Abstract
The mechanism underlying estrogen modulation of visceral pain remains unclear. Our previous studies indicate activation of estrogen receptor α (ERα) enhances visceral pain. The purpose of the present study was to investigate the role of estrogen receptor β (ERβ) activation in spinal processing of visceral stimuli. The effects of selective ERβ agonists on the visceromotor response (VMR) and dorsal horn neuronal responses to colorectal distention (CRD) were tested in ovariectomized and intact female rats. The magnitude of the VMR to CRD was significantly attenuated by ERβ agonists diarylpropionitrile (DPN) and WAY200070 four hours after subcutaneous injection. Pretreatment with the estrogen receptor antagonist ICI 182,780 obscured the DPN-evoked attenuation. There was no effect of DPN on the VMR at earlier time points. Subcutaneous and spinal administration of DPN attenuated the response of visceroceptive dorsal horn neurons with a comparable time course. DPN attenuated the VMR in intact rats regardless of estrous cycle stage. The timecourse of effect of ERβ activation on the visceromotor response and neuronal activity is consistent with transcriptional or translational modulation of neuronal activity.
Perspective
Activation of ERβ is antinociceptive in the colorectal distention model of visceral pain, which may provide a therapeutic target to manage IBS in the clinic.
Keywords: visceral pain, gonadal hormone, colorectal distention, ERβ agonist, spinal cord, estrogen receptor, visceromotor response
Introduction
Some painful disorders such as irritable bowel syndrome (IBS), temporomandibular disorder and fibromyalgia are more common in women 6,11,20,21. Gonadal hormone modulation of nociceptive sensitivity is likely a major factor underlying the female prevalence of these disorders. The incidence of IBS dramatically increases post menarche and decreases post menopause, suggesting that high or fluctuating serum levels of estrogen contributes to pain in IBS. Several retrospective studies suggest there is a correlation between the menstrual cycle and IBS symptoms, the increase in symptoms occurring most often during menses or in the perimenstrual period 24,30,49,53,70. Animal studies show that visceral sensitivity of rats fluctuates across the estrous cycle 23,27,60. Accumulating evidence suggests that estrogen is pronociceptive 1,4,5,10,14,31,39,56,57, but antinociceptive actions have also been reported 25,35,36,59. Several possibilities could account for these conflicting results including different functions mediated by different estrogen receptors (ER), ER expression fluctuating with the estrous cycle, or by the change in the ratio of different ERs in the tissue 12,41,48.
Two classical estrogen receptors, ERα and ERβ, have been cloned 34,37. Both are expressed in the peripheral and central nervous systems 15,46,54,61,64,65. We previously reported that selective activation of spinal ERα mimicked the pronociceptive effects of 17β-estradiol (E2) on visceral sensitivity 28. In contrast, selective activation of ERβ attenuated chemical or spinal nerve ligation-induced hyperalgesia and allodynia and chronic inflammatory pain induced by complete Freund’s adjuvant, while not affecting normal pain sensitivity 17,38,55, but the role of ERβ in visceral nociceptive processing is unknown.
The purpose of the present study was to investigate the role of ERβ in visceral nociceptive processing in a model of visceral pain, colorectal distention (CRD). Modulation of visceral sensitivity was determined by changes in the visceromotor response (VMR) and dorsal horn neuronal activity following application of ERβ selective agonists. A potential role for nuclear and/or membrane initiated signaling was determined by examining the timecourse of behavioral and neuronal responses to ERβ activation.
Materials and Methods
Animals
Experimental protocols were approved by the University of Maryland School of Dentistry Institutional Animal Care and Use Committee and adhered to guidelines for experimental pain in animals published by the International Association for the Study of Pain. Female Sprague-Dawley rats weighing 225–250 g were obtained from Harlan. Rats were housed in same sex pairs with free access to food and water at 25 °C with 12 h/12 h alternating light-dark cycle. Rats were anesthetized with isofluorane (2–5%) and ovariectomized by a dorsolateral approach. Rats were treated postoperatively with buprenorphine (0.03 mg/kg, s.c., twice per day) for 2 days. The VMR and neuronal responses were each tested under 2 time courses: 0–2 h and 4–7 h post agonist administration 10–14 days after surgery.
Visceromotor response (VMR)
Electromyogram (EMG) electrodes made from Teflon-coated 32 gauge stainless steel wire (Cooner Wire Company, Chatsworth, CA) were stitched into the ventrolateral abdominal wall at the same time as the ovariectomy surgery. The electrode leads were tunneled subcutaneously and exteriorized at the back of the neck. Rats were individually housed and were fasted for 18–24 h prior to testing. Water was available ad libitum.
On the day of testing, rats were briefly sedated with isofluorane and a 5–6 cm balloon attached to Tygon tubing was inserted into descending colon and rectum through the anus. The secured end of the balloon was at least 1 cm proximal to the external anal sphincter and the tubing was taped to the tail. Rats were loosely restrained in Plexiglas tubes and given 30 min to recover from sedation. The EMG signals were recorded with a CED 1401 plus and analyzed using Spike 2 for windows software (Cambridge Electronic Design, Cambridge, UK). CRD was produced by inflating the distention balloon with air. The pressure was monitored and kept constant by a pressure controller/timing device. For the first experiment at least three graded intensity stimulation trials (20, 40, 60, 80 mmHg CRD, 20 sec duration, 3 min interstimulus interval) were run to establish a stable baseline. Rats were then injected subcutaneously (s.c.) with an ERβ selective agonist (diarylpropionitrile, DPN: 1.5 mg/kg (n = 11), 3.0 mg/kg (n = 18), 5.0 mg/kg (n = 12) or WAY200070: 10 mg/kg (n = 12); Tocris Bioscience, Ellisville, MO, USA) or vehicle (dimethyl sulfoxide, DMSO, 100 μl, n = 10). Rats were returned to their cages and tested again 4 h after injection of agonist or vehicle. To verify the effects of ERβ activation, the nonselective estrogen receptor antagonist ICI- 182,780 (10 nmol, Tocris Bioscience, Ellisville, MO, USA) was intrathecally (i.t.) injected 15 minutes before s.c. administration of 5 mg/kg DPN (n = 11).
In the second experiment, the ability of ERβ activation to affect a rapid membrane-initiated response was examined. Rats were distended to 80 mmHg five times (10 sec duration and 2 min interstimulus interval) to establish the baseline VMR. DPN was injected (3 mg/kg, s.c., n = 12) and the VMR recorded 0, 30, 60, 90 and 120 minutes after injection.
The EMG was analyzed by rectifying the signal. The background activity of the same duration as the distention stimulus was subtracted from the response during the stimulus. Data were plotted as the mean response at each distention pressure or the area under the curve (AUC) for the entire trial.
Electrophysiology
Rats were anesthetized with Nembutal (50 mg/kg, i.p.). Recording procedures were similar to those previously described 26,28 . Briefly, the left jugular vein was catheterized for continuous infusion of Nembutal (5–10 mg/kg/hr). The left carotid artery was catheterized to monitor arterial blood pressure and bolus administration of pancuronium bromide (0.2 mg/kg/h). A tracheal cannula was inserted for artificial ventilation. End-tidal CO2 was maintained at 3.5–4.5%. Body temperature was maintained with a water-jacket heating pad and overhead lamp. The rat was placed in a head holder, suspended with thoracic vertebral and ischial clamps, and the lumbosacral (L6-S2; LS) spinal cord segments exposed by laminectomy. The dura matter was cut and the spinal cord was bathed in warm paraffin oil. The distention balloon was placed into the colon.
Tungsten microelectrodes (1-2 MΩ; Micro probe, Potomac, MD) were used for extracellular single-unit recording in the LS spinal segments (0–1.5 mm lateral to midline, 500–1300 μm ventral to spinal cord dorsum). Signals were amplified (model 1800 AC amplifier; A-M systems, Carlsborg, WA) and passed through a window discriminator (DDIS-1; BAK Electronics, Germantown, MD) to isolate a single unit. Data were collected with a CED micro 1401 and Spike 2 for Windows software for analysis.
Neurons with excitatory responses to CRD were classified as Abrupt or Sustained on the basis of their response to 80 mmHg CRD. Abrupt neuron activity increased at stimulus onset and ceased within 2 sec of stimulus cessation, activity dropping below the mean plus 2 standard deviations of the background activity. Sustained neurons had an afterdischarge that persisted longer than 4 sec, response terminating when it dropped below the mean plus 2 standard deviations of the background activity for 2 sec.
In the first experiment two graded intensity distention trials (20, 40, 60 and 80 mmHg, 20 sec duration, 3 min interstimulus interval) were run to test the response of dorsal horn neurons between 4 and 7 h after DPN s.c. injection (3 mg/kg, n = 22 for Abrupt neurons and n = 14 for Sustained neurons) or between 3 and 7 h after DPN applied directly to the surface of spinal cord (25 μl, 0.5 μg, n = 5 for Abrupt neurons and n = 6 for Sustained neurons). This protocol was designed as a population study in which the magnitude of response of neurons to graded intensities of CRD was compared between vehicle and DPN experimental groups.
In the second experiment, after identifying a neuron, rats were distended to 80 mmHg 5 times (10 sec duration, 2 min interstimulus interval) to establish the baseline response. DPN (3 mg/kg, 100 μl, n = 18 for Abrupt neurons and n = 17 for Sustained neurons) or vehicle (DMSO, n = 13 for Abrupt neurons and n = 11 for Sustained neurons) was injected s.c. and the response to CRD was recorded at 0, 30, 60, 90 and 120 min after injection. Only one neuron was studied per rat and the response post DPN/vehicle was compared to the baseline response.
Abrupt unit activity was quantified as the mean discharge frequency during the distention minus the mean background spontaneous activity in the preceding 10 or 20 sec. The response of Sustained neurons was quantified as the mean response starting from CRD onset until the afterdischarge ceased minus the mean background spontaneous activity (20 sec).
VMR and neuronal data are expressed as mean ± SEM. Data were analyzed by one or two way ANOVA as appropriate. p < 0.05 was considered significant.
Results
The visceromotor response to CRD
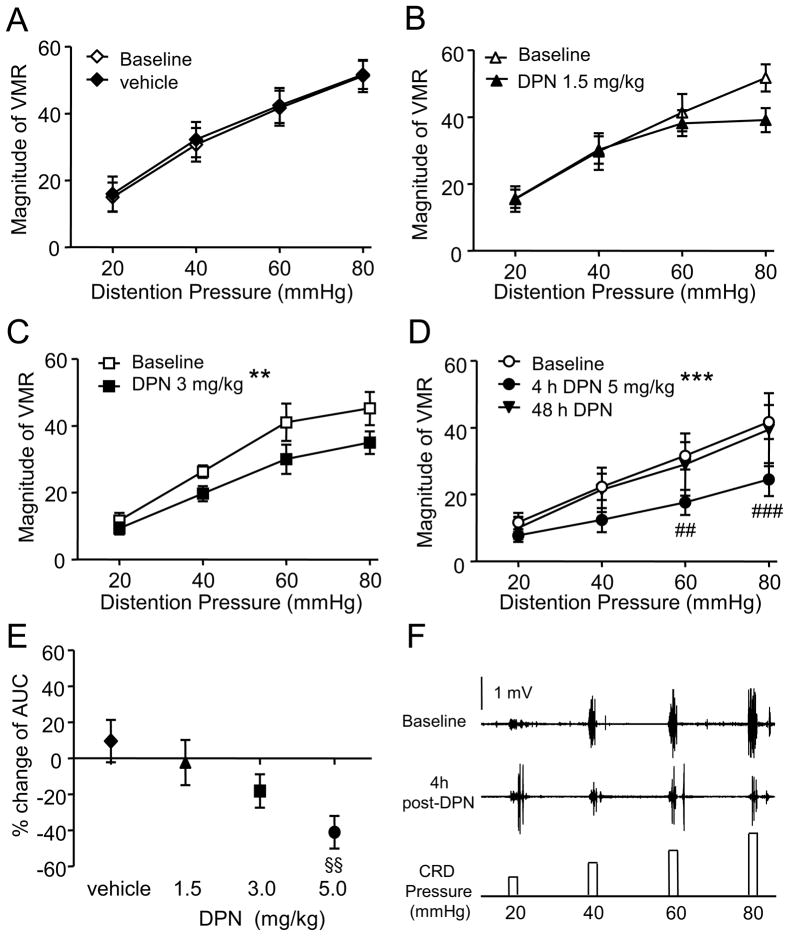
The VMR was recorded in 96 awake ovariectomized rats. In the first experiment the effect of DPN was examined starting 4 h after s.c. injection. Vehicle had no effect on the magnitude of the VMR (two way RM ANOVA, p = 0.998; Figure 1A). There was no difference in the overall magnitude of the VMR to CRD 4 h after injection of 1.5 mg/kg DPN (two way RM ANOVA, p = 0.088). Increasing the concentration of DPN significantly attenuated the VMR compared to baseline (two way RM ANOVA: 3 mg/kg, p = 0.002; 5 mg/kg, p < 0.0001; Figure 1C, D). Summing the graded responses (area under the curve, AUC) and comparing to baseline revealed a dose-dependent attenuation by DPN (one way ANOVA, p = 0.017; Figure 1E). Seven of the 12 rats tested with 5 mg/kg DPN were tested again at 48 h post DPN. At this later time point, the magnitude of the VMR returned to the level at baseline (two way RM ANOVA, p > 0.05; Figure 1D).
Figure 1.
The VMR to graded intensities colorectal distention are inhibited by ERβ activation. A-D: The VMR before and after subcutaneous injection of vehicle (A, n = 10) or DPN (B: 1.5 mg/kg, n = 11; C: 3 mg/kg, n = 18; D: 5 mg/kg, n = 12). ** p < 0.01, *** p < 0.001 compared with baseline. ## p < 0.01, ### p < 0.001 compared with baseline for same pressure. E, The percent change from baseline in the area under the curve (AUC) to graded intensities of colorectal distention for different doses of DPN. §§ p < 0.01 compared with the vehicle group. F, Original electromyogram recording shows the VMR in a rat was inhibited by 3 mg/kg DPN.
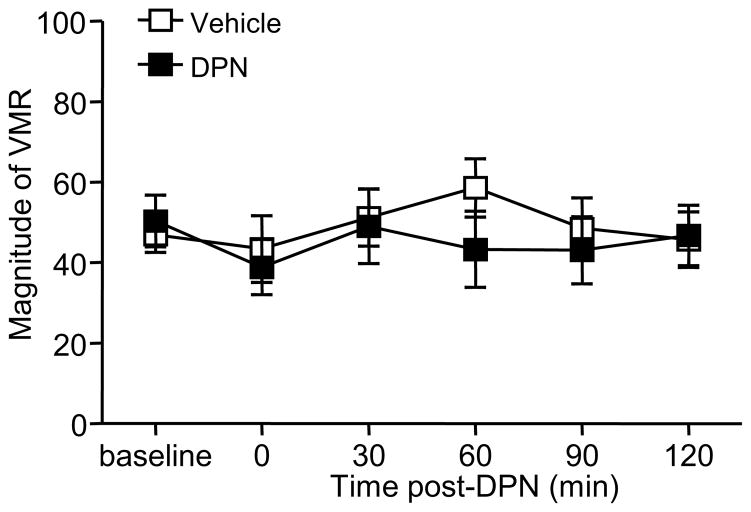
We previously reported that selective activation of ERα facilitated the VMR as soon as 15 minutes after agonist administration 28. To determine if ERβ activation affected the VMR through a potential membrane-initiated signaling mechanism, the VMR to 80 mmHg was recorded before and immediately following DPN injection through 2 h. There was no change in the VMR following either vehicle or 3 mg/kg DPN (one way RM ANOVA, p > 0.05; Figure 2).
Figure 2.
ERβ activation does not inhibit the VMR by membrane-initiated rapid signaling. The magnitude of the VMR remained constant when measured every 30 minutes for 2 h following injection of 3 mg/kg DPN (n=12) or vehicle (n=10).
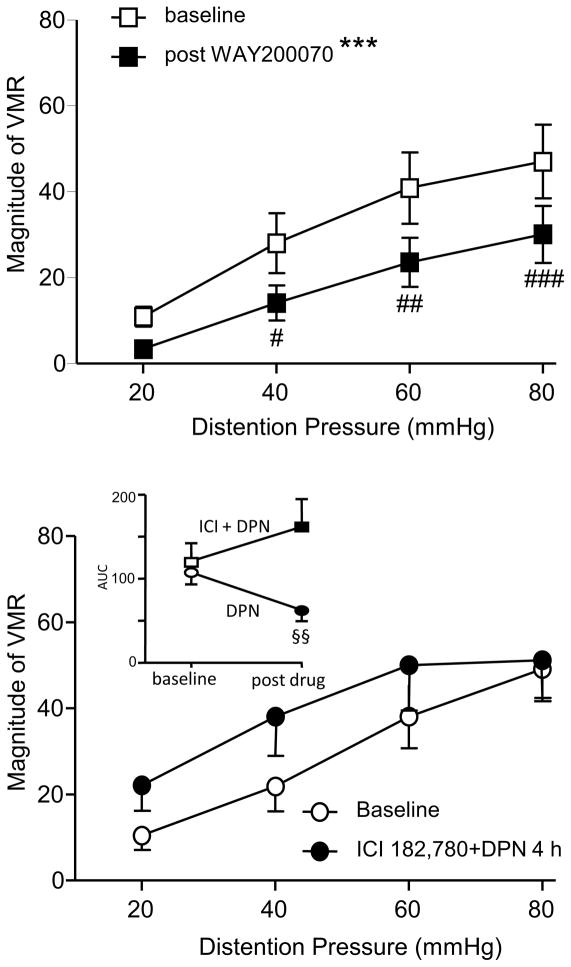
The inhibitory effect of ERβ activation on the VMR was confirmed two ways. First, a second ERβ agonist, WAY200070 (10 mg/kg), attenuated the VMR (two way RM ANOVA, p < 0.0001; Figure 3A). Second, a fifteen minute pretreatment with the estrogen receptor antagonist ICI 182,178 (10 nmol, i.t.) obscured the effects of 5 mg/kg DPN when tested 4 h later (two way RM ANOVA, p > 0.05; Figure 3B). There was no significant change in the total AUC of four graded intensities CRD at 4 h post ICI 182,178 plus DPN compared with baseline (paired t-test, p = 0.322), whereas 5 mg/kg DPN significantly attenuated the AUC (paired t-test, p = 0.003; Figure 3B inset).
Figure 3.
Confirmation of ERβ inhibition of visceral pain. A: The magnitude of the VMR to graded intensities colorectal distention before and 4 h after s.c. injection of the ERβ agonist WAY200070 (10 mg/kg, n = 12). *** p < 0.001 compared with the baseline. # p < 0.05, ## p < 0.01, ### p < 0.001 compared with baseline for same pressure. B: Fifteen minutes pretreatment with the estrogen receptor antagonist ICI 182,780 (10 nmol) obscured the inhibitory effect of 5 mg/kg DPN (n=11) at 4 h. The inset shows the AUC of four graded intensities distention at baseline and 4 h after 5 mg/kg DPN with or without ICI 182,780. §§ p < 0.01 compared with baseline in same group. For clarification, error bars are only shown in one direction in panel B.
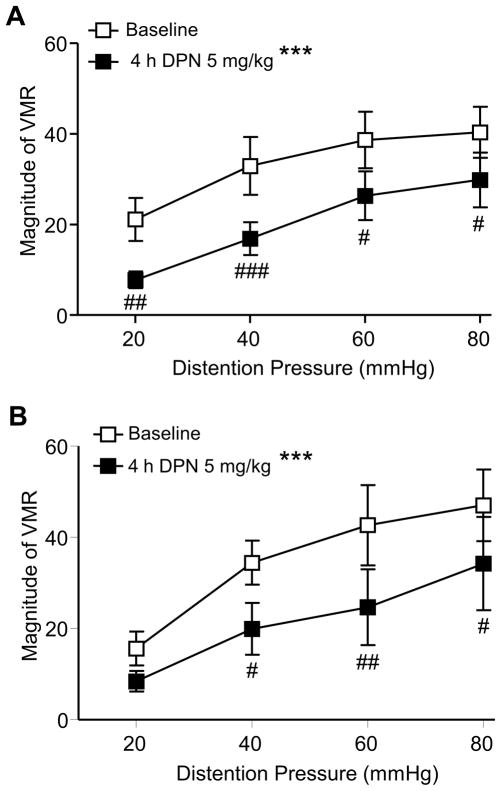
Estradiol facilitates the response to CRD in ovariectomized rats which is mimicked by selective activation of ERα 26,28. In addition, the magnitude of the VMR is greater when serum estradiol is high during proestrus 23,27,60. To determine if exogenous ERβ would obscure the pronociceptive effect of estrogen, DPN (5 mg/kg, s.c.) was tested in intact cycling rats. Based on vaginal smears taken daily for 2 weeks and the estrous phase on the day of testing, rats were divided into 2 groups, proestrus (n = 5) and non-proestrus (n = 15: 3 were in estrus and 12 were in diestrus). Four hours following DPN administration the VMR was significantly attenuated compared to baseline in both proestrus and non-proestrus rats (two way RM ANOVA, p < 0.0001 for both groups; Figure 4). In addition, the percent decrease in the VMR for the proestrus, non-proestrus and OVx rats treated with 5 mg/kg DPN was the same (-42±13, -37±6 and −41±9, respectively).
Figure 4.
Exogenous DPN inhibited the VMR in intact rats. The magnitude of the VMR to graded intensities colorectal distention before and 4 h after s.c. injection of ERβ agonist DPN (5 mg/kg) in non-proestrus rats (A, n = 15) and proestrus (B, n = 5) rats. *** p < 0.001 compared with baseline; # p < 0.05, ## p < 0.01, ### p < 0.001 compared with baseline for same pressure.
Response of spinal dorsal horn neurons to CRD
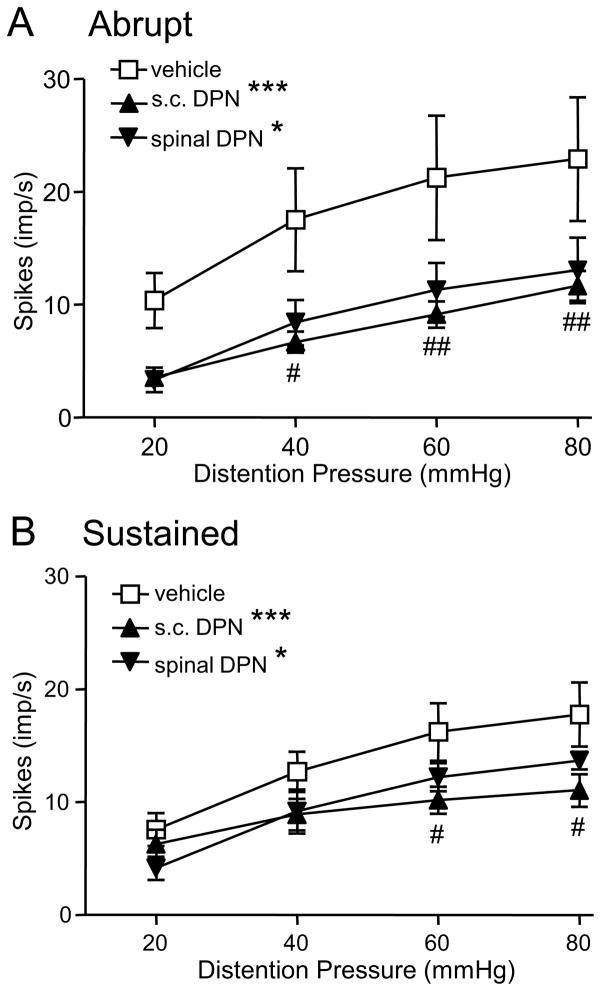
To test the hypothesis that ERβ acts at the level of the spinal cord to inhibit colorectal sensitivity to noxious and innocuous stimuli, the response of dorsal horn neurons to graded intensities of CRD was examined. Similar to the VMR study, the effects of DPN were examined between 4 and 7 h after injection and within the first 2 h. Four hours following injection there was no difference in the response of Abrupt neurons to s.c. injection of 1.5 and 3 mg/kg DPN (p = 0.949), therefore the data were pooled as the s.c. DPN group. Compared with vehicle, both s.c. DPN (two way ANOVA, p < 0.0001) and spinal administration of DPN (p < 0.02) significantly decreased the response of Abrupt neurons to CRD (Figure 5A). There was no difference in the response of Abrupt neurons between s.c. DPN and spinal application of DPN (two way ANOVA, p = 0.551), suggesting that the effects of s.c. DPN are mediated, at least partially, through modulation of dorsal horn neuron activity.
Figure 5.
The response of dorsal horn neurons to graded intensities of colorectal distention 4-7 h after subcutaneous injection of vehicle (n = 12 for Abrupt and n = 11 for Sustained) or DPN (3 mg/kg, n = 22 for Abrupt and n = 14 for Sustained), and after spinal application of DPN (n = 5 for Abrupt and n = 6 for Sustained). A, Abrupt neurons. B, Sustained neurons. * p < 0.05, *** p < 0.001 compared with the vehicle group. # p < 0.05, ## p < 0.01, ### p < 0.001 compared with the vehicle group for same pressure.
Similar to Abrupt neurons, the response of Sustained neurons to both s.c. DPN (pooled 1.5 and 3 mg/kg; two way ANOVA, p < 0.0006) and spinal administration (p < 0.05) of DPN was significantly lower than the vehicle group (Figure 5B). The responses of Sustained neurons to s.c. DPN and spinal DPN were similar (p = 0.739). In addition, there was no significant difference in background spontaneous discharges among different groups either in the response of Abrupt neurons (two way ANOVA, p = 0.462) or Sustained neurons (p = 0.069), indicating the inhibitory effects of ERβ activation on visceral pain are the result of evoked activity of spinal neurons and not due to differences in spontaneous activity.
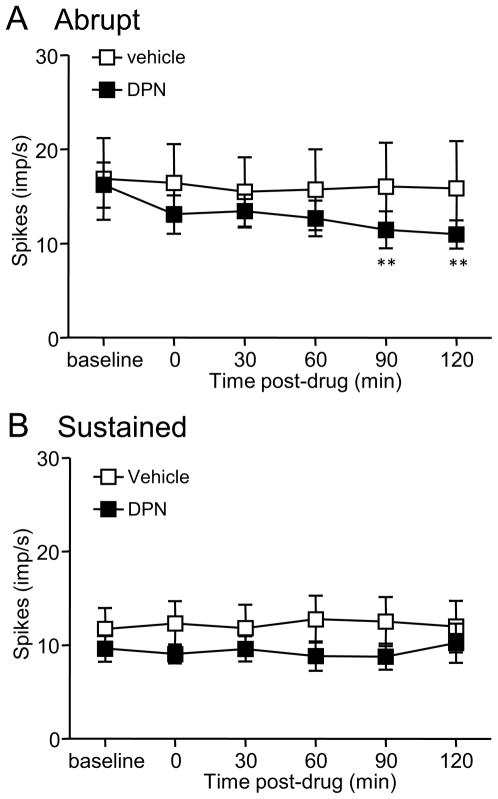
To investigate the time course of ERβ activation on CRD-evoked dorsal horn neuron activity, the response of dorsal horn neurons was recorded before and for 2 h immediately following DPN injection. S.c. injection of 3 mg/kg DPN inhibited the responses of Abrupt neurons starting 90 min after injection of DPN compared to baseline (one way ANOVA, p = 0.005; Figure 6A). Vehicle had no effect (p = 0.980). In contrast, neither DPN nor vehicle (one way ANOVA, p > 0.05) had any effect on the response of Sustained neurons within 2 h of administration (Figure 6B). To compare the background spontaneous activities before and after administration of DPN, there was no significant difference between pre-DPN and different time points post-DPN either in Abrupt neurons (one way ANOVA, p = 0.986) or Sustained neurons (one way ANOVA, p = 0.658). Vehicle had no effect on the background spontaneous activities also (one way ANOVA, p = 0.946 for Abrupt neurons and p = 0.981 for Sustained neurons).
Figure 6.
The response of dorsal horn neurons to constant pressure (80 mmHg) colorectal distention over the first 2 h following injection of 3 mg/kg DPN (n = 18 for Abrupt; n = 17 for Sustained) or vehicle (n = 13 for Abrupt; n = 11 for Sustained). A, Abrupt neurons. B, Sustained neurons. ** p < 0.01 compared with baseline.
Discussion
The present study reports s.c. injection of the ERβ selective agonist DPN attenuated the VMR to CRD 4 h after injection, but not at earlier time points. This visceral antinociception by ERβ activation was confirmed using a second agonist, WAY200070. In addition, excessive activation of ERβ in intact rats inhibited the VMR, further supporting the antinociceptive function of ERβ. The electrophysiology studies provide further confirmation and suggest this effect occurs at the level of the spinal cord. Finally, that antinociception was not apparent till 4 h following ERβ activation suggests a rapid membrane-initiated signaling mechanism is not involved.
Activation of ERβ inhibits visceral sensitivity
The quantifiable VMR to CRD is a sensitive pseudoaffective measure of visceral nociception in awake animals and is correlated to human psychophysical studies employing CRD 51. The decrease in the magnitude of the VMR by activation of ERβ in the present study is consistent with the inhibitory effect of ERβ agonists on neuropathic and inflammatory pain 17,38,55. The ERβ agonist ERb-131 alleviated tactile hyperalgesia induced by capsaicin, reversed tactile allodynia caused by spinal nerve ligation, and inhibited hyperalgesia induced by sulprostone, phenylephrine and NMDA 55. Another ERβ agonist, ERB-041 significantly blocked PGE2 and capsaicin-induced thermal hyperalgesia and reversed thermal hyperalgesia in a carrageenan-induced acute inflammation model 38. ERB-041 reversed the chronic diarrhea in HLA-B27 transgenic rats, improved histological disease scores in the colon and inhibited the expression of the majority of genes and proteins in the spleen, lymph nodes and liver altered in adjuvant-induced arthritis 16,19. These data along with the present results suggest that ERβ activation is antinociceptive or has a protective function. Because ERB-041 has poor blood-brain barrier permeability, the effects of ERB-041 are likely mediated by peripheral ERβ. In contrast, DPN and WAY200070 readily penetrate the blood-brain barrier 40,69 and ERβ is present in many regions of the brain and spinal cord related to pain transmission and modulation 15,54,61,65. Our electrophysiological data support the postulation that ERβ in the central nervous system may play an important role in visceral nociceptive processing.
In contrast to these apparent antinociceptive effects, ERβ appears pronociceptive in the formalin test. The interphase between phases 1 and 2 of the formalin test is associated with inhibitory mechanisms in the spinal cord 18,22. This interphase appears to be activated by ERβ since licking behavior was decreased during the interphase in ERβ knockout mice and increased in ovariectomized mice injected with DPN 12. Interestingly, ERβ modulation of the formalin response only occurred in females, there was no effect in males. In contrast, ERα activation in the formalin model was antinociceptive 12 while it was pronociceptive in the CRD model of visceral pain 28. The inconsistent results between the formalin study and studies in which ERβ activation is antinociceptive may be due to the differences in species, pain model, drug dosing, test time points or anti-inflammatory effects of estrogens.
Potential mechanisms underlying ERβ-mediated inhibition of visceral sensitivity
Estrogen receptors can modulate neuronal activity by several mechanisms. Classical estrogen receptor activity involves dimerization, nuclear translocation, binding to an estrogen response element and subsequent modulation of transcription, a process that takes a minimum of several hours 44,45. Estrogen receptors are also involved in membrane-initiated rapid signaling whereby membrane-bound estrogen receptors activate second messenger pathways to modulate receptor and ion channel activity. This process could be activated within seconds to affect synaptic transmission 9,33,43,47,58,63. Lastly, estrogen receptors could be activated in the plasma membrane or in the cytoplasm to activate second messenger mechanisms that indirectly modulate transcription, but also could modulate translation and trafficking, a process that could occur over minutes to hours 7,32,66.
In the present study the electrophysiology data are consistent with the behavioral data following activation of ERβ, providing a mechanism for spinal ERβ modulation of visceral sensitivity. Abrupt and Sustained neurons are the two most prominent phenotypes of dorsal horn neurons that are excited by CRD 26,29,50,52. ERβ is expressed in dorsal horn neurons 15,46,54,61, therefore the inhibition of visceral sensitivity by ERβ activation may be explained, at least partly, by the decrease of dorsal horn neuron activity.
Our previous study showed that selective activation of ERα facilitated the visceromotor response within 15 minutes, suggesting membrane-initiated rapid signaling was activated 28. However, in the present study inhibition of the VMR was not observed earlier than 4 h following ERβ activation, suggesting that membrane-initiated rapid signaling does not contribute to ERβ modulation of visceral sensitivity, at least through direct modulation of receptors or ion channels. However, the timing of the neuronal and behavioral modulation is not inconsistent with direct or indirect transcriptional modulation or post-translational modification 7,32,66.
Although the present data suggest a spinal location for the effects of ERβ, they do not differentiate between effects on spinal dorsal horn neurons and primary afferent terminals. ERα is not expressed in colonic afferents in intact female rats and ovariectomy had no effects on the response of colonic afferents to CRD 28. There are no specific data for ERβ in visceral pain, although ERβ is expressed in dorsal horn neurons and unlabeled DRG cells 15,46,54,61,64,65. Indeed, prolonged exposure of DRG neurons to 17β-estradiol reduces the TRPV1 response to capsaicin, which is mediated by intracellular ERβ, but the response is not affected by short exposure (within 1 h) to estradiol, suggesting a rapid signaling pathway is not involved in this model 71.
Alternatively, it cannot be concluded that the Abrupt and Sustained neurons in the present study express ERβ and the possibility exists that ERβ activation increases activity in inhibitory interneurons. Estradiol increased enkephalin in the spinal cord with a time course consistent with the present results 2. This increase in enkephalin was partially colocalized to neurons expressing ERα, but ERβ was never examined 3. Furthermore, activation of ERβ increases key synaptic proteins including the presynaptic marker synaptophysin, postsynaptic scaffold protein PSD-95 and the AMPA receptor subunit GluR1 in the hippocampus, and regulates GluR1 trafficking and phosphorylation 40. An increase in excitability of inhibitory interneurons could decrease activity in Abrupt and Sustained neurons leading to visceral antinociception.
Indeed, a recent study reported that activation of ERβ elevates tph2 mRNA expression in the dorsal raphe nuclei. Tryptophan hydroxylase-2 (TPH2) is a rate-limiting enzyme for 5-HT synthesis in the brain, suggesting ERβ may increase 5-HT synthesis in the dorsal raphe nuclei 13 enhancing the serotonergic (5-HT) descending inhibitory pathway. Anatomical studies show that a majority of ERβ expressing cortical neurons double label for GABAergic-associated calcium-binding protein parvalbumin, suggesting activation of ERβ can regulate neuronal excitability in brain through modulating inhibitory neurons 8. Although the inhibition by ERβ was confirmed using behavioral and electrophysiological methods in the present study, the anatomical evidence is currently difficult to obtain because the absence of a specific ERβ antibody 62 prevents colocalization of ERβ with other transmitter receptors to further investigate the underlying mechanisms of ERβ function in the nervous system.
Conclusion
IBS is a common functional gastrointestinal disorder and better therapeutic agents for IBS treatment are needed. The results of the present study showing ERβ’s antinociceptive effects on visceral pain may provide a therapeutic target to manage IBS in the clinic. Previous studies showing ERβ has anxiolytic and anti-depressive action 42,67-69 is also beneficial to IBS patients because stress may trigger or exacerbate IBS.
Acknowledgments
The authors wish to thank Ms. Sangeeta Pandya for technical assistance and Mr. Michael Keaser for setting up the program to analyze data.
Footnotes
Disclosures: This work was supported by National Institutes of Health (NIH) grant R01 NS 37424 to RJT. The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Allen AL, McCarson KE. Estrogen increases nociception-evoked brain-derived neurotrophic factor gene expression in the female rat. Neuroendocrinology. 2005;81:193–199. doi: 10.1159/000087002. [DOI] [PubMed] [Google Scholar]
- 2.Amandusson A, Hallbeck M, Hallbeck AL, Hermanson O, Blomqvist A. Estrogen-induced alterations of spinal cord enkephalin gene expression. Pain. 1999;83:243–248. doi: 10.1016/s0304-3959(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 3.Amandusson A, Hermanson O, Blomqvist A. Colocalization of oestrogen receptor immunoreactivity and preproenkephalin mRNA expression to neurons in the superficial laminae of the spinal and medullary dorsal horn of rats. Eur J Neurosci. 1996;8:2440–2445. doi: 10.1111/j.1460-9568.1996.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 4.Bereiter DA. Sex differences in brainstem neural activation after injury to the TMJ region. Cells Tissues Organs. 2001;169:226–237. doi: 10.1159/000047886. [DOI] [PubMed] [Google Scholar]
- 5.Bereiter DA, Okamoto K, Bereiter DF. Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil-induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain. 2005;117:58–67. doi: 10.1016/j.pain.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 7.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 8.Blurton-Jones M, Tuszynski MH. Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J Comp Neurol. 2002;452:276–287. doi: 10.1002/cne.10393. [DOI] [PubMed] [Google Scholar]
- 9.Boulware MI, Mermelstein PG. Membrane estrogen receptors activate metabotropic glutamate receptors to influence nervous system physiology. Steroids. 2009;74:608–613. doi: 10.1016/j.steroids.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradshaw HB, Berkley KJ. Estrous changes in responses of rat gracile nucleus neurons to stimulation of skin and pelvic viscera. J Neurosci. 2000;20:7722–7727. doi: 10.1523/JNEUROSCI.20-20-07722.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- 12.Coulombe MA, Spooner MF, Gaumond I, Carrier JC, Marchand S. Estrogen receptors beta and alpha have specific pro- and anti-nociceptive actions. Neuroscience. 2011;184:172–182. doi: 10.1016/j.neuroscience.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 13.Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evrard HC. Estrogen synthesis in the spinal dorsal horn: a new central mechanism for the hormonal regulation of pain. Am J Physiol Regul Integr Comp Physiol. 2006;291:R291–R299. doi: 10.1152/ajpregu.00930.2005. [DOI] [PubMed] [Google Scholar]
- 15.Fan X, Kim HJ, Warner M, Gustafsson JA. Estrogen receptor beta is essential for sprouting of nociceptive primary afferents and for morphogenesis and maintenance of the dorsal horn interneurons. Proc Natl Acad Sci U S A. 2007;104:13696–13701. doi: 10.1073/pnas.0705936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Follettie MT, Pinard M, Keith JC, Jr, Wang L, Chelsky D, Hayward C, Kearney P, Thibault P, Paramithiotis E, Dorner AJ, Harris HA. Organ messenger ribonucleic acid and plasma proteome changes in the adjuvant-induced arthritis model: responses to disease induction and therapy with the estrogen receptor-beta selective agonist ERB–041. Endocrinology. 2006;147:714–723. doi: 10.1210/en.2005-0600. [DOI] [PubMed] [Google Scholar]
- 17.Gardell LR, Hyldtoft L, Del Tredici AL, Andersen CB, Fairbairn LC, Lund BW, Gustafsson M, Brann MR, Olsson R, Piu F. Differential modulation of inflammatory pain by a selective estrogen receptor beta agonist. Eur J Pharmacol. 2008;592:158–159. doi: 10.1016/j.ejphar.2008.06.107. [DOI] [PubMed] [Google Scholar]
- 18.Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958:139–145. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- 19.Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, Keith JC., Jr Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 20.Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs. 2003;5:56–65. doi: 10.1177/1099800403005001006. [DOI] [PubMed] [Google Scholar]
- 21.Heitkemper MM, Jarrett M. Gender differences and hormonal modulation in visceral pain. Curr Pain Headache Rep. 2001;5:35–43. doi: 10.1007/s11916-001-0008-z. [DOI] [PubMed] [Google Scholar]
- 22.Henry JL, Yashpal K, Pitcher GM, Coderre TJ. Physiological evidence that the 'interphase' in the formalin test is due to active inhibition. Pain. 1999;82:57–63. doi: 10.1016/S0304-3959(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 23.Holdcroft A, Sapsed-Byrne S, Ma D, Hammal D, Forsling ML. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distention in male and female rats anesthetized with halothane. Br J Anaesth. 2000;85:907–910. doi: 10.1093/bja/85.6.907. [DOI] [PubMed] [Google Scholar]
- 24.Houghton LA, Lea R, Jackson N, Whorwell PJ. The menstrual cycle affects rectal sensitivity in patients with irritable bowel syndrome but not healthy volunteers. Gut. 2002;50:471–474. doi: 10.1136/gut.50.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter DA, Barr GA, Amador N, Shivers KY, Kemen L, Kreiter CM, Jenab S, Inturrisi CE, Quinones-Jenab V. Estradiol-induced antinociceptive responses on formalin-induced nociception are independent of COX and HPA activation. Synapse. 2011;65:643–651. doi: 10.1002/syn.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji Y, Murphy AZ, Traub RJ. Estrogen Modulates the Visceromotor Reflex and Responses of Spinal Dorsal Horn Neurons to Colorectal Stimulation in the Rat. J Neurosci. 2003;23:3908–3915. doi: 10.1523/JNEUROSCI.23-09-03908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji Y, Tang B, Traub RJ. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain. 2011:1182–1191. doi: 10.1016/j.pain.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji Y, Traub RJ. Differential effects of spinal CNQX on two populations of dorsal horn neurons responding to colorectal distention in the rat. Pain. 2002;99:217–222. doi: 10.1016/s0304-3959(02)00106-9. [DOI] [PubMed] [Google Scholar]
- 30.Kane SV, Sable K, Hanauer SB. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. Am J Gastroenterol. 1998;93:1867–1872. doi: 10.1111/j.1572-0241.1998.540_i.x. [DOI] [PubMed] [Google Scholar]
- 31.Kayser V, Berkley KJ, Keita H, Gautron M, Guilbaud G. Estrous and sex variations in vocalization thresholds to hindpaw and tail pressure stimulation in the rat. Brain Res. 1996;742:352–354. doi: 10.1016/s0006-8993(96)01108-0. [DOI] [PubMed] [Google Scholar]
- 32.Kelly MJ, Levin ER. Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol Metab. 2001;12:152–156. doi: 10.1016/s1043-2760(01)00377-0. [DOI] [PubMed] [Google Scholar]
- 33.Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Molecular and Cellular Endocrinology. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koike S, Sakai M, Muramatsu M. Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic Acids Res. 1987;15:2499–2513. doi: 10.1093/nar/15.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer PR, Bellinger LL. The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology. 2009;150:3680–3689. doi: 10.1210/en.2008-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuba T, Wu HB, Nazarian A, Festa ED, Barr GA, Jenab S, Inturrisi CE, Quinones-Jenab V. Estradiol and progesterone differentially regulate formalin-induced nociception in ovariectomized female rats. Horm Behav. 2006;49:441–449. doi: 10.1016/j.yhbeh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leventhal L, Brandt MR, Cummons TA, Piesla MJ, Rogers KE, Harris HA. An estrogen receptor-beta agonist is active in models of inflammatory and chemical-induced pain. Eur J Pharmacol. 2006;553:146–148. doi: 10.1016/j.ejphar.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 39.Liu B, Eisenach JC, Tong C. Chronic estrogen sensitizes a subset of mechanosensitive afferents innervating the uterine cervix. J Neurophysiol. 2005;93:2167–2173. doi: 10.1152/jn.01012.2004. [DOI] [PubMed] [Google Scholar]
- 40.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 41.Lu YP, Zeng M, Swaab DF, Ravid R, Zhou JN. Colocalization and alteration of estrogen receptor-alpha and -beta in the hippocampus in Alzheimer's disease. Hum Pathol. 2004;35:275–280. doi: 10.1016/j.humpath.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 43.Malyala A, Kelly MJ, Ronnekleiv OK. Estrogen modulation of hypothalamic neurons: activation of multiple signaling pathways and gene expression changes. Steroids. 2005;70:397–406. doi: 10.1016/j.steroids.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7:497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 46.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 47.Mhyre AJ, Dorsa DM. Estrogen activates rapid signaling in the brain: Role of estrogen receptor [alpha] and estrogen receptor [beta] in neurons and glia. Neuroscience. 2006;138:851–858. doi: 10.1016/j.neuroscience.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore J, Barlow D, Jewell D, Kennedy S. Do gastrointestinal symptoms vary with the menstrual cycle? Br J Obstet Gynaecol. 1998;105:1322–1325. doi: 10.1111/j.1471-0528.1998.tb10014.x. [DOI] [PubMed] [Google Scholar]
- 50.Ness TJ, Gebhart GF. Characterization of superficial T13-L2 dorsal horn neurons encoding for colorectal distention in the rat: comparison with neurons in deep laminae. Brain Res. 1989;486:301–309. doi: 10.1016/0006-8993(89)90516-7. [DOI] [PubMed] [Google Scholar]
- 51.Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distention as a noxious visceral stimulus. Pain. 1990;43:377–386. doi: 10.1016/0304-3959(90)90035-C. [DOI] [PubMed] [Google Scholar]
- 52.Olivar T, Cervero F, Laird JM. Responses of rat spinal neurones to natural and electrical stimulation of colonic afferents: effect of inflammation. Brain Res. 2000;866:168–177. doi: 10.1016/s0006-8993(00)02274-5. [DOI] [PubMed] [Google Scholar]
- 53.Palomba S, Orio F, Jr, Manguso F, Russo T, Falbo A, Lombardi G, Doldo P, Zullo F. Leuprolide acetate treatment with and without coadministration of tibolone in premenopausal women with menstrual cycle-related irritable bowel syndrome. Fertil Steril. 2005;83:1012–1020. doi: 10.1016/j.fertnstert.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304:193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- 55.Piu F, Cheevers C, Hyldtoft L, Gardell LR, Del Tredici AL, Andersen CB, Fairbairn LC, Lund BW, Gustafsson M, Schiffer HH, Donello JE, Olsson R, Gil DW, Brann MR. Broad modulation of neuropathic pain states by a selective estrogen receptor beta agonist. Eur J Pharmacol. 2008;590:423–429. doi: 10.1016/j.ejphar.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Robbins A, Berkley KJ, Sato Y. Estrous cycle variation of afferent fibers supplying reproductive organs in the female rat. Brain Res. 1992;596:353–356. doi: 10.1016/0006-8993(92)91572-v. [DOI] [PubMed] [Google Scholar]
- 57.Robbins MT, Mebane H, Ball CL, Shaffer AD, Ness TJ. Effect of estrogen on bladder nociception in rats. J Urol. 2010;183:1201–1205. doi: 10.1016/j.juro.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front Biosci. 2011;16:1560–1573. doi: 10.2741/3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanoja R, Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: A model of functional abdominal pain. Pain. 2005;118:243–253. doi: 10.1016/j.pain.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- 61.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 62.Snyder MA, Smejkalova T, Forlano PM, Woolley CS. Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. J Neurosci Methods. 2010;188:226–234. doi: 10.1016/j.jneumeth.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srivastava DP, Waters EM, Mermelstein PG, Kram+ír EA, Shors TJ, Liu F. Rapid Estrogen Signaling in the Brain: Implications for the Fine-Tuning of Neuronal Circuitry. The Journal of Neuroscience. 2011;31:16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J Neurosci Res. 1999;57:603–615. [PubMed] [Google Scholar]
- 65.VanderHorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- 66.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 68.Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 69.Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whitehead WE, Cheskin LJ, Heller BR, Robinson JC, Crowell MD, Benjamin C, Schuster MM. Evidence for exacerbation of irritable bowel syndrome during menses. Gastroenterology. 1990;98:1485–1489. doi: 10.1016/0016-5085(90)91079-l. [DOI] [PubMed] [Google Scholar]
- 71.Xu S, Cheng Y, Keast JR, Osborne PB. 17beta-estradiol activates estrogen receptor beta-signalling and inhibits transient receptor potential vanilloid receptor 1 activation by capsaicin in adult rat nociceptor neurons. Endocrinology. 2008;149:5540–5548. doi: 10.1210/en.2008-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]