Abstract
Inappropriate survival signaling after DNA damage may facilitate clonal expansion of genetically compromised cells, and it is known that protein tyrosine phosphatase (PTP) inhibitors activate key survival pathways. In this study we employed the genotoxicant, hexavalent chromium [Cr(VI)], which is a well-documented carcinogen of occupational and environmental concern. Cr(VI) induces a complex array of DNA damage, including DNA double strand breaks (DSBs). We recently reported that PTP inhibition bypassed cell cycle arrest and abrogated Cr(VI)-induced clonogenic lethality. Notably, PTP inhibition resulted in an increase in forward mutations at the HPRT locus, supporting the hypothesis that PTP inhibition in the presence of DNA damage may lead to genomic instability (GIN), via cell cycle checkpoint bypass. The aim of the present study was to determine the effect of PTP inhibition on DNA DSB formation and chromosomal integrity after Cr(VI) exposure. Diploid human lung fibroblasts were treated with Cr(VI) in the presence or absence of the PTP inhibitor, sodium orthovanadate, for up to 24 hours, and cells were analyzed for DNA DSBs and chromosomal damage. Cr(VI) treatment induced a rapid increase in DNA DSBs, and a significant increase in total chromosomal damage (chromatid breaks and gaps) after 24 hours. In sharp contrast, PTP inhibition abrogated both DNA DSBs and chromosomal damage after Cr(VI) treatment. In summary, PTP inhibition in the face of Cr(VI) genotoxic stress decreases chromosomal instability (CIN) but increases mutagenesis, which we postulate to be a result of error-prone DNA repair.
Keywords: Chromosomal stability, mutagenesis, PTP inhibition, Cr(VI)
INTRODUCTION
It is known that protein tyrosine phosphorylation plays a critical role in maintaining cell survival, particularly during neoplastic progression [1]. In general, cell survival is promoted by protein tyrosine kinases. Conversely, cell survival is reduced largely by protein tyrosine phosphatases (PTPs) [1;2]. Therefore, PTP inhibition promotes activation of key survival pathways [3]. We have previously reported that maintenance of protein tyrosine phosphorylation through PTP inhibition was associated with increased proliferation, clonogenic survival, and mutagenesis in normal diploid lung cells after exposure to hexavalent chromium [Cr(VI)], a well documented genotoxicant, certain forms of which are associated with respiratory carcinogenesis [4]. Indeed, the enhancement of clonogenic survival and mutagenesis by PTP inhibition after Cr(VI) exposure was the result of an override of the Cr-induced growth arrest at both G1/S and G2/M checkpoints [5;6].
Cr(VI)-induced DNA damage has been detailed by us and others (for review, see [7;8]), and has been shown to result in DNA double strand breaks (DSBs), which endanger genomic stability, and consequently, are potentially lethal (for review see [9]). Indeed, Cr(VI)-induced DNA DSBs are significant lesions that if left unrepaired, result in cell cycle arrest and cell death. Furthermore, proper repair of Cr(VI)-induced DNA DSBs plays an important role in protecting cells from Cr(VI)-induced chromosome instability (CIN) [10;11]. Studies in our laboratory have shown that exposure of normal human cells to Cr(VI) was associated with a prolonged G1/S and G2/M arrest [12]. In light of our recent reports that PTP inhibition bypasses the G2/M cell cycle checkpoint [6], and enhances clonogenic survival and mutagenesis after Cr(VI) exposure [4], we postulated that the override of Cr-induced cell cycle arrest by PTP inhibition was at the expense of proper DNA DSB repair. The objective of the present study was to investigate the effect of PTP inhibition on the formation of DNA DSBs after Cr(VI) exposure in normal human lung fibroblasts. We further investigated the effect of PTP inhibition on CIN as a hallmark of genomic instability (GIN) resulting from Cr(VI)-induced DNA DSBs. Our findings suggest that dysregulation of tyrosine phosphorylation may facilitate inappropriate DNA DSB repair after cell cycle checkpoint bypass following genotoxic insult.
MATERIALS AND METHODS
Materials and chemical reagents
Sodium chromate (Na2CrO4.4H2O; [Cr(VI)]) was purchased from J.T. Baker Chemicals, Phillipsburg, NJ, USA. Sodium orthovanadate (Na3VO4; SOV); was purchased from Sigma-Aldrich, St. Louis, MO. Other chemicals were from Fisher Scientific and/or Sigma-Aldrich, unless indicated otherwise.
Cell culture
The normal diploid human lung fibroblast (HLF) cell line, LL24, was obtained from ATCC (Manassas, VA) and was originally isolated from normal autopsy tissue of an 11-year-old male. Cells were maintained in Ham s F-12 nutrient mixture which was supplemented with 15% fetal bovine serum (Hyclone Laboratories, Inc., Logan, UT.) containing 50 units/ml penicillin and 50 ug/ml streptomycin at 37°C in a humidified incubator in an atmosphere containing 5% CO2. Medium was replaced every 48 hours.
Experimental treatment of cells
Both sodium chromate [Cr(VI)] and sodium orthovanadate (SOV) were respectively dissolved in deionized H2O and sterilized by passage through a 0.2 micron filter. Cells were treated with the indicated final concentration of Cr(VI) for the duration of the indicated time points in complete medium. In experiments in which SOV was co-incubated with Cr(VI), SOV was added 30 minutes prior to Cr(VI) addition.
CometAssay under neutral conditions to measure DNA DSBs
HLF cells were seeded in 60 mm dishes at a density of 300,000 cells for 24 hours prior to treatment. Cells were then treated without and with 10 uM SOV for 30 minutes, and then co-treated without and with 0 or 3 μM Cr(VI) for 15 minutes- 24 hours. Single cell gel electrophoresis was performed using the Trevigen CometAssay Kit under neutral conditions to detect mainly DNA DSBs, as previously described [13]. Briefly, collected cells were added to low melting point agarose and placed onto slides. The slides were incubated at 4°C in the dark for 30 minutes, placed in cold Lysis Solution at 4°C for 30 minutes, and then electrophoresed in a horizontal electrophoresis chamber. The slides were fixed in 70% ethanol, stained with SyBr® Green I, and DNA fragmentation was visualized with an Olympus BX-60 light/fluorescence microscope. The comets were analyzed with Image Pro Plus 5.1, which was used to circumscribe the head and tail regions of each comet and the integrated fluorescence values of each defined area were recorded. The tail:head fluorescence ratio was used as a relative measure of DNA fragmentation for each sample [13]. An average of 50 individual comets was scored per sample. The data were expressed as fold of respective untreated control.
Clastogenesis assay
Cells were seeded at a density of 250,000 cells per 100 mm dish and allowed to grow for 24 hours. Cells were pretreated with 10 μM SOV for 30 minutes and then co-treated with 0–3 μM Cr(VI) for 24 hours. Five hours prior to the end of the treatment time, 0.1 μg/ml colcemid was added to the dishes to block the cells in metaphase. After treatment, medium was collected and the cells were washed with PBS. Cells were harvested and re-suspended in 0.075 M potassium chloride (KCl) hypotonic solution to swell the cells, as previously described [14]. Then methanol: acetic acid fixative (3:1) was added to the hypotonic solution, and the cells were centrifuged for 5 minutes at 1000 rpm. The supernatant was removed and the pellet was re-suspended in methanol:acetic acid fixative, and were kept at 4 °C for at least 1 hour. Cells were centrifuged and the fixative was changed twice. The cells were dropped on a clean wet slide and stained with 5% Giemsa stain in Gurr s buffer. Each experiment was repeated at least three times. One hundred metaphases per data point were analyzed in each experiment. Chromosome aberrations were scored by standard criteria as described in detail [14;15].
RESULTS
PTP inhibition enhances the repair of genotoxin-induced DNA DSBs
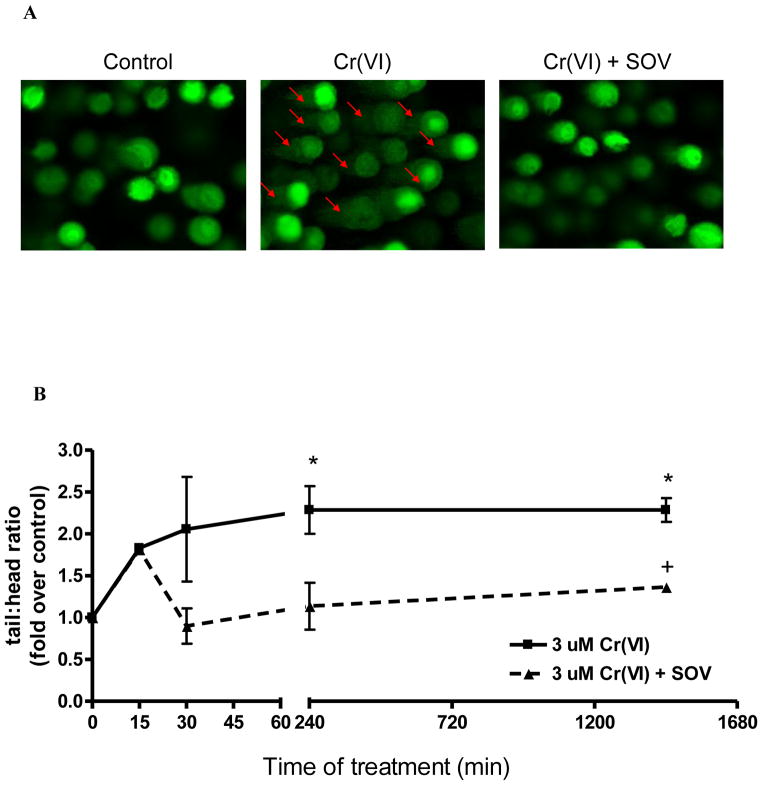
Our previous studies indicate that PTP inhibition reversed Cr(VI)-induced clonogenic lethality and bypassed the G2/M cell cycle checkpoint, resulting in increased mutation frequency, which was not a result of altered Cr-DNA adduct levels [4;6]. Therefore, we postulated that Cr(VI)-induced DNA DSB repair was enhanced by PTP inhibition, potentially by an error-prone mechanism, thereby increasing mutagenesis. We studied DNA DSB induction by Cr(VI) in the absence and presence of PTP inhibition with SOV by using the comet assay under neutral conditions to detect mainly DNA DSBs. As seen in Figure 1, 3 μM Cr(VI) exposure for 24 hours (1,440 minutes) induced an approximate 2 fold increase in DNA DSBs as early as 15 minutes. The Cr(VI)-induced increase in DNA DSBs significantly peaked at ~ 2.3 fold of control at 4 hours (240 minutes) exposure, which was maintained for 24 hours. SOV treatment alone, in the absence of Cr(VI) exposure, had no effect on DNA DSB formation (data not shown). Likewise, SOV co-treatment had no effect on Cr(VI)-induced DNA DSBs upon initial exposure (time 0, data not shown). Cr(VI)-induced DNA DSBs were maintained at 15 minutes of treatment in the presence of SOV, however, SOV co-treatment abrogated Cr(VI)-induced DNA DSBs from 30 minutes to 24 hours time-of-treatment (Figure 1).
Figure 1. PTP inhibition enhances the repair of Cr(VI)-induced DNA DSBs.
Single cell gel eletrophoresis was performed using Trevigen CometAssay Kit on HLF cells treated with the indicated concentrations of Cr(VI) ± 10 μM SOV for 30 minutes. DNA fragmentation was visualized with an Olympus BX-60 light/fluorescence microscope and comets were analyzed with Image Pro Plus 5.1. A) Representative comets at 30 min of treatment (arrows indicate comet “tails”) B) Data are means ± SD of 2 experiments expressed as fold of respective control. * indicates a statistically significant difference from the respective control at p < 0.05. + indicates a statistically significant difference between the samples treated with and without SOV at p < 0.05.
PTP inhibition decreases chromosomal damage after Cr(VI) exposure
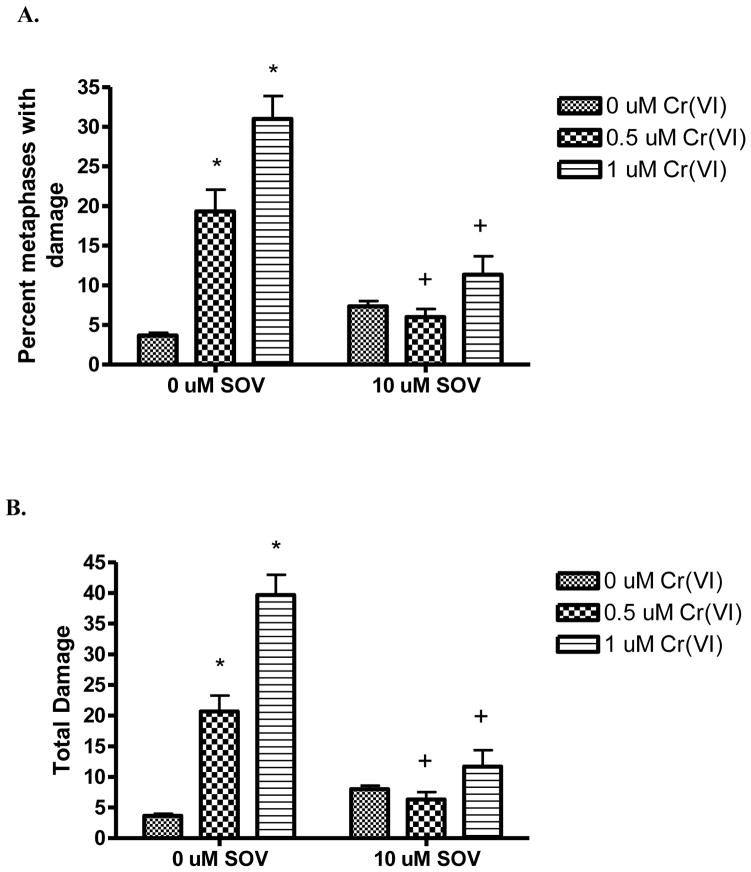
We have previously found that PTP inhibition increases Cr(VI)-induced mutagenesis[4]. In light of the ability of the PTP inhibitor to attenuate Cr(VI)-induced DNA DSBs, we postulated that PTP inhibition may enhance genomic instability, at the expense of chromosomal instability after Cr(VI) exposure. We investigated clastogenesis as a measure of chromosomal damage and chromosomal instability [14]. HLF cells were treated with 0, 0.5, and 1 μM Cr(VI) in the presence and absence of the PTP inhibitor, SOV, for 24 hours. Cells were blocked in metaphase and then harvested and analyzed for chromosomal aberrations. As seen in Figure 2A, Cr(VI) induced a concentration-dependent increase in the percent of metaphases with damage by 3.3-fold and 9.3-fold at 0.5 μM and 1 μM Cr(VI), respectively, as compared to control. Chromosomal analyses was not possible after 3 μM Cr(VI) exposure, due to insufficient metaphases, as a result of cell cycle arrest. Cr(VI) increased the total chromosomal damage by 6.1-fold and 11.9-fold at 0.5 μM and 1 μM Cr(VI), respectively as compared to control (Figure 2B). Notably, PTP inhibition significantly abrogated the number of metaphases with chromosomal aberrations observed with Cr(VI) treatment. Table 1 shows the spectrum of chromosome damage observed with 24 h Cr(VI) and SOV co-treatment. As previously reported, Cr(VI)-induced chromosomal damage consisted mainly of chromosome breaks and gaps, while all other types of damage were negligible [14]. Notably, the Cr(VI)-induced increase in chromosome lesions was completely abrogated by PTP inhibition.
Figure 2. PTP inhibition decreases chromosomal damage after Cr(VI) exposure.
Data are presented as the percent metaphases with damage (A), which reflects the number of metaphases with at least one aberration, and as total damage (B), which reflects the total amount of chromosome damage in 100 metaphases. The data are mean + SE of 3 independent experiments. * indicates a significant increase from untreated control at p < 0.05. + indicates a statistically significant difference between the samples treated with and without SOV at p < 0.05.
Table 1.
Effect of PTP inhibition on Cr(VI)-induced spectrum of chromosome aberrations in HLF cells.
| [Cr(VI)], μM | 0 | 0.5 | 1.0 | |||
| [SOV], μM | 0 | 10 | 0 | 10 | 0 | 10 |
| Chromatid breaks | 1.33 (0.67) | 3.00 (1.00) | 10.67 * (2.33) | 2.00 + (1.00) | 22.33 * # (1.45) | 4.67 + (1.76) |
| Chromatid gaps | 1.33 (0.33) | 2.33 (0.33) | 8.67 * (1.45) | 2.33 + (0.33) | 12.33 * (2.67) | 5.00 + (0) |
| Isochromatid breaks | nd | 0.33 (0.33) | 0.67 (0.67) | 0.67 (0.33) | 1.67 (0.88) | 1.00 (0.58) |
| Isochromatid gaps | nd | 1.00 (0.58) | 0.33 (0.33) | 0.67 (0.33) | 0.67 (0.33) | 0.33 (0.33) |
| Chromatid exchanges | 0.33 (0.33) | 1.00 (0) | 0.67 (0.67) | 0.33 (0.33) | 2.00 (1.16) | nd |
| Dicentrics | 0.33 (0.33) | nd | 0.33 (0.33) | nd | 0.67 | nd |
| Acentric fragments | 0.33 (0.33) | nd | nd | 0.33 (0.33) | 1.00 (0.58) | 0.33 (0.33) |
| Double minute chromosomes | nd | 0.33 (0.33) | 0.33 (0.33) | nd | 0.33 (0.33) | nd |
Data are the mean and (SEM) of 3 experiments. nd = not detected.
indicates a statistically significant difference from the respective untreated control at p < 0.05;
indicates a statistically significant difference between Cr(VI) concentrations at p<0.05;
indicates a statistically significant difference between the samples treated with and without SOV at p < 0.05.
DISCUSSION
There is a considerable amount of evidence that protein tyrosine phosphorylation is responsible for the maintenance of proliferative signals and is involved in the early stages of neoplasia [1]. Inhibitors of PTPs, such as SOV, promote the activation of key survival signaling pathways [3;16;17], while our recent report showed that PTP inhibition enhances clonogenic survival and Cr(VI)-induced mutation frequency in normal diploid mammalian cells [4]. This increase in survival and mutagenesis is associated with an override of Cr(VI)-induced growth arrest through the bypass of the G2/M checkpoint [6]. Therefore, we postulated that PTP inhibition may enhance/provoke error-prone DNA repair, and thus, genomic instability (GIN).
Due to the potential for PTP inhibition to enhance GIN as a result of Cr-induced G2/M checkpoint bypass, we investigated the ability of the PTP inhibitor to alter DNA DSB formation and repair after Cr(VI) exposure. It has been previously reported by us and others that Cr(VI) induces DNA DSBs in a replication-dependent fashion, presumably via replication fork collapse [13;18]. We found that DNA DSBs increased ~ 2 fold by 15 minutes of 3 μM Cr(VI) exposure in the absence or presence of the PTP inhibitor, indicating that the action of the PTP inhibitor is not to prevent DSB formation. However, Cr(VI)-induced DNA DSBs were abrogated by co-treatment with the PTP inhibitor from 30 minutes to 24 hours, indicating the ability of the PTP inhibitor to facilitate DNA DSB repair in the presence of Cr(VI). Moreover, PTP inhibition decreased Cr(VI)-induced chromosomal damage, in particular chromosome lesions, which is consistent with its effect on DNA DSBs. Notably, the PTP inhibitor-induced effect was not related to a difference in Cr-DNA binding, as PTP inhibition had no effect on either Cr uptake or Cr-DNA adduct levels, as previously reported [4].
All eukaryotic cells have conserved two primary mechanisms in which DNA DSBs are repaired: homologous recombination (HR) and non-homologous end-joining (NHEJ) [9;19–21]. NHEJ has been found to be the major repair pathway in mammalian cells for DNA DSBs and is known as the “error-prone” DNA DSB repair pathway [22;23]. Simply stated, NHEJ allows the broken ends of the DNA DSB to be juxtaposed and rejoined by DNA ligation, without regard for sequence fidelity [23]. Interestingly, Stackpole et al. found an increase in CIN (chromatid exchanges) of Cr(VI)-treated HR-deficient cells restricted to NHEJ DNA DSB repair [10]. A possible explanation as to why more exchanges were not found in the present study is most likely because the cells have proficient HR repair. Indeed, studies now show that NHEJ and HR can be cooperative systems and are not mutually exclusive [24]. Moreover, NHEJ has been found to proceed by different pathways [25]. Therefore we postulate that PTP inhibition may activate certain NHEJ pathways, consequently leading to a more mutagenic repair at the DNA sequence level, but that the proficient HR repair can cooperate with NHEJ to avoid the more complex chromosomal aberrations. In accordance with our data, the activation of pro-survival signaling through tyrosine phosphorylation-mediated pathways such as Akt/Erk/EGFR may enhance double strand break repair after DNA damage potentially through an increase in NHEJ activity [26]. Likewise inhibitors of Akt and Erk have been shown to inhibit NHEJ [27].
Concurrent with our data, PTP inhibition also abrogated the 2.5 fold increase in DNA DSBs induced by neocarzinostatin (NCS), a radiomimetic that produces DNA DSBs and induces a prolonged growth arrest, similar to Cr(VI) (data not shown). We have previously shown γH2AX (a marker for DNA DSBs) is expressed following Cr(VI) and neocarzinostatin exposure [12;13]. In contrast, PTP inhibition had no effect on the 2.5 fold increase in DNA DSBs induced by methyl methanesulfonate (MMS), an alkylating agent (data not shown). This is in keeping with our report that PTP inhibition enhanced clonogenic survival, induced prolonged growth arrest and increased mutagenesis in the presence of Cr(VI), but not MMS [4].
Taken together, our data suggest that the previously reported inappropriate cell cycle progression, enhanced survival and increased mutagenesis induced by PTP inhibition in the presence of Cr(VI) exposure [4] is associated with enhanced DNA DSB repair and a decrease in chromosomal damage. The PTP inhibitor-enhanced DNA repair that occurs after 15 minutes and persists for the duration of Cr(VI) exposure suggests a mechanism involving an error-prone end joining repair pathway since it results in increased Cr(VI)-induced mutagenesis [4]. We hypothesize that an error-prone NHEJ repair pathway could mediate fast repair of DNA DSBs induced by Cr(VI), which would result in increased mutagenesis and increased chromosomal stability as the ends of the DNA DSB are resolved. Recently, it was reported that Cr(VI)-induced chromosomal instability is partly due to an unscheduled activation of APC/C activities [28]. Further studies in our laboratory are currently underway to elucidate the mechanism of error-prone repair pathways after Cr(VI) exposure.
Acknowledgments
This work was supported in part by NIH grants CA107972 and ES017334 (S.C.); a PhRMA Foundation predoctoral fellowship (G.C.K.); NIH grant ES05304 (S.R.P.); NIEHS grant ES016893 (J.P.W.); and the Maine Center for Toxicology and Environmental Health at the University of Southern Maine.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 2.Chernoff J. Protein tyrosine phosphatases as negative regulators of mitogenic signaling. J Cell Physiol. 1999;180:173–181. doi: 10.1002/(SICI)1097-4652(199908)180:2<173::AID-JCP5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 3.Furlong F, Finlay D, Martin F. PTPase inhibition restores ERK1/2 phosphorylation and protects mammary epithelial cells from apoptosis. Biochem Biophys Res Commun. 2005;336:1292–1299. doi: 10.1016/j.bbrc.2005.08.260. [DOI] [PubMed] [Google Scholar]
- 4.Bae D, Camilli TC, Chun G, Lal M, Wright K, O'Brien TJ, Patierno SR, Ceryak S. Bypass of hexavalent chromium-induced growth arrest by a protein tyrosine phosphatase inhibitor: enhanced survival and mutagenesis. Mutat Res. 2009;660:40–46. doi: 10.1016/j.mrfmmm.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lal MA, Bae D, Camilli TC, Patierno SR, Ceryak S. AKT1 mediates bypass of the G1/S checkpoint after genotoxic stress in normal human cells. Cell Cycle. 2009;8:1589–1602. doi: 10.4161/cc.8.10.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun G, Bae DS, Nickens K, O'Brien T, Patierno SR, Ceryak S. Polo-like kinase 1 enhances survival and mutagenesis after genotoxic stress in normal cells through cell cycle checkpoint bypass. Carcinogenesis. 2010;31:785–793. doi: 10.1093/carcin/bgq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Wise SS, Holmes AL, Wise JP., Sr Hexavalent chromium-induced DNA damage and repair mechanisms. Rev Environ Health. 2008;23:39–57. doi: 10.1515/reveh.2008.23.1.39. [DOI] [PubMed] [Google Scholar]
- 9.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection [Review] [76 refs] Nature Genetics. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 10.Stackpole MM, Wise SS, Goodale BC, Duzevik EG, Munroe RC, Thompson WD, Thacker J, Thompson LH, Hinz JM, Wise JP., Sr Homologous recombination repair protects against particulate chromate-induced chromosome instability in Chinese hamster cells. Mutat Res. 2007;625:145–154. doi: 10.1016/j.mrfmmm.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes AL, Wise SS, Sandwick SJ, Wise JP., Sr The clastogenic effects of chronic exposure to particulate and soluble Cr(VI) in human lung cells. Mutat Res. 2006;610:8–13. doi: 10.1016/j.mrgentox.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Ha L, Ceryak S, Patierno SR. Chromium (VI) activates ataxia telangiectasia mutated (ATM) protein: Requirement of ATM for both apoptosis and recovery from terminal growth arrest. Journal of Biological Chemistry. 2003;278:17885–17894. doi: 10.1074/jbc.M210560200. [DOI] [PubMed] [Google Scholar]
- 13.Ha L, Ceryak S, Patierno SR. Generation of S phase-dependent DNA double strand breaks by Cr(VI) exposure: involvement of ATM in Cr(VI) induction of {gamma}-H2AX. Carcinogenesis. 2004 doi: 10.1093/carcin/bgh242. [DOI] [PubMed] [Google Scholar]
- 14.Wise JP, Sr , Wise SS, Little JE. The cytotoxicity and genotoxicity of particulate and soluble hexavalent chromium in human lung cells. Mutat Res. 2002;517:221–229. doi: 10.1016/s1383-5718(02)00071-2. [DOI] [PubMed] [Google Scholar]
- 15.Wise JP, Leonard JC, Patierno SR. Clastogenicity of lead chromate particles in hamster and human cells. Mutation Research. 1992;278:69–79. doi: 10.1016/0165-1218(92)90287-a. [DOI] [PubMed] [Google Scholar]
- 16.Chen WL, Harris DL, Joyce NC. Effects of SOV-induced phosphatase inhibition and expression of protein tyrosine phosphatases in rat corneal endothelial cells. Exp Eye Res. 2005;81:570–580. doi: 10.1016/j.exer.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto J, Morioka M, Hasegawa Y, Kawano T, Yoshinaga Y, Maeda T, Yano S, Kai Y, Fukunaga K, Kuratsu J. Sodium orthovanadate enhances proliferation of progenitor cells in the adult rat subventricular zone after focal cerebral ischemia. J Pharmacol Exp Ther. 2006;318:982–991. doi: 10.1124/jpet.106.104562. [DOI] [PubMed] [Google Scholar]
- 18.Xie H, Wise SS, Holmes AL, Xu B, Wakeman TP, Pelsue SC, Singh NP, Wise JP., Sr Carcinogenic lead chromate induces DNA double-strand breaks in human lung cells. Mutat Res. 2005;586:160–172. doi: 10.1016/j.mrgentox.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karagiannis TC, El Osta A. Double-strand breaks: signaling pathways and repair mechanisms. Cell Mol Life Sci. 2004;61:2137–2147. doi: 10.1007/s00018-004-4174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hefferin ML, Tomkinson AE. Mechanism of DNA double-strand break repair by non-homologous end joining. DNA Repair (Amst) 2005;4:639–648. doi: 10.1016/j.dnarep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Sonoda E, Hochegger H, Saberi A, Taniguchi Y, Takeda S. Differential usage of non-homologous end-joining and homologous recombination in double strand break repair. DNA Repair (Amst) 2006;5:1021–1029. doi: 10.1016/j.dnarep.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 23.Valerie K, Povirk LF. Regulation and mechanisms of mammalian double-strand break repair. Oncogene. 2003;22:5792–5812. doi: 10.1038/sj.onc.1206679. [DOI] [PubMed] [Google Scholar]
- 24.Mladenov E, Iliakis G. Induction and repair of DNA double strand breaks: the increasing spectrum of non-homologous end joining pathways. Mutat Res. 2011;711:61–72. doi: 10.1016/j.mrfmmm.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Iliakis G. Backup pathways of NHEJ in cells of higher eukaryotes: cell cycle dependence. Radiother Oncol. 2009;92:310–315. doi: 10.1016/j.radonc.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Golding SE, Morgan RN, Adams BR, Hawkins AJ, Povirk LF, Valerie K. Pro-survival AKT and ERK signaling from EGFR and mutant EGFRvIII enhances DNA double-strand break repair in human glioma cells. Cancer Biol Ther. 2009;8:730–738. doi: 10.4161/cbt.8.8.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kriegs M, Kasten-Pisula U, Rieckmann T, Holst K, Saker J, Dahm-Daphi J, Dikomey E. The epidermal growth factor receptor modulates DNA double-strand break repair by regulating non-homologous end-joining. DNA Repair (Amst) 2010;9:889–897. doi: 10.1016/j.dnarep.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Hu L, Liu X, Chervona Y, Yang F, Tang MS, Darzynkiewicz Z, Dai W. Chromium induces chromosomal instability, which is partly due to deregulation of BubR1 and Emi1, two APC/C inhibitors. Cell Cycle. 2011;10:2373–2379. doi: 10.4161/cc.10.14.16310. [DOI] [PMC free article] [PubMed] [Google Scholar]