Abstract
Study Objective
To determine if metformin has direct effects on ovarian theca-interstitial cell proliferation through activation of AMP-activated protein kinase (AMPK).
Design
In vitro experimental study.
Setting
Academic medical center laboratory.
Animal(s)
Immature Sprague-Dawley female rats
Interventions
Ovarian theca-interstitial (T-I) cells were isolated, purified and cultured in the absence (control) or presence of insulin (1mcg/mL) with or without metformin or other activators/inhibitors of AMPK (AICAR, Compound C).
Main outcome measure(s)
Proliferation was assessed by determination of expression levels of proteins involved in cell cycle progression, cyclin D3 and cyclin-dependent kinase 4 (CDK4) with Western blot analysis, and determination of DNA synthesis with bromodeoxyuridine (BrdU) incorporation assay. Activation of AMPK, Erk1/2 and S6K1 was determined by Western blot analysis with the use of antibodies specific for the phosphorylated (activated) forms.
Results
Metformin inhibited insulin-induced ovarian T-I cell proliferation and upregulation of cell cycle regulatory proteins, cyclin D3 and CDK4. Metformin independently activated AMPK in a dose-dependent manner. Treatment with metformin inhibited insulin-induced activation of Erk1/2 and S6K1. This effect was reversed with the addition of compound C, a known AMPK inhibitor.
Conclusions
Metformin directly inhibits proliferation of ovarian theca-interstitial cells via an AMPK-dependent mechanism. Present findings further validate potential benefits of metformin in the treatment of conditions associated with hyperinsulinemia and excessive growth of ovarian T-I cells (such as PCOS).
Keywords: metformin, AMPK, theca-interstitial cell, insulin, proliferation, PCOS
Introduction
Polycystic ovary syndrome (PCOS) is a multisystem, reproductive-metabolic disorder that affects 5–10% of women of childbearing age (1, 2). Hyperandrogenism is one of the most notable features of the syndrome and results in acne, hirsutism and ovulatory dysfunction. While both the ovary and adrenal glands contribute to the pool of increased circulating androgens, hyperplasia of the ovarian theca-interstitial (T-I) cell compartment is a key feature of the polycystic ovary phenotype and is the major driving factor of androgen excess (3, 4). Some investigators have related hyperandrogenemia to a greater intrinsic capability of producing androgens by T-I cells of women with PCOS; however, aberrant signaling with high tonic levels of LH and insulin compound this effect by leading to increased total number of T-I cells and greater mass effect (5–7). Several in vitro studies have demonstrated that LH and insulin directly stimulate proliferation of T-I cells leading to increased androgen production (8–10).
Current mainstays of therapy include birth control pills in women not attempting for a pregnancy and ovulation induction for those who do desire a pregnancy. However, longer term therapies (such as metformin) that address not only anovulation, but also other components of the syndrome (e.g., insulin-resistance and increased risk of cardiovascular disease) are still underutilized clinically.
Metformin (1,1-dimethylbiguanide hydrochloride) is an oral anti-hyperglycemic medication that was first approved for use in the United States in 1995 and has since become a mainstay in the treatment of type 2 diabetes. The medication has also proven to be useful in the treatment of polycystic ovary syndrome. In previous clinical studies of women with PCOS, metformin has been shown to induce regular menstrual cycles, improve hyperinsulinemia and reduce hyperandrogenemia (11–14). While its actions on regulation of glucose metabolism and insulin, through inhibition of hepatic gluconeogenesis, have been well-documented, the mechanism by which it improves ovarian function still remains unclear (15, 16). The systemic effects of insulin sensitization and improved metabolic control certainly are beneficial to women with PCOS and documented insulin-resistance; however, the variability with which it is capable of restoring ovulatory cycles independent of improvements in insulin levels seems to suggest adjunctive effects to these actions, possibly more locally at the level of the ovary (16, 17).
In vitro studies examining the mechanisms of action of metformin have pointed to its ability to activate AMP-activated protein kinase (AMPK), an ubiquitously expressed serine/threonine kinase important in the regulation of cellular energy (18). AMPK is a pleiotropic heterotrimeric protein kinase that acts as a fuel gauge for the cell in sensing fluctuations in the ratio of AMP to ATP. Under conditions of stress, AMPK blocks anabolic, ATP-consuming biosynthetic pathways through phosphorylation of downstream substrates in efforts to restore ATP levels (19, 20). In fact, several in vitro studies have shown processes such as cholesterol synthesis, protein synthesis, cell growth and proliferation all appear to be blunted when AMPK is activated. Studies of metformin’s ability to inhibit gluconeogenesis in the liver have shown the effect to be due, at least in part, to metformin activating AMPK (18).
Past studies of metformin for the treatment of PCOS have focused largely on its insulin-sensitizing effects or possibly on its effects on steroidogenesis (21–23). More recent in vitro studies with metformin have pointed to an anti-proliferative mechanism associated with activation of AMPK (24, 25). Given the predominance of hyperplasia of ovarian theca-interstitial (T-I) cells with PCOS, we hypothesized that metformin’s ability to improve ovarian function occurs, in part, through direct action on the T-I cell compartment by activating AMPK and thereby controlling the overall mass-effect of androgen producing cells.
Here, we studied the effect of metformin on the proliferation of T-I cells in response to insulin, a known mitogenic factor contributing to T-I cell hyperplasia, in primary cultured rat ovarian theca cells. These findings provide further insights into the mechanisms by which metformin acts at the level of the ovary and further validate its potential therapeutic benefit in conditions associated with theca-interstitial hyperplasia such as PCOS.
Materials and Methods
Chemicals, Hormones and Antibodies
Medium 199, McCoy’s 5A medium, L-glutamine, and HEPES buffer were purchased from Invitrogen/GIBCO (Carlsbad, CA). Penicillin-streptomycin was purchased from Roche Diagnostics (Indianapolis, IN). Collagenase (CLS I) and deoxyribonuclease I were obtained from Worthington Biochemical Corp. (Freehold, NJ). BSA, purified bovine insulin, metformin (1,1-Dimethylbiguanide hydrochloride), AICAR (5-Amino-1-β-D-ribofuranosyl-1H-imidazole-4-carboxamide), compound C (6-[4-(2-Piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo[1,5-a]pyrimidine), and β-tubulin antibody were purchased from Sigma Chemical Co. (St. Louis, MO). Antibodies against phosphorylated AMPK (Thr172), phosphorylated MAPK (Thr202/Tyr204) (Erk1/2), phosphorylated p70 S6 kinase (Thr 389), cyclin D3, Cdk4 were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against total AMPKα 1/2 and Erk 1/2 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antimouse, antirabbit IgG horseradish peroxidase conjugates, enhanced chemiluminescence using the Femto Supersignal Substrate System and Restore Western blot stripping buffer were purchased from Pierce (Rockford, IL). All other reagents used were conventional commercial products.
Animals
Sprague-Dawley female rats (25 d old) were purchased from Charles River Laboratories (Wilmington, MA). All the experimental protocols used in this study were approved by the University Committee on the Use and Care of Animals. Animals were housed in a temperature-controlled room with proper dark-light cycles as per the guidelines provided by the University Committee on the Use and Care of Animals (UCUCA). The animals were killed by CO2 asphyxiation. The ovaries were removed under sterile conditions and were processed immediately for the isolation of theca-interstitial (T-I) cells.
Isolation and culture of T-I cells
The T-I cells were isolated, dispersed, and cultured following a protocol previously published from our laboratory (26, 27). Briefly, freshly collected ovaries were placed in medium 199 containing 25 mM HEPES (pH 7.4), 2 mM L-glutamine, 1 mg/ml BSA, 100 U/ml penicillin, and 100 µg/ml streptomycin. The ovaries were freed from adhering fat and actively punctured with a 27-gauge needle under a dissecting microscope to release the granulosa and red blood cells. The remaining ovarian tissue was then washed three times with medium to release any remaining granulosa cells. The tissue was then minced and incubated for 30 min at 37 C in the same medium, supplemented with 0.65 mg/ml collagenase type 1 plus 10 µg/ml deoxyribonuclease. The dispersion was encouraged by mechanically pipetting the ovarian tissue suspension with a 10-ml pipette. The T-I cells released by this digestion process were centrifuged at 1000 rpm for 5 min and washed in medium two times to eliminate remaining collagenase. The dispersed cells were then resuspended in McCoy’s 5A medium containing 2 mM L-glutamine, 1 mg/ml BSA, 100 U/ml penicillin, and 100 µg/ml streptomycin and subjected to unit gravity sedimentation for 5 min to eliminate small fragments of undispersed ovarian tissue. Cell viability was assessed by trypan blue exclusion and was always above 90%. The dispersed cells were seeded in 60-mm plates (2 × 106 viable cells). The plated cells were maintained overnight in McCoy’s 5A medium containing 2 mM L-glutamine, 1 mg/ml BSA, 100 U/ml penicillin, and 100 µg/ml streptomycin in a humidified atmosphere of 95% air-5% CO2 at 37 C. After allowing cells to attach, they were treated with insulin and metformin for different time intervals, and inhibitors were used as indicated in the figure legends.
Western blot analysis
After various treatments described in the respective figure legends, cell monolayers were washed with Hanks’ balanced salt solution (1x) and then were solubilized using radioimmunoprecipitation assay buffer (PBS containing 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate). Cell lysates were then sonicated and centrifuged for 15 min at 14,000 × g. The protein content of the supernatants was determined using BCA reagent (Pierce). Proteins (50 µg/lane) were separated by electrophoresis using 12% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) before immunoblot analysis. Membranes were blocked with 5% fat-free milk in 20 mM Tris base (pH 7.45), 137 mM NaCl, and 0.1% Tween 20 (TBST) for 1 h at room temperature and then incubated overnight at 4 C with primary antibody in 5% fat-free milk/TBST. After three 5-min washes with TBST, membranes were incubated in appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Three additional 5-min washes with TBST were performed and subsequently membrane-bound antibodies were detected with the Femto Supersignal Substrate System Western blotting detection kit (Pierce). Protein loading was monitored by reprobing the same blots with appropriate antibodies (loading control) as indicated in the figure legends.
BrdU cell proliferation assay
Cell proliferation was evaluated by measuring the incorporation of BrdU using BrdU immunoassay kit (Calbiochem). In brief, T-I cells were seeded into 96-well plates and cultured overnight with 0.1% BSA-containing McCoy’s medium. After attachment, cells were treated with insulin (1 mcg/ml) for 24 h with or without metformin (3 mmol/L). Cells were labeled with BrdU during the above treatment periods. The reactions were terminated by removing the media, and cells were incubated with fixative/denaturing solution followed by BrdU antibody for 1 h at room temperature. Unbound antibody was washed, and then horseradish peroxidase-conjugated goat antimouse IgG was added for 30 min at room temperature. After washing three times, substrate was added and incubated in the dark for 15 min. The plates were read using a spectrophotometric plate reader.
Statistical analysis
Statistical analysis was carried out using ANOVA followed by the Tukey multiple comparison test using Prism software (GraphPad Prism, version 3.0; GraphPad, Inc., San Diego, CA). Values were considered statistically significant at P < 0.05. Each experiment was repeated at least three times, with similar results. Blots shown are representative of one experiment, and graphs represent the mean ± SE of three replicates.
Results
Metformin Inhibits T-I Cell Proliferation and Cell Cycle Regulatory Proteins
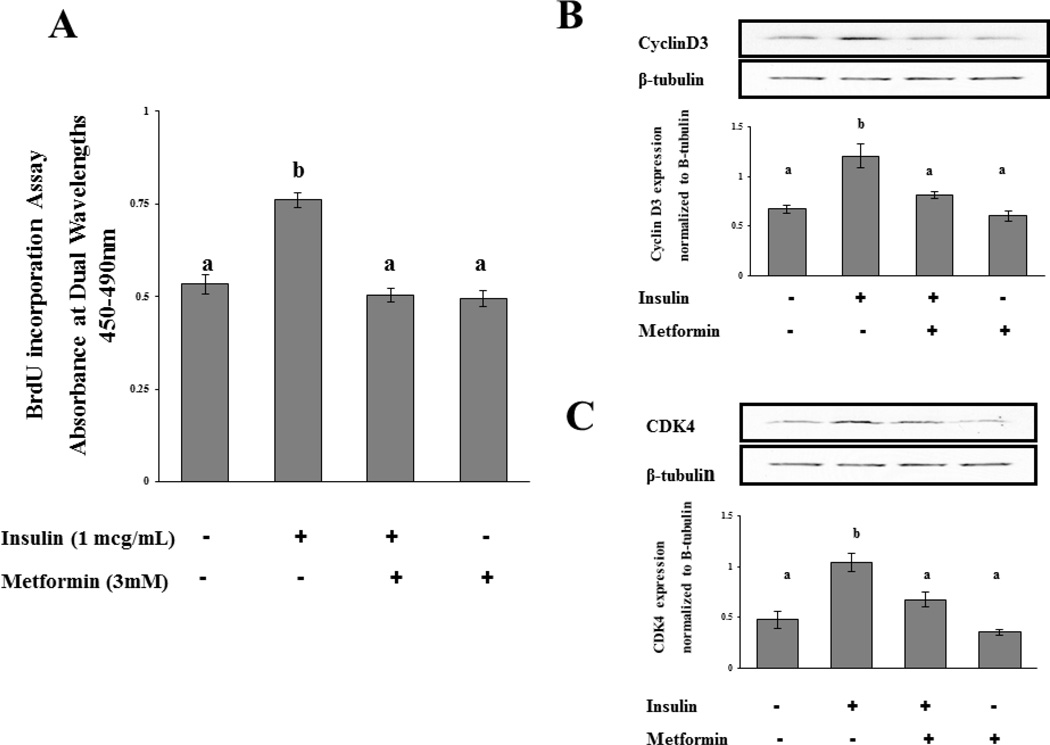
Cultured rat ovarian T-I cells were treated with insulin (1mcg/mL) for 24 hours with and without metformin (3mM) in the presence of bromodeoxyuridine (BrdU). The 3 mM concentration was chosen as this dose is similar to the daily dose of metformin used for women with hyperandrogenism due to PCOS (1500 milligrams/3 Liters plasma volume in average human) (28). Proliferation of T-I cells was assessed by BrdU incorporation assay as described in the Materials and Methods. Figure 1A shows that insulin stimulates a 50% increase in the incorporation of BrdU into newly synthesized DNA compared to control (p<0.05). The insulin-mediated stimulation of proliferation was completely reversed by the addition of metformin (p<0.05).
Figure 1.
Effect of metformin on insulin-induced cell proliferation and cell cycle regulatory protein expression. A, T-I cells were incubated in 0.1% BSA-containing McCoy’s media for 24 hours in 96 well plates at a concentration of 2 × 104 cells/well. Cells were labeled with BrdU, and cell proliferation was assessed by BrdU incorporation as described in Materials and Methods. B–C, Cells were treated without or with metformin (3mM) for 18 h in the presence or absence of insulin (1µg/mL). Cell lysates were analyzed by Western blotting for cyclin D3, CDK4, and for β-tubulin to verify equal protein loading. The graphs in B–C represent densitometric scans of cyclin D3 and CDK4, respectively, normalized for β-tubulin as seen in the representative Western blots. Blots are representative of one experiment, and the graphs represent the mean of three experiments. Error bars represent mean ± SE. Each mean is labeled with a superscript (a or b). b, p < 0.05 vs other treatment groups (a).
Expression of proteins integral to cell cycle progression was also examined as another surrogate marker of T-I cell proliferation to confirm metformin’s ability to attenuate the proliferative effects of insulin. Cell cycle progression from G1 to S phase is regulated by D-type cyclins, which interact and modulate cyclin-dependent kinases (Cdk). Cyclin D3 and Cdk4 have previously been shown as important cell cycle regulatory proteins in theca interstitial cells (8). To determine the inhibitory effect of metformin on insulin-induced cell cycle progression, cells were incubated for 18 hours with insulin (1 mcg/mL) with and without metformin (3mM). Preliminary time course experiments demonstrated peak expression of cell cycle regulatory proteins occurred after 18 hours of incubation with insulin (data not shown). Therefore, in subsequent studies this incubation period was chosen to investigate the effect of metformin on insulin-stimulated expression of cell cycle regulatory proteins. Cell lysates were examined for cyclin D3, Cdk4, and β-tubulin by Western blot analysis. The results show that insulin alone produced a significant increase in expression of cyclin D3 and Cdk4 compared to control (p<0.05). More importantly, our results show that metformin significantly decreased the insulin-induced expression of cyclin D3 and Cdk4 compared to insulin alone (p<0.05) (Figures 1B and 1C, respectively). Treatment with metformin alone showed no significant difference from the control group (p>0.05).
Metformin Independently Activates AMPK in T-I Cells
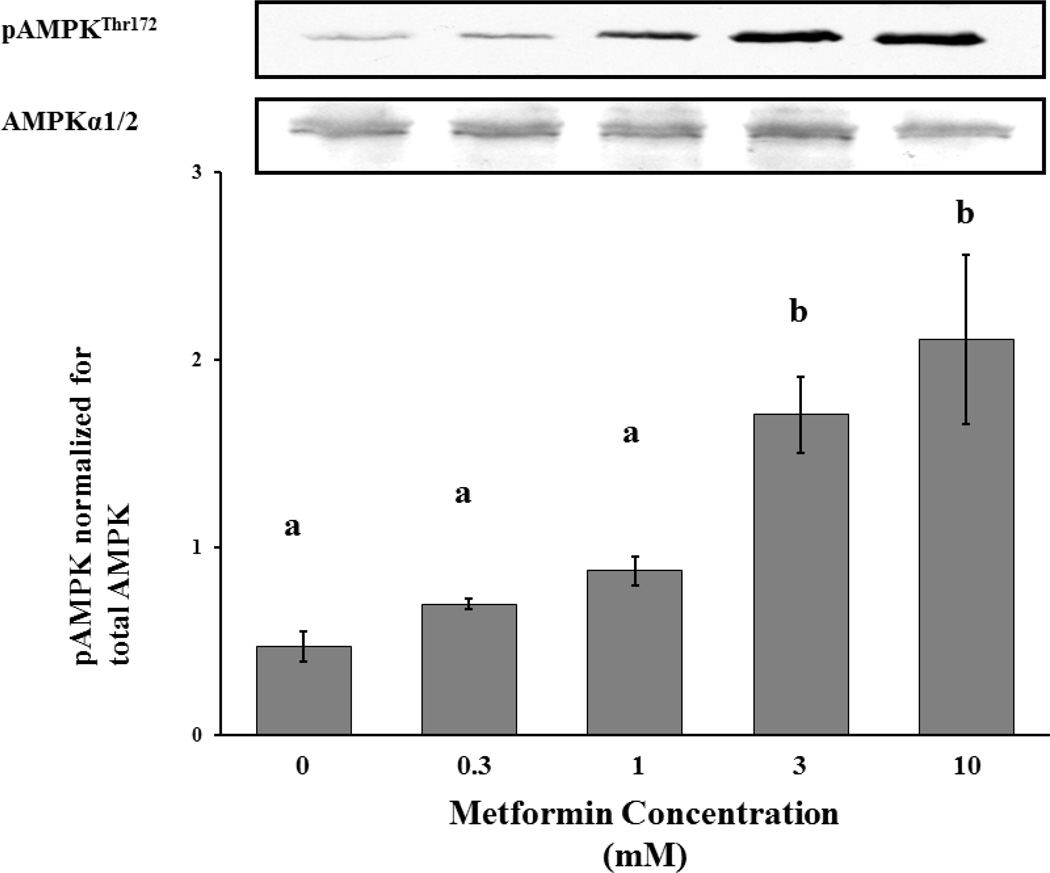
We next examined the underlying mechanism of metformin’s anti-proliferative effect in T-I cells by testing the effect of metformin on the activation of AMPK since previous studies have shown metformin activates this enzyme in the liver (18). Cells were incubated for 18 hours in the absence (McCoy’s 5A media alone) or presence of metformin at various concentrations (300 µM, 1mM, 3mM, 10mM). Eighteen hours was chosen as the incubation time based on experiments with metformin in other somatic cell cultures (24). Cell lysates were collected and examined by Western blot analysis for phosphorylated AMPK using phospho-specific antibody that recognizes AMPK phosphorylated at Thr172 as phosphorylation of this site is shown to be necessary for the activation of AMPK (29). The results presented in Figure 2 show that metformin independently activated AMPK in T-I cells in a dose-dependent manner.
Figure 2.
Dose-response study of effect of metformin on phosphorylation of AMPK. T-I cells (2 × 106 cells/plate) were incubated in 0.1% BSA-containing McCoy’s media for 24 hours and then treated with 0, 0.3, 1, 3, 10 mM Metformin for 18 hours. Cell lysates were analyzed by Western blotting for AMPK phosphorylated at Thr172 and protein loading was normalized for total AMPK as seen in the representative Western blots. Blots are representative of one experiment, and the graphs represent the mean of three experiments. Error bars represent mean ± SE. Each mean is labeled with a superscript (a, b or c). b, P < 0.05 vs a; c, P < 0.01 vs a.
Metformin Inhibits Activation of Erk 1/2 and S6K1
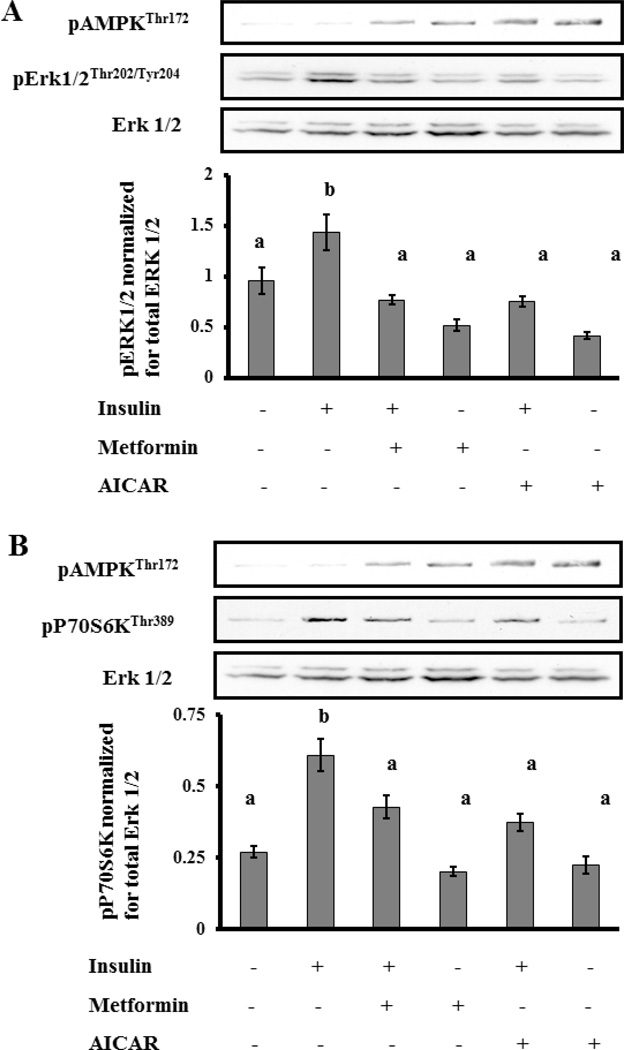
Insulin is known to induce proliferation in T-I cells via two important signal transduction pathways, Erk and Akt/mTOR pathways (30). Because activation of AMPK has been shown in other somatic cell lines to inhibit Akt/mTOR and Erk activation (31), we tested the ability of metformin to inhibit either or both of these two insulin-stimulated mitogenic signaling pathways. In addition, we compared this effect to AICAR, a pharmacological agent known specifically to activate AMPK (32). Cells were preincubated with or without metformin (3mM) for 18 hrs or AICAR (1mM) for 1 hr followed by stimulation with insulin (1mcg/mL) for 10 minutes. Cell lysates were subjected to Western blot analysis for phosphorylated Erk1/2Thr202/Tyr204, phosphorylated S6 kinase 1Thr389 (S6K1), and phosphorylated AMPKThr172. Protein loading was assessed and normalized with antibodies for total Erk1/2. In the absence of either metformin or AICAR, insulin rapidly stimulated phosphorylation of Erk 1/2 and S6K1 (p<0.05). Furthermore, both metformin and AICAR activated AMPK in the presence and absence of insulin (p<0.05). But most importantly, pretreatment with both metformin and AICAR resulted in a significant inhibition of insulin-dependent activation of both Erk 1/2 and S6K1 (p<0.05) (Figure 3A and 3B, respectively).
Figure 3.
Effect of metformin on insulin-induced phosphorylation of Erk1/2 and S6K1. T-I cells (2 × 106 cells/plate) were pretreated without or with metformin (3mM) for 18 h or AICAR (1mM), a specific AMPK activator, for 1 h followed by insulin (1µg/mL) treatment for 10 min. Control groups were treated with vehicle (acid water, pH 2). Cell lysates were analyzed for phospho-specific Erk1/2 (Thr202/Tyr204) in panel A and S6K (Thr389) in panel B and protein loading was normalized for Erk1/2 by Western blotting. Blots are representative of one experiment, and the graphs represent the mean of three experiments. Error bars represent mean ± SE. Each mean is labeled with a superscript (a or b). b, P < 0.05 vs other treatment groups (a).
Metformin’s Effect on Signaling Pathways is AMPK-Dependent
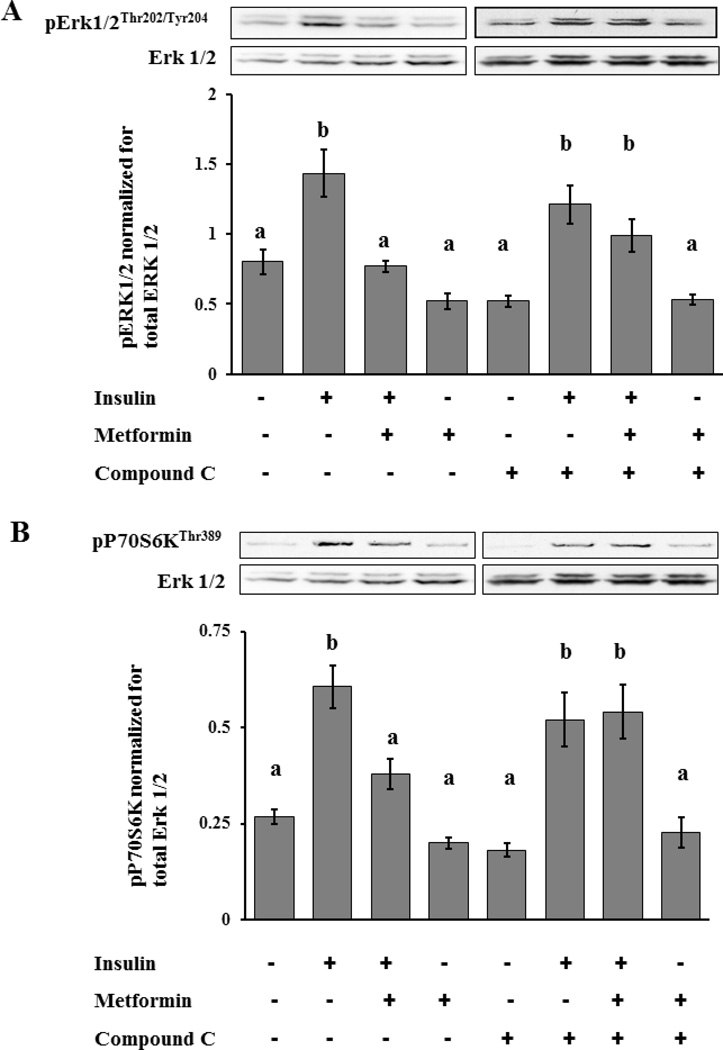
To determine whether metformin’s effect on the proliferation pathways is dependent upon AMPK, we utilized a known AMPK inhibitor, compound C. Compound C is a cell-permeable pyrrazolopyrimidine compound that acts as a potent, selective, reversible, and ATP-competitive inhibitor of AMPK (18). Cells were incubated with or without metformin (3mM) for 18 hrs in the presence or absence of compound C (20µM for 2 hrs) followed by stimulation with insulin (1 mcg/mL) for 10 minutes. Phosphorylation of the proteins important in the signaling cascades were then assayed by Western blot analysis. The addition of compound C had no effect on the ability of insulin to stimulate phosphorylation of either Erk1/2 or S6K1. More importantly, the results show that incubation with compound C, which inhibits AMPK, reversed the inhibitory effects of metformin on insulin signaling pathways. Figures 4A and 4B show that in the presence of compound C, metformin produced no significant inhibition of insulin-stimulated phosphorylation of Erk1/2 and S6K1 (Figure 4A and 4B, respectively) (p>0.05).
Figure 4.
Effect of metformin on insulin-induced phosphorylation of Erk1/2 and S6K1 in cells treated or not treated with compound C, a specific AMPK inhibitor. T-I cells (2 × 106 cells/plate) were incubated without or with metformin (3mM) for 18 hours followed by compound C (20µM) or vehicle (dimethylsulfoxide) for 2 h and insulin (1µg/mL) or vehicle (acid water, pH 2) for 10 min. Cell lysates were analyzed for phospho-specific Erk1/2 (Thr202/Tyr204) in panel A and S6K (Thr389) in panel B and protein loading was normalized for Erk1/2 by Western blotting. Blots are representative of one experiment, and the graphs represent the mean of three experiments. Error bars represent mean ± SE. Each mean is labeled with a superscript (a or b). b, P < 0.05 vs other treatment groups (a).
Discussion
This study presents new insights into the effects of metformin on intracellular signaling cascades, which characterize additional mechanisms of action of metformin in ovarian T-I cells. We show that in rat ovarian T-I cells, metformin: (1) inhibits insulin-induced cell proliferation, (2) activates AMPK in a dose-dependent manner, (3) antagonizes mitogenic insulin signaling cascades, and (4) acts via an AMPK-dependent manner.
The present findings indicate that metformin’s ability to attenuate androgen production by ovarian theca cells is, at least in part, also due to its effect on inhibiting signaling cascades that stimulate proliferation and hyperplasia. Metformin may thereby also act to regulate the overall size of the T-I compartment and subsequent mass effect of these steroidogenically active cells. While our study did not directly measure androgen levels, previous studies with metformin have shown a dose-dependent inhibition of androstenedione production by metformin in cultured T-I cells (33).
Our results clearly show that metformin’s actions are mediated through activation of AMPK. The observation that metformin alone exerted no effect on cell proliferation under unstimulated conditions shows that metformin produced no toxic effects to the cell at the concentrations used in the present study. The ability to activate AMPK was originally reported by investigators examining the mechanism of how metformin inhibits gluconeogenesis in hepatocytes. Since then, the ability of metformin to activate AMPK has been established for other tissues as well (18, 22, 24). Insulin is known to exert mixed effects on AMPK depending on the tissue of interest. In some cells, insulin antagonizes activation of AMPK (such as in cardiac muscle), while in others, such as skeletal muscle, both insulin and activators of AMPK stimulate glucose transport into the cell (34). In ovarian T-I cells, we observed metformin independently activates AMPK and the addition of insulin did not significantly alter this effect. This finding differs from that reported by Pellatt LJ et al who showed that in human granulosa-luteal cells collected from IVF retrieval fluid the stimulatory effect of metformin on AMPK was seen only in the presence of insulin (35). The reason for this discrepancy might be explained on the basis of intrinsic differences in the proliferative capacity of theca cells compared to granulosa-luteal cells.
Hyperinsulinemia has been implicated to be a stimulus of proliferation and subsequent hyperplasia of ovarian theca-interstitial cells, an essential impetus to the development of polycystic ovarian morphology and hyperandrogenism in women with PCOS. Metformin, in previous studies, has been shown to improve this, in part, by its effects on inhibiting gluconeogenesis in the liver and inhibition of ovarian androgen production, through decreases in StAR and cytochrome P450c17α activity (21, 23). Our present findings lend further credence to the ability of metformin to activate AMPK and support an additional mechanism of action with direct inhibitory effects in ovarian T-I cells through insulin-dependent mitogenic signaling pathways. The dose of insulin utilized in this investigation exceeded the saturation threshold for just the insulin receptor and likely reflected activation of the IGF-1 receptor pathway as well. Several studies have shown evidence that PCOS is associated with increased levels of bioavailable IGF-1, possibly due to a decrease in IGFBP-1. Thus our studies still provide an appropriate representative in vitro model for the signals leading to T-I cell hyperplasia associated with PCOS (36–38).
These observations further validate the potential benefits and clinical use of metformin in the treatment of conditions associated with hyperinsulinemia and excessive growth of ovarian theca-interstitial cells (such as PCOS). While clinical studies have shown metformin to have limited short-term clinical utility as an infertility treatment for women with PCOS with only modest improvements in pregnancy rates (39, 40), recent observational studies have demonstrated potential additional benefits of metformin (41, 42). Mechanistic studies such as ours provide biologically plausible explanations to clinical observations. As such, future clinical research should consider examining longer term end points such as change in ovarian morphology, risk of cancer and cardiovascular health. Metformin has recently been found in several epidemiologic studies to be associated with decreased risk of developing cancer (41, 42). Currently, several studies are being conducted to evaluate metformin’s ability to alter the risk of developing ovarian cancer and/or cancer-related outcomes (e.g., survival). Future clinical research on metformin’s ability to alter cancer-related health risks in women with PCOS, a population known to have a 2.5-fold relative risk, is warranted (43).
In summary, metformin has additional direct anti-proliferative effects on ovarian T-I cells. These observations further support its potential utility in the treatment of syndromes associated with theca-interstitial hyperplasia (such as PCOS).
Acknowledgements
This work was supported by NIH grant HD38424.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–2438. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 2.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 3.Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;326:1375–1379. doi: 10.1016/s0140-6736(85)92552-8. [DOI] [PubMed] [Google Scholar]
- 4.Longacre TA, Gilks CB. Nonneoplastic Lesions of the Ovary. In: Nucci MR, Oliva E, editors. Gynecologic Pathology, 1st ed. Orlando, Florida: Churchill Livingstone; 2009. pp. 367–391. [Google Scholar]
- 5.Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. 1994;79:1158–1165. doi: 10.1210/jcem.79.4.7962289. [DOI] [PubMed] [Google Scholar]
- 6.de Ziegler D, Steingold K, Cedars M, Lu JK, Meldrum DR, Judd HL, et al. Recovery of hormone secretion after chronic gonadotropin releasing hormon agonist administration in women with polycystic ovarian disease. J Clin Endocrinol Metab. 1989;68:1111–1117. doi: 10.1210/jcem-68-6-1111. [DOI] [PubMed] [Google Scholar]
- 7.Magoffin DA. The Role of the Ovary in the Genesis of Hyperandrogenism. In: Leung PCK, Adashi EY, editors. The Ovary: 2nd Ed. San Diego, CA: Elsevier; 2004. pp. 513–519. [Google Scholar]
- 8.Palaniappan M, Menon KM. Human chorionic gonadotropin stimulates theca-interstitial cell proliferation and cell cycle regulatory proteins by a cAMP-dependent activation of AKT/mTORC1 signaling pathway. Mol Endocrinol. 2010;24:1782–1793. doi: 10.1210/me.2010-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Effects of Insulin and Insulin-Like Growth Factors on Proliferation of Rat Ovarian Theca-Interstitial Cells. Biol Reprod. 1997;56:891–897. doi: 10.1095/biolreprod56.4.891. [DOI] [PubMed] [Google Scholar]
- 10.Duleba AJ, Spaczynski RZ, Olive DL. Insulin and insulin-like growth factor I stimulate the proliferation of human ovarian theca-interstitial cells. Fertil Steril. 1998;69:335–340. doi: 10.1016/s0015-0282(97)00473-1. [DOI] [PubMed] [Google Scholar]
- 11.Essah PA, Apridonidze T, Iuorno MJ, Nestler JE. Effects of short-term and long-term metformin treatment on menstrual cyclicity in women with polycystic ovary syndrome. Fertil Steril. 2006;86:230–232. doi: 10.1016/j.fertnstert.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 12.Palomba S, Orio F, Jr, Falbo A, Manguso F, Russo T, Cascella T, et al. Prospective parallel randomized, double-blind, double-dummy controlled clinical trial comparing clomiphene citrate and metformin as the first-line treatment for ovulation induction in nonobese anovulatory women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:4068–4074. doi: 10.1210/jc.2005-0110. [DOI] [PubMed] [Google Scholar]
- 13.Baillargeon JP, Jakubowicz DJ, Iuorno MJ, Jakubowicz S, Nestler JE. Effects of metformin and rosiglitazone, alone and in combination, in nonobese women with polycystic ovary syndrome and normal indices of insulin sensitivity. Fertil Steril. 2004;82:893–902. doi: 10.1016/j.fertnstert.2004.02.127. [DOI] [PubMed] [Google Scholar]
- 14.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139–146. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 15.Stumvoll M, Nurijhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 16.Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 17.Palomba S, Falbo A, Russo T, Orio F, Tolino A, Zullo F. Systemic and local effects of metformin administration in patients with polycystic ovary syndrome (PCOS): relationship to the ovulatory response. Human Reproduction. 2010;25:1005–1013. doi: 10.1093/humrep/dep466. [DOI] [PubMed] [Google Scholar]
- 18.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viollet B, Mounier R, Leclerc J, Yazigi A, Foretz M, Andreelli F. Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007;33:395–402. doi: 10.1016/j.diabet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Boyle JG, Salt IP, McKay GA. Meformin action on AMP-activated protein kinase: a translational research approach to understanding a potential new therapeutic target. Diabet Med. 2010;27:1097–1106. doi: 10.1111/j.1464-5491.2010.03098.x. [DOI] [PubMed] [Google Scholar]
- 21.Nestler JE, Jakubowicz DJ. Decreases in Ovarian Cytochrome P450c17alpha Activity and Serum Free Testosterone After Reduction of Insulin Secretion in Polycystic Ovary Syndrome. N Engl J Med. 1996;335:617–623. doi: 10.1056/NEJM199608293350902. [DOI] [PubMed] [Google Scholar]
- 22.Tosca L, Chabrolle C, Uzbekova S, Dupont J. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5'monophosphate-activated protein kinase (AMPK) Biol Reprod. 2007;76:368–378. doi: 10.1095/biolreprod.106.055749. [DOI] [PubMed] [Google Scholar]
- 23.Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril. 2001;76:517–524. doi: 10.1016/s0015-0282(01)01975-6. [DOI] [PubMed] [Google Scholar]
- 24.Ning J, Clemmons DR. AMP-activated protein kinase inhibits IGF-I signaling and protein synthesis in vascular smooth muscle cells via stimulation of insulin receptor substrate 1 S794 and tuberous sclerosis 2 S1345 phosphorylation. Mol Endocrinol. 2010;24:1218–1229. doi: 10.1210/me.2009-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tosca L, Rame C, Chabrolle C, Tesseraud S, Dupont J. Metformin decreases IGF1-induced cell proliferation and protein synthesis through AMP-activated protein kinase in cultured bovine granulosa cells. Reproduction. 2010;139:409–418. doi: 10.1530/REP-09-0351. [DOI] [PubMed] [Google Scholar]
- 26.Palaniappan M, Menon KM. Regulation of sterol regulatory element-binding transcription factor 1a by human chorionic gonadotropin and insulin in cultured rat theca-interstitial cells. Biol Reprod. 2009;81:284–292. doi: 10.1095/biolreprod.108.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisseha S, Towns R, Harada M, Peegel H, Menon KM. Inhibitory effect of valproic acid on ovarian androgen biosynthesis in rat theca-interstitial cells. Endocrine. 2010;37:187–193. doi: 10.1007/s12020-009-9287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pugeat M, Ducluzeau PH. Insulin resistance, polycystic ovary syndrome and metformin. Drugs. 1999;58(Suppl 1):41. doi: 10.2165/00003495-199958001-00010. [DOI] [PubMed] [Google Scholar]
- 29.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 30.Spiegel AM, Carter-Su C, Taylor SI, Kolkarni RN. Mechanism of Action of Hormones That Act at the Cell Surface. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams Textbook of Endocrinology, 12th ed. Philadelphia, PA: Elsevier; 2011. pp. 62–82. [Google Scholar]
- 31.Kim J, Yoon MY, Choi SL, Kang I, Kim SS, Kim YS, et al. Effects of stimulation of AMP-activated protein kinase on insulin-like growth factor 1- and epidermal growth factor-dependent extracellular signal-regulated kinase pathway. J Biol Chem. 2001;276:19102–19110. doi: 10.1074/jbc.M011579200. [DOI] [PubMed] [Google Scholar]
- 32.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 1995 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 33.Mansfield R, Galea R, Brincat M, Hole D, Mason H. Metformin has direct effects on human ovarian steroidogenesis. Fertil Steril. 2003;79:956–962. doi: 10.1016/s0015-0282(02)04925-7. [DOI] [PubMed] [Google Scholar]
- 34.Towler MC, Hardie DG. AMP-Activated Protein Kinase in Metabolic Control and Insulin Signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 35.Pellatt LJ, Rice S, Mason HD. Phosphorylation and Activation of AMP-Activated Protein Kinase (AMPK) by Metformin in the Human Ovary Requires Insulin. Endocrinology. 2011;152:1112–1118. doi: 10.1210/en.2009-1429. [DOI] [PubMed] [Google Scholar]
- 36.Iwashita M, Mimuro T, Watanabe M, Setoyama T, Matsuo A, Adachi T, et al. Plasma levels of insulin-like growth factor-I and its binding protein in polycystic ovary syndrome. Horm Res. 1990;33(Suppl 2):21–26. doi: 10.1159/000181561. [DOI] [PubMed] [Google Scholar]
- 37.Homburg R, Pariente C, Lunenfeld B, Jacobs HS. The role of insulin-like growth factor-1 (IGF-1) and IGF binding protein-1 (IGFBP-1) in the pathogenesis of polycystic ovary syndrome. Hum Reprod. 1992;7:1379–1383. doi: 10.1093/oxfordjournals.humrep.a137577. [DOI] [PubMed] [Google Scholar]
- 38.Suikkari AM, Ruutiainen K, Erkkola R, Seppala M. Low levels of low molecular weight insulin-like growth factor-binding protein in patients with polycystic ovarian disease. Hum Reprod. 1989;4:136–139. doi: 10.1093/oxfordjournals.humrep.a136858. [DOI] [PubMed] [Google Scholar]
- 39.Moll E, Bossuyt PM, Korevaar JC, Lambalk CB, van der Veen F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomized double blind clinical trial. BMJ. 2006;332:1485. doi: 10.1136/bmj.38867.631551.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356:551–566. doi: 10.1056/NEJMoa063971. [DOI] [PubMed] [Google Scholar]
- 41.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JMM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol. 1996;88:554–559. doi: 10.1016/0029-7844(96)00226-8. [DOI] [PubMed] [Google Scholar]