Abstract
Both taxonomic and thematic semantic relations have been studied extensively in behavioral studies and there is an emerging consensus that the anterior temporal lobe plays a particularly important role in the representation and processing of taxonomic relations, but the neural basis of thematic semantics is less clear. We used eye tracking to examine incidental activation of taxonomic and thematic relations during spoken word comprehension in participants with aphasia. Three groups of participants were tested: neurologically intact control participants (N=14), individuals with aphasia resulting from lesions in left hemisphere BA 39 and surrounding temporo-parietal cortex regions (N=7), and individuals with the same degree of aphasia severity and semantic impairment and anterior left hemisphere lesions (primarily inferior frontal gyrus and anterior temporal lobe) that spared BA 39 (N=6). The posterior lesion group showed reduced and delayed activation of thematic relations, but not taxonomic relations. In contrast, the anterior lesion group exhibited longer-lasting activation of taxonomic relations and did not differ from control participants in terms of activation of thematic relations. These results suggest that taxonomic and thematic semantic knowledge are functionally and neuroanatomically distinct, with the temporo-parietal cortex playing a particularly important role in thematic semantics.
Keywords: semantic knowledge, semantic deficit, thematic relation, aphasia, eye tracking
1. Introduction
Semantic knowledge consists of two broad kinds of relations: similarity and complementarity. Similarity is typically defined in terms of feature overlap, which can naturally give rise to categorical, or “taxonomic” structure (e.g., Rogers & McClelland, 2004; Rogers et al., 2004; O’Connor, Cree, & McCrae, 2009). Taxonomic semantic knowledge has been studied extensively in behavioral, neuropsychological, and functional imaging studies with an emerging consensus that anterior temporal lobe (ATL) structures play a particularly important role in the representation and processing of these relations (for a review, see Patterson, Nestor, & Rogers, 2007).
Complementary objects typically do not share features; rather they have complementary features that correspond to their complementary roles in events or situations. Complementarity can be defined as frequent occurrence in events or situations and we refer to these as “thematic” relations. Although thematic relations have been studied extensively in behavioral studies (for a review, see Estes, Golonka, & Jones, 2011), relatively little is known about their neural basis. Recent behavioral evidence suggests that taxonomic and thematic semantic knowledge may be somewhat distinct: Mirman and Graziano (in press) found that across 30 neurologically intact individuals, the incidental activation of taxonomically related concepts compared to thematically related concepts in a word recognition task predicted the tendency to choose the taxonomic option in an explicit, nonverbal semantic similarity judgment task. That is, individuals varied in their reliance on taxonomic vs. thematic knowledge across tasks (see also Simmons & Estes, 2008). This finding suggests that taxonomic and thematic knowledge comprise two parallel complementary semantic systems (for related work distinguishing concrete and abstract concepts see Crutch & Warrington, 2005; 2010). However, these results did not speak to the neural basis of these systems.
In a recent voxel-based lesion-symptom mapping (VLSM) study of picture naming errors produced by individuals with aphasia, Schwartz et al. (2011) found that participants generally produced far more taxonomic errors (coordinate, superordinate, or subordinate noun substitutions) than thematic errors (non-taxonomic errors that named an object that co-occurred with the target in the context of an action, event, or sentence), but the relative tendency to produce one error type vs. the other varied as a function of their lesion location. Individuals with lesions affecting the left anterior temporal lobe (ATL) produced a higher proportion of taxonomic errors relative to thematic errors and individuals with lesions affecting the left temporo-parietal cortex (TPC) produced a higher proportion of thematic errors relative to taxonomic errors.
The finding that ATL damage is associated with taxonomic errors is consistent with this region’s well-documented importance for taxonomic semantics (e.g., Patterson et al., 2007; Schwartz et al., 2009; Walker et al., 2011; individuals with ATL degeneration produce almost exclusively taxonomic errors, but deficits in thematic semantic knowledge become evident when thematic relations are explicitly tested, e.g., Bozeat et al., 2000; Butler, Brambati, Miller, & Gorno-Tempini, 2009). In contrast, the finding that TPC damage is associated with thematic errors is quite novel, though at least one functional imaging study has identified this region as important for processing thematic relations (Kalenine et al., 2009; for a recent review of the neural basis of semantic memory see Binder & Desai, 2011). Schwartz et al. (2011) proposed a complementary semantic systems account, but there are at least two alternative interpretations of this result. The first is that TPC involvement in production of thematic picture naming errors is specific to picture naming or, slightly more generally, word production tasks and does not reflect core thematic semantic processing.
The second alternative is that thematic errors in picture naming are symptomatic of a cognitive control deficit and TPC is involved in cognitive control, rather than thematic semantics (e.g., Jefferies & Lambon Ralph, 2006). There is strong evidence that at least some individuals with aphasia have relatively general cognitive control deficits (Corbett, Jefferies, & Lambon Ralph, 2011; Hoffman et al., 2011; Jefferies & Lambon Ralph, 2006; Mirman, Yee, Blumstein, & Magnuson, 2011; Noonan, Jefferies, Corbett, & Lambon Ralph, 2010; Novick, Kan, Trueswell, & Thompson-Schill, 2009; Robinson, Blair, & Cippoloti, 1998; Schnur et al., 2009; Warrington & Cippoloti, 1996). These deficits have been associated with damage to the inferior frontal gyrus (IFG; e.g., Schnur et al., 2009), but Jefferies, Lambon Ralph and their colleagues have proposed that TPC damage may also cause cognitive control deficits. This proposal emerged from several studies in which patients with lesions encompassing both inferior frontal (IFG) and temporo-parietal (TPC) regions exhibited semantic control deficits. Further, patients with lesions restricted to one region or the other tended to show similar behavioral patterns of semantic control deficits (Corbett et al., 2011; Hoffman et al., 2011; Jefferies & Lambon Ralph, 2006; Noonan et al., 2010). The strongest evidence for a unique role of TPC in cognitive control processes comes from a recent TMS study (Whitney et al., 2012), which suggested that this region is particularly important for top-down control of semantic retrieval, namely retrieving feature-specific information such as shape or color. Schwartz et al. (2011) considered this cognitive control account and ruled it out because (1) their measure of thematic error production controlled for controlled semantic retrieval as measured by the Camel and Cactus Test (Bozeat et al., 2000) and (2) production of the canonical “off-task” error type – semantic descriptions (horse –> “it goes neigh”) – was associated with ATL damage only, not TPC damage. Thus, their results do not appear attributable to a TPC-based cognitive control deficit, but the extent to which TPC is involved in thematic semantics versus cognitive control remains an open question. The present study was designed to test the claim that TPC is specifically involved in thematic semantics and to evaluate the two alternative accounts.
2. Experiment
We tested the hypothesis that TPC is specifically involved in thematic semantics and the two alternative accounts using eye tracking to examine incidental activation of taxonomic and thematic knowledge during spoken word comprehension. We used a passive version of the “visual world paradigm” (Tanenhaus, Spivey-Knowlton, Eberhard, & Sedivy, 1995; cf. Cooper, 1974) in which pictures of four objects are presented on a computer screen and participants hear a word that matches one of the pictures1. Participants tend to fixate the objects that correspond to the spoken word and, critically, objects that are related to the spoken word tend to be fixated more than objects that are unrelated to the spoken word. Importantly, because participants are simply listening to spoken words, this task involves minimal controlled retrieval demands (see Salverda & Altmann, 2011, for evidence that referents of spoken words automatically capture visual attention). Past studies have shown that this paradigm is sensitive to taxonomic similarity (e.g., Huettig & Altmann, 2005; Mirman & Magnuson, 2009), thematic similarity (Mirman & Graziano, in press; see also Yee & Sedivy, 2006), and more specific semantic relations (e.g., Kalenine, Mirman, Middleton, & Buxbaum, in press; Yee, Huffstetler, & Thompson-Schill, 2011).
This experimental paradigm allows us to test both of the alternative accounts: if TPC is specialized for thematic semantics, then we should see a specific impairment of activation of thematic semantic relations - but not taxonomic relations - for individuals with TPC lesions and not for individuals with lesions that spare TPC. Since this is a word comprehension task, we should not see this effect if the Schwartz et al. (2011) VLSM results were specific to word production. Since this task requires minimal semantic/cognitive control or controlled retrieval, we should also not see this effect if TPC is involved in general semantic control (or even more generally, executive control) rather than thematic semantics. Indeed, a cognitive control deficit that impairs response selection may produce increased or extended activation of semantically related competitors, rather than decreased or delayed activation (for a more detailed discussion of cognitive control and competition in the visual world paradigm see Mirman et al., 2011).
2.1 Methods
2.1.1 Materials
The critical stimuli consisted of 20 taxonomically related image pairs and 20 thematically related image pairs. For each critical related pair, two phonologically and semantically unrelated pictures were selected to serve as unrelated distractors. An additional 30 sets of 4 unrelated pictures were selected to serve as practice (10) and filler (20) trials. The critical relations were assigned based on the coding scheme used by Schwartz et al. (2011) to code picture naming errors: taxonomically related pairs were members of the same category and thematically related pairs frequently participated in an event or scenario and were not members of the same category. A separate semantic relatedness norming study (for details see Mirman & Graziano, in press) showed that our materials matched the differential and somewhat asymmetric taxonomic and thematic relations used by Schwartz et al. (2011): average ratings on the thematic dimension were only slightly higher for thematic (4.4) than taxonomic (4.3) pairs, whereas ratings on the taxonomic dimension were substantially higher for taxonomic (4.1) than thematic (3.4) pairs (the interaction between pair type and rating type was highly significant both by items and by subjects, both F > 10, p < 0.01). Since our primary focus was on thematic semantics, it is sufficient that the thematically related pairs were primarily thematically (not taxonomically) related and that there was a differential relationship in the taxonomic and thematic pairs. Unrelated items received low relatedness ratings on both dimensions (taxonomic: 1.2; thematic: 1.3). Target and competitor words were matched on word frequency, familiarity, length, neighborhood density, and association strength (Nelson, McEvoy, & Schreiber, 2004) across the two conditions (all p > 0.15).
Picture stimuli were drawn from a normed set of 260 color drawings of common objects (Rossion & Pourtois, 2004, available at http://stims.cnbc.cmu.edu/Image%20Databases/TarrLab/Objects/). Images were scaled to a maximum size of 200 × 200 pixels such that at least one dimension was 200 pixels. The full list of stimuli is provided in the Appendix. Stimulus words were recorded by a native English speaker at 44.1kHz. The individual words were edited to eliminate silence at the beginning and end of each sound file.
Appendix.
Experiment Stimuli
| Condition | Target | Competitor | Unrelated 1 | Unrelated 2 |
|---|---|---|---|---|
| thematic | anchor | sailboat | French horn | grasshopper |
| thematic | ashtray | cigarette | rhino | lettuce |
| thematic | balloon | clown | rolling pin | donkey |
| thematic | barn | pig | jello | ironing board |
| thematic | bird | tree | honey | guitar |
| thematic | eye | glasses | seal | chisel |
| thematic | football | helmet (football) | beetle | harp |
| thematic | hair | comb | drum | corn |
| thematic | hammer | nail | chicken | flag |
| thematic | hand | glove | leaf | mushroom |
| thematic | hanger | blouse | cherry | doll |
| thematic | kettle | stove | cat | door |
| thematic | lamp | table | box | chain |
| thematic | lock | key | pear | belt |
| thematic | monkey | banana | bicycle | house |
| thematic | needle | thread | piano | caterpillar |
| thematic | sheep | sweater | light switch | frying pan |
| thematic | sock | foot | seahorse | cake |
| thematic | toaster | bread | snowman | baby carriage |
| thematic | vase | flower | sled | bow |
| taxonomic | airplane | helicopter | swan | well |
| taxonomic | ant | spider | asparagus | book |
| taxonomic | bat | racket | celery | dresser |
| taxonomic | bus | train | peacock | refrigerator |
| taxonomic | cigar | pipe | fish | garbage can |
| taxonomic | cup | glass | iron | kangaroo |
| taxonomic | deer | cow | light bulb | coat |
| taxonomic | ear | nose | accordion | windmill |
| taxonomic | fork | knife | ostrich | purse |
| taxonomic | gun | cannon | spinning wheel | artichoke |
| taxonomic | leg | arm | strawberry | turtle |
| taxonomic | moon | sun | envelope | doorknob |
| taxonomic | motorcycle | car | umbrella | tomato |
| taxonomic | necklace | ring | plug | saltshaker |
| taxonomic | owl | eagle | ladder | nail file |
| taxonomic | paintbrush | pen | mountain | onion |
| taxonomic | top | ball | ruler | skunk |
| taxonomic | violin | flute | potato | clothespin |
| taxonomic | watch | clock | grapes | heart |
| taxonomic | wrench | pliers | roller skate | rooster |
2.1.2 Apparatus
Participants were seated approximately 24 inches away from a 17-inch monitor with the resolution set to 1024×768 dpi. Stimuli were presented using E-Prime Professional 2.0 experimental design software. A remote Eyelink 1000 eye tracker was used to record participants’ left eye gaze position at 250 Hz.
2.1.3 Procedure
Each trial began with a 1s fixation screen, followed by a 1300ms preview of a four-image display in which each image was near one of the screen corners. Each critical display contained a target object image, a semantic competitor (taxonomically or thematically related), and two unrelated distractors. The position of the four pictures was randomized for each trial for each participant. During the last 300ms of the preview, a red circle appeared in the center of the screen in order to draw attention back to the neutral central location. After the preview, participants heard the target word through speakers and the objects remained on the screen for 4s. There were a total of 70 trials: 10 practice trials, 20 trials with taxonomic competitors, 20 trials with thematic competitors, and 20 filler trials where none of the images were related to each other. Trial order for the 60 non-practice trials was randomized. Participants were told that their eye movements would be recorded and the testing session began with a calibration, but they were not instructed to move their eyes in any particular way (aside from the general instruction to look at the screen). To improve data reliability, participants with aphasia completed 2 replications of the experiment at least 12 weeks apart (max: 24 weeks).
At the time of testing, the participants with aphasia also completed the 10-item Transitive Gesture to Sight Test of limb apraxia (Buxbaum, Giovannetti, & Libon, 2000). This test assessed their ability to correctly produce common transitive gestures (e.g., “show me how to wind a watch”), while imagining they are holding and using the specified item with their left hand. Items were in view while the gesture was produced. Gesture productions were scored on five components: content, hand posture, arm posture, amplitude, and timing.
2.1.4 Participants
Thirteen participants with aphasia (46% females; 54% Caucasian, 46% African American) completed the study. Their mean age was 56 (range = 33–74) and mean years of education was 15 (range = 12–20). Participants with aphasia were recruited from the Neuro-Cognitive Rehabilitation Research Patient Registry at the Moss Rehabilitation Research Institute (Schwartz et al., 2005). Lesion location was assessed by a previously administered MRI or CT scan (see Schwartz et al., 2009; Walker et al., 2011 for lesion analysis details) and performance on background tests was drawn from the Moss Aphasia Psycholinguistic Project Database (Mirman et al., 2010). Detailed information about the participants with aphasia is provided in Table 1.
Table 1.
Demographic and clinical descriptions of participants with aphasia
| ID | Lesion Group | Age | Education | Lesion Vol. (cc) | WAB AQ | PNT (%) | Syn. Trip. (%) | CCT (%) | PPVT | Gesture To Sight (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1857 | Anterior | 74 | 12 | 17.86 | 90.2 | 82 | 83 | 80 | 77 | 75 |
| 1238 | Anterior | 53 | 14 | 172.21 | 67.8 | 67 | 87 | 81 | 96 | 98 |
| 1687 | Anterior | 74 | 20 | 40.95 | 74.5 | 82 | 93 | 86 | 101 | 83 |
| 2172 | Anterior | 61 | 16 | 73.10 | 35.4 | 30 | 90 | 89 | 93 | 53 |
| 2350 | Anterior | 48 | 14 | 55.69 | 73.7 | 83 | 57 | 77 | 76 | 63 |
| 0419 | Anterior | 43 | 12 | 51.86 | 91.5 | 91 | 87 | 77 | 83 | 93 |
|
| ||||||||||
| 1846 | Posterior | 51 | 14 | 31.43 | 68 | 67 | 90 | 78 | 88 | 88 |
| 2083 | Posterior | 62 | 14 | 51.53 | 91.5 | 86 | 100 | 88 | 102 | 90 |
| 2006 | Posterior | 50 | 14 | 5.38 | 65 | 55 | 83 | 81 | 86 | 50 |
| 2180 | Posterior | 67 | 14 | 67.16 | 41.4 | 25 | 73 | 72 | 51 | 60 |
| 2221 | Posterior | 33 | 18 | 63.92 | 78.7 | 93 | 97 | 81 | 81 | 80 |
| 2284 | Posterior | 65 | 19 | 19.47 | 94.5 | 98 | 93 | 95 | 92 | 95 |
| 1862 | Posterior | 53 | 12 | NA | 76.6 | 75 | 93 | 64 | 88 | 80 |
Note: WAB AQ = Western Aphasia Battery Aphasia Quotient; PNT = Philadelphia Naming Test; Syn. Trip. = Synonymy Triplets; CCT = Camel & Cactus Test; PPVT = Peabody Picture Vocabulary Test.
All participants in the Posterior lesion location group (N=7) had lesions that included BA 39 and surrounding TPC regions, but not extending into anterior regions (specifically IFG and ATL). BA 39 was chosen as the focus region because this area had the largest concentration of suprathreshold voxels in the Schwartz et al. (2011) VLSM study of thematic errors in picture naming. All participants in the Anterior lesion location group (N=6) had lesions that did not include BA 39, but included anterior regions associated with semantic processing and semantic control (IFG and ATL). Two Anterior group participants (1238 and 2172) had primarily anterior lesions with minor extension along the Sylvian fissure into the posterior temporal and inferior parietal areas (BA 40, 41, and 42), but analyses excluding these participants did not change any of the results, so the more inclusive analyses are reported here. Figure 1 shows the lesion overlap for the two groups. As shown in Table 1, the two groups were matched (all p > 0.25) on age, education, aphasia severity (Western Aphasia Battery Aphasia Quotient [Kertesz, 1982] and Philadelphia Naming Test [Roach et al., 1996]), lesion volume, overall semantic impairment (synonymy triplets [Martin, Schwartz, & Kohen, 2006] and Camel and Cactus Test [Bozeat et al., 2000]), word-to-picture matching (Peabody Picture Vocabulary Test [Dunn & Dunn, 1997]), and object-related action knowledge (Gesture to Sight Test [Buxbaum et al., 2000]).
Figure 1.
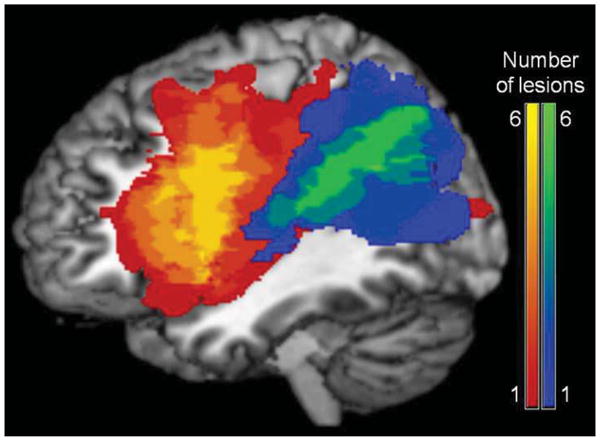
Lesion overlap maps for the participants with aphasia. The anterior group is shown in the red-yellow scale and the posterior group is shown in the blue-green scale.
For comparison, we also report results from 14 neurologically intact control participants (46% females; 92% Caucasian, 8% African American) who were approximately matched in age (M = 69, range = 58–78) and education (M = 16, range = 12–21) to the participants with aphasia. The control participants scored in the normal range (M = 28, range = 27–29) on the Mini Mental State Exam (Folstein, Folstein, & McHugh, 1975), confirming they had no cognitive impairments. Results from this group have been previously reported as part of a study of individual differences in taxonomic and thematic relations (Mirman & Graziano, in press).
All participants were right-handed and had English as their native language. None of the participants had any major psychiatric or neurologic co-morbidities. Participants were compensated for their participation and reimbursed for travel and related expenses. All were living in the community at the time of testing.
2.2 Data analysis
The fixation time course data were analyzed using Growth Curve Analysis (GCA) with fourth-order orthogonal polynomials, which is a multi-level modeling technique specifically designed to capture change over time (for detailed description of this method’s application to eye tracking data see Mirman, Dixon, & Magnuson, 2008). In the GCA approach, the Level 1 model captures the overall fixation time course with the intercept term reflecting average overall fixation proportion, the linear term reflecting a monotonic change in fixation proportion (similar to a linear regression of fixation proportion as a function of time), the quadratic term reflecting the symmetric rise and fall rate around a central inflexion point, and the cubic and quartic terms similarly reflecting the steepness of the curve around inflexion points. The Level 2 submodels capture the effects of experimental manipulations on the Level 1 time terms. In this experiment, we were interested in how semantic competition effects differed between participant groups, so the Level 2 models included effects of competitor relatedness (Object: Related vs. Unrelated), participant group (Group: Control vs. Anterior and Control vs. Posterior), and, critically, the difference in competitor relatedness effects across groups (Object-x-Group interaction).
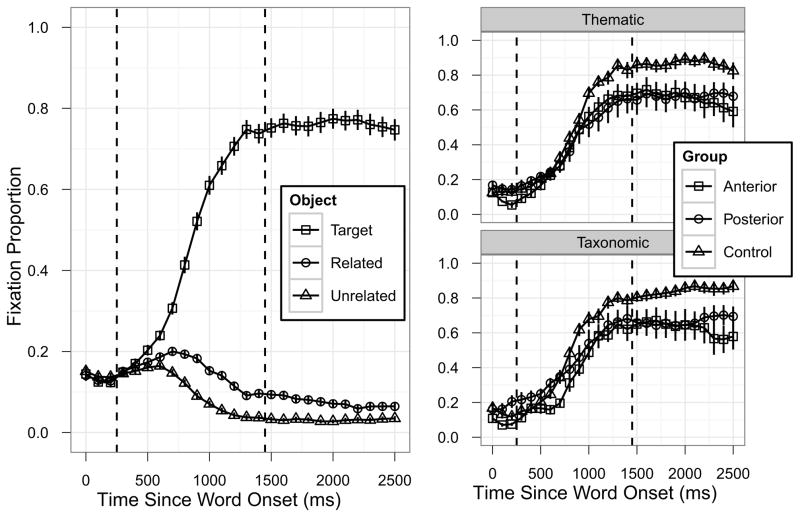
The focus of the experiment was on incidental activation of semantically related concepts during spoken word comprehension, so we used the time course of spoken word comprehension – target fixation – to identify the critical time window for analysis. The time window was defined to begin shortly before the target fixation curve started to separate from the non-target fixation curves (indicating that the spoken word was beginning to drive fixations) and end when the target fixation curve reached its asymptote (indicating that the spoken word had been recognized). Visual inspection of the overall target fixation curve averaged across all experimental conditions and participant groups (Figure 2, left panel) indicated that a window starting 300ms after word onset and ending 1400ms after word onset met these criteria. Examination of target fixation curves separately by participant group and semantic relatedness type (Figure 2, right panels) confirmed that this time window was appropriate for each of the individual cells in the study design. Growth curve analyses confirmed this: there were no effects of condition or group (all p > 0.1) in the 300ms following the time window cut-off (1500–1700ms after word onset, and the same results were obtained when this test window was extended to 1900ms after word onset). Most critical was the complete absence of any effects on the linear term (all p > 0.15), indicating that there was no significant increase in target fixation (i.e., target recognition had reached asymptote) following our time window cut-off and no significant differences in target fixations between conditions or groups. Note that by using target fixation time course to define the analysis time window we avoided biasing the competitor fixation time course analyses. Further robustness analyses revealed that changing the time window by 100–300ms in either direction did not affect the qualitative pattern of results.
Figure 2.
Selecting the analysis time window. The left panel shows overall fixation time course averaged across all critical conditions and participant groups. The right panels show target fixation curves separately for each of the participant groups and semantic relatedness types. Error bars indicate ±1SE. The dashed vertical lines indicate the time window (300–1400ms) identified for the critical competitor analyses.
The critical data analyses considered related and unrelated competitor fixation curves during the 300–1400ms time window, separately for the Taxonomic and Thematic conditions. The full GCA model contained all five Level 1 time terms (intercept, linear, quadratic, cubic, and quartic) and the Level 2 submodels for each of those time terms contained fixed effects of Object, Group, and the critical Object-x-Group interaction and random effects for individual participants and participant-x-object. The unrelated competitor was considered the baseline level for the Object factor and parameters were estimated for the related competitor; the Control group was considered the baseline level for the Group factor and parameters were estimated for each of the two aphasic participant groups relative to the control group.
2.3 Results
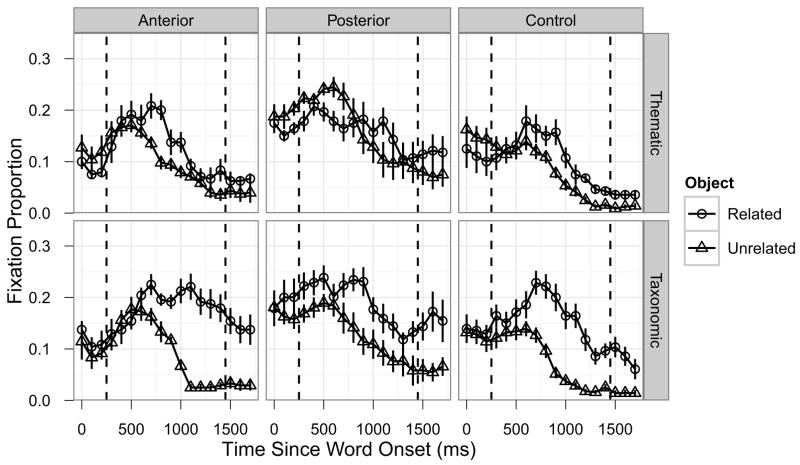
Figure 3 shows the competitor fixation time courses separately for each of the three participant groups for the Thematic and Taxonomic condition. The full GCA results for the critical Object-x-Group interaction terms are shown in Table 2. In the Thematic condition, the Posterior group exhibited less overall semantic competition compared to the Control group (Intercept term: Estimate = −0.036, SE = 0.016, p < 0.05) and the competition emerged later in the time window (Cubic term: Estimate = −0.084, SE = 0.022, p < 0.001). In contrast, Thematic competition for the Anterior group was generally very similar to the Control group, though there was a very weak trend suggesting that the effect may have emerged earlier in the time window (Cubic term: Estimate = 0.039, SE = 0.024, p = 0.098). In the Taxonomic condition, the Posterior group did not differ reliably from the Control group (all p > 0.14), but the Anterior group exhibited longer-lasting competition (Linear term: Estimate = 0.157, SE = 0.054, p < 0.01; Quartic term: Estimate = −0.052, SE = 0.023, p < 0.05).
Figure 3.
Time course of fixations by participant group and semantic relatedness type. The columns correspond to participant groups (left: Anterior; middle: Posterior; right: Control), the rows correspond to semantic relatedness types (top: Taxonomic; bottom: Thematic). Error bars indicate ±1SE, dashed vertical lines indicate the analysis time window.
Table 2.
GCA results: Object-x-Group interaction terms. The values correspond to parameter estimates (SE in parentheses) for each of the aphasic groups relative to the control group for the related competitor relative to the unrelated competitor.
| Time | Thematic | Taxonomic | ||
|---|---|---|---|---|
|
|
||||
| Term | Anterior | Posterior | Anterior | Posterior |
|
|
||||
| Intercept | 0.002 (0.017) | −0.036 (0.016)* | 0.0002 (0.022) | −0.022 (0.021) |
| Linear | −0.009 (0.048) | 0.064 (0.045) | 0.157 (0.054)** | −0.049 (0.051) |
| Quadratic | −0.006 (0.048) | 0.048 (0.046) | 0.081 (0.049)~ | 0.069 (0.047) |
| Cubic | 0.039 (0.024)~ | −0.084 (0.022)*** | −0.035 (0.023) | 0.031 (0.022) |
| Quartic | 0.031 (0.024) | −0.015 (0.022) | −0.052 (0.023)* | −0.007 (0.022) |
p < 0.1,
p < 0.05,
p < 0.01,
p < 0.001
3. General Discussion
We used eye tracking to examine incidental activation of taxonomic and thematic relations during spoken word comprehension in participants with aphasia. Compared to neurologically intact control participants, individuals with lesions in BA 39 and surrounding temporo-parietal cortex (TPC) regions showed reduced and delayed activation of thematic relations, but not taxonomic relations. In contrast, individuals with the same degree of aphasia severity and semantic impairment resulting from lesions that primarily affected the inferior frontal gyrus (IFG) and anterior temporal lobe (ATL) regions and spared BA 39 exhibited longer-lasting activation of taxonomic relations and did not differ from controls in terms of activation of thematic relations. These results are consistent with the hypothesis that the TPC is particularly important for thematic semantics.
Because the spoken word comprehension task had such minimal task demands (just listening to spoken words with no explicit response required), it is not clear how a cognitive control deficit could account for the observed thematic semantic specificity of the effect of TPC damage. Conversely, a cognitive control impairment caused by damage to IFG may explain why the anterior group exhibited longer-lasting taxonomic competition. The taxonomic competitors in our study were strongly related to the targets and damage to IFG may have impaired these participants’ ability to exclude the taxonomic competitor from consideration2 (for a related account in the phonological domain, see Mirman et al., 2011). Note that the difficulty was specific to taxonomic competitors, not thematic competitors, and the posterior group did not show this effect, so this account is consistent with the view that IFG - not TPC - plays a critical role in selection from competing alternatives (e.g., Schnur et al., 2009; Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997).
The present results converge with a recent voxel-based lesion-symptom mapping study of picture naming errors in aphasia (Schwartz et al., 2011), which showed that TPC lesions were associated with a disproportionally increased rate of thematic semantic errors. Since Schwartz et al. examined word production and the current study examined word comprehension, together, the two studies provide strong converging evidence that the TPC is particularly important for thematic semantics. Schwartz et al. also found that ATL lesions were associated with a disproportionally increased rate of taxonomic semantic errors in picture naming and theories of the role of ATL in semantic cognition have almost exclusively considered taxonomic semantics (e.g., Patterson et al., 2007; Rogers et al., 2004), albeit typically without distinguishing between taxonomic and thematic semantics. However, in explicit tests, ATL degeneration affects both taxonomic and thematic semantic knowledge (Bozeat et al., 2000; Butler et al., 2009) and the anterior group in the present study had lesions primarily in the IFG with only small extension into the superior portion of ATL (which is not the site of maximal atrophy in semantic dementia [e.g., Binney et al., 2010], nor the region most strongly associated with taxonomic errors in Schwartz et al.), so it remains unclear what role the ATL plays in thematic semantics (for a recent review of the neural basis of semantic memory see Binder & Desai, 2011).
There is substantial evidence that ATL serves as a “hub” for taxonomic semantic knowledge (e.g., Patterson et al., 2007), might TPC play a similar role for thematic semantic knowledge? A full answer to this question certainly requires further research, but several lines of research suggest that TPC may play just such a role. First, the TPC is located close to temporo-parietal regions involved in motion, action, and spatial processing (e.g., Kable & Chatterjee, 2006; Kable et al., 2005; Kalenine, Buxbaum, & Coslett, 2010; Kemmerer, 2006; Noppeney, 2008), which are likely to be important for extracting event- and situation-based thematic semantic knowledge. Second, this region has been identified as important for successful assignment of subject-object grammatical roles: individuals with damage to TPC have particular difficulty choosing the correct picture to match a sentence such as “The girl washes the boy” when the options include a picture of a girl washing a boy and a picture of a boy washing a girl (Thothathiri, Kimberg, & Schwartz, 2011; Wu, Waller, & Chatterjee, 2007). This sort of “thematic role assignment” may rely on a similar processing architecture as event processing. Finally, preliminary evidence suggests that TPC may have hub-like connectivity to surrounding regions that would allow it to extract thematic relations (Jouen et al., 2011; see also Turken & Dronkers, 2011).
The documented involvement of posterior and inferior parietal regions in functions outside the specific domain of semantic memory may also support the specialization of TPC for thematic semantics. For example, this region appears to be important for autobiographical memory, particularly free recall of rich event memories (e.g., Berryhill et al., 2007). The same neural substrate that is involved in formation and retrieval of memories of life events is likely to be involved in processing of events for the extraction of thematic semantic knowledge. In addition, this region appears to be important for short-term and working memory (e.g., Baldo & Dronkers, 2006; Hoffman et al., 2011; Olson & Berryhill, 2009). The serial-order and sequencing demands of short-term and working memory tasks may also be important for event and sentence processing functions that would underlie thematic semantics (e.g., Botvinick & Plaut, 2006). Although speculative (post-hoc analyses did not reveal statistically reliable effects of action knowledge, sentence comprehension, or short-term memory on thematic competition), this perspective points toward a way to integrate TPC specialization for thematic semantics with other functions that are known to depend on this broad cortical region.
In sum, our results show that temporo-parietal cortex is particularly important for thematic semantic knowledge: individuals with lesions in left TPC exhibited reduced activation of thematic relations and normal activation of taxonomic relations during spoken word recognition. A comparison group of individuals with lesions that included left IFG and ATL, but not TPC, exhibited longer-lasting activation of taxonomic relations and minimally different activation of thematic relations. These results support a complementary semantic systems view (Mirman & Graziano, in press; Schwartz et al., 2011; see also Crutch & Warrington, 2005; 2010) in which taxonomic and thematic semantic knowledge are supported by somewhat distinct semantic systems with TPC playing a critical role in thematic semantics.
Highlights.
Examined activation of taxonomic and thematic semantic relations using eye-tracking
Compared participants with fronto-temporal or temporo-parietal lesions to controls
Posterior lesion group had reduced activation of thematic, not taxonomic, relations
Anterior lesion group had extended activation of taxonomic, not thematic, relations
Left temporo-parietal cortex is specialized for thematic semantic knowledge
Acknowledgments
This work was supported by National Institutes of Health grant R01DC010805 to DM and the Moss Rehabilitation Research Institute. A preliminary report was presented as a poster at the 2011 Neurobiology of Language Conference. We thank Myrna Schwartz and the Language and Aphasia lab at MRRI for helpful discussions and insightful suggestions.
Footnotes
In the more common version of the task participants are instructed to click on the picture that matches the spoken word. We chose a passive version in which a click response was not required in order to further reduce response selection demands, make the task easier for participants with aphasia (for whom hemiparesis may make manual responses somewhat difficult), and because previous studies suggest that a manual response is not required for the effects to emerge (e.g., Altmann & Kamide, 1999; Mirman & Graziano, in press).
Post-hoc analyses did not reveal a modulatory effect of lesion location on the association between semantic relation strength and amount of competition, possibly due to the resticted range of item-level variability in relation strength.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006;20(5):529–38. doi: 10.1037/0894-4105.20.5.529. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. The Journal of Neuroscience. 2007;27(52):14415–23. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJM, Lambon Ralph MA. The Ventral and Inferolateral Aspects of the Anterior Temporal Lobe Are Crucial in Semantic Memory: Evidence from a Novel Direct Comparison of Distortion-Corrected fMRI, rTMS, and Semantic Dementia. Cerebral Cortex. 2010;20(11):2728–2738. doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Plaut DC. Short-term memory for serial order: A recurrent neural network model. Psychological Review. 2006;113(2):201–233. doi: 10.1037/0033-295X.113.2.201. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson KE, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38(9):1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Butler CR, Brambati SM, Miller BL, Gorno-Tempini ML. The neural correlates of verbal and nonverbal semantic processing deficits in neurodegenerative disease. Cognitive and Behavioral Neurology. 2009;22(2):73–80. doi: 10.1097/WNN.0b013e318197925d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ, Giovannetti T, Libon D. The Role of the Dynamic Body Schema in Praxis: Evidence from Primary Progressive Apraxia. Brain and Cognition. 2000;44:166–191. doi: 10.1006/brcg.2000.1227. [DOI] [PubMed] [Google Scholar]
- Cooper RM. The control of eye fixation by the meaning of spoken language: A new methodology for the real-time investigation of speech perception, memory, and language processing. Cognitive Psychology. 1974;6(1):84–107. [Google Scholar]
- Corbett F, Jefferies E, Lambon Ralph MA. Deregulated semantic cognition follows prefrontal and temporo-parietal damage: Evidence from the impact of task constraint on nonverbal object use. Journal of Cognitive Neuroscience. 2011;23(5):1125–1135. doi: 10.1162/jocn.2010.21539. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK. Abstract and concrete concepts have structurally different representational frameworks. Brain. 2005;128(3):615–627. doi: 10.1093/brain/awh349. [DOI] [PubMed] [Google Scholar]
- Crutch SJ, Warrington EK. The differential dependence of abstract and concrete words upon associative and similarity-based information: Complementary semantic interference and facilitation effects. Cognitive Neuropsychology. 2010;27(1):46–71. doi: 10.1080/02643294.2010.491359. [DOI] [PubMed] [Google Scholar]
- Dunn Lloyd M, Dunn Leota M. Examiner’s manual for the Peabody Picture Vocabulary-Test III (PPVT-III) Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Estes Z, Golonka S, Jones LL. Thematic thinking: The apprehension and consequences of thematic relations. Psychology of Learning and Motivation. 2011;54:249–294. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Jefferies E, Lambon Ralph MA. Explaining semantic short-term memory deficits: evidence for the critical role of semantic control. Neuropsychologia. 2011;49(3):368–81. doi: 10.1016/j.neuropsychologia.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettig F, Altmann GTM. Word meaning and the control of eye fixation: Semantic competitor effects and the visual world paradigm. Cognition. 2005;96(1):B23–B32. doi: 10.1016/j.cognition.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jouen A-L, Ellmore TM, Madden C, Pallier C, Dominey PF, Ventre-Dominey J. A common “meaning” system for language and visual images revealed by fMRI and DTI. Poster presented at the 2011 Neurobiology of Language Conference; Annapolis, MD, USA. 2011. [Google Scholar]
- Kable JW, Chatterjee A. Specificity of action representations in the lateral occipitotemporal cortex. Journal of Cognitive Neuroscience. 2006;18(9):1498–1517. doi: 10.1162/jocn.2006.18.9.1498. [DOI] [PubMed] [Google Scholar]
- Kable JW, Kan IP, Wilson A, Thompson-Schill SL, Chatterjee A. Conceptual representations of action in the lateral temporal cortex. Journal of Cognitive Neuroscience. 2005;17(12):1855–1870. doi: 10.1162/089892905775008625. [DOI] [PubMed] [Google Scholar]
- Kalénine S, Buxbaum LJ, Coslett HB. Critical brain regions for action recognition: lesion symptom mapping in left hemisphere stroke. Brain. 2010;133(11):3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalénine S, Mirman D, Middleton EL, Buxbaum LJ. Temporal dynamics of activation of thematic and functional action knowledge during auditory comprehension of artifact words. Journal of Experimental Psychology: Learning, Memory, and Cognition. doi: 10.1037/a0027626. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalénine S, Peyrin C, Pichat C, Segebarth C, Bonthoux F, Baciu M. The sensory-motor specificity of taxonomic and thematic conceptual relations: A behavioral and fMRI study. NeuroImage. 2009;44(3):1152–1162. doi: 10.1016/j.neuroimage.2008.09.043. [DOI] [PubMed] [Google Scholar]
- Kemmerer D. The semantics of space: integrating linguistic typology and cognitive neuroscience. Neuropsychologia. 2006;44(9):1607–1621. doi: 10.1016/j.neuropsychologia.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery. New York: Grune & Stratton; 1982. [Google Scholar]
- Martin N, Schwartz MF, Kohen FP. Assessment of the ability to process semantic and phonological aspects of words in aphasia: A multi-measurement approach. Aphasiology. 2006;20(2–4):154–166. [Google Scholar]
- Mirman D, Dixon JA, Magnuson JS. Statistical and computational models of the visual world paradigm: Growth curves and individual differences. Journal of Memory and Language. 2008;59(4):475–494. doi: 10.1016/j.jml.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Graziano KM. Individual differences in the strength of taxonomic versus thematic relations. Journal of Experimental Psychology: General. doi: 10.1037/a0026451. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Magnuson JS. Dynamics of activation of semantically similar concepts during spoken word recognition. Memory & Cognition. 2009;37(7):1026–1039. doi: 10.3758/MC.37.7.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Strauss TJ, Brecher A, Walker GM, Sobel P, Dell GS, Schwartz MF. A large, searchable, web-based database of aphasic performance on picture naming and other tests of cognitive function. Cognitive Neuropsychology. 2010;27(6):495–504. doi: 10.1080/02643294.2011.574112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirman D, Yee E, Blumstein SE, Magnuson JS. Theories of spoken word recognition deficits in aphasia: Evidence from eye-tracking and computational modeling. Brain and Language. 2011;117(2):53–68. doi: 10.1016/j.bandl.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida free association, rhyme, and word fragment norms. Behavior Research Methods, Instruments & Computers. 2004;36(3):402–407. doi: 10.3758/bf03195588. Retrieved from http://www.usf.edu/FreeAssociation/ [DOI] [PubMed] [Google Scholar]
- Noonan KA, Jefferies E, Corbett F, Lambon Ralph MA. Elucidating the Nature of Deregulated Semantic Cognition in Semantic Aphasia: Evidence for the Roles of Prefrontal and Temporo parietal Cortices. Journal of Cognitive Neuroscience. 2010;22(7):1597–1613. doi: 10.1162/jocn.2009.21289. [DOI] [PubMed] [Google Scholar]
- Noppeney U. The neural systems of tool and action semantics: a perspective from functional imaging. Journal of Physiology- Paris. 2008;102(1–3):40–49. doi: 10.1016/j.jphysparis.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Novick JM, Kan IP, Trueswell JC, Thompson-Schill SL. A case for conflict across multiple domains: Memory and language impairments following damage to ventrolateral prefrontal cortex. Cognitive Neuropsychology. 2009;26(6):527–567. doi: 10.1080/02643290903519367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor CM, Cree GS, McRae K. Conceptual Hierarchies in a Flat Attractor Network: Dynamics of Learning and Computations. Cognitive Science. 2009;33(1):1–44. doi: 10.1111/j.1551-6709.2009.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Berryhill ME. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiology of Learning and Memory. 2009;91(2):155–165. doi: 10.1016/j.nlm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson KE, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Roach A, Schwartz MF, Martin N, Grewal RS, Brecher AR. The Philadelphia Naming Test: Scoring and rationale. Clinical Aphasiology. 1996;24:121–133. [Google Scholar]
- Robinson G, Blair J, Cipolotti L. Dynamic aphasia: an inability to select between competing verbal responses? Brain. 1998;121:77–89. doi: 10.1093/brain/121.1.77. [DOI] [PubMed] [Google Scholar]
- Rogers TT, McClelland JL. Semantic Cognition: A Parallel Distributed Processing Approach. Cambridge, MA: MIT Press; 2004. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson KE. Structure and Deterioration of Semantic Memory: A Neuropsychological and Computational Investigation. Psychological Review. 2004;111(1):205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart’s object pictorial set: The role of surface detail in basic-level object recognition. Perception. 2004;33(2):217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Salverda AP, Altmann GTM. Attentional capture of objects referred to by spoken language. Journal of Experimental Psychology: Human Perception and Performance. 2011;37(4):1122–1133. doi: 10.1037/a0023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnur TT, Schwartz MF, Kimberg DY, Hirshorn E, Coslett HB, Thompson-Schill SL. Localizing interference during naming: Convergent neuroimaging and neuropsychological evidence for the function of Broca’s area. Proceedings of the National Academy of Sciences. 2009;106(1):322–327. doi: 10.1073/pnas.0805874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Brecher AR, Whyte J, Klein MG. A patient registry for cognitive rehabilitation research: A strategy for balancing patients’ privacy rights with researchers’ need for access. Archives of Physical Medicine and Rehabilitation. 2005;86(9):1807–1814. doi: 10.1016/j.apmr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Brecher AR, Faseyitan O, Dell GS, Mirman D, Coslett HB. A neuroanatomical dissociation for taxonomic and thematic knowledge in the human brain. Proceedings of the National Academy of Sciences. 2011;108(20):8520–8524. doi: 10.1073/pnas.1014935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher AR, Dell GS, Coslett HB. Anterior temporal involvement in semantic word retrieval: Voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132(12):3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons S, Estes Z. Individual differences in the perception of similarity and difference. Cognition. 2008;108(3):781–795. doi: 10.1016/j.cognition.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Tanenhaus MK, Spivey-Knowlton MJ, Eberhard KM, Sedivy JC. Integration of visual and linguistic information in spoken language comprehension. Science. 1995;268(5217):632–634. doi: 10.1126/science.7777863. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thothathiri M, Kimberg DY, Schwartz MF. The Neural Basis of Reversible Sentence Comprehension: Evidence from Voxel-based Lesion Symptom Mapping in Aphasia. Journal of Cognitive Neuroscience. 2012;24(1):212–22. doi: 10.1162/jocn_a_00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011 Feb;5:1–20. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GM, Schwartz MF, Kimberg DY, Faseyitan O, Brecher AR, Dell GS, Coslett HB. Support for anterior temporal involvement in semantic error production in aphasia: New evidence from VLSM. Brain and Language. 2011;117(3):110–122. doi: 10.1016/j.bandl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, Cipolotti L. Word comprehension: The distinction between refractory and storage impairments. Brain. 1996;119(2):611–625. doi: 10.1093/brain/119.2.611. [DOI] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O’Sullivan J, Lambon Ralph Ma, Jefferies E. Executive Semantic Processing Is Underpinned by a Large-scale Neural Network: Revealing the Contribution of Left Prefrontal, Posterior Temporal, and Parietal Cortex to Controlled Retrieval and Selection Using TMS. Journal of Cognitive Neuroscience. 2012;24(1):133–147. doi: 10.1162/jocn_a_00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DH, Waller S, Chatterjee A. The functional neuroanatomy of thematic role and locative relational knowledge. Journal of Cognitive Neuroscience. 2007;19(9):1542–1555. doi: 10.1162/jocn.2007.19.9.1542. [DOI] [PubMed] [Google Scholar]
- Yee E, Sedivy JC. Eye Movements to Pictures Reveal Transient Semantic Activation During Spoken Word Recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32(1):1–14. doi: 10.1037/0278-7393.32.1.1. [DOI] [PubMed] [Google Scholar]
- Yee E, Huffstetler S, Thompson-Schill SL. Function follows Form: Activation of Shape and Function Features During Word Recognition. Journal of Experimental Psychology: General. 2011;140(3):348–363. doi: 10.1037/a0022840. [DOI] [PMC free article] [PubMed] [Google Scholar]