Abstract
Background
The analgesic properties and mechanisms of loperamide hydrochloride, a peripherally acting opioid receptor agonist, in neuropathic pain warrant further investigation.
Methods
We examined the effects of systemic or local administration of loperamide on heat and mechanical hyperalgesia in rats after an L5 spinal nerve ligation (SNL).
Results
1) Systemic loperamide (0.3–10 mg/kg, subcutaneous in the back) dose-dependently reversed heat hyperalgesia in SNL rats, but did not produce thermal analgesia. Systemic loperamide (3 mg/kg) did not induce thermal antinociception in naïve rats; 2) Systemic loperamide-induced anti-heat hyperalgesia was blocked by pretreatment with intraperitoneal naloxone methiodide (5 mg/kg), but not by intraperitoneal naltrindole (5 mg/kg) or intrathecal naltrexone (20 μg/10 μL); 3) Local administration of loperamide (150 μg), but not vehicle, into plantar or dorsal hind paw tissue induced thermal analgesia in SNL rats and thermal antinociception in naïve rats; 4) The analgesic effect of intraplantar loperamide (150 μg/15 μL) in SNL rats at 45 min, but not 10 min, post-injection was blocked by pretreatment with an intraplantar injection of naltrexone (75 μg/10 μL); 5) Systemic (3.0 mg/kg) and local (150 μg) loperamide reduced the exaggerated duration of hind paw elevation to noxious pinprick stimuli in SNL rats. Intraplantar injection of loperamide also decreased the frequency of pinprickevoked response in naïve rats.
Conclusions
These findings suggest that both systemic and local administration of loperamide induce an opioid receptor-dependent inhibition of heat and mechanical hyperalgesia in nerve-injured rats, but that local paw administration of loperamide also induces thermal and mechanical antinociception.
1. Introduction
The treatment of neuropathic pain is challenging as it is often refractory to most clinically available agents or limited by the adverse effects of these systemically administered drugs. Centrally acting opiates can be effective at reducing neuropathic pain symptoms. However, since they bind to opioid receptors that are widely expressed throughout the central nervous system (CNS), dose-limiting adverse effects secondary to their CNS actions (sedation, dizziness, cognitive dysfunction, opioid-induced hyperalgesia) and the perceived risks of addiction and abuse significantly limit their clinical usefulness (McCleane and Smith, 2007; Zollner and Stein, 2007). Hence, it is important to find alternative therapies that can help minimize the adverse effects associated with centrally acting opiates for the treatment of neuropathic pain. Increasing evidence suggest that peripherally acting opiates may represent a promising therapeutic approach for alleviating pathological pain (Tegeder et al., 2003; Zhang et al., 2008; Stein and Lang, 2009; Vadivelu et al., 2011).
Loperamide hydrochloride is a mu-opioid receptor (MOR)-preferring agonist that does not cross the blood–brain barrier after systemic administration (DeHaven-Hudkins et al., 1999). Hence, it may not produce the central side effects commonly associated with the systemic use of opiates. We reported earlier that systemic and local (intraplantar) administration of loperamide alleviated mechanical allodynia (i.e., mechanical hypersensitivity to non-noxious tactile stimuli) during the development and maintenance phases of neuropathic pain in rats (Guan et al., 2008). A previous study suggested that the intraperitoneal or subcutaneous administration of loperamide also alleviated neuropathic heat hyperalgesia (Shinoda et al., 2007). However, various manifestations/symptoms of neuropathic pain (e.g., tactile allodynia, mechanical hyperalgesia, heat hyperalgesia) may be mediated through different peripheral and central mechanisms (Chen et al., 2006; Cavanaugh et al., 2009), and they may show different sensitivities to peripherally acting opioid analgesics. In addition, since loperamide may act at different targets after systemic and local drug administration into the testing area, the analgesic properties and the underlying mechanisms of loperamide may also differ depending on the route of drug administration. Therefore, the therapeutic actions and mechanisms of peripherally acting opioids in neuropathic pain is worthy of further investigation.
As an extension of our previous observations (Guan et al., 2008), we sought to examine the effects of systemic and local (hind paw) administration of loperamide on both heat and mechanical hyperalgesia in a rodent model of neuropathic pain (L5 spinal nerve ligation, SNL), using noxious heat and mechanical stimuli (Hargreaves et al., 1988; Tal and Bennett, 1994). Within the same experimental setting, we also determined whether loperamide acts exclusively as an antihyperalgesic agent or also has analgesic effect following systemic and local drug administration in nerve-injured rats. The current study expands our knowledge of peripheral opioidergic mechanisms in neuropathic pain by: 1) Characterizing and differentiating the pain-inhibitory actions of systematic versus locally administrated loperamide in SNL rats, using both thermal and mechanical hyperalgesia as outcome measures; 2) Examining the dose-response function, time-course, opioidergic mechanism, and site of action (spinal versus peripheral) of systemic loperamide-induced attenuation of hyperalgesia.
2. Methods
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee as consistent with the National Institutes of Health Guide for the Use of Experimental Animals. To avoid causing stress, drug injections were made with animals under brief inhalation anesthesia (isoflurane, 1.5%). Nerve-injured rats were euthanized at the end of the experiment. The investigator who performed the behavioral tests was blinded to the drug assignment.
2.1. Animal surgery
For inducing nerve injury, the left L5 spinal nerve of male Sprague-Dawley rats (200–350 g, Harlan, Indianapolis, IN) was ligated with a 6-0 silk suture and cut distally (Kim et al., 1997; Guan et al., 2008). For intrathecal catheter implantation, a small slit was cut on the atlanto-occipital membrane, into which a saline-filled PE-10 tubing (6–7 cm) was inserted. After completing the experiment, intrathecal drug delivery was confirmed by injecting lidocaine (400 μg/20 μL, Hospira, Lack Forest, IL) which resulted in a temporary motor paralysis of the lower limbs (Dobos et al., 2003).
2.2. Animal behavioral tests
2.2.1. Hargreaves test
Paw withdrawal latency (PWL) to radiant heat stimuli (cut-off time: 20 seconds) was measured with a plantar stimulator analgesia meter (IITC model 390, Woodland Hills, CA) (Hargreaves et al., 1988). Rats were habituated for >30 min on a heated glass floor (30°C) before testing. Both hind paws were tested three times (2 min interval). The average PWL of the three trials was used for analysis.
2.2.2. Pinprick test
Mechanical hyperalgesia was determined by pressing the plantar surface of the hind paw with a custom-designed pinprick stimulator, which produces a quick reflex withdrawal response in normal animals but is insufficient to damage the skin. Both sides were tested three times (5 min interval). The frequency of paw withdrawal was calculated as [(the number of withdrawal)/3]x100. The duration of paw holding/elevation after stimulation was timed in SNL rats with a stopwatch, but it was often too short to be timed accurately in normal animals, so a duration of 0.5 second was assigned (Tal and Bennett, 1994; Suter et al., 2003). The average duration of the three trials was used for analysis.
2.3. Drugs
Loperamide hydrochloride, naloxone methiodide, naloxone hydrochloride, naltrexone hydrochloride, naltrindole hydrochloride, and 2-hydroxypropyl-beta-cyclodextrin (CDEX) were purchased from Sigma-Aldrich (St. Louis, MO). Loperamide was dissolved in 20% CDEX, made by diluting the 40% CDEX/water solution (isotonic) with saline.
2.4. Experimental design
Study 1: To establish the dose-response function by which systemic loperamide attenuates heat hyperalgeisa
After pre-drug baseline test in rats at 5–7 days post-SNL, we injected rats subcutaneously (s.c.) in the back with vehicle (20% CDEX, n=13) or loperamide (0.3 mg/kg, n=13; 1.0 mg/kg, n=14; 1.5 mg/kg, n=16; 3.0 mg/kg, n=13; 10 mg/kg, n=8; volume: 1 mL/kg). Measurements of PWL were obtained at 30, 60, 90, and 150 min post-injection. Animals were randomly assigned a drug treatment regimen. Different doses were tested in different groups of rats. We also examined whether 3.0 mg/kg loperamide (n=10, s.c.) or vehicle (n=10) induces thermal antinociception in naïve rats.
Study 2: To determine if the anti-heat hyperalgesia of systemic loperamide can be blocked by pretreatment with systemic or intrathecal administration of opioid antagonists
In rats on day 5–7 post-SNL, naloxone methiodide (a peripherally-acting opioid receptor antagonist, 5 mg/kg, n=8), naltrindole [a selective delta-opioid receptor (DOR) antagonist, 5 mg/kg, n=8], or saline (n=4) was injected intraperitoneally (i.p.) at 5 min before systemic injection of loperamide (1.5 mg/kg, s.c.). The dose for each antagonist was based on earlier reports of the dose that effectively reversed the agonist effect (Portoghese et al., 1988; Svensson et al., 2003; Sevostianova et al., 2005; Brainin-Mattos et al., 2006; Guan et al., 2008).
In a different group of rats on day 5 post-SNL, we pretreated animals with an intrathecal naltrexone (20 μg/10 μL, i.th.) to achieve a prolonged blockade of spinal opioid receptors, followed 5 min later by a systemic injection of loperamide (1.5 mg/kg, n=3, s.c.) or vehicle (n=3). Naltrexone (10–15 μg, i.th.) at the lower doses antagonized the analgesia of 1–10 μg morphine (i.th.) (Danzebrink et al., 1995; Yamamoto and Sakashita, 1999). Rats were tested again on day 7 post-SNL, using a cross-over design so that each animal was exposed to both loperamide and vehicle. The data from the two days were combined for analysis. We measured PWL before antagonist treatment and at 30 min post-loperamide/vehicle injection.
Study 3: To examine the effect of locally injected loperamide on heat hyperalgesia
We injected SNL rats in the intraplantar (i.pl.) region of the left hind paw (ipsilateral to SNL) with vehicle (n=4) or loperamide (150 μg/15 μL, n=4). This dose of loperamide reversed mechanical allodynia without inducing a systemic effect (Guan et al., 2008). Since intraplantar injection of 50 μL vehicle increased PWL for over 30 min in our pilot study, presumably due to decreased heat penetration/conduction, the injection volume was decreased to 15 μL to limit the “volume effect”. Rats were tested two days later with switching the drug assignment, and data were combined for analysis. In a separate study, we examined whether injecting loperamide (150 μg/50 μL) into the dorsal aspect of the left hind paw (Intrapaw, i.pw.) also alleviates heat hyperalgesia in SNL rats (n=9) and induces thermal antinociception in naïve rats (n=13). Injection of vehicle in naïve rats was used as a control (n=13). Intrapaw injection has shown to be effective for local drug administration (DeHaven-Hudkins et al., 1999). Intrapaw injection of 50 μl vehicle did not change PWL. We measured PWL before and at 30 min post-injection.
Study 4: To determine if local loperamide-induced thermal analgesia is sensitive to opioid receptor antagonism
We pretreated SNL rats with injection of naltrexone (75 μg/10 μL, i.pl., n=8) and naloxone hydrochloride (100 μg/30 μL, i.pw., n=9) into left hind paw, respectively, followed by intraplantar (15 μL) and intrapaw (50 μL) injection of 150 μg loperamide 5 min later. Intrapaw saline pretreatment (n=5) was used as a control. We measured PWL before antagonist treatment and at 30 min post-loperamide/vehicle injection.
In a separate study, we further examined the time course over which naltrexone pretreatment blocks local loperamide-induced analgesia. At day 5–7 post-SNL, rats were pretreated with an intraplantar injection of naltrexone (75 μg/10 μL, n=7) or saline (n=6) followed by intraplantar injection of loperamide (150 μg/15 μL) 5 min later. To control for the “volume effect” and residual anesthesia, the third group of rats received intraplantar saline pretreatment followed by intraplantar vehicle injection (n=6). We examined PWL before antagonist treatment and at 10–25 min and 45–60 min post-loperamide/vehicle injection.
Study 5: To study if systemic and local loperamide attenuate mechanical hyperalgesia
Nerve-injured rats often showed prolonged paw holding/elevation to a pinprick of the left hind paw (ipsilateral to SNL), indicating the presence of mechanical hyperalgesia. Since we aimed to examine whether loperamide alleviates mechanical hyperalgesia, SNL rats with an average paw elevation duration of >1.0 s on the left side were included for drug testing. Rats were injected subcutaneously in the back with vehicle (n=10) or loperamide (3.0 mg/kg, n=7). To examine if systemic loperamide also affects normal pinprick response (paw withdrawal frequency), we tested the same dose of loperamide (n=7, s.c.) in naïve rats. The pinprick test was performed before and at 20–45 min post-loperamide/vehicle injection.
To examine the effect of local loperamide on pinprick response, we injected a separate group of rats in the plantar region of the left hind paw with loperamide (150 μg/50 μL, n=12 SNL, 6 naïve) or vehicle (n=8 SNL, 4 naïve). Finally, we examined the pinprick response in SNL rats that were pretreated with an intraplantar injection of naloxone hydrochloride (100 μg/30 μL) followed 5 min later by intraplantar injection of loperamide (150 μg/50 μL, n=5) or vehicle (n=6). The test was performed before antagonist treatment and at 20–45 min post-loperamide/vehicle injection.
2.5. Statistical analysis
To compare between groups in study 1, the PWLs of SNL rats were normalized to their respective pre-SNL baselines, and the PWLs of naïve rats were normalized to the pre-injection value. To compare the peak drug effects, we calculated the %Reversal of the ipsilateral PWL at 60 min post-injection as [(post-injection PWL – pre-injection PWL)/(pre-SNL PWL – pre-injection PWL)]x100. In studies 1–3, a one-way repeated measures ANOVA was used to compare the PWLs between different time points in each group, and a one-way ANOVA was used to compare the %Reversals. In study 4, data from different groups were compared by using a two-way mixed model ANOVA. The Tukey honestly significant difference post-hoc test was used to compare specific data points in ANOVA. Paired t-test was used to examine the drug effect in pinprick testing. STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK, USA) was used for analysis. Data are expressed as means ± SEM; P < 0.05 was considered significant.
3. Results
3.1. Systemic loperamide dose-dependently reversed heat hyperalgesia in nerve-injured rats but did not induce thermal antinociception in naïve rats
Loperamide (1.0 mg/kg, s.c.) significantly increased the ipsilateral PWL of SNL rats from the pre-drug level at 30–150 min post-injection (P<0.05–0.01, Fig. 1a), but did not alter the contralateral PWL (Fig. 1b). The vehicle did not affect the PWL of SNL rats (Fig. 1a–d) or naïve rats (Fig. 1e). Loperamide (3.0 mg/kg, s.c.) also did not change the PWL in naïve rats (Fig. 1e). The ipsilateral PWLs (as % pre-SNL) at 30 and 60 min after injection of loperamide at 1.0, 1.5, and 10 mg/kg doses were significantly increased from the pre-injection level, but not from the pre-SNL baseline (Fig. 1c). The contralateral PWLs were not changed after injection (Fig. 1d). The %Reversal of the ipsilateral PWL for 1.0, 1.5, and 10 mg/kg doses were significantly greater than that of vehicle (P<0.01, Fig. 1f).
Figure 1.
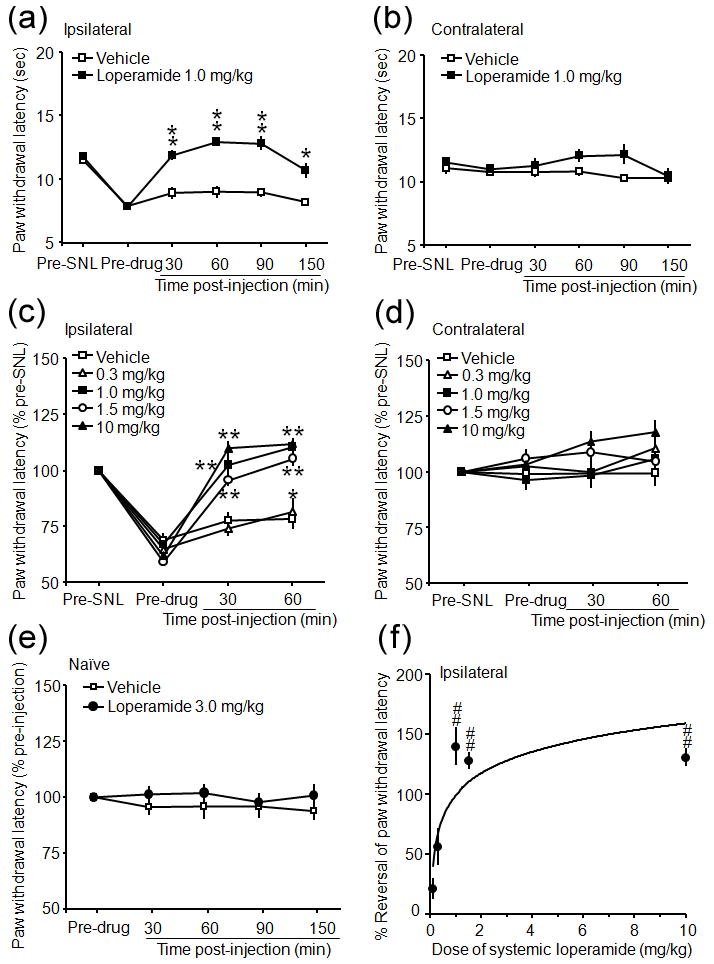
Systemic loperamide dose dependently attenuated heat hyperalgesia in rats after an L5 spinal nerve ligation (SNL). (a) The time course of systemic loperamide-induced anti-heat hyperalgesia in rats on days 5–7 post-SNL. (b) The PWLs on the contralateral side were not significantly changed by loperamide or vehicle injection. (c) Systemic loperamide dose dependently reversed heat hyperalgesia in the ipsilateral (left) hind paw at 30 min and 60 min post-injection. (d) However, paw withdrawal latencies (PWLs) on the contralateral side were not significantly changed from the pre-drug injection levels. (e) Neither loperamide at 3.0 mg/kg nor vehicle changed PWL in naïve rats. (f) Percent reversal (%Reversal) of the ipsilateral PWL was calculated as [(post-drug PWL – pre-drug PWL)/(pre-SNL PWL – pre-drug PWL)]x100 at 60 min post-injection and used to compare drug effect at different doses. Data are presented as means ± SEM. *P < 0.05, **P < 0.01 versus pre-injection level. ##P < 0.01 versus vehicle (20% CDEX) treatment.
3.2. Systemic loperamide-induced anti-heat hyperalgesia was blocked by naloxone methiodide
Pretreatment with intraperitoneal injection of the peripheral opioid antagonist, naloxone methiodide (5 mg/kg, Fig. 2a), but not the DOR antagonist, naltrindole (5 mg/kg, Fig. 2b) or saline (data not shown), blocked the anti-heat hyperalgesic effect of systemic loperamide (1.5 mg/kg, s.c.). Pretreatment with intrathecal injection of naltrexone (20 μg/10 μL) failed to reverse the systemic loperamide-induced anti-heat hyperalgesia (Fig. 2c), and did not alter the PWL in SNL rats that received vehicle injection (Fig. 2d).
Figure 2.
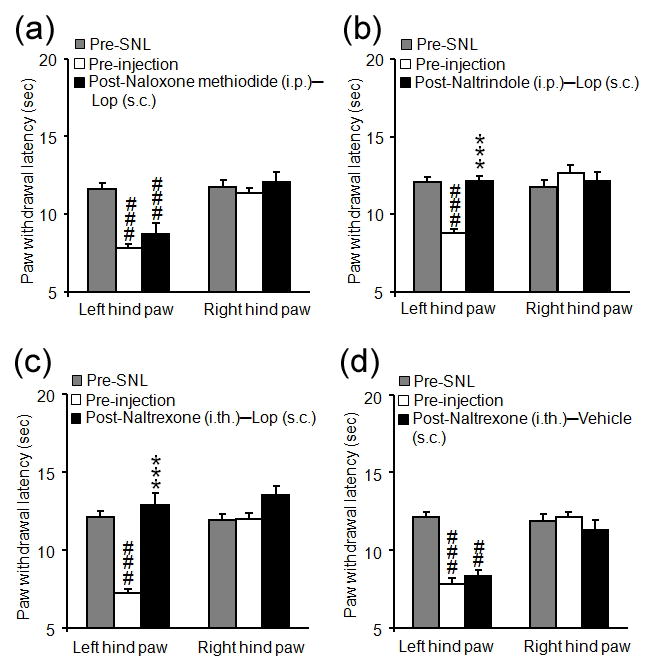
Peripherally acting opioid receptor antagonists blocked systemic loperamide-induced anti-heat hyperalgesia in rats after an L5 spinal nerve ligation (SNL). (a–b) The antihyperalgesic effect of systemic loperamide was blocked by systemic pretreatment with intraperitoneal (i.p.) injection of naloxone methiodide (a, 5 mg/kg, i.p.), but was not blocked by delta-opioid receptor antagonist naltrindole (b, 5.0 mg/kg, i.p.) or saline (data not shown). SNL rats were pre-treated with saline or opioid receptor antagonist followed 5 min later by subcutaneous (s.c.) loperamide (1.5 mg/kg). Paw withdrawal latency (PWL) was examined at pre- and 30 min after loperamide injection. (c) Systemic loperamide-induced anti-heat hyperalgesia was not blocked by pretreatment with intrathecal (i.t.) injection of naltrexone (20 μg/10 μL). (d) Intrathecal injection of naltrexone (20 μg/10 μL), followed by systemic administration of 20% CDEX (vehicle for loperamide, s.c.), did not change PWLs. Data are presented as means ± SEM. ***P < 0.001 versus pre-injection level. ##P < 0.01, ###P < 0.001 versus pre-SNL.
3.3. Local injection of loperamide into the hind paw induced thermal analgesia in nerve-injured and naïve rats
Intraplantar injection of loperamide (150 μg/15 μL), but not vehicle, into the ipsilateral paw of SNL rats significantly increased the PWL from both pre-injection and pre-SNL levels at 30 min post-injection (P<0.001, Fig. 3a, b). The analgesic effect at 30 min post-injection was blocked by pretreatment with intraplantar naltrexone (Fig. 3c), but not saline (data not shown). The contralateral PWLs were not changed after drug treatment. Intrapaw injection of loperamide (150 μg/50 μL) also induced thermal analgesia in SNL rats (Fig. 3d), and exerted thermal antinociception in naive rats (P<0.001, Fig. 3e). Local naloxone hydrochloride pretreatment partially blocked the analgesic effect of intrapaw loperamide: the PWL was significantly lower than that of the saline-pretreated group (P<0.05) but remained significantly higher than the pre-injection level (P<0.001, Fig. 3f). Naloxone hydrochloride by itself did not change the PWL, which was 95.6 ± 7.3% of pre-injection value.
Figure 3.
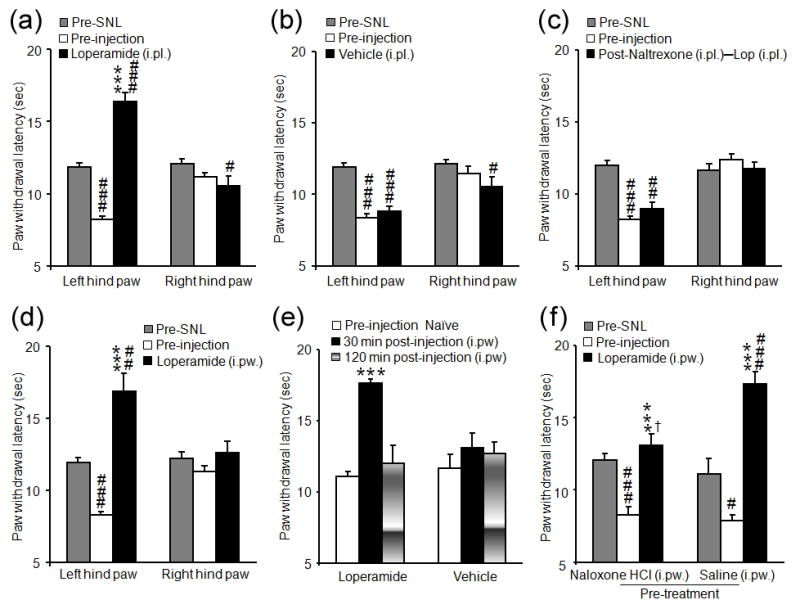
Local paw administration of loperamide induced thermal analgesia in rats after an L5 spinal nerve ligation (SNL). (a–b) In SNL rats, intraplantar (i.pl.) injection of loperamide (a, 150 μg/15 μL), but not vehicle (b, 20% CDEX, 15 μL), into the ipsilateral hind paw significantly increased paw withdrawal latency (PWL) after 30 min, as compared to both pre-SNL and pre-injection levels. (c) The inhibitory effect of local loperamide (150 μg/15 μL, i.pl.) at 30 min post-injection was blocked by pretreatment with i.pl. naltrexone (75 μg/10 μL), but not saline (data not shown). (d) Injection of loperamide (150 μg/50 μL, n=9) into the dorsal aspect of the ipsilateral hind paw (intrapaw, i.pw.) also significantly increased the PWL after 30 min, as compared to both pre-SNL and pre-injection values. (e) Intrapaw injection of loperamide (150 μg/50 μL, n=13) significantly increased the PWL in naïve rats at 30 min but not at 120 min post-injection. By contrast, vehicle injection (n=13) had no effect on PWL. (f) Pretreatment with an i.pw. injection of naloxone hydrochloride (naloxone HCl, 100 μg/30 μL, n=9) partially blocked the inhibitory effect of i.pw. loperamide (150 μg/50 μL) in SNL rats at 30 min as compared to saline pretreatment (30 μL, n=5). Data are presented as means ± SEM. ***P < 0.001 versus pre-injection level, #P < 0.05, ##P < 0.01, ###P < 0.001 versus pre-SNL baseline, † P < 0.05 versus the saline-pretreated group.
In SNL rats that received intraplantar saline injection (n = 6), intraplantar loperamide (150 μg/15 μL) significantly increased the ipsilateral PWLs at 10–25 min and 45–60 min post-injection from both the pre-injection and pre-SNL levels (Fig. 4a). Pretreatment with intraplantar naltrexone completely blocked the loperamide-induced analgesia at 45–60 min (P<0.001, n=7), but not at 10–25 min post-injection (P=0.458, Fig. 4a). The ipsilateral PWL increased from the pre-injection level in saline-vehicle group at 10–25 min (P<0.05, n=6). The contralateral PWLs were not significantly changed after drug treatment (Fig. 4b).
Figure 4.
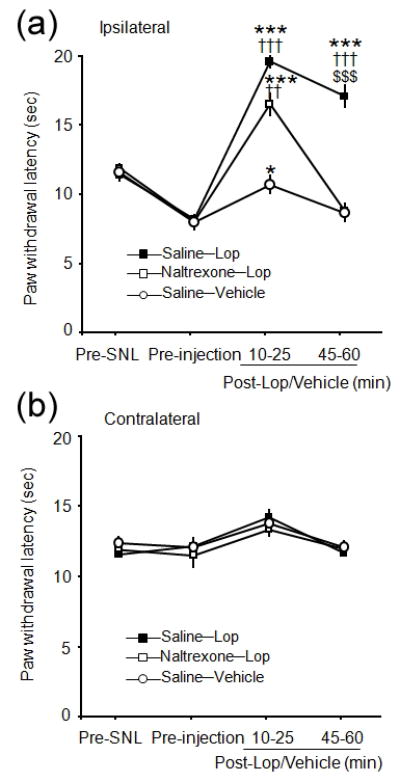
Effect of local pretreatment with naltrexone on intraplantar loperamide-induced thermal analgesia in rats after an L5 spinal nerve ligation (SNL). (a) There was no significant difference of the thermal analgesic effect of intraplantar (i.pl.) loperamide (Lop, 150 μg/15 μL) on the ipsilateral hind paw between naltrexone-pretreated (75 μg/10 μL, i.pl.) and saline-pretreated (i.pl.) SNL rats early after loperamide treatment (10–25 min). However, the thermal analgesic effect of intraplantar loperamide was significantly attenuated in the naltrexone-pretreated SNL rats at 45–60 min post-treatment. (b) The contralateral paw withdrawal latencies (PWLs) were not significantly changed. Data are presented as means ± SEM. ††P < 0.01, †††P < 0.001 versus saline-vehicle group and $$$P < 0.001 versus naltrexone-loperamide group at the same time point. *P < 0.05, ***P < 0.001 versus pre-injection level.
3.4. Systemic and local loperamide attenuated mechanical hyperalgesia in nerve-injured rats
Mechanical stimulus applied with the pinprick stimulator (Fig. 5e) elicited a quick paw withdrawal response in both naïve and SNL rats. The frequencies of response were comparable between the two groups (94% vs.100%). However, the duration of paw withdrawal holding/elevation on the ipsilateral side was much longer than on the contralateral side in SNL rats (Fig. 5a, b), indicative of mechanical hyperalgesia. In SNL rats, systemic (3.0 mg/kg, P<0.05) and intraplantar loperamide (150 μg/50 μL, P<0.01) significantly reduced the duration of paw elevation (Fig. 5a), but not the frequency of response. Yet, intraplantar loperamide decreased the paw withdrawal frequency in naïve rats (P<0.05, Fig. 5d). Pretreatment with intraplantar naloxone hydrochloride (100 μg) blocked the antihyperalgesic effect of loperamide (Fig. 5c).
Figure 5.
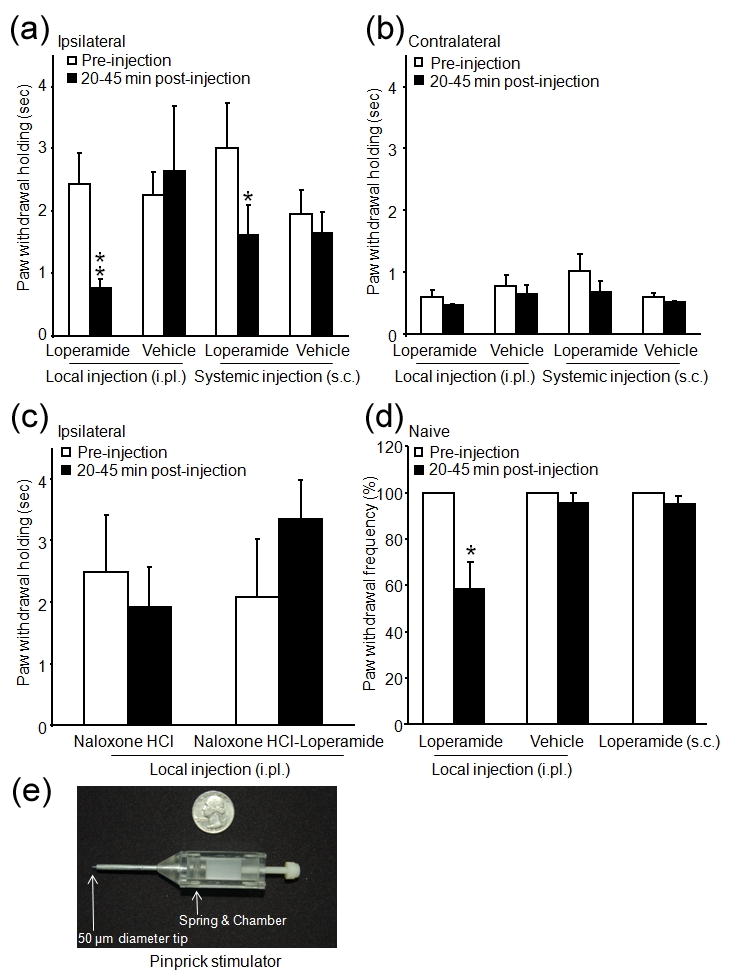
Effects of systemic and local administration of loperamide on mechanical hyperalgesia to pinprick stimuli. (a) The duration of paw withdrawal holding/elevation to pinprick stimulus was significantly decreased at 20–45 min after intraplantar (150 μg/50 μL, i.pl.) or systemic [3.0 mg/kg, subcutaneous (s.c.) injection in the back] injection of loperamide in SNL rats. (b) The contralateral hind paw (right) response to pinprick stimulus was not affected by loperamide. (c) Intraplantar injection of naloxone hydrochloride (naloxone HCl, 100 μg/50 μL) did not change the duration of paw withdrawal holding, but it blocked the antihyperalgesic effect of intraplantar loperamide (150 μg/50 μL). (d) In naïve rats, the frequency of paw withdrawal response to pinprick stimulus was significantly decreased by intraplantar injection of loperamide (150 μg/50 μL), but not by vehicle or systemic loperamide injection (3.0 mg/kg, s.c.). (e) The custom-designed pinprick stimulator has a spring-loaded steel stylus with a blunted tip (50 μm diameter). It exerts a mean force of 47.5 g and produces a quick reflex withdrawal response in normal animals. Data are presented as means ± SEM. *P < 0.05, **P < 0.01 versus pre-injection level.
4. Discussion and conclusions
Systemic loperamide reversed heat hyperalgesia, but did not produce thermal analgesia (i.e., increases PWL above pre-injury baseline) in SNL rats. Furthermore, 3.0 mg/kg loperamide did not affect baseline thermal nociception in naïve animals. Previously, systemic loperamide inhibited capsaicin-induced thermal hypersensitivity, but did not induce thermal antinociception (Butelman et al., 2004; Sevostianova et al., 2005). Therefore, loperamide may act as a selective antiallodynic/antihyperalgesic agent when administered systemically. Although pharmacokinetic data is unavailable for subcutaneous loperamide administration in nerve-injured rats, the available evidence indicates that loperamide does not accumulate in appreciable amounts in the central nervous system (CNS) after systemic administration (DeHaven-Hudkins et al., 1999; Nozaki-Taguchi and Yaksh, 1999; Shannon and Lutz, 2002; Baker, 2007). Although SNL might increase blood-spinal cord barrier permeability (Gordh et al., 2006) and the risk of drug penetration into CNS grows as the dose of loperamide is increased (Labuz et al., 2007), our results suggest that peripheral opioid mechanisms are important to systemic loperamide-induced antihyperalgesia at the dose-range examined in the current study. The unchanged contralateral PWL of SNL rats after systemic loperamide treatment supports this notion, as bilateral antinociception would be expected if systemically administrated opiates activate central opioid receptors. The lack of inhibition on the contralateral PWL also suggests that systemic loperamide administration did not interfere with the motor functions. Importantly, the systemic loperamide-induced anti-heat hyperalgesia was blocked by pretreatment of intraperitoneal injection of naloxone methiodide, a peripheral opioid receptor antagonist, but was unaffected by intrathecal naltrexone (20 μg) which effectively blocks spinal opioid receptors over a prolonged period of time (Tiseo and Yaksh, 1993; Danzebrink et al., 1995).
Loperamide shows a considerably higher binding affinity to MOR than DOR (Ki = 3.3 versus 48 nmol/L) (DeHaven-Hudkins et al., 1999; Menendez et al., 2005). Intraperitoneal injection of naloxone methiodide, but not naltrindole (5mg/kg), blocked systemic loperamide-induced anti-heat hyperalgesia. Since naltrindole at lower doses effectively blocked the analgesic effects of DOR agonists (Portoghese et al., 1988; Svensson et al., 2003), our findings suggest that opioid receptor subtypes other than DOR may mediate the observed drug actions. Similarly, MOR, but not DOR, antagonist blocked loperamide-induced inhibition on formalin pain(Shannon and Lutz, 2002), bone cancer pain, and mechanical allodynia induced by herpes simplex virus type-1 and SNL (Menendez et al., 2005; Sasaki et al., 2007; Guan et al., 2008). However, the current results are inconsistent with a previous observation that naltrindole (1 mg/kg, s.c.) inhibited systemic loperamide-induced anti-heat hyperalgesia (Shinoda et al., 2007). The reason for this discrepancy is unclear, but may be related to differences in drug preparation (e.g., 20% CDEX to increase the water solubility and reduce the clearance rate versus 10% DMSO) (Jang et al., 1992), routes of drug delivery, testing protocol (e.g., high rate versus low rate of skin heating), post-injury condition and post-drug time point for behavioral testing. There may be dynamic changes of DOR expression in peripheral nervous system after nerve injury (Robertson et al., 1999; Stone et al., 2004), which may partially underlie the differential involvement of DOR versus MOR in loperamide-induced analgesia at different post-injury time points. Activation of peripheral DORs or combining DOR agonists with other analgesics was shown to induce synergistic analgesic effect (Kabli and Cahill, 2007; Obara et al., 2009).
Local paw injection of loperamide in SNL rats not only inhibited allodynia (Guan et al., 2008) and hyperalgesia, but also exerted an analgesic effect. Furthermore, it induced antinociception in naïve rats. Similarly, application of 5% loperamide cream on a normal paw induced antinociception (Nozaki-Taguchi and Yaksh, 1999). These findings support the premise that morphine and loperamide produced potent analgesia and antinociception when administered locally (Stein et al., 2003; Obara et al., 2007; Obara et al., 2009; Stein and Lang, 2009). The specific mechanisms underlying the discrepant analgesic properties of loperamide following local and systemic administration are not full clear. A possible explanation is that the tissue concentrations of loperamide following systemic and local administration may differ significantly. Peak plasma concentration in mice after intravenous injection of 1mg/kg loperamide was 0.4–0.5 μM (Chu et al., 2011), and in rats after intravenous infusion of loperamide at rate of 0.95 mg/kg/min for 5 min (Elkiweri et al., 2009) or after subcutaneous injection of 50 mg/kg was approximately 2 μM (≈1000 ng/ml, MW: 513.5)(Kalvass et al., 2007). Based on these studies, the plasma concentration resulting from systemic administration of loperamide at the highest dose (10mg/kg) tested in this study is estimated to be 0.4–4 μM. A pharmacokinetic study, using 14C-labeled loperamide, suggested that the peak concentration in local tissue occurred at 30 min after topical application (Nozaki-Taguchi and Yaksh, 2002). Here, the concentration of loperamide injected into the paw was 150 μg/15–50μL or 5.8–19.8 mM. Based on an estimated 10-fold dilution of the injected drug in tissue, the expected drug concentration in paw tissue would be about 0.6–2.0 mM. Although loperamide may accumulate in paw tissue after systemic administration and result in a higher concentration than the plasma concentration, systemic loperamide remains unlikely to reach the equivalent local concentration obtained following intraplantar/intrapaw injection, which is several orders of magnitude higher than that after systemic administration. Thus, the mechanisms for local loperamide-induced analgesia may differ from that resulting from systemic drug administration. Injection of loperamide into the skin receptive field quickly induced non-opioid receptor mediated inhibition on the response properties of unmyelinated nociceptors, and application of loperamide to the nerve trunk produced a conduction block in unmyelinated afferents (Ringkamp et al., 2011). Local naltrexone pretreatment did not block the thermal analgesic effect of intraplantar loperamide at 10–25 min post-injection, also indicative of an early local anesthetic effect and non-opioidergic mechanisms. Loperamide is known to have several opioid receptorindependent activities, such as blocking voltage-sensitive calcium channels, interacting with N-methyl-D-aspartate receptors, and inhibiting hyperpolarization-activated current (Hagiwara et al., 2003; Sevostianova et al., 2005; Baker, 2007; Menendez et al., 2007; Vasilyev et al., 2007). Recent studies also implicate the role of nitric oxide signaling pathway in the peripheral antinociceptive actions of opioids (Menendez et al., 2007; Cunha et al., 2010). Yet, the non-opioidergic mechanisms may not underlie the main inhibitory effect at the later time points after local administration, since local naltrexone significantly attenuated intraplantar loperamide induced analgesia at 45–60 min post-injection. The partial reversal of intrapaw loperamide-induced analgesia with naloxone could have resulted from an insufficient dose and limited diffusion to plantar tissue. The increased ipsilateral PWL of saline-pretreated rats at 10–25 min after intraplantar CDEX injection may be due to both a “volume effect” and incomplete recovery from anesthesia.
Systemic loperamide reduced the duration of paw holding/elevation, but not the response frequency to pinprick in SNL rats. Thus, SNL rats can still detect the abrupt/sharp mechanical stimulation mediated by A-fibers, but it may not feel as painful as that in pre-drug condition. The prolonged paw holding/elevation after SNL may involve a combination of functional changes (e.g., sensitization) in PNS and CNS. Although the exaggerated response is centrally mediated and involves supraspinal mechanisms, it may also depend on peripheral afferent activation. We speculate that loperamide may exert a stronger inhibition on peripheral C-fibers than on A-fibers, and hence it reduces the duration of paw elevation by inhibiting the peripheral noxious barrage mediated by hyperexcitable C-fibers to reach CNS. Yet, intraplantar loperamide decreased the incidence of pinprick-evoked response in naïve rats. Although the reason for this difference is unclear, it may be partially due to nerve injury-induced changes in local tissue environment (e.g., loss of peripheral opioid receptors, nerve degeneration, changes in intracellular signaling) (Kim et al., 2001; Chao et al., 2008; Obara et al., 2009).
Our study suggests that both systemic and local administration of loperamide reversed behavioral hypersensitivity to noxious heat and mechanical stimuli in SNL rats. Additionally, locally administered loperamide also exerted an analgesic effect in neuropathic rats and induced antinociception in naïve rats. These findings corroborate and extend our previous observation that loperamide induced antiallodynic effects after being delivered via either route in nerve-injured rats. Since the protective baseline heat and mechanical nociception are largely preserved after systemic drug administration, systemic route may be favored for giving loperamide as an anti-hyperalgesic/anti-allodynic selective agent to treat neuropathic pain.
Analgesic efficacy of peripherally-restricted opioid agonist has also been shown in clinical studies. For example, activating peripheral MOR with morphine-6-beta-glucuronide (M6G) induced antihyperalgesia in experimental human pain models (Tegeder et al., 2003), and M6G inhibited post-operative pain in patients (Dahan et al., 2008; Binning et al., 2011). A potential disadvantage is that systemic loperamide is likely to share the gastrointestinal side effects common to mu opioids (Hanauer, 2008). Yet, the 50% effective dose (1.6 mg/kg) of systemic loperamide in inhibiting gastrointestinal motility is higher than that to attenuate neuropathic pain (0.78 mg/kg) (Tan-No et al., 2003; Guan et al., 2008). In addition, selective overexpression of MORs in dorsal root ganglion neurons and a better understanding of the mechanisms (e.g., intracellular signaling) involved in the pain-relief actions of peripherally acting opioids like loperamide may help to improve their analgesic efficacy and reduce their adverse effects.
Bulleted statements.
What’s already known about this topic?
Peripherally acting opiates (here loperamide) may represent a promising therapeutic approach for alleviating pathological pain. The analgesic properties and the underlying mechanisms of loperamide may differ, however, depending on the route of drug administration.
What does this study add?
Systemic and local paw administration of loperamide hydrochloride induced an opioid receptor-dependent inhibition of heat and mechanical hyperalgesia in nerve-injured rats. In addition, local administration induced antinociception.
Acknowledgments
The authors would like to thank Claire Levine, MS, for editing the manuscript.
Funding sources: This study was supported by grants from the NIH (NS26363, NS70814) and the Johns Hopkins Blaustein Pain Research Fund for Y.G.
Footnotes
Disclosures: The authors declare no conflict of interest.
Author contributions
C.C., A.F.C., A.D.M. and F.Y. performed animal surgery, drug injection, animal behavioral testing, and data analysis. S.N.R, M.R., and X.D contributed to the experimental design and the interpretation of the results. T.V.H helped with drug preparation. Y.G. designed the experiments and oversaw the overall execution of the project. C.C., S.N.R. and Y.G. prepared the manuscript. All authors discussed the results and commented on the manuscript.
References
- Baker DE. Loperamide: a pharmacological review. Rev Gastroenterol Disord. 2007;7(Suppl 3):S11–S18. [PubMed] [Google Scholar]
- Binning AR, Przesmycki K, Sowinski P, Morrison LM, Smith TW, Marcus P, Lees JP, Dahan A. A randomised controlled trial on the efficacy and side-effect profile (nausea/vomiting/sedation) of morphine-6-glucuronide versus morphine for post-operative pain relief after major abdominal surgery. Eur J Pain. 2011;15:402–408. doi: 10.1016/j.ejpain.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Brainin-Mattos J, Smith ND, Malkmus S, Rew Y, Goodman M, Taulane J, Yaksh TL. Cancer-related bone pain is attenuated by a systemically available delta-opioid receptor agonist. Pain. 2006;122:174–181. doi: 10.1016/j.pain.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek MJ. Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. J Pharmacol Exp Ther. 2004;311:155–163. doi: 10.1124/jpet.104.068411. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao T, Pham K, Steward O, Gupta R. Chronic nerve compression injury induces a phenotypic switch of neurons within the dorsal root ganglia 189. J Comp Neurol. 2008;506:180–193. doi: 10.1002/cne.21537. [DOI] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. doi: 10.1016/j.neuron.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Chu X, Zhang Z, Yabut J, Horwitz S, Levorse J, Li XQ, Zhu L, Lederman H, Ortiga R, Strauss J, Li X, Owens KA, Dragovic J, Vogt TF, Evers R, Shin MK. Characterization of Mdr1a/P-glycoprotein Knockout Rats Generated by Zinc Finger Nucleases. Mol Pharmacol. 2011 doi: 10.1124/mol.111.074179. [DOI] [PubMed] [Google Scholar]
- Cunha TM, Roman-Campos D, Lotufo CM, Duarte HL, Souza GR, Verri WA, Jr, Funez MI, Dias QM, Schivo IR, Domingues AC, Sachs D, Chiavegatto S, Teixeira MM, Hothersall JS, Cruz JS, Cunha FQ, Ferreira SH. Morphine peripheral analgesia depends on activation of the PI3Kgamma/AKT/nNOS/NO/KATP signaling pathway. Proc Natl Acad Sci U S A. 2010;107:4442–4447. doi: 10.1073/pnas.0914733107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, van DE, Smith T, Yassen A. Morphine-6-glucuronide (M6G) for postoperative pain relief. Eur J Pain. 2008;12:403–411. doi: 10.1016/j.ejpain.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Danzebrink RM, Green SA, Gebhart GF. Spinal mu and delta, but not kappa, opioid-receptor agonists attenuate responses to noxious colorectal distension in the rat. Pain. 1995;63:39–47. doi: 10.1016/0304-3959(94)00275-J. [DOI] [PubMed] [Google Scholar]
- DeHaven-Hudkins DL, Burgos LC, Cassel JA, Daubert JD, DeHaven RN, Mansson E, Nagasaka H, Yu G, Yaksh T. Loperamide (ADL 2-1294), an opioid antihyperalgesic agent with peripheral selectivity. J Pharmacol Exp Ther. 1999;289:494–502. [PubMed] [Google Scholar]
- Dobos I, Toth K, Kekesi G, Joo G, Csullog E, Klimscha W, Benedek G, Horvath G. The significance of intrathecal catheter location in rats. Anesth Analg. 2003;96:487–92. doi: 10.1097/00000539-200302000-00035. table. [DOI] [PubMed] [Google Scholar]
- Elkiweri IA, Zhang YL, Christians U, Ng KY, Tissot van Patot MC, Henthorn TK. Competitive substrates for P-glycoprotein and organic anion protein transporters differentially reduce blood organ transport of fentanyl and loperamide: pharmacokinetics and pharmacodynamics in Sprague-Dawley rats. Anesth Analg. 2009;108:149–159. doi: 10.1213/ane.0b013e31818e0bd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordh T, Chu H, Sharma HS. Spinal nerve lesion alters blood-spinal cord barrier function and activates astrocytes in the rat. Pain. 2006;124:211–221. doi: 10.1016/j.pain.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M, Meyer RA, Raja SN. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;138:318–329. doi: 10.1016/j.pain.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara K, Nakagawasai O, Murata A, Yamadera F, Miyoshi I, Tan-No K, Tadano T, Yanagisawa T, Iijima T, Murakami M. Analgesic action of loperamide, an opioid agonist, and its blocking action on voltage-dependent Ca2+ channels. Neurosci Res. 2003;46:493–497. doi: 10.1016/s0168-0102(03)00126-3. [DOI] [PubMed] [Google Scholar]
- Hanauer SB. The role of loperamide in gastrointestinal disorders. Rev Gastroenterol Disord. 2008;8:15–20. [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Jang J, Yaksh TL, Hill HF. Use of 2-hydroxypropyl-beta-cyclodextrin as an intrathecal drug vehicle with opioids. J Pharmacol Exp Ther. 1992;261:592–600. [PubMed] [Google Scholar]
- Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 2007;127:84–93. doi: 10.1016/j.pain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Olson ER, Cassidy MP, Selley DE, Pollack GM. Pharmacokinetics and pharmacodynamics of seven opioids in P-glycoprotein-competent mice: assessment of unbound brain EC50, u and correlation of in vitro, preclinical, and clinical data. J Pharmacol Exp Ther. 2007;323:346–355. doi: 10.1124/jpet.107.119560. [DOI] [PubMed] [Google Scholar]
- Kim DS, Yoon CH, Lee SJ, Park SY, Yoo HJ, Cho HJ. Changes in voltage-gated calcium channel alpha(1) gene expression in rat dorsal root ganglia following peripheral nerve injury 139. Brain Res Mol Brain Res. 2001;96:151–156. doi: 10.1016/s0169-328x(01)00285-6. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models 157. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- Labuz D, Mousa SA, Schafer M, Stein C, Machelska H. Relative contribution of peripheral versus central opioid receptors to antinociception. Brain Res. 2007;1160:30–38. doi: 10.1016/j.brainres.2007.05.049. [DOI] [PubMed] [Google Scholar]
- McCleane G, Smith HS. Opioids for persistent noncancer pain. Med Clin North Am. 2007;91:177–197. doi: 10.1016/j.mcna.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Menendez L, Juarez L, Garcia V, Hidalgo A, Baamonde A. Involvement of nitric oxide in the inhibition of bone cancer-induced hyperalgesia through the activation of peripheral opioid receptors in mice. Neuropharmacology. 2007;53:71–80. doi: 10.1016/j.neuropharm.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Menendez L, Lastra A, Meana A, Hidalgo A, Baamonde A. Analgesic effects of loperamide in bone cancer pain in mice. Pharmacol Biochem Behav. 2005;81:114–121. doi: 10.1016/j.pbb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Yaksh TL. Characterization of the antihyperalgesic action of a novel peripheral mu-opioid receptor agonist--loperamide. Anesthesiology. 1999;90:225–234. doi: 10.1097/00000542-199901000-00029. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Yaksh TL. Spinal and peripheral mu opioids and the development of secondary tactile allodynia after thermal injury. Anesth Analg. 2002;94:968–74. doi: 10.1097/00000539-200204000-00036. table. [DOI] [PubMed] [Google Scholar]
- Obara I, Makuch W, Spetea M, Schutz J, Schmidhammer H, Przewlocki R, Przewlocka B. Local peripheral antinociceptive effects of 14-O-methyloxymorphone derivatives in inflammatory and neuropathic pain in the rat. Eur J Pharmacol. 2007;558:60–67. doi: 10.1016/j.ejphar.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain. 2009;141:283–291. doi: 10.1016/j.pain.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Takemori AE. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Tal M, Hartke T, Schaefer K, Guan Y, Raja S, Meyer R. Loperamide produces a conduction block in unmyelinated afferents. Soc Neurosci Abstr. 2011;274.22 [Google Scholar]
- Robertson B, Schulte G, Elde R, Grant G. Effects of sciatic nerve injuries on delta-opioid receptor and substance P immunoreactivities in the superficial dorsal horn of the rat. Eur J Pain. 1999;3:115–129. doi: 10.1053/eujp.1998.0104. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Nakashima Y, Takasaki I, Andoh T, Shiraki K, Kuraishi Y. Effects of loperamide on mechanical allodynia induced by herpes simplex virus type-1 in mice. J Pharmacol Sci. 2007;104:218–224. doi: 10.1254/jphs.fp0070294. [DOI] [PubMed] [Google Scholar]
- Sevostianova N, Danysz W, Bespalov AY. Analgesic effects of morphine and loperamide in the rat formalin test: interactions with NMDA receptor antagonists. Eur J Pharmacol. 2005;525:83–90. doi: 10.1016/j.ejphar.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Lutz EA. Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology. 2002;42:253–261. doi: 10.1016/s0028-3908(01)00173-3. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Hruby VJ, Porreca F. Antihyperalgesic effects of loperamide in a model of rat neuropathic pain are mediated by peripheral delta-opioid receptors. Neurosci Lett. 2007;411:143–146. doi: 10.1016/j.neulet.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Lang LJ. Peripheral mechanisms of opioid analgesia. Curr Opin Pharmacol. 2009;9:3–8. doi: 10.1016/j.coph.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Stein C, Schafer M, Machelska H. Attacking pain at its source: new perspectives on opioids. Nat Med. 2003;9:1003–1008. doi: 10.1038/nm908. [DOI] [PubMed] [Google Scholar]
- Stone LS, Vulchanova L, Riedl MS, Williams FG, Wilcox GL, Elde R. Effects of peripheral nerve injury on delta opioid receptor (DOR) immunoreactivity in the rat spinal cord. Neurosci Lett. 2004;361:208–211. doi: 10.1016/j.neulet.2003.12.067. [DOI] [PubMed] [Google Scholar]
- Suter MR, Papaloizos M, Berde CB, Woolf CJ, Gilliard N, Spahn DR, Decosterd I. Development of neuropathic pain in the rat spared nerve injury model is not prevented by a peripheral nerve block. Anesthesiology. 2003;99:1402–1408. doi: 10.1097/00000542-200312000-00025. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Rew Y, Malkmus S, Schiller PW, Taulane JP, Goodman M, Yaksh TL. Systemic and spinal analgesic activity of a delta-opioid-selective lanthionine enkephalin analog. J Pharmacol Exp Ther. 2003;304:827–832. doi: 10.1124/jpet.102.039750. [DOI] [PubMed] [Google Scholar]
- Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve 588. Pain. 1994;57:375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Tan-No K, Niijima F, Nakagawasai O, Sato T, Satoh S, Tadano T. Development of tolerance to the inhibitory effect of loperamide on gastrointestinal transit in mice. Eur J Pharm Sci. 2003;20:357–363. doi: 10.1016/j.ejps.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Meier S, Burian M, Schmidt H, Geisslinger G, Lotsch J. Peripheral opioid analgesia in experimental human pain models. Brain. 2003;126:1092–1102. doi: 10.1093/brain/awg115. [DOI] [PubMed] [Google Scholar]
- Tiseo PJ, Yaksh TL. Dose-dependent antagonism of spinal opioid receptor agonists by naloxone and naltrindole: additional evidence for delta-opioid receptor subtypes in the rat. Eur J Pharmacol. 1993;236:89–96. doi: 10.1016/0014-2999(93)90230-f. [DOI] [PubMed] [Google Scholar]
- Vadivelu N, Mitra S, Hines RL. Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag. 2011;7:55–68. doi: 10.5055/jom.2011.0049. [DOI] [PubMed] [Google Scholar]
- Vasilyev DV, Shan Q, Lee Y, Mayer SC, Bowlby MR, Strassle BW, Kaftan EJ, Rogers KE, Dunlop J. Direct inhibition of Ih by analgesic loperamide in rat DRG neurons. J Neurophysiol. 2007;97:3713–3721. doi: 10.1152/jn.00841.2006. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sakashita Y. Differential effects of intrathecally administered morphine and its interaction with cholecystokinin-B antagonist on thermal hyperalgesia following two models of experimental mononeuropathy in the rat. Anesthesiology. 1999;90:1382–1391. doi: 10.1097/00000542-199905000-00023. [DOI] [PubMed] [Google Scholar]
- Zhang G, Mohammad H, Peper BD, Raja S, Wilson SP, Sweitzer SM. Enhanced peripheral analgesia using virally mediated gene transfer of the mu-opioid receptor in mice. Anesthesiology. 2008;108:305–313. doi: 10.1097/01.anes.0000299836.61785.79. [DOI] [PubMed] [Google Scholar]
- Zollner C, Stein C. Opioids Handb Exp Pharmacol. 2007:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]


