Abstract
Brain-derived neurotrophic factor (BDNF) is the most studied neurotrophin involved in synaptic plasticity processes that are required for long-term learning and memory. Specifically, BDNF gene expression and activation of its high-affinity TrkB receptor are necessary in the amygdala, hippocampus and prefrontal cortex for the formation of emotional memories, including fear memories. Among the psychiatric disorders with altered fear processing there is Post-traumatic Stress Disorder (PTSD) which is characterized by an inability to extinguish fear memories. Since BDNF appears to enhance extinction of fear, targeting impaired extinction in anxiety disorders such as PTSD via BDNF signalling may be an important and novel way to enhance treatment efficacy. The aim of this review is to provide a translational point of view that stems from findings in the BDNF regulation of synaptic plasticity and fear extinction. In addition, there are different systems that seem to alter fear extinction through BDNF modulation like the endocannabionoid system and the hypothalamic-pituitary adrenal axis (HPA). Recent work also finds that the pituitary adenylate cyclase-activating polypeptide (PACAP) and PAC1 receptor, which are upstream of BDNF activation, may be implicated in PTSD. Especially interesting are data that exogenous fear extinction enhancers such as antidepressants, histone deacetylases inhibitors (HDACi) and D-cycloserine, a partial NMDA agonist, may act through or in concert with the BDNF-TrkB system. Finally, we review studies where recombinant BDNF and a putative TrkB agonist, 7,8-DHF, may enhance extinction of fear. These approaches may lead to novel agents that improve extinction in animal models and eventually humans.
Keywords: Neurotrophic factor, TrkB, anxiety, depression, fear, PTSD, brain derived neurotrophic factor
1. General Introduction
1.1. BDNF, synaptic plasticity and long-term potentiation
Neurotrophins are a group of proteins that support different neuronal processes, such as synaptic plasticity and its electrophysiological correlate, long-term potentiation (LTP) (Chao 2003). Synaptic plasticity is the process by which connections between two neurons, or synapses, change in strength. These changes involve structural and functional alterations of synapses which underlie LTP (Xu et al., 2000; Minichiello 2009). The most abundant neurotrophin in the central nervous system related to these processes is brain-derived neurotrophic factor (BNDF) which has two receptors, its high-affinity receptor Tropomyosin-related Kinase B (TrkB) and the low-affinity receptor p75NTR which is a common nonspecific receptor for all neurotrophins (Reichardt 2006). BDNF and TrkB are found in the central nervous system (CNS) in different areas such as cortex, hypothalamus, hippocampus and amygdala. BDNF is essential for synaptic plasticity processes which are required for long-term learning and memory (Minichiello 2009). Because of the breadth of the current literature, we will mainly consider studies in this review that include LTP and/or fear extinction in humans and rodents where BDNF and TrkB are known or hypothesized to play a key role.
1.2. Fear learning and memory, structures involved and PTSD
Among learning and memory processes, fear memories are crucial in some psychiatric disorders like post-traumatic stress disorder (PTSD). PTSD is an anxiety disorder that results in some individuals after repeated or single exposure to an extreme traumatic event directly or by witnessing these situations in others. The traumatic event is often re-experienced following trauma reminders and recurrent nightmares. PTSD is also generally accompanied by hyperarousal symptoms (DSM-IV-TR 2000). Moreover, persistent highly aversive memories related to the trauma and the inability of these memories to be extinguished is a main characteristic of this disorder. The most effective learning-based psychotherapy involves the extinction of these highly aversive memories by the acquisition of an incompatible or inhibitory competing memory (Choi et al., 2010b). The formation of these aversive memories and fear extinction has been studied according to the Pavlovian learning paradigm. This associative learning process consists of the pairing of a neutral conditioned stimulus (CS) with an aversive unconditioned stimulus (US) eliciting a conditioned fear response (see Myers and Davis 2007 for a review). The CS can be a cue (e.g. a tone) or a context (e.g. a room). Examples of conditioned fear responses are increased fear potentiated startle and heart rate. Fear extinction consists of a new inhibitory learning after repeated or prolonged CS presentations, without the US, which causes a gradual decrease in the magnitude and/or frequency of the conditioned response (Myers and Davis 2007; Choi et al., 2010b). However, the fear memory after extinction is not erased, but inhibited, base on its re-appearance in three different phenomena: spontaneous recovery, renewal and reinstatement (Myers and Davis 2007). Spontaneous recovery refers to the CS re-emerging after extinction with the passage of time. Renewal consists in the extinguished CS fear return when the CS is presented outside of the extinction context. Reinstatement denotes unsignaled exposure to the US after extinction which leads to reappearance of the CS.
Interestingly, the most important cerebral structures involved in both contextual and cue-dependent fear regulation are also relevant in PTSD; those more involved in emotional processes: amygdala, hippocampus and the prefrontal Cortex (PFC) (Myers and Davis 2007; Mahan and Ressler 2012). The amygdala is the storage structure for fear memories and the hippocampus is especially important for the processing of contextual information being both necessary for fear acquisition and extinction (Myers and Davis et al., 2007; Sierra-Mercado et al., 2011). The most important substructures related to fear processing in the PFC are the dorsal anterior cingulate cortex (dACC) (Prelimbic cortex, PL, in rodents) and the ventromedial prefrontal cortex (vmPFC) (Infralimbic cortex, IL, in rodents). PL and IL, both substructures of the medial prefrontal cortex (mPFC), appear to have opposing influences on fear processes. Muscimol inactivation suggests that PL is necessary for fear acquisition but not extinction whereas IL is required for fear extinction but not fear acquisition (Sierra-Mercado et al., 2011).
Fear learning due to externally experienced traumas represents a critical environmentally-determined risk for the development of PTSD. However, evidence also suggests that risk for PTSD is in part genetically determined (Koenen et al., 2003; Koenen et al., 2006; Norrholm and Ressler 2009; Cornelis et al., 2010; Skelton et al., 2012). Thus it is also important to study genetic variability which may help to explain why some individuals develop the disorder while others do not.
1.3. BDNF Single-nucleotide polymorphisms, PTSD and fear extinction
The study of single-nucleotide polymorphisms (SNP) related to PTSD is useful to establish genetic risk factors for the onset and development of the disorder and improve its treatment (Mahan and Ressler 2012). Examples of genetic variation related to PTSD risk are FKBP5, Serotonin transporter gene variants SLC6A4 and 5-HTTLPR, and RGS2 (Binder et al., 2008; Amstadter et al., 2009; Koenen et al., 2009; Mehta et al., 2011; Mercer et al., 2012; Wang et al., 2011). A SNP that has also been suggested to be relevant for PTSD is the Val66Met polymorphism which consists in the substitution of Met for Val at position 66 in the pro-region of BDNF (Frielingsdorf et al., 2010). The Val66Met SNP causes decreased hippocampal volume, deficits in declarative memory and impaired fear extinction (Egan et al., 2003; Bueller et al., 2006; Soliman et al., 2010). Concordantly, studies in transgenic mice containing a knock-in allele of the human Val66Met allele and cell cultures show reduced: neuronal BDNF availability, neuronal survival, hippocampal dendritic arborization, hippocampal volume and LTP which may result in the declarative memory and fear extinction deficits found in these BDNF Met66 knock-in mice (Chen et al., 2004; Chen et al., 2005; Chen et al., 2006; Cao et al., 2007; Yu et al., 2009; Frielingsdorf et al., 2010; Ninan et al., 2010; Soliman et al., 2010; Spencer et al., 2010).
Unfortunately, there are few specific studies addressing the association between PTSD and the BDNF Val66Met polymorphism (Rakofsky et al., 2012). These reports show no effect although they have a small number of participants, probably given the low percentage of homozygous individuals in the population (Lee et al., 2006; Zhang et al., 2006; Valente et al., 2011). More studies are needed to clarify this lack of effect because there are other studies suggesting a relationship between blood BDNF levels and PTSD. Peripheral BDNF blood levels have been found to be altered in PTSD patients (Dell’osso et al., 2009; Hauck et al., 2010) and low BDNF levels predict a higher recovery rate for PTSD symptoms under selective serotonin reuptake inhibitor (SSRI) treatment (Berger et al., 2010). However, the meaning of BDNF in peripheral blood levels is unclear because the relationship between peripheral concentrations and brain function is not known. All these data on BDNF and SNP studies suggest that altered BDNF levels are related to extinction learning deficits. This could be relevant for developing new PTSD biological markers and improving the treatment by enhancing BDNF activity. These studies are in agreement with the findings that BDNF is necessary in the amygdala for fear conditioning acquisition and consolidation of fear extinction (Rattiner et al., 2004a; Chhatwal et al., 2006). Moreover, in vitro administration of recombinant BDNF (rBDNF) or 7,8-dihydroxyflavone (7,8-DHF), a putative TrkB agonist, lowers the threshold of LTP induction in the amygdala (Li et al., 2011). Concordantly, rBDNF administered in the hippocampus in vivo or in slices induces long-lasting enhancement of synaptic plasticity (Kang et al., 1995; Messaoudi et al., 2002; Ying et al., 2002). Also, genetic alterations of BDNF and TrkB in animal models have given interesting insight in the study of synaptic plasticity and fear memories, as discussed below.
1.4. Genetic manipulations of BDNF/TrkB in synaptic plasticity and fear extinction
BDNF or TrkB homozygous knockout mice rarely survive beyond the third week of life and if they survive have serious health problems and extreme phenotype abnormalities (Klein et al., 1993; Ernfors et al. 1994). However, BDNF heterozygous knockout mice present a viable, but altered, phenotype including impaired hippocampus-LTP and deficits in the acquisition of contextual fear conditioning, which are both rescued by rBDNF infusion (Korte et al., 1995; Patterson et al., 1996; Liu et al., 2004). On the contrary, transgenic mice overexpressing TrkB receptors have enhanced contextual fear conditioning (Koponen et al., 2004). Together, these knockout mouse lines demonstrate that BDNF and TrkB are necessary for CNS development and BDNF/TrkB play a key role in synaptic plasticity and the formation of fear memories. Since this altered gene expression during the lifespan could present compensations that might occur during development, the generation of conditional knockout mice is more desirable. Deletion of BDNF by injecting cre recombinase expressing lentivirus into specific brain regions of floxed BDNF transgenic mice has shown that fear extinction effects are regional dependent. While this BDNF deletion in the hippocampus leads to cue-dependent fear extinction deficits (Heldt et al., 2007), no effect is found in extinction of cue-dependent fear when the BDNF deletion is restricted to the PL (Choi et al., 2010a). However this latter study also shows that BDNF in the PL is necessary for cue-dependent fear acquisition and the deficit in learned fear is rescued by 7,8-DHF, which mimics endogenous BDNF presumably by activating TrkB receptor.
1.5. Epigenetic regulation of BDNF in the extinction of fearful memories
The BDNF gene in rodents has at least nine 5′ noncoding exons each containing a unique promoter region and a 3′ coding exon (IX), which codes for the BDNF prepropeptide (see Musumeci and Minichiello 2011 for a review). It has been recently reported that epigenetic regulation of BDNF gene could be crucial in depression (Fuchikami et al., 2011). Specifically, DNA methylation of the CpG island at the promoter 1 of the BDNF gene might be a biological marker of depression although the study is limited by a low number of subjects and needs to be replicated. Animal models of PTSD suggest that epigenetic regulation of the BDNF gene may be also crucial for this disorder. For example, specific exon-containing BDNF mRNAs seem differentially regulated in fear processes in rats depending on the procedure (cue-dependent or contextual conditioning), previous stress, and which brain region is analyzed. Note that BDNF exon nomenclature of the papers reviewed below follow the new nomenclature proposed by Aid and colleagues (Aid et al., 2007).
In brief, cue-dependent fear acquisition increases BDNF exon I and IV mRNA in the amygdala (Rattiner et al., 2004b), while re-exposure to the contextual fear conditioning box causes increased exon IV mRNA in the hippocampus (Lubin et al., 2008). Interestingly, contextual fear extinction elicits an increase in BDNF exon I and IV mRNA in the mPFC, although in this study PL and IL were not differentiated (Bredy et al., 2007). A recent study suggests that single prolonged stress (SPS) causes an increase in the levels of BDNF exon I, IV and IX mRNAs in the hippocampus after contextual fear conditioning when compared to animals with similar fear conditioning exposure but no stress (Takei et al., 2011). These data suggest that BDNF gene transcriptional changes could be modulated by previous exposure to stress which might be relevant for understanding PTSD.
Thus, BDNF gene expression is needed for fear learning processes. In addition, chromatin structure remodelling and dynamic changes in the nucleosomal packaging of DNA appears to have a crucial role in its regulation (Musumeci and Minichiello 2011). Chromatin structural remodelling that includes reversible histone acetylation/deacetylation of the lysine residues in the N-terminal tail are mediated by histone acetyltransferases (HATs) and histone deacetylases (HDACs), respectively. Specific studies of fear processes suggest that histone H3 acetylation and phosphoacetylation levels are enhanced and histone H4 acetylation levels decreased in promoter 4 in the hippocampus after contextual fear conditioning (Lubin et al., 2008; Takei et al., 2011). Again, regional differences might explain an increase in H4 acetylation in the promoter 4 within the PFC after contextual fear extinction (Bredy et al., 2004).
Moreover, cue-dependent fear reconsolidation is accompanied by increased levels of acetylated histone H3 but not H4 in the lateral amygdala (LA) (Maddox and Schafe 2011). The use of drugs that act as histone deacetylase inhibitors (HDACi) as agents that may enhance fear extinction is reviewed below.
2. Fear extinction modulators which act or may act through BDNF
Seeing that BDNF is extremely important in fear learning, and extinction, and is also regulated by these processes, BDNF-trkB modulation might be clinically relevant. In this section, we will review some of the endogenous and exogenous compounds that modulate fear extinction in both humans and rodents, either directly or indirectly via BDNF/TrkB signalling mechanisms. In particular, we review effective fear extinction enhancers that mediate or may mediate its actions through the BDNF system accompanied by fear extinction training: Antidepressants, HDACi, rBDNF, TrkB agonists and D-cycloserine (Figure 1). Moreover the potential role of the endocannabinoid system and the HPA axis as BDNF modulators in fear extinction is also reviewed. Suffice to say, these compounds and their modes of action have implications for PTSD. Pharmacotherapy is important for PTSD treatment because it has been shown that some molecules can enhance extinction learning and memory potentiating the effects of psychotherapies based on fear extinction (Choi et al., 2010b).
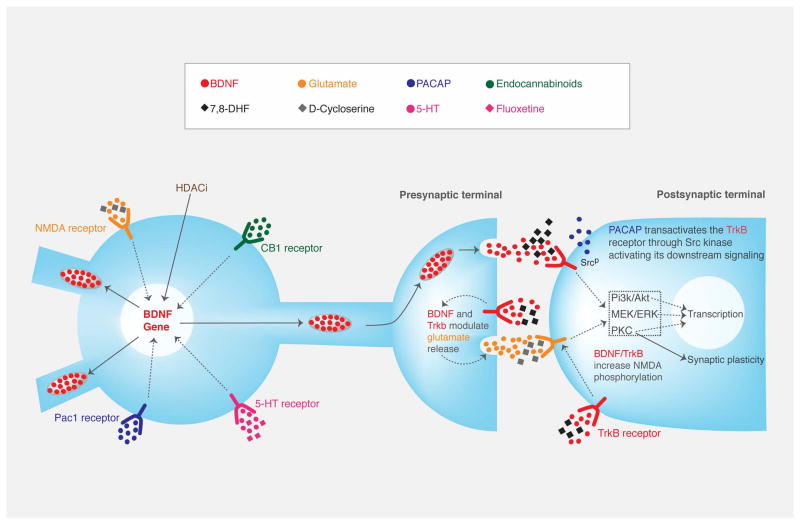
Figure 1.
Simplified schematic diagram of BDNF modulators acting at the synaptic level during fear extinction processes. NMDA receptor, HDACi, CB1 receptor, 5-HT receptor and Pac1 receptor activation lead to epigenetic BDNF gene changes which affect BDNF synthesis. At a presynaptic level, BDNF is released from vesicles and its binding of the postsynaptic TrkB receptor leads to autophosphorylation. This causes activation of downstream signaling pathways (Pi3K/Akt, MEK/ERK and PKC), leading to gene transcription changes and synaptic plasticity. In addition, PACAP has been suggested to transactivate the TrkB receptor through Src and causing the activation of TrkB downstream signaling. Moreover, BDNF and TrkB modulate glutamate release and NMDA phosphorylation.
2.1. Antidepressants
The most common pharmacological treatment for PTSD is antidepressants alone and there are few studies combining antidepressants with exposure psychotherapy (Choi et al., 2010b; Hetrick et al., 2010). Results are disappointing in PTSD clinical trials using antidepressants alone and when combined with psychotherapy there is not enough data to support or refute an increased effectiveness (Choi et al., 2010b; Hetrick et al., 2010). There are two rodent studies related to fear processing suggesting that the antidepressant fluoxetine (SSRI) enhances synaptic plasticity in wild-type mice animals but not in mice with a genetic alteration of the BDNF functioning. The first study shows that fluoxetine enhances synaptic plasticity and fear extinction through BDNF in the amygdala and hippocampus in mice (Karpova et al., 2011). The second one shows how the BDNF Met66 knock-in mice present impaired fluoxetine-induced enhancement of hippocampal synaptic plasticity (Bath et al., 2012). Thus, altered BDNF functioning in PTSD patients, as suggested above, may explain why antidepressants have not been shown to be as effective a pharmacological treatment as hoped for in this disorder.
2.2. Histone deacetylases inhibitors
Pharmacological HDACi catalytic activity appears to enhance emotional learning processes (Bredy and Barad 2008, Maddox and Schafe 2011). The use of compounds such as valproic acid (VPA), trichostatin A or sodium butyrate, seem to enhance contextual fear extinction when injected systemically and when infused in the hippocampus (Lattal et al., 2007; Bredy et al., 2007; Bredy et al., 2008). Nonetheless, trichostatin A enhances cue-dependent fear memory reconsolidation when injected in the LA (Maddox and Schafe 2011). In contrast, in the same study, the DNA methyltransferase (DNMT) inhibitor 5-AZA impairs cue-dependent fear memory reconsolidation. In addition, systemic VPA induces increased mRNA BDNF levels in exon IV within the mPFC and increases histone 4 acetylated levels around the promoter 1 and 4 after fear extinction (Bredy et al., 2007). This is concordant with an in vitro study where VPA is shown to strongly activate promoter 4 of BDNF in cortical neurons (Yasuda et al., 2009). More studies are needed in this direction to elucidate the epigenetic changes elicited by HDACi in structures involved in emotional processing. Several HDACi could be tested to enhance fear extinction in humans since they are approved for the treatment of mood disorders and as anti-epileptics.
2.3. Recombinant BDNF and the putative TrkB agonist, 7,8-dihydroxyflavone
rBDNF has been used extensively in rodent studies. For example, an interesting experiment showed that a single rBDNF infusion in the IL of rats enhances cue-dependent extinction in rats, even without extinction training. This BDNF effect is dependent on N-methyl D-aspartate (NMDA) receptors (Peters et al., 2010). In contrast, as mentioned above, BDNF deletion in the PL causes deficits in cue-dependent fear acquisition but not extinction or innate fear (Choi, et al., 2010a). Thus, these data support the widely accepted idea explained above that there is a clear dissociation in the role of different mPFC areas in fear learning and memory processes, with IL involved in fear extinction and PL in fear acquisition. Moreover, these two studies show how BDNF is a key modulator in both of them. In contrast to the enhanced extinction found when rBDNF is injected in the IL, rBDNF injected in the dorsal CA1 region of the hippocampus causes impairment in the consolidation of contextual fear extinction (Kirtley et al., 2010). This latter apparently contradictory study and the paper by Heldt and colleagues 2007, where hippocampal BDNF deletion causes impairment of cue-dependent fear extinction, may suggest that BDNF levels in the hippocampus functions follow an inverted-U shape function. Extremely high and low BDNF levels in the hippocampus may impair its functions in relation to memory processes.
Notably, rBDNF in humans seems to have limited or no therapeutic properties since clinical trials of acute ischemic stroke or the neurodegenerative disease studies reported lack of effect, probably due to the poor pharmacokinetic properties of BDNF (Wu 2005). An interesting alternative was identified with a potential small-molecule TrkB receptor agonist, 7,8-DHF (Jang et al., 2010b). This flavone can be obtained from the plant Godmania aesculifolia or through chemical synthesis. Most recent studies have used the commercially available synthetic version. When systemically administered in mice, 7,8-DHF crosses the blood brain barrier (BBB), provokes TrkB receptor dimerization and autophosphorylation, and activation of downstream signaling (Jang et al., 2010b). Moreover, 7,8-DHF presents better pharmacokinetic properties than BDNF and higher TrkB binding affinity. A single systemic dose of 7,8-DHF activates TrkB receptors within the amygdala and enhances both fear acquisition and extinction in naïve mice (Andero et al., 2011). More importantly, in the same study, a systemic 7,8-DHF injection rescues a deficit in fear extinction in a potential mouse model of PTSD. In addition, other recent studies have suggested interesting actions of 7,8-DHF in rodents. 7,8-DHF blocks long-term declarative memory deficits elicited by acute stress immobilization (Andero et al., 2012), prevents depressive symptoms and structural changes caused by a chronic stress procedure (Blugeot et al., 2011) and reverses memory deficits in an Alzheimer mouse model (Devi and Ohno 2012). For all these reasons, 7,8-DHF seems an interesting candidate that ought to be tested for its potential to enhance fear extinction in humans.
Other flavonoid-derivatives have been described also as relatively specific TrkB agonists and present antidepressant-like actions in mice, including 4′-dimethylamino-7,8-dihydroxyflavone (Liu et al., 2010) and Deoxygedunin (Jang et al., 2010a). Moreover, Deoxygedunin also enhances cue-dependent fear conditioning learning similarly as 7,8-DHF (Jang et al., 2010a).
LM22A-4 appears to be another potential TrkB agonist, which when chronically administered intranasally in rodents crosses the BBB and activates its downstream signaling (Massa et al., 2010). In addition, treatment with LM22A-4 for two weeks restores motor learning in a traumatic brain injury model in rats. We have no knowledge of fear learning experiments performed with this agent. More studies involving potential beneficial activities of this compound in vivo and more detailed pharmacodynamics would be desirable.
2.4. D-Cycloserine
BDNF and TrkB are found in glutamatergic neurons at both presynaptic and postsynaptic sites (see Minichiello 2009 for a review). Moreover, there are interactions between BDNF/TrkB and glutamate receptors. For example, BDNF increases glutamate release, enhances ionotropic glutamate receptor NMDA subtype activity, and increases phosphorylation of NMDA receptor subunits (Minichiello 2009). All these processes may influence synaptic plasticity.
Nonetheless, studies involving NMDA antagonists in rodents impair fear extinction and LTP while agonists enhance those processes (see Myers et al., 2011 for a review). D-cycloserine (DCS) is an antibiotic that enhances excitatory neurotransmission mediated by NMDA receptors, by binding to the strychnine-insensitive glycine recognition site of the NMDA receptor complex and increasing calcium influx without causing neurotoxicity. Thus, it acts as a NMDA receptor partial agonist and it has been shown to be an effective drug to facilitate consolidation of fear extinction in different anxiety disorders (Ressler et al., 2004; Hoffman et al., 2006; Kushner et al., 2007; Guastella et al., 2008; Wilhelm et al., 2008; Otto et al., 2010; Storch et al., 2010). DCS is especially effective in patients with maladaptive fear and rodents with previous exposure to stress (Myers et al., 2011). Specifically, DCS enhances fear memory extinction when systemically injected and when locally infused into the basolateral amygdala (BLA), mPFC and hippocampus of rats suggesting regional specific effects (Myers et al., 2011). In addition, BDNF Met66 knock-in mice have impaired NMDA receptor dependent synaptic plasticity (Ninan et al., 2010) and delayed fear extinction which is rescued by a systemic DCS injection (Yu et al., 2009).
At a molecular level, in vitro studies suggest that NMDA receptor activation leads to increased expression of BDNF (Marini et al., 1998, Yaka et al., 2003). In particular, NMDA receptor stimulation leads to specific activation of the BDNF promoter 3 (Tabuchi et al., 2000). Moreover, DCS enhancement of fear extinction is dependent on activation of mitogen activated protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI3K) signalling cascades in the BLA (Yang and Lu 2005). Interestingly, these pathways are also two of the ones by which TrkB receptors exert its neurotrophic actions, including learning and memory processes. So, taking all these data together, there are important bidirectional interactions between BDNF/TrkB and NMDA receptors, which may be key for fear extinction although more studies are needed in this direction to further clarify this issue.
2.5. Endocannabionoid system
The cannabinoid type 1 receptor (CB1) is part of the endogenous endocannabionoid system. CB1 has been related to anxiety and required for the extinction of conditioned fear (see Chhatwal and Ressler 2007 for a review). Recent findings have found interesting interactions between the CB1 receptors and BDNF/TrkB. For example, endocannabinoids have been suggested to be released as retrograde messengers induced by postsynaptic TrkB activation (Lemtiri-Chlieh and Levine 2010). Interestingly, BDNF incubation increases the expression of CB1 receptor transcripts and protein (Maison et al., 2009). In addition, BDNF incubation causes an increased sensitivity of the CB1 receptor to endocannabinoid agonists, 2-arachidonylglycerol (2-AG) and noladin in cerebellar cell culture. Also, BDNF release triggered by CB1 receptor stimulation mediates the neuroprotective effects of cannabinoids in hippocampal cultures (Khaspekov et al., 2004). Although there is limited evidence of in vivo functional interaction of endocannabionoids and BDNF, there is a study where CB1 receptor knockout mice present decreased basal BDNF levels in the hippocampus and rBDNF infusion rescues despair-like behaviour (Aso et al., 2008). In humans, it has been suggested that habitual δ-9 tetrahydrocannabinol (δ-9-THC) users have decreased serum BDNF levels (D’Souza et al., 2009), although this study is limited by a low number of subjects. Taken together, these data suggest that there are interactions between the endocannabinoid and BDNF/TrkB systems which would be important for the modulation of fear extinction.
2.6. Hypothalamic-pituitary adrenal axis and pituitary adenylate cyclase-activating polypeptide
One of the physiological systems that more strongly alter BDNF is the hypothalamic-pituitary adrenal axis (HPA) which is activated in response to stressful situations. There are a myriad studies in rodents showing how stress alters BDNF and TrkB levels. Although the picture is complex because these stress-induced BDNF/TrkB alterations are regionally dependent and influenced by several factors, such as stress paradigm, stress duration and sex (Smith et al., 1995; Molteni et al., 2001; Marmigere et al., 2003; Greenwood et al., 2007; Lin et al., 2009; Molteni et al., 2010). All these changes in BDNF/TrkB might have in common the later alteration of extinction processing. Corticosterone and adenylate cyclase-activating polypeptide (PACAP) are discussed below as potential modulators of BDNF, fear extinction and PTSD.
There is only one study about the HPA axis-BDNF regulation combined with fear extinction (Gourley et al., 2009). The authors found that 3 weeks of exposure to a high dose of corticosterone in rats leads to impaired fear extinction to contextual freezing. Moreover, cortical BDNF mRNA was decreased in the Orbitofrontal Cortex (OF) but not in mPFC, although this subarea has been extensively related to fear processes. A possible explanation of this lack of effect on the BDNF mRNA levels in the mPFC after chronic stress exposure might be that stress differentially regulates PL and IL BDNF levels. Then, when analyzing BDNF in the whole mPFC this effect may be masked. More specific studies are needed for a more deep understanding of the HPA regulation of BDNF and fear extinction processes, a cornerstone of PTSD.
A recent study, also related to stress, shows how PACAP and its specific G protein-coupled PAC1 receptor (PAC1R) are associated with PTSD (Ressler et al., 2011). In addition, experiments with animal models suggest that PACAP and PAC1R in the bed nucleus of the stria terminalis (BNST) and the amygdala are related to fear conditioning and chronic stress (Hammack et al., 2009; Ressler et al., 2011). The BNST is a structure of the HPA axis key for stress and anxiety responses whereas the amygdala, besides being crucial for fear processes as discussed above, is an important HPA axis modulator. The first study suggest that 2 hours after fear conditioning in mice, PAC1R gene (ADCYAP1R1) levels are increased in the amygdala and positively correlated to freezing behavior (Ressler et al., 2011). The second study shows increased PACAP levels in the dorsal BNST in rats after chronic stress exposure (Hammack et al., 2009).
The potential PACAP effects on BDNF and fear extinction processes come from in vitro and in vivo studies. In vitro studies suggest that hippocampal neurons incubated with PACAP enhance the activity of the NMDA receptor channel which in turn increases BDNF activity, specifically the BDNF transcript IV (Yaka et al., 2003; Kidane et al., 2008). Also, PACAP has been suggested to transactivate the TrkB receptor trough Src kinase activating its downstream signalling, crucial for learning and memory, in the absence of neurotrophins (Minichiello 2009). In addition, there is an in vivo study showing interaction between PAC1R and BDNF since PAC1R knockout mice present decreased transcriptional regulation of BDNF in the hippocampus, maybe trough altered excitatory transmission (Zink et al., 2004). Taking all these data together, it can be hypothesized that deregulation in PACAP and PAC1R affecting BDNF/TrkB might be critical in fear extinction processes although more studies are needed in this direction.
3. Conclusions
Herein we have reviewed the known and hypothesized roles of BDNF in the synaptic plasticity processes involved in fear learning and memory, including human and translational genetic and epigenetic studies examining these processes. We then reviewed the results of a number of experimental studies demonstrating that exogenous enhancers of fear extinction may act, at least in part, through BDNF/TrkB processes. The BDNF/TrkB neurotrophin system is clearly critical to the regulation of emotional learning and memory. Further understanding of the pathways mediating BDNF/TrkB action along with potential specific small molecule agonists and antagonists of this system may provide exciting and important future approaches to understanding the neurobiology of fear as well as in treating or preventing fear-related disorders in humans.
Acknowledgments
The authors would like to thank Nicole Gouws for her help designing the figure 1. Support was provided by NIH (F32MH085443, R01DA01962), the Burroughs Wellcome Fund, and by the National Center for Research Resources P51RR165 and is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132.
Footnotes
Disclosures:
Dr. Ressler is a founding member of Extinction Pharmaceuticals/Therapade Technologies, which have provided no equity or income, but which exist to develop d-Cycloserine for use to augment the effectiveness of psychotherapy.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. Journal of neuroscience research. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, Gelernter J. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. Journal of anxiety disorders. 2009;23:369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Daviu N, Escorihuela RM, Nadal R, Armario A. 7,8-dihydroxyflavone, a TrkB receptor agonist, blocks long-term spatial memory impairment caused by immobilization stress in rats. Hippocampus. 2012;22:399–408. doi: 10.1002/hipo.20906. [DOI] [PubMed] [Google Scholar]
- Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. The American journal of psychiatry. 2011;168:163–172. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso E, Ozaita A, Valdizán EM, Ledent C, Pazos A, Maldonado R, Valverde O. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB1 knockout mice. Journal of neurochemistry. 2008;105:565–572. doi: 10.1111/j.1471-4159.2007.05149.x. [DOI] [PubMed] [Google Scholar]
- Bath KG, Jing DQ, Dincheva I, Neeb CC, Pattwell SS, Chao MV, Lee FS, Ninan I. BDNF Val66Met Impairs Fluoxetine-Induced Enhancement of Adult Hippocampus Plasticity. Neuropsychopharmacology. 2012 Jan 4; doi: 10.1038/npp.2011.318. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W, Mehra A, Lenoci M, Metzler TJ, Otte C, Tarasovsky G, Mellon SH, Wolkowitz OM, Marmar CR, Neylan TC. Serum brain-derived neurotrophic factor predicts responses to escitalopram in chronic posttraumatic stress disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2010;34:1279–1284. doi: 10.1016/j.pnpbp.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA: the journal of the American Medical Association. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blugeot A, Rivat C, Bouvier E, Molet J, Mouchard A, Zeau B, Bernard C, Benoliel JJ, Becker C. Vulnerability to depression: from brain neuroplasticity to identification of biomarkers. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:12889–12899. doi: 10.1523/JNEUROSCI.1309-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learning & memory (Cold Spring Harbor, NY) 2008;15:39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learning & memory (Cold Spring Harbor, NY) 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta JK. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biological psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Cao L, Dhilla A, Mukai J, Blazeski R, Lodovichi C, Mason CA, Gogos JA. Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. Current biology: CB. 2007;17:911–921. doi: 10.1016/j.cub.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nature reviews Neuroscience. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Ressler KJ. Modulation of fear and anxiety by the endogenous cannabinoid system. CNS spectrums. 2007;12:211–220. doi: 10.1017/s1092852900020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nature neuroscience. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proceedings of the National Academy of Sciences of the United States of America. 2010a;107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Rothbaum BO, Gerardi M, Ressler KJ. Pharmacological enhancement of behavioral therapy: focus on posttraumatic stress disorder. Current topics in behavioral neurosciences. 2010b;2:279–299. doi: 10.1007/7854_2009_10. [DOI] [PubMed] [Google Scholar]
- Cornelis MC, Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: review and recommendations for genome-wide association studies. Current psychiatry reports. 2010;12:313–326. doi: 10.1007/s11920-010-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’osso L, Carmassi C, Del Debbio A, Dell’osso MC, Bianchi C, da Pozzo E, Origlia N, Domenici L, Massimetti G, Marazziti D, Piccinni A. Brain-derived neurotrophic factor plasma levels in patients suffering from post-traumatic stress disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:899–902. doi: 10.1016/j.pnpbp.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Devi L, Ohno M. 7,8-Dihydroxyflavone, a Small-Molecule TrkB Agonist, Reverses Memory Deficits and BACE1 Elevation in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:434–44. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Pittman B, Perry E, Simen A. Preliminary evidence of cannabinoid effects on brain-derived neurotrophic factor (BDNF) levels in humans. Psychopharmacology. 2009;202:569–578. doi: 10.1007/s00213-008-1333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2000. DSM-IV-TR. [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ, Lee FS. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2010;1208:150–157. doi: 10.1111/j.1749-6632.2010.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M, Morinobu S, Segawa M, Okamoto Y, Yamawaki S, Ozaki N, Inoue T, Kusumi I, Koyama T, Tsuchiyama K, Terao T. DNA Methylation Profiles of the Brain-Derived Neurotrophic Factor (BDNF) Gene as a Potent Diagnostic Biomarker in Major Depression. PLoS ONE. 2011;6:e23881–e23881. doi: 10.1371/journal.pone.0023881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Thompson RS, Fleshner M. Learned helplessness is independent of levels of brain-derived neurotrophic factor in the hippocampus. Neuroscience. 2007;144:1193–1208. doi: 10.1016/j.neuroscience.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds MR. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck S, Kapczinski F, Roesler R, de Moura Silveira E, Magalhães PV, Kruel LRP, Schestatsky SS, Ceitlin LHF. Serum brain-derived neurotrophic factor in patients with trauma psychopathology. Progress in neuro-psychopharmacology & biological psychiatry. 2010;34:459–462. doi: 10.1016/j.pnpbp.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Molecular psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetrick SE, Purcell R, Garner B, Parslow R. Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD) Cochrane Database Syst Rev. 2010:CD007316. doi: 10.1002/14651858.CD007316.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JAJ, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Archives of general psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Jang SW, Liu X, Chan CB, France SA, Sayeed I, Tang W, Lin X, Xiao G, Andero R, Chang Q, Ressler KJ, Ye K. Deoxygedunin, a natural product with potent neurotrophic activity in mice. PloS one. 2010a;5:e11528–e11528. doi: 10.1371/journal.pone.0011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science (New York, NY) 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A, Antila H, Popova D, Akamine Y, Sullivan R, Hen R, Drew LJ, Castren E. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaspekov LG, Brenz Verca MS, Frumkina LE, Hermann H, Marsicano G, Lutz B. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. The European journal of neuroscience. 2004;19:1691–1698. doi: 10.1111/j.1460-9568.2004.03285.x. [DOI] [PubMed] [Google Scholar]
- Kidane AH, Roubos EW, Jenks BG. Pituitary adenylate cyclase-activating polypeptide regulates brain-derived neurotrophic factor exon IV expression through the VPAC1 receptor in the amphibian melanotrope cell. Endocrinology. 2008;149:4177–4182. doi: 10.1210/en.2008-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtley A, Thomas KL. The exclusive induction of extinction is gated by BDNF. Learning & memory (Cold Spring Harbor, NY) 2010;17:612–619. doi: 10.1101/lm.1877010. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- Koenen KC, Aiello AE, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R, Kilpatrick DG, Gelernter J, Galea S. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. American journal of epidemiology. 2009;169:704–711. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, Stroud L, Niaura R, McCaffery J, Goldberg J, Eisen SA, True W, Tsuang M. Posttraumatic stress disorder and late-onset smoking in the Vietnam era twin registry. Journal of consulting and clinical psychology. 2006;74:186–190. doi: 10.1037/0022-006X.74.1.186. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Lyons MJ, Goldberg J, Simpson J, Williams WM, Toomey R, Eisen SA, True WR, Cloitre M, Wolfe J, Tsuang MT. A high risk twin study of combat-related PTSD comorbidity. Twin Res. 2003;6:218–226. doi: 10.1375/136905203765693870. [DOI] [PubMed] [Google Scholar]
- Koponen E, Võikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H, Taira T, Castrén E. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Molecular and cellular neurosciences. 2004;26:166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behavioral neuroscience. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Kang R, Lim S, Paik J, Choi M, Lee M. No association between the brain-derived neurotrophic factor gene Val66Met polymorphism and post-traumatic stress disorder. Stress and Health. 2006;22:115–119. [Google Scholar]
- Lemtiri-Chlieh F, Levine ES. BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. Journal of neurophysiology. 2010;104:1923–1932. doi: 10.1152/jn.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Dabrowska J, Hazra R, Rainnie DG. Synergistic Activation of Dopamine D1 and TrkB Receptors Mediate Gain Control of Synaptic Plasticity in the Basolateral Amygdala. PloS one. 2011;6:e26065–e26065. doi: 10.1371/journal.pone.0026065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Ter Horst GJ, Wichmann R, Bakker P, Liu A, Li X, Westenbroek C. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cerebral cortex (New York, NY: 1991) 2009;19:1978–1989. doi: 10.1093/cercor/bhn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu IYC, Lyons WE, Mamounas LA, Thompson RF. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:7958–7963. doi: 10.1523/JNEUROSCI.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chan C-B, Jang S-W, Pradoldej S, Huang J, He K, Phun LH, France S, Xiao G, Jia Y, Luo HR, Ye K. A Synthetic 7,8-Dihydroxyflavone Derivative Promotes Neurogenesis and Exhibits Potent Antidepressant Effect. Journal of medicinal chemistry. 2010 Nov 12; doi: 10.1021/jm101206p. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Schafe GE. Epigenetic alterations in the lateral amygdala are required for reconsolidation of a Pavlovian fear memory. Learn Mem. 2011;18:579–593. doi: 10.1101/lm.2243411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends in Neurosciences. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison P, Walker DJ, Walsh FS, Williams G, Doherty P. BDNF regulates neuronal sensitivity to endocannabinoids. Neuroscience letters. 2009;467:90–94. doi: 10.1016/j.neulet.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Marini AM, Rabin SJ, Lipsky RH, Mocchetti I. Activity-dependent release of brain-derived neurotrophic factor underlies the neuroprotective effect of N-methyl-D-aspartate. J Biol Chem. 1998;273:29394–29399. doi: 10.1074/jbc.273.45.29394. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–655. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN, Nehama D, Rajadas J, Longo FM. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. The Journal of clinical investigation. 2010;120:1774–1785. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Gonik M, Klengel T, Rex-Haffner M, Menke A, Rubel J, Mercer KB, Pütz B, Bradley B, Holsboer F, Ressler KJ, Müller-Myhsok B, Binder EB. Using Polymorphisms in FKBP5 to Define Biologically Distinct Subtypes of Posttraumatic Stress Disorder: Evidence From Endocrine and Gene Expression Studies. Archives of general psychiatry. 2011;68:901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer KB, Orcutt HK, Quinn JF, Fitzgerald CA, Conneely KN, Barfield RT, Gillespie CF, Ressler KJ. Acute and Posttraumatic Stress Symptoms in a Prospective Gene × Environment Study of a University Campus Shooting. Archives of general psychiatry. 2012;69:89–97. doi: 10.1001/archgenpsychiatry.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Ying SW, Kanhema T, Croll SD, Bramham CR. Brain-derived neurotrophic factor triggers transcription-dependent, late phase long-term potentiation in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:7453–7461. doi: 10.1523/JNEUROSCI.22-17-07453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nature reviews Neuroscience. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Chourbaji S, Brandwein C, Racagni G, Gass P, Riva MA. Depression-prone mice with reduced glucocorticoid receptor expression display an altered stress-dependent regulation of brain-derived neurotrophic factor and activity-regulated cytoskeleton-associated protein. Journal of psychopharmacology (Oxford, England) 2010;24:595–603. doi: 10.1177/0269881108099815. [DOI] [PubMed] [Google Scholar]
- Molteni R, Lipska BK, Weinberger DR, Racagni G, Riva MA. Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Molecular psychiatry. 2001;6:285–292. doi: 10.1038/sj.mp.4000865. [DOI] [PubMed] [Google Scholar]
- Musumeci G, Minichiello L. BDNF-TrkB signalling in fear learning: from genetics to neural networks. Reviews in the neurosciences. 2011;22:303–315. doi: 10.1515/RNS.2011.031. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS, Chao MV. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:8866–8870. doi: 10.1523/JNEUROSCI.1405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ressler KJ. Genetics of anxiety and trauma-related disorders. Neuroscience. 2009;164:272–287. doi: 10.1016/j.neuroscience.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, Hofmann SG, Eisenmenger K, Krystal JH, Pollack MH. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science (New York, NY) 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakofsky JJ, Ressler KJ, Dunlop BW. BDNF function as a potential mediator of bipolar disorder and post-traumatic stress disorder comorbidity. Molecular psychiatry. 2012;17:22–35. doi: 10.1038/mp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004a;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learning & memory (Cold Spring Harbor, NY) 2004b;11:727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Archives of general psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. PTSD and gene variants: New pathways and new thinking. Neuropharmacology. 2012;62:628–37. doi: 10.1016/j.neuropharm.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A Genetic Variant BDNF Polymorphism Alters Extinction Learning in Both Mouse and Human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Milner TA, Lee FS, McEwen BS. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc Natl Acad Sci U S A. 2010;107:4395–4400. doi: 10.1073/pnas.0915105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Goodman WK, Geffken GR, Lewin AB, Henin A, Micco JA, Sprich S, Wilhelm S, Bengtson M, Geller DA. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010;68:1073–1076. doi: 10.1016/j.biopsych.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, Kuraishi Y, Tsuda M. Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-D-aspartate receptor Ca2+ channels. J Biol Chem. 2000;275:17269–17275. doi: 10.1074/jbc.M909538199. [DOI] [PubMed] [Google Scholar]
- Takei S, Morinobu S, Yamamoto S, Fuchikami M, Matsumoto T, Yamawaki S. Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. Journal of psychiatric research. 2011;45:460–468. doi: 10.1016/j.jpsychires.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Valente NLM, Vallada H, Cordeiro Q, Miguita K, Bressan RA, Andreoli SB, Mari JJ, Mello MF. Candidate-gene approach in posttraumatic stress disorder after urban violence: association analysis of the genes encoding serotonin transporter, dopamine transporter, and BDNF. Journal of molecular neuroscience: MN. 2011;44:59–67. doi: 10.1007/s12031-011-9513-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Baker DG, Harrer J, Hamner M, Price M, Amstadter A. The relationship between combat-related posttraumatic stress disorder and the 5-HTTLPR/rs25531 polymorphism. Depression and anxiety. 2011;28:1067–1073. doi: 10.1002/da.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- Wu D. Neuroprotection in experimental stroke with targeted neurotrophins. NeuroRx: the journal of the American Society for Experimental NeuroTherapeutics. 2005;2:120–128. doi: 10.1602/neurorx.2.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003;278:9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- Yang YL, Lu KT. Facilitation of conditioned fear extinction by d-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Molecular psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- Ying SW, Futter M, Rosenblum K, Webber MJ, Hunt SP, Bliss TVP, Bramham CR. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, Bath KG, Lee FS, Chen ZY. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, Charney DS, Price LH, Southwick S, Yang BZ, Rasmussen A, Gelernter J. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. American journal of medical genetics. Part B, Neuropsychiatric genetics: the official publication of the International Society of Psychiatric Genetics. 2006;141B:387–393. doi: 10.1002/ajmg.b.30332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink M, Otto C, Zörner B, Zacher C, Schütz G, Henn FA, Gass P. Reduced expression of brain-derived neurotrophic factor in mice deficient for pituitary adenylate cyclase activating polypeptide type-I-receptor. Neuroscience letters. 2004;360:106–108. doi: 10.1016/j.neulet.2004.01.030. [DOI] [PubMed] [Google Scholar]