Abstract
The objective of this prospective surveillance study was to quantify colonization with antimicrobial-resistant organisms (AROs) and infections attributable to indwelling devices in skilled nursing facility (SNF) residents. The study was conducted in 15 SNFs in Southeast Michigan. Residents with (n = 90) and without (n = 88) an indwelling device were enrolled and followed for 907 resident-months. Residents were cultured monthly from multiple anatomic sites and data on infections were obtained. The device-attributable rate was calculated by subtracting the infection rate in the device group from the infection rate in the non-device group. A total of 197 new infections occurred during the study period; 87 in the device group (incidence rate [IR] = 331/1,000 resident-months) and 110 infections in the non-device group (IR = 171/1,000 resident-months), with a relative risk of 1.9 (95% confidence interval [CI]: 1.4–2.6). The attributable rate of excess infections among residents in the device group was 160/1,000 resident-months, with an attributable fraction of 48% (95% CI: 31–61%). Prevalence rates for all AROs were higher in the device group compared with the no-device group. The prevalence of the number of AROs per 1,000 residents cultured increased from no-device to those with only feeding tubes, followed by those with only urinary catheters and both these devices. In conclusion, the presence of indwelling devices is associated with higher incidence rates for infections and prevalence rates for AROs. Our study quantifies this risk and shows that approximately half of all infections in SNF residents with indwelling devices can be eliminated with device removal. Effective strategies to reduce infections and AROs in these residents are warranted.
Introduction
Urinary catheters and feeding tubes are often used in skilled nursing facilities (SNFs). Prevalence estimates of these devices range between 6 and 40%, depending on the SNF population studied [1–7]. Prior cross-sectional studies have shown that SNF residents with indwelling devices are more likely to be colonized with antimicrobial-resistant organisms (AROs), such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and resistant Gram-negative bacteria (R-GNB), when compared with the residents without a device [8–11].
SNF residents with indwelling devices are also at a higher risk of having infections. Approximately 3–7% of SNF residents with an indwelling urinary catheter will acquire a urinary tract infection (UTI) with each day that the catheter remains in place. By day 30 following catheter insertion, the risk of a catheter-associated bacteriuria is almost 100% [1]. It is estimated that 50% of SNF residents with a urinary catheter will have symptomatic catheter-related UTIs. In addition, residents with urinary catheters for longer than 30 days have a mortality rate higher than residents without a catheter [2, 12, 13]. Similarly, SNF residents with feeding tubes are susceptible to aspiration pneumonia (PNA), local skin and soft tissue infections, and other mechanical complications. The mortality rate due to PNA is higher in tube-fed patients than in orally-fed patients [14].
AROs cause infections that are difficult and expensive to treat. Colonization is a prerequisite for infection [15], and the same colonizing ARO strain can also lead to infection [16, 17]. Risk factors for ARO colonization and infection include age, functional status, longer term institutionalization, prior antibiotic exposure, presence of an indwelling device, and comorbidities [8–11, 18–20].
The objective of our prospective surveillance study was to extend our prior work and assess how indwelling device use contributes to new infection rate and ARO prevalence in the SNF population. A cohort of SNF residents with indwelling devices with a concurrent cohort of no-device residents provided a unique opportunity to examine the quantitative aspect of this relationship. We were also interested in defining the discrepancies between SNF infections diagnosed clinically versus those that meet published SNF appropriate infection surveillance and minimum diagnostic criteria [21–23].
Methods
Study design and population
We conducted a prospective surveillance study involving 15 community-based SNFs in Southeast Michigan. This study was approved by the University of Michigan Institutional Review Board, the Veterans Affairs Institutional Review Board, and the individual SNFs. Any SNF resident in these facilities with or without a device was eligible for the study. Device use in this study was defined as the presence of an indwelling urinary catheter and/or a feeding tube. A random numbers table was used to identify no-device residents. Written consent to obtain cultures and to conduct chart review was obtained from either the resident or their durable power of attorney before including them in the study. Of the 483 residents with indwelling devices and their randomly selected no-device residents, 178 (37%) were enrolled in the study. The main reasons for non-enrollment were refusal to give informed consent by the residents (23%) or their family or legal guardian (32%), inability to contact family or legal guardian (21%), and discharge from the facility or device discontinuation by the time of enrollment (23%).
Clinical and demographic data were obtained by chart review. The enrolled residents were cultured on a monthly basis at various anatomical sites for up to a year unless other factors removed them from the study, such as death, discharge from the facility, voluntary self-removal, and, in the case of the device group, discontinuation of the device.
Microbiologic methods
Cultures were obtained monthly from multiple anatomic sites, including the nares, oropharynx, groin, and perianal area. If present, wounds, feeding tube site, as well as supra-pubic catheter sites were also sampled using Culturette™ swabs (Becton Dickinson Inc., Sparks, MD). Standard microbiologic methods were used to positively identify colonization with Staphylococcus aureus, vancomycin-resistant Enterococcus spp. (VRE), and GNB. S. aureus isolates were further tested for methicillin resistance using oxacillin screening plates. To test for GNB isolates, swabs were streaked on MacConkey agar. Phenotypically different colonies were identified to the species level using API 20E test strips (Analytab Products, Plainview, NY). GNB isolates were screened for ceftazidime resistance (CAZ-R) and ciprofloxacin resistance (CIP-R) by the disc diffusion method using 10-μg/mL CAZ discs and 5-μg/mL CIP discs on Mueller–Hinton agar (BD BBL™ Sensi-Disc , Sparks, MD). Both full and intermediate resistance were considered to be positive for GNB.
Data collection
Age, Charlson’s comorbidity score [24], and functional status using Lawton and Brody’s Physical Self Maintenance Scale (PSMS) [25] were recorded on every participant. Monthly data on infections were obtained by chart review. Infections were defined using three separate criteria: (1) clinical definition: clinical notes documenting an infection followed by the prescription of a systemic antibiotic for 5 or more days to treat that infection [21]; (2) McGeer’s criteria for the surveillance of infections in SNFs [22]; and (3) minimum criteria to initiate antibiotics in SNFs [23] (Table 1).
Table 1.
| Pneumonia (PNA) definition | Urinary tract infection (UTI) definition |
|---|---|
| Clinical definition: A clinician’s note with a diagnosis of pneumonia followed by a prescription of antibiotics for more than 5 days |
Clinical definition: A clinician’s note with a diagnosis of UTI followed by a prescription of antibiotics for more than 5 days A. For residents without an indwelling urinary catheter |
|
McGeer’s criteria Both of the following criteria must be met:
|
McGeer’s criteria Must have at least three of the following:
|
|
Minimum criteria A. Febrile resident If resident has temperature >38.9°C (102°F), must have at least one of the following:
If resident has temp >37.9°C (100°F) (or a 1.5°C [2.4°F] increase over the baseline temperature) but ≤38.9°C, must include the presence of cough and at least one of the following:
B. Afebrile resident If afebrile resident has COPD, must include:
If afebrile resident does not have COPD, must have presence of new cough with purulent sputum production and at least one of the following:
In the setting of new infiltrate on chest radiograph thought to represent PNA, any one of the following constitute appropriate minimum criteria:
|
Minimum criteria
And at least one of the following:
B. For residents with indwelling urinary catheter McGeer’s criteria Must have at least two of the following:
Minimum criteria Include at least one of the following:
|
Statistical methods
We used Stata v.9 for our statistical analyses. The incidence rate (IR) of infection was defined as the number of first infections per follow-up month per 1,000 residents (i.e., 1,000 resident-months). The relative risk of infection was calculated as the incidence rate of infection in the device group (IRe) divided by the incidence rate of infection in the no-device group (IRu), which expresses a relative measure of the effect of an indwelling device. The attributable rate was calculated by subtracting the incidence rate among the no-device group from the incidence rate in the device group to measure the excess rate of infections in both groups. The attributable fraction was calculated by dividing the attributable rate to the incidence rate of infection in the device group, which demonstrates the proportion of infections among the device group that could be prevented by device removal. For overall colonization, we defined the point prevalence rate of colonization as the number of AROs per 1,000 resident-months. Colonization density was defined as the number of unique AROs/resident. Poisson regression was used to calculate the relative risk of new infections. Risk factors included in the analysis included age, PSMS, Charlson’s comorbidity score, and device use.
Results
Study population characteristics
Of the 178 residents enrolled in the study, 90 had an indwelling device (device group) and 88 did not (no-device group). In the device group, 48 had a urinary catheter, 30 had a feeding tube, and 12 had both a feeding tube and urinary catheter. The 90 device group participants had a total follow-up of 263 resident-months. The 88 no-device group participants had a total follow-up of 644 resident-months. Residents with urinary catheters had an overall follow-up time of 128 resident-months, while those with feeding tubes had a total follow-up of 100 resident-months and those with both a feeding tube and urinary catheter had 35 resident-months of follow-up. Residents in the device group were generally younger, had a higher comorbidity score, had a shorter follow-up period, were more functionally dependent, and more likely to be male, as previously reported [26].
Overall infection incidence rates
A total of 197 clinical infections, as defined by our clinical definition, occurred in both groups (Table 2). In the device group, 87 overall clinical infections (IR = 331 infections/ 1,000 resident-months) occurred over 263 resident-months and 110 overall clinical infections occurred over 644 resident-months (IR = 171 infections/1,000 resident-months) in the no-device group (relative risk [RR] = 1.9; p<0.001). The attributable rate of infections in the device group is 160 more infections per 1,000 resident-months compared to those in the no-device group; a 48% increase in the infection rate. Using Poisson regression, the relative risk of infection for the device group was 1.3 (95% confidence interval [CI]: 1.1–1.5, p = 0.002), adjusting for age, functional status (PSMS), and Charlson’s comorbidity score.
Table 2.
Incidence and attributable risk of infection
| Number of infections
|
Incidence rate (infections/1,000 resident-months)
|
Relative risk (95%) | p-value | Attributable rate | Attributable fraction (AR%) | |||
|---|---|---|---|---|---|---|---|---|
| Device (263 f/u-mon) | Non-device (644 f/u-mon) | Device (IRe) | No-device (IRu) | (IRe – IRu) | ([IRe – IRu]/IRe) × 100 | |||
| Total infectionsa | 87 | 110 | 331 | 171 | 1.9 (1.4–2.6) | <0.001 | 160 | 48 |
| Urinary tract infectionsa | 49 | 54 | 186 | 84 | 2.2 (1.5–3.3) | <0.001 | 102 | 55 |
| Pneumoniaa | 23 | 20 | 87 | 31 | 2.8 (1.5–5.4) | 0.0004 | 56 | 64 |
| Other infectionsb | 15 | 36 | 57 | 56 | 1.0 (0.5–1.9) | 0.47 | 5.7 | 2 |
| McGeer–s criteriac | 8 | 15 | 30 | 23 | 1.3 (0.5–3.3) | 0.27 | 7.1 | 23 |
| Minimum criteriac | 12 | 10 | 46 | 16 | 2.9 (1.2–7.6) | 0.007 | 30 | 66 |
| McGeer’s or minimum criteriac | 15 | 18 | 57 | 28 | 2.0 (1.0–4.3) | 0.02 | 29 | 51 |
Clinical defintion
Includes skin and soft tissue infections, Clostridium difficile colitis, conjunctivitis, upper respiratory, and lower respiratory tract infections
Includes pneumonia and/or urinary tract infections
The device group had 49 UTIs over 263 resident-months (IR = 186 UTIs/1,000 resident-months) compared to 54 UTIs over 644 resident-months (IR = 84 UTIs/1,000 resident-months) in the no-device group (RR = 2.2; p<0.001). The attributable rate of having an infection in the device group is 102 more UTIs per 1,000 resident-months, a 55% increase. Similarly, the device group had 23 PNAs (IR = 87 PNAs/1,000 resident-months) compared to 20 (IR = 3 PNAs/1,000 resident-months) in the no-device group (RR = 2.8; p = 0.0004); the attributable rate being a 64% increase in PNAs.
The device group also had 15 infections other than UTI and PNA (IR = 57 infections/1,000 resident-months) compared to 36 infections other than UTI and PNA (IR = 56 infections/ 1,000 resident-months) in the no-device group (RR = 1.0; p = 0.47); the attributable rate was a 2% increase in infections, which was statistically insignificant, suggesting that these other infections, including skin and soft tissue infections, Clostridium difficile infection, conjunctivitis, and respiratory infections (excluding pneumonia), are not affected by the use of indwelling devices.
Of the 87 infections in the device group, only 8 (IR = 30 infections/1,000 resident-months) defined using clinical criteria met the criteria using McGeer’s surveillance definitions compared to 15 (IR = 23 infections/1,000 resident-months) in the no-device group (RR = 1.3; p = 0.27). Twelve infections (IR = 46 infections/1,000 resident-months) in the device group defined using clinical criteria met the minimum criteria for initiating antibiotics compared to 10 (IR = 16 infections/1,000 resident-months) in the no-device group (RR = 2.9; p = 0.007). Finally, 15 infections (IR = 57 infections/1,000 resident-months) defined using clinical definitions in the device group met either McGeer’s or minimum criteria compared to 18 (IR = 28 infections/1,000 resident-months) in the no-device group (RR = 2.0; p = 0.02); a 51% increase in the infection rate.
Infection rates by device type
Seventeen (57%) of 30 residents with a feeding tube developed 23 infections over 100 resident-months of follow-up, for a rate of 170 infections/1,000 resident-months. These infections included 11 PNA, seven UTIs, and five infections other than UTI or PNA. Thirty-four (71%) of 48 residents with a urinary catheter developed 52 infections over 128 resident-months of follow-up, for a rate of 266 infections/1,000 resident-months. These 52 infections included 35 UTIs, eight PNAs, and nine infections other than UTI or PNA. Ten (83%) of 12 residents with both a feeding tube and urinary catheter developed 12 infections over 35 resident-months of follow-up, for a rate of 286 infections/1,000 resident-months. These 12 infections included seven UTIs, four PNAs, and one infection other than UTI or PNA. Overall, residents with both a feeding tube and urinary catheter were more likely to have an infection compared to those with either a urinary catheter or feeding tube alone (Table 3).
Table 3.
Number of residents with infections according to device type
| Incidence of Infection | Percent of residents with infection | Follow-up time (resident-months) | Rate (infections/1,000 resident-months) | |
|---|---|---|---|---|
| No device (n=88) | 50 | 57 | 644 | 78 |
| Feeding tube (n=30) | 17 | 57 | 100 | 170 |
| Urinary catheter (n=48) | 34 | 71 | 128 | 266 |
| Feeding tube and urinary catheter (n=12) | 10 | 83 | 35 | 286 |
ARO colonization density
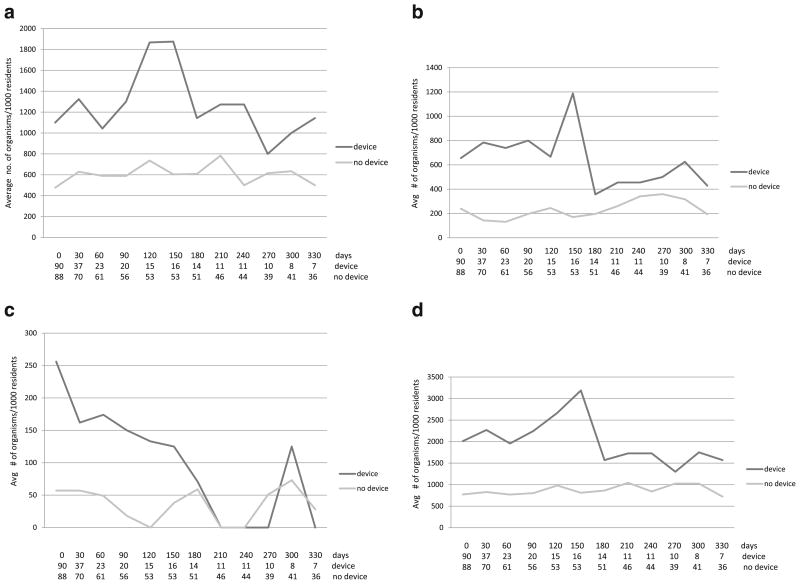
Compared with the no-device group, residents with indwelling devices had a consistently higher colonization density with MRSA, VRE, and R-GNB. For example, on their first visit, 49 of 90 (54%) residents in the device group were colonized with 181 AROs (colonization density = 2.01 AROs/resident). In contrast, 35 of 88 (40%) residents in the no-device group were colonized with 68 AROs (colonization density = 0.77 AROs/ resident, p<0.01) [Fig. 1].
Fig. 1.
Distribution of antimicrobial-resistant organisms (AROs) per 1,000 residents over time (days), with the number of residents cultured at each follow-up time in the device and no-device groups shown at the bottom. a Resistant Gram-negative bacteria, b Methicillin-resistant Staphylococcus aureus, c Vancomycin-resistant Enterococci, d All antimicrobial-resistant organisms
The prevalence of the number of AROs per 1,000 residents cultured increased from the no-device group to those with only feeding tubes, followed by those with only urinary catheters, such that residents with both feeding tubes and urinary catheters had the highest numbers of ARO colonization (Table 4).
Table 4.
Device-specific distribution of antimicrobial-resistant organisms (AROs)
| Number of organisms isolated (per 1,000 resident-months of follow-up)
|
||||
|---|---|---|---|---|
| Total ARO | MRSA | VRE | R-GNB | |
| No device | 859 | 222 | 37 | 599 |
| Feeding tube | 1,890 | 750 | 130 | 1,010 |
| Urinary catheter | 2,047 | 594 | 109 | 1,344 |
| Feeding tube and urinary catheter | 2,743 | 857 | 429 | 1,457 |
Discussion
In this prospective study in 15 SNFs, irrespective of the definitions used, we found an excess rate of infections among study participants with indwelling devices. We also found that participants with an indwelling device were more likely to be colonized with AROs than those with no devices and over prolonged periods of follow-up. Our study also showed that the prevalence rates of ARO colonization are incremental from the no-device group to participants with feeding tubes to those with urinary catheters. Residents with both devices had the highest rates of colonization, suggesting an additive effect.
Our data also showed that participants with indwelling devices have twice the risk of infections when compared with the no-device group. This risk remained statistically significant after adjusting for age, functional status, and comorbidity. The relative risk of UTI (2.2 times the risk) and PNA (2.8 times the risk) infections was significant when comparing the device group to the no-device group, while the relative risk of infections other than UTI and PNA were not significant. As expected, participants with urinary catheters have a higher incidence rate of UTI than those with feeding tubes and those with feeding tubes have a higher incidence rate of PNA infection than those with urinary catheters, indicating a relationship between the infection type and the device type. Assuming this causality, our study suggests that 50% of infections in the device group can be prevented by removing the device. Similar to ARO colonization, the incidence rate of infection was highest in participants with both a urinary catheter and a feeding tube compared to those with only one device, again, suggesting a dose–response type of relationship with the number of devices used and infection.
Infection data were collected from available medical records when a clinician’s note indicated an infectious diagnosis followed by an antibiotic prescription for 5 or more days. When the generally accepted surveillance or diagnostic criteria for infections was applied to this data, only 17% of these infections met either McGeer’s or minimum criteria for infection. However, the attributable rate of infection related to device use remained high using any of the three definitions. Our data show that most of the clinical infections would not meet McGeer’s and minimum criteria, and lead to antibiotic overuse in these residents. This is consistent with prior studies which demonstrate that multiple definitions for infection are used in SNFs, resulting in varied infection rates and inappropriate antibiotic use [21, 27]. Our study points to opportunities to institute both an effective intervention to reduce antibiotic usage and further refinement of the diagnostic criteria used to define infections in this setting.
Our study also shows that participants with indwelling devices not only had higher prevalence rates of AROs, similarly described by other studies [8–11], but they also had a higher density of colonization over time. This suggests that device residents are at a higher risk of getting subsequent infections with AROs, as well as serving as a reservoir for potential transmission to others. We also show that R-GNB is emerging as a major threat in SNFs and the infection rates surpass those of MRSA and VRE, similar to other studies [28–30].
One of the best ways to prevent ARO colonization and infection in SNF residents is to remove the indwelling device. Our study shows that, if all urinary catheter and feeding tube devices were removed, it could represent up to a 50% reduction in the risk of infection in those residents. SNFs should continually assess the appropriateness of urinary-catheter and feeding-tube use in order to maximize the risk-reduction potential. In cases where an indwelling device is medically necessary, strict adherence to enhanced barrier precautions may help to prevent the transmission of AROs to this at-risk group, reducing the risk of infection.
Our study has several limitations. The cost per device-related infection was not assessed and is beyond the scope of the study. Although we have reported data on healthcare workers’ knowledge, attitudes, and practices pertaining to device-related care in the past [31], we were not able to perform direct observations, which could provide an insight into the infection prevention practices at the point-of-care. While we conducted multivariate analyses to adjust for other risk factors, such as functional status, age, and comorbidity, there could be other underlying factors in both groups that could explain the differences in outcomes between them.
Overall, defining and quantifying ARO colonization and infection risk over time is a major strength of our study. We report the preventable fraction of infections with implications that will inform the effective and medically-necessary use of either a feeding tube or a urinary catheter in this population.
Acknowledgments
The authors would like to thank all the participating facilities, staff, and residents for their continued support of this research.
Funding source Funding source for the study was provided by NIA K23 AG028943, ASP/AGS T. Franklin Williams Research Scholarship, NIA R01AG032298, and Claude D. Pepper Older Americans Independence Center and Michigan Institute for Clinical & Health Research, University of Michigan Pilot Grant Program.
Contributor Information
L. Wang, Division of Geriatric Medicine, University of Michigan Medical School, Ann Arbor, MI, USA
B. Lansing, Division of Geriatric Medicine, University of Michigan Medical School, Ann Arbor, MI, USA
K. Symons, Infectious Diseases Section, University of Michigan School of Public Health, Ann Arbor, MI, USA
E. L. Flannery, Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, MI, USA
J. Fisch, Division of Geriatric Medicine, University of Michigan Medical School, Ann Arbor, MI, USA
K. Cherian, Division of Geriatric Medicine, University of Michigan Medical School, Ann Arbor, MI, USA
S. E. McNamara, Division of Geriatric Medicine, University of Michigan Medical School, Ann Arbor, MI, USA
L. Mody, Email: lonamody@umich.edu, Division of Geriatric Medicine, University of Michigan Medical School, Ann Arbor, MI, USA, Geriatrics Research Education and Clinical Center (GRECC), Veteran Affairs Ann Arbor Healthcare System, 11-G GRECC, 2215 Fuller Rd., Ann Arbor, MI 48105, USA
References
- 1.Warren JW, Tenney JH, Hoopes JM, et al. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J Infect Dis. 1982;146:719–723. doi: 10.1093/infdis/146.6.719. [DOI] [PubMed] [Google Scholar]
- 2.Kunin CM, Douthitt S, Dancing J, et al. The association between the use of urinary catheters and morbidity and mortality among elderly patients in nursing homes. Am J Epidemiol. 1992;135:291–301. doi: 10.1093/oxfordjournals.aje.a116283. [DOI] [PubMed] [Google Scholar]
- 3.Ahronheim JC, Mulvihill M, Sieger C, et al. State practice variations in the use of tube feeding for nursing home residents with severe cognitive impairment. J Am Geriatr Soc. 2001;49:148–152. doi: 10.1046/j.1532-5415.2001.49035.x. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell SL, Teno JM, Roy J, et al. Clinical and organizational factors associated with feeding tube use among nursing home residents with advanced cognitive impairment. JAMA. 2003;290:73–80. doi: 10.1001/jama.290.1.73. [DOI] [PubMed] [Google Scholar]
- 5.Teno JM, Mor V, DeSilva D, et al. Use of feeding tubes in nursing home residents with severe cognitive impairment. JAMA. 2002;287:3211–3212. doi: 10.1001/jama.287.24.3211. [DOI] [PubMed] [Google Scholar]
- 6.Teno JM, Feng Z, Mitchell SL, et al. Do financial incentives of introducing case mix reimbursement increase feeding tube use in nursing home residents? J Am Geriatr Soc. 2008;56:887–890. doi: 10.1111/j.1532-5415.2008.01647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsan L, Davis C, Langberg R, et al. Prevalence of nursing home-associated infections in the Department of Veterans Affairs nursing home care units. Am J Infect Control. 2008;36:173–179. doi: 10.1016/j.ajic.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136:834–844. doi: 10.7326/0003-4819-136-11-200206040-00013. [DOI] [PubMed] [Google Scholar]
- 9.Mody L, Maheshwari S, Galecki A, et al. Indwelling device use and antibiotic resistance in nursing homes: identifying a high-risk group. J Am Geriatr Soc. 2007;55:1921–1926. doi: 10.1111/j.1532-5415.2007.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mody L, Kauffman CA, Donabedian S, et al. Epidemiology of Staphylococcus aureus colonization in nursing home residents. Clin Infect Dis. 2008;46:1368–1373. doi: 10.1086/586751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dommeti P, Wang L, Flannery EL, et al. Patterns of ciprofloxacin-resistant gram-negative bacteria colonization in nursing home residents. Infect Control Hosp Epidemiol. 2011;32:177–180. doi: 10.1086/657946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Platt R, Polk BF, Murdock B, et al. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307:637–642. doi: 10.1056/NEJM198209093071101. [DOI] [PubMed] [Google Scholar]
- 13.Kunin CM, Chin QF, Chambers S. Morbidity and mortality associated with indwelling urinary catheters in elderly patients in a nursing home—confounding due to the presence of associated diseases. J Am Geriatr Soc. 1987;35:1001–1006. doi: 10.1111/j.1532-5415.1987.tb04003.x. [DOI] [PubMed] [Google Scholar]
- 14.Mamun K, Lim J. Role of nasogastric tube in preventing aspiration pneumonia in patients with dysphagia. Singapore Med J. 2005;46:627–631. [PubMed] [Google Scholar]
- 15.Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis. 2004;39:219–226. doi: 10.1086/422002. [DOI] [PubMed] [Google Scholar]
- 16.Pacio GA, Visintainer P, Maguire G, et al. Natural history of colonization with vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, and resistant gram-negative bacilli among long-term-care facility residents. Infect Control Hosp Epidemiol. 2003;24:246–250. doi: 10.1086/502201. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Ami R, Schwaber MJ, Navon-Venezia S, et al. Influx of extended-spectrum β-lactamase-producing enterobacteriaceae into the hospital. Clin Infect Dis. 2006;42:925–934. doi: 10.1086/500936. [DOI] [PubMed] [Google Scholar]
- 18.Eun SH, Lee YS, Cha JO, et al. The point prevalence and associated factors of nasal methicillin-resistant Staphylococcus aureus colonisation in eight geriatric hospitals in Korea. Clin Microbiol Infect. 2006;12:81–83. doi: 10.1111/j.1469-0691.2005.01313.x. [DOI] [PubMed] [Google Scholar]
- 19.Pop-Vicas A, Mitchell SL, Kandel R, et al. Multidrug-resistant gram-negative bacteria in a long-term care facility: prevalence and risk factors. J Am Geriatr Soc. 2008;56:1276–1280. doi: 10.1111/j.1532-5415.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 20.Benenson S, Cohen MJ, Block C, et al. Vancomycin-resistant enterococci in long-term care facilities. Infect Control Hosp Epidemiol. 2009;30:786–789. doi: 10.1086/598345. [DOI] [PubMed] [Google Scholar]
- 21.Nicolle LE, Mubareka S, Simor A, et al. Variation in mortality rates among long-term care facilities for residents with lower respiratory tract infection. Infect Control Hosp Epidemiol. 2008;29:754–759. doi: 10.1086/590123. [DOI] [PubMed] [Google Scholar]
- 22.McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19:1–7. doi: 10.1016/0196-6553(91)90154-5. [DOI] [PubMed] [Google Scholar]
- 23.Loeb M, Bentley DW, Bradley S, et al. Development of minimum criteria for the initiation of antibiotics in residents of long-term-care facilities: results of a consensus conference. Infect Control Hosp Epidemiol. 2001;22:120–124. doi: 10.1086/501875. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 26.Flannery EL, Wang L, Zöllner S, et al. Wounds, functional disability, and indwelling devices are associated with cocolonization by methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci in southeast Michigan. Clin Infect Dis. 2011;53:1215–1222. doi: 10.1093/cid/cir733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juthani-Mehta M, Quagliarello V, Perrelli E, et al. Clinical features to identify urinary tract infection in nursing home residents: a cohort study. J Am Geriatr Soc. 2009;57:963–970. doi: 10.1111/j.1532-5415.2009.02227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trick WE, Weinstein RA, DeMarais PL, et al. Colonization of skilled-care facility residents with antimicrobial-resistant pathogens. J Am Geriatr Soc. 2001;49:270–276. doi: 10.1046/j.1532-5415.2001.4930270.x. [DOI] [PubMed] [Google Scholar]
- 29.Pop-Vicas A, Strom J, Stanley K, et al. Multidrug-resistant gram-negative bacteria among patients who require chronic hemodialysis. Clin J Am Soc Nephrol. 2008;3:752–758. doi: 10.2215/CJN.04651107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Fallon E, Pop-Vicas A, D’ E. The emerging threat of multidrug-resistant gram-negative organisms in long-term care facilities. J Gerontol A Biol Sci Med Sci. 2009;64:138–141. doi: 10.1093/gerona/gln020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mody L, Saint S, Galecki A, et al. Knowledge of evidence-based urinary catheter care practice recommendations among healthcare workers in nursing homes. J Am Geriatr Soc. 2010;58:1532–1537. doi: 10.1111/j.1532-5415.2010.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]