Abstract
We tested the hypothesis that sodium nitrite treatment reverses large elastic artery stiffening in old mice via reductions in collagen I, increases in elastin and/or decreases in advanced glycation endproducts (AGEs) mediated by reduced oxidative stress. Aortic pulse wave velocity (aPWV), a measure of large elastic artery stiffness, was greater in old (26–28 mo) compared with young (4–6 mo) control animals (520 ± 9 vs. 405 ± 6 cm/s, p<0.05), and this was reversed by 3 weeks of sodium nitrite treatment (50 mg/L) (435 ± 17 cm/s). Age-related increases (p<0.05) in aortic superoxide production were associated with greater total and adventitial nitrotyrosine staining, all of which were reversed by nitrite treatment. Total and adventitial transforming growth factor β and collagen I were increased, and total and medial elastin were reduced with aging (p<0.05), but were unaffected by sodium nitrite. Aorta from old mice had increased total, adventitial and medial AGEs (p<0.05 vs. young), which were normalized by sodium nitrite treatment. In aortic segments from young mice in vitro, pyrogallol (10 µM), a superoxide generator, induced an “aging-like” increase in AGEs, and direct treatment with AGEs induced vascular stiffening; these effects were prevented by incubation with sodium nitrite. De-stiffening of aged large elastic arteries by short-term sodium nitrite therapy is mediated in part by normalization of AGEs secondary to amelioration of oxidative stress.
Keywords: arterial stiffness, collagen, elastin, nitrotyrosine, superoxide
Cardiovascular diseases (CVD) are the leading cause of mortality in modern societies (Lloyd-Jones and others 2010), and advancing age is the primary risk factor for CVD (Lakatta 2002; Lakatta and Levy 2003). The influence of aging on CVD is attributable in part to stiffening of the large elastic arteries (aorta and carotid arteries), which, in turn, has several pathophysiological effects on the heart and other organs (Lakatta and Levy 2003; Mitchell 2009). Indeed, aortic pulse wave velocity (aPWV), the gold standard clinical measure of large elastic artery stiffness (Vlachopoulos and others 2010), has emerged as a strong independent risk factor for incident CVD among older adults (Mattace-Raso and others 2006; Mitchell and others 2010; Sutton-Tyrrell and others 2005; Willum Hansen and others 2006). As such, establishing treatments that can reduce aPWV in older adults and identifying the associated mechanisms of action have important clinical implications for the prevention of age-associated CVD.
The nitrite anion, a metabolite of the cardiovascular-protective molecule nitric oxide (NO), is stored in the circulation and tissues and, when needed, can be enzymatically or non-enzymatically reduced to NO to increase NO (Webb and others 2008; Lundberg and others 2009). We recently demonstrated that 3-weeks of treatment with sodium nitrite essentially normalizes aPWV in old mice, while having no effect on young animals (Sindler and others 2011). However, the mechanisms associated with this de-stiffening effect of sodium nitrite on large elastic arteries of old mice are unknown.
Several changes to the walls of large elastic arteries are thought to contribute to their stiffening with aging including increases in collagen content, fragmentation and loss of elastin and increases in advanced glycation end-products (AGEs), the latter causing cross-linking of structural proteins (Diez 2007). The development of oxidative stress is believed to play an important role in some or all of these changes with aging (Fleenor and others 2010; Fleming and others 2011; Klemm and others 2011). Therefore, it is possible that the de-stiffening effect of sodium nitrite with aging is associated with reduced oxidative stress and changes in one or more of these structural factors. Accordingly, here we tested the hypothesis that reductions in aPWV with sodium nitrite treatment in old mice would be associated with reductions in collagen, increases in elastin and/or decreases in AGEs in the aorta and, if so, whether any such change could be linked to reduced oxidative stress.
In a recent study (Fleenor and others 2010), we showed that another intervention that reduces large elastic artery stiffness in old mice, voluntary wheel running, decreased expression of collagen I, the primary load-bearing structural protein in arteries, and that this was linked to decreases in transforming growth factor β (TGFβ), a pro-fibrotic cytokine. Moreover, we found that changes in structural proteins with aging and voluntary wheel running, were, in some cases, specific to the medial (middle) or adventitial (outer) layers of the artery. Therefore, in the present study we also determined if sodium nitrite treatment reduces TGFβ and collagen I, and if changes in the structural factors in question are observed in the medial and/or adventitial layers.
To do so, we measured these structural factors in both whole aortic lysates (western blotting) and specifically in the medial and adventitial layers (immunohistochemical analysis of cross-sections of the aortic wall). To assess the role of oxidative stress, we measured aortic superoxide production directly via electron paramagnetic resonance spectroscopy, assessed nitrotyrosine, a cellular marker of oxidation of proteins (Radi 2004), and conducted complementary in vitro aortic ring experiments. Additional in vitro experiments were conducted to assess the mechanistic role of AGES.
Methods
Animals
Male C57BL/6 mice were obtained from the National Institute of Aging rodent colony. Young (4–6 months) and old (26–28 months) mice were housed in an animal care facility at the University of Colorado at Boulder on a 12:12 light:dark cycle and fed normal rodent chow. All animals had access to water. Control mice had regular drinking water and treated mice had nitrite-supplemented water (50 mg/L) for three weeks as described previously (Sindler and others 2011). All procedures were approved by the campus Animal Care and Use Committee and conformed to the US National Institutes of Health guidelines “Guide to the Care and Use of Laboratory Animals” (NIH publication no. 85-23, revised 1996).
aPWV
aPWV was assessed as previously described (Sindler and others 2011). Briefly, anesthetized mice (2% isoflurane) were placed supine on a heating board and legs secured to ECG electrodes. Doppler probes were placed on the transverse aortic arch and abdominal aorta with pre-ejection time, the time between the R-wave of the ECG to foot of the Doppler signal, determined for each site. PWV was calculated by dividing distance between the probes by the difference in the pre-ejection times of the thoracic and abdominal regions.
Aortic superoxide production
Aortic superoxide production was measured by electron paramagnetic resonance (EPR) spectrometry as previously described (Rippe and others 2010; Sindler and others 2011). Aortic rings (2 mm) were incubated for 60 minutes at 37° C in Krebs-HEPES buffer (200 µl) wit h the spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (Alexis Biochemicals) and immediately analyzed on an MS300 X-band EPR spectrometer (Magnettech).
Western blotting
Whole artery protein expression was determined by western blotting as described previously (Fleenor and others 2010; Rippe and others 2010; Sindler and others 2011). The aorta was removed, cleaned of perivascular fat, frozen in liquid nitrogen and stored at −80°C. The aorta was pulverized and then homogenized in ice-cold RIPA lysis buffer containing protease and phosphatase inhibitors (Roche) and 0.01% phosphatase inhibitor cocktail (Sigma). Equal volume (10 micrograms) of protein was loaded on a polyacrylamide gradient gel (4–12%), separated by electrophoresis and transferred to a nitrocellulose membrane. Primary antibodies for specific proteins of interest include: nitrotyrosine (1:100, Abcam), TGFβ (1:200, Santa Cruz), collagen type I (1:1000, Millipore), alpha elastin (1:100, Abcam), AGEs (1:1000, GeneTex) and GAPDH (1:1000, Cell Signaling). Bands were analyzed with ImageJ software (NIH), normalized to GAPDH and expressed relative to the control group.
Immunhistochemistry
Immunohistochemistry and quantification were performed as described previously (Fleenor and others 2010). Thoracic aorta segments were excised and frozen in optimal cutting temperature compound (Fisher Scientific) in liquid nitrogen cooled isopentane. Seven-micron sections were fixed with acetone and washed in Tris Buffer. All slides were stained in one batch with the Dako EnVision+System-HRP-DAB kit according to the manufacturer’s protocol (Dako) using the following primary antibodies: nitrotyrosine (1:2000, Millipore), TGFβ (1:1000, Santa Cruz), collagen type I (1:4000, Millipore), alpha elastin (1:25, Abcam) and AGEs (1:200, GeneTex). Primary antibodies were incubated for 1 hour at 4° C. The labeled polymer secondary was applied for 30 minutes and staining was visualized after a 2-minute exposure to diaminobenzidine. Slides were subsequently dehydrated and cover slipped. Digital photomicrographs were obtained using a Nikon Eclipse TS100 photomicroscope, and quantification was performed with Image-Pro Plus software (Media Cybernetics). The adventitial and medial layers for each sample were quantified independently and summed to determine the whole vessel expression.
In vitro aortic ring studies
The aorta was excised from young mice (6 months) and perivascular fat removed from the artery. The vessel was cut into equal segments and placed in a 6-well plate containing DMEM (with antibiotics), DMEM + pyrogallol (10 µM), a superoxide generating chemical, DMEM + pyrogallol (10 µM) + sodium nitrite (50 mM or 100 µM) for 72 hours. The media for each condition was changed daily. The vessels were lysed and AGEs (1:1000, GeneTex) protein expression was assessed via western blot. Because the pyrogallol treatment induced significant changes in our normalizing proteins, AGEs was normalized to total protein for each lane on the membrane that was determined with a reversible protein stain (Thermo Scientific) as previously described (Moningka and others 2011).
In vitro intrinsic mechanical properties
Aortic segments (1–2 mm in length) from young mice (6 months) were cleaned of perivascular fat and placed in a 6-well plate containing DMEM (with antibiotics, Sigma), DMEM + bovine serum albumin (BSA, MBL international), DMEM + sodium nitrite (100 µM, Sigma), DMEM + AGEs-BSA (100 µg/mL, MBL international) or DMEM + AGEs-BSA (100 µg/mL) + sodium nitrite (100 µM) for 72 hours that was changed daily. After treatment arterial segments were placed in a pre-heated (37° C) and calibrated wire myograp h chamber (DMT) containing calcium-free phosphate buffered saline. Aortic samples were loaded onto the wire myograph and pre-stretched for 3 minutes to a 1 mm luminal diameter displacement that was returned to the non-stretched baseline, and this was repeated 2 times. To begin the experiment after pre-stretching, segments were stretched to a baseline force of 1 mN and luminal displacement was increased incrementally (~10% increase) every 3 minutes. At the end of every 3 minute increment the force was recorded and displacement increased until mechanical failure of the tissue occurred, defined by an observed transient decrease in force. Stress and strain were calculated where stress was defined as: t = λL / 2HD. t = one-dimensional stress, λ = strain, L = one-dimensional load applied, H = wall thickness, D = length of vessel. Strain was defined as: λ = Δd / d(i). λ = strain, Δd = change in diameter, d(i) = initial diameter. The slope of the stress-strain curve was used to determine the elastic modulus (Humphrey 2002).
Statistical Analysis
Data are presented as mean ± S.E.M. All analyses were performed with PASW 18. A two-way ANOVA was used to analyze pulse wave velocity, superoxide, western blots and immunohistochemistry. The in vitro ring studies were analyzed with a one-way ANOVA. Post hoc tests were used where appropriate. Significance was set at p<0.05.
Results
Animal characteristics
Animal characteristics of the groups are presented in Table 1. Body and heart mass were greater in the old compared with the young control groups (p<0.05). Sodium nitrite treatment had no effect on these characteristics in either young or old mice.
Table 1.
Animal characteristics
| YC | OC | YN | ON | |
|---|---|---|---|---|
| Body mass (g) | 27.7 ± 0.7 | 33.2 ± 1.0* | 27.1 ± 1.1 | 31.7 ± 1.1* |
| Heart mass (g) | 138 ± 5 | 186 ± 8* | 136 ± 2 | 187 ± 8* |
Values are mean ± S.E.M.
P < 0.05, Main effect of age
aPWV
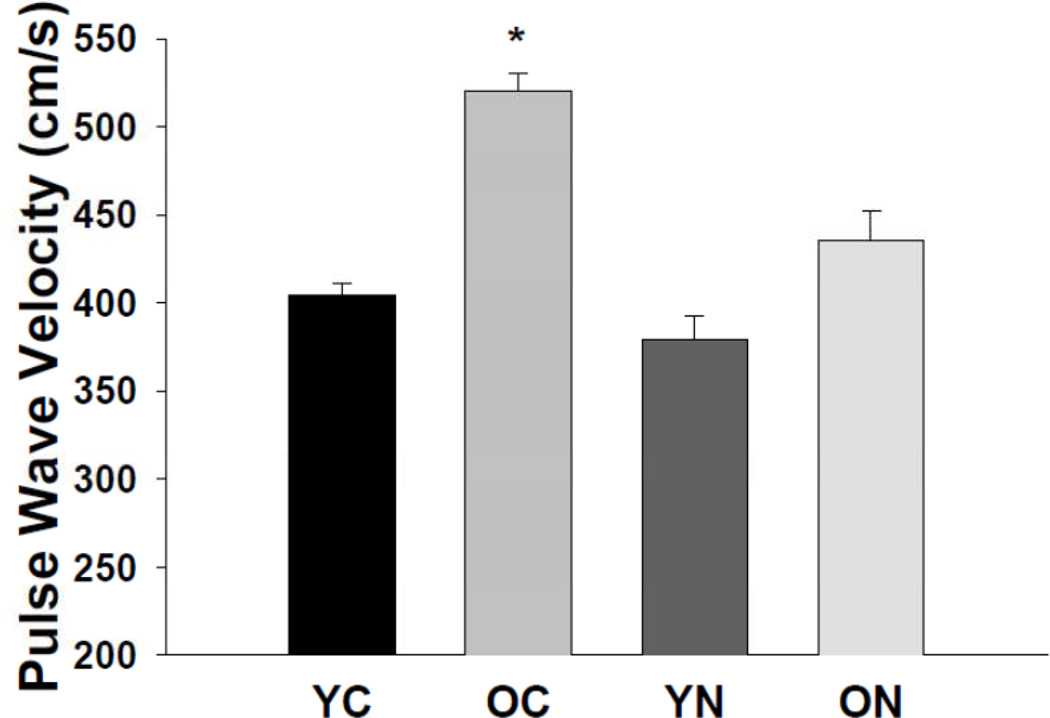
aPWV was greater in old compared with young control mice (p<0.05, Figure 1). Sodium nitrite reduced aPWV in old mice (p<0.05) to levels not significantly different from young controls, without affecting aPWV in young mice.
Figure 1. Aortic pulse wave velocity.
Aortic pulse wave velocity in young and old control (YC and OC) and nitrite supplemented (YN and ON) mice (n = 7–9 per group). Values are mean ± S.E.M. * p<0.05 vs. all.
Aortic superoxide production and nitrotyrosine abundance
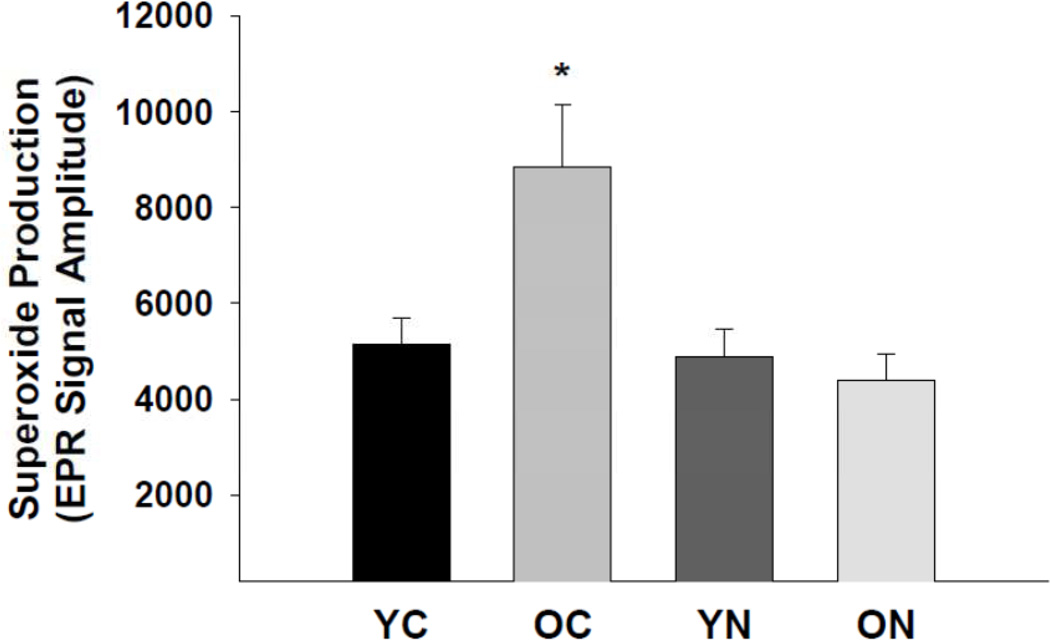
Superoxide production was greater in the aorta of old compared with young control mice (p<0.05, Figure 2). Sodium nitrite treatment normalized aortic superoxide production in the old mice (p<0.05), but had no effect in the young mice.
Figure 2. Aortic superoxide production.
Mean electron paramagnetic resonance signal in young and old control (YC and OC) and nitrite supplemented (YN and ON) mice (n = 6–8 per group). Values are mean ± S.E.M. * p<0.05 vs. all.
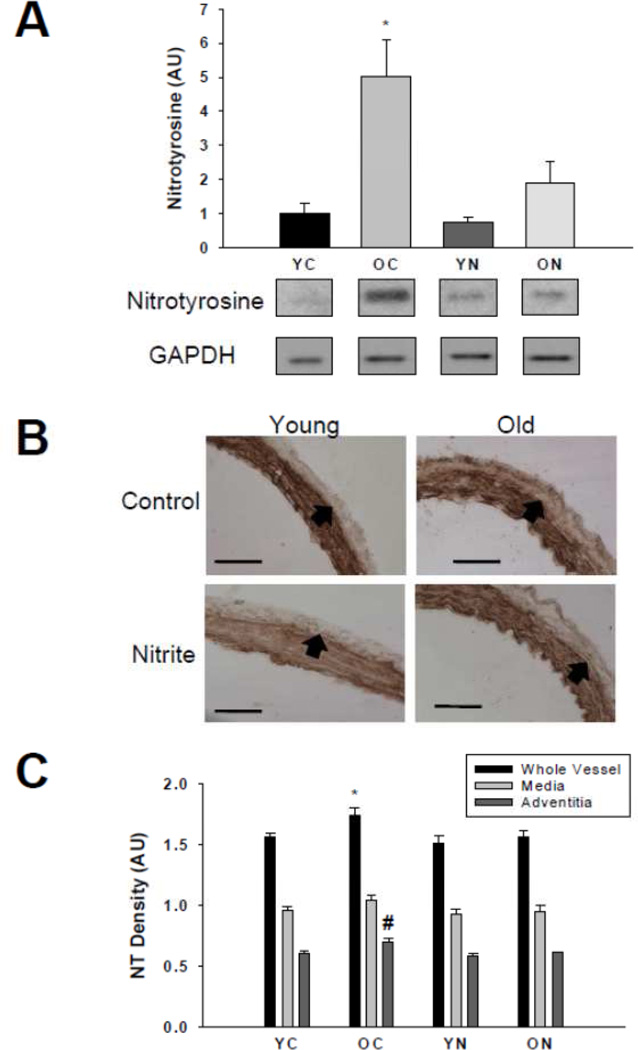
Nitrotyrosine abundance was greater in the whole aorta (Figure 3A) and adventitial layer (Figure 3B and C) of old compared with young control mice (p<0.05), whereas no significant differences were observed in the medial layer (p=0.10, Figure 3B and C). Sodium nitrite treatment reduced nitrotyrosine in the whole aorta and adventitial layer in old mice to levels not different from young controls, without affecting the abundance in the medial layer of old animals (p=0.36 old nitrite vs. old controls) or any segment of young mice.
Figure 3. Nitrotyrosine abundance.
Thoracic aorta nitrotyrosine abundance assessed by (A) western blot of whole artery lysates and (B) histological cross-sections with (C) quantification from young and old control (YC and OC) and nitrite supplemented (YN and ON) mice (n = 4–8 per group). Values are mean ± S.E.M. * p<0.05 vs. whole vessel YC, YN, and ON; # p<0.05 vs. adventitia YC, YN and ON. Arrows demarcate the medial-adventitial border; Bar = 100 µm.
Aortic TGFβ and collagen I expression
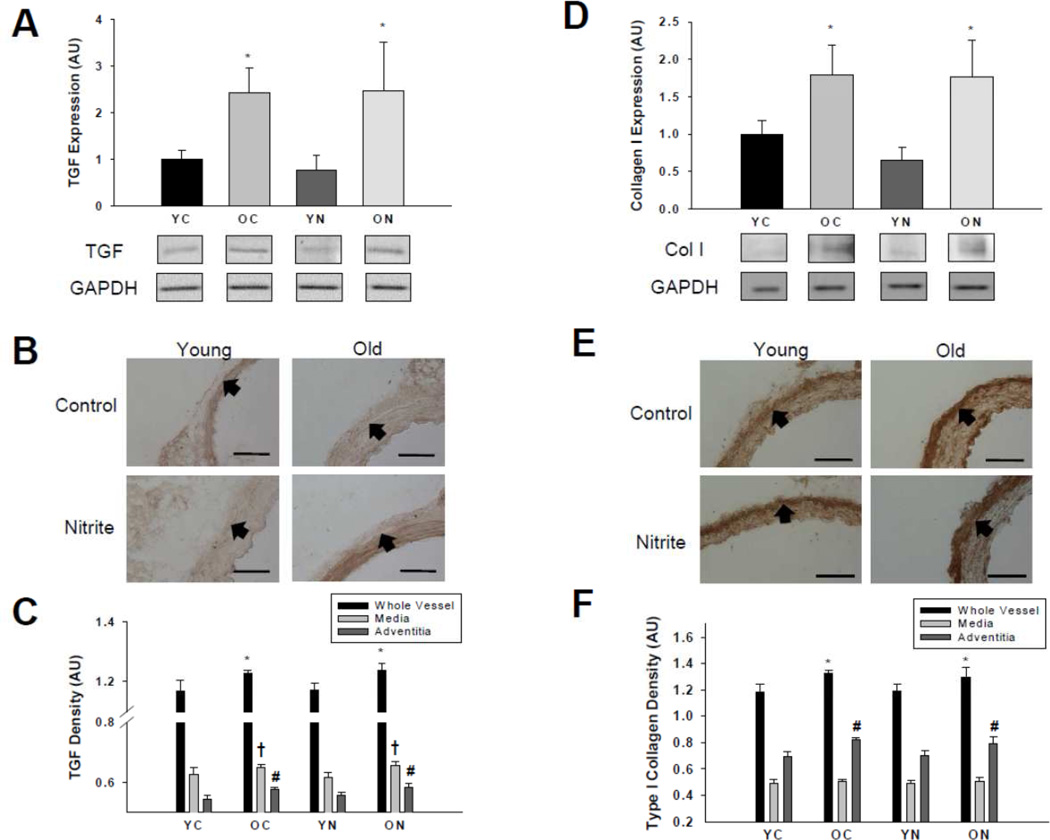
TGFβ expression was greater in the whole aorta (Figure 4A), medial and adventitial layers (Figure 4B and C) of old compared with young control mice (all p<0.05). Sodium nitrite treatment had no effect on TGFβ expression in the whole aorta or either layer of the aorta in either age group.
Figure 4. Transforming growth factor β and type I collagen protein expressions.
Thoracic aorta expression of transforming growth factor β (A, B, C) and type I collagen (D, E, F) assessed by (A, D) western blot of whole artery lysates and (B, E) histological cross-sections with (C, F) quantification from young and old control (YC and OC) and nitrite supplemented (YN and ON) mice (n = 4–8 per group). Values are mean ± S.E.M. * p<0.05 vs. whole vessel YC and YN; † p<0.05 vs. media YC and YN; # p<0.05 vs. adventitia YC and YN. Arrows demarcate the medial-adventitial border; Bar = 100 µm.
Expression of collagen I was greater in the whole aorta (Figure 4D) and the adventitial layer, but not the media (Figure 4E and F), of old control mice compared with the young animals (p<0.05). Sodium nitrite did not affect collagen I in the whole aorta or either layer in either age group.
Aortic elastin expression
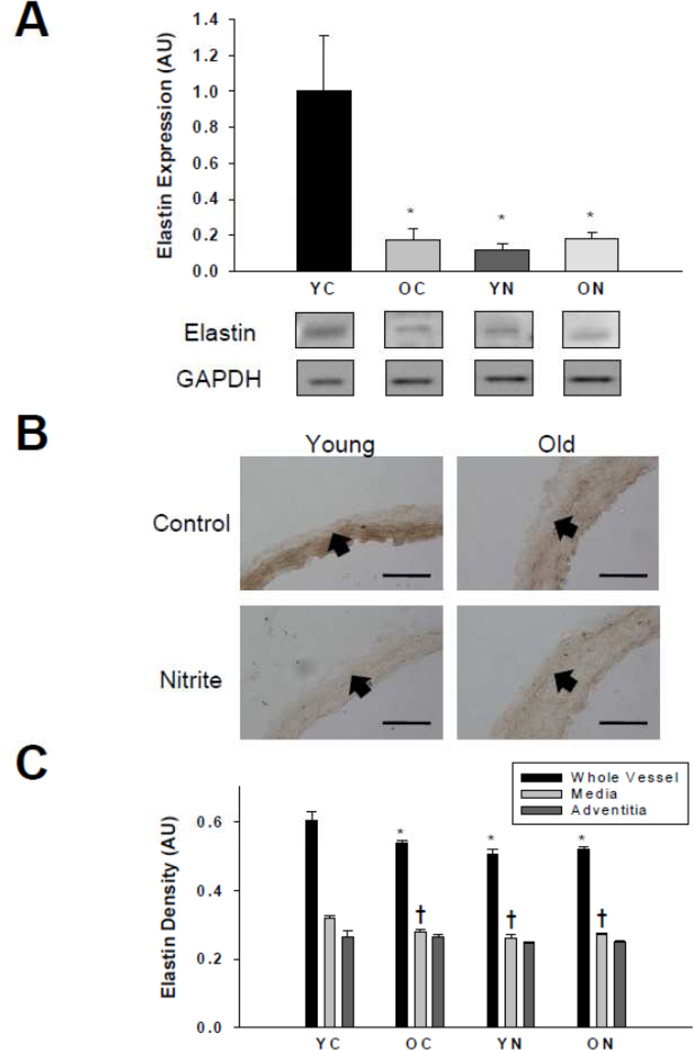
Elastin was lower in the whole aorta (Figure 5A) and medial layer (Figure 5B and C), of old compared with young control mice (p<0.05). Sodium nitrite treatment had no effect on elastin in the whole aorta or any layer in the old mice, although reduced expression was observed in the young nitrite-treated animals.
Figure 5. Elastin protein expression.
Thoracic aorta expression of elastin assessed by (A) western blot of whole artery lysates and (B) histological cross-sections with (C) quantification from young and old control (YC and OC) and nitrite supplemented (YN and ON) mice (n = 4–8 per group).Values are mean ± S.E.M. * p<0.05 vs. whole vessel YC; † p<0.05 vs. media YC. Arrows demarcate the medial-adventitial border; Bar = 100 µm.
Aortic AGEs
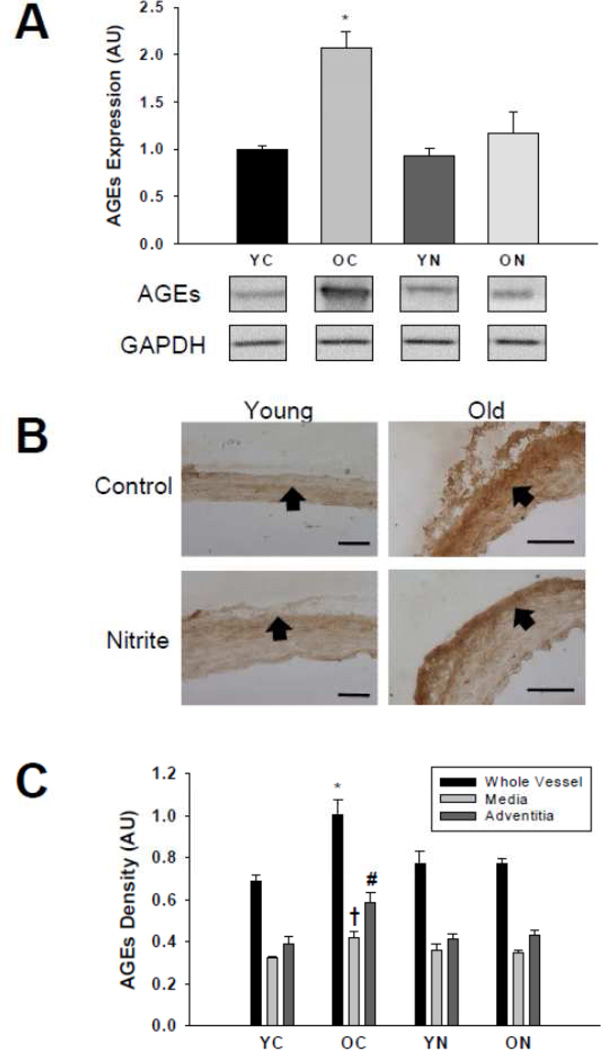
The abundance of AGEs was greater in the whole aorta, medial and adventitial layers (Figure 6A, B and C) of old compared with young control animals (p<0.05). Sodium nitrite treatment reversed AGEs accumulation in the whole aorta, media and adventitia in old mice (p<0.05) to levels not different from young controls, without affecting accumulation in young treated mice.
Figure 6. AGEs protein expression.
Thoracic aorta expression of AGEs assessed by (A) western blot of whole artery lysates and (B) histological cross-sections with (C) quantification from young and old control (YC and OC) and nitrite supplemented (YN and ON) mice (n = 4–8 per group). Values are mean ± S.E.M. * p<0.05 vs. whole vessel YC, YN and ON; † p<0.05 vs. media YC, YN and ON; # p<0.05 vs. adventitia YC, YN and ON. Arrows demarcate the medial-adventitial border; Bar = 100 µm.
Effects of superoxide and sodium nitrite on AGEs in aortic tissue
Because sodium nitrite treatment reduced AGEs accumulation throughout the vessel wall of old mice, in vitro experiments were performed to establish more directly that sodium nitrite inhibits oxidative stress-induced accumulation of AGEs in aortic tissue. Aortic rings from young mice treated with the superoxide generator, pyrogallol, showed an ~120% increase in AGEs (p<0.05, Figure 7A and B). Treatment of aortic rings with either 50 mM (Figure 7A) or 100 µM (Figure 7B) sodium nitrite inhibited AGEs accumulation induced by pyrogallol (p<0.05). These in vitro data support the concept that our short-term sodium nitrite treatment in vivo inhibited superoxide-induced aortic AGEs expression in our old mice.
Figure 7. AGEs protein expression and stiffening effects in aortic segments in vitro.
Effects of pyrogallol (Pyr) and (A) 50 mM and (B) 100 µM sodium nitrite on AGEs expression in cultured aortic segments obtained from young mice (n = 4–8 per group). (C) Effects of AGEs (100 µg/mL) and 100 µM sodium nitrite on elastic modulus, a measure of intrinsic arterial stiffness, in cultured aortic segments from young mice (n = 3–6 per group). Values are mean ± S.E.M. * p<0.05 vs. all.
Effects of AGEs and sodium nitrite on intrinsic mechanical properties in aortic segments
To determine if AGEs directly contributes to increases in arterial stiffness and sodium nitrite prevents this stiffening, additional in vitro experiments were performed assessing intrinsic mechanical properties. Aortic segments from young mice treated with AGEs demonstrated greater stiffness compared with controls (p<0.05, Figure 7C). Sodium nitrite treatment completely prevented AGEs-induced stiffening (Figure 7C).
Discussion
The major new finding of the present study is that the reduction in large elastic artery stiffness in old mice observed after treatment with sodium nitrite is associated with normalization of AGEs, which are, in turn, related to amelioration of excessive superoxide production and oxidative stress. Importantly, results from complementary in vitro experiments directly link nitrite treatment-induced reductions in superoxide (oxidative stress) and AGEs to destiffening of the aorta. Finally, our data indicate that the de-stiffening effects of sodium nitrite on large elastic arteries of old mice are not obviously associated with reductions in collagen I or TGFβ, or increases in elastin.
The present study confirms our recent finding that even short-term oral administration of sodium nitrite to old mice lowers aPWV, the most clinically important measure of large elastic artery stiffness and a strong independent risk factor for incident CVD in older adults (Mattace-Raso and others 2006; Mitchell and others 2010; Sutton-Tyrrell and others 2005; Willum Hansen and others 2006). Here we provide the first insight into the potential mechanisms by which nitrite confers its de-stiffening effects. Specifically, we found that sodium nitrite treatment ameliorated the age-related increase in aortic concentrations of AGEs, a key factor mediating the increase in large elastic artery stiffening with aging (Kass and others 2001). Moreover, our results show that nitrite therapy reduced AGEs in the whole artery (western blot analysis), as well as in both the medial and adventitial layers (immunohistochemical analysis) of aorta of old mice. These results from our primary in vivo nitrite treatment study were further extended to show a direct effect of AGEs on the mechanical properties of isolated aortic segments in vitro that was prevented with sodium nitrite treatment.
Using a combination of ex vivo biochemical analysis of the whole aortas and in vitro treatments of aortic rings from our mice, we also present evidence that the reductions in large elastic artery stiffness and AGEs in old mice treated with sodium nitrite are linked to decreases in oxidative stress. We first show that nitrite therapy completely reversed the marked age-associated increase in aortic superoxide production, confirming our recent observations (Sindler and others 2011). Next, we demonstrated that nitrotyrosine, a cellular marker of oxidative stress, was increased 5-fold in the whole aorta of old compared with young mice and that this marked age-related increase was completely reversed by nitrite treatment (Sindler and others 2011). We also show that this effect of sodium nitrite was observed in both the medial and adventitial layers of the arterial wall. In addition, sodium nitrite added to the bath of aortic rings completely inhibited the formation of AGEs produced by stimulation of oxidative stress with the superoxide-generating agent pyrogallol. Finally, sodium nitrite prevented the AGEs-induced stiffening in isolated arterial segments. Taken together, these results strongly support the idea that sodium nitrite reduces oxidative stress, which reduces AGEs accumulation and lowers aortic stiffness. Our results also are consistent with the results of a clinical trial showing that ALT-711, an AGEs cross-link breaking compound, improves large elastic artery stiffness in older adults with elevated systolic blood pressure (Kass and others 2001).
The oxidative stress-lowering influence of sodium nitrite with aging can be attributed in part to both oxidant producing and antioxidant mechanisms. We recently showed that the age-related increase in aortic expression of the pro-oxidant enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase was reversed by short-term sodium nitrite supplementation (Sindler 2011 and others). Previous findings show that sodium nitrite also can reduce NADPH oxidase activity, but does not directly scavenge superoxide in an animal model of hypertension (Montenegro and others 2011). Moreover, nitrite treatment attenuates the age-related decrease in expression of the antioxidant enzyme manganese superoxide dismutase (SOD) as well as SOD activity (Sindler 2011). Collectively, these observations suggest that sodium nitrite reduces age-associated oxidative stress, at least in part, by lowering pro-oxidant and increasing antioxidant enzyme expression and activity.
In the present study we also investigated the possibility that sodium nitrite induces de-stiffening of large elastic arteries in old animals by reversing other age-associated changes in structural proteins known to contribute to increases in stiffness. We confirmed our recent findings that stiffening of large elastic arteries with aging in mice is associated with increases in collagen I, the primary load-bearing protein (Diez 2007), and TGFβ, a pro-fibrotic cytokine that we recently showed to stimulate collagen I production in adventitial fibroblasts (Fleenor and others 2010). However, sodium nitrite did not influence whole aortic nor layer-specific expression of these proteins despite inducing clear reductions in aPWV. Moreover, although we confirmed our previous observations of age-related reductions in aortic elastin (Fleenor and others 2010), the main structural protein conferring elasticity (Diez 2007), sodium nitrite treatment had no effect on this protein either in the whole aorta nor in the medial or adventitial layers.
In the present study, sodium nitrite therapy reduced elastin expression in the aorta of young mice, an observation made previously in vitro (Paik and others 1997). Despite this nitrite-associated reduction in elastin, large elastic artery stiffness assessed by aPWV was unaffected in young animals. Thus, sodium nitrite does not adversely affect arterial stiffness in young mice and is an effective therapy for reducing age-related arterial stiffening.
In conclusion, the findings of the present study present the first evidence concerning the mechanisms by which oral sodium nitrite treatment may act to reverse large elastic artery stiffening with age. Our results indicate that the de-stiffening effects of nitrite therapy are associated with reduced superoxide-related oxidative stress and normalization of AGEs accumulation in the whole aorta, including both the medial and adventitial layers. Moreover, observations from our in vitro studies provide direct evidence that nitrite prevents oxidative stress-induced AGEs formation and consequent effects on mechanical properties of the aorta leading to stiffening. In contrast, sodium nitrite treatment does not obviously influence aortic expression of the major structural proteins collagen I or elastin. These preclinical observations provide the experimental basis for assessing the potential therapeutic effects of sodium nitrite for reducing arterial oxidative stress-associated accumulation of AGEs, improving large elastic stiffness and reducing the risk of CVD in older adults.
Highlights.
In old animals, sodium nitrite therapy:
De-stiffens large elastic arteries
Reverses arterial superoxide production and oxidative stress
Attenuates oxidant induced advanced glycation end-products formation
Prevents advanced glycation end-products induced arterial stiffening
Sodium nitrite therapy is a promising treatment for arterial stiffness with aging.
Acknowledgements
We thank Natasha Marvi, Nadia Claassen and Hylke Snieder for technical assistance. Supported by AG013038, AG000279, HL007822
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Diez J. Arterial stiffness and extracellular matrix. Advances in Cardiology. 2007;44:76–95. doi: 10.1159/000096722. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. The Journal of Physiology. 2010;588:3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TH, Humpert PM, Nawroth PP, Bierhaus A. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process - A mini-review. Gerontology. 2011;57:435–443. doi: 10.1159/000322087. [DOI] [PubMed] [Google Scholar]
- Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, deGroof RC, Lakatta EG. Improved Arterial Compliance by a Novel Advanced Glycation End-Product Crosslink Breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- Humphrey JD. Cardiovascular solid mechanics: cells, tissues, and organs. Springer; 2002. [Google Scholar]
- Klemm DJ, Majka SM, Crossno JT, Jr, Psilas JC, Reusch JEB, Garat CV. Reduction of Reactive Oxygen Species Prevents Hypoxia-induced CREB Depletion in Pulmonary Artery Smooth Muscle Cells. Journal of Cardiovascular Pharmacology. 2011;58:181–191. doi: 10.1097/FJC.0b013e31821f2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG. Age-associated Cardiovascular Changes in Health: Impact on Cardiovascular Disease in Older Persons. Heart Failure Reviews. 2002;7:29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part I: Aging Arteries: A "Set Up" for Vascular Disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. on behalf of the American Heart Association Statistics Committee and Stroke Statistics, S. Heart Disease and Stroke Statistics-2010 Update: A Report From the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman JM, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nature Chemical Biology. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace-Raso FUS, van der Cammen TJM, Hofman A, van Popele NM, Bos ML, Schalekamp MADH, Asmar R, Reneman RS, Hoeks APG, Breteler MMB, Witteman JCM. Arterial Stiffness and Risk of Coronary Heart Disease and Stroke: The Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- Mitchell GF. Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk. Artery Research. 2009;3:56–64. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial Stiffness and Cardiovascular Events: The Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moningka NC, Sindler AL, Muller-Delp JM, Baylis C. Twelve Weeks of Treadmill Exercise Does Not Alter Age-Dependent Chronic Kidney Disease in the Fisher 344 Male Rat. The Journal of Physiology. 2011 doi: 10.1113/jphysiol.2011.214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro MF, Amaral JH, Pinheiro LC, Sakamoto EK, Ferreira GC, Reis RI, Marçal DMO, Pereira RP, Tanus-Santos JE. Sodium nitrite downregulates vascular NADPH oxidase and exerts antihypertensive effects in hypertension. Free Radical Biology and Medicine. 2011;51:144–152. doi: 10.1016/j.freeradbiomed.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Paik D, Ramey W, Dillon J, Tilson M. The nitrite/elastin reaction: implications for in vivo degenerative effects. Connective Tissue Research. 1997;36:241–251. doi: 10.3109/03008209709160224. [DOI] [PubMed] [Google Scholar]
- Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proceedings of the National Academy of Sciences. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging Cell. 2010;9:304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. for the Health A.B.C.S. Elevated Aortic Pulse Wave Velocity, a Marker of Arterial Stiffness, Predicts Cardiovascular Events in Well-Functioning Older Adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of Cardiovascular Events and All-Cause Mortality With Arterial Stiffness: A Systematic Review and Meta-Analysis. Journal of the American College of Cardiology. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- Webb AJ, Milsom AB, Rathod KS, Chu WL, Qureshi S, Lovell MJ, Lecomte FMJ, Perrett D, Raimondo C, Khoshbin E, Ahmed Z, Uppal R, Benjamin N, Hobbs AJ, Ahluwalia A. Mechanisms Underlying Erythrocyte and Endothelial Nitrite Reduction to Nitric Oxide in Hypoxia: Role for Xanthine Oxidoreductase and Endothelial Nitric Oxide Synthase. Circ Res. 2008;103:957–964. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willum Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen Jr. Prognostic Value of Aortic Pulse Wave Velocity as Index of Arterial Stiffness in the General Population. Circulation. 2006;113:664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]