Abstract
Objective
The study aimed determining whether assessment of COMP degradation products could serve as a serological disease course and therapeutic response predictor in arthritis.
Methods
We generated a panel of monoclonal antibodies against COMP fragments and developed a novel capture ELISA for detecting COMP fragments in patients with osteoarthritis (OA) and rheumatoid arthritis (RA). This test was also used to monitor COMP fragments in surgically induced OA, collagen induced arthritis (CIA), and TNF transgenic animal models.
Results
Compared with a commercial COMP ELISA kit that detected no significant difference in COMP levels between OA and control groups, a significant increase of the COMP fragments were noted in the serum of OA patients assayed by this newly established ELISA. In addition, serum COMP fragment levels were well correlated with severity in OA patients and the progression of surgically induced OA in murine models. Furthermore, the serum levels of COMP fragments in RA patients, mice with CIA, and TNF transgenic mice were significantly higher when compared with their controls. Interestingly, treatment with TNFα inhibitors and methotrexate led to a significant decrease of serum COMP fragments in RA patients. Additionally, administration of Atsttrin (Tang, et al, Science, 2011 332(6028):478) also resulted in a significant reduction in COMP fragments in arthritis mice models.
Conclusion
A novel sandwich ELISA is capable of reproducibly measuring serum COMP fragments in both arthritic patients and rodent arthritis models. This test also provides a valuable means to utilize serum COMP fragments for monitoring the effects of interventions in arthritis.
INTRODUCTION
Arthritis is a group of chronic diseases that is characterized by the damage of joints and the inflammation of soft tissue around the joints[1]. The impact of arthritic conditions is expected to grow because of increased longevity of the population and increased environmentally induced diseases[2]. The lack of effective non-surgical treatment modalities for arthritis may probably be attributed to late detection of disease, in particular late detection of cartilage degeneration, at which point the patient’s chondrocytes are no longer effective in stopping or reversing joint destruction[3–5]. Thus, tremendous efforts have been taken to identify a specific biological marker, which can help to make an early diagnosis of altered joint cartilage metabolism, to assess the disease progression, and to monitor the effects of drug treatment. Cartilage oligomeric matrix protein (COMP), a prominent non-collagenous component of cartilage, accounts for approximately 1% of the wet weight of articular tissue[6, 7]. Proteolytic fragments of COMP have been detected in the degenerating cartilage, synovial fluid, and serum of patients with knee injuries, post-traumatic and primary OA or RA[8–10]. COMP is a putative substrate for matrix metalloproteinases (MMPs), including MMP-1[11], MMP-13[12], MMPs 19 and 20[13], ADAMTS-4 [11], -7[14], and -12[15]. Increased fragments of COMP have been reported in patients with OA, RA, and joint injury[16–18], and show great potential to serve as valuable biological markers of cartilage breakdown in arthritis[10, 19, 20]. Herein we report a novel COMP ELISA for measuring the levels of proteolytic COMP fragments in serum, and exploit the possibility of employing COMP fragments as a biomarker for monitoring the arthritic progression and the effects of therapeutic interventions.
MATERIALS AND METHODS
Human serum samples
Osteoarthritis Cohort
Patients with OA were recruited as part of a NIH–funded study and 57 serum samples from these patients were available for this study. All patients had symptomatic knee OA according to the ACR criteria[21]. Twenty one age-matched healthy control subjects were also recruited. In all patients, radiographs of the knee were obtained in lateral and antero-posterior planes. and the radiographic findings were scored according to the K&L scale[22]. Table 1 describes the demographic and clinical characteristics of OA and healthy controls.
Table 1.
Demographic characteristic of OA cohort study
| Groups | n | Median Age | Female/Male | Median BMI | Race (White/Black/Others) |
|---|---|---|---|---|---|
| Controls | 21 | 51 (31–67) | 12/9 | 21.4 (19.1–24.7) | 14/5/2 |
| OA | 57 | 64 (39–81) | 39/18 | 26.8 (15.4–32.6) | 24/19/14 |
Rheumatoid Arthritis Cohort
Samples from patients affected by RA, who met the ACR criteria[23], were collected at the Departmental Tissue Bank of the Division of Rheumatology. Patients were evaluated using the Disease Activity Score (DAS-28)[24]. We selected serum samples from sixty-one RA patients that were not prescribed any DMARDs at the time of blood collection. In addition, sera from RA patients at baseline and after three or five months treatment were also collected, thirty-three pairs of samples from RA patients treated with TNF inhibitors (etanercept, infliximab or adalimumab) and eighteen pairs of samples from RA patients treated with methotrexate (MTX) were obtained and stored at −80°C. Table 2 describes the demographic and clinical characteristics of RA patients. All patient samples were approved for use in this study by the Human Research Ethics Board of New York University Medical Center.
Table 2.
Demographic characteristic of RA cohort study
| Groups | n | Median Age | Gender (F/M) | Median DAS28 (Baseline) | Median DAS28 (post-treatment) |
|---|---|---|---|---|---|
| Drug Naïve | 61 | 47 (18–73) | 51/10 | 5.56 (1.88–7.85) | N/A |
| TNF-inhibitor | 33 | 51 (25–79) | 31/2 | 5.60 (1.67–8.40) | 5.04 (1.54–7.88) |
| Methotrexate | 18 | 45 (18–65) | 12/6 | 5.36 (3.62–7.82) | 3.95 (2.02–6.37) |
Construction of the expression plasmids encoding His-tagged human and mouse COMP type III domain
The DNA fragment encoding human COMP type III domain was generated by 3 cycles of PCR using a set of primers: forward primer P1 (5′-gcgcgtccggacgcgacactgacctagac-3′), forward primer P2 (5′-gcgttcttctgctcaccctggctgccctcggcgcgtccggacgcga-3′), forward primer P3 (5′-aggatccactatggtccccgacaccgcctgcgttcttctgctcacc-3′), reverse primer (5′-actcgaggagcgtgacttcagcgttc-3′). For the mouse COMP type III domain construct, mouse cDNA was used as template and the following primers were used: forward primer P1 (5′-gggcgacaggccgcgacacggacctgga-3′), forward primer P2 (5′-gcgttctagtgctcgccctggctatcctgc gggcgacaggccgcga-3′), forward primer P3 (5′-aggatccatgggccccactgcctgcgttctagtgctcgcc-3′), reverse primer (5′-cctcgagctcggggcacacatcgatct-3′). BamH I and Xho I sites are underlined and the italic letters represent three parts of the signal peptide sequence of COMP. After 3 cycles, these sequences were joined together to generate the intact N-terminal signal peptide, which facilitated the recombinant protein secretion into the culture medium. The amplified DNA fragment was ligated into the BamH I and Xho I sites of pCDNA3.1-myc-his A (Invitrogen).
Expression and purification of recombinant COMP type III in HEK 293EBNA cells
HEK 293EBNA cells were transfected with 20 μg DNA using Lipofectamine 2000 (Invitrogen). The stable lines were generated by G418 (Sigma) selection. The serum-free conditioned medium was mixed with Probond™ Nickel-Chelating Resin (Invitrogen). The bound proteins in the washed resin were eluted with 250 mM Imidazole. The secretion of recombinant protein was confirmed by SDS-PAGE. Additionally, the recombinant proteins were analyzed by Western blotting using a rabbit anti-COMP polyclonal antiserum (pAb) or anti-COMP type III monoclonal antibody (mAb) 2127F5, respectively.
Generation and partial characterization of mAbs against the COMP type III domain
Female Balb/c mice were immunized with the recombinant type III domain of COMP (aa 266–526) and hybridomas were produced according to standard procedures. Procedures for spleen fusion and clone selection were as previously described[25].
Recombinant human COMP was incubated with MMP13 catalytic domain (Abcam) or purified catalytic domain of ADAMTS-7 in digestion buffer (50 mM Tris-HCl, 100 mM NaCl, 5 mM CaCl2, 2 mM ZnCl2, and 0.05% Brij-35, pH 7.5, substrate and enzyme molar ratio 6:1) at 37°C for 16 hrs. The digested products were analyzed by Western blotting using selected mAbs or the pAb against COMP.
Epitope mapping of anti-COMP mAb 2127F5
An overlapping peptide library for human COMP type III domain was designed and generated by Genscript Inc (Piscataway, New Jersey). Each peptide of the library was 15 amino acid long with 10 amino acids overlapping with its adjacent peptide. All cysteines in the peptides were replaced with serine. A total of 51 biotinylated overlapping peptides were synthesized and screened by ELISA with mAb 2127F5.
Immunoblotting for COMP in serum
A volume of 10 μl of hyaluronidase (100 U in 0.05 M sodium acetate buffer, pH 5.8) from bovine testis (Sigma) was added to 50 μl of serum from normal control, OA patients, and RA patients. After incubation at 37°C for 2 hrs, samples were precipitated by adding 1 volume of 100% Trichloroacetic acid to 4 volumes of serum, and incubated on ice for 20 min. The supernatant was spun and the pellet washed first with ice-cold acetone twice and then dissolved in non-reducing SDS-PAGE loading buffer. Soluble material was subjected to SDS-PAGE and detected with either the anti-COMP pAb or mAb 2127F5.
Immunohistochemistry
Specimens of articular cartilage from OA and RA patients and normal controls were fixed in 4% PBS buffered PFA at 4°C overnight and then decalcified in 10% EDTA for 4 weeks. After blocking, tissue sections were incubated with mAb 2127F5 at 4°C overnight, followed by incubation with a biotinylated secondary anti-mouse IgG for 1 hr at room temperature. Following intermittent rinses in PBS, avidin-biotin-peroxidase complex (Vector Elite ABC Kit; Vectastain) was applied for 1 hr and signal detection was performed by using diaminobenzidine for 10 min (Sigma-Aldrich) as the substrate, followed by a counterstaining with methyl green.
Sandwich ELISA
Briefly, the anti-COMP pAb[26] was used as a capture antibody, whereas mAb 2127F5 was used as a detection antibody. Ninety-six well ELISA plates were coated with 50 μl/well of anti-COMP pAb diluted with PBS to 2 μg/ml and kept overnight at 4°C. Coated wells were washed with PBST (PBS with 0.5% Tween-20) and blocked with 5% BSA (w/v) in PBS for 1.5 hr at room temperature. Human or mouse type III standards (6.25, 12.5, 25, 50, 100 ng/ml) and serum samples (1/50 dilution for human, 1/10 for mouse) diluted with 0.5% BSA in PBST were transferred to BSA pre-saturated wells at 100 μl/well and incubated for 2 hrs at room temperature. Plates were washed with PBST and 100 μl of diluted HRP conjugated detection antibody (diluted to 1 μg/ml with 0.5%BSA in PBST) was added. The plates were incubated for 2 hrs at room temperature and washed with PBST. Peroxidase substrate TMB solution (eBioscience) was applied to plates at 100 μl/well. The plates were incubated for 20 min and color development stopped by 100 μl 2M sulfuric acid. The COMP concentrations in the serum were calculated from the linear range of a standard curve. To determine intra- and inter-assay variance, six fresh aliquots of one serum sample on each plate were stored at −80°C. Each day a plate was thawed and measured with six repeats on one plate for six consecutive plates.
For comparison, serum COMP levels of twenty-one normal controls and fifty-seven OA patients were measured by Cartilage Oligomeric Matrix Protein Human ELISA kit (BioVendor) according to the manufacturer’s protocol.
Arthritis animal models
An instability-induced OA model was created in 8-week-old C57BL/6 mice. The animals were anesthetized and the right knee joint was destabilized by transection of the medial meniscotibial ligament followed by removal of the cranial half of the medial meniscus. Control mice were sham-operated using the same approach but without any ligament transection or meniscectomy. Mice were kept for an additional 12 weeks after surgery. For generation of a collagen-induced arthritis (CIA) model, eight-weeks old male DBA1/J mice were immunized via a 0.1 ml intradermal injection at the base of the tail with 100 μg chicken type II collagen (Chondrex) emulsified with an equal volume of complete Freund’s adjuvant (Chondrex). Four weeks after the first immunization, 2.5 mg/kg etanacept, rhPGRN, or Atsttrin were administered intraperitoneally twice every week, and the COMP fragment levels were measured by ELISA 4 weeks after treatment.
TNF-Tg mice with mild (clinical score 4–6) arthritis were administered intraperitoneal PBS or Atsttrin (2.5 mg/kg body weight) twice per week. The clinical score and serum COMP fragment levels were determined every week post-treatment for 4 weeks.
All animal studies were performed in compliance with the Institutional Animal Care and Use Committee approved protocols.
Statistical Analysis
The non-parametric Mann-Whitney test (two-tailed) was used to compare COMP serum levels in two unpaired groups, and the Wilcoxon’s matched pairs test (two-tailed) was used to compare the results of baseline and end COMP levels in individual patients. Correlations were assessed using the Spearman’s correlation coefficient. Associations between continuous and qualitative variables were evaluated by the analysis of variance (ANOVA). All P values were two sided, and the P value <0.05 was considered to be statistically significant. All statistics were carried out using SPSS version 11.0 (SPSS Inc., Chicago, IL).
RESULTS
mAb 2127F5 recognizes degradation fragments of COMP present in serum of arthritic patients
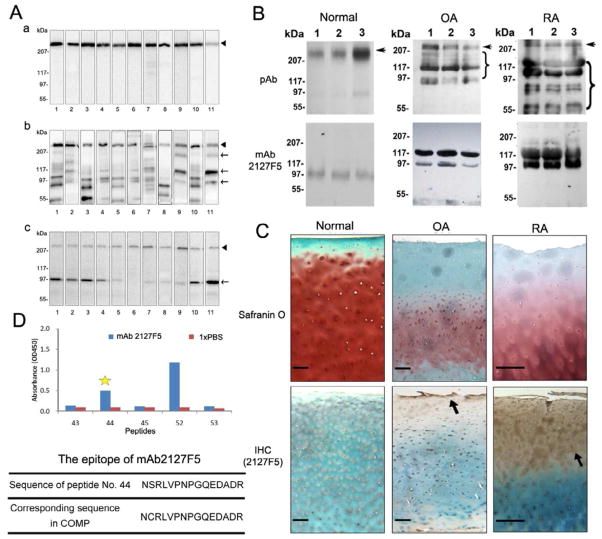
An immunoblotting screening was performed in order to identify mAbs that might preferentially recognize degradation products of COMP. As shown in the panel “a” of Fig. 1A, ten of selected anti-COMP mAbs recognized intact human COMP, while mAb 2127F5 exhibited lower reactivity towards intact COMP; however this mAb showed enhanced reactivity against fragmented COMP produced by in vitro digestion with either MMP13 (Fig. 1A, panel b) or ADAMTS-7 (Fig. 1A, panel c). mAb 2127F5 was then selected for detection of COMP fragments in the serum of patients with arthritis (Fig. 1B). The major immunoactive COMP fragments recognized by mAb 2127F5 had apparent Mr:s ranging from 97 kDa to 117 kDa, whereas the anti-COMP pAb recognized a broad range of COMP polypeptides ranging from intact pentamers (>500 kDa) to degraded lower molecular weight fragments. Immunohistochemistry carried out on human normal, OA, and RA articular cartilage (Fig. 1C) revealed that COMP was enriched in the peri-cellular matrices through the whole middle zone of normal cartilage, while it exhibited a substantially different expression and distribution pattern in arthritic cartilage. COMP fragments were heavily immunolocalized and showed the most abundant deposition in the superficial zone of OA cartilage. Accumulation of COMP fragments was seen in both the superficial and middle zones of RA cartilage. Peptide-based mapping of using a purposely designed peptide library (Supplementary Data) pinpointed the epitope sequence recognized by mAb 2127F5 at amino acids 483–497 (NCRLVPNPGQEDADR) of COMP (Fig. 1D).
Figure 1. Characteristics of the anti-COMP type III monoclonal antibody, mAb 2127F5.
(A) Characterization of the COMP monoclonal antibodies using in vitro digestion assay. Intact COMP (a), or digested with either MMP13 catalytic domain (b) or ADAMTS-7 catalytic domain (c), were resolved in 10% non-reduced SDS-PAGE and detected with a panel of antibodies against COMP (1, rabbit pAb; 2–11, mAb: HC487E10H6, HCR6G5E12, HC644A8, HC8H7, HC490H10C8, H483A10G11, 2111A6, 2117B2, 2130E2, 2127F5). Arrow heads represent the intact COMP, arrows indicate the digestive fragments. (B) Determination of the efficacy of mAB 21275 in the detection of COMP fragments by Western blotting. The serum samples from three each healthy donors, osteoarthritis (OA), and rheumatoid arthritis (RA) patients were first digested by hyaluronidase and precipitated by TCA. They were then resolved in a 4–15% non-reducing gradient gel, and visualized by immunoassay using rabbit anti-COMP pAb or anti-COMP typeIII mAb2127F5. Arrows indicate the full length COMP and braces indicate degraded COMP fragments. (C) Histological and immunostaining assay for COMP of normal, OA, and RA cartilage. The cartilage specimens were assayed using Safranin O-fast green staining, and the immunohistochemistry for COMP using the mAb2127F5B6. Representative pictures are shown and black arrows indicate the expression of COMP in the cartilage. Scale bars=100μm. (D) Epitope mapping of the mAb 2127F5. 51 peptides were synthesized and tested by ELISA using 2127F5, No.44 peptide showed the positive signal, No.52 and No. 53 were positive control and negative control respectively (For each library peptide reaction to mAb2127F5, see Supplementary Data).
Sandwich ELISA based upon mAb 2127F5 reveals a significant increase of COMP degradation products in the serum of OA and RA patients
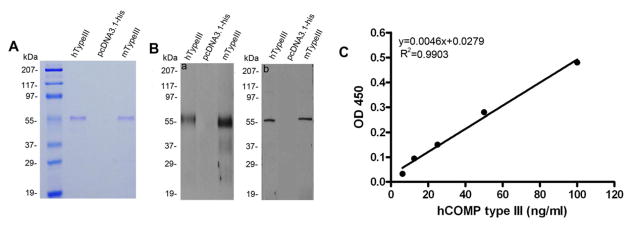
In order to develop an ELISA procedure for specific assessments of circulating COMP fragments, we generated expression plasmids encoding the human and mouse type III domain of COMP. Purified type III domains were examined by SDS-PAGE (Fig. 2A) and were confirmed to exhibit an apparent Mr of 55 kDa, which was much higher than the predicted Mr of 29 kDa and may presumably be associated with post-translational modifications characterizing COMP [6, 27]. In addition, identity of these recombinant type III proteins was further verified by Western blotting using both the anti-COMP pAb and mAb 2127F5 (Fig. 2B). A sandwich ELISA was then devised using the recombinant COMP type III fragments as a standard. A representative typical standard curve is shown in Fig. 2C, having a linear range of 10–100 ng/ml. The overall intra-assay and inter-assay coefficient of variation were estimated to be 7.2% and 8.7%, respectively. To validate our ELISA protocol, serum COMP levels from 57 patients diagnosed with OA and 21 healthy controls were comparatively measured with our assay and a commercial COMP ELISA kit. While the latter did not detect significant differences in COMP levels between symptomatic knee OA (OA) and non-OA control groups (P=0.128), a significant increase of the COMP fragments was noted in the serum of OA patients assayed with our ELISA setting (P=0.005) (Fig. 3A). In addition to OA, the COMP serum level in RA patients measured with our sandwich ELISA was significantly higher compared with healthy controls (2390 ng/ml (95CI: 2124.7 to 2655.3 ng/ml) vs 153.5 ng/ml (95CI: 120.5 to 188.9 ng/ml), P<0.0001; Fig. 3C). According to these data, the sensitivity of this newly established ELISA protocol for distinguishing RA from healthy individuals was 87.72%, while the specificity of the assay could reach to 100%.
Figure 2. Characterization of recombinant COMP type III domain and the establishment of the sandwich ELISA using recombinant COMP type III as a standard.
(A) The HEK293-EBNA cells were transfected with either expression plasmid encoding His-tagged human COMP type III (lane 1), His-tagged mouse COMP type III (lane 3), or empty vector as a control (lane 2) and the conditioned media collected. Purified recombinant proteins using Ni-NTA resin was separated by non-reducing 10% SDS-PAGE gel and visualized with Coomassie Brilliant blue staining. (B) After purification with Ni-NTA resin, the expression of recombinant human COMP type III (lane 1) and mouse COMP type III (lane 3) was verified by western blot using anti-COMP pAb (a) or anti-COMP mAb2127F5 (b). No band was detected in the control media (lane 2). (C) The standard curve was generated by the serial concentration of diluted recombinant human COMP type III plotted against the OD450 value; the blank has been subtracted from all the absorbances.
Figure 3. COMP fragment levels were gradually elevated in the progression of both human OA and RA.
(A) Measurements of serum COMP levels from OA patients and normal controls using both the Commercial ELISA kit and the newly established sandwich ELISA. The bottom and top of the box indicating values between 2.5th and 97.5th percentile, the horizontal band in the box represents the median value, the ends of the whiskers represent 10th and 90th percentile, respectively. The P value was calculated when compared with the normal group. (B) Adjusted serum COMP levels (ln scale) were correlated with the Kellgren&Lawrence grade score in OA patients and normal controls: K&L grade 0 group (n=21); K&L grade 1 group (n =31); K&L grade 2 group (n =29); grade 3 group (n =35); and K&L grade 4 group (n =32). The whiskers indicates the minimum and maximum values, the box indicating values between 2.5th and 97.5th percentile, the horizontal band in the box represents the median value, *P=0.046, **P=0.009, ***P<0.001 when compared with K&L grade 0 group, ANOVA followed by post hoc Dunnett’s multiple comparison test. (C) Serum COMP level was markedly higher in RA patients (n=60) compared with the normal control (n=21). ***P<0.001. The COMP levels increased in the progression of OA in the mice model. Surgical OA was induced in wild-type C57BL/6 mice at the age of 8 weeks and the knee joints harvested 4, 8, and 12 weeks after surgery (n = 6–8 mice per group). Articular cartilage loss was evaluated by the modified Mankin Score, and the serum COMP levels were determined pre-surgery and 4 weeks, 8 weeks, and 12 weeks after surgery. Sham-operated mice were used as control. Error bars show 95% Confidence Interval(CI).., ***P<0.001 when compared with sham group at matched time point.
Serum levels of COMP fragments correlate with the Kellgren & Lawrence grade score in OA patients and with the progression of surgically induced OA in murine models
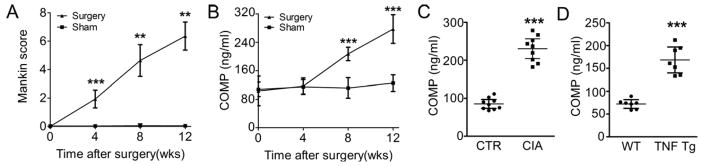
To better explore the potential of COMP as a biomarker of clinical utility, OA patients were divided into five groups according to the radiological changes of their joints using the Kellgren and Lawrence (K&L) grading scale and the serum COMP levels were assessed with our ELISA protocol. As shown in Fig. 3B, patients with higher K&L grades also displayed significantly higher serum COMP levels. Importantly, our COMP ELISA was also able to detect murine COMP fragments in operated mice showing gradual articular cartilage loss due to the instability of the knee joint. In these mice, the change in histology was indicated by the modified Mankin Score (Fig. 4A), while sham-operated mice remained unaffected. In these mice OA models, the serum COMP levels were determined at different time points after surgery; the experimental group showed significantly higher levels of COMP at 8 and 12 weeks post surgery compared with the control, while COMP levels remain unchanged in sham-operated mice (Fig. 4B).
Figure 4. COMP fragment levels were significantly increased in mouse surgically-induced osteoarthritis model, the mouse collagen-induced arthritis (CIA) model, and TNFα transgenic mice.
(A, B) The COMP levels increased in the progression of OA in the mice model. Surgical OA was induced in wild-type C57BL/6 mice at the age of 8 weeks and the knee joints harvested 4, 8, and 12 weeks after surgery (n = 6–8 mice per group). Articular cartilage loss was evaluated by the modified Mankin Score, and the serum COMP levels were determined pre-surgery and 4 weeks, 8 weeks, and 12 weeks after surgery. Sham-operated mice were used as contro, **P<0.01, ***P<0.001 when compared with sham group at matched time point. (C)Eight-weeks-old DBA1/J mice were immunized with chicken collagen II to induce inflammatory arthritis, and serum COMP was measured in CIA and PBS control groups 15 weeks after primary immunization (n=9). (D) TNFα transgenic mice spontaneously started to develop inflammatory arthritis at the age of 14 weeks. The serum was harvested and the COMP level was determined. n=7 for each group, Error bars show 95% Confidence Interval.,***P<0.001.
Alterations in serum levels of COMP fragments in mice with collagen-induced arthritis, and TNF-α transgenic mice
The CIA mouse model[28, 29] shares both immunological and pathological features with human rheumatoid arthritis and we therefore analyzed in a similar manner COMP levels in the CIA model. As indicated in Fig. 4C, significantly increased COMP levels were observed in the CIA group compared to the PBS control group at 4 weeks after the first immunization. TNF-Tg mice are known to develop an inflammatory arthritis phenotype spontaneously[30, 31]. COMP fragment levels were also significantly higher in those mice compared with the wild type control mice (Fig. 4D).
Treatments with TNFα inhibitors and methotrexate lead to a significant decrease of COMP fragmentation detected in the blood of RA patients
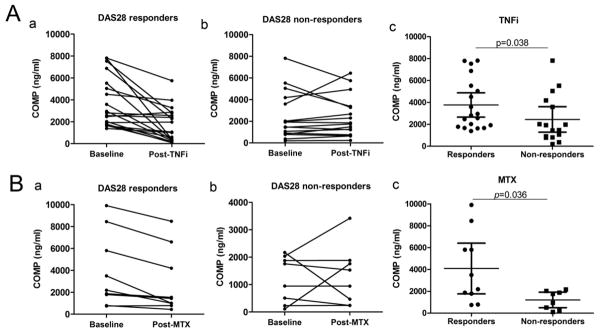
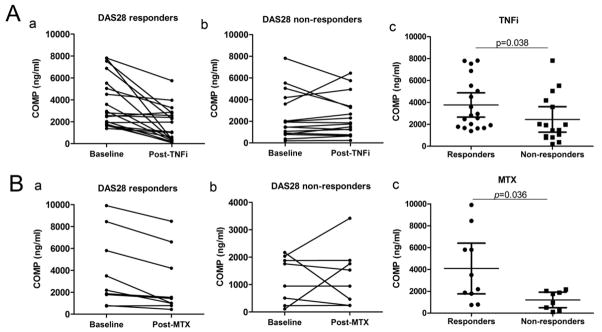
In order to determine whether alterations in the serum COMP level can be used for monitoring the efficacy of therapeutic agents administered to RA patients, a total of 33 RA patients who were treated with TNF-a inhibitors were examined for their serum levels of COMP. All the RA patients in the follow-up study were divided into two groups: responders and non-responders according to European League Against Rheumatism (EULAR) DAS 28 response criteria[24]. 19 responders showed significant reduction in COMP levels (Fig. 5A panel a, P=0.0004), while in the non-responders group (n=14) no significant difference of COMP levels was detected (Fig. 5A panel b). Ten RA patients classified as responders treated with methotrexate (MTX) for a period of 5 months exhibited a significant reduction in COMP levels compared with the baseline level (Fig. 5B panel a, P=0.008) and no significant change of COMP levels was seen in non-responders (n=8, Fig. 5B, panel b). In addition, the basal levels of COMP fragments in responders were significantly higher than those in non-responders (Fig. 5A, B panel c, P=0.038 and 0.036 respectively).
Figure 5. The serum COMP fragment levels at baseline and post treatment with TNFα inhibitors and methotrexate (MTX) in RA patients.
(A) Patients diagnosed with RA were treated with TNFα inhibitors (TNFi) (n=33); after 12 weeks of treatment, patients were divided into responders and non-responders group, each individual’s serum COMP level was compared with the baseline level (panel a, b, P=0.0004 and 0.884 respectively). The serum COMP levels at baseline between the two groups were compared (panel c, P=0.038). (B) For RA patients treated with MTX (n=18) for 5 months, serum COMP levels were compared with the baseline level and the treatment end (panel a, b, P=0.008 and 0.782 respectively). The serum COMP levels at baseline between the two groups were compared (panel c, P=0.036). Error bars show 95% Confidence Interval.
Administration of progranulin and its derived Atsttrin also significantly reduces serum levels of COMP fragments in inflammatory arthritis mice models
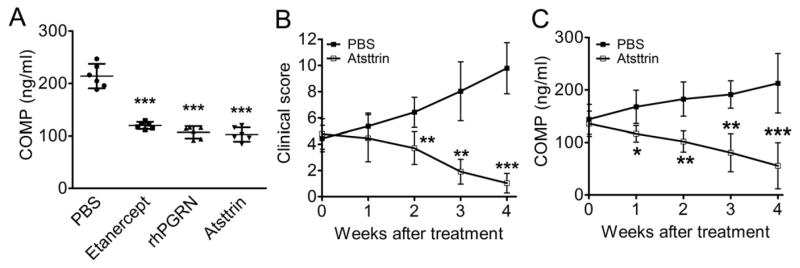
We recently reported that the growth factor progranulin (PGRN) binds to TNF receptors and is therapeutic against inflammatory arthritis[32]. Importantly, Atsttrin, an engineered protein composed of three PGRN fragments, potently prevents inflammation in multiple arthritis mouse models[32]. We then sought to determine whether the treatment efficacy of Atsttrin also correlated to the levels of COMP fragments. Mice with CIA were treated with etanercept, recombinant human PGRN (rhPGRN), Atsttrin, or PBS as a control, and COMP levels were measured 4 weeks after treatment. As shown in Fig. 6A, the three treatment groups showed markedly reduced COMP levels compared with the PBS group. We also used TNFα transgenic mice that spontaneously develop inflammatory arthritis. TNFα transgenic mice at a clinical score between 4 and 6 were treated with Atsttrin, and COMP fragment levels were determined every week post treatment for 4 weeks. The PBS group showed increasingly severe inflammation as indicated by clinical score, while inflammation in the Atsttrin-treated group was reversed (Fig. 6B). COMP levels gradually decreased along with the administration of Atsttrin, while the PBS group showed significantly increasing levels of COMP (Fig. 6C).
Figure 6. Mice with established inflammatory arthritis were treated with PGRN or its derived protein Atsttrin.
(A) Mice with CIA were treated with 2.5mg/kg of etanacept, rhPGRN, or Atsttrin twice a week, and the COMP fragment levels were measured by ELISA 4 weeks after treatment, ***P<0.0001 versus the control PBS group. (B) TNFα transgenic mice with a clinical score between 4 and 6 were treated with 2.5mg/kg bodyweight of Atsttrin, twice a week. The clinical score and COMP fragment levels were determined every week post treatment for 4 weeks. n=6 each group, Error bars show 95% Confidence Interval, *P<0.05, **P<0.01, ***P<0.001 versus the control PBS group.
DISCUSSION
COMP shows great promise as a biological marker of cartilage metabolism in arthritis[10, 33] and increased fragmentation of COMP has been reported in patients with arthritis and joint injury[9, 19]. The monitoring of COMP levels in synovial fluid or serum has been suggested to be a helpful method for assessing the presence and progression of arthritis. Significant efforts have therefore been undertaken to design sensitive and highly specific serological procedures for the measurements of COMP released into synovial fluid or serum. Numerous antibodies against COMP have been generated and different ELISA protocols for assessing body fluid levels of COMP have been described during the years and in many cases made commercially available. pAbs and mAbs used in those assays were in most cases raised against intact COMP[9, 19] and were suboptimal since they apparently exhibit a low affinity for proteolytic fragments of COMP, or entirely fail to recognize epitopes within critically important regions of the COMP molecule prone to degradation during cartilage destruction. We thus generated a unique panel of anti-COMP mAbs by immunizing mice with recombinant fragments corresponding to distinct COMP domains. One such mAb, denoted 2127F5 and directed against the COMP type III domain, was found to show higher affinity against a COMP fragment found in human serum and containing this domain than against the intact molecule. We then took advantage of this unique reactivity and utilized this immunological reagent to develop a novel assay to quantify proteolytic fragments of COMP circulating in body fluids. Epitope mapping of this mAb showed it recognized a 15 amino acid-long sequence within the type III domain of COMP. Although intact COMP also contains this epitope, it is plausible that the mAb may more readily access its epitope in fragmented COMP because of a possible unfolding of the epitope upon degradation of the pentameric COMP molecule or due to conformation changes of proteolytic fragments (Fig. 1). A more profound understanding of the modes of binding and antigen affinity modulation of mAb 2127F5 remains to be acquired by more detailed proteomic analysis of the protein region containing its epitope. In addition, whether serum peptides and proteins not yet detected may bind to this antibody and influence the assay signal in a yet undetermined way remains to be delineated.
The majority of COMP released into the serum of healthy people is in the intact form, while catabolism of COMP is significantly increased in patients with either OA or RA. We find no significant difference between serum COMP levels in OA using a COMP detection kit from BioVendor (Karasek, Czech Republic), whereas previous reports using the same antibody pair as that composing the Biovendor kit revealed that serum COMP levels were increased in OA patients and correlated with the OA progression[34, 35], as well as that higher baseline levels of COMP correlated with incident OA[36]. This discrepancy may be due to the limited sample size of the study or to a different compositions of the examined patient cohorts[34]. The COMP ELISA protocol developed here reveals a significant difference in serum COMP levels between arthritic patients and non-arthritic control subjects (Fig. 3). In addition, the serum levels of COMP were even higher in patients with RA than those with OA (Fig. 3), which probably resulted from the different degree and pattern of articular cartilage degradation by various inflammatory cytokines and proteolytic enzymes operating in the pathogenesis of OA and RA.
Using our method, we found a significant correlation between levels of serum COMP fragments and the K&L grade (Fig. 3). Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index, which quantifies the pain, stiffness, and limitation of function in OA patients, is another frequently used scoring system. However, no significant correlation between the serum COMP levels and the WOMAC score was observed. This is probably due to the fact that the WOMAC score only reflects the degree of symptoms, which not necessarily correlates with the degree of degeneration of the articular cartilage. Our findings suggest that the serum levels of COMP fragmentation are a direct indicator of cartilage degradation in OA and that this novel sandwich COMP ELISA has the full potential for being employed as an accurate and reproducible test for the clinical evaluation of OA patients. It is worthwhile to note that this approach can also be used for detecting the COMP fragment level in the mouse models of surgically-induced OA, collagen-induced RA, and TNF transgenic inflammatory arthritis (Figs. 4 and 6), while most available ELISA kits only work for certain species. Thus, this provides another level of utility of the assay which, accordingly, can be exploited for basic and applied research on arthritis, especially that involving drug development.
Methotrexate (MTX) has been a traditional, standard disease-modifying anti-rheumatic drug (DMARD) for patients who have moderate to severe rheumatoid arthritis. New generation biological drugs, which are represented by TNFα inhibitors, such as etanercept, infliximab, and adalimumab, have been recently adopted as the more effective anti-inflammatory therapeutics for treatment of arthritis[37]. Efficacious use of conventional and novel anti-TNF drugs in RA therapy strongly rely upon the identification of precise prognostic factors capable of predicting the disease course of RA and monitor the therapeutic response of patients undergoing such therapeutic treatments. In this study, we found that 39% and 53%, respectively, of the patients treated with MTX and anti-TNF drugs showed a significant reduction in COMP fragment levels (Fig. 5). These findings were in accordance with a recent report revealing that COMP level in RA decreases after therapy with TNF inhibitors[38]. In addition, the higher serum levels of COMP fragments at the baseline indicated the good response to treatment, suggesting that serum abundance of COMP fragments can be a useful indicator for the evaluation of the disease progression and in monitoring the therapeutic response at an early stage in RA treatment. They may therefore be valuable in the stratification of arthritic patients according to treatment-independent disease progression and responsiveness to treatment and, hence, assist the clinician in the choice of more individualized treatment protocols. This notion is in part supported by the reported higher COMP levels detected in sera predicted responders to TNF inhibition therapy[39]. Interestingly, Atsttrin, an engineered protein derived from progranulin growth factor and known to have potent anti-inflammatory activity in mice[32], also effectively inhibited the specific COMP degradation measured with our ELISA protocol in the course of inflammatory arthritis in mice (Fig. 6). The full clinical utility of our immune-serological assay and its spectrum of applicability in the management of arthritic patients will be determined by forthcoming larger case studies.
Supplementary Material
No.1–51 represent the 51 overlapping peptides, No. 52 and 54 are positive controls, No.53 are negative controls.
Acknowledgments
This work was funded by NIH research grants AR061484, AR053210 (to C. J. Liu.), and AR052873 (to SB Abramson) and institutional funds of the National Cancer Center, CRO-IRCCS, in Aviano, Italy.
Abbreviations
- COMP
cartilage oligomeric matrix protein
- ELISA
Enzyme-linked immunosorbent assay
- OA
osteoarthritis
- RA
rheumatoid arthritis
- ECM1
Extracellular matrix protein 1
- ADAMTS
A Disintegrin And Metalloproteinase with Thrombospondin Motifs
- MTX
methotrexate
- TNF-α
Tumor Necrosis Factor α
- IL-1β
interleukin-1β
- PGRN
progranulin
- Atsttrin
antagonist of TNF/TNFR signaling via targeting to TNF receptors
- K&L grade
Kellgren & Lawrence grade
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
- DAS28
Disease Activity Score including a 28-joint count
Footnotes
Author contributions
Study concept and design: Chanju Liu, Yongjie Lai
Acquisition of data: Yongjie Lai
Analysis and interpretation of data: Yongjie Lai, Yuying Zhang, Qingyun Tian, Haicheng Song
Human sera samples provider: Mukundan Attur, Steven Abramson, Jonathan Samuels, Svetlana Krasnokutsky, Jeffrey D. Greenberg
Monoclonal antibodies preparation and characterization: Maria Teresa Mucignat, Roberto Perris
Statistical analysis: Yongjie Lai
Drafting of the manuscript: Yongjie Lai, Chuanju Liu, Xiuping Yu, Paul E. Di Cesare
Conflict of interest:
We herein declare that we have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huskisson EC. Nimesulide, a balanced drug for the treatment of osteoarthritis. Clin Exp Rheumatol. 2001;19(1 Suppl 22):S21–5. [PubMed] [Google Scholar]
- 2.Gouze JN, Ghivizzani SC, Gouze E, Palmer GD, Betz OB, Robbins PD, et al. Gene therapy for rheumatoid arthritis. Hand Surg. 2001;6(2):211–9. doi: 10.1142/s0218810401000709. [DOI] [PubMed] [Google Scholar]
- 3.Campbell JR. Nonfracture injuries to the canine elbow. J Am Vet Med Assoc. 1969;155(5):735–44. [PubMed] [Google Scholar]
- 4.Lukoschek M, Schaffler MB, Burr DB, Boyd RD, Radin EL. Synovial membrane and cartilage changes in experimental osteoarthrosis. J Orthop Res. 1988;6(4):475–92. doi: 10.1002/jor.1100060403. [DOI] [PubMed] [Google Scholar]
- 5.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460–6. [PubMed] [Google Scholar]
- 6.DiCesare P, Hauser N, Lehman D, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994;354(2):237–40. doi: 10.1016/0014-5793(94)01134-6. [DOI] [PubMed] [Google Scholar]
- 7.Hedbom E, Antonsson P, Hjerpe A, Aeschlimann D, Paulsson M, Rosa-Pimentel E, et al. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267(9):6132–6. [PubMed] [Google Scholar]
- 8.Di Cesare PE, Carlson CS, Stolerman ES, Hauser N, Tulli H, Paulsson M. Increased degradation and altered tissue distribution of cartilage oligomeric matrix protein in human rheumatoid and osteoarthritic cartilage. J Orthop Res. 1996;14(6):946–55. doi: 10.1002/jor.1100140615. [DOI] [PubMed] [Google Scholar]
- 9.Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Hauselmann HJ. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol. 1997;36(11):1151–60. doi: 10.1093/rheumatology/36.11.1151. [DOI] [PubMed] [Google Scholar]
- 10.Saxne T, Heinegard D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992;31(9):583–91. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson SC, Vankemmelbeke MN, Buttle DJ, Rosenberg K, Heinegard D, Hollander AP. Cleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifs. Matrix Biol. 2003;22(3):267–78. doi: 10.1016/s0945-053x(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 12.Ganu V, Goldberg R, Peppard J, Rediske J, Melton R, Hu SI, et al. Inhibition of interleukin-1alpha-induced cartilage oligomeric matrix protein degradation in bovine articular cartilage by matrix metalloproteinase inhibitors: potential role for matrix metalloproteinases in the generation of cartilage oligomeric matrix protein fragments in arthritic synovial fluid. Arthritis Rheum. 1998;41(12):2143–51. doi: 10.1002/1529-0131(199812)41:12<2143::AID-ART9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Stracke JO, Fosang AJ, Last K, Mercuri FA, Pendas AM, Llano E, et al. Matrix metalloproteinases 19 and 20 cleave aggrecan and cartilage oligomeric matrix protein (COMP) FEBS Lett. 2000;478(1–2):52–6. doi: 10.1016/s0014-5793(00)01819-6. [DOI] [PubMed] [Google Scholar]
- 14.Liu CJ, Kong W, Ilalov K, Yu S, Xu K, Prazak L, et al. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. Faseb J. 2006;20(7):988–90. doi: 10.1096/fj.05-3877fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CJ, Kong W, Xu K, Luan Y, Ilalov K, Sehgal B, et al. ADAMTS-12 associates with and degrades cartilage oligomeric matrix protein. J Biol Chem. 2006;281(23):15800–8. doi: 10.1074/jbc.M513433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohmander LS, Saxne T, Heinegard DK. Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann Rheum Dis. 1994;53(1):8–13. doi: 10.1136/ard.53.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersson IF, Boegard T, Svensson B, Heinegard D, Saxne T. Changes in cartilage and bone metabolism identified by serum markers in early osteoarthritis of the knee joint. Br J Rheumatol. 1998;37(1):46–50. doi: 10.1093/rheumatology/37.1.46. [DOI] [PubMed] [Google Scholar]
- 18.Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegard D, et al. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995;34(4):306–10. doi: 10.1093/rheumatology/34.4.306. [DOI] [PubMed] [Google Scholar]
- 19.Forslind K, Eberhardt K, Jonsson A, Saxne T. Increased serum concentrations of cartilage oligomeric matrix protein. A prognostic marker in early rheumatoid arthritis. Br J Rheumatol. 1992;31(9):593–8. doi: 10.1093/rheumatology/31.9.593. [DOI] [PubMed] [Google Scholar]
- 20.Kraus VB, Huebner JL, Fink C, King JB, Brown S, Vail TP, et al. Urea as a passive transport marker for arthritis biomarker studies. Arthritis Rheum. 2002;46(2):420–7. doi: 10.1002/art.10124. [DOI] [PubMed] [Google Scholar]
- 21.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 22.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35(4):745–57. vii–viii. doi: 10.1016/j.rdc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Kong L, Tian Q, Guo F, Mucignat MT, Perris R, Sercu S, et al. Interaction between cartilage oligomeric matrix protein and extracellular matrix protein 1 mediates endochondral bone growth. Matrix Biol. 2010;29(4):276–86. doi: 10.1016/j.matbio.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiCesare PE, Morgelin M, Carlson CS, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein: isolation and characterization from human articular cartilage. J Orthop Res. 1995;13(3):422–8. doi: 10.1002/jor.1100130316. [DOI] [PubMed] [Google Scholar]
- 27.DiCesare PE, Morgelin M, Mann K, Paulsson M. Cartilage oligomeric matrix protein and thrombospondin 1. Purification from articular cartilage, electron microscopic structure, and chondrocyte binding. Eur J Biochem. 1994;223(3):927–37. doi: 10.1111/j.1432-1033.1994.tb19070.x. [DOI] [PubMed] [Google Scholar]
- 28.Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9(10):1245–50. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 29.Deng GM, Zheng L, Chan FK, Lenardo M. Amelioration of inflammatory arthritis by targeting the pre-ligand assembly domain of tumor necrosis factor receptors. Nat Med. 2005;11(10):1066–72. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]
- 30.Li P, Schwarz EM. The TNF-alpha transgenic mouse model of inflammatory arthritis. Springer Semin Immunopathol. 2003;25(1):19–33. doi: 10.1007/s00281-003-0125-3. [DOI] [PubMed] [Google Scholar]
- 31.Thwin MM, Douni E, Aidinis V, Kollias G, Kodama K, Sato K, et al. Effect of phospholipase A2 inhibitory peptide on inflammatory arthritis in a TNF transgenic mouse model: a time-course ultrastructural study. Arthritis Res Ther. 2004;6(3):R282–94. doi: 10.1186/ar1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ, Liu GY, et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science. 2011;332(6028):478–84. doi: 10.1126/science.1199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mansson B, Carey D, Alini M, Ionescu M, Rosenberg LC, Poole AR, et al. Cartilage and bone metabolism in rheumatoid arthritis. Differences between rapid and slow progression of disease identified by serum markers of cartilage metabolism. J Clin Invest. 1995;95(3):1071–7. doi: 10.1172/JCI117753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan JM, Luta G, Stabler T, Renner JB, Dragomir AD, Vilim V, et al. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003;48(3):675–81. doi: 10.1002/art.10822. [DOI] [PubMed] [Google Scholar]
- 35.Vilim V, Olejarova M, Machacek S, Gatterova J, Kraus VB, Pavelka K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10(9):707–13. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 36.Golightly YM, Marshall SW, Kraus VB, Renner JB, Villaveces A, Casteel C, et al. Biomarkers of incident radiographic knee osteoarthritis: do they vary by chronic knee symptoms? Arthritis Rheum. 2011;63(8):2276–83. doi: 10.1002/art.30412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nurmohamed MT, Dijkmans BA. Efficacy, tolerability and cost effectiveness of disease-modifying antirheumatic drugs and biologic agents in rheumatoid arthritis. Drugs. 2005;65(5):661–94. doi: 10.2165/00003495-200565050-00006. [DOI] [PubMed] [Google Scholar]
- 38.Tiderius CJ, Sandin J, Svensson J, Dahlberg LE, Jacobsson L. Knee cartilage quality assessed with dGEMRIC in rheumatoid arthritis patients before and after treatment with a TNF inhibitor. Acta Radiol. 2010;51(9):1034–7. doi: 10.3109/02841851.2010.510482. [DOI] [PubMed] [Google Scholar]
- 39.Kawashiri SY, Kawakami A, Ueki Y, Imazato T, Iwamoto N, Fujikawa K, et al. Decrement of serum cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis (RA) patients achieving remission after 6 months of etanercept treatment: comparison with CRP, IgM-RF, MMP-3 and anti-CCP Ab. Joint Bone Spine. 2010;77(5):418–20. doi: 10.1016/j.jbspin.2010.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
No.1–51 represent the 51 overlapping peptides, No. 52 and 54 are positive controls, No.53 are negative controls.