Abstract
BAFF (B-cell–activating factor) is a critical survival factor for transitional and mature B cells and is a promising therapeutic target for systemic lupus erythematosus (SLE). In 2010–2011, two phase 3 clinical trials showed that the addition of the anti-BAFF antibody belimumab to standard-of-care therapy in patients with moderately active SLE results in a better outcome at 52 weeks than standard-of-care therapy alone. Belimumab has been US Food and Drug Administration approved for the treatment of SLE, and other drugs that target BAFF are now in various stages of clinical testing. This review describes the function of BAFF and its homolog APRIL (a proliferation-inducing ligand) and addresses the rationale for the treatment of SLE with BAFF/APRIL inhibitors.
Keywords: Systemic lupus erythematosus, SLE, B cells, BAFF, APRIL, Belimumab, Atacicept, B-cell selection, Murine models, Inhibition, Clinical trials
Introduction
BAFF (B-cell–activating factor) and APRIL (a proliferation-inducing ligand) are homotrimers belonging to the tumor necrosis factor family that are widely expressed by many cell types, including hematopoietic and stromal cells. Increased serum levels of BAFF and/or its homolog APRIL are found in patients with autoimmune diseases, including systemic lupus erythematosus (SLE), and both cytokines can be elaborated in inflammatory sites. The appreciation that BAFF overexpression causes SLE and that BAFF inhibition delays SLE onset in murine models has spurred the development of therapeutic agents for inhibiting BAFF and APRIL. The monoclonal anti-BAFF antibody belimumab is the first new drug in 50 years to be US Food and Drug Administration approved for the treatment of SLE, and the clinical efficacy of several other inhibitors of BAFF and/or APRIL is currently being tested. Although two large phase 3 studies of belimumab, added to standard-of-care therapy showed modest benefit over standard-of-care therapy alone for moderately active SLE, the primary clinical end point was no longer met after 1 year of therapy. Further investigation should help clarify whether there is a subset of patients who respond to therapy and identify the appropriate way to use BAFF/APRIL inhibition within the SLE therapeutic armamentarium.
The Physiology of BAFF and APRIL and Their Receptors
B Cells
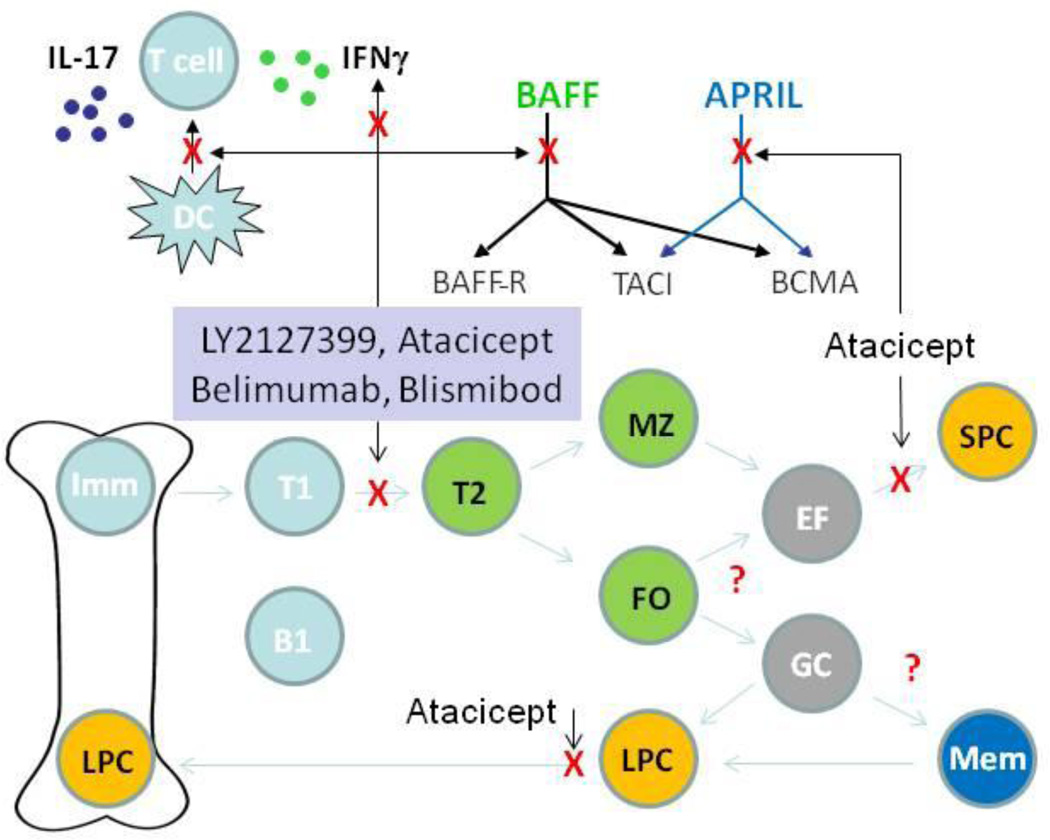
BAFF and APRIL have three receptors—BAFF-R, transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), and B-cell maturation factor Ag (BCMA)—each of which is differentially expressed by B cells during their ontogeny. BAFF-R is expressed at the late transitional (T2) B-cell stage and on all mature B cells, is downregulated on germinal center B cells, is re-expressed on memory cells, and is absent on plasma cells. TACI is expressed on B cells after the T2 stage and on plasmablasts and plasma cells, whereas BCMA is upregulated exclusively on plasmablasts and plasma cells. BAFF-R is specific for BAFF and signals through the alternative nuclear factor-κB (NF-κB) pathway to enhance B-cell survival by upregulating anti-apoptotic proteins and through mTOR and Pim2 to promote cell growth. TACI and BCMA bind to both BAFF and APRIL and signal through the classic NF-κB pathway and other pathways to counteract apoptosis and to drive immunoglobulin class switching (Fig. 1) [1, 2•].
Fig 1.
Proposed mechanisms of action of human BAFF and APRIL inhibitors: BAFF and APRIL bind differently to the three receptors (BAFF-R, TACI, and BCMA). Selective BAFF inhibitors block the interaction between BAFF and its receptors, leaving APRIL functions intact, whereas the dual BAFF/APRIL inhibitor atacicept (TACI-Ig) blocks the interaction of both BAFF and APRIL with all three receptors. The effect of the inhibitors on B-cell subsets is shown. Survival of cells in blue is not affected by BAFF or APRIL inhibition. Survival of cells in green is inhibited by selective BAFF inhibition. Survival of cells in orange is inhibited by dual BAFF/APRIL inhibition. The effect of BAFF inhibition on cells in gray is not known. BAFF inhibition may also alter the function of dendritic cells (DCs) and T cells and alter T-cell cytokine secretion. B1, B1 B cell; EF, extrafollicular focus; FO, follicular B cell; GC, germinal center; IL, interleukin; Imm, immature B cell; LPC, long-lived plasma cell; Mem, memory B cell; MZ, marginal zone B cell; SPC, short-lived plasma cell; T1, transitional type 1; T2, transitional type 2
BAFF is cleaved from the cell surface to form a soluble homotrimer [3], whereas APRIL is cleaved intracellularly and secreted as a soluble protein. A small proportion of circulating BAFF forms soluble 60-mer multimers, whereas APRIL multimerizes on cell surfaces by attaching to proteoglycans. Circulating BAFF homotrimers bind well to BAFF-R, but binding of both BAFF and APRIL to TACI or BCMA is markedly improved by multimerization [4]. Other forms of the cytokines and receptors can be generated by alternative splicing. Of these, the best studied is ΔBAFF, an isoform that cannot be cleaved from the cell surface and appears to act as a dominant negative inhibitor of BAFF [5].
Mice deficient in BAFF or BAFF-R have a profound decrease in mature B2 cells. This is because the interaction of BAFF with BAFF-R is essential to the survival of B cells past the early transitional (T1) stage, with only a minor contribution from TACI and none from APRIL or BCMA [6–8]. T1 cells are subject to deletion or anergy induction when they receive a BCR signal because their immature rafts contain insufficient cholesterol to assemble signaling molecules. In the T2 stage, BCR signaling through the classical NF-kB pathway upregulates expression of BAFF-R and also generates p100, an essential substrate for the nonclassical NF-B signaling pathway used by BAFF-R [9]. Upon receiving both BCR- and BAFF-mediated signals, T2 cells differentiate and migrate to the marginal zone or to the B-cell follicles, where they require a source of BAFF for their continued survival. Autoreactive B cells that have downregulated their BCR as a consequence of antigen stimulation at the T1 stage produce less p100 and compete poorly for BAFF as they progress to the T2 stage. When B-cell numbers and BAFF levels are normal, stringent deletion of autoreactive B cells occurs. However, an increase in serum BAFF levels, such as occurs during B-cell lymphopenia or perhaps during inflammatory states, results in relaxation of B-cell selection, with survival of more autoreactive B cells [10, 11]. Importantly, however, BAFF excess has less effect on B-cell selection if physiologic competition is provided by non-autoreactive B cells [12•]. A few studies have addressed the fate of autoreactive B cells within a diverse repertoire under conditions of BAFF excess or BAFF inhibition. Findings in several different autoreactive B-cell transgenic models suggest that the effect of excess BAFF on naïve B-cell selection can be quite variable, and that not all autoreactive B cells are equally susceptible to BAFF inhibition at the transitional B-cell checkpoint [13, 14]. It is therefore important to further dissect the factors that determine BAFF responsiveness of autoreactive B cells so as to find a means of determining which individuals are most likely to be responsive to BAFF inhibition.
In SLE, class switching of autoreactive B cells from IgM to more pathogenic IgG is a critical checkpoint in the initiation of clinical disease. BAFF collaborates with cytokines and Toll-like receptor (TLR) signals to promote increased TLR expression, T-independent Ig class switching, and plasma cell differentiation [15, 16]. APRIL also, by binding to TACI, can mediate class switching but preferentially supports switching to IgA [2•]. In SLE, autoreactive B cells internalize nucleic acid–containing immune complexes or apoptotic material that can activate TLRs, thereby inducing increased expression of TACI [15, 17]. High serum levels of BAFF may therefore preferentially support the survival and induce class switching of autoreactive cells that recognize nucleic acids. In support of this notion, marginal zone B cells undergo T-independent class switching in BAFF transgenic mice and secrete antinuclear autoantibodies that cause SLE [17]. Some SLE patients have twofold to fivefold increases in serum BAFF levels [18]; this could be due to B-cell lymphopenia, BAFF production from inflammatory sites, or induction by increased type 1 interferons (IFNs). It is not yet clear whether this degree of increase in BAFF levels is responsible for aberrant selection or class switching of naïve autoreactive B cells in SLE, or whether such abnormalities are reversed by BAFF inhibition. Of note, baseline levels of serum BAFF did not correlate with the clinical response to belimumab in human clinical trials [19•].
Although there are abundant data to indicate that excess BAFF may alter the selection of naïve B cells and induce a more autoreactive naïve repertoire, the role of BAFF during antigen-induced B-cell responses has not been as well-studied. This is because the generalized B-cell–depleting effect of BAFF deficiency or inhibition in murine models makes it difficult to study the later steps of B-cell differentiation. After antigen encounter, marginal zone B cells form extrafollicular foci in which short-lived plasma cells are generated, whereas follicular B cells interact with T cells and then move to extrafollicular foci or to germinal centers. The survival of short-lived plasma cells generated in the extrafollicular foci is highly dependent on the interaction of BAFF or APRIL with TACI [20]. For this reason, immune responses to T-independent antigens such as encapsulated bacteria are more severely impaired by BAFF and APRIL inhibition than by selective BAFF inhibition and are markedly decreased in TACI-deficient individuals [21].
Within the germinal center (GC) microenvironment, B cells compete for antigen and for growth and costimulatory factors provided by T cells, resulting in selection of those high-affinity B cells that receive help, but death of B cells that receive low-affinity BCR signals or BCR signals without cognate T-cell help [22]. Downregulation of BCR and BAFF-R expression on GC B cells, low levels of BAFF, and high expression of Fas all help ensure that high-affinity B cells that receive additional help from T cells are preferentially selected for clonal expansion and further differentiation into memory or plasma cells. The role of BAFF in regulating selection of the antigen-experienced repertoire is not clearly defined. BAFF-deficient mice fail to form a mature GC follicular dendritic cell (FDC) network, resulting in premature termination of the GC response [23, 24]. Whether this is a direct consequence of competition for BAFF or due to generalized B-cell depletion and a secondary deficiency of B-cell–derived LTα1β2 that is required for an optimal GC response remains to be determined. In contrast, APRIL appears to have a minimal role in GCs [23]. Although somatic mutation and affinity maturation in response to hapten antigens can still occur in BAFF-deficient mice, the magnitude of the antibody response to T-dependent antigens is significantly attenuated [24]. While it is a plausible hypothesis that GC B-cell selection might be impaired by BAFF deficiency, this has not yet been shown experimentally. Our own studies in a transgenic SLE-prone mouse have shown that inhibition of both BAFF and APRIL with TACI-Ig does not prevent clonal expansion or plasma cell differentiation of B cells with germline-encoded autoreactivity. Further studies will be needed to determine whether B cells that acquire autoreactivity as a consequence of somatic mutation are regulated in the GC by BAFF/APRIL inhibition [13].
Neither BAFF nor APRIL is required for the maintenance or reactivation of memory B cells. BAFF has, however, been shown to collaborate with interleukin (IL)-17 or IL-21 in promoting human memory B-cell proliferation and differentiation in vitro [25, 26], suggesting that it might play a role in spontaneous reactivation of memory B cells during inflammation. Either BAFF or APRIL is sufficient to support plasma cells, especially IgM plasma cells [27]. While APRIL has been shown to be necessary and sufficient for the support of bone marrow plasma cells in neonates [28], presumably through its preferential interaction with BCMA, plasma cells become less dependent on BAFF and APRIL in older mice, and in some lupus-prone strains, inhibition of both BAFF and APRIL does not deplete long-lived bone marrow plasma cells [27]. This is most likely because of the complexity of bone marrow niches that support plasma cells, with multiple contributions from other stromal components and even perhaps from memory T cells that accumulate with age.
T Cells
BAFF-R is expressed on some T cells and may modulate T-cell activation and promote IFN-γ and IL-17 production. Studies in SLE-prone mice deficient in the transcription factor Lyn have shown that absence of BAFF-R on T cells directly inhibits their activation and decreases their secretion of IFN-γ [29•]. Similar findings were reported in a murine transplant model [30].
Dendritic Cells
BAFF induces dendritic cells to produce IL-12 and supports both the survival of monocytes and their differentiation into activated macrophages [31]. In a murine model of arthritis, synovial dendritic cells transduced with an siRNA that silences BAFF remained in an immature state and failed to produce IL-6, resulting in a decrease in the differentiation of pathogenic Th17 [32]. Activated human monocytes and dendritic cells express intracellular TACI, but cell surface TACI expression may be induced during inflammatory conditions [31]. Thus, BAFF appears to have a proinflammatory role; it helps dendritic cells and monocytes to activate and recruit immune cells and directly enhances the proinflammatory activity of T cells. These functions are important because BAFF is often expressed in target organs in autoimmune diseases and may therefore contribute to the amplification of inflammation in these sites.
Clinical Studies of BAFF Inhibitors
Murine Studies
Inhibition of BAFF and APRIL can be achieved using blocking antibodies or fusion proteins of the receptors. Multiple studies of BAFF and APRIL inhibition have been performed in SLE-prone mice; these have recently been reviewed [33] and are not described in detail here. In sum, these studies show that BAFF inhibition is more effective at preventing than treating disease, although there is considerable heterogeneity between models. The administration of type 1 IFNs confers increasing resistance to BAFF inhibition, suggesting that environmental as well as genetic factors will determine responsiveness to this intervention. Both types of factors therefore need to be considered when performing analyses to identify responders to therapy or when considering combination therapies. In most, but not all SLE models, long-lived plasma cells are not depleted by blockade of both BAFF and APRIL; in these models, selective inhibition of BAFF is as effective as inhibition of both BAFF and APRIL and can be therapeutic despite failure to modulate the anti-DNA response. Nevertheless, in the MRL/lpr SLE-prone model in which there is a dominant extrafollicular response, short-lived plasma cells are rapidly depleted by TACI-Ig, resulting in a precipitous drop in anti-DNA antibodies. These findings in sum show that there is still much to learn about the role of BAFF in the effector phase of inflammation, and suggest that considerable heterogeneity among humans in their responses to BAFF and BAFF/APRIL inhibition can be expected.
Human Studies
Clinical trials in lupus patients have been performed with four reagents (Table 1). Belimumab (Human Genome Sciences) is a fully human monoclonal antibody that inhibits soluble, but not membrane bound BAFF. A-623 (Blisibimod; Anthera Pharmaceuticals) is a BAFF antagonist peptibody based on BAFF-R, and LY2127399 (Eli Lilly and Company) is a human antibody to BAFF; both these reagents inhibit both soluble and membrane-bound BAFF. TACI-Ig (atacicept; Merck Serono) is a fusion protein that inhibits both BAFF and APRIL (Fig. 1).
Table 1.
Currently available BAFF/APRIL-inhibiting drugs (see http://www.clinicaltrials.gov)
| Drug | Company | Structure | Target | Trials |
|---|---|---|---|---|
| Belimumab | Human Genome Sciences |
Monoclonal antibody |
Soluble BAFF | Two phase 3 trials completed for general SLE |
| US Food and Drug Administration approved | ||||
| Further trials in SLE subsets planned/ongoing | ||||
| Atacicept | EMD Serono |
Fusion protein (TACI-Ig) |
BAFF and APRIL | Phase 2/3 general SLE ongoing (1° outcome: % patients who flare) |
| Dose-ranging phase 2 trial planned (1° outcome: SLEDAI- 2K Responder Index-50) | ||||
| Blisibimod (A-623) |
Anthera | Peptibody | Soluble and membrane BAFF |
Phase 2 in progress; phase 3 planned in general SLE (1° outcome: SLE Responder Index) |
| LY2127399 | Eli Lilly and Company |
Monoclonal antibody |
Soluble and membrane BAFF |
Phase 3 general SLE ongoing (1° outcome: SLE Responder Index) |
Belimumab and atacicept were first tested in rheumatoid arthritis. While the clinical end points were met for belimumab, only approximately 30% of patients achieved an American College of Rheumatology (ACR) 20, compared with 17% of controls [34]. Primary end points were not met in the atacicept trial. In contrast, in a phase 2 study, LY2127399 achieved similar response rates in rheumatoid arthritis as other currently used biologic agents (M Genovese, abstract 1923 presented at ACR Meeting, Philadelphia, 2009). The reason for the enhanced efficacy of LY2127399 in rheumatoid arthritis compared with belimumab or atacicept is not yet known. Although it is possible that blocking both membrane and soluble BAFF is more effective than blocking soluble BAFF alone, other explanations could include differences in the affinity of LY2127399 for BAFF, or the potential ability of LY2127399 to mediate antibody-dependent cytotoxicity (ADCC) of cells expressing surface BAFF. Whether the enhanced efficacy of LY2127399 compared with belimumab and atacicept will also apply to SLE awaits the results of a phase 3 study that is currently under way. Similarly, a phase 2 study of Blisibimod in active SLE is in process, and a phase 3 study is planned. Although the patient recruitment criteria and the primary and secondary outcomes of these trials are somewhat different from each other, it will be of great interest to compare the results with those of belimumab. A phase 2/3 trial of atacicept in active nonrenal, non–central nervous system lupus is in its final stages. This is the only clinically available drug that inhibits both BAFF and APRIL and is expected to have a more profound effect on plasma cells than the other drugs.
Preliminary mechanistic studies of belimumab and atacicept have been reported. While in vitro studies of human B cells have suggested that BAFF alone (without any BCR signal) is not sufficient to promote survival of transitional and naïve B cells, in vivo studies have clearly shown that inhibition of BAFF by belimumab induces marked depletion of transitional and naïve B cells that is maximal by 3 to 6 months and is then sustained during treatment. While circulating plasmablasts, particularly IgM plasmablasts, decrease during belimumab treatment, class-switched memory cells are not affected and may even increase in number during the first few months of treatment. In accordance with these findings, serum IgG levels decreased by only 10% [35•, 36], and baseline titers to tetanus and pneumococcus were not affected [19•]. A similar degree of peripheral naïve B-cell depletion has been reported with atacicept. As expected, however, atacicept has a more profound effect on serum levels of immunoglobulins than does belimumab, particularly on levels of IgM. Despite a 20% decrease in serum levels of IgG in patients taking a short course of atacicept, titers of antibodies to tetanus toxoid were not affected, suggesting that other factors apart from BAFF and APRIL can maintain plasma cells in adult patients [37, 38]. Nevertheless, a trial of combination therapy with mycophenolic acid and atacicept had to be halted almost immediately because of the occurrence of hypogammaglobulinemia in several patients, and the high-dose arm of the current atacicept trial was stopped early because of adverse events. This suggests that plasma cells from some individuals may manifest more dependence on BAFF and APRIL than others, and that combination of this drug with immunosuppressive agents that target activated lymphocytes will need to be approached with caution.
The first large, 52-week phase 2 study of belimumab in moderately active SLE failed to meet its primary end points, namely a percent change in the SELENA-SLEDAI score at week 24 and a decrease in time to first flare. Post-hoc analyses of the results of the trial showed that responders were more likely to be serologically active (positive ANA or anti-DNA), and that responses were better at week 52 than at week 24 [36]. Given these results and considering the known slow kinetics of B-cell depletion by BAFF inhibition in humans, two subsequent phase 3 studies of patients with moderately active SLE (BLISS 52, carried out in Eastern Europe, Asia, and South America, and BLISS 76, carried out in North and Central America and Western/Central Europe) were designed to be of relatively long duration (52–76 weeks), and dose increases in other immunosuppressive treatments and corticosteroids were allowed during the first 6 months of treatment. Only serologically active patients were enrolled, and a new composite disease outcome measure (the SLE Responder Index) was used as the primary outcome. This outcome measure requires a four-point reduction in SELENA-SLEDAI score, with no significant new disease activity in any organ system or change in the physicians’ global disease assessment [39••]. Using this design, both trials met their primary end point at 52 weeks, and there were also improvements in secondary outcomes, including a decrease in the frequency of severe flares and in concurrent steroid dosage. Furthermore, the safety profile was acceptable and comparable with the placebo arm [40••, 41••].
Because individuals in the control arm in both trials received standard-of-care therapy for the first 6 months, during which increases in immunosuppressive therapy and steroids were allowed, the response rates in this arm were high, but there was a clear additional benefit of belimumab, especially in the BLISS 52 trial (response rates, 58% vs 44% in BLISS 52 and 43% vs 33.5% in BLISS 76). Disappointingly, however, belimumab did not demonstrate a significant benefit over standard-of-care therapy before 52 weeks or at 76 weeks (response rates, 39.1% vs 32.4%), although several secondary outcomes were still achieved, including a reduction in steroid dose and in the number of severe flares. Given the rather low rate of response in either treatment arm at 76 weeks, it is not yet clear how to determine whether an individual patient has benefited and for how long to use the drug. Serologic improvements were noted in belimumab-treated patients with correction of hypergammaglobulinemia, increases in serum complement levels in 30% to 40% of belimumab-treated patients (compared with 20% of standard-of-care–treated patients), and 30% to 50% decreases in median titers of anti-DNA antibodies, compared with only a 15% median decrease in serum IgG. A small subset (<20%) of patients became anti-DNA negative by week 52, but these decreases in anti-DNA antibodies occurred only in patients with low titers of autoantibodies at baseline. A 30% median decrease in total serum IgM levels, compared with only a 10% to 15% decrease in IgG antibodies, reflects the greater dependence on BAFF of IgM- than IgG-producing cells. Although the median decrease in anti-DNA antibodies was higher than that of total IgG, it is not possible to know whether this truly is due to an effect of belimumab on B-cell selection, or whether this is due to a greater dependence of short-lived than long-lived plasma cells on BAFF. Nevertheless, those patients in whom there was an early serologic response to belimumab and a greater degree of naïve B-cell depletion were also more likely to have a clinical response [19•]. Differences in serologic responses and in the degree of B-cell depletion in patients taking the same dose of belimumab could reflect individual pharmacokinetic, genetic, or environmental influences.
As expected, belimumab treatment caused a decrease in circulating B cells, but not T cells, beginning 8 to 12 weeks after treatment initiation. The most significant decreases occurred in the naïve and transitional subsets, but decreases were also noted in activated CD69+ B cells, in the CD27+/IgM+ population that contains marginal zone B cells and human B1 cells, and in plasmablasts, particularly IgM-secreting plasmablasts [19•, 35•]. As previously reported, belimumab treatment resulted in rapid expansion of the memory B-cell pool [36, 42]. Whether this is due to homeostatic proliferation of memory B cells or their mobilization into the circulation is not yet known but is important to understand the reasons for this increase, as it would be counterproductive to deplete the naïve B-cell population while expanding an autoreactive memory-cell population.
Conclusions
There has already been extensive clinical experience with belimumab, but there is still much to learn. Approximately 2,000 patients with moderately active SLE have been treated in the belimumab clinical trials, and the results with respect to B-cell–depleting effects, serologic changes, and clinical responses as measured by the responder index have consistently shown benefit compared with current standard-of-care therapies [19•]. Although the controls also improved, most likely as a result of adjustments in background treatments allowed during the study, they did so in the setting of a higher background dose of steroids. It is now crucial to determine whether these benefits will be sustained over longer periods and whether there will therefore be a decrease in disease- and corticosteroid-related damage over time in belimumab-treated patients. Even small changes could cumulatively result in significant benefits over many years of disease. While patients from the phase 2 study have now been followed for greater than 5 years, allowing an evaluation of long-term immunologic effects and safety profile of BAFF inhibition in a selected group of individuals, there is no longer a placebo arm for these patients, making it impossible to evaluate long-term efficacy in these patients. It is also crucial to understand whether it is possible to identify responders and nonresponders to this drug and whether there are any adverse immunologic consequences of long-term administration or of stopping the drug. While the published data only show changes in median values of biologic markers, more rigorous analysis of the mechanistic data collected during the clinical trials may help shed light on these issues; it is incumbent on the drug sponsors to make these data available in a timely fashion. Finally, the efficacy of belimumab has still not been shown in certain patient subsets, including those with active renal or neurologic disease or in certain ethnic minorities (eg, African Americans). Clinical trials to address these questions are planned or ongoing.
It has been puzzling that B-cell depletion with rituximab has not been as effective as BAFF inhibition for the treatment of moderately active SLE [43]. It is difficult to directly compare the results of these two sets of clinical trials because they were differently designed, and the belimumab trials had larger numbers of enrolled patients, resulting in more power to detect small differences in outcome. Nevertheless, the marked improvement in clinical outcome in patients with multiple sclerosis who were treated with Rituxan compared with rapid disease worsening in patients treated with atacicept (http://www.clinicaltrials.gov) suggests that there are differences in the biologic effects of these two classes of drugs. One possible explanation could be differential effects of the two drugs on certain B-cell subsets such as regulatory B cells or memory cells. There are no data as of yet that address this hypothesis. Another explanation is the direct effect of BAFF inhibition, but not Rituxan, on cell types other than B cells, such as activated T cells or dendritic cells. Murine studies are strongly supportive of this hypothesis. For example, we have recently shown that treatment of a stringent SLE model with TACI-Ig has no effect on autoantibody production, systemic T-cell activation, or renal deposition of immune complexes but inhibits renal migration of activated macrophages and results in a decreased renal inflammatory response [44].
A final question that needs to be addressed is whether BAFF inhibition will synergize with other lupus therapies, including TLR-directed therapy that targets type 1 IFNs, therapies that inhibit new GC formation, or during the recovery phase after B-cell depletion. This question can be addressed initially in animal models but will ultimately require human clinical trials.
Acknowledgment
Dr. Davidson has received grant support from the National Institutes of Health.
Glossary
- APRIL
a proliferation-inducing ligand
- BAFF
B-cell-activating factor
- SLE
systemic lupus erythematosus
- TACI
transmembrane activator and calcium modulator and cyclophilin ligand interactor
Footnotes
Disclosure Dr. Davidson has served as a consultant for Merck Serono.
References
- 1.Mackay F, Figgett WA, Saulep D, et al. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 2. Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. This is an excellent review about BAFF and APRIL and their receptors.
- 3.Gross JA, Johnston J, Mudri S, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404(6781):995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 4.Bossen C, Cachero TG, Tardivel A, et al. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111:1004–1012. doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- 5.Gavin AL, Duong B, Skog P, et al. {Delta}BAFF, a splice isoform of BAFF, opposes full-length baff activity in vivo in transgenic mouse models. J Immunol. 2005;175:319–328. doi: 10.4049/jimmunol.175.1.319. [DOI] [PubMed] [Google Scholar]
- 6.Mackay F, Schneider P, Rennert P, et al. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003;21:231–264. doi: 10.1146/annurev.immunol.21.120601.141152. [DOI] [PubMed] [Google Scholar]
- 7.Seshasayee D, Valdez P, Yan M, et al. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18:279–288. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 8.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 9.Stadanlick JE, Kaileh M, Karnell FG, et al. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lesley R, Xu Y, Kalled SL, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 11.Thien M, Phan TG, Gardam S, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 12. Liu Z, Davidson A. BAFF and selection of autoreactive B cells. Trends Immunol. 2011;32:388–394. doi: 10.1016/j.it.2011.06.004. This is a review of how BAFF influences selection of autoreactive naïve B cells.
- 13.Huang W, Moisini I, Bethunaickan R, et al. BAFF/APRIL inhibition decreases selection of naive but not antigen-induced autoreactive B cells in murine systemic lupus erythematosus. J Immunol. 2011;187:6571–6580. doi: 10.4049/jimmunol.1101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikbakht N, Migone TS, Ward CP, et al. Cellular competition independent of BAFF/B lymphocyte stimulator results in low frequency of an autoreactive clonotype in mature polyclonal B cell compartments. J Immunol. 2011;187:37–46. doi: 10.4049/jimmunol.1003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treml LS, Carlesso G, Hoek KL, et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 16.Katsenelson N, Kanswal S, Puig M, et al. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur J Immunol. 2007;37:1785–1795. doi: 10.1002/eji.200636800. [DOI] [PubMed] [Google Scholar]
- 17.Groom JR, Fletcher CA, Walters SN, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stohl W, Metyas S, Tan SM, et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum. 2003;48:3475–3486. doi: 10.1002/art.11354. [DOI] [PubMed] [Google Scholar]
- 19. Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement, and reduces select B-cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012 doi: 10.1002/art.34400. (in press, Jan 24). This is a post-hoc analysis of the two phase 3 belimumab trials that attempts to identify characteristics of responders.
- 20.Balazs M, Martin F, Zhou T, et al. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 21.Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008;19:263–276. doi: 10.1016/j.cytogfr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol. 2010;126:898–907. doi: 10.1016/j.jaci.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Kalled SL. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol. 2006;18:290–296. doi: 10.1016/j.smim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Rahman ZS, Rao SP, Kalled SL, et al. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med. 2003;98:1157–1169. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ettinger R, Sims GP, Robbins R, et al. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J Immunol. 2007;178:2872–2882. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
- 26.Doreau A, Belot A, Bastid J, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 27.Ramanujam M, Wang X, Huang W, et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116:724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belnoue E, Pihlgren M, McGaha TL, et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 29. Scapini P, Hu Y, Chu CL, et al. Myeloid cells, BAFF, and IFN-{gamma} establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–1773. doi: 10.1084/jem.20100086. This was a murine model of SLE in which BAFF-R expression on T cells influenced cytokine production.
- 30.Ye Q, Wang L, Wells AD, et al. BAFF binding to T cell-expressed BAFF-R costimulates T cell proliferation and alloresponses. Eur J Immunol. 2004;34:2750–2759. doi: 10.1002/eji.200425198. [DOI] [PubMed] [Google Scholar]
- 31.Chang SK, Mihalcik SA, Jelinek DF. B lymphocyte stimulator regulates adaptive immune responses by directly promoting dendritic cell maturation. J Immunol. 2008;180:7394–7403. doi: 10.4049/jimmunol.180.11.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, et al. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci USA. 2008;105:14993–14998. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Davidson A. BAFF inhibition: A new class of drugs for the treatment of autoimmunity. Exp Cell Res. 2011;317:1270–1277. doi: 10.1016/j.yexcr.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen SB. Updates from B Cell Trials: Efficacy. J Rheumatol Suppl. 2006;77:12–17. [PubMed] [Google Scholar]
- 35. Jacobi AM, Huang W, Wang T, et al. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase, II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2010;62:201–210. doi: 10.1002/art.27189. This was a mechanistic study of the effect of belimumab on human B cells.
- 36.Wallace DJ, Stohl W, Furie RA, et al. A phase, II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 2009;61:1168–1178. doi: 10.1002/art.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pena-Rossi C, Nasonov E, Stanislav M, et al. An exploratory dose-escalating study investigating the safety, tolerability, pharmacokinetics and pharmacodynamics of intravenous atacicept in patients with systemic lupus erythematosus. Lupus. 2009;18:547–555. doi: 10.1177/0961203309102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dall'Era M, Chakravarty E, Wallace D, et al. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. 2007;56:4142–4150. doi: 10.1002/art.23047. [DOI] [PubMed] [Google Scholar]
- 39. Furie R, Petri M, Wallace DJ, et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum. 2009;61:1143–1151. doi: 10.1002/art.24698. This was the first description of the SLE Responder Index that is being used as a primary outcome measure in several SLE clinical trials.
- 40. Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–731. doi: 10.1016/S0140-6736(10)61354-2. This was the first phase 3 study of belimumab to be completed.
- 41. Furie R, Petri M, Zamani O, et al. A phase, III randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. This was the second phase 3 belimumab study that involved patients in the United States and a 76-week treatment window.
- 42.Nestorov I, Munafo A, Papasouliotis O, et al. Pharmacokinetics and biological activity of atacicept in patients with rheumatoid arthritis. J Clin Pharmacol. 2008;48:406–417. doi: 10.1177/0091270008315312. [DOI] [PubMed] [Google Scholar]
- 43.Merrill JT, Neuwelt CM, Wallace DJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z, Bethunaickan R, Huang W, et al. IFN-{alpha} confers resistance of systemic lupus erythematosus nephritis to therapy in NZB/W F1 mice. J Immunol. 2011;187:1506–1513. doi: 10.4049/jimmunol.1004142. [DOI] [PMC free article] [PubMed] [Google Scholar]