Abstract
This review discusses the function of neural crest as they relate to cardiovascular defects. The cardiac neural crest cells are a subpopulation of cranial neural crest discovered nearly 30 years ago by ablation of premigratory neural crest. The cardiac neural crest cells are necessary for normal cardiovascular development. We begin with a description of the crest cells in normal development, including their function in remodeling the pharyngeal arch arteries, outflow tract septation, valvulogenesis, and development of the cardiac conduction system. The cells are also responsible for modulating signaling in the caudal pharynx, including the second heart field. Many of the molecular pathways that are known to influence specification, migration, patterning and final targeting of the cardiac neural crest cells are reviewed. The cardiac neural crest cells play a critical role in the pathogenesis of various human cardiocraniofacial syndromes such as DiGeorge, Velocardiofacial, CHARGE, Fetal Alcohol, Alagille, LEOPARD, and Noonan syndromes, as well as Retinoic Acid Embryopathy. The loss of neural crest cells or their dysfunction may not always directly cause abnormal cardiovascular development, but are involved secondarily because crest cells represent a major component in the complex tissue interactions in the head, pharynx and outflow tract. Thus many of the human syndromes linking defects in the heart, face and brain can be better understood when considered within the context of a single cardiocraniofacial developmental module with the neural crest being a key cell type that interconnects the regions.
Keywords: cardiac neural crest, aortic arch arteries, persistent truncus arteriosus, congentital heart defects, DiGeorge Syndrome, Retinoic Acid, second heart field
Introduction and historical perspective
The neural crest is a multipotent cell population that arises on the dorsal neural tube during embryonic development. The crest cells delaminate and migrate throughout the body forming peripheral nervous system and melanocytes. Crest cells from the cranial neural tube also have the potential to give rise to ectomesenchyme. The ectomesenchyme is the source of many of the hard and soft tissues of the head and neck, such as bones, teeth, and connective tissue. By the early 1980s the scientific community had established a long list of neural crest derivatives that largely matches our current understanding of neural crest potency, save for neural crest derivatives in the heart (Le Douarin, 1982).
In a chick study of parasympathetic innervation of the heart, Margaret Kirby and colleagues ablated neural crest and serendipitously discovered that the embryos lacked aorticopulmonary septation (Kirby et al., 1983). The subregion of cranial neural crest ablated by Dr. Kirby has been called the “cardiac neural crest”, not because the cells of this region migrate solely to the heart, but for the importance of crest-derived ectomesenchyme in cardiovascular development. To date there are hundreds of publications on cardiac neural crest in the US National Library of Medicine database, PubMed. This review will highlight some of the major findings since Dr. Kirby opened the field nearly 30 years ago. This review will not focus on the regulation of initial specification and delamination of the neural crest from the neural tube, but rather on the function of neural crest as they relate to cardiovascular defects.
The initial studies of the role of cardiac neural crest in cardiovascular development were done in the avian models using quail to chick chimeras and cell ablation studies largely due to the accessibility of the neural crest. A significant advance in understanding the contributions of mammalian cardiac neural crest came with transgenic methods that allowed the neural crest cells to be marked genetically by neural crest-specific promoters driving lacZ or GFP expression, as well as by cre recombinase (cre-lox) technology to lineage trace neural crest cells in mouse embryos. The most commonly used promoters to drive cre recombinase are the Wnt1-cre, Pax3-cre, P0-cre, and PlexinA2-cre (Brown et al., 2001; Jiang et al., 2000; Lee et al., 1997). While there are some differences in the patterns of labeled neural crest cells in these models, all of the models confirm the major patterns of migration and contributions of cardiac neural crest to cardiovascular development that were described in quail-chick chimeras.
Cardiac neural crest derivatives in aortic arch arteries
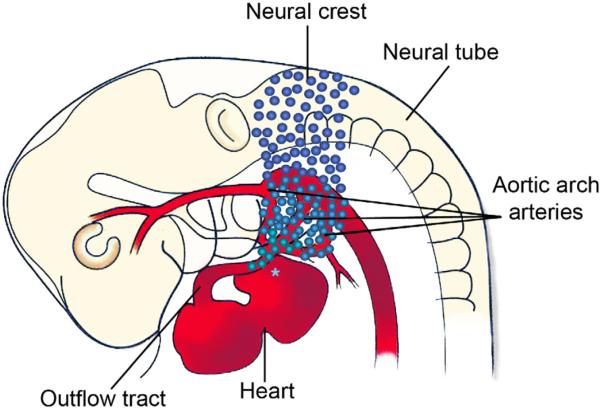
The cardiac neural crest cells originate from the dorsal neural tube between the mid-otic placode to the posterior border of somite three (Fig. 1). The neural crest cells migrate ventrally then pause in the circumpharyngeal ridge before continuing into pharyngeal arch three, four, and then six as each arch forms sequentially. The cells migrate between the pharyngeal ectoderm and endoderm, proliferate, and envelope the endothelial strands of the nascent aortic arch arteries. The pharyngeal arch arteries form as a bilaterally symmetrical series of arteries that connect the aortic sac to the paired dorsal aortas. Later in development aortic arch arteries 3–6 are remodeled into the asymmetric great arteries, including the common carotid, definitive aortic arch, and the ductus arteriosus. The cardiac neural crest cells are not required for aortic arch artery formation, but are required for their repatterning (Bockman et al., 1987) and will form the smooth muscle tunica media of the arteries (Bergwerff et al., 1998) (Fig. 2).
Figure 1.
Migratory path of the cardiac neural crest cells as defined by the chick cardiac neural crest ablation model. Cardiac neural crest cells originate from the neural tube extending from the axial level of the mid otic placode to the third somite in chick. The cells then migrate from the neural tube into the caudal pharyngeal arches (3, 4 and 6). Some neural crest cells remain in the pharynx to support aortic arch artery development, while a subpopulation continues on to migrate into the outflow tract of the heart. Retroviral studies in chick and cre-lineage experiments in mouse show a neural crest contribution to the inflow near the cardiac conduction system (asterisk). The migration path and timing are uncertain. (Adapted from Kirby and Hutson, 2010).
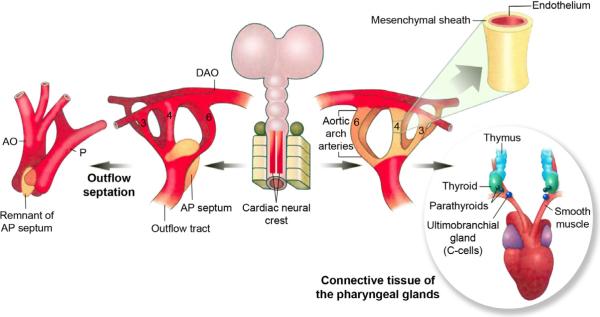
Figure 2.
Derivatives of the cardiac neural crest cells. Cardiac neural crest cells support development of and patterning of the persisting aortic arch arteries into the great arteries of the thorax and form their smooth muscle tunics. In addition, the cells contribute to the development of the thymus, parathyroids and thyroid glands, and provide stromal cells after gland development. (Adapted from Kirby and Hutson, 2010).
Outflow septation
In the chick, several days after the neural crest cells have emigrated from the dorsal neural tube to the pharynx, a subpopulation of cells migrate into the cardiac outflow cushions. Here they will condense to form the aorticopulmonary septation complex (Waldo et al., 1998; Waldo et al., 1999) dividing the common arterial outflow into the aorta and pulmonary trunk (Figs. 2, 3). The cushions are cardiac jelly-filled ridges in a spiral conformation within the outflow tract that eventually become populated by three different mesenchymal cell populations. In the proximal outflow tract the conal myocardium induces an epithelial to mesenchymal transformation (EMT) of the underlying endocardium. These mesenchymal cells will form the bulk of the semilunar valve leaflets. Distally, there are some mesenchymal cells derived from the pharynx that populate the cushions prior to the arrival of the neural crest cells (Ward et al., 2005). Later neural crest cells migrate into the cushions and form two centrally placed columns or “prongs”, of condensed cells in the distal (truncal) outflow. The prongs join more distally through a shelf of mesenchymal cells protruding into the dorsal wall of the aortic sac forming a horseshoe or an upside down “U”-shaped septation complex with the prongs making up the arms of the U. The shelf is situated between the origins of the fourth and sixth pairs of arch arteries, which will be remodeled into the arch of the aorta and the ductus arteriosus, respectively. Thus, the shelf is perfectly positioned to divide the systemic and pulmonary outflow. As septation proceeds the shelf lengthens through the distal outflow at the expense of the prongs. Due to the spiral conformation of the prongs, the septation spirals as well. Once the prongs are expended and the aortic sac and truncus are septated, the proximal outflow septum forms in a distal to proximal progression closing toward the ventricles. Septation of the conus is accomplished by myocardialization in which invading myocardial cells cause the cushions to bulge into the lumen (van den Hoff et al., 1999). The endocardial layer covering the cushions breaks down, allowing mixing of mesenchyme and myocardium from opposing cushions to form the septum. Cardiac neural crest cells are also found in the proximal outflow in the subendocardial space. After fusion of the cushions, they appear as a seam in the septum and it is not clear if they play an active role in the fusion of the cushions (Waldo et al., 1998; Waldo et al., 1999).
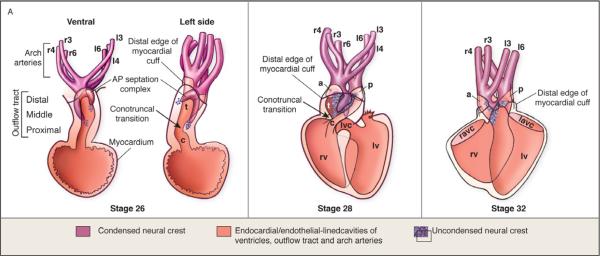
Figure 3.
Stages of outflow septation as reported in quail-chick chimeras. (A) U-shaped condensed mesenchyme of the aorticopulmonary septation complex is continuous with the smooth muscle tunica media of the aortic arch arteries. (B) Septation of the distal and middle portions of the arterial pole by the aorticopulmonary septation complex. The septum forms at the expense of the prongs. (C) Septation of the conus leaves a seam of subendocardial cardiac neural crest cells. (Adapted from Kirby and Hutson, 2010).
Outflow septation in the mouse appears to occur in a similar fashion but with some notable differences in the timing of events and distribution of the cardiac neural crest. For example, cardiac neural crest arrive in the distal outflow in the mouse at E9.5 approximately one day after emigration from the neural tube, while in the chick the neural crest-derived cells enter the outflow tract 3.5 days after leaving the neural tube. Another notable difference is in how the cardiac neural crest cells enter the outflow tract cushions prior to septation (Waldo et al., 1999). In quail-chick chimeras, the cardiac neural crest-derived cells enter by two distinct routes, subendocardially and submyocardially. This is in contrast to a single subendocardial route taken by the cardiac neural crest cells as shown in the Cx43-lacZ-marked cardiac neural crest cells in the mouse. In addition the cardiac neural cells in the mouse outflow tract extend into the distal conus, whereas they only just reach the conus in the chick (Waldo et al., 1999). These differences are likely due to differences in timing of some of the developmental events in cardiovascular development along with morphological differences between mammals and birds.
Valvulogensis
After outflow tract septation, the cushion mesenchyme is remodeled to form the aortic and pulmonary semilunar valves, each with three cusps or leaflets. As mentioned above the bulk of the leaflets is made up of endocardial-derived mesenchymal cells. It is not clear to what extent the cardiac neural crest cells participate in semilunar valve formation. Neural crest cells have been found at the tips of the leaflets in chick and mouse during early stages of valve development (Nakamura et al., 2006; Waldo et al., 1998) but neural crest-derived cells were not found in the mature leaflets in the mouse (Jiang et al., 2000). Recent studies have indicated that semilunar valve leaflets are derived exclusively from endocardium (de Lange et al., 2004). These findings suggest that endocardial-derived mesenchyme replaces neural crest-derived cells at later stages of valve development. A recent study has shown that the neural crest contribution to the outflow endocardial cushions is required for late gestation valve remodeling, mesenchymal apoptosis, and proper valve architecture in mouse (Jain et al., 2011). Flow though the developing valve region is another important component of valve remodeling. Because the outflow tract is abnormal after cardiac neural crest perturbations (see below), the relative requirements of blood flow versus the presence of the crest cells to valve remodeling remains to be determined.
Neural crest and cardiac innervation and conduction
Neural crest cells give rise to all parasympathetic innervation of the heart (Kirby et al., 1983). There is some controversy concerning the extent to which neural crest cells contribute to the development of the cardiac conduction system, which is known to be of myogenic origin (Miquerol et al., 2011). Retroviral studies in chick and cre-lineage analysis in mouse show a contribution of neural crest cells to the inflow region near the cardiac conduction system (Poelmann and Gittenberger-de Groot, 1999; Poelmann et al., 2004). These cells are likely to migrate in through the inflow, but the exact timing and path of migration are unknown. A neural crest contribution to the inflow has not been demonstrated in quail-chick chimeras (Poelmann et al., 1998; Waldo et al., 1998). A genetic lineage analysis of neural crest cells in mouse identified a small proportion of the His-Purkinje conduction system that arises from Mesp1-negative cells, implying that a some cells within the system are not of mesodermal origin and may be neural crest cells (Kitajima et al., 2006). Another lineage analysis using a Wnt1-Cre mouse suggested that the neural crest cells may contribute to the central conduction system (Nakamura et al., 2006). Miquerol and colleagues maintain that as yet there is no convincing evidence that neural crest cells make cellular contributions to the His-Purkinje conduction system (Miquerol et al., 2011).
While the neural crest cells may not contribute directly to the His-Purkinje conduction system, they are required for its normal development. Following ablation of cardiac neural crest cells in chick embryos, conduction system bundles fail to compact, leaving them uninsulated from surrounding myocardium. This leads to an inhibited or delayed maturation of conduction system function (Gurjarpadhye et al., 2007). In viral and genetic mapping studies cardiac neural crest cells are found in close proximity with the developing conduction system (Nakamura et al., 2006; Poelmann and Gittenberger-de Groot, 1999; Poelmann et al., 2004). Deletion of Hf1b from neural crest cells resulted in atrial and atrioventricular conduction dysfunction due to deficiencies in the neurotrophin receptor trkC (St Amand et al., 2006). Taken together, these results suggest that NCCs have a role in normal development and maturation of the cardiac conduction system.
Neural crest ablation phenotype
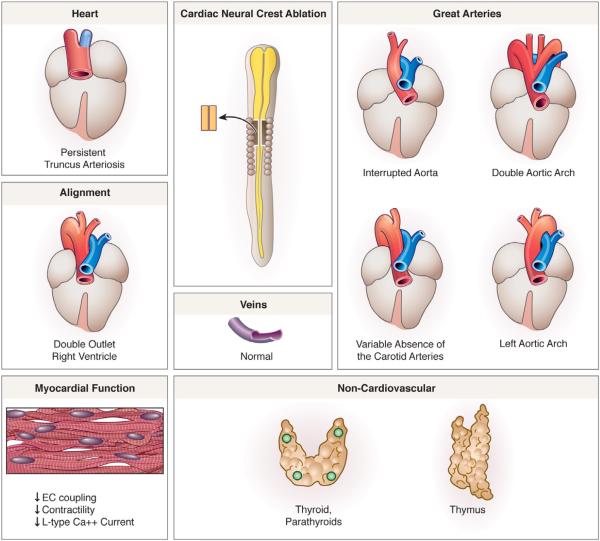
Most genes associated with cardiac neural crest function have been characterized through transgenic manipulation of neural crest-related gene expression in mouse models, while much of what is known regarding the specific neural crest contribution to cardiovascular development has been obtained from avian models: quail to chick chimeras and cardiac neural crest ablation studies. Cardiac neural crest ablation defects include defective outflow tract septation (persistent truncus arteriosus), abnormal patterning of the aortic arch arteries and great arteries (such as interrupted aorta and double aortic arch), hypoplasia/aplasia of the thymus, thyroid and parathyroids, abnormal heart tube looping leading to arterial pole alignment defects (such as double outlet right ventricle), ventricular septal defects, and abnormal myocardial function resulting in decreased excitation-contraction (EC) coupling, contractility and L-type Ca+2 current (Fig. 4) (Besson et al., 1986; Bockman et al., 1987; Creazzo et al., 1997; Kirby et al., 1983).
Figure 4.
The cardiac neural crest ablation phenotype. Persistent truncus arteriosus, is observed in 90% of neural crest-ablated embryos while 10% exhibit arterial pole misalignment defects such as double-outlet right ventricle. Abnormal myocardial function, mispatterning of the arch arteries and glandular defects occur in 100% of embryos after cardiac neural crest ablation. (Adapted from Kirby and Hutson, 2010).
Targeted gene knockouts in mouse have led to the development of many mouse models that have recapitulated portions or all of the neural crest ablation phenotype in chick, many of which are detailed below. Recently the neural crest cells have been temporally-spatially ablated in PuΔTK:Wnt1-Cre mice, leading to a phenotype similar to cardiac neural crest ablation in chick (Porras and Brown, 2008). Important differences include cases of pulmonary valvar and infundibular stenosis observed in mouse but not in chick. It is not known whether these differences are in fact species-specific or an inherent characteristic of the mouse ablation system.
The neural crest-related defects observed in both animal models can be largely classified into defects which are directly the result of an absence of a structural contribution by the crest cells, or those which are indirectly caused by absence of the crest cells in the caudal pharynx, resulting in abnormal signaling and/or tissue interactions. The loss of neural crest cells or their dysfunction may not always directly cause abnormal cardiovascular development, but may be involved secondarily because crest cells represent a major component in the complex tissue interactions in the caudal pharynx and outflow tract.
Cardiac neural crest cells regulate FGF signaling in the secondary heart field and caudal pharynx
Neural crest ablation experiments in chick revealed that crest cells are required not only for outflow septation, but also for completion of normal heart looping. Abnormal looping is one of the earliest defects seen after cardiac neural crest ablation, and can be observed before the crest cells reach the outflow. Defective looping is caused by a failure of addition of myocardium to the heart tube from the secondary heart field (Yelbuz et al., 2002), a population of cells located in the ventral caudal pharynx, just behind the outflow attachment to the pharynx. The secondary heart field, a subpopulation of the second heart field1, has been shown to be required for lengthening the outflow tract, which eventually leads to the proper alignment of the aorta and pulmonary trunk over the left and right ventricle. The abnormally shortened loop results in arterial pole malalignment defects such as double outlet right ventricle (DORV) and overriding aorta. Significantly, the undivided outflow tract in neural crest-ablated embryos is also dextroposed suggesting a secondary heart field-related defect. In neural crest-ablated embryos cell proliferation in the secondary heart field is elevated, indicating that the presence of the neural crest cells in the pharynx is necessary for normal secondary heart field development (Waldo et al., 2005). How the neural crest or the absence of the neural crest could affect the secondary heart field, which is located at a distance from the crest cells, has been a confounding research question. One hypothesis is that the neural crest regulates or modulates the activity of secreted signaling factors in the pharynx that then become dysregulated after neural crest perturbation. FGF8 appears to be one of these factors. FGF8 signals through a tyrosine kinase receptor and has been shown to influence proliferation, differentiation, cell survival and cell migration in a context dependent manner. FGF8 signaling is upregulated in the pharynx after neural crest ablation. This elevated signaling is concomitant with the timing of addition of myocardium from the secondary heart field to the heart tube (Hutson et al., 2006). When excess FGF signaling is neutralized using the FGFR1 receptor blocker, SU5402, or an FGF8 blocking antibody, secondary heart field development, cardiac looping and outflow alignment are all rescued. These embryos do however, still exhibit persistent truncus arteriosus, as the cardiac neural crest cells are still absent and are required for outflow septation.
It was once thought that outflow septation and outflow alignment were interdependent processes, but more recent experiments, including those mentioned just above, have shown that they can be separated and are, in fact, independent processes. Alignment and septation involve distinct cell populations and separate events during development. Arterial pole alignment is reliant on lengthening of the of the heart tube, which allows the inflow and outflow poles to converge with adequate length in the loop for the outflow tract to rotate and wedge correctly such that the atria and outflow vessels are in concordance with ventricles. Heart tube lengthening is largely a function of addition of myocardium from the secondary heart field to the outflow pole. Outflow septation, by contrast, is dependent on ingression of neural crest cells that form the aorticopulmonary septum. As mentioned above the persistent truncus arteriosus in neural crest-ablated embryos overrides the right ventricle, but reducing FGF signaling to more normal levels leads to persistent truncus arteriosus with correct alignment (Hutson et al., 2006). Knockdown of HIRA, a gene found within the DiGeorge critical region (see below), results in persistent truncus but with proper alignment of the arterial pole (Farrell et al., 1999). Thus the secondary heart field and cardiac neural crest cells are distinct cell populations with different roles in arterial pole formation but because both cell populations are located in the pharynx during critical stages of cardiac outflow formation, any disruption of development within the pharynx can affect both populations.
In addition to arterial pole alignment defects, cardiac neural crest ablation also compromises myocardial function, and the effect is again observed prior to the arrival of the neural crest cells at the outflow (Leatherbury et al., 1990). The myocardium exhibits reduced ejection fraction, with the underlying cause being that calcium transient, L-type calcium current, excitation-contraction coupling and calcium sensitivity of the contractile apparatus are all reduced. Embryos that survive do so by ventricular dilation to maintain cardiac output in a normal range (Creazzo et al., 1998; Leatherbury et al., 1991). Upregulated FGF signaling in neural crest ablated embryos also appears to effect myocardial function; normal myocardial calcium transients can be restored when FGF signaling is reduced to normal levels (Farrell et al., 2001; Hutson et al., 2006). The direct relationship of FGF signaling and myocardial function at the early looping stages is unclear.
Cardiac Congenital Abnormalities Involving the Neural Crest in Humans and Animal Models
22q11.2 deletion syndromes
Cardiac neural crest ablation phenocopies many of the cardiocraniofacial defects found in DiGeorge (DGS) and velocardiofacial syndrome (VCFS) patients. There is considerable overlap between VCFS and DGS-associated defects, and both syndromes are often caused by a chromosomal 22q11.2 deletion. The syndromes are characterized by interrupted aortic arch type B, outflow tract malformations that include persistent truncus arteriosus, tetralogy of Fallot (pulmonary atresia/stenosis) or double outlet right ventricle, hypoplastic thymus with some degree of immunodeficiency, and hypoparathyroidism. Mild craniofacial defects, ventricular septal defect, aberrant origin of the right subclavian artery, hypothyroidism, psychosis, and in the case of VCFS, cleft lip and cleft palate are frequently seen. The diagnosis of DGS or VFCS depends on the features of the disorder when it is first diagnosed. Children diagnosed with the immunological or cardiac defects are more likely to be diagnosed as having DGS, while patients with a cleft palate or psychiatric issues are more likely to be diagnosed with VCFS. The syndromes have an expansive phenotype with over 180 clinical features reported (Shprintzen, 2008). Most cases of these syndromes are caused by hemizygous deletions within chromosome band 22q11.2. However, DGS and VCFS can also be caused by other deletions or translocations involving chromosome 22q11, and some cases do not appear to involve any deletion (Scambler, 2010). Variable phenotypic expressivity is common, and reports exist of phenotypic discordance between monozygotic twins with identical deletions (Goodship et al., 1995; Singh et al., 2002).
Patients with clinically similar phenotypes have been found with non-overlapping deletions within 22q11.2 (Amati et al., 1999; McQuade et al., 1999; O'Donnell et al., 1997; Scambler, 2000). Therefore, the human deletion map has not been helpful in identifying candidate genes (Lindsay et al., 2001). Deletion of proximal mouse chromosome 16, termed Df1 for deficiency 1, was the first targeted deletion of the region homologous to human 22q11, and thus the first true model of 22q11 deletion syndrome (Lindsay et al., 1999). Among many other murine genes from the typically deleted region in humans, the Df1 region includes Tbx1, a T-box transcription factor. Df1/+ mice are nearly identical to Tbx1+/− mice. Deletion and complementation studies in mice have indicated that Tbx1 insufficiency recapitulates cardiovascular phenotypes of DGS (Jerome and Papaioannou, 2001; Lindsay et al., 2001; Merscher et al., 2001).
Although genetic disruptions of Tbx1 in mouse models have been powerful in implicating Tbx1 as the key gene disruption in DGS and VCFS, there are fundamental differences between the mouse models and human etiology. Gene dosage requirements for mouse and human appear to be different; Tbx1 mRNA levels in mouse must be decreased to approximately 20% of the normal level before outflow tract defects are penetrant (Zhang and Baldini, 2008). Further, human mutations in Tbx1 in the absence of a 22q11 deletion are a rare cause of DGS (Scambler, 2010). In addition, deletions on chromosomes other than 22 have been found in association with DGS (Prescott et al., 2005), implying that the syndrome is multigenic in etiology.
Recent studies have begun to elucidate the mechanisms through which Tbx1 impacts cardiac neural crest cell development. Tbx1 is expressed in the pharyngeal ectoderm, endoderm and second heart field, but not by neural crest cells (Garg et al., 2001; Vitelli et al., 2002a). Because Tbx1 is a transcription factor, loss of Tbx1 does not directly affect the neural crest cells, but rather impacts tissue-specific gene expression altering the environment through which the neural crest cells migrate. This in turn impacts signaling between the crest cells and surrounding tissues, and altered migration is one of the consequences (Calmont et al., 2009; Frank et al., 2002; Kochilas et al., 2002; Vitelli et al., 2002a). Conditional Tbx1 mutants show aorticopulmonary septal defects, which are caused by a reduced number of neural crest-derived cells migrating through the fourth arch (Xu et al., 2004). Tbx1 regulates expression of the transcription factor, Gbx2, which in turn regulates the expression of Slit, a guidance molecule, in the pharyngeal endoderm and ectoderm. In an environment of decreased Slit expression, cardiac neural crest cells migrate into arches three and six, but fail to migrate into arch four. Without the sheath of cardiac neural crest cells supporting the endothelial cells of the fourth arch artery, the vessel becomes disorganized, and results in interrupted aortic arch (Calmont et al., 2009).
Fgf8 has also emerged as a critical target of Tbx1, and likely contributes to the etiology of DGS/VCFS (Frank et al., 2002; Hu et al., 2004; Moon et al., 2006; Vitelli et al., 2002b; Zhang et al., 2005). Fgf8 is not within the typically deleted 22q11 region, nonetheless Fgf8 expression is diminished in Tbx1 mutant mice (Vitelli et al., 2002b) and Fgf8 hypomorphic mutant mice phenocopy DiGeorge and VCFS. As the cardiac neural crest migrate into the pharynx, FGF8 is highly expressed in the pharyngeal ectoderm and endoderm and is expressed at lower levels in the splanchnic mesoderm. The phenotypes observed in Fgf8 hypomorphs are likely due to disrupted FGF8 signaling from the epithelia of the caudal pharyngeal arches (3–6) to the cardiac neural crest cells migrating through these arches (Frank et al., 2002). Fgf8 hypomorphic and conditional mutant mice show abnormal apoptosis of the cardiac neural crest (Abu-Issa et al., 2002; Frank et al., 2002; Macatee et al., 2003). FGF8 has recently been shown to be chemokinetic for the cardiac neural crest (Sato et al., 2011). In FGF8 overexpression experiments the neural crest cells migrated faster and in greater numbers into the pharyngeal arches. This suggests that FGF8 influences both neural crest survival and the timing/targeting of neural crest migration into the pharynx.
Fgf8 has tissue-specific roles in outflow tract formation and remodeling. Mesodermal Fgf8 is required for correct alignment of the outflow tract, whereas Fgf8 produced by the pharyngeal endoderm regulates outflow tract septation (Park et al., 2006). Arterial pole alignment defects without persistent truncus arteriosus are observed in chick embryos after FGF8 levels are reduced to abnormally low levels during secondary heart field migration (Hutson et al., 2006). Production of FGF8 in the pharyngeal ectoderm, in particular, is required for normal fourth arch artery development (Macatee et al., 2003). However, whereas Fgf8 expression is absent in the pharyngeal endoderm of Tbx1 null embryos (Vitelli et al., 2002b), ectodermal expression of Fgf8 is robust, so it is unlikely that Tbx1 regulates Fgf8 in ectoderm (Zhang et al., 2005). Fgf8 is additionally required in the cardiac and pharyngeal mesoderm for normal pharyngeal arch artery and outflow tract development (Watanabe et al., 2010).
Other genes have been shown to be dysregulated upon the reduction or absence of Tbx1 and play an important role in cardiovascular development (Ivins et al., 2005; Liao et al., 2008). Expression of Hes1, a gene encoding a basic helix-loop-helix protein, is decreased in Tbx1 null cells, and has been demonstrated to be required for the development of structures affected in DGS and VCFS (van Bueren et al., 2010). Retinoic acid signaling, which will be discussed in further detail below, is up-regulated in Tbx1 null mice (Roberts et al., 2006). Inhibition of the retinoic acid degrading enzyme, Cyp26, phenocopies DGS in chick (Roberts et al., 2006), and retinoic acid signaling is locally aberrant in Crkl+/−;Tbx1+/− embryos (Guris et al., 2006). Other T-box family members such as Tbx2 and Tbx3 are individually required for outflow tract and pharyngeal development, and Tbx1 deficiency affects Tbx2 and Tbx3 expression in neural crest-derived cells and pharyngeal mesoderm (Mesbah et al., 2008; Mesbah et al., 2011). Vascular Endothelial Growth Factor, Vegf, and Chrd, a BMP antagonist, have been proposed to be modifiers of DGS (Choi and Klingensmith, 2009; Stalmans et al., 2003). Other possible genetic modifiers, including Fgf10, Pitx2, Shh, and Tgfβ, have been reviewed recently (Aggarwal and Morrow, 2008).
Although Tbx1 remains the leading gene candidate for DGS and VCFS, other genes from the 22q11 region have proved important in cardiovascular development as well. As its name implies, DiGeorge Critical Region Gene 8 (Dgcr8) is annotated to the 22q11.2 microdeletion (Shiohama et al., 2003). The gene encodes a double stranded RNA-binding protein essential for miRNA synthesis (Seitz and Zamore, 2006). Conditional inactivation of Dgcr8 in neural crest cells, results in a variety of cardiovascular defects, including persistent truncus arteriosus, interrupted aortic arch, aberrant origin of the right subclavian artery and ventricular septal defect (Chapnik et al., 2011). These defects stem from elevated apoptosis of neural crest cells in the caudal pharyngeal arches just before crest cells enter the outflow.
Crkl, an adaptor protein activated in receptor tyrosine kinase intracellular signaling cascades, is also located within the 22q11-deleted region. In mouse Crkl protein is expressed at high levels in neural crest cell derivatives. Disruption of Crkl causes the cardiovascular, craniofacial, and glandular defects characteristic of DGS and VCFS (Guris et al., 2001). Crkl deficiency has been shown to disrupt Fgf8 signaling and alter MAPK activation (Moon et al., 2006). Dose-sensitive interaction of Crkl and Tbx1 has been demonstrated, supporting the assertion that in the majority of cases DGS/VCFS is a contiguous gene syndrome (Guris et al., 2006). Crkl has been identified as an essential component of an FGF8-induced feed forward loop permissive for efficient activation of the mitogen-activated protein kinase Erk1/2, which is also necessary for cardiac neural crest cell development (see below). Crkl is essential for Fgf8 to sustain growth and survival in the absence of cell adhesion (Seo et al., 2009). In culture experiments Crkl is required for Fgf8-induced chemotaxis (Moon et al., 2006), raising intriguing implications for the role of Crkl in cardiac neural crest migration.
Other genes near to or within the typically deleted DiGeorge critical region play essential roles in cardiovascular development and appear to affect neural crest cell behavior: Erk2/Mapk1 is expressed within the pharyngeal arches and mouse mutants show craniofacial and cardiovascular defects, including ventricular septal defects, persistent truncus arteriosus, double outlet right ventricle, as well as thyroid and thymus developmental defects (Corson et al., 2003; Newbern et al., 2008). DiGeorge Critical Region Gene 6 (Dgcr6) is expressed in neural crest cell derivatives, and mutants exhibit subarterial ventricular septal defects, double outlet right ventricle, and absence of the fourth pharyngeal arches (Hierck et al., 2004). HIRA, a human homolog of yeast genes that encode repressors of histone gene transcription, is also expressed in neural crest-derived tissues and the pharyngeal arches. Knockdown of HIRA in chick embryos results in persistent truncus arteriosus (Farrell et al., 1999; Roberts et al., 1997; Roberts et al., 2002).
CHARGE Syndrome
The CHARGE acronym refers to this syndrome's characteristic phenotype: coloboma, heart anomaly, atresia of choanae, retardation of physical and mental development, genital hypoplasia and ear anomalies and/or deafness (Siebert et al., 1985). The collective anomalies associated with CHARGE syndrome overlap with DGS/VCFS-associated defects. The thyroid and parathyroid glands are often absent and accompany aortic arch artery and outflow defects suggesting neural crest development is affected in this syndrome. The range and severity of organ systems affected suggest that development is impacted early or that the involved gene has a broader expression pattern than those implicated in DGS/VCFS. Malformation of the foregut, reproductive organs, kidneys, limbs, digits, and brain, including pituitary gland, with lung abscesses and focal hepatic necrosis have all been observed. Over 90% of individuals affected with CHARGE syndrome have heterozygous mutations in CHD7, a member of the chromodomain helicase DNA-binding gene family (Bergman et al., 2011). CHARGE syndrome can also be phenocopied by exposure to teratogens, such as retinoic acid or thalidomide. Like DGS and VCFS, the CHARGE phenotype is highly variable, even within families carrying the same mutation.
Knockdown of CHD7 in Xenopus embryos recapitulates all major features of CHARGE syndrome in humans (Bajpai et al., 2010), and mice with heterozygous loss of function mutations in Chd7 are a model of CHARGE syndrome (Layman et al., 2010). CHD7 is required for activation of critical neural crest transcriptional circuitry, including Sox9, Twist, and Slug (Bajpai et al., 2010). CHD7 is responsible for chromatin remodeling and is widely expressed during development, helping to explain the broad range of congenital defects observed. The role of CHD7 in chromatin remodeling reinforces the emerging view that epigenetic regulation plays an important role in neural crest cell development (Liu and Xiao, 2011).
A subset of CHARGE cases have reported 22q11 deletions, and a recent paper explored the embryological relationship between CHARGE and VCFS (Randall et al., 2009). Chd7 and Tbx1 were found to interact synergistically in the development of the fourth pharyngeal arch artery, thymus, and ear. Pharyngeal arch artery morphogenesis could not be rescued by restoring neural crest Chd7 expression. Instead, biallelic expression of Chd7 and Tbx1 were required in the pharyngeal ectoderm.
Semaphorin 3E (SEMA3E) has also been proposed as a candidate gene for CHARGE syndrome, although it appears to be a rare cause, as only two patients with the mutation have been described (Bergman et al., 2011; Lalani et al., 2004; Martin et al., 2001). Sema3E is involved in vascular patterning, including formation of the first embryonic blood vessels (Gu et al., 2005; Meadows et al., 2012). It is unknown if Sema3E has a role in the neural crest cells, but other semaphorins, such as Sema3C (see below) have been shown to be particularly important in targeting cardiac neural crest cells to the outflow tract (Feiner et al., 2001).
Retinoic Acid Embryopathy and Retinoic Acid Signaling
When isotretinoin, a retinoid commercially known as Accutane, came into use for severe acne, many pregnant women were inadvertently exposed to it. Isotretinoin is a vitamin A derivative and an analog of retinoic acid. Shortly thereafter, Retinoic Acid Embryopathy was recognized among babies whose mothers were prescribed the drug during pregnancy. Features of the syndrome include anomalies of the cranium and face, central nervous system, heart and thymus–a constellation of defects reminiscent of neural crest-related syndromes. Cardiac defects include conotruncal heart defects (transposition of the great vessels, tetralogy of Fallot, double outlet right ventricle, truncus arteriosus, and ventricular septal defect) and aortic arch abnormalities (Type B interrupted aortic arch, retroesophageal right subclavian artery, and aortic arch hypoplasia) (Coberly et al., 1996; Lammer et al., 1985). Large amounts of vitamin A are also known to be teratogenic during pregnancy (Lammer et al., 1985; Mulder et al., 2000). An increased risk of congenital cardiovascular defects, including conotruncal defects, has been reported for human pregnancies when excess vitamin A (> 10,000 IU) is consumed in the form of retinol from supplements (Botto et al., 2001; Jenkins et al., 2007; Rothman et al., 1995).
Whereas isotretinoin or excess vitamin A exposure during gestation results in abnormally high retinoic acid signaling and associated birth defects, vitamin A deficiency also causes congenital anomalies, but due to reduced retinoic acid signaling (Zile, 2001). Cardiovascular defects observed in animal models of vitamin A deficiency include persistent truncus arteriosus, ventricular septal defects, and aortic arch artery defects (Pan and Baker, 2007; Wilson et al., 1953; Wilson and Warkany, 1950).
A number of gene mutations result in aberrant retinoic acid signaling. Human mutations in ALDH1A2 (formerly RALDH2), an enzyme responsible for the final step of endogenous retinoic acid synthesis, are associated with tetralogy of Fallot (Pavan et al., 2009). Aldh1a2 null mutant mice die at early embryonic stages but can be rescued from early lethality by transient maternal retinoic acid supplementation. These rescued mice consistently exhibit persistent truncus arteriosus suggesting Aldh1a2 is specifically required for normal outflow septation. (Niederreither et al., 2001). STRA6 codes for a membrane receptor which specifically binds the vitamin A-retinol binding protein (RBP) complex for cellular uptake (Kawaguchi et al., 2007). Human homozygous mutations in STRA6 cause a pleiotropic, multisystem malformation syndrome characterized by bilateral anophthalmia, mild facial dysmorphism, early lethality in most cases, and a variety of malformations of the lungs, diaphragm, heart, and urogenital system (Pasutto et al., 2007). Of the varied congenital heart defects in these patients, the authors report patent ductus arteriosus, coarctation of the aorta, ventricular and atrial septal defects, pulmonary atresia, right aortic arch, persistent truncus arteriosus, and tetralogy of Fallot.
Retinoic acid signaling occurs through two families of nuclear receptors, retinoid acid receptors (RAR) and retinoid X receptors (RXR) that act as transcriptional regulators when complexed with the retinoic acid ligand (Chen et al., 1996a). The RAR family is activated by all-trans-retinoic acid and 9-cis-retinoic acid, whereas the RXR family is activated by 9-cis-retinoic acid, as well as a number of other non-retinoid ligands. Each receptor family comprises three receptor isoforms designated alpha, beta, and gamma, and differential splicing produces further variants. Each isoform exhibits a specific spatio-temporal distribution during embryogenesis. RAR-RXR heterodimers initiate transcription of retinoic acid gene targets (Chen et al., 1996a; Lu et al., 1997). Compound mutations in RAR isoforms mimic anomalies observed in vitamin A deficiency including failure of outflow tract septation and abnormal arch artery patterning (Ghyselinck et al., 1998).
There are few studies that have revealed the cellular and molecular mechanisms in the cardiac neural crest cells that lead to abnormal outflow tract septation in retinoid receptor mouse mutants. Increased apoptosis is observed in outflow tract cushions of Rxrα null embryos (Kubalak et al., 2002). While the specific cell types undergoing apoptosis were not determined, the location is consistent with the neural crest cells. TGFβ2 is also widely upregulated in the hearts of Rxrα−/− mutants, and when crossed with mice heterozygous for a TGFβ2-null allele, the abnormal apoptosis, as well as outflow tract septation can be partially rescued. When RARα and RARβ are conditionally knocked out in neural crest cells, the number, migration and terminal fate of the cardiac crest are normal. However, the ability of these cells to form the aorticopulmonary septum is impaired (Jiang et al., 2002). Thus, retinoic acid signaling is likely required by other cell types in the outflow tract that then allows the neural crest cells to initiate and form the aorticopulmonary septum.
The second heart field progenitor cells, which produce the myocardium of the outflow tract, are particularly sensitive to retinoic acid deficiency (Ryckebusch et al., 2008). As mentioned above, normal second heart field development is intertwined with the normal function of the neural crest. A retinoic acid response element (RARE-Cre;R26R) mouse has been developed to lineage trace retinoic acid activity in both the second heart lineage and in the cardiac neural crest (Dollé et al., 2010). Marked retinoic acid-responsive cells are found in the caudal pharyngeal arches, aortic wall and the outflow tract myocardium and cushions. A recent study by Li et al. (2010) shows that retinoic acid receptor mutant mice display a shortened outflow tract and malalignment defects due to failure of the second heart field to contribute to the outflow. As an additional consequence, the outflow tract is misspecified along its proximal-distal axis, which results in ectopic expression of TGFβ2 and ectopic mesenchymal transformation of the endocardium. Reduction of TGFβ2 gene dosage in the retinoic acid receptor-deficient background restores septation but does not rescue alignment defects, indicating that excess TGFβ causes septation defects. The failure of the neural crest cells to septate the outflow may be due to the excess TGFβ acting on the neural crest and/or the inability of the neural crest to divide the misspecified outflow tract.
Retinoic acid directly regulates Hoxa3, a homeobox-containing transcription factor, in embryonic pharyngeal endoderm, cardiac neural crest and a subdomain of the second heart field (Diman et al., 2011). Hoxa3 is required for normal development of the third arch artery but not for cardiac development (Chisaka and Capecchi, 1991; Kirby et al., 1997). These results indicate that third arch crest cells carry information regarding arch artery patterning, but the same patterning instructions are not required for outflow septation.
Fetal Alcohol Syndrome
Fetal Alcohol Syndrome (FAS), the most extreme presentation of Fetal Alcohol Spectrum Disorders (FASD), is characterized by pre- and postnatal growth deficiency, microcephaly, developmental delay, fine motor dysfunction, specific facies, cleft plate, joint anomalies, altered palmar creases, and cardiac defects (Jones, 2011). FAS has been linked to disruption of neural crest cell development and structures derived from them. Exposure to alcohol during the period when neural crest cells are populating the caudal pharyngeal arches can phenocopy DGS in animal models, and alcohol has been proposed as a tetratogen involved in DGS (Sulik et al., 1986). Epidemiological studies suggest that consumption of alcoholic beverages by pregnant women more than once weekly during the periconceptional period can increase the risk of conotruncal defects in offspring by 2- to 2.5-fold (Carmichael et al., 2003).
Ethanol can exert a teratogenic effect through a number of proposed mechanisms. Disruption of microtubules and microfilaments by ethanol interferes with cell migration (Hassler and Moran, 1986). Newborn mice treated with ethanol in utero show abnormalities in cardiac mitochondria with decreased mitochondrial respiration (Nyquist-Battie and Freter, 1988; Satiroglu-Tufan and Tufan, 2004). Select cell populations, including cranial neural crest cells, exhibit increased apoptosis upon treatment with ethanol (Dunty et al., 2001). Increased cell death may be caused by heightened membrane fluidity (Chen et al., 1996b). Other authors have proposed that apoptosis is mediated by rapid depletion of beta-catenin in neural crest cell progenitors (Flentke et al., 2011). Gene expression is also affected in neural crest cells exposed to toxic levels of ethanol. Cranial neural crest downregulate certain Hox genes upon exposure to ethanol, which may be of etiological importance in FAS (Wentzel and Eriksson, 2009). Ethanol exposure also appears to influence retinoic acid signaling by antagonizing retinol (Vitamin A) and retinal conversion to retinoic acid, reducing retinoic acid levels to below that required for normal development (Yelin et al., 2005). Ethanol-induced anomalies in chick embryos are ameliorated by application of all-trans-retinoic acid, further indicating that retinoic acid signaling is reduced in FAS (Satiroglu-Tufan and Tufan, 2004).
Alagille Syndrome
Alagille syndrome, or arteriohepatic dysplasia, is a multisystem disorder caused by mutations in the Notch signaling pathway, most commonly in JAGGED1 (Alagille syndrome type 1), but in a small number of cases mutation in NOTCH2 (Alagille syndrome type 2). The primary defects and pathological features include chronic cholestasis due to fewer than normal intrahepatic bile ducts, peripheral pulmonary artery stenosis, minor vertebral segmentation abnormalities, characteristic facies, posterior embryotoxon/anterior segment anomalies, pigmentary retinopathy, and dysplastic kidneys (Turnpenny and Ellard, 2011). Common cardiac defects include tetralogy of Fallot and ventricular septal defects (Eldadah et al., 2001; Krantz et al., 1999; McElhinney et al., 2002). Alagille syndrome follows autosomal dominant inheritance, but penetrance is often reduced and phenotypic variability is frequently observed. Phenotypic discordance between monozygotic twins with the same JAG1 mutation indicates that the clinical variability observed cannot be explained by genotypic variation alone (Kamath et al., 2002).
Animal models have begun to elucidate the role of the Notch signaling pathway in cardiovascular development. Notch receptors and ligands are widely expressed in the remodeling outflow tract and aortic arch arteries (High et al., 2007; Loomes et al., 2002). Mice with targeted inhibition of Notch signaling in cardiac neural crest derivatives exhibit aortic arch patterning defects, pulmonary artery stenosis and ventricular septal defects (High et al., 2007; Loomes et al., 2002). Notch is essential for differentiation of cardiac neural crest-derived cells into the vascular smooth muscle of the aortic arch arteries. Interestingly however, Notch inactivation does not affect crest cell proliferation or migration into the outflow tract (High et al., 2007). Conversely, second heart field-specific Cre drivers targeting Jagged or Notch inhibition in the outflow precursors in mice have double outlet right ventricle and persistent truncus arteriosus, indicating that Jag1 is an essential Notch ligand in outflow development (High et al., 2009). Most recently disruption of Notch signaling in the second heart field was shown to result in semilunar valve defects, which the authors postulate is due to abnormal patterning of the cardiac neural crest migrating in apposition with second heart field derivatives (Jain et al., 2011).
Waardenburg Syndrome
Waardenburg syndrome is a disorder characterized by pigmentary abnormalities, congenital hearing loss, and facioskeletal anomalies. Cardiac defects have been observed in some Waardenburg patients (Banerjee, 1986; Khaldi et al., 1990; Mathieu et al., 1990). A number of genes are involved in this syndrome that are known to impact neural crest development. Heterozygous mutations in PAX3 (encoding the paired box 3 transcription factor) in humans give rise to Waardenburg syndrome type 1 and 3 (Tassabehji et al., 1994). Pax3 has a demonstrated role in cardiovascular development in animal models, and has been studied in the naturally occurring Splotch mutant mouse (Conway et al., 1997a; Conway et al., 1997b; Conway et al., 1997c). Global Pax3 deficiency results in a decrease in the number of migrating cardiac neural crest cells, which ultimately leads to a lack of cardiac neural crest-derived cells in the outflow tract septum and persistent truncus arteriosus (Conway et al., 2000). Splotch mice also exhibit myocardial dysfunction and aortic arch artery defects similar to what is seen in neural crest-ablated chick embryos (Conway et al., 1997a; Conway et al., 1997b). Pax3 is not required during migration or outflow tract septation, but is essential for all early neural crest progenitor formation, including cardiac neural crest (Olaopa et al., 2011). Loss of Pax3 and subsequent deletion of the cardiac neural crest has recently been shown to result in abnormal semilunar valve morphology (Jain et al., 2011).
It is of note that more cardiac defects are not observed in patients deficient for PAX3, given that Pax3 has been shown to be critical for cardiovascular development in animal models. It may be that species-specific differences in genetic background are important in determining phenotypic readout of Pax3 mutation. It may also be that severe mutations in PAX3 in humans are generally not conducive to life, and therefore cardiac defects are rarely observed.
LEOPARD and Noonan Syndromes
Noonan and LEOPARD syndromes are allelic with considerable phenotypic overlap (Tartaglia et al., 2011). Noonan syndrome is characterized by postnatally reduced growth, distinctive dysmorphic facial features, skeletal abnormalities, webbing of the neck, hypertrophic cardiomyopathy, and congenital heart defects. Pulmonary stenosis and septal defects are the most common cardiac defects, but aortic coarctation and a wide variety of other anomalies are also observed (Burch et al., 1993; Marino et al., 1999). LEOPARD syndrome's acronymic name refers to its major features: Lentigines, ECG conduction abnormalities, Ocular hypertelorism, Pulmoic stenosis, Abnormal genitalia, Retardation of growth, and sensorineural Deafness (Gorlin et al., 1971). Roughly half of all individuals affected have heart defects similar to those seen in Noonan syndrome, but with different frequencies. ECG anomalies, progressive conduction defects and hypertrophic cardiomyopathy are most frequently observed (Tartaglia et al., 2011).
Approximately half of all cases of Noonan syndrome are caused by heterozygous mutations in PTPN11, a gene encoding the protein-tyrosine phosphatase SHP2 (Tartaglia et al., 2002; Zenker et al., 2004). SHP2 positively regulates the RAS and mitogen-activated protein kinase (MAPK) signal transduction pathway. Mutations in PTPN11 that cause Noonan syndrome increase SHP2 phosphatase activity and signaling through the RAS-MAPK pathway (Fragale et al., 2004; Keilhack et al., 2005; Martinelli et al., 2008; Oishi et al., 2006; Tartaglia et al., 2006). Other disease genes include SOS1, KRAS, NRAS, RAF1, BRAF, SHOC2, MEK1 and CBL, all of which affect RAS-MAPK signaling. The vast majority of individuals with LEOPARD syndrome carry mutations in PTPN11 (Digilio et al., 2002; Legius et al., 2002), which produce catalytically inactive SHP2 with dominant negative effects (Kontaridis et al., 2006). In a small number of cases LEOPARD syndrome has also been linked to RAF1 or BRAF mutations (Koudova et al., 2009; Pandit et al., 2007; Sarkozy et al., 2009). While LEOPARD syndrome alleles engender loss-of-function effects, Noonan syndrome mutations result in gain-of-function. These biochemical findings prompt the question of how PTPN11 mutations with opposite functional effects result in similar disorders (Edouard et al., 2007; Oishi et al., 2009). Recent studies suggest that mutations resulting in LEOPARD syndrome are not simply dominant negative in nature, and that Shp2 plays phosphatase-independent roles (Edouard et al., 2010; Martinelli et al., 2008; Oishi et al., 2009; Stewart et al., 2010).
Noonan, LEOPARD as well as some of the syndromes and genes discussed above are linked by the common theme of aberrant ERK signaling as a result of alterations in upstream effectors. For example, FGF stimulates the ERK1/2 cascade by promoting the association of Shp2 with small G proteins that are activators of Ras leading to increased ERK1/2 signaling (Böttcher and Niehrs, 2005). Similarly, Crkl and Fgf8 interact to regulate the expression of Fgf8 target genes in the pharyngeal arches, and Crkl deficiency disrupts ERK1/2 activation (Moon et al., 2006). ERK1/2 phosphorylation is reduced in mice with reduced or eliminated FGF8 expression and these animals have craniofacial and cardiovascular defects similar to those noted in Tbx1- and Crkl-mutant mice, respectively (Abu-Issa et al., 2002; Park et al., 2006). In the second heart field Tbx1 is required for Fgf8 expression, and activation of ERK1/2 is significantly reduced in Tbx1−/− cells (Vitelli et al., 2010). Shp2 is important for the normal development of cardiac neural crest cells. Ablation of PTPN11 specifically from the premigratory neural crest cells results initially in normal migration and proliferative patterns, but cardiac crest cells fail to enter the developing outflow tract. Embryos display persistent truncus arteriosus, septal defects and abnormalities of the great vessels (Nakamura et al., 2009). Interestingly, Shp2 null mutation in the neural crest of mice more closely phenocopies the 22q11 deletion syndrome than LEOPARD syndrome, indicating that complete absence of Shp2 has different consequences than the expression of a LEOPARD syndrome-causing mutant. A recent study identified a role for Shp2 in neural crest specification and migration in zebrafish embryos carrying mRNA for LEOPARD syndrome alleles. Shp2 was also shown to prevent p53-mediated apoptosis of neural crest cells (Stewart et al., 2010). In contrast, another study employing an inducible knock-in approach demonstrated that all cardiac defects in Noonan syndrome result from mutant Shp2 expression in the endocardium, not in neural crest cells. These authors showed that increasing Erk MAPK activation affects the endocardial to mesenchymal transformation by extending the interval during which cardiac endocardial cells undergo EMT (Araki et al., 2009). As discussed above, proper EMT is critical for development of the endocardial cushions, which interact with cardiac neural crest cells during septation.
Endothelin Signaling
Mutations in the endothelin-1 (ET1) pathway in mouse models result in patterning defects of the great arteries and outflow despite normal migration of the neural crest cells (Clouthier et al., 1998; Kurihara et al., 1994; Yanagisawa et al., 1998). ET1 is expressed in the arch artery epithelium and binds its receptor, endothelin A (ETA), present on neural crest cells in the pharyngeal arches. Chimera studies in mice have shown that embryonic stem cells null for ETA are excluded from the walls of the pharyngeal arch arteries (Clouthier et al., 2003). This result suggests that neural crest cells require ETA to interact with the endothelium of the forming arteries. In addition, endothelin signaling appears to affect patterning of particular cardiac neural crest lineages. Constitutive expression of exogenous ET1 in the neural crest cells results in selective expansion of the outermost, adventitial cell layer of the great vessels, while the smooth muscle layer is unaffected (Ballard and Mikawa, 2002). Human mutations in endothelin signaling can result in Hirschsprung Disease, which is primarily a disorder of the enteric nervous system. These patients can exhibit cardiac malformations, including patent ductus arteriosus and ventricular septal defects, as well as problems with parasympathetic and sympathetic innervation of the heart (Hofstra et al., 1999; Staiano et al., 1999). Mutations in endothelin pathway genes can also result in Waardenburg syndrome (Pingault et al., 2010).
Semaphorin 3C
Semaphorin 3C, a secreted ligand used in axon guidance, is also important in the migration and targeting of cardiac neural crest cells to the outflow tract. Sema3C-null mice exhibit interrupted aortic arch and persistent truncus arteriosus (Feiner et al., 2001). Sema3C is particularly required by cardiac neural crest because all other neural crest derivatives appear to be normal. The myocardium of the undivided outflow tract expresses Sema3C while the neural crest cells express the receptors for semaphorin ligands which are multimeric complexes of Plexins and neuropilin (Np)1 and/or Np2. Semaphorin ligands bind neuropilin and plexin receptors which lead to alterations in the cytoskeleton and microtubule network (Yu and Kolodkin, 1999). A complex of Np1 and PlexinA2 is likely the functional receptor in the cardiac neural crest, as either PlexinA2 or Np1-null mice have persistent truncus arteriosus and interrupted aortic arch (Brown et al., 2001; Kawasaki et al., 1999). GATA6, a transcription factor expressed by vascular smooth muscle, has been shown to regulate Sema3C expression in the outflow tract and vascular smooth muscle. Mice with targeted deletion of GATA6 in the vascular smooth muscle also have interrupted aortic arch and persistent truncus arteriosus (Lepore et al., 2006). Two GATA6 mutations have been identified in some patients with persistent truncus arteriosus (Kodo et al., 2009). Both of the human GATA6 mutant proteins failed to transactivate gene expression of SEMA3C and PLXNA2, suggesting an underlying molecular basis for the outflow defect.
Forkhead Transcription Factors
Members of the forkhead family of transcription factors, Foxc1 and Foxc2, are required for arch artery patterning, outflow septation and second heart field development in mice. These genes are coexpressed in the second heart field, the cardiac neural crest cells, the endocardium, and proepicardium. Embryos lacking either Foxc1 or Foxc2 alone or compound heterozygotes, have coarctation or interrupted aortic arch (Iida et al., 1997; Kume et al., 2001; Seo and Kume, 2006; Winnier et al., 1999). The cardiac neural crest cells in Foxc1 and Foxc2 compound heterozygous mice undergo abnormal apoptosis leading to outflow septation defects. FOXC1 mutations in humans are associated with the dominantly inherited Axenfeld-Rieger anomaly (Honkanen et al., 2003; Mears et al., 1998; Winnier et al., 1999). Some patients have congenital heart defects such as mitral valve dysplasia and atrial septal defects. Human FOXC2 mutations are linked to the autosomal dominant syndrome, lymphedema-distichiasis, in which 15% of patients also have conotruncal cardiac defects (Fang et al., 2000).
TGFβ/BMP Superfamily
BMP2 and 4, have been implicated as regulators of neural crest cell induction, migration, differentiation and survival. One of the BMP receptors, Bmpr1A is expressed in the neural tube early enough to be involved in neural crest specification and/or migration. Mice in which the Bmpr1A receptor has been ablated from the neural crest prior to their migration display a shortened cardiac outflow tract and defective septation but do not have defects in the induction, delamination or initial migration of the neural crest (Stottmann et al., 2004). These mice die at midgestation due to acute heart failure. A more recent study shows that neural crest-specific deletion of Bmpr1A is associated with hypoplasia of the outflow cushions (Nomura-Kitabayashi et al., 2009). Deletion of Alk4, another BMP receptor, in the cardiac neural crest also results in arch artery patterning defects and persistent truncus arteriosus (Kaartinen et al., 2004). In these mice not enough neural crest cells reach the outflow to effect normal septation. In TGFβ2 knockout mice neural crest cell migration and differentiation into smooth muscle cells is normal. However, these mutants do display fourth pharyngeal arch artery interruptions and innervation of the fourth arch artery is diminished (Molin et al., 2004). Smad7 is a negative regulator of both TGFβ and BMP intracellular signaling. Smad7 knockout mouse embryos have cardiovascular defects including outflow tract malformations (Chen et al., 2009). When the inhibitory Smad7 is overexpressed within the neural crest lineages, neural crest-derived craniofacial, pharyngeal and cardiac outflow tract cushion cells are considerably reduced in number, and the mice display craniofacial defects, an undivided outflow tract and thickened outflow valves (Tang et al., 2010). As in the BMP receptor mutant mice, initial migration of the neural crest cells is not affected. Interestingly, even though there is elevated cell death in the pharyngeal arches and colonization of the outflow tract is significantly reduced, the pharyngeal arch arteries have normal smooth muscle tunics and are patterned normally.
Thus a common theme emerges: too much or not enough signaling in a particular pathway (eg. TGFβ/BMP, FGF, retinoic acid) results in similar cardiovascular defects by perturbing the development of the neural crest cells as they migrate from the neural tube, into the pharynx and eventually the heart. The analysis of these pathways is complicated by the fact that they play multiple roles at multiple times of development and impact (positively or negatively) other signaling pathways. For example, interrupting FGF signaling in second heart field mesoderm causes altered myocardial production of BMP and TGFβ that secondarily perturbs mesenchymal transformation of endocardium as well as the ability of the cardiac neural crest to populate the outflow tract (Park et al., 2008). Consequently there is a delicate balance of these signaling pathways needed to orchestrate the development of the neural crest as well as the other surrounding cell types in the pharynx and heart.
The cardiocraniofacial developmental module
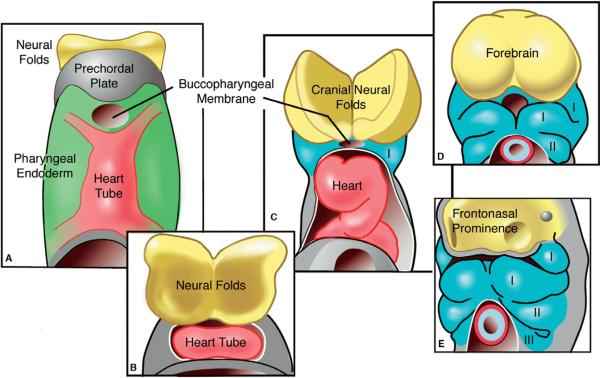
Cardiac neural crest cells communicate during their migration with myriad cell types in their environment: pharyngeal endoderm, ectoderm, and mesoderm, endothelial cells, and cells of the outflow tract. The crest cells provide an additional link between the head (neural folds and later face) and the cardiovascular system. Syndromes linking the face, brain, and heart can best be understood when these regions are considered as a single developmental module (Fig. 5). The cardiocraniofacial module is comprised initially of cranial neural plate ectoderm (including the neural crest), the mesendodermal prechordal plate, pharyngeal endoderm and lateral plate mesoderm in which the bilateral heart fields exist (Kirby, 2006). As the anterior intestinal portal moves caudally and the pharynx forms, the bilateral heart fields merge into a midline heart tube that covers the region that will become the face. The cranial neural folds grow forward forming the forebrain, also pushing the heart ventrally. As the pharyngeal arches interpose between the heart and neural folds and the frontonasal prominence forms, the heart is moved further caudally. Pharyngeal mesoderm, the mesodermal core within the arches, broadly includes paraxial and splanchnic mesoderm and gives rise to significant parts of the head muscles and heart (Tzahor and Evans, 2011). The neural crest migrates from the neural tube and intermingles with the pharyngeal mesoderm. Pharyngeal mesoderm, pharyngeal endoderm, ectoderm, and neural crest cells are all in apposition with each other, and all these tissues influence the development of one another.
Figure 5.
Schematic representation of the cardiocraniofacial module. (A) The cranial neural plate (yellow), prechordal plate (grey) and fused midline heart tube (pink) are in close apposition with the ventral pharyngeal endoderm (green) in the region in which the face will develop. As development proceeds (B–E), the cranial neural folds grow forward forming the forebrain, while the heart is pushed ventrally. The heart moves farther caudally as the pharyngeal arches (blue) and the frontonasal prominence develop to form the primordia that will shape the face. (Adapted from Hutson and Kirby, 2003).
Many of the syndromes discussed above are likely the result of disruptions in the cardiocraniofacial module. The phenotypes of any of the cardiocraniofacial syndromes vary widely, even among genetically similar family members and between monozygotic twins. This phenomenon can be partly explained by considering the number of interacting and interdependent tissues within the module. The varied outcomes of a single gene mutation, a chromosomal microdeletion or an environmental assault on the module can result from only small modifications in the crosstalk among components of the module. A gene mutation affecting transcription or signaling could alter the dialogue between cells in the module, which would destabilize constituents of the module (Kirby, 2006).
For example, DGS is caused by absence of or mutations in TBX1. It has been shown in animal models that Tbx1 is expressed in the endoderm and that it controls Fgf8 expression in the pharyngeal endoderm at a time when important patterning and differentiation events are occurring in the pharyngeal arches. The arches contain the migrating neural crest cells, pharyngeal pouches, and secondary heart field. A reduction in this critical signaling component would lead to abnormal development among multiple elements of the module.
Acknowledgments
ALK is funded by NIH NRSA grant HD070631-01. MRH is funded by the George and Jean Brumley, Jr. Neonatal-Perinatal Research Institute of Duke University, AHA grants AHA0830464N, BGIA7370008 and NIH grant HL084413.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We use “secondary” to refer to the specific subpopulation of the second heart field that contributes the myocardial to smooth muscle junction at the level of the outflow valves. We use “second” to refer to the broader heart field that contributes both inflow (venous pole) and outflow. See Dyer and Kirby (2009) for a discussion of the second and secondary heart fields.
Works Cited
- Abu-Issa R, Smyth G, Smoak I, Yamamura K.-i., Meyers EN. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development (Cambridge, England) 2002;129:4613–4625. doi: 10.1242/dev.129.19.4613. [DOI] [PubMed] [Google Scholar]
- Aggarwal VS, Morrow BE. Genetic modifiers of the physical malformations in velo-cardio-facial syndrome/DiGeorge syndrome. Dev Disabil Res Rev. 2008;14:19–25. doi: 10.1002/ddrr.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati F, Conti E, Novelli A, Bengala M, Diglio MC, Marino B, Giannotti A, Gabrielli O, Novelli G, Dallapiccola B. Atypical deletions suggest five 22q11.2 critical regions related to the DiGeorge/velo-cardio-facial syndrome. European journal of human genetics : EJHG. 1999;7:903–909. doi: 10.1038/sj.ejhg.5200399. [DOI] [PubMed] [Google Scholar]
- Araki T, Chan G, Newbigging S, Morikawa L, Bronson RT, Neel BG. Noonan syndrome cardiac defects are caused by PTPN11 acting in endocardium to enhance endocardial-mesenchymal transformation. Proc Natl Acad Sci USA. 2009;106:4736–4741. doi: 10.1073/pnas.0810053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, Helms J, Chang C-P, Zhao Y, Swigut T, Wysocka J. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–962. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard VLT, Mikawa T. Constitutive expression of preproendothelin in the cardiac neural crest selectively promotes expansion of the adventitia of the great vessels in vivo. Developmental biology. 2002;251:167–177. doi: 10.1006/dbio.2002.0818. [DOI] [PubMed] [Google Scholar]
- Banerjee AK. Waardenburg's syndrome associated with ostium secundum atrial septal defect. J R Soc Med. 1986;79:677–678. doi: 10.1177/014107688607901121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman JEH, Janssen N, Hoefsloot LH, Jongmans MCJ, Hofstra RMW, van Ravenswaaij-Arts CMA. CHD7 mutations and CHARGE syndrome: the clinical implications of an expanding phenotype. J Med Genet. 2011;48:334–342. doi: 10.1136/jmg.2010.087106. [DOI] [PubMed] [Google Scholar]
- Bergwerff M, Verberne ME, DeRuiter MC, Poelmann RE, Gittenberger-de Groot AC. Neural crest cell contribution to the developing circulatory system: implications for vascular morphology? Circulation research. 1998;82:221–231. doi: 10.1161/01.res.82.2.221. [DOI] [PubMed] [Google Scholar]
- Besson WT, Kirby ML, Van Mierop LH, Teabeaut JR. Effects of the size of lesions of the cardiac neural crest at various embryonic ages on incidence and type of cardiac defects. Circulation. 1986;73:360–364. doi: 10.1161/01.cir.73.2.360. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Redmond ME, Waldo K, Davis H, Kirby ML. Effect of neural crest ablation on development of the heart and arch arteries in the chick. The American journal of anatomy. 1987;180:332–341. doi: 10.1002/aja.1001800403. [DOI] [PubMed] [Google Scholar]
- Böttcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Botto LD, Loffredo C, Scanlon KS, Ferencz C, Khoury MJ, David Wilson P, Correa A. Vitamin A and cardiac outflow tract defects. Epidemiology. 2001;12:491–496. doi: 10.1097/00001648-200109000-00005. [DOI] [PubMed] [Google Scholar]
- Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, Epstein JA. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development (Cambridge, England) 2001;128:3071–3080. doi: 10.1242/dev.128.16.3071. [DOI] [PubMed] [Google Scholar]
- Burch M, Sharland M, Shinebourne E, Smith G, Patton M, McKenna W. Cardiologic abnormalities in Noonan syndrome: phenotypic diagnosis and echocardiographic assessment of 118 patients. J Am Coll Cardiol. 1993;22:1189–1192. doi: 10.1016/0735-1097(93)90436-5. [DOI] [PubMed] [Google Scholar]
- Calmont A, Ivins S, Van Bueren KL, Papangeli I, Kyriakopoulou V, Andrews WD, Martin JF, Moon AM, Illingworth EA, Basson MA, Scambler PJ. Tbx1 controls cardiac neural crest cell migration during arch artery development by regulating Gbx2 expression in the pharyngeal ectoderm. Development (Cambridge, England) 2009;136:3173–3183. doi: 10.1242/dev.028902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Yang W, Lammer EJ. Maternal periconceptional alcohol consumption and risk for conotruncal heart defects. Birth Defects Res Part A Clin Mol Teratol. 2003;67:875–878. doi: 10.1002/bdra.10087. [DOI] [PubMed] [Google Scholar]
- Chapnik E, Sasson V, Blelloch R, Hornstein E. Dgcr8 controls neural crest cells survival in cardiovascular development. Developmental biology. 2011 doi: 10.1016/j.ydbio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Chen JY, Clifford J, Zusi C, Starrett J, Tortolani D, Ostrowski J, Reczek PR, Chambon P, Gronemeyer H. Two distinct actions of retinoid-receptor ligands. Nature. 1996a;382:819–822. doi: 10.1038/382819a0. [DOI] [PubMed] [Google Scholar]
- Chen Q, Chen H, Zheng D, Kuang C, Fang H, Zou B, Zhu W, Bu G, Jin T, Wang Z, Zhang X, Chen J, Field LJ, Rubart M, Shou W, Chen Y. Smad7 is required for the development and function of the heart. J Biol Chem. 2009;284:292–300. doi: 10.1074/jbc.M807233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SY, Yang B, Jacobson K, Sulik KK. The membrane disordering effect of ethanol on neural crest cells in vitro and the protective role of GM1 ganglioside. Alcohol. 1996b;13:589–595. doi: 10.1016/s0741-8329(96)00073-0. [DOI] [PubMed] [Google Scholar]
- Chisaka O, Capecchi MR. Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox-1.5. Nature. 1991;350:473–479. doi: 10.1038/350473a0. [DOI] [PubMed] [Google Scholar]
- Choi M, Klingensmith J. Chordin is a modifier of tbx1 for the craniofacial malformations of 22q11 deletion syndrome phenotypes in mouse. PLoS Genet. 2009;5:e1000395. doi: 10.1371/journal.pgen.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development (Cambridge, England) 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Williams SC, Hammer RE, Richardson JA, Yanagisawa M. Cell-autonomous and nonautonomous actions of endothelin-A receptor signaling in craniofacial and cardiovascular development. Developmental biology. 2003;261:506–519. doi: 10.1016/s0012-1606(03)00128-3. [DOI] [PubMed] [Google Scholar]
- Coberly S, Lammer E, Alashari M. Retinoic acid embryopathy: case report and review of literature. Pediatr Pathol Lab Med. 1996;16:823–836. [PubMed] [Google Scholar]
- Conway SJ, Bundy J, Chen J, Dickman E, Rogers R, Will BM. Decreased neural crest stem cell expansion is responsible for the conotruncal heart defects within the splotch (Sp(2H))/Pax3 mouse mutant. Cardiovasc Res. 2000;47:314–328. doi: 10.1016/s0008-6363(00)00098-5. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Godt RE, Hatcher CJ, Leatherbury L, Zolotouchnikov VV, Brotto MA, Copp AJ, Kirby ML, Creazzo TL. Neural crest is involved in development of abnormal myocardial function. J Mol Cell Cardiol. 1997a;29:2675–2685. doi: 10.1006/jmcc.1997.0499. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Henderson DJ, Copp AJ. Pax3 is required for cardiac neural crest migration in the mouse: evidence from the splotch (Sp2H) mutant. Development (Cambridge, England) 1997b;124:505–514. doi: 10.1242/dev.124.2.505. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Henderson DJ, Kirby ML, Anderson RH, Copp AJ. Development of a lethal congenital heart defect in the splotch (Pax3) mutant mouse. Cardiovasc Res. 1997c;36:163–173. doi: 10.1016/s0008-6363(97)00172-7. [DOI] [PubMed] [Google Scholar]
- Corson LB, Yamanaka Y, Lai K-MV, Rossant J. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development (Cambridge, England) 2003;130:4527–4537. doi: 10.1242/dev.00669. [DOI] [PubMed] [Google Scholar]
- Creazzo TL, Brotto MA, Burch J. Excitation-contraction coupling in the day 15 embryonic chick heart with persistent truncus arteriosus. Pediatr Res. 1997;42:731–737. doi: 10.1203/00006450-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Creazzo TL, Godt RE, Leatherbury L, Conway SJ, Kirby ML. Role of cardiac neural crest cells in cardiovascular development. Annu Rev Physiol. 1998;60:267–286. doi: 10.1146/annurev.physiol.60.1.267. [DOI] [PubMed] [Google Scholar]
- de Lange FJ, Moorman AFM, Anderson RH, Männer J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJB, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95:645–654. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- Digilio MC, Conti E, Sarkozy A, Mingarelli R, Dottorini T, Marino B, Pizzuti A, Dallapiccola B. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002;71:389–394. doi: 10.1086/341528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diman NYS-G, Remacle S, Bertrand N, Picard JJ, Zaffran S, Rezsohazy R. A retinoic Acid responsive hoxa3 transgene expressed in embryonic pharyngeal endoderm, cardiac neural crest and a subdomain of the second heart field. PLoS ONE. 2011;6:e27624. doi: 10.1371/journal.pone.0027624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollé P, Fraulob V, Gallego-Llamas J, Vermot J, Niederreither K. Fate of retinoic acid-activated embryonic cell lineages. Dev Dyn. 2010;239:3260–3274. doi: 10.1002/dvdy.22479. [DOI] [PubMed] [Google Scholar]
- Dunty WC, Chen SY, Zucker RM, Dehart DB, Sulik KK. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol Clin Exp Res. 2001;25:1523–1535. [PubMed] [Google Scholar]
- Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Developmental biology. 2009;336:137–144. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edouard T, Combier J-P, Nédélec A, Bel-Vialar S, Métrich M, Conte-Auriol F, Lyonnet S, Parfait B, Tauber M, Salles J-P, Lezoualc'h F, Yart A, Raynal P. Functional effects of PTPN11 (SHP2) mutations causing LEOPARD syndrome on epidermal growth factor-induced phosphoinositide 3-kinase/AKT/glycogen synthase kinase 3beta signaling. Mol Cell Biol. 2010;30:2498–2507. doi: 10.1128/MCB.00646-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edouard T, Montagner A, Dance M, Conte F, Yart A, Parfait B, Tauber M, Salles JP, Raynal P. How do Shp2 mutations that oppositely influence its biochemical activity result in syndromes with overlapping symptoms? Cell Mol Life Sci. 2007;64:1585–1590. doi: 10.1007/s00018-007-6509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldadah ZA, Hamosh A, Biery NJ, Montgomery RA, Duke M, Elkins R, Dietz HC. Familial Tetralogy of Fallot caused by mutation in the jagged1 gene. Human molecular genetics. 2001;10:163–169. doi: 10.1093/hmg/10.2.163. [DOI] [PubMed] [Google Scholar]
- Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, Seaver LH, Glover TW. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet. 2000;67:1382–1388. doi: 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Burch JL, Wallis K, Rowley L, Kumiski D, Stadt H, Godt RE, Creazzo TL, Kirby ML. FGF-8 in the ventral pharynx alters development of myocardial calcium transients after neural crest ablation. J Clin Invest. 2001;107:1509–1517. doi: 10.1172/JCI9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Stadt H, Wallis KT, Scambler P, Hixon RL, Wolfe R, Leatherbury L, Kirby ML. HIRA, a DiGeorge syndrome candidate gene, is required for cardiac outflow tract septation. Circ Res. 1999;84:127–135. doi: 10.1161/01.res.84.2.127. [DOI] [PubMed] [Google Scholar]
- Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, Mombaerts P, Epstein JA, Raper JA. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development (Cambridge, England) 2001;128:3061–3070. doi: 10.1242/dev.128.16.3061. [DOI] [PubMed] [Google Scholar]
- Flentke GR, Garic A, Amberger E, Hernandez M, Smith SM. Calcium-mediated repression of β-catenin and its transcriptional signaling mediates neural crest cell death in an avian model of fetal alcohol syndrome. Birth Defects Res Part A Clin Mol Teratol. 2011;91:591–602. doi: 10.1002/bdra.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale A, Tartaglia M, Wu J, Gelb BD. Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Human mutation. 2004;23:267–277. doi: 10.1002/humu.20005. [DOI] [PubMed] [Google Scholar]
- Frank D, Fotheringham L, Brewer J, Muglia L, Tristani-Firouzi M, Capecchi M, Moon A. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development (Cambridge, England) 2002;129:4591–4603. doi: 10.1242/dev.129.19.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V, Yamagishi C, Hu T, Kathiriya IS, Yamagishi H, Srivastava D. Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Developmental biology. 2001;235:62–73. doi: 10.1006/dbio.2001.0283. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB, Wendling O, Messaddeq N, Dierich A, Lampron C, Decimo D, Viville S, Chambon P, Mark M. Contribution of retinoic acid receptor beta isoforms to the formation of the conotruncal septum of the embryonic heart. Developmental biology. 1998;198:303–318. [PubMed] [Google Scholar]
- Goodship J, Cross I, Scambler P, Burn J. Monozygotic twins with chromosome 22q11 deletion and discordant phenotype. J Med Genet. 1995;32:746–748. doi: 10.1136/jmg.32.9.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlin RJ, Anderson RC, Moller JH. The Leopard (multiple lentigines) syndrome revisited. Birth Defects Orig Artic Ser 07. 1971:110–115. [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert D, Mann F, Merte J, Henderson C, Jessell T, Kolodkin A, Ginty D. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science (New York, N.Y. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- Guris DL, Duester G, Papaioannou VE, Imamoto A. Dose-dependent interaction of Tbx1 and Crkl and locally aberrant RA signaling in a model of del22q11 syndrome. Developmental cell. 2006;10:81–92. doi: 10.1016/j.devcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Guris DL, Fantes J, Tara D, Druker BJ, Imamoto A. Mice lacking the homologue of the human 22q11.2 gene CRKL phenocopy neurocristopathies of DiGeorge syndrome. Nature genetics. 2001;27:293–298. doi: 10.1038/85855. [DOI] [PubMed] [Google Scholar]
- Gurjarpadhye A, Hewett KW, Justus C, Wen X, Stadt H, Kirby ML, Sedmera D, Gourdie RG. Cardiac neural crest ablation inhibits compaction and electrical function of conduction system bundles. Am J Physiol Heart Circ Physiol. 2007;292:H1291–1300. doi: 10.1152/ajpheart.01017.2006. [DOI] [PubMed] [Google Scholar]
- Hassler JA, Moran DJ. Effects of ethanol on the cytoskeleton of migrating and differentiating neural crest cells: possible role in teratogenesis. J Craniofac Genet Dev Biol Suppl. 1986;2:129–136. [PubMed] [Google Scholar]
- Hierck BP, Molin DGM, Boot MJ, Poelmann RE, Gittenberger-de Groot AC. A chicken model for DGCR6 as a modifier gene in the DiGeorge critical region. Pediatr Res. 2004;56:440–448. doi: 10.1203/01.PDR.0000136151.50127.1C. [DOI] [PubMed] [Google Scholar]
- High FA, Jain R, Stoller JZ, Antonucci NB, Lu MM, Loomes KM, Kaestner KH, Pear WS, Epstein JA. Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development. J Clin Invest. 2009;119:1986–1996. doi: 10.1172/JCI38922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, Epstein JA. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest. 2007;117:353–363. doi: 10.1172/JCI30070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstra RM, Valdenaire O, Arch E, Osinga J, Kroes H, Löffler BM, Hamosh A, Meijers C, Buys CH. A loss-of-function mutation in the endothelin-converting enzyme 1 (ECE-1) associated with Hirschsprung disease, cardiac defects, and autonomic dysfunction. Am J Hum Genet. 1999;64:304–308. doi: 10.1086/302184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen RA, Nishimura DY, Swiderski RE, Bennett SR, Hong S, Kwon YH, Stone EM, Sheffield VC, Alward WLM. A family with Axenfeld-Rieger syndrome and Peters Anomaly caused by a point mutation (Phe112Ser) in the FOXC1 gene. Am J Ophthalmol. 2003;135:368–375. doi: 10.1016/s0002-9394(02)02061-5. [DOI] [PubMed] [Google Scholar]
- Hu T, Yamagishi H, Maeda J, McAnally J, Yamagishi C, Srivastava D. Tbx1 regulates fibroblast growth factors in the anterior heart field through a reinforcing autoregulatory loop involving forkhead transcription factors. Development (Cambridge, England) 2004;131:5491–5502. doi: 10.1242/dev.01399. [DOI] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. Neural crest and cardiovascular development: a 20-year perspective. Birth Defects Res C Embryo Today. 2003;69:2–13. doi: 10.1002/bdrc.10002. [DOI] [PubMed] [Google Scholar]
- Hutson MR, Zhang P, Stadt HA, Sato AK, Li Y-X, Burch J, Creazzo TL, Kirby ML. Cardiac arterial pole alignment is sensitive to FGF8 signaling in the pharynx. Developmental biology. 2006;295:486–497. doi: 10.1016/j.ydbio.2006.02.052. [DOI] [PubMed] [Google Scholar]
- Iida K, Koseki H, Kakinuma H, Kato N, Mizutani-Koseki Y, Ohuchi H, Yoshioka H, Noji S, Kawamura K, Kataoka Y, Ueno F, Taniguchi M, Yoshida N, Sugiyama T, Miura N. Essential roles of the winged helix transcription factor MFH-1 in aortic arch patterning and skeletogenesis. Development (Cambridge, England) 1997;124:4627–4638. doi: 10.1242/dev.124.22.4627. [DOI] [PubMed] [Google Scholar]
- Ivins S, Lammerts van Beuren K, Roberts C, James C, Lindsay E, Baldini A, Ataliotis P, Scambler PJ. Microarray analysis detects differentially expressed genes in the pharyngeal region of mice lacking Tbx1. Developmental biology. 2005;285:554–569. doi: 10.1016/j.ydbio.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Jain R, Engleka KA, Rentschler SL, Manderfield LJ, Li L, Yuan L, Epstein JA. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J Clin Invest. 2011;121:422–430. doi: 10.1172/JCI44244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR, Elixson M, Warnes CA, Webb CL, Young A.H.A.C.o.C.D.i.t. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:2995–3014. doi: 10.1161/CIRCULATIONAHA.106.183216. [DOI] [PubMed] [Google Scholar]
- Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nature genetics. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- Jiang X, Choudhary B, Merki E, Chien KR, Maxson RE, Sucov HM. Normal fate and altered function of the cardiac neural crest cell lineage in retinoic acid receptor mutant embryos. Mechanisms of development. 2002;117:115–122. doi: 10.1016/s0925-4773(02)00206-x. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development (Cambridge, England) 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Jones KL. The Effects of Alcohol on Fetal Development. Birth Defects Res C. 2011;93:3–11. doi: 10.1002/bdrc.20200. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development (Cambridge, England) 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- Kamath BM, Krantz ID, Spinner NB, Heubi JE, Piccoli DA. Monozygotic twins with a severe form of Alagille syndrome and phenotypic discordance. Am J Med Genet. 2002;112:194–197. doi: 10.1002/ajmg.10610. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science (New York, N.Y. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development (Cambridge, England) 1999;126:4895–4902. doi: 10.1242/dev.126.21.4895. [DOI] [PubMed] [Google Scholar]
- Keilhack H, David FS, McGregor M, Cantley LC, Neel BG. Diverse biochemical properties of Shp2 mutants. Implications for disease phenotypes. The Journal of biological chemistry. 2005;280:30984–30993. doi: 10.1074/jbc.M504699200. [DOI] [PubMed] [Google Scholar]
- Khaldi F, Serbegi M, Mokadem H, Lazzem B, Bennaceur B. Waardenburg syndrome. Report of a familial case. Ann Pediatr (Paris) 1990;37:55–58. [PubMed] [Google Scholar]
- Kirby ML. Cardiac Development. Oxford University Press; Oxford, UK: 2006. [Google Scholar]
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aorticopulmonary septation. Science (New York, N.Y. 1983;220:1059–1061. doi: 10.1126/science.6844926. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Hunt P, Wallis K, Thorogood P. Abnormal patterning of the aortic arch arteries does not evoke cardiac malformations. Dev Dyn. 1997;208:34–47. doi: 10.1002/(SICI)1097-0177(199701)208:1<34::AID-AJA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Hutson MR. Role of Cardiac Neural Crest in the Development of the Caudal Pharyngeal Arches, the Cardiac Outflow and Disease. In: Rosenthal N, Harvey RP, editors. Heart Development and Regeneration. Academic Press; London: 2010. pp. 441–462. [Google Scholar]
- Kitajima S, Miyagawa-Tomita S, Inoue T, Kanno J, Saga Y. Mesp1-nonexpressing cells contribute to the ventricular cardiac conduction system. Dev Dyn. 2006;235:395–402. doi: 10.1002/dvdy.20640. [DOI] [PubMed] [Google Scholar]
- Kochilas L, Merscher-Gomez S, Lu MM, Potluri V, Liao J, Kucherlapati R, Morrow B, Epstein JA. The role of neural crest during cardiac development in a mouse model of DiGeorge syndrome. Developmental biology. 2002;251:157–166. doi: 10.1006/dbio.2002.0819. [DOI] [PubMed] [Google Scholar]
- Kodo K, Nishizawa T, Furutani M, Arai S, Yamamura E, Joo K, Takahashi T, Matsuoka R, Yamagishi H. GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc Natl Acad Sci USA. 2009;106:13933–13938. doi: 10.1073/pnas.0904744106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontaridis MI, Swanson KD, David FS, Barford D, Neel BG. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J Biol Chem. 2006;281:6785–6792. doi: 10.1074/jbc.M513068200. [DOI] [PubMed] [Google Scholar]
- Koudova M, Seemanova E, Zenker M. Novel BRAF mutation in a patient with LEOPARD syndrome and normal intelligence. Eur J Med Genet. 2009;52:337–340. doi: 10.1016/j.ejmg.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Krantz ID, Smith R, Colliton RP, Tinkel H, Zackai EH, Piccoli DA, Goldmuntz E, Spinner NB. Jagged1 mutations in patients ascertained with isolated congenital heart defects. Am J Med Genet. 1999;84:56–60. [PubMed] [Google Scholar]
- Kubalak SW, Hutson DR, Scott KK, Shannon RA. Elevated transforming growth factor beta2 enhances apoptosis and contributes to abnormal outflow tract and aortic sac development in retinoic X receptor alpha knockout embryos. Development (Cambridge, England) 2002;129:733–746. doi: 10.1242/dev.129.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes & development. 2001;15:2470–2482. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao WH, Kamada N. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- Lalani S, Safiullah A, Molinari L, Fernbach S, Martin D, Belmont J. SEMA3E mutation in a patient with CHARGE syndrome. J Med Genet. 2004;41:e94. doi: 10.1136/jmg.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammer EJ, Chen DT, Hoar RM, Agnish ND, Benke PJ, Braun JT, Curry CJ, Fernhoff PM, Grix AW, Lott IT. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Layman WS, Hurd EA, Martin DM. Chromodomain proteins in development: lessons from CHARGE syndrome. Clinical genetics. 2010;78:11–20. doi: 10.1111/j.1399-0004.2010.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM. The Neural Crest. 1 ed. Cambride University Press; Cambridge: 1982. [Google Scholar]
- Leatherbury L, Connuck DM, Gauldin HE, Kirby ML. Hemodynamic changes and compensatory mechanisms during early cardiogenesis after neural crest ablation in chick embryos. Pediatr Res. 1991;30:509–512. doi: 10.1203/00006450-199112000-00002. [DOI] [PubMed] [Google Scholar]
- Leatherbury L, Gauldin HE, Waldo K, Kirby ML. Microcinephotography of the developing heart in neural crest-ablated chick embryos. Circulation. 1990;81:1047–1057. doi: 10.1161/01.cir.81.3.1047. [DOI] [PubMed] [Google Scholar]
- Lee M, Brennan A, Blanchard A, Zoidl G, Dong Z, Tabernero A, Zoidl C, Dent MA, Jessen KR, Mirsky R. P0 is constitutively expressed in the rat neural crest and embryonic nerves and is negatively and positively regulated by axons to generate non-myelin-forming and myelin-forming Schwann cells, respectively. Mol Cell Neurosci. 1997;8:336–350. doi: 10.1006/mcne.1996.0589. [DOI] [PubMed] [Google Scholar]
- Legius E, Schrander-Stumpel C, Schollen E, Pulles-Heintzberger C, Gewillig M, Fryns J-P. PTPN11 mutations in LEOPARD syndrome. J Med Genet. 2002;39:571–574. doi: 10.1136/jmg.39.8.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore JJ, Mericko PA, Cheng L, Lu MM, Morrisey EE, Parmacek MS. GATA-6 regulates semaphorin 3C and is required in cardiac neural crest for cardiovascular morphogenesis. J Clin Invest. 2006;116:929–939. doi: 10.1172/JCI27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Pashmforoush M, Sucov HM. Retinoic acid regulates differentiation of the secondary heart field and TGFbeta-mediated outflow tract septation. Developmental cell. 2010;18:480–485. doi: 10.1016/j.devcel.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Aggarwal VS, Nowotschin S, Bondarev A, Lipner S, Morrow BE. Identification of downstream genetic pathways of Tbx1 in the second heart field. Developmental biology. 2008;316:524–537. doi: 10.1016/j.ydbio.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay EA, Botta A, Jurecic V, Carattini-Rivera S, Cheah YC, Rosenblatt HM, Bradley A, Baldini A. Congenital heart disease in mice deficient for the DiGeorge syndrome region. Nature. 1999;401:379–383. doi: 10.1038/43900. [DOI] [PubMed] [Google Scholar]
- Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xiao A. Epigenetic regulation in neural crest development. Birth Defects Res Part A Clin Mol Teratol. 2011;91:788–796. doi: 10.1002/bdra.20797. [DOI] [PubMed] [Google Scholar]
- Loomes KM, Taichman DB, Glover CL, Williams PT, Markowitz JE, Piccoli DA, Baldwin HS, Oakey RJ. Characterization of Notch receptor expression in the developing mammalian heart and liver. American journal of medical genetics. 2002;112:181–189. doi: 10.1002/ajmg.10592. [DOI] [PubMed] [Google Scholar]
- Lu HC, Eichele G, Thaller C. Ligand-bound RXR can mediate retinoid signal transduction during embryogenesis. Development (Cambridge, England) 1997;124:195–203. doi: 10.1242/dev.124.1.195. [DOI] [PubMed] [Google Scholar]
- Macatee TL, Hammond BP, Arenkiel BR, Francis L, Frank DU, Moon AM. Ablation of specific expression domains reveals discrete functions of ectoderm- and endoderm-derived FGF8 during cardiovascular and pharyngeal development. Development (Cambridge, England) 2003;130:6361–6374. doi: 10.1242/dev.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino B, Digilio MC, Toscano A, Giannotti A, Dallapiccola B. Congenital heart diseases in children with Noonan syndrome: An expanded cardiac spectrum with high prevalence of atrioventricular canal. J Pediatr. 1999;135:703–706. doi: 10.1016/s0022-3476(99)70088-0. [DOI] [PubMed] [Google Scholar]
- Martin DM, Sheldon S, Gorski JL. CHARGE association with choanal atresia and inner ear hypoplasia in a child with a de novo chromosome translocation t(2;7)(p14;q21.11) Am J Med Genet. 2001;99:115–119. doi: 10.1002/1096-8628(2000)9999:999<00::aid-ajmg1126>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Martinelli S, Torreri P, Tinti M, Stella L, Bocchinfuso G, Flex E, Grottesi A, Ceccarini M, Palleschi A, Cesareni G, Castagnoli L, Petrucci TC, Gelb BD, Tartaglia M. Diverse driving forces underlie the invariant occurrence of the T42A, E139D, I282V and T468M SHP2 amino acid substitutions causing Noonan and LEOPARD syndromes. Human molecular genetics. 2008;17:2018–2029. doi: 10.1093/hmg/ddn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu M, Bourges E, Caron , Piussan C. Waardenburg's syndrome and severe cyanotic cardiopathy. Arch Fr Pediatr. 1990;47:657–659. [PubMed] [Google Scholar]
- McElhinney DB, Krantz ID, Bason L, Piccoli DA, Emerick KM, Spinner NB, Goldmuntz E. Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation. 2002;106:2567–2574. doi: 10.1161/01.cir.0000037221.45902.69. [DOI] [PubMed] [Google Scholar]
- McQuade L, Christodoulou J, Budarf M, Sachdev R, Wilson M, Emanuel B, Colley A. Patient with a 22q11.2 deletion with no overlap of the minimal DiGeorge syndrome critical region (MDGCR) Am J Med Genet. 1999;86:27–33. [PubMed] [Google Scholar]
- Meadows SM, Fletcher PJ, Moran C, Xu K, Neufeld G, Chauvet S, Mann F, Krieg PA, Cleaver O. Integration of repulsive guidance cues generates avascular zones that shape Mammalian blood vessels. Circulation research. 2012;110:34–46. doi: 10.1161/CIRCRESAHA.111.249847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears AJ, Jordan T, Mirzayans F, Dubois S, Kume T, Parlee M, Ritch R, Koop B, Kuo WL, Collins C, Marshall J, Gould DB, Pearce W, Carlsson P, Enerbäck S, Morissette J, Bhattacharya S, Hogan B, Raymond V, Walter MA. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. Am J Hum Genet. 1998;63:1316–1328. doi: 10.1086/302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merscher S, Funke B, Epstein JA, Heyer J, Puech A, Lu MM, Xavier RJ, Demay MB, Russell RG, Factor S, Tokooya K, Jore BS, Lopez M, Pandita RK, Lia M, Carrion D, Xu H, Schorle H, Kobler JB, Scambler P, Wynshaw-Boris A, Skoultchi AI, Morrow BE, Kucherlapati R. TBX1 is responsible for cardiovascular defects in velocardio-facial/DiGeorge syndrome. Cell. 2001;104:619–629. doi: 10.1016/s0092-8674(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Mesbah K, Harrelson Z, Théveniau-Ruissy M, Papaioannou VE, Kelly RG. Tbx3 is required for outflow tract development. Circ Res. 2008;103:743–750. doi: 10.1161/CIRCRESAHA.108.172858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesbah K, Rana MS, Francou A, van Duijvenboden K, Papaioannou VE, Moorman AF, Kelly RG, Christoffels VM. Identification of a Tbx1/Tbx2/Tbx3 genetic pathway governing pharyngeal and arterial pole morphogenesis. Human molecular genetics. 2011 doi: 10.1093/hmg/ddr553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Beyer S, Kelly RG. Establishment of the mouse ventricular conduction system. Cardiovasc Res. 2011;91:232–242. doi: 10.1093/cvr/cvr069. [DOI] [PubMed] [Google Scholar]
- Molin DG, Poelmann RE, DeRuiter MC, Azhar M, Doetschman T. Gittenberger-de Groot, A.C., 2004. Transforming growth factor beta-SMAD2 signaling regulates aortic arch innervation and development. Circulation research. 95:1109–1117. doi: 10.1161/01.RES.0000150047.16909.ab. [DOI] [PubMed] [Google Scholar]
- Moon AM, Guris DL, Seo J.-h., Li L, Hammond J, Talbot A, Imamoto A. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Developmental cell. 2006;10:71–80. doi: 10.1016/j.devcel.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder GB, Manley N, Grant J, Schmidt K, Zeng W, Eckhoff C, Maggio-Price L. Effects of excess vitamin A on development of cranial neural crest-derived structures: a neonatal and embryologic study. Teratology. 2000;62:214–226. doi: 10.1002/1096-9926(200010)62:4<214::AID-TERA7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Gulick J, Colbert MC, Robbins J. Protein tyrosine phosphatase activity in the neural crest is essential for normal heart and skull development. Proc Natl Acad Sci USA. 2009;106:11270–11275. doi: 10.1073/pnas.0902230106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern J, Zhong J, Wickramasinghe RS, Li X, Wu Y, Samuels I, Cherosky N, Karlo JC, O'Loughlin B, Wikenheiser J, Gargesha M, Doughman YQ, Charron J, Ginty DD, Watanabe M, Saitta SC, Snider WD, Landreth GE. Mouse and human phenotypes indicate a critical conserved role for ERK2 signaling in neural crest development. Proc Natl Acad Sci USA. 2008;105:17115–17120. doi: 10.1073/pnas.0805239105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development (Cambridge, England) 2001;128:1019–1031. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- Nomura-Kitabayashi A, Phoon CKL, Kishigami S, Rosenthal J, Yamauchi Y, Abe K, Yamamura K.-i., Samtani R, Lo CW, Mishina Y. Outflow tract cushions perform a critical valve-like function in the early embryonic heart requiring BMPRIA-mediated signaling in cardiac neural crest. Am J Physiol Heart Circ Physiol. 2009;297:H1617–1628. doi: 10.1152/ajpheart.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist-Battie C, Freter M. Cardiac mitochondrial abnormalities in a mouse model of the fetal alcohol syndrome. Alcohol Clin Exp Res. 1988;12:264–267. doi: 10.1111/j.1530-0277.1988.tb00192.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell H, McKeown C, Gould C, Morrow B, Scambler P. Detection of an atypical 22q11 deletion that has no overlap with the DiGeorge syndrome critical region. Am J Hum Genet. 1997;60:1544–1548. doi: 10.1016/S0002-9297(07)64250-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Gaengel K, Krishnamoorthy S, Kamiya K, Kim IK, Ying H, Weber U, Perkins LA, Tartaglia M, Mlodzik M, Pick L, Gelb BD. Transgenic Drosophila models of Noonan syndrome causing PTPN11 gain-of-function mutations. Human molecular genetics. 2006;15:543–553. doi: 10.1093/hmg/ddi471. [DOI] [PubMed] [Google Scholar]
- Oishi K, Zhang H, Gault WJ, Wang CJ, Tan CC, Kim I-K, Ying H, Rahman T, Pica N, Tartaglia M, Mlodzik M, Gelb BD. Phosphatase-defective LEOPARD syndrome mutations in PTPN11 gene have gain-of-function effects during Drosophila development. Human molecular genetics. 2009;18:193–201. doi: 10.1093/hmg/ddn336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaopa M, Zhou H.-m., Snider P, Wang J, Schwartz RJ, Moon AM, Conway SJ. Pax3 is essential for normal cardiac neural crest morphogenesis but is not required during migration nor outflow tract septation. Developmental biology. 2011;356:308–322. doi: 10.1016/j.ydbio.2011.05.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Baker KM. Retinoic acid and the heart. Vitam Horm. 2007;75:257–283. doi: 10.1016/S0083-6729(06)75010-5. [DOI] [PubMed] [Google Scholar]
- Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, Martinelli S, Pogna EA, Schackwitz W, Ustaszewska A, Landstrom A, Bos JM, Ommen SR, Esposito G, Lepri F, Faul C, Mundel P, López Siguero JP, Tenconi R, Selicorni A, Rossi C, Mazzanti L, Torrente I, Marino B, Digilio MC, Zampino G, Ackerman MJ, Dallapiccola B, Tartaglia M, Gelb BD. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nature genetics. 2007;39:1007–1012. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai C-L, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development (Cambridge, England) 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Watanabe Y, Smyth G, Miyagawa-Tomita S, Meyers E, Klingensmith J, Camenisch T, Buckingham M, Moon AM. An FGF autocrine loop initiated in second heart field mesoderm regulates morphogenesis at the arterial pole of the heart. Development (Cambridge, England) 2008;135:3599–3610. doi: 10.1242/dev.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nürnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernández-Martínez L, Keating S, Mortier G, Hennekam RCM, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nürnberg P, Reis A, Rauch A. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan M, Ruiz VF, Silva FA, Sobreira TJ, Cravo RM, Vasconcelos M, Marques LP, Mesquita SMF, Krieger JE, Lopes AAB, Oliveira PS, Pereira AC, Xavier-Neto J. ALDH1A2 (RALDH2) genetic variation in human congenital heart disease. Bmc Med Genet. 2009;10:113. doi: 10.1186/1471-2350-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingault V, Ente D, Dastot-Le Moal F, Goossens M, Marlin S, Bondurand N. Review and update of mutations causing Waardenburg syndrome. Hum Mutat. 2010;31:391–406. doi: 10.1002/humu.21211. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Gittenberger-de Groot AC. A subpopulation of apoptosis-prone cardiac neural crest cells targets to the venous pole: multiple functions in heart development? Developmental biology. 1999;207:271–286. doi: 10.1006/dbio.1998.9166. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Mikawa T, Gittenberger-de Groot AC. Neural crest cells in outflow tract septation of the embryonic chicken heart: differentiation and apoptosis. Developmental dynamics : an official publication of the American Association of Anatomists. 1998;212:373–384. doi: 10.1002/(SICI)1097-0177(199807)212:3<373::AID-AJA5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Jongbloed MRM, Molin DGM, Fekkes ML, Wang Z, Fishman GI, Doetschman T, Azhar M, Gittenberger-de Groot AC. The neural crest is contiguous with the cardiac conduction system in the mouse embryo: a role in induction? Anat Embryol. 2004;208:389–393. doi: 10.1007/s00429-004-0401-6. [DOI] [PubMed] [Google Scholar]
- Porras D, Brown CB. Temporal-spatial ablation of neural crest in the mouse results in cardiovascular defects. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237:153–162. doi: 10.1002/dvdy.21382. [DOI] [PubMed] [Google Scholar]
- Prescott K, Woodfine K, Stubbs P, Super M, Kerr B, Palmer R, Carter NP, Scambler P. A novel 5q11.2 deletion detected by microarray comparative genomic hybridisation in a child referred as a case of suspected 22q11 deletion syndrome. Hum Genet. 2005;116:83–90. doi: 10.1007/s00439-004-1195-6. [DOI] [PubMed] [Google Scholar]
- Randall V, McCue K, Roberts C, Kyriakopoulou V, Beddow S, Barrett AN, Vitelli F, Prescott K, Shaw-Smith C, Devriendt K, Bosman E, Steffes G, Steel KP, Simrick S, Basson MA, Illingworth E, Scambler PJ. Great vessel development requires biallelic expression of Chd7 and Tbx1 in pharyngeal ectoderm in mice. The Journal of clinical investigation. 2009;119:3301–3310. doi: 10.1172/JCI37561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts C, Daw SC, Halford S, Scambler PJ. Cloning and developmental expression analysis of chick Hira (Chira), a candidate gene for DiGeorge syndrome. Human molecular genetics. 1997;6:237–245. doi: 10.1093/hmg/6.2.237. [DOI] [PubMed] [Google Scholar]
- Roberts C, Ivins S, Cook AC, Baldini A, Scambler PJ. Cyp26 genes a1, b1 and c1 are down-regulated in Tbx1 null mice and inhibition of Cyp26 enzyme function produces a phenocopy of DiGeorge Syndrome in the chick. Human molecular genetics. 2006;15:3394–3410. doi: 10.1093/hmg/ddl416. [DOI] [PubMed] [Google Scholar]
- Roberts C, Sutherland HF, Farmer H, Kimber W, Halford S, Carey A, Brickman JM, Wynshaw-Boris A, Scambler PJ. Targeted mutagenesis of the Hira gene results in gastrulation defects and patterning abnormalities of mesoendodermal derivatives prior to early embryonic lethality. Mol Cell Biol. 2002;22:2318–2328. doi: 10.1128/MCB.22.7.2318-2328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake. N Engl J Med. 1995;333:1369–1373. doi: 10.1056/NEJM199511233332101. [DOI] [PubMed] [Google Scholar]
- Ryckebusch L, Wang Z, Bertrand N, Lin SC, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkozy A, Carta C, Moretti S, Zampino G, Digilio MC, Pantaleoni F, Scioletti AP, Esposito G, Cordeddu V, Lepri F, Petrangeli V, Dentici ML, Mancini GMS, Selicorni A, Rossi C, Mazzanti L, Marino B, Ferrero GB, Silengo MC, Memo L, Stanzial F, Faravelli F, Stuppia L, Puxeddu E, Gelb BD, Dallapiccola B, Tartaglia M. Germline BRAF mutations in Noonan, LEOPARD, and cardiofaciocutaneous syndromes: molecular diversity and associated phenotypic spectrum. Hum Mutat. 2009;30:695–702. doi: 10.1002/humu.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satiroglu-Tufan NL, Tufan AC. Amelioration of ethanol-induced growth retardation by all-trans-retinoic acid and alpha-tocopherol in shell-less culture of the chick embryo. Reprod Toxicol. 2004;18:407–412. doi: 10.1016/j.reprotox.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Sato A, Scholl AM, Kuhn EB, Stadt HA, Decker JR, Pegram K, Hutson MR, Kirby ML. FGF8 signaling is chemotactic for cardiac neural crest cells. Developmental biology. 2011;354:18–30. doi: 10.1016/j.ydbio.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambler PJ. The 22q11 deletion syndromes. Human molecular genetics. 2000;9:2421–2426. doi: 10.1093/hmg/9.16.2421. [DOI] [PubMed] [Google Scholar]
- Scambler PJ. 22q11 deletion syndrome: a role for TBX1 in pharyngeal and cardiovascular development. Pediatr Cardiol. 2010;31:378–390. doi: 10.1007/s00246-009-9613-0. [DOI] [PubMed] [Google Scholar]
- Seitz H, Zamore PD. Rethinking the microprocessor. Cell. 2006;125:827–829. doi: 10.1016/j.cell.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Seo J.-h., Suenaga A, Hatakeyama M, Taiji M, Imamoto A. Structural and functional basis of a role for CRKL in a fibroblast growth factor 8-induced feed-forward loop. Mol Cell Biol. 2009;29:3076–3087. doi: 10.1128/MCB.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Kume T. Forkhead transcription factors, Foxc1 and Foxc2, are required for the morphogenesis of the cardiac outflow tract. Developmental biology. 2006;296:421–436. doi: 10.1016/j.ydbio.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Shiohama A, Sasaki T, Noda S, Minoshima S, Shimizu N. Molecular cloning and expression analysis of a novel gene DGCR8 located in the DiGeorge syndrome chromosomal region. Biochemical and biophysical research communications. 2003;304:184–190. doi: 10.1016/s0006-291x(03)00554-0. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: 30 Years of study. Dev Disabil Res Rev. 2008;14:3–10. doi: 10.1002/ddrr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JR, Graham JM, MacDonald C. Pathologic features of the CHARGE association: support for involvement of the neural crest. Teratology. 1985;31:331–336. doi: 10.1002/tera.1420310303. [DOI] [PubMed] [Google Scholar]
- Singh SM, Murphy B, O'Reilly R. Monozygotic twins with chromosome 22q11 deletion and discordant phenotypes: updates with an epigenetic hypothesis. J Med Genet. 2002;39:e71. doi: 10.1136/jmg.39.11.e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Amand TR, Lu JT, Zamora M, Gu Y, Stricker J, Hoshijima M, Epstein JA, Ross JJ, Ruiz-Lozano P, Chien KR. Distinct roles of HF-1b/Sp4 in ventricular and neural crest cells lineages affect cardiac conduction system development. Developmental biology. 2006;291:208–217. doi: 10.1016/j.ydbio.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Staiano A, Santoro L, De Marco R, Miele E, Fiorillo F, Auricchio A, Carpentieri ML, Celli J, Auricchio S. Autonomic dysfunction in children with Hirschsprung's disease. Dig Dis Sci. 1999;44:960–965. doi: 10.1023/a:1026608630301. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Lambrechts D, De Smet F, Jansen S, Wang J, Maity S, Kneer P, von der Ohe M, Swillen A, Maes C, Gewillig M, Molin DGM, Hellings P, Boetel T, Haardt M, Compernolle V, Dewerchin M, Plaisance S, Vlietinck R, Emanuel B, Gittenberger-de Groot AC, Scambler P, Morrow B, Driscol DA, Moons L, Esguerra CV, Carmeliet G, Behn-Krappa A, Devriendt K, Collen D, Conway SJ, Carmeliet P. VEGF: a modifier of the del22q11 (DiGeorge) syndrome? Nat Med. 2003;9:173–182. doi: 10.1038/nm819. [DOI] [PubMed] [Google Scholar]
- Stewart RA, Sanda T, Widlund HR, Zhu S, Swanson KD, Hurley AD, Bentires-Alj M, Fisher DE, Kontaridis MI, Look AT, Neel BG. Phosphatase-dependent and - independent functions of Shp2 in neural crest cells underlie LEOPARD syndrome pathogenesis. Developmental cell. 2010;18:750–762. doi: 10.1016/j.devcel.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development (Cambridge, England) 2004;131:2205–2218. doi: 10.1242/dev.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Daft PA, Russell WE, Dehart DB. Fetal alcohol syndrome and DiGeorge anomaly: critical ethanol exposure periods for craniofacial malformations as illustrated in an animal model. Am J Med Genet Suppl. 1986;2:97–112. doi: 10.1002/ajmg.1320250614. [DOI] [PubMed] [Google Scholar]
- Tang S, Snider P, Firulli AB, Conway SJ. Trigenic neural crest-restricted Smad7 over-expression results in congenital craniofacial and cardiovascular defects. Developmental biology. 2010;344:233–247. doi: 10.1016/j.ydbio.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Gelb BD, Zenker M. Noonan syndrome and clinically related disorders. Best Pract Res Clin Endocrinol Metab. 2011;25:161–179. doi: 10.1016/j.beem.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Kalidas K, Shaw A, Song X, Musat DL, van der Burgt I, Brunner HG, Bertola DR, Crosby A, Ion A, Kucherlapati RS, Jeffery S, Patton MA, Gelb BD. PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. Am J Hum Genet. 2002;70:1555–1563. doi: 10.1086/340847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia M, Martinelli S, Stella L, Bocchinfuso G, Flex E, Cordeddu V, Zampino G, Burgt I, Palleschi A, Petrucci TC, Sorcini M, Schoch C, Foa R, Emanuel PD, Gelb BD. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. American Journal of Human Genetics. 2006;78:279–290. doi: 10.1086/499925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M, Newton VE, Leverton K, Turnbull K, Seemanova E, Kunze J, Sperling K, Strachan T, Read AP. PAX3 gene structure and mutations: close analogies between Waardenburg syndrome and the Splotch mouse. Human molecular genetics. 1994;3:1069–1074. doi: 10.1093/hmg/3.7.1069. [DOI] [PubMed] [Google Scholar]
- Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2011 doi: 10.1038/ejhg.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Evans SM. Pharyngeal mesoderm development during embryogenesis: implications for both heart and head myogenesis. Cardiovascular research. 2011;91:196–202. doi: 10.1093/cvr/cvr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bueren KL, Papangeli I, Rochais F, Pearce K, Roberts C, Calmont A, Szumska D, Kelly RG, Bhattacharya S, Scambler PJ. Hes1 expression is reduced in Tbx1 null cells and is required for the development of structures affected in 22q11 deletion syndrome. Developmental biology. 2010;340:369–380. doi: 10.1016/j.ydbio.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoff MJ, Moorman AF, Ruijter JM, Lamers WH, Bennington RW, Markwald RR, Wessels A. Myocardialization of the cardiac outflow tract. Developmental biology. 1999;212:477–490. doi: 10.1006/dbio.1999.9366. [DOI] [PubMed] [Google Scholar]
- Vitelli F, Morishima M, Taddei I, Lindsay EA, Baldini A. Tbx1 mutation causes multiple cardiovascular defects and disrupts neural crest and cranial nerve migratory pathways. Human molecular genetics. 2002a;11:915–922. doi: 10.1093/hmg/11.8.915. [DOI] [PubMed] [Google Scholar]
- Vitelli F, Taddei I, Morishima M, Meyers EN, Lindsay EA, Baldini A. A genetic link between Tbx1 and fibroblast growth factor signaling. Development (Cambridge, England) 2002b;129:4605–4611. doi: 10.1242/dev.129.19.4605. [DOI] [PubMed] [Google Scholar]
- Vitelli F, Lania G, Huynh T, Baldini A. Partial rescue of the Tbx1 mutant heart phenotype by Fgf8: genetic evidence of impaired tissue response to Fgf8. J Mol Cell Cardiol. 2010;49:836–840. doi: 10.1016/j.yjmcc.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Developmental biology. 1998;196:129–144. doi: 10.1006/dbio.1998.8860. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Hutson MR, Stadt HA, Zdanowicz M, Zdanowicz J, Kirby ML. Cardiac neural crest is necessary for normal addition of the myocardium to the arterial pole from the secondary heart field. Developmental biology. 2005;281:66–77. doi: 10.1016/j.ydbio.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Lo CW, Kirby ML. Connexin 43 expression reflects neural crest patterns during cardiovascular development. Developmental biology. 1999;208:307–323. doi: 10.1006/dbio.1999.9219. [DOI] [PubMed] [Google Scholar]
- Ward C, Stadt H, Hutson M, Kirby ML. Ablation of the secondary heart field leads to tetralogy of Fallot and pulmonary atresia. Developmental biology. 2005;284:72–83. doi: 10.1016/j.ydbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Miyagawa-Tomita S, Vincent SD, Kelly RG, Moon AM, Buckingham ME. Role of mesodermal FGF8 and FGF10 overlaps in the development of the arterial pole of the heart and pharyngeal arch arteries. Circ Res. 2010;106:495–503. doi: 10.1161/CIRCRESAHA.109.201665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzel P, Eriksson UJ. Altered gene expression in neural crest cells exposed to ethanol in vitro. Brain Res. 2009;1305(Suppl):S50–60. doi: 10.1016/j.brainres.2009.08.057. [DOI] [PubMed] [Google Scholar]
- WILSON JG, ROTH CB, WARKANY J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. The American journal of anatomy. 1953;92:189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- WILSON JG, WARKANY J. Congenital anomalies of heart and great vessels in offspring of vitamin A-deficient rats. Am J Dis Child. 1950;79:963. [PubMed] [Google Scholar]
- Winnier GE, Kume T, Deng K, Rogers R, Bundy J, Raines C, Walter MA, Hogan BL, Conway SJ. Roles for the winged helix transcription factors MF1 and MFH1 in cardiovascular development revealed by nonallelic noncomplementation of null alleles. Developmental biology. 1999;213:418–431. doi: 10.1006/dbio.1999.9382. [DOI] [PubMed] [Google Scholar]
- Xu H, Morishima M, Wylie JN, Schwartz RJ, Bruneau BG, Lindsay EA, Baldini A. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development (Cambridge, England) 2004;131:3217–3227. doi: 10.1242/dev.01174. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Hammer RE, Richardson JA, Williams SC, Clouthier DE, Yanagisawa M. Role of Endothelin-1/Endothelin-A receptor-mediated signaling pathway in the aortic arch patterning in mice. J Clin Invest. 1998;102:22–33. doi: 10.1172/JCI2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelbuz TM, Waldo KL, Kumiski DH, Stadt HA, Wolfe RR, Leatherbury L, Kirby ML. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation. 2002;106:504–510. doi: 10.1161/01.cir.0000023044.44974.8a. [DOI] [PubMed] [Google Scholar]
- Yelin R, Schyr RB-H, Kot H, Zins S, Frumkin A, Pillemer G, Fainsod A. Ethanol exposure affects gene expression in the embryonic organizer and reduces retinoic acid levels. Developmental biology. 2005;279:193–204. doi: 10.1016/j.ydbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Yu HH, Kolodkin AL. Semaphorin signaling: a little less per-plexin. Neuron. 1999;22:11–14. doi: 10.1016/s0896-6273(00)80672-8. [DOI] [PubMed] [Google Scholar]
- Zenker M, Buheitel G, Rauch R, Koenig R, Bosse K, Kress W, Tietze H-U, Doerr H-G, Hofbeck M, Singer H, Reis A, Rauch A. Genotype-phenotype correlations in Noonan syndrome. J Pediatr. 2004;144:368–374. doi: 10.1016/j.jpeds.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Baldini A. In vivo response to high-resolution variation of Tbx1 mRNA dosage. Human molecular genetics. 2008;17:150–157. doi: 10.1093/hmg/ddm291. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cerrato F, Xu H, Vitelli F, Morishima M, Vincentz J, Furuta Y, Ma L, Martin J, Baldini A, Lindsay E. Tbx1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development (Cambridge, England) 2005;132:5307–5315. doi: 10.1242/dev.02086. [DOI] [PubMed] [Google Scholar]
- Zile MH. Function of vitamin A in vertebrate embryonic development. J Nutr. 2001;131:705–708. doi: 10.1093/jn/131.3.705. [DOI] [PubMed] [Google Scholar]