Abstract
Task-induced decreases in blood flow and the widespread use of “resting” baselines produced unexpected and discrepant results in early cognitive imaging studies, especially in language comprehension experiments. Here I describe from a personal perspective some of the events and thought processes leading to the first hypothesis-driven fMRI study of the “resting” state.
Keywords: functional MRI, resting state, mind-wandering, semantics, memory
A (Very) Brief History of Task-Induced “Negative Signal Changes”
Scientists using functional brain imaging techniques in the early 1990’s began regularly to notice and comment on unexpected negative-going signals. Of course, any signal that goes up in one condition relative to another must come down again, but these “deactivations” were notable because they were induced by active tasks relative to “resting” states. From our current vantage point it may be difficult to understand why this phenomenon was so surprising. The reaction at the time, however, was influenced by longstanding assumptions, shared across many experimental fields, that the main function of the nervous system is to respond to external stimuli (cf. behaviorist psychology, classical neurophysiology). The “resting” state, unperturbed by external stimuli or explicit task demands, was often naively viewed as a low-level baseline characterized by minimal “background” neural activity.
The earliest reports of task-induced deactivation were from PET blood flow studies (Frith et al., 1991; Haxby et al., 1994; Kawashima et al., 1995; Warburton et al., 1996). The main hypothesis put forward was that the phenomenon represented cross-modal inhibition (Haxby et al., 1994; Kawashima et al., 1995). According to this theory, active inhibition of modal sensory cortex not involved in task performance (e.g., inhibition of visual and auditory cortex during a somatosensory task) serves to improve performance by suppressing background noise. In retrospect, it is interesting to note that the “visual areas” inhibited by somatosensory discrimination tasks in the study by Kawashima et al. included the angular gyrus and precuneus, areas now known to be deactivated across tasks in all modalities, and that these same areas had also been “inhibited” during visual tasks (Haxby et al., 1994).
A PET study by Nancy Andreasen and colleagues (Andreasen et al., 1995), published in the American Journal of Psychiatry in 1995, offered an entirely different, and at the time largely overlooked, hypothesis. These authors came from a psychoanalytic and cognitive psychology tradition in which the “resting” state is viewed as highly active, involving free association, autobiographical recollection, daydreaming, creativity, and planning (see (Freud, 1999; Ingvar, 1985; James, 1890; Proust, 1981) for some literary and scientific precedents). Their study included a “resting” state (“lie quietly with your eyes closed”), however they considered this state to be an active episodic memory task, which they dubbed, tongue partly in cheek, “Random Episodic Silent Thinking” (REST). Compared to a phonological word generation task (“say words starting with C”), the REST condition “activated” the angular gyri bilaterally, precuneus/posterior cingulate region, and right ventromedial prefrontal cortex. The authors attributed these activations to the episodic and autobiographical retrieval processes they considered central to REST. Because they viewed this state as an active task, they did not mention the notion of task-induced deactivation/inhibition.
Task-induced decreases in blood flow were largely ignored, however, until the landmark meta-analysis by Shulman and colleagues at Washington University in 1997 (Shulman et al., 1997), discussed in detail elsewhere in this issue by Randy Buckner. Shulman et al. established beyond a doubt that task-induced decreases in blood flow, relative to resting and passive stimulation, were a common phenomenon in PET activation studies. More importantly, it was established that many of these decreases occurred in consistent brain locations across tasks. The authors considered a number of possible explanations for the phenomenon, including (i) cross-modal sensory inhibition; (ii) more general inhibition effects from increased arousal in the active tasks; (iii) inhibition of emotion regulation systems in order to facilitate cognitive processing; and (iv) suspension of processes ongoing during the passive state. Possible ongoing passive-state processes considered by the authors included: unconstrained verbally mediated thoughts, monitoring of the external environment for novel events, monitoring body position and spatial orientation, and monitoring of emotional state. Each of these explanations could account for some of the data, and the models based on monitoring the external environment, body position, and emotional state seemed to find the most favor. The earlier work of Andreasen et al. (Andreasen et al., 1995) was not mentioned.
A Problem With Baselines
My initial interest in this area had little to do with task-induced decreases in blood flow, but rather with a need to understand some highly discordant results that were emerging from language imaging studies. Like others at the time interested in this field, I was puzzled by two recent PET studies -- by Richard Wise and colleagues in 1991 (Wise et al., 1991) and Jean-Francois Démonet and colleagues in 1992 (Démonet et al., 1992) – that had suggested very different neural substrates for language comprehension. Wise et al. reported activations in the superior temporal gyrus (STG) bilaterally during an auditory word comprehension task. In contrast, Démonet et al. reported activations in a widely distributed, left-lateralized network of inferior temporal, inferior parietal, and prefrontal regions. The comprehension tasks in the two studies were not identical, but these small differences seemed inadequate to explain such large discrepancies in activation patterns. A more salient factor, perhaps, was the difference in baseline conditions that were used. Wise et al. used a “resting” baseline, whereas Démonet et al. used an active tone discrimination task. The latter authors pointedly expressed concern about activation paradigms in which “too vague requirements are made (e.g., ‘passive’ listening)” and designed their experiment “to keep subjects fully engaged in the relevant cognitive task.”
The discrepancies between these studies were no small matter for those of us trying to understand the neurobiology of language. At that time there was still a lingering belief that the left STG held most of the circuitry for language comprehension (Geschwind, 1971; Mayeux and Kandel, 1985; Mesulam, 1990). On the other hand, considerable evidence had accumulated indicating that semantic knowledge and comprehension deficits could occur from lesions in more inferior regions of the temporal lobe (Alexander et al., 1989; Damasio, 1989; Hart and Gordon, 1990; Hodges et al., 1992; Kertesz et al., 1982; Lüders et al., 1991; Silveri and Gainotti, 1988; Sirigu et al., 1991). The STG had also been strongly implicated in auditory sensory and speech perception processes (Buchman et al., 1986; Galaburda and Sanides, 1980; Henschen, 1918–1919). Was it possible that the bilateral STG activation observed by Wise et al. simply represented activation of the cortical auditory system that was not controlled for by a resting baseline? On the other hand, if the inferior temporal, parietal, and prefrontal activations observed by Démonet et al. represented semantic networks supporting word comprehension, why were these networks not observed by Wise et al.?1
Weighing the facts of the lesion data, I was strongly inclined to accept the Démonet results. Like many others new to the functional neuroimaging field, I had casually wondered about the nature of the resting state and the validity of this condition as a baseline. As a subject myself in numerous early fMRI experiments, I could attest in detail to the stream of complex thoughts that entered my mind during “resting” blocks in the scanner. Willing myself to “clear your mind” or “avoid thinking of words” (Rueckert et al., 1994; Yetkin et al., 1995) seemed to make no difference. In our first fMRI language experiment, in which we loosely adapted the tasks of Démonet et al. but also included a resting state, we noticed several brain regions that showed negative-going signal changes during the tone discrimination task relative to the resting condition. Intriguingly, these regions were also activated during the word comprehension task relative to the tone task (Binder et al., 1995).
It seemed to me an inescapable conclusion that the “resting” state was not what many researchers steeped in animal neurophysiology had claimed it to be. Instead of a low-level baseline characterized by minimal, unorganized background activity, what was called “resting” was actually an active, cognitively complex state marked by internal dialogue, imagery, emotion, retrieval of episodic memories, problem solving, and planning. Moreover, it appeared that what many of these processes shared in common was their reliance on internal stores of knowledge, i.e., learned facts about the world, properties of objects, how people behave and why, what works and what doesn’t work, how one feels in a given situation, and which goals are most important. For lack of a better term, I refer to these processes that use internal stores of knowledge as “conceptual” and apply this descriptor both to the knowledge stores themselves – which include semantic, episodic, autobiographical, social, and emotional content – and to the specific control processes that retrieve, select, and manipulate this knowledge. Of course, these conceptual processes also underlie language comprehension, providing a simple explanation for the discrepant results reported by Wise et al. and Démonet et al. If conceptual processes are a central feature of both word comprehension and “resting,” then the use of a “resting” baseline will mask these processes in a standard subtraction design. In contrast, the active tone discrimination task used by Démonet et al. is sufficiently demanding of attentional resources that conceptual processes must be interrupted, much as one’s “train of thought” is interrupted by a novel or attentionally demanding external event (Pope and Singer, 1976). This interruption of ongoing conceptual processes is critically necessary for identifying these processes in a task contrast. It also provides a simple explanation for “task-induced decreases” relative to “resting” and “passive” states.
Testing a Cognitive Model of the Conscious Resting State
Scientific theories founded mainly on introspection and intuition can be less than compelling (Skinner, 1975). A central problem with any claim about the “resting” state is the difficulty of documenting the condition with anything other than a subjective description. In considering the effects of different baselines on functional activation results, however, it became clear that the classic subtraction model provides a way around this problem. An explicit task can only produce deactivation by interrupting ongoing processes if the explicit task itself does not also engage those processes. More generally, task-induced deactivation should not occur, or should be much weaker, when the explicit task engages the same processes that are engaged during “resting.” To understand the processes active during “resting,” it is only necessary to find a task that does NOT cause deactivation.
In their initial meta-analysis, Shulman et al. had examined a range of visual and language tasks and found no general relationship between the degree of blood flow decrease and the type of active task used (language vs. non-language) or degree of task difficulty (Shulman et al., 1997). There were task effects in some brain areas. Decreases were more pronounced in the right parietal lobe and precuneus during language tasks than during non-language tasks, and more pronounced in a left inferior frontal region during non-language tasks. All tasks produced deactivation in the usual areas, however, leaving unclear what sort of task might activate these regions as much as they are active at “rest.”
The model of “resting” described above suggested that such a task would need to involve retrieval and manipulation of internal stores of knowledge. The semantic decision we had developed based on Démonet et al. seemed like a good candidate, particularly as the model being tested was motivated by that study. The semantic task requires retrieval and manipulation of relatively complex factual knowledge (Is it found in the United States? Is it useful to people?) in response to hearing names of animals. If the model is correct, this task should engage some of the same conceptual, problem solving processes that are active during a typical “resting” state, resulting in little or no deactivation. In contrast, a purely perceptual task with no requirement for knowledge retrieval, if sufficiently demanding of attentional resources, should interrupt (i.e., suspend, disengage) ongoing conceptual processes, producing robust deactivation. The tone discrimination task we had developed, also inspired by Démonet et al., fit the bill. In our version, subjects listen to short trains of tones and must mentally keep track of how many oddball (higher pitch) tones occur. Thus the task focuses attention on a physical (as opposed to semantic) property of the stimuli and requires that this information be maintained in working memory, thereby preventing the subject from returning to task-unrelated thoughts.
A final element needed to test these predictions was the inclusion of a phonological control. Our model claimed that the areas showing task-induced deactivation are involved in ongoing conceptual processes. A comparison of the semantic decision and tone discrimination tasks is not adequate to test this hypothesis, however, because the resulting activation could be due to perceptual processing and short-term maintenance of complex speech sounds (i.e., phonemes comprising the animal names) compared to simple non-speech sounds (tones). In the phonological control task, once again based on Démonet et al., participants monitored 3-syllable pseudowords for the occurrence of target phonemes (/b/ and /d/). Like the tone discrimination task, this task focuses attention on a physical (as opposed to semantic) property of the stimuli and requires that this information be maintained in working memory. Activation resulting from the contrast between semantic decision and phonological control cannot be due to differences in phonological input and must be conceptual in nature.
It was during the planning of this experiment that I stumbled across the somewhat neglected work of John Antrobus, Jerome Singer, and colleagues, who several decades earlier had investigated behavioral methods for studying task-unrelated thoughts (Antrobus, 1968; Antrobus et al., 1966; Singer, 1993). In an early archetypal experiment, participants were required to perform a tone discrimination task in which they pressed one of several keys in response to different combinations of tones. At random intervals, the task would suddenly stop, and participants were to report on the contents of their mind at the moment the task stopped. The tone task was made easier or more difficult by varying the trial rate and number of tones presented on each trial. The results showed a high prevalence of task-unrelated thoughts (e.g., content involving another place or time) when the tone task was very easy, and a simple monotonic decrease in this “mind-wandering” as the task became more demanding. I had been aware of William James’ introspective writing on the “stream of consciousness” (James, 1890), but the “discovery” of this more modern experimental literature gave me some much-needed encouragement to test these ideas in an fMRI study.2 It also occurred to me that the tone task we had developed for inducing fMRI deactivation was very similar to the tone task used by Antrobus et al., and that the same methods for quantifying task-unrelated thoughts could be used to supplement the fMRI study with relevant behavioral data.
Data collection was carried out during 1995 and 1996. From a technical standpoint, the study was ambitious because we wanted to scan the entire brain, as opposed to the limited coverage typical of fMRI studies at the time, and to apply stereotaxic normalization and group averaging of data, as opposed to the single subject analyses that had been used up to then (Binder et al., 1995; Hinke et al., 1993; McCarthy et al., 1993; Rueckert et al., 1994). I was lucky to have brilliant collaborators at the Medical College of Wisconsin including the physicists Peter Bandettini, James Hyde, and Andrzej Jesmanowicz, and the applied mathematician Robert Cox, who had recently developed the first incarnation of AFNI in response to local demands for stereotaxic normalization and other 3D image processing tools. Results from the straightforward contrast between semantic decision and tone discrimination tasks were published in 1997 (Binder et al., 1997).
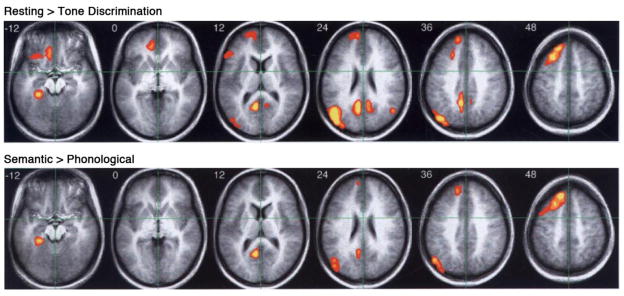
Other results from the study were strongly supportive of our “resting” state model. In a behavioral study outside the scanner, participants reported “task-unrelated thoughts” on 63% of probe trials during the “resting” condition and on only 11% of probe trials during the tone discrimination task. In the scanner, the tone discrimination task produced decreased BOLD signal, relative to “resting,” in all the areas reported previously by Shulman et al., including medial prefrontal cortex, posterior cingulate gyrus and precuneus, angular gyrus, medial temporal lobe, and left lateral orbital frontal cortex (Figure 1, top). The deactivations were more left-lateralized than those reported by Shulman et al., presumably because the active task was non-linguistic.
Figure 1.
Overlap of regions showing “task-induced deactivation” and regions involved in semantic knowledge retrieval. Top row: Higher levels of BOLD signal during a “resting” state compared to a perceptual tone discrimination task. Bottom row: Higher levels of BOLD signal during a semantic decision task compared to a phonological control task. Overlapping regions include the medial temporal lobe, posterior cingulate gyrus, angular gyrus, and dorsomedial prefrontal cortex. Adapted from Binder et al. (1999).
In keeping with the main hypothesis, the semantic decision task produced no significant deactivation in these regions, except for a small focus in the right posterior cingulate, suggesting that, unlike the tone task, the semantic task engaged these regions. Even more striking to us was the close similarity between areas showing deactivation and areas showing relative activation in the semantic-phonological contrast (Figure 1, bottom). As predicted by the model, many of the areas that are active during “rest” and deactivated by a perceptual task appear to be engaged specifically in conceptual processes. These results were robust, but outside the mainstream thinking of the time. An oral abstract was presented in 1996 (Binder et al., 1996), yet I struggled for several years with how best to formulate the interpretation and place it in the context of previous work. In the process I encountered very helpful research in primate cognitive evolution (Barkow et al., 1992; Corballis, 1991; Donald, 1991) and “spontaneous thought” (Antrobus, 1968; Pope and Singer, 1976; Solomon et al., 1961). An important turning point was the realization that ongoing conceptual processing has played a profound adaptive role in human survival. Unlike other animals, our ability to retrieve and manipulate information “outside of time” enables people to solve problems, create cultural and technological artifacts, and plan the future independent from immediate ongoing perceptual experience.3 A paper emphasizing these aspects of the study was finally published in 1999 (Binder et al., 1999).
Subsequent Research
The idea that “resting” is an active cognitive state has become widely accepted among functional imaging researchers over the ensuing decade, and the adaptive significance of “resting” cognition is increasingly recognized (Andrews-Hanna, 2011; Buckner et al., 2008; Raichle, 2011; Spreng et al., 2010). The hypothesis that deactivation results from reallocation of attentional resources from internal to external sources of information has been broadly supported by imaging studies of task-unrelated thought modulation (Mason et al., 2007; McKiernan et al., 2006; McKiernan et al., 2003) and the demonstration of large-scale anti-correlated networks associated with intrinsic and extrinsic information processing (Fox et al., 2005; Golland et al., 2007; Kelly et al., 2008).
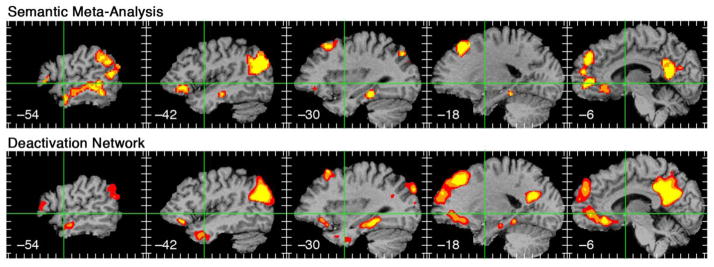
Recognition that the “resting” state is highly active has also led to ongoing efforts to better understand “resting state” cognitive processes. A large number of specific functions have been ascribed to the deactivation network, including episodic memory, autobiographical memory, self-projection, prospection, scene construction, theory-of-mind, emotion monitoring, self-referential processes, and so on (Andreasen et al., 1995; Buckner and Caroll, 2007; Gusnard et al., 2001; Hassabis and Maguire, 2007; Schacter and Addis, 2007; Spreng et al., 2009). My colleagues and I recently performed a voxel-based meta-analysis of activation foci from 120 neuroimaging studies of semantic processing (Binder et al., 2009). Foci were included only if the contrast that produced them included adequate controls for a variety of non-semantic processes (attention, perception, phonology, etc.). As shown in Figure 2, the network identified by statistically overlapping these foci is strikingly similar to the network typically deactivated by perceptual tasks. The contrasts included in this meta-analysis focused on general semantic knowledge retrieval (typically knowledge about object concepts) and did not emphasize episodic, autobiographical, social, emotional, self, or any other specific knowledge domain. It thus seems likely that the same conceptual network underlies all of these specific types of knowledge (see Buckner et al., 2008; Spreng et al., 2009 for further evidence).
Figure 2.
Overlap of left hemisphere regions identified in a large-scale meta-analysis of 120 semantic imaging experiments (top) and regions showing task-induced deactivation in a comparison of “resting” and a perceptual tone discrimination task (bottom). Similarity between the maps is striking given that they are derived using very different types of data and analysis methods. Reprinted from Binder et al. (2009).
For lack of a better term, regions that show higher levels of activity at “rest” are commonly referred to as the “default mode” network (Raichle et al., 2001). The term nicely captures the fact that these regions seem to return to an active state spontaneously whenever attention is not directed to an extrinsic input. On the other hand, the label says nothing about the nature of the information processing that characterizes this state, nor does it capture anything about the adaptive value of engaging these processes during resting and predictable states. The term is also a misnomer because the cognitive processes that characterize this state are not unique to “resting” and “passive” conditions, but are also clearly engaged during many active tasks. Perhaps as these processes become better understood, a more descriptive and precise term – such as “conceptual network,” “knowledge network,” or “semantic network” – can be substituted for the current default term.
Footnotes
I apologize again to Wise et al. for seeming to single out this study. It was by far the best designed and executed of the many language imaging studies in those years that used resting or passive baselines, and was the most influential. Unlike other similar studies, the results were robust and unambiguous – and therefore provide an easier target. Wise and several of his co-authors also deserve appreciation for having helped carry out the Démonet et al. study soon afterward, providing a comparison that has yielded valuable insights into language comprehension networks and the resting state.
I was, regrettably, unaware of the 1995 paper on REST by Andreasen et al. that had recently appeared, though knowledge of it might have made conception of the fMRI study much easier.
As pointed out by Andreasen et al., cognitive activity during REST “is a resource not only for the creative process, but also for meditational states, religious experiences, and dreams.” I remained unaccountably ignorant of this prescient paper until several years later.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander MP, Hiltbrunner B, Fischer RS. Distributed anatomy of transcortical sensory aphasia. Archives of Neurology. 1989;46:885–892. doi: 10.1001/archneur.1989.00520440075023. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD. Remembering the past: Two facets of episodic memory explored with positron emission tomography. American Journal of Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. Neuroscientist. 2011 doi: 10.1177/1073858411403316. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus JS. Information theory and stimulus-independent thought. British Journal of Psychology. 1968;59:423–430. [Google Scholar]
- Antrobus JS, Singer JL, Greenberg S. Studies in the stream of consciousness: Experimental enhancement and suppression of spontaneous cognitive processes. Perceptual and Motor Skills. 1966;23:399–417. [Google Scholar]
- Barkow JH, Cosmides L, Tooby J, editors. The adapted mind. Oxford University Press; New York: 1992. [Google Scholar]
- Binder JR, Desai R, Conant LL, Graves WW. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. Journal of Cognitive Neuroscience. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional MRI. Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW. Left hemisphere activation at rest: a functional MRI study. Neurology. 1996;46(Suppl 2):A423. [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Frost JA, Bandettini PA, Jesmanowicz A, Hyde JS. Lateralized human brain language systems demonstrated by task subtraction functional magnetic resonance imaging. Archives of Neurology. 1995;52:593–601. doi: 10.1001/archneur.1995.00540300067015. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Garron DC, Trost-Cardamone JE, Wichter MD, Schwartz D. Word deafness: one hundred years later. Journal of Neurology, Neurosurgery, and Psychiatry. 1986;49:489–499. doi: 10.1136/jnnp.49.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Caroll DC. Self-projection and the brain. Trends in Cognitive Sciences. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The lopsided ape: Evolution of the generative mind. Cambridge University Press; Cambridge, UK: 1991. [Google Scholar]
- Damasio H. Neuroimaging contributions to the understanding of aphasia. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Elsevier; Amsterdam: 1989. pp. 3–46. [Google Scholar]
- Démonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Donald M. Origins of the modern mind. Harvard University Press; Cambridge, MA: 1991. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud S. Complete psychological works, standard edition. Vintage Books; New York: 1999. [Google Scholar]
- Frith CD, Friston KJ, Liddle PF, Frackowiak RSJ. A PET study of word finding. Neuropsychologia. 1991;29:1137–1148. doi: 10.1016/0028-3932(91)90029-8. [DOI] [PubMed] [Google Scholar]
- Galaburda A, Sanides F. Cytoarchitectonic organization of the human auditory cortex. Journal of Comparative Neurology. 1980;190:597–610. doi: 10.1002/cne.901900312. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Aphasia. New England Journal of Medicine. 1971;284:654–656. doi: 10.1056/NEJM197103252841206. [DOI] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, Hasson U, Malach R. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cerebral Cortex. 2007;17:766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomic correlation. Annals of Neurology. 1990;27:226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA. Deconstructing episodic memory with construction. Trends in Cognitive Sciences. 2007;11:299–306. doi: 10.1016/j.tics.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Grady CL. The functional organization of human extrastriate cortex: A PET-rCBF study of selective attention to faces and locations. Journal of Neuroscience. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschen SE. On the hearing sphere. Acta Oto-laryngologica. 1918–1919;1:423–486. [Google Scholar]
- Hinke RM, Hu X, Stillman AE, Kim SG, Merkle H, Salmi R, Ugurbil K. Functional magnetic resonance imaging of Broca’s area during internal speech. Neuroreport. 1993;4:675–678. doi: 10.1097/00001756-199306000-00018. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Ingvar DH. Memory of the future: an essay on the temporal organization of conscious awareness. Human Neurobiology. 1985;4:127–136. [PubMed] [Google Scholar]
- James W. Principles of psychology. Vol. 1. Dover Publications; New York: 1890. The stream of consciousness; pp. 224–290. [Google Scholar]
- Kawashima R, O’Sullivan BT, Roland PE. Positron-emission tomography studies of corss-modality inhibition in selective attentional tasks: Closing the “mind’s eye”. Proceedings of the National Academy of Sciences USA. 1995;92:5969–5972. doi: 10.1073/pnas.92.13.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Sheppard A, MacKenzie R. Localization in transcortical sensory aphasia. Archives of Neurology. 1982;39:475–478. doi: 10.1001/archneur.1982.00510200017002. [DOI] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, Godoy J. Basal temporal language area. Brain. 1991;114:743–754. doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, Kandel ER. Natural language, disorders of language, and other localizable disorders of cognitive function. In: Kandel ER, Schwartz J, editors. Principles of Neural Science. Elsevier Science Publishing Co; New York: 1985. pp. 688–703. [Google Scholar]
- McCarthy G, Blamire AM, Rothman DL, Gruetter R, Shulman RG. Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. Proceedings of the National Academy of Science, USA. 1993;90:4952–4956. doi: 10.1073/pnas.90.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: An fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Pope KS, Singer JL. Regulation of the stream of consciousness: Toward a theory of ongoing thought. In: Schwartz GE, Shapiro D, editors. Consciousness and self-regulation. Plenum Press; New York: 1976. pp. 101–135. [Google Scholar]
- Proust M. Remembrance of Things Past (1913–1927) Random House; New York: 1981. [Google Scholar]
- Raichle ME. The restless brain. Brain Connectivity. 2011;1:3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, McLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert L, Appollonio I, Grafman J, Jezzard P, Johnson R, Le Bihan D, Turner R. Magnetic resonance imaging functional activation of left frontal cortex during covert word production. Journal of Neuroimaging. 1994;4:67–70. doi: 10.1111/jon19944267. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philosophical Transactions of the Royal Society of London: Series B. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Meizin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Silveri CM, Gainotti G. Interaction between vision and language in category-specific semantic impairment. Cognitive Neuropsychology. 1988;5:677–709. [Google Scholar]
- Singer JL. Experimental and theoretical studies of consciousness. John Wiley & Sons; Chichester: 1993. Experimental studies of ongoing conscious experience; pp. 100–122. [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Poncet M. The role of sensorimotor experience in object recognition. Brain. 1991;114:2555–2573. doi: 10.1093/brain/114.6.2555. [DOI] [PubMed] [Google Scholar]
- Skinner BF. The steep and thorny way to a science of behavior. American Psychologist. 1975;30:42–49. doi: 10.1037//0003-066x.30.1.42. [DOI] [PubMed] [Google Scholar]
- Solomon P, Kubzansky PE, Leiderman PH, Mendelson JH, Trumbull R, Wexler D. Sensory deprivation. Harvard University Press; Cambridge, MA: 1961. [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton E, Wise RJS, Price CJ, Weiller C, Hadar U, Ramsay S, Frackowiak RSJ. Noun and verb retrieval by normal subjects. Studies with PET. Brain. 1996;119:159–179. doi: 10.1093/brain/119.1.159. [DOI] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R. Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain. 1991;114:1803–1817. doi: 10.1093/brain/114.4.1803. [DOI] [PubMed] [Google Scholar]
- Yetkin FZ, Hammeke TA, Swanson SJ, Morris GL, Mueller WM, McAuliffe TL, Haughton VM. A comparison of functional MR activation patterns during silent and audible language tasks. American Journal of Neuroradiology. 1995;16:1087–1092. [PMC free article] [PubMed] [Google Scholar]