Abstract
Purpose
To determine the rates of keratoplasty for corneal endothelial disease (CED) from 2001–2009 in a large managed care network in the United States, factors that affect which patients undergo this procedure, and surgical outcomes.
Design
A retrospective review of data from a longitudinal cohort study.
Participants
Beneficiaries with CED aged ≥40 years who were receiving eye care during 2001–2009.
Methods
Rates of keratoplasty for CED were determined at 6-month intervals from January 2001 through December 2009. Mean number of postoperative visits and rates of severe adverse events in the year following keratoplasty surgery were monitored over the course of the decade. Univariate and multivariable logistic regression were performed to identify sociodemographic and other factors associated with undergoing keratoplasty for CED.
Main Outcome Measures
Odds of undergoing keratoplasty with 95% confidence intervals, changes in the number of postoperative visits and rates of adverse events in the year following keratoplasty.
Results
Of the 38,648 enrollees who met the inclusion criteria, 2,187 persons underwent one or more keratoplasty surgeries from January 2001 to December 2009. After adjustment for confounding factors, individuals with CED had a 47% increased odds of undergoing keratoplasty during 2007–2009 relative to 2001–2006. The mean number of postoperative visits to eye-care providers in the year following keratoplasty declined from 12.6 in 2001–2006 to 10.5 in 2007–2008. There was no difference in the proportion of enrollees who developed adverse events following keratoplasty over time.
Conclusions
In this analysis of claims data, from 2001–2009, a period during which there was a rise in the rate of endothelial keratoplasty, we observed a trend of greater rates of keratoplasty in patients with CED and fewer visits for postoperative care in the later years of the decade compared with the earlier years, along with no change in rates of severe adverse events.
INTRODUCTION
Keratoplasty is one of the most common intraocular surgeries performed in the United States (US). Since its inception over 100 years ago, keratoplasty has undergone significant refinements with changes in surgical techniques, instrumentation, medical treatment, and eye banking. Its recent evolution has included innovations to specifically target the dysfunctional region(s) of the cornea, with growing numbers of lamellar keratoplasties being performed worldwide. Due to the introduction and rapid growth of endothelial keratoplasty techniques in recent years, we thought the years from 2001–2009 would be particularly informative time period to study1.
In the US, corneal endothelial disease (CED), which includes Fuchs’ dystrophy and post-surgical corneal edema, is the leading indication for keratoplasty1. According to the Eye Bank Association of America (EBAA), CED accounted for 48% of all keratoplasty procedures performed in 20101. Comparable rates have been reported at academic medical centers in the US2–4 and worldwide5–6. CED is primarily a condition affecting older individuals, and with the aging population in the US and other developed countries, rates of keratoplasty for CED are likely to rise in the coming years.
Little is known about the characteristics of individuals with CED and the factors that influence their propensity to undergo keratoplasty surgery. In this longitudinal cohort study of 38,648 individuals with CED who were enrolled in a large managed-care network in the US from 2001 to 2009, we determined the proportion of patients with CED each year who underwent keratoplasty and how these numbers changed over the decade. In addition, we sought to determine sociodemographic and other factors that increased or decreased the likelihood of undergoing keratoplasty for CED, and assessed the safety profile of this surgical procedure in this patient population.
METHODS
Data Source
The i3 InVision Data Mart database (Ingenix, Eden Prairie, MN) contains detailed fully de-identified records of all beneficiaries in United Healthcare, a large managed care network in the United States. We had access to data for 10,324,334 beneficiaries in the Data Mart database who had any form of eye care from January 1, 2001 through December 31, 2009. This subset consisted of beneficiaries who had one or more International Classification of Diseases (ICD-9-CM) code for any eye-related diagnosis (360–379.9), or Current Procedural Terminology (CPT-4) code for any eye-related visits, diagnostic or therapeutic procedures (65091–68899 or 92002–92499), or any other ICD-9-CM or CPT codes submitted by an ophthalmologist or optometrist during their time in the medical plan. We had access to all beneficiaries’ medical claims (inpatient, outpatient, skilled nursing facility) for ocular and non-ocular medical conditions. The database also contains detailed records of demographic (age, sex, race, race/ethnicity) and socioeconomic (education, household net worth) information for each beneficiary.
Patients
We identified all persons age 40 or older in the i3 InVision Data Mart database for at least one year continuously. Individuals with non-continuous enrollment were excluded. Next, we identified all individuals diagnosed with CED (N=38,648) at any point during their time in the medical plan based on the receipt of ≥1 of the following ICD-9-CM diagnosis codes: 371.57, 371.2, 371.20, or 371.23 (Figure 1 and Table 1, both available at http://aaojournal.org). Among patients diagnosed with CED, we next identified those who underwent ≥1 keratoplasty surgeries during their time in the plan. Keratoplasty was identified based on the following CPT codes: 65730, 65750, 65755, and 65756 (Table 1, available at http://aaojournal.org). Before 2009, endothelial keratoplasty (EK) did not have a unique CPT code, and therefore it was not possible to ascertain from the claims data whether penetrating keratoplasty (PK) or EK was performed.
Analyses of Rates of Keratoplasty for CED
For each six month period from January 1, 2001 and December 31, 2009, we determined the proportion of individuals with CED who underwent keratoplasty. For this calculation, to be eligible to be counted in the denominator in a given 6 month interval, a patient must have been diagnosed with CED before or during that interval and must have been enrolled in the plan throughout the entire 6 month interval. The proportion of eligible patients who underwent keratoplasty during each interval was determined. Enrollees with CED could undergo keratoplasty in more than one 6 month interval. For those who had >1 keratoplasty, we were unable to determine from the claims data whether they underwent more than one keratoplasty in the same eye or whether each eye underwent keratoplasty.
Postoperative Visits
For those enrollees with CED who underwent keratoplasty we determined the number of visits to eye-care providers (ophthalmologists or optometrists) during the year following the date of surgery. Next, we determined the mean number of postoperative visits for patients who had keratoplasty during each interval. For enrollees who underwent more than one keratoplasty, only the first surgery was considered for this analysis. This analysis was not performed for patients who had surgery in 2001 (since data from the year 2000 was not available to us), and in 2009 (since these individuals did not have a full year of post-operative follow-up).
Adverse Events
Severe, potentially sight-threatening postoperative adverse events (endophthalmitis, suprachoroidal hemorrhage, and rhegmatogenous retinal detachment) were identified based on ICD-9-CM codes. To exclude non-incident adverse events (those which occurred prior to keratoplasty) we employed a 1 year look-back period to assess whether enrollees had ≥1 codes for any of these adverse events. Enrollees, who had record of one of these events in the year prior to their surgery were excluded from this analysis. We stratified these three postoperative complications into those that occurred within the first 90 days following surgery and those that occurred between day 91 and 365. Severe adverse events were identified starting the year 2002 (since there was an inadequate look-back period to identify non-incident complications for those who had surgery in 2001). Individuals who underwent their first keratoplasty during 2009 did not have 1 full year of enrollment in the plan following their surgery to assess for complications, so they were also excluded from this analysis. For enrollees who underwent more than one keratoplasty, only the first keratoplasty was considered for this analysis.
Multivariable Regression
All analyses were performed by using Stata™ version 11 (College Station, TX). Participant characteristics were summarized for the entire sample by using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Next, a logistic regression model was developed to determine factors affecting the odds of undergoing keratoplasty for CED. Univariate and multivariable models were performed. Year was the key predictor of interest in the model, enabling us to evaluate for change in the rate of keratoplasty over time. We aggregated years 2001 to 2006, a period when patients with CED were treated primarily with penetrating keratoplasty, versus 2007 to 2009, a period in which the rate of endothelial keratoplasty to treat CED was rapidly increasing1. In the multivariable models, adjustments were made for age, sex, race, region of residence within the US, education level, household net worth, plan type (eg: health maintenance organization vs preferred provider organization), density of ophthalmologists in the state of residence, and the following medical and ocular conditions: cataract, pseudophakia or aphakia, open-angle glaucoma, macular degeneration, diabetic retinopathy, dementia, depression, and Charlson Comorbidity Index, an overall measure of health (Table 1, available at http://aaojournal.org). Logistic regression models with repeated measures were used to determine factors affecting the odds of undergoing keratoplasty for CED. Generalized estimating equations with robust (empirical) variance estimation were performed for the logistic regression model. Quasi-likelihood information criterion (QIC) was used for model selection7.
For all analyses, a p-value of < 0.05 was considered statistically significant. The University of Michigan Institutional Review Board determined this study was exempt from requiring IRB approval.
RESULTS
A total of 38,648 enrollees met the inclusion criteria and had one or more diagnoses of CED during their time in the plan. The mean length of time these individuals were enrolled in the plan was 53 ± 27 months. Among the 38,648 individuals with CED, 2,187 beneficiaries (5.7%) underwent one or more keratoplasty procedures during their time in the plan. The mean age of those with CED who underwent keratoplasty was (63.1 ± 11.6 years), which was 2.7 years older than the mean age of the overall study sample of individuals with CED (60.4 ± 11.7 years). There were 1,678 whites, 119 blacks, 89 Latinos, and 39 Asian Americans who underwent keratoplasty for CED. Those who underwent keratoplasty had more comorbid ocular and systemic diseases relative to those who did not undergo surgery (Table 2).
Table 2.
Characteristics of Individuals with Corneal Endothelial Disease in Study Sample
| Patients with CED who underwent keratoplasty | Patients with CED who did not undergo keratoplasty | Total with CED | ||
|---|---|---|---|---|
| Total N | 2187 | 36461 | 38648 | |
|
| ||||
| Age at plan enrollment (years) | 40–49 | 300 (13.7%) | 7931 (21.8%) | 8231 (21.3%) |
| 50–59 | 602 (27.5%) | 11048 (30.3%) | 11650 (30.1%) | |
| 60–69 | 563 (25.7%) | 8585 (23.6%) | 9148 (23.7%) | |
| 70–79 | 530 (24.2%) | 6639 (18.2%) | 7169 (18.6%) | |
| ≥ 80 | 192 (8.8%) | 2258 (6.2%) | 2450 (6.3%) | |
|
| ||||
| Sex | Female | 1129 (51.6%) | 22551 (61.9%) | 23680 (61.3%) |
| Male | 1058 (48.4%) | 13910 (38.2%) | 14968 (38.7%) | |
|
| ||||
| Race | White | 1678 (86.2%) | 27778 (88.1%) | 29456 (88.0%) |
| Black | 119 (6.1%) | 1712 (5.4%) | 1831 (5.5%) | |
| Latino | 89 (4.6%) | 1242 (3.9%) | 1331 (4.0%) | |
| Asian American | 39 (2.0%) | 541 (1.7%) | 580 (1.7%) | |
| Other | 22 (1.1%) | 242 (0.8%) | 264 (0.8%) | |
|
| ||||
| Education | < High School | 38 (1.9%) | 456 (1.4%) | 494 (1.4%) |
| High School Diploma | 766 (37.7%) | 11991 (36.1%) | 12757 (36.2%) | |
| Some College | 767 (37.8%) | 12829 (38.6%) | 13596 (38.6%) | |
| College Diploma | 456 (22.5%) | 7855 (23.7%) | 8311 (23.6%) | |
| Advanced Degree | 3 (0.2%) | 74 (0.2%) | 77 (0.2%) | |
|
| ||||
| United States Region of Residence | Northeast | 268 (12.3%) | 4861 (13.3%) | 5129 (13.3%) |
| Southeast | 926 (42.4%) | 16863 (46.3%) | 17789 (46.1%) | |
| Midwest | 708 (32.4%) | 11449 (31.4%) | 12157 (31.5%) | |
| West | 281 (12.9%) | 3244 (8.9%) | 3525 (9.1%) | |
| Other | 2 (0.1%) | 31 (0.1%) | 33 (0.1%) | |
|
| ||||
| Plan Type | HMO | 543 (24.8%) | 9287 (25.5%) | 9830 (25.4%) |
| EPO | 160 (7.3%) | 3128 (8.6%) | 3288 (8.5%) | |
| Indemnity | 338 (15.5%) | 3666 (10.1%) | 4004 (10.4%) | |
| POS | 740 (33.8%) | 14299 (39.2%) | 15039 (38.9%) | |
| PPO | 387 (17.7%) | 5853 (16.1%) | 6240 (16.2%) | |
| Other | 19 (0.9%) | 228 (0.6%) | 247 (0.6%) | |
|
| ||||
| Net Worth | $0 – $24,999 | 179 (9.3%) | 2546 (8.0%) | 2725 (8.1%) |
| $25,000 – $74,999 | 131 (6.8%) | 2048 (6.4%) | 2179 (6.4%) | |
| $75,000 – $149,999 | 236 (12.2%) | 4004 (12.5%) | 4240 (12.5%) | |
| $150,000 – $499,999 | 874 (45.3%) | 14730 (46.2%) | 15604 (46.1%) | |
| ≥ $500,000 | 510 (26.4%) | 8592 (26.9%) | 9102 (26.9%) | |
|
| ||||
| Ocular Comorbidities | Non-exudative AMD | 289 (13.2%) | 4498 (12.3%) | 4787 (12.4%) |
| Exudative AMD | 55 (2.5%) | 696 (1.9%) | 751 (1.9%) | |
| NPDR | 115 (5.3%) | 1663 (4.6%) | 1778 (4.6%) | |
| PDR | 48 (2.2%) | 624 (1.7%) | 672 (1.7%) | |
| OAG | 740 (33.8%) | 4807 (13.2%) | 5547 (14.4%) | |
| Cataract | 1361 (62.2%) | 19829 (54.4%) | 21190 (54.8%) | |
| Pseudophakia/Aphakia | 1233 (56.4%) | 8013 (22.0%) | 9246 (23.9%) | |
Note: For some variables, the n’s will not add up to the total N due to missing data
CED= Corneal endothelial disease; HMO= health maintenance organization; EPO= exclusive provider organization; POS= point of service; PPO= preferred provider organization; AMD=age-related macular degeneration; NPDR=non-proliferative diabetic retinopathy; PDR=proliferative diabetic retinopathy; OAG=open-angle glaucoma
Trends in Utilization of Keratoplasty Surgery, 2001–2009
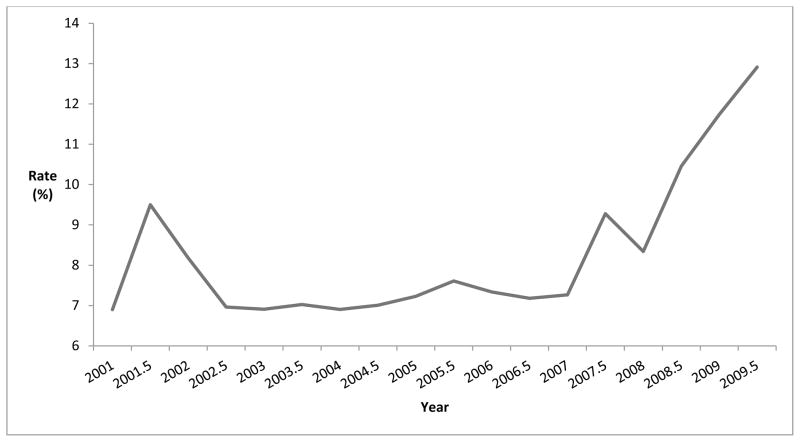
Among the 2,187 persons with CED who underwent keratoplasty, a total of 2,696 keratoplasty surgeries were performed from January 2001 through December 2009 (range: 89–198 procedures for each 6 month interval). In that time period, 1757 persons (65.2%) had 1 keratoplasty, 728 (27.0%) had two keratoplasties, and 211 persons (7.8%) had more than 2 keratoplasties. Rates of keratoplasty were relatively stable from 2002 to 2006 (6.9% to 8.2%) for each 6 month interval. Keratoplasty rates for CED rose considerably (7.3% to 12.9%) from 2007–2009 (Figure 2). After adjustment for confounding factors in multivariable analysis (Table 3), individuals with CED enrolled in the medical plan during the years 2007–2009 had a 47% increased odds of undergoing keratoplasty (adjusted OR = 1.47, 95% CI 1.35–1.59) relative to those in the plan during the years 2001–2006.
Figure 2. Rate of Keratoplasty for Corneal Endothelial Disease.
Keratoplasty Rate = Number of enrollees with corneal endothelial disease in each six month interval who underwent keratoplasty divided by the total number of individuals with corneal endothelial disease in plan during that six month interval
Table 3.
Univariate and Multivariable Logistic Regression to Identify Factors Associated with Undergoing Keratoplasty for Corneal Endothelial Disease
| Logistic Regression | Univariate | Multivariable | |||
|---|---|---|---|---|---|
|
| |||||
| Covariates | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Year in Plan | 2001–2006 | 0.71** | 0.66–0.77 | (Ref) | - |
| 2007–2009 | 1.40** | 1.30–1.51 | 1.47** | 1.35–1.59 | |
|
| |||||
| Age at plan enrollment (years) | 40–49 | 0.99 | 0.89–1.11 | (Ref) | - |
| 50–59 | 0.98 | 0.91–1.06 | 1.09 | 0.95–1.24 | |
| 60–64 | 1.09 | 0.99–1.19 | 1.18* | 1.02–1.36 | |
| 65–69 | 1.05 | 0.94–1.16 | 1.21* | 1.04–1.41 | |
| 70–74 | 1.01 | 0.91–1.12 | 1.20* | 1.02–1.41 | |
| 75–79 | 0.97 | 0.88–1.07 | 1.16* | 0.98–1.36 | |
| 80–84 | 0.93 | 0.84–1.03 | 1.11 | 0.93–1.31 | |
| ≥ 85 | 0.91 | 0.75–1.11 | 1.02 | 0.80–1.30 | |
|
| |||||
| Sex | Female | 1.03 | 0.98–1.09 | 1.02 | 0.96–1.08 |
|
| |||||
| Race | White | 0.81** | 0.75–0.87 | (Ref) | - |
| Black | 1.16* | 1.02–1.32 | 1.05 | 0.92–1.21 | |
| Latino | 1.14* | 1.02–1.28 | 1.08 | 0.96–1.22 | |
| Asian | 1.12 | 0.90–1.39 | 1.19 | 0.95–1.49 | |
| Other | 1.02 | 0.79–1.32 | 1.07 | 0.82–1.38 | |
|
| |||||
| Education | < High School | 1.11 | 0.87–1.42 | (Ref) | – |
| High School Diploma | 1.09** | 1.03–1.16 | 0.82** | 0.72–0.93 | |
| Some College | 0.91** | 0.86–0.96 | 0.76** | 0.67–0.87 | |
| College Diploma | 0.88** | 0.82–0.94 | 0.76** | 0.66–0.87 | |
| Advanced Degree | 0.76 | 0.43–1.35 | 0.64* | 0.42–0.98 | |
|
| |||||
| Net Worth | $0 – $24,999 | 1.21** | 1.09–1.34 | (Ref) | - |
| $25,000 – $74,999 | 1.03 | 0.92–1.16 | 0.88* | 0.77–1.00 | |
| $75,000 – $149,999 | 0.98 | 0.90–1.07 | 0.81** | 0.73–0.90 | |
| $150,000 – $499,999 | 0.95 | 0.90–1.01 | 0.84** | 0.77–0.92 | |
| $500,000 | 0.85** | 0.80–0.91 | 0.80** | 0.72–0.89 | |
|
| |||||
| Plan Type | HMO | 1.14** | 1.07–1.22 | (Ref) | - |
| EPO | 1.03 | 0.92–1.14 | 0.85* | 0.75–0.97 | |
| Indemnity | 0.81** | 0.76–0.87 | 0.74** | 0.68–0.81 | |
| POS | 0.96 | 0.91–1.02 | 0.82** | 0.75–0.89 | |
| PPO | 1.10* | 1.02–1.19 | 0.88** | 0.80–0.97 | |
| Other | 0.82 | 0.65–1.05 | 0.81 | 0.64–1.03 | |
|
| |||||
| United States Region of Residence | Northeast | 0.96 | 0.88–1.04 | (Ref) | - |
| Southeast | 1.17** | 1.11–1.24 | 1.06 | 0.96–1.17 | |
| Midwest | 0.91** | 0.86–0.97 | 0.94 | 0.85–1.04 | |
| West | 0.89** | 0.83–0.96 | 0.93 | 0.83–1.04 | |
| Other | 1.37** | 1.33–1.41 | 1.19 | 1.00–1.42 | |
|
| |||||
| Ocular comorbidities | Non-exudative AMD | 0.84** | 0.78–0.91 | 0.87** | 0.81–0.95 |
| Exudative AMD | 1.00 | 0.84–1.18 | 1.17 | 0.99–1.37 | |
| PDR | 0.79** | 0.66–0.93 | 0.86 | 0.71–1.04 | |
| NPDR | 0.86* | 0.77–0.97 | 0.99 | 0.86–1.13 | |
| OAG | 0.92** | 0.86–0.97 | 0.91** | 0.86–0.97 | |
| Cataract | 0.87** | 0.84–0.94 | 0.87** | 0.82–0.92 | |
| Pseudophakia/aphakia | 0.95 | 0.90–1.01 | 0.99 | 0.94–1.06 | |
|
| |||||
| Other comorbidities | CC Index Score | 0.98** | 0.97–0.99 | 0.99** | 0.98–1.00 |
| Dementia | 0.99 | 0.86–1.15 | 1.12 | 0.97–1.29 | |
| Depression | 0.93 | 0.84–1.03 | 0.95 | 0.85–1.05 | |
|
| |||||
| Ophthalmologist density | 1.00 | 0.93–1.07 | 1.01 | 0.92–1.11 | |
p<0.05;
p<0.01
CI= confidence interval; HMO= health maintenance organization; EPO= exclusive provider organization; POS= point of service plan; PPO= preferred provider organization; AMD=age-related macular degeneration; NPDR=non-proliferative diabetic retinopathy; PDR=proliferative diabetic retinopathy; OAG=open-angle glaucoma; CC index= Charlson comorbidity index
Ophthalmologist density calculated as number of ophthalmologists per 10000 residents
Postoperative Visits Following Keratoplasty
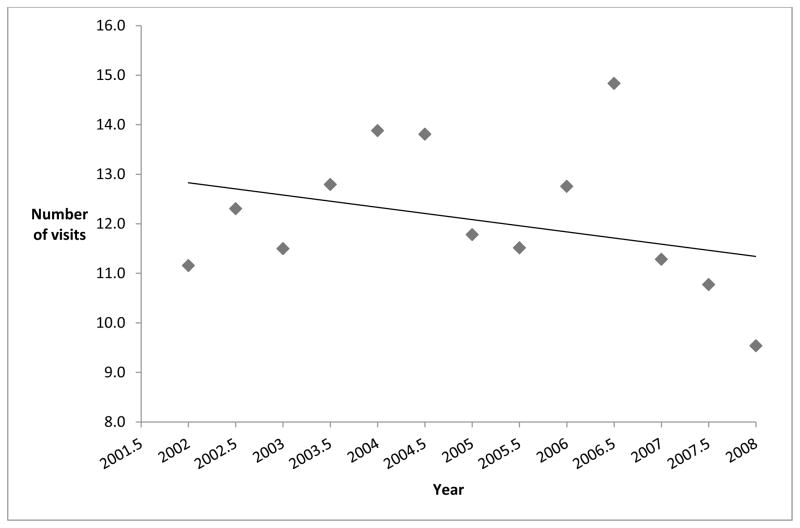
Figure 3 shows the mean number of post-operative visits to eye care providers in the year following initial keratoplasty at each 6-month time interval. From 2002–2006, patients had a mean of 12.6 post-operative visits in the year following keratoplasty. From 2007–2008, the mean number of postoperative visits to eye care providers in the year following keratoplasty dropped to 10.5 post-operative visits.
Figure 3. Mean Number of Eye Provider Visits in the Year Following Keratoplasty.
Eye provider visits include visits to ophthalmologists or optometrists
For enrollees who underwent more than one keratoplasty, only the first keratoplasty was considered for this analysis
Individuals who underwent keratoplasty in 2009 did not have a full year of postoperative follow-up following the surgery so that year was not included in the figure.
Other Factors Associated with Undergoing Keratoplasty
Multivariable logistic regression analysis was performed to determine factors affecting the odds of undergoing keratoplasty for CED (Table 3). There was no significant difference in the odds of undergoing keratoplasty for CED among individuals age 50–59 relative to those aged 40–49 (adjusted OR = 1.09, CI 0.95–1.24). Individuals with CED aged 60–64, 65–69, and 70–74 all had 18–21% higher odds of undergoing keratoplasty relative to persons age 40–49 (p<0.03 for all comparisons). Individuals aged 75 and older also had higher odds of undergoing keratoplasty as compared to persons aged 40–49, though these findings did not reach statistical significance (p>0.05). There was no difference in the odds of undergoing keratoplasty for CED among blacks (adjusted OR = 1.05, 95% CI 0.92–1.21), Latinos (adjusted OR = 1.08, 95% CI 0.96 – 1.22), or Asian Americans (adjusted OR = 1.19, 95% CI 0.95–1.49) relative to whites. There was no significant difference in the odds of undergoing keratoplasty for CED among males as compared with females, or based on US region of residence. The presence of the following ocular comorbidities reduced the odds of undergoing keratoplasty for CED: nonexudative age-related macular degeneration (adjusted OR = 0.87, 95% CI 0.81–0.95), open-angle glaucoma (adjusted OR = 0.91, 95% CI 0.86–0.97), and cataract (adjusted OR = 0.87, 95% CI 0.82–0.92). For every additional medical comorbidity (as captured using the Charlson Index score), the odds of undergoing keratoplasty for CED decreased 1.5% (adjusted OR = 0.99, 95% CI 0.98–1.00). Density of ophthalmologists in a given US state did not affect the odds of undergoing keratoplasty for CED. Compared to persons with less than high school education, those with a high school diploma had an 18% decreased odds of undergoing keratoplasty, those with some college education or a college diploma had a 24% decreased odds of keratoplasty, and those with an advanced degree had a 36% decreased odds of undergoing keratoplasty for CED. Likewise, compared with enrollees who had a household net worth of < $25,000, individuals with higher household net worth levels had a 12–20% decreased odds of undergoing keratoplasty for CED (p<0.05 for all comparisons) (Table 3).
Adverse Events Following Keratoplasty
Table 4 shows the number and proportion of patients who experienced serious adverse events in the first 90 days and the first year following keratoplasty. Fifty-nine individuals (3.45%) from 1,699 keratoplasties experienced a severe postoperative event (endophthalmitis, suprachoroidal hemorrhage, and retinal detachment) from 2002–2008. Of these 59 severe complications, 30 occurred in the first 90 days following surgery and 29 occurred in the remainder of the first postoperative year. There were a total of 20 individuals (1.18%) who experienced endophthalmitis, 10 (0.59%) of these cases occurred in the immediate postoperative period (first 90 days) and 10 (0.59%) in the later postoperative period. There were 12 individuals (0.71%) with suprachoroidal hemorrhage, and 36 patients (2.08%) who developed a retinal detachment in the first postoperative year. There was no difference in the proportion of persons who developed endophthalmitis (p=0.55), suprachoroidal hemorrhage (p=0.18), or retinal detachment (p=0.16), or any severe adverse event (p=.19) in the keratoplasties performed from 2002–2006 versus those performed during 2007–2008. Despite the large number of keratoplasties assessed for these secondary outcomes, the small number of adverse events made the power to detect statistically significant differences low.
Table 4.
Adverse Events Associated with Keratoplasty for Corneal Endothelial Disease
| Year | # of keratoplasties | Severe Adverse Events* | Endophthalmitis | Suprachoroidal Hemorrhage | Rhegmatogenous Retinal Detachment | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||||||||||
| total | ≤90 days | >90 days | total | ≤90 days | >90 days | total | ≤90 days | >90 days | total | ≤90 days | >90 days | ||||||||||||||
|
| |||||||||||||||||||||||||
| # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | # | % | ||
| 2002 | 128 | 6 | 4.69 | 2 | 1.56 | 4 | 3.13 | 1 | 0.78 | 1 | 0.78 | 0 | 0 | 1 | 0.78 | 1 | 0.78 | 0 | 0 | 5 | 3.91 | 0 | 0 | 5 | 3.91 |
| 2002.5 | 105 | 3 | 2.86 | 2 | 1.9 | 1 | 0.95 | 2 | 1.9 | 1 | 0.95 | 1 | 0.95 | 1 | 0.95 | 1 | 0.95 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2003 | 121 | 4 | 3.31 | 3 | 2.48 | 1 | 0.83 | 1 | 0.83 | 0 | 0 | 1 | 0.83 | 1 | 0.83 | 1 | 0.83 | 0 | 0 | 2 | 1.65 | 2 | 1.65 | 0 | 0 |
| 2003.5 | 112 | 4 | 3.57 | 4 | 3.57 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1.79 | 1 | 0.89 | 1 | 0.89 | 3 | 2.68 | 2 | 1.79 | 1 | 0.89 |
| 2004 | 116 | 5 | 4.31 | 1 | 0.86 | 4 | 3.45 | 1 | 0.86 | 1 | 0.86 | 0 | 0 | 1 | 0.86 | 0 | 0 | 1 | 0.86 | 4 | 3.45 | 0 | 0 | 4 | 3.45 |
| 2004.5 | 113 | 5 | 4.42 | 2 | 1.77 | 3 | 2.65 | 1 | 0.88 | 1 | 0.88 | 0 | 0 | 1 | 0.88 | 0 | 0 | 1 | 0.88 | 4 | 3.54 | 1 | 0.88 | 3 | 2.65 |
| 2005 | 126 | 5 | 3.97 | 1 | 0.79 | 4 | 3.17 | 2 | 1.59 | 0 | 0 | 2 | 1.59 | 2 | 1.59 | 0 | 0 | 2 | 1.59 | 1 | 0.79 | 1 | 0.79 | 0 | 0 |
| 2005.5 | 135 | 6 | 4.44 | 3 | 2.22 | 3 | 2.22 | 3 | 2.22 | 2 | 1.48 | 1 | 0.74 | 1 | 0.74 | 1 | 0.74 | 0 | 0 | 2 | 1.48 | 0 | 0 | 2 | 1.48 |
| 2006 | 116 | 5 | 4.31 | 3 | 2.59 | 2 | 1.72 | 3 | 2.59 | 2 | 1.72 | 1 | 0.86 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1.72 | 1 | 0.86 | 1 | 0.86 |
| 2006.5 | 103 | 1 | 0.97 | 0 | 0 | 1 | 0.97 | 1 | 0.97 | 0 | 0 | 1 | 0.97 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2007 | 123 | 4 | 3.25 | 3 | 2.44 | 1 | 0.81 | 2 | 1.63 | 2 | 1.63 | 0 | 0 | 1 | 0.81 | 1 | 0.81 | 0 | 0 | 3 | 2.44 | 2 | 1.63 | 1 | 0.81 |
| 2007.5 | 142 | 4 | 2.82 | 2 | 1.41 | 2 | 1.41 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 2.82 | 2 | 1.41 | 2 | 1.41 |
| 2008 | 126 | 4 | 3.17 | 2 | 1.59 | 2 | 1.59 | 1 | 0.79 | 0 | 0 | 1 | 0.79 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2.38 | 1 | 0.79 | 2 | 1.59 |
| 2008.5 | 133 | 3 | 2.26 | 2 | 1.5 | 1 | 0.75 | 2 | 1.5 | 0 | 0 | 2 | 1.5 | 1 | 0.75 | 1 | 0.75 | 0 | 0 | 3 | 2.26 | 2 | 1.5 | 1 | 0.75 |
|
| |||||||||||||||||||||||||
| 2002–2008 | 1,699 | 59 | 3.45 | 30 | 1.76 | 29 | 1.69 | 20 | 1.18 | 10 | 0.59 | 10 | 0.59 | 12 | 0.71 | 7 | 0.41 | 5 | 0.30 | 36 | 2.08 | 14 | 0.81 | 22 | 1.27 |
Severe postoperative adverse events include endophthalmitis, suprachoroidal hemorrhage, and rhegmatogenous retinal detachment.
DISCUSSION
Our investigation of 38,648 individuals with CED enrolled in a large, national, US managed-care network demonstrates that rates of keratoplasty for CED have increased considerably in recent years. After adjusting for potential confounding factors, an individual with CED was nearly twice as likely to undergo keratoplasty in 2009 as compared with 2001. Over the course of the decade, rates of postoperative visits in the year following the surgery declined from a mean of 12.6 visits in 2002–2006 to 10.5 in 2007–2008. Rates of severe, potentially sight-threatening, post-operative adverse events following keratoplasty were relatively stable from 2001 through 2009.
Posterior lamellar keratoplasty techniques targeted to specifically treat CED were introduced between 1998 and 20018,9. Surgical techniques were further refined with the introduction of the Descemet’s stripping endothelial keratoplasty procedure in 2004–200510–12. In the next few years, EK gained mainstream popularity with a marked increase in corneal tissue utilization for EK from 6,027 in 2006 to 14,159 in 20071. This increase has continued, with 19,159 corneal donor tissues used for EK in 2010. In this study, among individuals with CED, rates of keratoplasty were relatively stable from 2002 to 2006 but substantially increased from 2007 to 2009 (Figure 2). This increase corresponds temporally to the widespread adoption of EK surgical procedures. Since the specific ICD-9-CM billing code for EK was only introduced in 2009, it is not possible to distinguish those who underwent EK versus PK in this study. But given the time course, we suspect that the widespread adoption of EK since 2007 plays a large role in the increased rates of keratoplasty for CED we are observing.
Another noteworthy finding in this study is the decrease in postoperative visits following keratoplasty. The mean number of postoperative visits to eye-care providers in the year following keratoplasty declined from 12.6 in 2002–2006 to 10.5 in 2007–2008 (Figure 3). Once again, the reduction in post-operative visits may correspond, in part, to the introduction and widespread use of EK. Compared with PK, EK requires fewer sutures and produces less astigmatism, better refractive stability, and earlier visual recovery13, all of which likely contribute to reduced need for post-operative evaluations. Fewer visits reduce the burden on patients and their family members in the peri-operative period. In addition to modifications in surgical technique, another factor affecting rates of postoperative visits may be related, in part, to the changing health care environment in the US that encourages increased attention to efficiency and minimization of unnecessary medical visits.
In this study we demonstrate a stable, low rate of vision-threatening complications following keratoplasty. Among all individuals who underwent keratoplasty from 2002–2009, the rate of endophthalmitis was 0.59% in the first 90 postoperative days. The rates of suprachoroidal hemorrhage and rhegmatogenous retinal detachment were 0.41% and 0.81%, respectively. There was no difference in the proportion of patients who developed adverse events following keratoplasties performed during 2002–2006 (when PK was the mainstay technique for corneal transplantation) versus those performed during 2007–2008 (when EK was gaining popularity and had become more prevalent); however, the overall severe adverse event rates are quite low and this analysis is not adequately powered to identify differences in severe adverse events that are statistically significant. Our results are consistent with previous studies: in an evaluation of 40,351 Medicare beneficiaries from 1984 to 1987, Aiello et al. determined the risk of re-hospitalization for endophthalmitis within 6 months of PK was 0.77% and the risk of re-hospitalization for RD within 2 years of PK was 1.85%14. In a meta-analysis that included 90,549 patients throughout the US from 1963 to 2003, Taban et al. calculated the incidence of endophthalmitis after PK to be even lower at 0.382%15. Direct comparison of actual percentages of adverse events in these studies with ours is difficult due to differences in study design, patient age, underlying corneal diagnosis, and other factors. However, the above mentioned studies, including ours, demonstrate low rates of vision-threatening complications following keratoplasty. Unlike previous reports in the literature, our analysis has the advantage of including more recent data and therefore likely better captures rates of complications associated with EK. This suggests that the transition from PK to EK has not been associated with any significant increase in rates of adverse events. Corneal graft failure and repeat keratoplasty are also significant risks associated with keratoplasty surgery. Unfortunately, we are unable to identify eye laterality or whether a surgery represents a primary or repeat graft by using claims data alone, so these complications could not be assessed.
In our analyses, older individuals (age 60–79) with CED have a statistically significant increased odds of undergoing keratoplasty relative to younger ones (age 40–49). Considering that older patients often have more advanced disease and higher rates of corneal decompensation, this finding is not surprising. Those older than 79 years who have CED actually have lower odds of undergoing keratoplasty relative to 40–49 year olds suggesting that at older ages, surgeons and patients may decide that the potential risks of the surgery may outweigh potential benefits expected from surgery. Since our regression models adjusted for age, it is unlikely that the observed difference is due to a cohort effect. While there is evidence of racial and sex disparities in access to eye care for other ophthalmic conditions16–18, our study indicates no statistically significant association between race or sex and the likelihood of undergoing keratoplasty for CED. We found that those with CED who are less educated and more economically disadvantaged have higher odds of keratoplasty relative to others with higher levels of education and wealth. A possible explanation for this somewhat surprising finding is that economically disadvantaged individuals tend to seek medical care at a later point in their disease process because of barriers to routine health care. Outside of the specialty of ophthalmology, there is evidence of higher utilization of expensive medical services among socioeconomically disadvantaged individuals relative to other groups19. Our study also shows that individuals with CED who have ocular comorbidities, such as macular degeneration or open-angle glaucoma, are less likely to undergo keratoplasty. Similarly, individuals with more systemic illnesses (as captured using the Charlson comorbidity index) are less likely to undergo keratoplasty. These findings suggest that ocular comorbidities (which may limit visual potential) and overall health are factors that influence the decision of whether individuals with CED undergo keratoplasty.
Study Strengths and Limitations
Strengths of this study include a large sample size of persons with CED and longitudinal follow-up of these individuals for an average follow-up of 4.5 years which allowed us to ascertain characteristics of persons with CED who were most likely to undergo keratoplasty. Given the large number of individuals with CED, it was possible to compare those who underwent keratoplasty to those who did not. This is a unique perspective compared to previous epidemiologic analyses of keratoplasty, which only report characteristics of the subset of patients with CED who actually underwent keratoplasty. In addition, this large sample contained an adequate representation of individuals of different sociodemographic backgrounds and allowed us to build complex regression models adjusting for an array of potential confounding factors. Furthermore, this analysis includes beneficiaries from across a diverse array of US communities, so findings are potentially more generalizable as compared to data exclusively from one specific academic medical center.
Several limitations need to be acknowledged. The data used in this study are extracted from billing records and therefore we cannot confirm, with certainty, that all of these enrollees did indeed have CED. Some enrollees may have been misdiagnosed or miscoded with this condition. Second, claims data do not contain information on important clinical factors such as indication for surgery, visual acuity, corneal examination findings, and some other important post-keratoplasty complications. Finally, caution must be taken when generalizing our findings to uninsured or underinsured individuals or individuals in other countries with CED who may have different levels of access to care than those in this analysis.
This is a unique analysis that longitudinally followed patients with CED from 2001 to 2009 to analyze the rates of keratoplasty and factors that increased or decreased the odds of undergoing this surgery. We show increasing rates of keratoplasty for CED since 2007 which corresponds to the popularization of EK. The trends revealed by this study highlight the transformation in our surgical management for CED. With the creation of a unique billing code for EK in 2009, replicating this study in several years will likely reveal further trends in the utilization of keratoplasty for CED.
Supplementary Material
Figure 1 Study Participant Inclusion and Exclusion Criteria (Online Only)
Acknowledgments
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (EY013511 and EY017885), American Glaucoma Society Clinician Scientist Grant, Blue Cross Blue Shield of Michigan Foundation, an unrestricted grant from Research to Prevent Blindness.
Footnotes
Presented in part at the 2011 Association for Research in Vision and Ophthalmology meeting, May 5, 2011.
The authors have no proprietary or commercial interest in any material discussed in this manuscript
This article contains online-only material. The following should appear online-only: Table 1 and Figure 1
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eye Bank Association of America. Eye Banking Statistical Report. Washington, D.C: The Association; 2010. [Google Scholar]
- 2.Ghosheh FR, Cremona FA, Rapuano CJ, et al. Trends in penetrating keratoplasty in the United States 1980–2005. Int Ophthalmol. 2008;28:147–53. doi: 10.1007/s10792-007-9177-z. [DOI] [PubMed] [Google Scholar]
- 3.Dobbins KR, Price FW, Jr, Whitson WE. Trends in the indications for penetrating keratoplasty in the midwestern United States. Cornea. 2000;19:813–6. doi: 10.1097/00003226-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Kang PC, Klintworth GK, Kim T, et al. Trends in the indications for penetrating keratoplasty, 1980–2001. Cornea. 2005;24:801–3. doi: 10.1097/01.ico.0000157407.43699.22. [DOI] [PubMed] [Google Scholar]
- 5.Jeganathan SV, Ghosh S, Jhanji V, et al. Resuturing following penetrating keratoplasty: a retrospective analysis. Br J Ophthalmol. 2008;92:893–5. doi: 10.1136/bjo.2007.133421. [DOI] [PubMed] [Google Scholar]
- 6.Siganos CS, Tsiklis NS, Miltsakakis DG, et al. Changing indications for penetrating keratoplasty in Greece, 1982–2006: a multicenter study. Cornea. 2010;29:372–4. doi: 10.1097/ICO.0b013e3181bd44a1. [DOI] [PubMed] [Google Scholar]
- 7.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57:120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 8.Melles GR, Eggink FA, Lander F, et al. A surgical technique for posterior lamellar keratoplasty. Cornea. 1998;17:618–26. doi: 10.1097/00003226-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Terry MA, Ousley PJ. Deep lamellar endothelial keratoplasty in the first United States patients: early clinical results. Cornea. 2001;20:239–43. doi: 10.1097/00003226-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Melles GR, Lander F, Nieuwendaal C. Sutureless, posterior lamellar keratoplasty: a case report of a modified technique. Cornea. 2002;21:325–7. doi: 10.1097/00003226-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Price FW, Jr, Price MO. Descemet’s stripping with endothelial keratoplasty in 50 eyes: a refractive neutral corneal transplant. J Refract Surg. 2005;21:339–45. doi: 10.3928/1081-597X-20050701-07. [DOI] [PubMed] [Google Scholar]
- 12.Melles GR, Wijdh RH, Nieuwendaal CP. A technique to excise the Descemet membrane from a recipient cornea (descemetorhexis) Cornea. 2004;23:286–8. doi: 10.1097/00003226-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Lee WB, Jacobs DS, Musch DC, et al. Ophthalmic Technology Assessment Committee Cornea and Anterior Segment Disorders Panel. Descemet’s stripping endothelial keratoplasty: safety and outcomes. A report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–30. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Aiello LP, Javitt JC, Canner JK. National outcomes of penetrating keratoplasty: risks of endophthalmitis and retinal detachment. Arch Ophthalmol. 1993;111:509–13. doi: 10.1001/archopht.1993.01090040101041. [DOI] [PubMed] [Google Scholar]
- 15.Taban M, Behrens A, Newcomb RL, et al. Incidence of acute endophthalmitis following penetrating keratoplasty: a systematic review. Arch Ophthalmol. 2005;123:605–9. doi: 10.1001/archopht.123.5.605. [DOI] [PubMed] [Google Scholar]
- 16.Devgan U, Yu F, Kim E, Coleman AL. Surgical undertreatment of glaucoma in black beneficiaries of Medicare. Arch Ophthalmol. 2000;118:253–6. doi: 10.1001/archopht.118.2.253. [DOI] [PubMed] [Google Scholar]
- 17.Vajaranant TS, Nayak S, Wilensky JT, Joslin CE. Gender and glaucoma: what we know and what we need to know. Curr Opin Ophthalmol. 2010;21:91–9. doi: 10.1097/ICU.0b013e3283360b7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abou-Gareeb I, Lewallen S, Bassett K, Courtright P. Gender and blindness: a meta-analysis of population-based prevalence surveys. Ophthalmic Epidemiol. 2001;8:39–59. doi: 10.1076/opep.8.1.39.1540. [DOI] [PubMed] [Google Scholar]
- 19.Alter DA, Stukel T, Chong A, Henry D. Lesson from Canada’s universal care: socially disadvantaged patients use more health services, still have poorer health. Health Aff (Millwood) 2011;30:274–83. doi: 10.1377/hlthaff.2009.0669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1 Study Participant Inclusion and Exclusion Criteria (Online Only)