagonist in vivo.
Fig. 4.
SAB attenuated lipid accumulation and CD36 expression in hyperlipidemic ApoE KO mice. (A) Primary macrophage from ApoE KO mice were seeded in a 4 chamber slides and then incubated with 20 μg/ml oxLDL and/or different concentration of SAB for 48 h. Lipids were stained with oil red O and examined by microscopy; representative images are shown as a-d. a, veh; b, 25 μM SAB treatment; c, 20 μg/ml oxLDL and d, 20 μg/ml oxLDL+25 μM SAB. Scale bar, 50 μm. e&f, Quantification for foam cell assay in primary macrophage. Intensity measured as integrated optical density refers to the degree of lipid accumulation (e); Area, refers to the average size of foam cells (f). The results were from two independent experiments that triplicated within each experiment. (B) The uptake of DiI-labeled acLDL was reduced in SAB-treated macrophages collected from hyperlipidemic ApoE KO mice. Macrophages were treated with 2 μg/ml DiI-labeled acLDL for 12 h with 100 μM SAB or vehicle (veh). The uptake of DiI-acLDL was detected in 10,000 cells from the whole portion of each group by flow cytometry. blank, cells only control; veh, 2 μg/ml DiI-acLDL treated cells; SAB, 2 μg/ml DiI-acLDL and 100 μM SAB treated cells. (C) CD36 expression was determined in peritoneal macrophages from vehicle or SAB-treated ApoE KO mice with high fat diet for 8 weeks. β-actin was used as internal control, and veh groups were normalized as 1. *, p<0.05, **, p<0.01 and ***, p<0.001, n=5 (veh) and 8 (SAB). (D) The lipid contents of aortic vessels were determined from ApoE KO mice with high fat diet that were treated with vehicle or SAB for 8 weeks. p=0.054, n=7 (veh) and 8 (SAB).
4. Discussion
CD36 has been implicated in the pathology of atherosclerosis through foam cell formation and lipoprotein deposit in the vessel wall [4]. Although the role for CD36 in atherosclerosis in vivo was initially controversial, several genetic and pharmacological studies support CD36's role in developing atherosclerosis [4,47]. Accordingly, strategies to inhibit CD36 have been suggested to ameliorate or delay the disease progression [4]. Using SAB as a potential CD36 antagonist candidate through our previous high throughput screening [42], the current study investigates and validates the efficacy and specificity of SAB to inhibit CD36 pathway in vitro and in vivo. We found that not only SAB attenuated lipid uptake in a CD36-dependent manner through direct binding to CD36, CD36 expression and lipid uptake were also reduced in the cultures and hyperlipidemic ApoE KO mice treated with SAB.
SAB has been reported to possess antiatherogenic and antioxidant capability [35,37–39]. In this study, we showed the dose dependent inhibitory effect of SAB in the uptake of mLDL in PMA-stimulated THP-1 and RAW 264.7 cells (Fig. 1) as well as in mouse peritoneal macrophages obtained from hyperlipidemic ApoE KO mice (Fig. 4). This inhibitory effect of lipid uptake was demonstrated to be CD36-dependent by investigating the influence of knockdown and overexpression of CD36 on mLDL uptake by macrophages (Fig. 2). The data provide evidence of the specific involvement of SAB in inhibiting mLDL uptake through CD36, which may contribute to its anti-atherosclerotic effects. Of note, the uptake of DiI-acLDL was not significantly affected by CD36 siRNA treatment on THP-1 cells compared with scrambled siRNA. This observation indicated that CD36 may not be the unique receptor for the lipid uptake and its activity was mostly compensated by other scavenger receptors when CD36 was knockdown (Fig. 2A). These results were consistent with the increased SR-A (macrophage scavenger receptor 1, MSR1 or scavenger receptor class A) mRNA level when CD36 expression was suppressed by CD36 siRNA (data not shown).
The study also addressed the physical binding property of SAB with CD36 and compared to the known CD36 ligand hexarelin by SPR analyses (Fig. 2D). Hexarelin was previously reported to interfere with the CD36-mediated uptake of oxLDL by macrophages, featuring its cardioprotective effects [23,24,48]. The SPR-derived kinetic and binding affinity data showed that SAB is a more potent CD36 antagonist than hexarelin (Fig. 2D). Previous in vivo study of EP 80317, a CD36 ligand derived from hexarelin, showed anti-atherosclerotic effects of CD36-dependent reduced lesion size in ApoE KO mice fed an atherogenic diet [11]. Consistent with this, SAB treated hyperlipidemic ApoE KO mice showed reduced lipid uptake in peritoneal macrophages (Fig. 4), representing SAB as another class of potential anti-atherosclerotic agent.
Recently, CD36 on macrophages and platelets has been reported to mediate related signaling pathways linking hyperlipidemia and inflammation [49,50]. The mechanism by which SAB effectively inhibits the oxLDL-induced CD36 mRNA levels in cultures and ApoE KO mice (Fig. 3, 4C) is not clear at present. Since CD36 activation triggers signaling, it is possible that SAB may antagonize CD36-related signal pathways. OxLDL was reported to bind CD36 and trigger the activation of transcription factor PPARγ, which leads to an increased presentation of the receptor to the macrophage surface that may contribute to atherogenesis [46]. Our findings suggested a possibility that the binding of SAB to CD36 block oxLDL binding to the receptor and subsequently suppress the oxLDL-induced feed-forward loop for PPARγ-dependnet CD36 transcription. Additionally, SAB has been reported to decrease several inflammatory factors [41,51,52], and attenuate some possible CD36-associated signal pathways including NF-κB and TGF-β/Smad [40,41].
SAB is the most abundant and bioactive hydrophilic component of Danshen, and an injection of “Depsides salts from Salvia miltiorrhiza”, which contains about 80% of magnesium SAB, has been approved in 2005 in China as a modernized Chinese medicinal treatment against coronary heart disease and angina pectoris [53]. In this study, we identified that SAB is a new class of nonpeptide small molecular CD36 antagonist in vitro and in vivo. Further development of SAB or its derivatives/analogs may serve as a potential anti-atherogenic strategy to treat atherosclerosis and other vascular/inflammatory diseases.
SAB reduces DiI-acLDL uptake in RAW 264.7 and THP-1 cells.
SAB attenuates lipid uptake in a CD36-dependent manner by directly binding to CD36.
SAB reduces oxLDL-induced CD36 expression in macrophages.
SAB suppresses lipid uptake and CD36 expression in hyperlipidemic ApoE KO mice.
Acknowledgements
This work was supported by the National Natural Science Foundation of China 90813027 (to B. H.) and 30801401 (to Y. Y.), the China Ministry of Science and Technology 2012ZX09301002-001 (to B. H.) and 2012ZX09301002-003 (to S. S.) and National Institute Health HL82511 (to S. C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J Lipid Res. 2009;50(Suppl):S282–S286. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- [3].Kuchibhotla S, Vanegas D, Kennedy DJ, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moore KJ, Kunjathoor VV, Koehn SL, et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Guy E, Kuchibhotla S, Silverstein R, et al. Continued inhibition of atherosclerotic lesion development in long term Western diet fed CD36o /apoEo mice. Atherosclerosis. 2007;192:123–130. doi: 10.1016/j.atherosclerosis.2006.07.015. [DOI] [PubMed] [Google Scholar]
- [7].Febbraio M, Guy E, Silverstein RL. Stem cell transplantation reveals that absence of macrophage CD36 is protective against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2333–2338. doi: 10.1161/01.ATV.0000148007.06370.68. [DOI] [PubMed] [Google Scholar]
- [8].Witztum JL. You are right too! J Clin Invest. 2005;115:2072–2075. doi: 10.1172/JCI26130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim E, Tolhurst AT, Qin LY, et al. CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci. 2008;28:4661–4670. doi: 10.1523/JNEUROSCI.0982-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cho S, Park EM, Febbraio M, et al. The class B scavenger receptor CD36 mediates free radical production and tissue injury in cerebral ischemia. J Neurosci. 2005;25:2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marleau S, Harb D, Bujold K, et al. EP 80317, a ligand of the CD36 scavenger receptor, protects apolipoprotein E-deficient mice from developing atherosclerotic lesions. FASEB J. 2005;19:1869–1871. doi: 10.1096/fj.04-3253fje. [DOI] [PubMed] [Google Scholar]
- [12].Bodart V, Febbraio M, Demers A, et al. CD36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart. Circ Res. 2002;90:844–849. doi: 10.1161/01.res.0000016164.02525.b4. [DOI] [PubMed] [Google Scholar]
- [13].Endemann G, Stanton LW, Madden KS, et al. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- [14].Huh HY, Pearce SF, Yesner LM, et al. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020–2028. [PubMed] [Google Scholar]
- [15].Abumrad NA, el-Maghrabi MR, Amri EZ, et al. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- [16].Bolin RB, Medina F, Cheney BA. Glycoprotein changes in fresh vs. room temperature-stored platelets and their buoyant density cohorts. J Lab Clin Med. 1981;98:500–510. [PubMed] [Google Scholar]
- [17].Swerlick RA, Lee KH, Wick TM, et al. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J Immunol. 1992;148:78–83. [PubMed] [Google Scholar]
- [18].Van Nieuwenhoven FA, Verstijnen CP, Abumrad NA, et al. Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochem Biophys Res Commun. 1995;207:747–752. doi: 10.1006/bbrc.1995.1250. [DOI] [PubMed] [Google Scholar]
- [19].Ryeom SW, Sparrow JR, Silverstein RL. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J Cell Sci. 1996;109(Pt 2):387–395. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- [20].Ge Y, Elghetany MT. CD36: a multiligand molecule. Lab Hematol. 2005;11:31–37. [PubMed] [Google Scholar]
- [21].Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007;39:2012–2030. doi: 10.1016/j.biocel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Demers A, McNicoll N, Febbraio M, et al. Identification of the growth hormone-releasing peptide binding site in CD36: a photoaffinity cross-linking study. Biochem J. 2004;382:417–424. doi: 10.1042/BJ20040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Avallone R, Demers A, Rodrigue-Way A, et al. A growth hormone-releasing peptide that binds scavenger receptor CD36 and ghrelin receptor up-regulates sterol transporters and cholesterol efflux in macrophages through a peroxisome proliferator-activated receptor gamma-dependent pathway. Mol Endocrinol. 2006;20:3165–3178. doi: 10.1210/me.2006-0146. [DOI] [PubMed] [Google Scholar]
- [25].Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006;8:E277–E283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao K, Zhao GM, Wu D, et al. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- [27].Cho S, Szeto HH, Kim E, et al. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem. 2007;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- [28].Fuhrman B, Volkova N, Aviram M. Oxidative stress increases the expression of the CD36 scavenger receptor and the cellular uptake of oxidized low-density lipoprotein in macrophages from atherosclerotic mice: protective role of antioxidants and of paraoxonase. Atherosclerosis. 2002;161:307–316. doi: 10.1016/s0021-9150(01)00646-3. [DOI] [PubMed] [Google Scholar]
- [29].Venugopal SK, Devaraj S, Jialal I. RRR-alpha-tocopherol decreases the expression of the major scavenger receptor, CD36, in human macrophages via inhibition of tyrosine kinase (Tyk2) Atherosclerosis. 2004;175:213–220. doi: 10.1016/j.atherosclerosis.2004.03.012. [DOI] [PubMed] [Google Scholar]
- [30].Ricciarelli R, Zingg JM, Azzi A. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation. 2000;102:82–87. doi: 10.1161/01.cir.102.1.82. [DOI] [PubMed] [Google Scholar]
- [31].Lian TW, Wang L, Lo YH, et al. Fisetin, morin and myricetin attenuate CD36 expression and oxLDL uptake in U937-derived macrophages. Biochim Biophys Acta. 2008;1781:601–609. doi: 10.1016/j.bbalip.2008.06.009. [DOI] [PubMed] [Google Scholar]
- [32].Lo YH, Pan MH, Li S, et al. Nobiletin metabolite, 3',4'-dihydroxy-5,6,7,8-tetramethoxyflavone, inhibits LDL oxidation and down-regulates scavenger receptor expression and activity in THP-1 cells. Biochim Biophys Acta. 2010;1801:114–126. doi: 10.1016/j.bbalip.2009.10.002. [DOI] [PubMed] [Google Scholar]
- [33].Wu YJ, Hong CY, Lin SJ, et al. Increase of vitamin E content in LDL and reduction of atherosclerosis in cholesterol-fed rabbits by a water-soluble antioxidant-rich fraction of Salvia miltiorrhiza. Arterioscler Thromb Vasc Biol. 1998;18:481–486. doi: 10.1161/01.atv.18.3.481. [DOI] [PubMed] [Google Scholar]
- [34].Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- [35].O K, Lynn EG, Vazhappilly R, et al. Magnesium tanshinoate B (MTB) inhibits low density lipoprotein oxidation. Life Sci. 2001;68:903–912. doi: 10.1016/s0024-3205(00)00989-9. [DOI] [PubMed] [Google Scholar]
- [36].Kang DG, Oh H, Sohn EJ, et al. Lithospermic acid B isolated from Salvia miltiorrhiza ameliorates ischemia/reperfusion-induced renal injury in rats. Life Sci. 2004;75:1801–1816. doi: 10.1016/j.lfs.2004.02.034. [DOI] [PubMed] [Google Scholar]
- [37].Yang TL, Lin FY, Chen YH, et al. Salvianolic acid B inhibits low-density lipoprotein oxidation and neointimal hyperplasia in endothelium-denuded hypercholesterolaemic rabbits. J Sci Food Agric. 2011;91:134–141. doi: 10.1002/jsfa.4163. [DOI] [PubMed] [Google Scholar]
- [38].Chen YL, Hu CS, Lin FY, et al. Salvianolic acid B attenuates cyclooxygenase-2 expression in vitro in LPS-treated human aortic smooth muscle cells and in vivo in the apolipoprotein-E-deficient mouse aorta. J Cell Biochem. 2006;98:618–631. doi: 10.1002/jcb.20793. [DOI] [PubMed] [Google Scholar]
- [39].Lin SJ, Lee IT, Chen YH, et al. Salvianolic acid B attenuates MMP-2 and MMP-9 expression in vivo in apolipoprotein-E-deficient mouse aorta and in vitro in LPS-treated human aortic smooth muscle cells. J Cell Biochem. 2007;100:372–384. doi: 10.1002/jcb.21042. [DOI] [PubMed] [Google Scholar]
- [40].Wang QL, Tao YY, Yuan JL, et al. Salvianolic acid B prevents epithelial-to-mesenchymal transition through the TGF-beta1 signal transduction pathway in vivo and in vitro. BMC Cell Biol. 2010;11:31. doi: 10.1186/1471-2121-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang SX, Hu LM, Gao XM, et al. Anti-inflammatory activity of salvianolic acid B in microglia contributes to its neuroprotective effect. Neurochem Res. 2010;35:1029–1037. doi: 10.1007/s11064-010-0151-1. [DOI] [PubMed] [Google Scholar]
- [42].Wang L, Bao Y, Yang Y, et al. Discovery of antagonists for human scavenger receptor CD36 via an ELISA-like high-throughput screening assay. J Biomol Screen. 2010;15:239–250. doi: 10.1177/1087057109359686. [DOI] [PubMed] [Google Scholar]
- [43].Xu Y, Wang J, Bao Y, et al. Identification of two antagonists of the scavenger receptor CD36 using a high-throughput screening model. Anal Biochem. 2010;400:207–212. doi: 10.1016/j.ab.2010.02.003. [DOI] [PubMed] [Google Scholar]
- [44].Bao Y, Yang Y, Wang L, et al. Identification of trichostatin A as a novel transcriptional up-regulator of scavenger receptor BI both in HepG2 and RAW 264.7 cells. Atherosclerosis. 2009;204:127–135. doi: 10.1016/j.atherosclerosis.2008.08.041. [DOI] [PubMed] [Google Scholar]
- [45].Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- [46].Collot-Teixeira S, Martin J, rmott-Roe C, et al. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007;75:468–477. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- [47].Makinen PI, Lappalainen JP, Heinonen SE, et al. Silencing of either SR-A or CD36 reduces atherosclerosis in hyperlipidaemic mice and reveals reciprocal upregulation of these receptors. Cardiovasc Res. 2010;88:530–538. doi: 10.1093/cvr/cvq235. [DOI] [PubMed] [Google Scholar]
- [48].Demers A, Rodrigue-Way A, Tremblay A. Hexarelin Signaling to PPARgamma in Metabolic Diseases. PPAR Res. 2008;2008:364784. doi: 10.1155/2008/364784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhou Z, Liu Y, Miao AD, et al. Salvianolic acid B attenuates plasminogen activator inhibitor type 1 production in TNF-alpha treated human umbilical vein endothelial cells. J Cell Biochem. 2005;96:109–116. doi: 10.1002/jcb.20567. [DOI] [PubMed] [Google Scholar]
- [52].Chen YH, Lin SJ, Ku HH, et al. Salvianolic acid B attenuates VCAM-1 and ICAM-1 expression in TNF-alpha-treated human aortic endothelial cells. J Cell Biochem. 2001;82:512–521. doi: 10.1002/jcb.1176. [DOI] [PubMed] [Google Scholar]
- [53].Jia JY, Lu YL, Li XC, et al. Pharmacokinetics of Depside Salts From Salvia miltiorrhiza in Healthy Chinese Volunteers: A Randomized, Open-Label, Single-Dose Study. Curr Ther Res. 2010;71:260–271. doi: 10.1016/j.curtheres.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]