Abstract
Engineered cartilage based on adult mesenchymal stem cells (MSCs) is an alluring goal for the repair of articular defects. However, efforts to date have failed to generate constructs with sufficient mechanical properties to function in the demanding environment of the joint. Our findings with a novel photocrosslinked hyaluronic acid (HA) hydrogel suggest that stiff gels (high HA concentration, 5% w/vol) foster chondrogenic differentiation and matrix production, but limit overall functional maturation due to the inability of formed matrix to diffuse away from the point of production and form a contiguous network. In the current study, we hypothesized that increasing the MSC seeding density would decrease the required diffusional distance, and so expedite the development of functional properties. To test this hypothesis, bovine MSCs were encapsulated at seeding densities of either 20 or 60 million cells per mL in 1%, 3%, and 5% (w/vol) hyaluronic acid (HA) hydrogels. Counter our hypothesis, higher concentration HA gels (3% and 5%) did not develop more rapidly with increased MSC seeding density. However, the biomechanical properties of low concentration (1%) HA constructs increased markedly (nearly 3-fold with a 3-fold increase in seeding density). To ensure that optimal nutrient access was delivered, we next cultured these constructs under dynamic culture conditions (orbital shaking) for 9 weeks. Under these conditions, 1% HA seeded at 60 million MSCs per mL reached a compressive modulus in excess of 1 MPa (compared to 0.3-0.4MPa for free swelling constructs). This is the highest level we have reported to date in this HA hydrogel system, and represents a significant advance towards functional stem cell-based tissue engineered cartilage.
Keywords: cartilage, hydrogel, tissue engineering, hyaluronic acid / hyaluronan, mesenchymal stem cell
Introduction
Articular cartilage injuries and disease result in focal defects with limited intrinsic capacity for regeneration. The presence of a defect requires that adjacent cartilage bear an increased proportion of joint load [1], which increases local stresses and the likelihood of continued degeneration and development of osteoarthritis [2, 3]. An ideal repair material would completely integrate to fill the defect with a cartilage-like material possessing functional load-bearing characteristics [4]. However, meeting of this high benchmark for functional repair remains an elusive goal. Current regenerative strategies that deliver ex-vivo expanded autologous chondrocytes (ACI/ACT) [5] or promote endogenous healing via bone marrow stimulation (microfracture) [6] may improve patient outcomes in the short term, but functional restoration of the tissue has yet to be demonstrated [7].
An alternative approach is to engineer de novo cartilage in vitro for implantation within a cartilage defect. Indeed, recent work utilizing chondrocytes in specialized media conditions and 3D hydrogels has produced constructs that match or exceed native tissue values for equilibrium modulus and proteoglycan (PG) content [8–11]. However, the clinical shortage of healthy chondrocytes and the co-morbidity associated with their harvest [12] are considerable limitations. Mesenchymal stem cells (MSCs) are a precursor cell population that can be obtained from patient bone marrow and expanded in vitro to clinically relevant numbers without losing their ability to undergo chondrogenic differentiation [13]. MSCs have been combined with countless biomaterials for cartilage tissue engineering [14], but no such combination has yet achieved mechanical properties that approach native tissue or engineered chondrocyte-based cartilage [15, 16].
One approach for improving the functional maturation of MSC-based engineered cartilage may be to increase the initial cell density within a construct. Here, the rationale is that with more point sources for matrix production, the functional contiguity of matrix should occur at an earlier time in culture, and formed matrix should be concentrated to a greater extent. With chondrocytes cultured in alginate and agarose, when provided a sufficient supply of nutrients, increasing seeding densities does increase mechanical and biochemical outcomes [17, 18]. MSCs likewise depend on seeding density, where up to ~10 million MSCs/mL increases expression of cartilage matrix associated genes compared to lower densities [19]. However, recent studies using both agarose and alginate hydrogels show no improvement in mechanics at higher MSC densities with continual exposure to pro-chondrogenic media [20–22]. Indeed, in alginate gels, there appeared to be a maximum in matrix production per cell occurring in the range of 25 million cells/mL, with both higher and lower densities leading to inferior outcomes [20].
Additional cues from the microenvironment, including biomolecular identity of the supporting 3D network [20] as well as its biophysical properties [23] can influence functional matrix elaboration. Our recent work with a photo-polymerizing hyaluronic acid (HA) hydrogel [24, 25] showed that when MSCs were encapsulated (at a density of 20 million cells/ml) in hydrogels of 1, 2, and 5% (w/v) macromer concentrations, the most robust constructs developed in the 1% formulation. This improved matrix functionality occurred despite the fact that higher levels of cartilage matrix-related gene expression and matrix synthesis (per construct) occurred in the higher macromer density constructs [26, 27]. Histological analysis showed that in high density gels, discrete lacunae of poorly distributed matrix formed, while in 1% gels a well distributed and contiguous ECM was established. Overcoming these limitations in the distribution of matrix may increase the potential of higher HA macromer density hydrogels for functional development while also taking advantage of their greater initial strength and dimensional stability.
To test this hypothesis, the objective of this work was to determine whether an increase in MSC seeding density would enhance tissue engineered cartilage properties in high macromer concentration HA hydrogels, and specifically whether this increase would be mediated by improved matrix connectivity (Fig. 1). Towards this end, HA hydrogels of 1, 3, and 5% macromer density were seeded at either 20 or 60 million MSCs/mL and cultured for 4 and 8 weeks in a chemically defined pro-chondrogenic media formulation. At each time point, construct maturation was evaluated via assessment of biomechanical, biochemical, and histological properties. Further, under the best conditions derived above, we evaluated growth of high seeding density constructs under dynamic culture (orbital shaking) conditions.
Figure 1.
Diffusion of formed cartilage matrix is limited within HA hydrogels of higher macromer density (left). Increasing MSC seeding density may accelerate and improve matrix connectivity (right) and so enhance the functional development of tissue engineered cartilage.
Methods
Hyaluronic Acid Hydrogel Synthesis
Methacrylated HA (MeHA) macromer was synthesized by reacting methacrylic anhydride (Sigma, St. Louis, MO) and 74 kDa HA (Lifecore, Chaska, MN) followed by 1H NMR characterization (25% methacrylated) as previously described [24]. Lyophilized MeHA was sterilized by exposure to a biocidal UV lamp for 15 minutes. Macromer was dissolved to 1, 3, and 5% (mass/volume) in sterile PBS with 0.05% photoinitiator Irgacure-2959 (2-methyl-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone; Ciba-Geigy, Tarrytown, NY).
MSC Isolation, Expansion, and 3D Culture
MSCs were isolated from three to five juvenile bovine femurs as in [15], expanded through passage 3, combined, and encapsulated at 20 or 60 million cells/mL in 1%, 3%, and 5% (w/v) MeHA. Gels were polymerized via UV exposure (10 min) between glass plates separated by 2.25 mm [28] and 4 mm diameter punches used to create MSC-laden hydrogel cylinders. MSCs were also encapsulated within agarose (Ag; 2% w/v; Type VII, Sigma, St. Louis, MO) as a well established control [15]. All constructs (1 ml/construct) were cultured in a chemically defined medium supplemented with TGF-β3 (10 ng/ml, R&D Systems, Minneapolis, MN) [28]. Constructs were cultured in non-tissue culture treated 6-well plates with media changes occurring thrice weekly. In a second series of studies, using only the high density 1% HA formulation, constructs were cultured on an orbital shaker (Bellco, Model #7744, Vineland, NJ) rotating at 1.2 rpm for the entire culture duration [29]. This ‘dynamic culture’ group was accompanied by a ‘static culture’ control group treated identically.
Mechanical and Biochemical Analysis
At defined time points (4 and 8 weeks for macromer study, 3, 6, and 9 weeks for shaking study), construct mechanical properties and biochemical content was assessed. The unconfined equilibrium compressive modulus was derived from a stress relaxation test (10% strain; 1000 sec relaxation) [30]. After equilibration, the dynamic modulus was determined by applying 5 sinusoidal cycles of compression at 1 Hz (1% strain amplitude) [31]. After testing, each construct was weighed and digested in papain before analysis of DNA, sulfated glycosaminoglycan (sGAG), and collagen content [15]. DNA content was analyzed using the Picogreen dsDNA assay kit (Molecular Probes, Eugene, OR), sulfated glycosaminoglycan (sGAG) using the 1,9-dimethylmethylene blue (DMMB) dye binding assay, and the orthohydroxyproline (OHP) was measured and converted to collagen as previously described [32–34].
Histological analysis
To assess viability, samples were halved diametrically and stained with calcein AM and ethidium homodimer (LIVE/DEAD kit; Invitrogen). Additional constructs were fixed in 4% paraformaldehyde, paraffin embedded, and sectioned (8 μm). Sections were stained for collagens (picrosirius red) and proteoglycan (alcian blue) before imaging at 100X magnification.
Statistical Analysis
Statistical analyses were performed using SYSTAT (v13, San Jose, CA). Three-way ANOVA was used with formulation (1, 3, 5% MeHA, and Ag), seeding density (20 or 60 M/mL), and time (0, 4, and 8 weeks) as independent variables. Two-way ANOVA was used with time (3, 6, and 9 weeks) and culture condition (dynamic and static) as independent variables. Fisher’s post hoc tests were used to make comparisons between groups, with p<0.05 indicating significant differences. Experiments were repeated in full at least once, with consistent findings between replicates; data from one replicate are presented here.
Results
Construct formation and mechanical properties with increasing seeding density
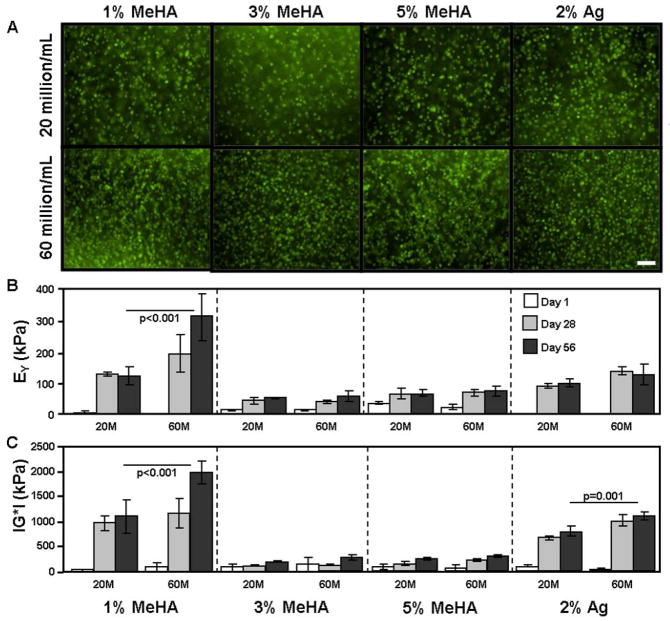
Increasing the initial MSC seeding density from 20 (20M) to 60 million cells/mL (60M) increased viable cell density (Fig. 2A, Day 1 images shown). Increased cell number did not compromise viability at any HA concentration at later time points (not shown). While an increase in cellularity was achieved, our starting hypothesis was not borne out by experimental findings. Namely, the compressive properties of higher macromer density (i.e., 3% and 5%) HA constructs did not increase with an increase in MSC seeding density. While the modulus (EY) of 20M 3% HA constructs increased to 51 kPa by 8 weeks, tripling the seeding density to 60M did not change construct properties (56 kPa) (Fig. 2B). In 5% HA gels, EY reached 66 kPa at 20M and 72 kPa at 60M (Fig. 2B). However, EY of 1% HA constructs reached 121 kPa at 20M, and was nearly 3-fold greater (313 kPa) at 60M (p<0.05; Fig. 2B). Consistent with our previous findings [22], Ag control constructs did not increase at higher seeding densities, reaching 138 and 126 kPa at 20M and 60M, respectively (Fig. 2B). The dynamic modulus followed a similar trend for all groups (where 3% and 5% HA constructs increased with time, but did not increase further at higher MSC seeding densities, Fig. 2C). The dynamic modulus of 20M 1% HA constructs reached 1.10 MPa while their 60M counterparts reached 1.97 MPa at 8 weeks (p<0.05; Fig. 2C). Ag controls increased with time and seeding density, reaching 0.78 MPa (20M) and 1.11 MPa (60M) after 8 weeks (p=0.001).
Figure 2.
(A) Calcein AM fluorescence 1 day after encapsulation confirmed differences in cell seeding density while demonstrating high initial viability in both 20M (top) and 60M (bottom) seeding density groups (100X magnification; 100 μm scale bar). (B) Equilibrium (EY) and (C) dynamic modulus (|G*|) of MSC-laden methacrylated HA (MeHA) and agarose (Ag) hydrogels at seeding densities of 20 million MSCs/mL (20M) and 60 million (60M) MSCs/mL after 1 (white), 28 (grey), and 56 (dark grey) days of in vitro culture in a chemically defined chondrogenic medium with TGF-β3 (10 ng/mL). (n=4 constructs per group; bars indicate p<0.05)
Biochemical content and distribution with increasing seeding density
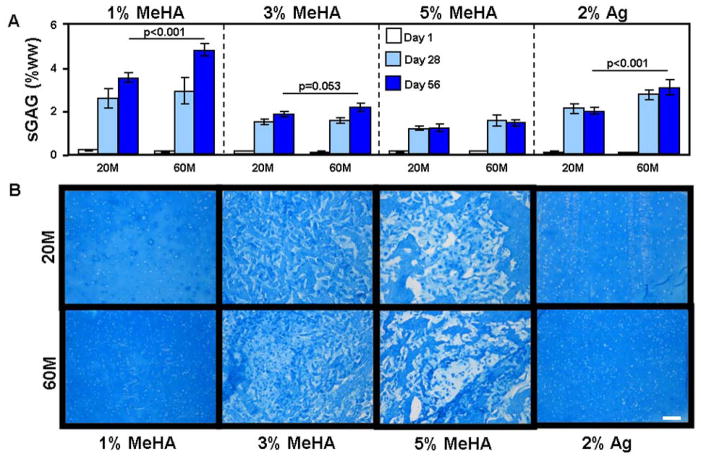
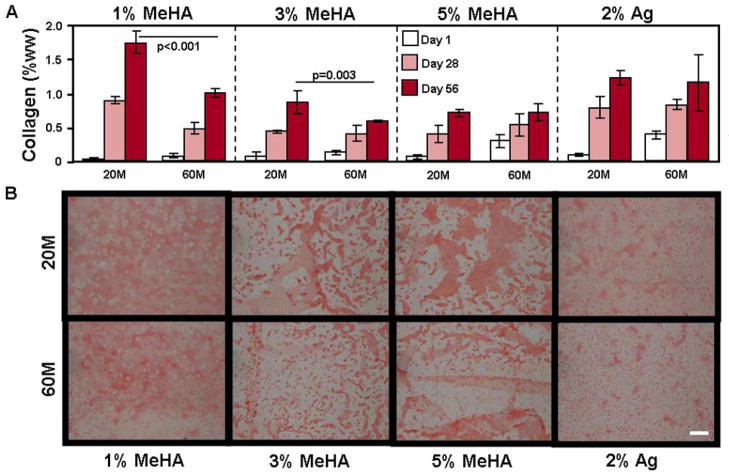
Consistent with these observed changes in functional properties, sGAG content in 20M 1% HA constructs reached 3.5% wet weight (%ww) while 60M constructs reached 4.8% ww, a value similar to native bovine cartilage (Fig. 3A) [35]. The 3% HA constructs reached 1.8% ww (20M) and 2.1% ww (60M) sGAG content, while the 5% HA constructs reached 1.2% ww (20M) and 1.4% ww (60M) sGAG content (Fig. 3A). sGAG content in the 2% Ag constructs reached 1.9% ww (20M) and 3.0% ww (60M). Collagen content showed differing trends; in 60M 1% HA constructs, collagen reached 1.0% ww, a level significantly less than in the 20M constructs (1.8% ww, p<0.001, Fig. 4A). Similarly, 60M 3% HA reached 0.6% collagen while 20M constructs reached 0.9%. Conversely, 20M and 60M 5% HA and 2% Ag constructs were equivalent at 0.7% and 1.2% collagen, respectively (Fig. 4A).
Figure 3.
(A) Concentration of sulfated glycosaminoglycan (sGAG) as a percent of the construct wet weight (%ww) in MSC-laden methacrylated HA (MeHA) and agarose (Ag) hydrogels at seeding densities of 20 million MSCs/mL (20M) and 60 million (60M) MSCs/mL after 1 (white), 28 (grey), and 56 (dark grey) days of in vitro culture in a chemically defined chondrogenic medium with TGF-β3 (10 ng/mL). (n=4 constructs per group; bars indicate p<0.05) (B) Alcian blue staining of proteoglycans after 56 days in MSC-laden HA and Ag constructs at 20M and 60M seeding densities. (100X magnification; 200 μm scale bar)
Figure 4.
(A) Collagen concentration as a percent of the construct wet weight (%ww) in MSC-laden methacrylated HA (MeHA) and agarose (Ag) hydrogels after 56 days at seeding densities of 20 million MSCs/mL (20M) and 60 million (60M) MSCs/mL after 1 (white), 28 (grey), and 56 (dark grey) days of in vitro culture in a chemically defined chondrogenic medium with TGF-β3 (10 ng/mL). (n=4 constructs per group; bars indicate p<0.05) (B) Picrosirius red staining of collagen in day 56 sections of MSC-laden HA and Ag constructs at 20M and 60M seeding densities. (100X magnification; 200 μm scale bar)
Consistent with biochemical measures, proteoglycan staining in 60M 1% HA was more intense than in the 20M group, while collagen staining was more intense for 20M samples (Fig. 3B and 4B). No differences in staining were observed in 3% or 5% HA, and increasing MSC seeding density did not result in less aggregation of accumulated matrix proteins (Fig. 3B and 4B). Similar to 1% HA, the 60M 2% Ag control constructs were stained more intensely for proteoglycan than their 20M counterparts (Fig. 3B).
Maturation of high density constructs with orbital shaking
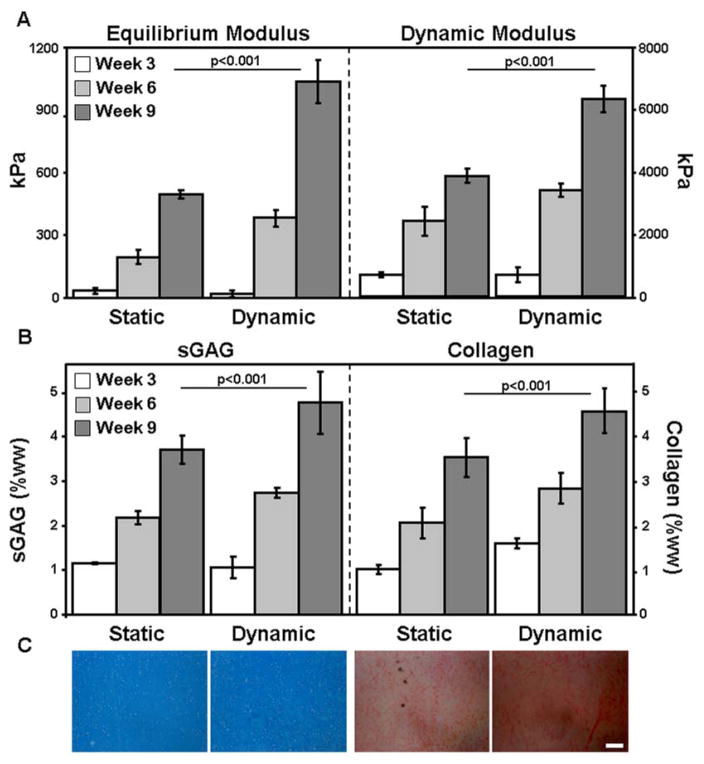
Gentle mixing of the culture medium had a profound effect on the maturation of high MSC density 1% HA constructs. Both the equilibrium and dynamic moduli of these constructs doubled with dynamic culture, reaching over 1 MPa and 6 MPa, respectively, by 9 weeks (Fig. 5A). sGAG and collagen content reached 4.8% (sGAG) and 4.5% (collagen), levels 30% and 29% greater than ‘static culture’ controls (Fig. 5B). Histological analyses confirmed high levels of proteoglycan and collagen deposition in all groups, with slightly higher collagen staining intensity in the ‘dynamic culture’ constructs (Fig. 5C).
Figure 5.
(A) Equilibrium (EY) and dynamic modulus (|G*|) of static and dynamic culture of 1% methacrylated HA (MeHA) constructs seeded at 60 million MSCs/mL after 3 (white), 6 (grey), and 9 (dark grey) weeks of in vitro culture. (B) sGAG and collagen concentration after 3 (white), 6 (grey), and 9 (dark grey) weeks. (n=4–5 constructs per group; bars indicate p<0.05) (C) Proteoglycan (left) and collagen staining (right) of week 9 constructs. (100X magnification; 200 μm scale bar)
Discussion
Engineered articular cartilage may be ideal for the restoration of focal defects, but only if it has the capacity to achieve mechanical properties matching that of native tissue. The objective of this study was to determine if increasing the seeding density of MSCs in HA hydrogels would enhance construct maturation, and whether the density of the surrounding polymer chains would influence this process. We hypothesized that a greater MSC density would be particularly important in higher macromer density HA (3% and 5%) gels, where spatial distribution of large macromolecules is limited. This limited diffusivity of formed matrix has been noted in other hydrogel systems as well (e.g., in fibrin/alginate composite constructs of increasing concentration [36]). Contrary to our original hypothesis, increased MSC density in 3% and 5% HA constructs did not improve matrix distribution, accumulation, or the development of functional properties. Our previous work showed that higher macromer concentrations of HA are less permissive to formed matrix distribution [26] and the current findings indicate that even a 3-fold increase in MSC density does not enable the formation of a functionally contiguous matrix in these higher macromer concentration hydrogels. Conversely, a higher initial MSC density (60M) in low macromer concentration (1%) HA constructs did increase the functional properties, with a nearly 3-fold increase in equilibrium properties to 313 kPa (Fig. 2B) after 8 weeks of culture. Interestingly, and in keeping with previous work, agarose constructs were independent of seeding density [22]. These results highlight the fundamental differences between HA and agarose hydrogels, and establish that functional gains can be achieved with higher seeding densities, but that these changes are highly dependent on the material formulation employed.
In this work, we used a modified version of hyaluronic acid (HA) to form the stable, covalently crosslinked backbone of the hydrogel. HA plays a critical role in anchoring large proteoglycans in the cartilage extracellular matrix [37, 38]. Cells also interact directly with HA through CD44 receptors, and this interaction can modulate cell migration, proliferation, differentiation, and HA degradation [39]. Interestingly, HA added to human MSCs in alginate increases cartilage matrix production [20], suggesting a direct biologic role for this molecule as well. Human MSCs possess abundant CD44 receptors and undergo chondrogenesis to a greater extent in these crosslinked HA networks compared to similarly crosslinked (but bioinert) poly(ethylene glycol) (PEG) gels [25]. Like PEG, agarose is a bioinert microenvironment that permits MSC chondrogenesis, but does not provide natural adhesion sites and is not degradable and so precludes cell-mediated remodeling. This may in part explain why increasing MSC density in HA constructs leads to greater functional properties than agarose constructs.
The ability for cells to remodel their microenvironment within the HA constructs is particularly relevant when considering the biochemical and biomechanical differences between the 20M and 60M 1% HA groups. The equilibrium modulus was ~3-fold greater in the 60M group, while the sGAG concentration was only ~25% greater, and the collagen concentration was lower, ~50% of the 20M constructs (Fig. 2–4). This disparity between mechanical properties and biochemical constituents indicates that other factors may be responsible for the significant increase in function observed. As cartilage develops, collagen becomes more organized [35] and is better crosslinked, resulting in increases in cartilage mechanical properties [40]. Likewise, expression analyses showed that MSCs differentiate towards a chondrocyte phenotype in agarose, but that hundreds of genes remain differentially expressed between the two cell types [16]. Therefore, the observed increase in mechanics in low macromer density HA constructs may result from cell-mediated matrix remodeling, or a contribution from other matrix constituents that were more highly expressed in this natural HA microenvironment. Further analysis is warranted to identify these key mediators of mechanical function.
The ability to remodel the surrounding matrix may also be critical for enhanced mechanical function with increasing MSC density. Along these lines, increasing initial MSC seeding density increased chondrogenesis on a per cell basis in a gelatin foam material, though mechanical properties were not assessed in that study [21]. Similarly, Wang and colleagues seeded umbilical cord MSCs at 5, 25, and 50 million/mL in a non-woven polyglycolic acid mesh and reported that matrix accumulation and mechanical integrity increased as a function of density [41]. Maher et al seeded 30 and 60 million MSCs/mL in a self-assembling peptide hydrogel to promote integration in a gap model of cartilage repair. They reported that hydrogel seeded at a higher MSC density formed a more cartilage-like material and increased the integration strength [42]. Similar to the HA gel employed in the present work, these studies were conducted in materials that are permissive to matrix remodeling and/or degradation, which may offer insight into why they benefit from high MSC density unlike agarose or other non-degradable materials. Current studies, using degradable linkages [25] within our HA network will further analyze this important parameter.
It has also been noted that MSCs are particularly sensitive to nutrient supply [43]. To address this concern, we cultured our best performing high density constructs (1% HA, 60 million cells/mL) in medium with continual agitation. This simple modification to the culture environment resulted in profound increases in bulk mechanics and matrix accumulation. Under these conditions, equilibrium properties reached levels in excess of 1 MPa, and sGAG content of 4.8% of the construct wet weight. These values match or exceed native tissue levels, and represent the highest ever achieved in this HA system. While this exact technique has not reportedly been used in conjunction with any other MSC-based cartilage tissue engineering approach, perfusion and rotating wall bioreactors have been utilized to increase nutrient transport for chondrocyte-based systems [44, 45]. Vunjak-Novakovic et al observed significant increases in all biochemical and mechanical metrics when chondrocyte seeded fibrous polyglycolic acid scaffolds were cultured in a rotating wall bioreactor [46]. Conversely, work by Sheehy et al reported an adverse effect on the growth of MSCs in agarose gels in a rotating wall bioreactor over 3 weeks [47]. In alginate, Hannouche et al and found that MSC chondrogenesis was delayed compared to the same MSCs in a collagen hydrogel [48] under rotational culture. These observations indicate that material environments not only differentially regulate MSC differentiation and matrix assembly, but also their response to dynamic culture conditions. Indeed, even in the case of dynamic compression, MSCs do not initially respond favorably to this stimulus when encased within an agarose hydrogel, but given time to mature and synthesize pericellular matrix, a robust response follows [49]. Conversely, in HA gels, it appears that compressive loading can be initiated at the outset of culture without any deleterious early effects, and in the long term this loading can promote more robust growth [50]. In the present study, dynamic culture was initiated at the time constructs were formed, though the degree of mixing was likely less than would occur in a rotating bioreactor system. The relationship between material microenvironment and dynamic fluid environments requires additional study to optimize this growth potential, and to understand the mechanisms governing this response.
Conclusions
HA hydrogels formed at a macromer concentration of 1% offer a permissive microenvironment to encapsulate MSCs at a high density (60 million/mL), resulting in constructs with a compressive modulus of 313 kPa at 8 weeks, ~50% greater than our best MSC-based results reported to date [22]. Dynamic culture accelerated the maturation of these high MSC density 1% HA constructs, with native tissue mechanical (~1MPa) and sGAG (4.8%) levels reached within 9 weeks of in vitro culture. These findings represent a significant step towards the development of functional MSC-based engineered tissue for cartilage repair.
Acknowledgments
This work was supported by the National Institutes of Health (R01 EB008722), the Penn Center for Musculoskeletal Disorders, and a Graduate Research Fellowship from the National Science Foundation (IEE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. Osteochondral Defects in the Human Knee. Am J Sports Med. 2004;32:1451–8. doi: 10.1177/0363546504263234. [DOI] [PubMed] [Google Scholar]
- 2.Magnussen RA, Mansour AA, Carey JL, Spindler KP. Meniscus status at anterior cruciate ligament reconstruction associated with radiographic signs of osteoarthritis at 5- to 10-year follow-up: a systematic review. J Knee Surg. 2009;22:347–57. doi: 10.1055/s-0030-1247773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding L, Heying E, Nicholson N, Stroud NJ, Homandberg GA, Buckwalter JA, et al. Mechanical impact induces cartilage degradation via mitogen activated protein kinases. Osteoarthritis Cartilage. 2005;18:1509–17. doi: 10.1016/j.joca.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ateshian GA, Soltz MA, Mauck RL, Basalo IM, Hung CT, Lai WM. The role of osmotic pressure and tension-compression nonlinearity in the frictional response of articular cartilage. Transport Porous Med. 2003;50:5–33. [Google Scholar]
- 5.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 6.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001:S362–9. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 7.Jones CW, Willers C, Keogh A, Smolinski D, Fick D, Yates PJ, et al. Matrix-induced autologous chondrocyte implantation in sheep: objective assessments including confocal arthroscopy. J Orthop Res. 2008;26:292–303. doi: 10.1002/jor.20502. [DOI] [PubMed] [Google Scholar]
- 8.Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025–33. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ng KW, Lima EG, Bian L, O'Conor CJ, Jayabalan PS, Stoker AM, et al. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16:1041–51. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient Exposure to Transforming Growth Factor Beta 3 Under Serum-Free Conditions Enhances the Biomechanical and Biochemical Maturation of Tissue-Engineered Cartilage. Tissue Eng Part A. 2008;14:1821–34. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bian L, Fong JV, Lima EG, Stoker AM, Ateshian GA, Cook JL, et al. Dynamic Mechanical Loading Enhances Functional Properties of Tissue-Engineered Cartilage Using Mature Canine Chondrocytes. Tissue Eng Part A. 2010;16:1781–90. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CR, Grodzinsky AJ, Hsu HP, Martin SD, Spector M. Effects of harvest and selected cartilage repair procedures on the physical and biochemical properties of articular cartilage in the canine knee. J Orthop Res. 2000;18:790–9. doi: 10.1002/jor.1100180517. [DOI] [PubMed] [Google Scholar]
- 13.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 14.Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. Journal of Biomechanics. 2010;43:128–36. doi: 10.1016/j.jbiomech.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauck RL, Yuan X, Tuan RS. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage. 2006;14:179–89. doi: 10.1016/j.joca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Huang AH, Stein A, Mauck RL. Evaluation of the Complex Transcriptional Topography of Mesenchymal Stem Cell Chondrogenesis for Cartilage Tissue Engineering. Tissue Engineering Part A. 2010;16:2699–708. doi: 10.1089/ten.tea.2010.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879–90. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Chang SC, Rowley JA, Tobias G, Genes NG, Roy AK, Mooney DJ, et al. Injection molding of chondrocyte/alginate constructs in the shape of facial implants. J Biomed Mater Res. 2001;55:503–11. doi: 10.1002/1097-4636(20010615)55:4<503::aid-jbm1043>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 19.Huang CY, Reuben PM, D'Ippolito G, Schiller PC, Cheung HS. Chondrogenesis of human bone marrow-derived mesenchymal stem cells in agarose culture. Anat Rec. 2004;278A:428–36. doi: 10.1002/ar.a.20010. [DOI] [PubMed] [Google Scholar]
- 20.Kavalkovich KW, Boynton RE, Murphy JM, Barry FP. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 2002;38:457–66. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. Journal of Biomedical Materials Research. 2000;52:246–55. doi: 10.1002/1097-4636(200011)52:2<246::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Huang AH, Stein A, Tuan RS, Mauck RL. Transient Exposure to Transforming Growth Factor Beta 3 Improves the Mechanical Properties of Mesenchymal Stem Cell-Laden Cartilage Constructs in a Density-Dependent Manner. Tissue Engineering Part A. 2009;15:3461–72. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–26. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386–91. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung C, Burdick JA. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243–54. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erickson IE, Huang AH, Sengupta S, Kestle S, Burdick JA, Mauck RL. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17:1639–48. doi: 10.1016/j.joca.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30:4287–96. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041–52. doi: 10.1089/ten.tea.2008.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrell MJ, Comeau ES, Mauck RL. Dynamic Culture Improves Mechanical Functionality of MSC-Laden Tissue Engineered Constructs in a Depth-Dependent Manner. Proceedings of the ASME 2010 Summer Bioengineering Conference; Farmingtion, Pennsylvania. 2011. [Google Scholar]
- 30.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 31.Park S, Nicoll S, Mauck R, Ateshian G. Cartilage mechanical response under dynamic compression at physiological stress levels following collagenase digestion. Ann Biomed Eng. 2008;36:425–34. doi: 10.1007/s10439-007-9431-6. [DOI] [PubMed] [Google Scholar]
- 32.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 33.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 34.Neuman RE, Logan MA. The determination of hydroxypoline. J Biol Chem. 1949:299–306. [PubMed] [Google Scholar]
- 35.Erickson IE, Van Veen SC, Sengupta S, Kestle RS, Mauck RL. Cartilage Matrix Formation by Bovine Mesenchymal Stem Cells in Three-dimensional Culture Is Age-dependent. Clin Orthop Relat Res. 2011 doi: 10.1007/s11999-011-1869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho ST, Cool SM, Hui JH, Hutmacher DW. The influence of fibrin based hydrogels on the chondrogenic differentiation of human bone marrow stromal cells. Biomaterials. 2010;31:38–47. doi: 10.1016/j.biomaterials.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 37.Mankin HJ, Mow VC, Buckwalter JA, Iannotti JP, Ratcliffe A. Form and Function of Articular Cartilage. In: Simon SR, editor. Orthopaedic Basic Science. Rosemont, Il: AAOS; 1994. pp. 1–44. [Google Scholar]
- 38.Knudson C. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol. 1993;120:825–34. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Embry JJ, Knudson W. G1 domain of aggrecan cointernalizes with hyaluronan via a CD44-mediated mechanism in bovine articular chondrocytes. Arthritis & Rheumatism. 2003;48:3431–41. doi: 10.1002/art.11323. [DOI] [PubMed] [Google Scholar]
- 40.Guilak F, Sah RL, Setton LA. Physical regulation of cartilage metabolism. In: Mow VC, Hayes WC, editors. Basic orthopaedic biomechanics. 2. Philadelphia: Lippincott-Raven; 1997. pp. 179–207. [Google Scholar]
- 41.Wang L, Seshareddy K, Weiss ML, Detamore MS. Effect of Initial Seeding Density on Human Umbilical Cord Mesenchymal Stromal Cells for Fibrocartilage Tissue Engineering. Tissue Engineering Part A. 2009;15:1009–17. doi: 10.1089/ten.tea.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maher SA, Mauck RL, Rackwitz L, Tuan RS. A nanofibrous cell-seeded hydrogel promotes integration in a cartilage gap model. J Tissue Eng Regen Med. 2009;4:25–9. doi: 10.1002/term.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pattappa G, Heywood HK, de Bruijn JD, Lee DA. The metabolism of human mesenchymal stem cells during proliferation and differentiation. Journal of Cellular Physiology. 2010 doi: 10.1002/jcp.22605. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 44.Chen HC, Lee HP, Sung ML, Liao CJ, Hu YC. A novel rotating-shaft bioreactor for two-phase cultivation of tissue-engineered cartilage. Biotechnol Prog. 2004;20:1802–9. doi: 10.1021/bp049740s. [DOI] [PubMed] [Google Scholar]
- 45.Sittinger M, Bujia J, Minuth WW, Hammer C, Burmester GR. Engineering of cartilage tissue using bioresorbable polymer carriers in perfusion culture. Biomaterials. 1994;15:451–6. doi: 10.1016/0142-9612(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 46.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130–8. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 47.Sheehy EJ, Buckley CT, Kelly DJ. Chondrocytes and bone marrow-derived mesenchymal stem cells undergoing chondrogenesis in agarose hydrogels of solid and channelled architectures respond differentially to dynamic culture conditions. J Tissue Eng Regen Med. 2011 doi: 10.1002/term.385. [DOI] [PubMed] [Google Scholar]
- 48.Hannouche D, Terai H, Fuchs JR, Terada S, Zand S, Nasseri BA, et al. Engineering of Implantable Cartilaginous Structures from Bone Marrow†“Derived Mesenchymal Stem Cells. Tissue Engineering. 2007;13:87–99. doi: 10.1089/ten.2006.0067. [DOI] [PubMed] [Google Scholar]
- 49.Huang AH, Farrell MJ, Kim M, Mauck RL. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur Cell Mater. 2010;19:72–85. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA. Dynamic Compressive Loading Enhances Cartilage Matrix Synthesis and Distribution and Suppresses Hypertrophy in hMSC-Laden Hyaluronic Acid Hydrogels. Tissue Eng Part A. doi: 10.1089/ten.tea.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]