Abstract
Background
Provider-based research networks (PBRNs) – collaborative research partnerships between academic centers and community-based practitioners – are a promising model for accelerating the translation of research into practice; however, empirical evidence of accelerated translation is limited. Oxaliplatin in adjuvant combination chemotherapy is an innovation with clinical trial-proven survival benefit compared to prior therapies. The goal of this study is to examine the diffusion of oxaliplatin into community practice, and whether affiliation with the National Cancer Institute’s (NCI’s) Community Clinical Oncology Program (CCOP) – a nationwide cancer-focused PBRN – is associated with accelerated innovation adoption.
Design, Setting, and Participants
This retrospective observational study used linked SEER-Medicare and NCI-CCOP data to examine Medicare participants with stage III colon cancer initiating treatment in 2003 through 2006, the years surrounding oxaliplatin’s FDA approval. A fixed-effects analysis examined chemotherapy use among patients treated outside academic centers at CCOP-affiliated practices compared to non-CCOP practices. Two-group modeling controlled for multiple levels of clustering, year of chemotherapy initiation, tumor characteristics, patient age, race, comorbidity, Medicaid dual-eligibility status, and education.
Results
Of 4,055 community patients, 35% received 5-FU, 20% received oxaliplatin, 7% received another chemotherapy, and 38% received no chemotherapy. 25% of CCOP patients received oxaliplatin, compared to 19% of non-CCOP patients. In multivariable analysis, CCOP exposure was associated with higher odds of receiving guideline-concordant treatment in general, and oxaliplatin specifically.
Conclusions
These findings contribute to a growing set of evidence linking PBRNs with a greater probability of receiving treatment innovations and high quality cancer care, with implications for clinical and research policy.
Keywords: colon cancer, translational research, diffusion of innovation, organization and administration, provider-based research networks
INTRODUCTION
For well over a decade, the Institute of Medicine (IOM) has focused intensively on improving health care quality through application of the best scientific knowledge. 1–3 Aspirations of crossing a quality chasm have motivated the development of strategies for linking research findings with treatment implementation, and developing strategies for dissemination and knowledge transfer.3,4 Despite this, many important new discoveries take years if not decades to enter routine practice.5,6 For example, a meaningful cancer innovation – tamoxifen for treatment of hormone receptor positive breast cancer – took 10 years to reach 70% utilization.7 Clearly, there is room for improvement.
The National Institutes of Health (NIH) embraced the IOM’s focus on quality through the NIH Roadmap.8 Recognizing that less than one percent of Americans seek healthcare through academic medical centers, the major hubs of clinical research,9 the Roadmap emphasizes practice-based research networks (PBRNs) and accelerating science while facilitating the translation of research into practice. 8 Facilitating research outside of academic centers, PBRNs are collaborative research partnerships between academic investigators and community-based practitioners, providing critical, stable infrastructure that supports community-based clinical research studies.8,10 Westfall and colleagues eloquently characterized PBRNs as “blue highways,” connecting the major academic centers to physicians and patients across the US.6 Through “blue highway” research, PBRNs accelerate science by improving access to research participation where the vast majority of the population seeks care – in the community. There, PBRNS serve as conduits for two-way information flow: community practitioners inform and improve clinical trials through communication of practical design and implementation considerations, while their research participation promotes a sense of familiarity, trust, and acceptance of research results, and strengthens their commitment to acting on findings. 4,11,12 Despite the urgent need to improve health care quality and the promise of PBRNs as a model for accelerating the translation of research into practice, compelling empirical evidence of such a link is exceptionally limited.13–15
Colon Cancer, Oxaliplatin, and the NCI CCOP Program
In 2010, approximately 103,000 people in the United States were diagnosed with colon cancer (CC) and over 51,000 died from it; worldwide mortality rates generally exceed those in the US.16 Since 1990, consensus guidelines have called for post-surgical adjuvant chemotherapy as the standard of care for stage III CC.17 From 1990 to 2004, the combination of 5-fluoruracil (5FU) plus leucovorin (LV) was the standard of care based on substantial survival improvement compared to surgery alone.17–19 In 2004, oxaliplatin was approved by the Food and Drug Administration (FDA) based on substantially better survival associated with its use in combination therapy with 5FU and LV, compared to 5FU and LV alone.20,21 Despite the survival benefit associated with oxaliplatin-containing therapy, community adoption of this innovation has been uneven.18
One of the two randomized controlled trials (RCTs) demonstrating the benefit of oxaliplatin was conducted through NCI CCOP practices.21 Briefly, CCOP is a cancer-focused PBRN connecting NCI Cooperative Groups (researchers at academic centers who develop clinical trials) with a nationwide network of community physicians treating cancer patients outside of academic medical centers and enrolling many of them on NCI clinical trials.22 CCOP is large, with nearly 3,500 community physicians and 390 hospitals in 34 states. Between 1999 and 2008, CCOP contributed 36% of all NCI trial enrollment.22 CCOP organizational goals specifically include enrolling patients on trials and accelerating the translation of evidence-based innovations into practice.22,23 As such, CCOP is an ideal research network for studying the role of PBRNs in facilitating the translation of research into practice.
This study responds to guidance from the IOM and the National Cancer Policy Board to assess patterns of care and factors associated with receipt of high quality care.2,3 Specifically, the objective of the study is to examine the association between PBRN research participation and innovation adoption, focusing on the case of oxaliplatin-containing adjuvant chemotherapy. It hypothesizes that provider affiliation with the National Cancer Institute’s (NCI’s) Community Clinical Oncology Program (CCOP) – a nationwide cancer-focused PBRN – is associated with accelerated diffusion of this innovation among community practices.
METHODS
Study Design and Population
This study employs a retrospective observational design with a two-group, multi-level primary analytic approach. Inclusion criteria reflect consensus guidelines regarding adjuvant therapy for Stage III CC.17,20,24 The study sample included individuals age 65 and older with stage III colon adenocarcinoma that was surgically resected within 180 days of diagnosis. Because CCOP engages community providers and not major academic medical centers, the study sample was restricted to those receiving primary surgery at a hospital that was unaffiliated or had a “Limited” affiliation with a medical school according to the Accrediting Council of Graduate Medical Education. Patients in Health Maintenance Organizations (HMOs) were excluded because HMO claims do not provide requisite procedure-level detail. To assure complete claims, participants included only those with continuous Part A and Part B enrollment from 12 months pre- through 12 months post-diagnosis. The study examined years 2003 through 2006, the years of the first international conference presentation of oxaliplatin’s efficacy in this population, through two years after FDA approval.
Data
The study sample is drawn from the NCI Surveillance Epidemiology and End Results (SEER)-Medicare program, linked to data on physician and hospital CCOP-affiliation data from the NCI CCOP Program. Briefly, SEER-Medicare is a collaboration between the NCI’s SEER Program of cancer registries and the Centers for Medicare and Medicaid Services (CMS).25 Cancer registry data are linked to administrative and claims data for individuals in the registries and also in Medicare. SEER data include demographic and incident cancer characteristics including stage and grade, among others, for approximately 25% of the US cancer population. Medicare provides health insurance for approximately 97% of Americans age 65 and over, and captures health services utilization and comorbid health conditions. Relevant to this study, approximately 64% of CC cases are diagnosed among those aged 65 and older, who experience approximately 73% of CC deaths.26
Main Outcome and Exposure
Receipt of adjuvant chemotherapy was the primary outcome of interest, measured as chemotherapy initiated within 120 days of surgery. Oxaliplatin-containing regimens were defined as those including oxaliplatin within 35 days of the first chemotherapy dose. Non-oxaliplatin containing regimen were defined and examined in two ways: first, as any form of 5-FU including capecitibine (and no oxaliplatin), and second, as any non-oxaliplatin chemotherapy.
Patients were designated as being “CCOP Patients” if they received cancer treatment from a CCOP-affiliated physician or hospital between diagnosis and first date of chemotherapy. Functional form was examined as continuous, proportion (semi-continuous), quadratic, cubit, binary (any vs. no CCOP exposure), and four-group categorical. To capture the degree of patient exposure to the CCOP, this measure was operationalized as the proportion of claims from CCOP-affiliated physicians or hospitals out of all claims (CCOP and non-CCOP, cancer and non-cancer) within the same surveillance window used to determine the primary outcome.
Covariates
Covariates were defined through previous research and a modified directed-acyclic graph (DAG), as-informed by theorized or actual association with care-seeking behaviors, treatment selection, or cancer outcomes.27,28 Person-level characteristics include age, race, gender, comorbidity, tumor grade, primary tumor site, Medicaid dual-eligibility status (i.e., the patient has limited financial resources and is eligible for Medicaid), census-level education, and year of first chemotherapy. Comorbidity was measured using the NCI Combined Comorbidity Index (CCI), and with individual constituent health conditions in the CCI.29 Hospitals were characterized by teaching status, designation as an NCI cancer center, and independent affiliation with NCI Cooperative Groups. Organizational affiliation with the American College of Surgeons Oncology Group (ACOSOG) was examined because the majority of ACOSOG sites are accredited by the ACOS Commission on Cancer (CoC) – a marker of program quality – and ACOSOG affiliation has been associated with accelerated innovation adoption.15,30
Analysis
Bivariate analysis examined crude associations among all variables to characterize measure relevance and independence, and included examination of variables’ functional form (e.g., continuous, categorical, etc.) to optimize the full use of information within the data while balancing statistical appropriateness and interpretability of results. Observations were examined independently and also clustered at the hospital level. Marginal mean models and generalized linear mixed models with maximum likelihood estimates were examined using Generalized Estimating Equations (implemented as the GENMOD and NLMIXED procedures, respectively in SAS 9.2). The marginal mean approach was selected to optimize model fit and interpretation of fixed effects. Modeling began by examining factors associated with the receipt of any adjuvant chemotherapy vs. no chemotherapy. Logistic models generated predicted probabilities to construct figures reflecting differential receipt of oxaliplatin-containing regimens across time. The main analytic model focused on factors associated with the receipt of oxaliplatin-containing regimens compared to 5-FU only regimens. Due to collinearity, dual-eligibility status was retained in lieu of census-level income measures. Fixed effects were examined as cooperative group affiliation, year of first chemotherapy, age, race, dual-eligibility status, and education. Effect modification was explored among measures of affiliation, race, and treatment as informed by our hypothesized causal model (DAG) and previous research.15,27,31 Final model selection was guided by theory and practical understanding of cancer care delivery, as well as statistical considerations such as effect estimate stability and statistical measures of model fit, while balancing interpretability and transparency.
RESULTS
As presented in Table 1, 5,732 individuals met study inclusion criteria, of whom 4,055 (71%) received surgery at a community hospital. Of these, 1,432 (35%) received 5FU, 821 (20%) received oxaliplatin, 264 (7%) received another or an unspecified chemotherapy, and 1,538 (38%) received no chemotherapy. Compared to other treatment groups, those receiving oxaliplatin were younger, more likely to have poorly differentiated disease, slightly healthier, wealthier and more educated. Those receiving no chemotherapy were the oldest (median age, 81.5), least healthy, and more likely than those receiving chemotherapy to be dually-eligible. Oxaliplatin use increased steadily during all study years (Table 2, Figure 1), and was more prevalent among those seeing CCOP providers, as was chemotherapy use overall.
Table 1.
Descriptive Characteristics of Overall Study Sample.
| All stage III Colon Cancer Patients | Received 5-Fu | Received Oxaliplatin | Received other, unknown chemo | Received No Chemo | Oxaliplatin vs. 5-FU3 | Any vs. no Chemo4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |||
| Patient Characteristics | 4054 | 1432 | (35.3%) | 821 | (20.3%) | 264 | (6.5%) | 1537 | (37.9%) | |||
|
| ||||||||||||
| Gender | ||||||||||||
| Male | 1728 | (42.6%) | 626 | (43.7%) | 405 | (49.3%) | 120 | (45.5%) | 577 | (37.5%) | *** | *** |
| Female | 2326 | (57.4%) | 806 | (56.3%) | 416 | (50.7%) | 144 | (54.5%) | 960 | (62.5%) | ||
| Race | ||||||||||||
| Caucasian American | 3519 | (86.8%) | 1257 | (87.8%) | 709 | (86.4%) | 217 | (82.2%) | 1336 | (86.9%) | ||
| African American | 316 | (7.8%) | 95 | (6.6%) | 58 | (7.1%) | 30 | (11.4%) | 133 | (8.7%) | ||
| Other/Unknown | 219 | (5.4%) | 80 | (5.6%) | 54 | (6.6%) | 17 | (6.4%) | 68 | (4.4%) | ||
| Age (in years) | ||||||||||||
| Mean (SD); (Range) | 77.6 (6.95) | (66–105) | 75.9 (5.72) | (66–94) | 72.9 (4.87) | (66–88) | 77.9 (6.89) | (66–95) | 81.5 (6.82) | (66–105) | ||
| 65–69 | 597 | (14.7%) | 224 | (15.6%) | 260 | (31.7%) | 38 | (14.4%) | 75 | (4.9%) | ||
| 70–74 | 862 | (21.3%) | 372 | (26.0%) | 254 | (30.9%) | 53 | (20.1%) | 183 | (11.9%) | ||
| 75–79 | 1022 | (25.2%) | 448 | (31.3%) | 216 | (26.3%) | 61 | (23.1%) | 297 | (19.3%) | ||
| 80+ | 1573 | (38.8%) | 388 | (27.1%) | 91 | (11.1%) | 112 | (42.4%) | 982 | (63.9%) | ||
| Income1 | ||||||||||||
| Quartile 1: lowest income | 1056 | (26.0%) | 389 | (27.2%) | 174 | (21.2%) | 74 | (28.0%) | 420 | (27.3%) | *** | ** |
| Quartile 2: low-med income | 1039 | (25.6%) | 344 | (24.0%) | 204 | (24.8%) | 69 | (26.1%) | 423 | (27.5%) | ||
| Quartile 3: med-high income | 1021 | (25.2%) | 378 | (26.4%) | 214 | (26.1%) | 71 | (26.9%) | 356 | (23.2%) | ||
| Quartile 4: highest income | 938 | (23.1%) | 321 | (22.4%) | 229 | (27.9%) | 50 | (18.9%) | 338 | (22.0%) | ||
| Education2 | ||||||||||||
| Quartile 1: lowest education | 1017 | (25.1%) | 362 | (25.3%) | 189 | (23.0%) | 70 | (26.5%) | 396 | (25.8%) | *** | |
| Quartile 2: low-med education | 1047 | (25.8%) | 371 | (25.9%) | 207 | (25.2%) | 79 | (29.9%) | 390 | (25.4%) | ||
| Quartile 3: med-high education | 1050 | (25.9%) | 389 | (27.2%) | 194 | (23.6%) | 64 | (24.2%) | 406 | (26.4%) | ||
| Quartile 4: highest education | 940 | (23.2%) | 310 | (21.6%) | 231 | (28.1%) | 51 | (19.3%) | 345 | (22.4%) | ||
| NCI combined comorbidity index | ||||||||||||
| Mean (SD); (Range) | 0.27 (0.46) | (0 – 3.66) | 0.22 (0.42) | (0 – 3.66) | 0.16 (0.34) | (0 – 2.05) | 0.33 (0.49) | (0 – 2.22) | 0.36 (0.52) | (0 – 2.70) | *** | *** |
| Primary site of tumor | ||||||||||||
| Cecum, Ascending | 2042 | (50.4%) | 715 | (49.9%) | 408 | (49.7%) | 123 | (46.6%) | 796 | (51.8%) | ** | |
| Flexures, Transverse/Descending | 939 | (23.2%) | 318 | (22.2%) | 177 | (21.6%) | 72 | (27.3%) | 372 | (24.2%) | ||
| Signmoid; Large intestine/NOS | 1073 | (26.5%) | 399 | (27.9%) | 236 | (28.7%) | 69 | (26.1%) | 369 | (24.0%) | ||
| Tumor grade | ||||||||||||
| Well-differentiated | 186 | (4.6%) | 76 | (5.3%) | 37 | (4.5%) | 15 | (5.7%) | 58 | (3.8%) | * | |
| Moderately differentiated | 2537 | (62.6%) | 920 | (64.2%) | 498 | (60.7%) | 169 | (64.0%) | 950 | (61.8%) | ||
| Poorly differentiated | 1152 | (28.4%) | 384 | (26.8%) | 252 | (30.7%) | 65 | (24.6%) | 451 | (29.3%) | ||
| Unknown | 179 | (4.4%) | 52 | (3.6%) | 34 | (4.1%) | 15 | (5.7%) | 78 | (5.1%) | ||
| Year of Chemo | ||||||||||||
| no chemo | 1537 | (37.9%) | - | - | - | - | - | - | 1537 | (100.0%) | *** | |
| 2003 | 775 | (19.1%) | 591 | (41.3%) | 126 | (15.3%) | 58 | (22.0%) | 0 | (0%) | *** | |
| 2004 | 813 | (20.1%) | 465 | (32.5%) | 264 | (32.2%) | 84 | (31.8%) | 0 | (0%) | ||
| 2005 | 800 | (19.7%) | 323 | (22.6%) | 364 | (44.3%) | 110–120 | (42–43%) | 0 | (0%) | ||
| 2006 (partial year examined) | 129 | (3.2%) | 53 | (3.7%) | 67 | (8.2%) | <11 | (<4%) | 0 | (0%) | ||
| Dual eligibility | ||||||||||||
| Yes | 707 | (17.4%) | 196 | (13.7%) | 95 | (11.6%) | 62 | (23.5%) | 354 | (23.0%) | *** | |
|
| ||||||||||||
| Organization characteristics (treating hospital) | ||||||||||||
|
| ||||||||||||
| Cooperative Group Affiliation | ||||||||||||
| Mean (SD); (Range) | 1.47 (1.62) | (0–9) | 1.41 (1.57) | (0–9) | 1.58 (1.75) | (0–8) | 1.33 (1.61) | (0–8) | 1.49 (1.60) | (0–8) | * | |
| No affiliation | 1649 | (40.7%) | 591 | (41.3%) | 318 | (38.7%) | 119 | (45.1%) | 621 | (40.4%) | ||
| Affiliated with 1+ C.G. | 2405 | (59.3%) | 841 | (58.7%) | 503 | (61.3%) | 145 | (54.9%) | 916 | (59.6%) | ||
| Medical School Affiliation | ||||||||||||
| Unaffiliated | 3237 | (79.8%) | 1146 | (80.0%) | 658 | (80.1%) | 205 | (77.7%) | 1228 | (79.9%) | ||
| Limited | 817 | (20.2%) | 286 | (20.0%) | 163 | (19.9%) | 59 | (22.3%) | 309 | (20.1%) | ||
| NCI-center affiliated | ||||||||||||
| Yes | <11 | (<1%) | <11 | (<1%) | <11 | (<1%) | <11 | (<1%) | <11 | (<1%) | ||
|
| ||||||||||||
| Primary Exposure | ||||||||||||
|
| ||||||||||||
| Any CCOP MD/hospital affiliated claims | ||||||||||||
| Yes | 1029 | (25.4%) | 361 | (25.2%) | 255 | (31.1%) | 76 | (28.8%) | 337 | (21.9%) | *** | *** |
| Proportion of claims from CCOP-affiliated MD or Hospital | ||||||||||||
| Mean (SD); (Range) | 0.11 (0.25) | (0–1) | 0.13 (0.27) | (0–1) | 0.18 (0.33) | (0–1) | 0.10 (0.22) | (0–0.96) | 0.05 (0.14) | (0–0.92) | *** | *** |
|
| ||||||||||||
| Outcome | ||||||||||||
|
| ||||||||||||
| 5-Fu | 1432 | (35.3%) | ||||||||||
| Folfox/Oxaliplatin | 821 | (20.3%) | ||||||||||
| Other/Unknown Chemotherapy | 264 | (6.5%) | ||||||||||
| No chemo | 1537 | (37.9%) | ||||||||||
Median household income in patient’s census tract.
Proportion of patient’s census tract population with high school education or greater.
Chi-square test (categorial) or t-test (continuous):
p<0.01: ***,
p<0.05: **,
p<0.10: *
-SEER regions were examined but are not reported. They were found to be statistically different (p <0.10) by chemotherapy use.
-Specific comorbidities were examined but not reported. Oxaliplatin patients were less likely to have renal disease (p <0.05) or history of cardio/cerebrovascular disease (p <0.01). Patients receiving chemotherapy were less likely to have dementia or paralysis (p <0.01), history of cardio/cerebrovascular disease (p <0.01), neruropathy (p <0.05), or diabetes (p <0.10).
-Per SEER-Medicare data use requirements, methods were used to suppress cell sizes smaller than 11, and included collapsing some categories of measures, using “<11” to reflect small numbers, and partially masking values to prevent mathematical derivation of small numbers.
Table 2.
Descriptive Characteristics of Study Sample, by CCOP Exposure.
| All stage III Colon Cancer Patients | CCOP Patients | non CCOP Patients | CCOP vs. non-CCOP3 | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n | (%) | n | (%) | n | (%) | ||
| Patient Characteristics | 4054 | 1029 | (25.4%) | 3025 | (74.6%) | ||
|
| |||||||
| Gender | |||||||
| Male | 1728 | (42.6%) | 430 | (41.8%) | 1298 | (42.9%) | |
| Female | 2326 | (57.4%) | 599 | (58.2%) | 1727 | (57.1%) | |
| Race | |||||||
| Caucasian American | 3519 | (86.8%) | 881 | (85.6%) | 2638 | (87.2%) | |
| African American | 316 | (7.8%) | 84 | (8.2%) | 232 | (7.7%) | |
| Other/Unknown | 219 | (5.4%) | 64 | (6.2%) | 155 | (5.1%) | |
| Age (in years) | |||||||
| Mean (SD); (Range) | 77.56 (6.95) | (66–105) | 76.86 (6.79) | (66–98) | 77.79 (6.99) | (66–105) | |
| 65–69 | 597 | (14.7%) | 165 | (16.0%) | 432 | (14.3%) | |
| 70–74 | 862 | (21.3%) | 256 | (24.9%) | 606 | (20.0%) | |
| 75–79 | 1022 | (25.2%) | 249 | (24.2%) | 773 | (25.6%) | |
| 80–84 | 889 | (21.9%) | 208 | (20.2%) | 681 | (22.5%) | |
| 85+ | 684 | (16.9%) | 151 | (14.7%) | 533 | (17.6%) | |
| Income1 | |||||||
| Quartile 1: lowest income | 1056 | (26.0%) | 246 | (23.9%) | 810 | (26.8%) | *** |
| Quartile 2: low-med income | 1039 | (25.6%) | 229 | (22.3%) | 810 | (26.8%) | |
| Quartile 3: med-high income | 1021 | (25.2%) | 277 | (26.9%) | 744 | (24.6%) | |
| Quartile 4: highest income | 938 | (23.1%) | 277 | (26.9%) | 661 | (21.9%) | |
| Education2 | |||||||
| Quartile 1: lowest education | 1017 | (25.1%) | 222 | (21.6%) | 795 | (26.3%) | ** |
| Quartile 2: low-med education | 1047 | (25.8%) | 285 | (27.7%) | 762 | (25.2%) | |
| Quartile 3: med-high education | 1050 | (25.9%) | 275 | (26.7%) | 775 | (25.6%) | |
| Quartile 4: highest education | 940 | (23.2%) | 247 | (24.0%) | 693 | (22.9%) | |
| NCI combined comorbidity index | |||||||
| Mean (SD); (Range) | 0.27 (0.46) | (0–3.66) | 0.26 (0.46) | (0–2.61) | 0.27 (0.46) | (0–3.66) | |
| Primary site of tumor | |||||||
| Cecum, Ascending | 2042 | (50.4%) | 534 | (51.9%) | 1508 | (49.9%) | ** |
| Flexures, Transv./Descend. | 939 | (23.2%) | 253 | (24.6%) | 686 | (22.7%) | |
| Large intestine/NOS | 79 | (1.9%) | 12 | (1.2%) | 67 | (2.2%) | |
| Sigmoid | 994 | (24.5%) | 230 | (22.4%) | 764 | (25.3%) | |
| Tumor grade | |||||||
| Well-differentiated | 186 | (4.6%) | 55 | (5.3%) | 131 | (4.3%) | * |
| Moderately differentiated | 2537 | (62.6%) | 640 | (62.2%) | 1897 | (62.7%) | |
| Poorly differentiated | 1152 | (28.4%) | 277 | (26.9%) | 875 | (28.9%) | |
| Unknown | 179 | (4.4%) | 57 | (5.5%) | 122 | (4.0%) | |
| Year of Chemo | |||||||
| no chemo | 1537 | (37.9%) | 337 | (32.8%) | 1200 | (39.7%) | *** |
| 2003 | 775 | (19.1%) | 215 | (20.9%) | 560 | (18.5%) | |
| 2004 | 813 | (20.1%) | 228 | (22.2%) | 585 | (19.3%) | |
| 2005 | 800 | (19.7%) | 218 | (21.2%) | 582 | (19.2%) | |
| 2006 | 129 | (3.2%) | 31 | (3.0%) | 98 | (3.2%) | |
| Dual eligibility | |||||||
| Yes | 707 | (17.4%) | 141 | (13.7%) | 566 | (18.7%) | *** |
|
| |||||||
| Organization characteristics (of treating hospital) | |||||||
|
| |||||||
| Cooperative Group Affiliation | |||||||
| Mean (SD); (Range) | 1.47 (1.62) | (0–9) | 1.68 (1.64) | (0–9) | 1.39 (1.61) | (0–8) | *** |
| No affiliation | 1649 | (40.7%) | 368 | (35.8%) | 1281 | (42.3%) | *** |
| Affiliated with 1+ C.G. | 2405 | (59.3%) | 661 | (64.2%) | 1744 | (57.7%) | |
| Medical School affiliation | |||||||
| Unaffiliated | 3237 | (79.8%) | 831 | (80.8%) | 2406 | (79.5%) | |
| Limited | 817 | (20.2%) | 198 | (19.2%) | 619 | (20.5%) | |
| NCI-center affiliated | |||||||
| Yes | <11 | (<1%) | <11 | (<1%) | <11 | (<1%) | |
|
| |||||||
| Outcome | |||||||
|
| |||||||
| 5-Fu | 1432 | (35.3%) | 361 | (35.1%) | 1071 | (35.4%) | *** |
| Folfox/Oxaliplatin | 821 | (20.3%) | 255 | (24.8%) | 566 | (18.7%) | |
| Other or Unknown Chemotherapy | 264 | (6.5%) | 76 | (7.4%) | 188 | (6.2%) | |
| No chemo | 1537 | (37.9%) | 337 | (32.8%) | 1200 | (39.7%) | |
Median household income in patient’s census tract.
Proportion of patient’s census tract population with high school education or greater.
Chi-square test (categorial) or t-test (continuous):
p<0.01: ***,
p<0.05: **,
p<0.10: *
-SEER regions were examined but are not reported. The distribution of patients seen by CCOPs vs. non-CCOPs differed by region (p<0.01)
-Specific comorbidities were examined but not reported. There were no differences among patients seen by CCOPs vs. non-CCOPs
-Per SEER-Medicare data use requirements, methods were used to suppress cell sizes smaller than 11, and included collapsing some categories of measures, using “<11” to reflect small numbers, and partially masking values to prevent mathematical derivation of small numbers.
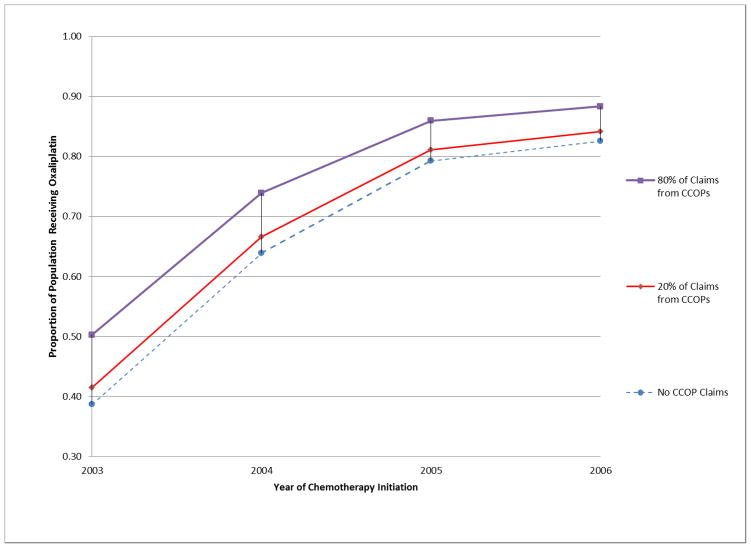
Figure 1.
Proportion of population receiving oxaliplatin-containing regimen, by year of treatment initiation and extent of CCOP interaction.
As presented in Table 2, CCOP patients were more likely to include minorities, and were slightly younger than non-CCOP patients. Non-CCOP patients were more likely to have the primary tumor located in the sigmoid colon, have poorly differentiated disease, be dually-eligible for Medicaid coverage and live in lower education areas. Fewer CCOP patients had a recent history of cardiac or pulmonary disease, though otherwise were similar to non-CCOP patients in terms of comorbidities.
In univariate regression (Table 3a), the degree to which patients interfaced with CCOPs was strongly associated with the probability of chemotherapy receipt (OR 10.85, 96% CI: 7.25–16.25). For example, a 10% increase in the proportion of a patient’s health care claims from a CCOP provider was associated with a 27% increase in the probability of receiving adjuvant chemotherapy (calculated from OR in Table 3; 95% CI: 1.22 – 1.32). Among those receiving chemotherapy, 10% increase in CCOP exposure was associated with a 7% increase in the probability of receiving oxaliplatin (95% CI: 1.03 – 1.10). Diffusion of oxaliplatin into practice is reflected in Table 3b, where the probability of receiving oxaliplatin among those initiating chemotherapy in 2006 was 2.22 times greater than those doing so in 2004 (95% CI: 1.52–3.24).
Table 3.
Univariate regression/Crude odds ratios in overall sample.
| 3a. Probability of outcome given the degree of exposure to CCOP MD or Hospital, among 2003–2005 cases treated at surgical hospital with no/limited medical school affiliation.
| ||||||
|---|---|---|---|---|---|---|
| Variable/Exposure | Outcome | β | (SE) | OR | 95% CI | p value |
| Proportion of claims from either CCOP MD or hospital | Chemo vs. no chemo | 2.384 | (0.206) | 10.85 | [7.25– 16.25] | <.001 |
|
| ||||||
| Proportion of claims from either CCOP MD or hospital | Oxaliplatin vs. 5-Fu | 0.659 | (0.168) | 1.93 | [1.39– 2.68] | <.001 |
| Proportion of claims from either CCOP MD or hospital | Oxaliplatin vs. any other chemo | 0.736 | (0.165) | 2.09 | [1.51– 2.88] | <.001 |
| 3b. Probability of Oxaliplatin receipt by year of chemotherapy initiation, among 2003–2005 cases treated at surgical hospital with no/limited medical school affiliation.
| ||||||
|---|---|---|---|---|---|---|
| Variable/Exposure | Outcome | β | (SE) | OR | 95% CI | p value |
| Year of chemo 2003 vs. 2004 | Oxaliplatin vs. 5-Fu | −0.983 | 0.119 | 0.37 | [0.30– 0.47] | <.001 |
| Year of chemo 2005 vs. 2004 | 0.667 | 0.109 | 1.95 | [1.57– 2.41] | <.001 | |
| Year of chemo 2006 vs. 2004 | 0.796 | 0.194 | 2.22 | [1.52– 3.24] | <.001 | |
|
| ||||||
| Year of chemo 2003 vs. 2004 | Oxaliplatin vs. any other chemo | −0.904 | 0.119 | 0.40 | [0.32– 0.51] | <.001 |
| Year of chemo 2005 vs. 2004 | 0.539 | 0.102 | 1.71 | [1.41 2.09] | <.001 | |
| Year of chemo 2006 vs. 2004 | 0.815 | 0.185 | 2.26 | [1.57– 3.25] | <.001 | |
In multivariable analysis (Table 4), CCOP exposure was associated with oxaliplatin receipt: a 10% increase in the proportion of a patient’s claims from a CCOP provider is associated with a 6% increase in the probability of that patient receiving oxaliplatin (calculated from OR in Table 4). Those initiating chemotherapy in 2006 were 2.7 times more likely to receive oxaliplatin than those initiating chemotherapy in 2004. The probability of receiving oxaliplatin decreased as patient age increased. Those with a history of renal disease, acute cardiovascular history, or diabetes were less likely to receive oxaliplatin. Those living in the highest education areas had a greater probability of receiving oxaliplatin.
Table 4.
Regression: Probability of receipt of innovative therapy (oxaliplatin-containing regimen) among those receiving any chemotherapy, 2003–2006.
| Patient Characteristics | β | (SE) | p | OR | 95% CI |
|---|---|---|---|---|---|
| Gender (ref = Male) | |||||
| Female | −0.204 ** | (0.099) | 0.039 | 0.815 | (0.672 – 0.990) |
| Race (ref = Caucasian) | |||||
| African American | 0.240 | (0.211) | 0.256 | 1.272 | (0.840 – 1.925) |
| Other/Unknown | −0.033 | (0.234) | 0.887 | 0.967 | (0.611 – 1.530) |
| Age (ref = 65–69) | |||||
| 70–74 | −0.555 *** | (0.125) | <.001 | 0.574 | (0.450 – 0.733) |
| 75–79 | −0.939 *** | (0.121) | <.001 | 0.391 | (0.309 – 0.496) |
| 80–84 | −1.543 *** | (0.179) | <.001 | 0.214 | (0.151 – 0.303) |
| 85+ | −2.657 *** | (0.375) | <.001 | 0.070 | (0.034 – 0.146) |
| Education1 (ref=Quartile 1) | |||||
| Quartile 2: low-med education | 0.061 | (0.131) | 0.643 | 1.063 | (0.822 – 1.374) |
| Quartile 3: med-high education | −0.016 | (0.143) | 0.912 | 0.984 | (0.744 – 1.302) |
| Quartile 4: highest education | 0.342 ** | (0.152) | 0.025 | 1.408 | (1.045 – 1.898) |
| Primary site of tumor (ref=Cecum, ascending) | |||||
| Flexures, Transverse/Descending | −0.135 | (0.133) | 0.308 | 0.874 | (0.674 – 1.133) |
| Large intestine/NOS | −0.427 | (0.332) | 0.199 | 0.653 | (0.340 – 1.251) |
| Sigmoid | −0.094 | (0.121) | 0.434 | 0.910 | (0.718 – 1.153) |
| Tumor grade (ref=poorly differentiated) | |||||
| Well-differentiated | −0.402 | (0.270) | 0.136 | 0.669 | (0.395 – 1.135) |
| Moderately differentiated | −0.241 ** | (0.114) | 0.034 | 0.786 | (0.629 – 0.982) |
| Unknown | −0.103 | (0.284) | 0.718 | 0.903 | (0.517 – 1.576) |
| Year of Chemo (ref=2004) | |||||
| 2003 | −1.031 *** | (0.125) | <.001 | 0.357 | (0.279 – 0.455) |
| 2005 | 0.771 *** | (0.118) | <.001 | 2.162 | (1.715 – 2.725) |
| 2006 | 0.984 *** | (0.218) | <.001 | 2.675 | (1.745 – 4.101) |
| Dual eligibility (ref=no) | |||||
| Yes | −0.183 | (0.171) | 0.282 | 0.833 | (0.596 – 1.163) |
| Comorbidity conditions (ref=no, for each) | |||||
| Liver disease | 0.912 | (0.823) | 0.268 | 2.488 | (0.496 – 12.479) |
| Rheumatoid disease or AIDs | −0.136 | (0.558) | 0.808 | 0.873 | (0.293 – 2.606) |
| Renal disease | −1.887 ** | (0.746) | 0.012 | 0.152 | (0.035 – 0.655) |
| Dementia or paralysis | −0.371 | (0.678) | 0.585 | 0.690 | (0.183 – 2.608) |
| CHF or acute MI or CVD or COPD | −0.245 * | (0.129) | 0.058 | 0.782 | (0.607 – 1.008) |
| Neuropathy | −0.223 | (0.375) | 0.551 | 0.800 | (0.384 – 1.667) |
| Diabetes | −0.278 ** | (0.135) | 0.040 | 0.757 | (0.581 – 0.987) |
|
| |||||
| Organization characteristics (of treating hospital) | |||||
|
| |||||
| Proportion of claims from CCOP-affiliated MD or Hospital | 0.589 ** | (0.234) | 0.012 | 1.803 | (1.141 – 2.849) |
| Any Direct Cooperative group affiliation (ref=no) | 0.100 | (0.131) | 0.444 | 1.106 | (0.855 – 1.430) |
| Any Medical School Affiliation (ref=unaffiliated) | −0.132 | (0.149) | 0.376 | 0.877 | (0.655 – 1.173) |
| NCI -center affiliated (ref=no) | 0.627 | (0.884) | 0.478 | 1.873 | (0.331 – 10.592) |
|
| |||||
| Intercept | 0.571 *** | (0.212) | 0.007 | ||
|
| |||||
| Estimates of Model Fit | |||||
| QIC | 2592.84 | ||||
| QICu | 2585.32 | ||||
| Sample Size | 2,253 | ||||
Significance:
.10;
.05;
.01
Proportion of patient’s census tract population with high school education or greater.
-SEER regions were examined but are not reported.
Among sensitivity analyses, the binary measure of CCOP exposure was significant in all analyses, and model results were similar to those using the proportion of claims with a CCOP provider. Model fit was more stable and substantially stronger using the continuous measure (proportion of claims), reflecting the non-linear relationship between CCOP exposure and probability of oxaliplatin receipt demonstrated in univariate models of functional form and illustrated in Figure 1. To examine the prospective effect of ongoing clinical trial enrollment as it may differentially influence oxaliplatin use, models examined data restricted to years following FDA approval. Findings were nearly identical in effect size, though with substantially smaller sample size, confidence intervals for some measures were sometimes wider.
Discussion
To investigate whether PBRNs are associated with more rapid translation of research innovations into practice, this study examined the use of adjuvant chemotherapy for stage III CC among patients treated at NCI CCOP-affiliated practices compared to other community-based practices. Oxaliplatin-containing adjuvant therapy has yielded trial-proven improved survival compared to the prior standard therapy. 20–22,24 Our results suggest that, compared to patients who received their care elsewhere in the community, patients who received their care from CCOP providers were more likely to receive this innovative therapy reflecting the best current scientific knowledge. Notably, a preponderance of the difference in CCOP versus non-CCOP oxaliplatin adoption appears to have occurred prior to FDA approval, likely due to clinical trial enrollment; however, this CCOP “advantage” remains persistent in all study years thereafter, including after FDA approval. These findings contribute to a growing set of evidence linking PBRNs to high quality cancer care.
In the short-term, these results demonstrate an association with improved quality of care for current cancer patients – receipt of trial-proven state-of-the-art therapy in lieu of inferior treatment options. At the same time, CCOP community-based practices also enroll approximately one-third of patients to NCI cancer treatment trials. 22 By doing so, CCOPs help accelerate enrollment and thus study completion times, and thus achieve the goals of the not only the Roadmap, but also more recent efforts such as the NCI Operational Efficiency Working Group, a goal of which is to identify strategies to “increase the percentage of studies that reach their accrual targets in a timely fashion.” 32 In turn, accelerated trial completion is anticipated to contribute to longer-term benefits, including a shortened time to the discovery of the next innovation and prospectively better outcomes for a larger number of future cancer patients.
The specific finding regarding the diffusion of oxaliplatin is important; however, the larger issue of adherence to consensus guidelines also merits discussion. Adjuvant therapy has been recommended for CC since 1990, and is an element of quality metrics defined by the American Society of Clinical Oncology Quality Oncology Practice Initiative (ASCO QOPI). 17,33 In our study population, a surprising 38% of patients did not receive any adjuvant chemotherapy. Many factors are likely contributory, including greater age, poorer health, and greater prevalence of specific contraindications among the untreated. For these patients, oxaliplatin’s potential survival benefit may not merit the risks of its associated additional toxicity, though they could appropriately receive 5-FU as a less toxic alternative.34 When controlling for such contraindications in this community-based study sample, patients receiving care from CCOP providers had substantially greater odds of receiving guideline concordant care vis-à-vis any adjuvant therapy (OR 12.97, 95% CI: 7.39 – 22.77 – Appendix Table 1), though the details of this care may have varied according to other measured and unmeasured factors in this population.
Appendix Table 1.
Regression: Probability of receipt of adjuvant therapy among those with surgical resection, 2003–05.
| Patient Characteristics | β | (SE) | p | OR | 95% CI |
|---|---|---|---|---|---|
| Gender (ref = Male) | |||||
| Female | −0.100 | 0.078 | 0.202 | 0.905 | (0.776–1.055) |
| Race (ref = Caucasian) | |||||
| African American | −0.198 | 0.193 | 0.304 | 0.820 | (0.562–1.197) |
| Other/Unknown | 0.309 | 0.226 | 0.172 | 1.362 | (0.874–2.122) |
| Age (ref = 65–69) | |||||
| 70–74 | −0.613 *** | 0.155 | <.001 | 0.542 | (0.400–0.734) |
| 75–79 | −1.011 *** | 0.151 | <.001 | 0.364 | (0.271–0.490) |
| 80–84 | −2.002 *** | 0.157 | <.001 | 0.135 | (0.099–0.184) |
| 85+ | −3.064 *** | 0.166 | <.001 | 0.047 | (0.034–0.065) |
| Education1 (ref=Quartile 1) | |||||
| Quartile 2: low–med education | −0.009 | 0.112 | 0.935 | 0.991 | (0.795–1.235) |
| Quartile 3: med–high education | −0.057 | 0.107 | 0.595 | 0.945 | (0.767–1.165) |
| Quartile 4: highest education | 0.066 | 0.116 | 0.572 | 1.068 | (0.850–1.341) |
| Primary site of tumor (ref=Cecum, | |||||
| Flexures, Transverse/Descending | −0.158 * | 0.092 | 0.087 | 0.854 | (0.713–1.023) |
| Large intestine/NOS | 0.291 | 0.271 | 0.283 | 1.337 | (0.787–2.273) |
| Sigmoid | −0.064 | 0.093 | 0.490 | 0.938 | (0.782–1.125) |
| Tumor grade (ref=poorly differentiated) | |||||
| Well-differentiated | 0.005 | 0.214 | 0.980 | 1.005 | (0.660–1.530) |
| Moderately differentiated | −0.070 | 0.089 | 0.429 | 0.932 | (0.783–1.109) |
| Unknown | −0.461 ** | 0.198 | 0.020 | 0.631 | (0.428–0.931) |
| Year of Diagnosis (ref=2003) | |||||
| 2004 | −0.169 * | 0.090 | 0.058 | 0.844 | (0.708–1.006) |
| 2005 | −0.160 * | 0.094 | 0.088 | 0.853 | (0.710–1.024) |
| Dual eligibility (ref=no) | |||||
| Yes | −0.726 *** | 0.105 | <.001 | 0.484 | (0.394–0.594) |
| Comorbidity conditions (ref=no, for | |||||
| Liver disease | 0.328 | 0.563 | 0.560 | 1.388 | (0.460–4.184) |
| Rheumatoid disease or AIDs | 0.461 | 0.329 | 0.161 | 1.585 | (0.832–3.019) |
| Renal disease | −0.098 | 0.366 | 0.788 | 0.906 | (0.442–1.857) |
| Dementia or paralysis | −0.410 | 0.289 | 0.156 | 0.664 | (0.376–1.170) |
| CHF or acute MI or CVD or COPD | −0.563 *** | 0.089 | <.001 | 0.570 | (0.479–0.678) |
| Neuropathy | −0.159 | 0.262 | 0.546 | 0.854 | (0.511–1.427) |
| Diabetes | −0.148 | 0.101 | 0.144 | 0.863 | (0.708–1.052) |
|
| |||||
| Organization characteristics (of treating hospital) | |||||
|
| |||||
| Proportion of claims from CCOP-affiliated MD or Hospital | 2.550 *** | 0.285 | <.001 | 12.806 | (7.319–22.406) |
| Any Direct Cooperative group affiliation (ref=no) | −0.184 * | 0.099 | 0.064 | 0.832 | (0.685–1.011) |
| Any Medical School Affiliation (ref=unaffiliated) | 0.011 | 0.116 | 0.928 | 1.011 | (0.805–1.268) |
| NCI-center affiliated (ref=no) | 0.021 | 0.798 | 0.979 | 1.021 | (0.214–4.875) |
|
| |||||
| Intercept | 2.588 *** | 0.223 | <.001 | 13.296 | (8.585–20.594) |
|
| |||||
| Estimates of Model Fit | |||||
| QIC | 4380.10 | ||||
| QICu | 4367.54 | ||||
| Sample Size | 4,054 | ||||
Significance:
.10;
.05;
.01
Proportion of patient’s census tract population with high school education or greater
-SEER regions were examined but are not reported.
Related to this, among limitations, the impact of several factors on treatment decisions is difficult to qualify using claims data. For example, a patient may have a comorbid condition, but the claims data do not characterize that condition’s severity or its immediate relevance. Moreover, the data do not measure some relevant factors, such as patient preference. By focusing primarily on patients who were healthy enough for surgery and received some form of chemotherapy, while excluding those who did not, this study took a conservative approach in order to minimize potential bias associated with treatment selection.
Characterizing the influence of a particular provider over treatment decisions is similarly challenging. We used the proportion of insurance claims from a CCOP provider as a proxy for the influence of the CCOP on treatment decisions. However, it is possible that a treatment plan could be defined by one physician who submitted a single insurance claim, though the plan was implemented in its entirety by another, who submitted all other claims. Sensitivity analyses using multiple forms of the CCOP exposure measure were consistent, strengthening our confidence in this study’s summary findings. Notably, a binary measure characterized patients as having any CCOP claims compared to those with no CCOP claims (Appendix 2). The effect estimate of CCOP exposure in this model was very similar in magnitude and direction as our primary model (Appendix 2: OR 1.38, 95% CI: 1.03–1.84; Table 4: OR 1.80, 95% CI: 1.14–2.85), suggesting that having any CCOP exposure may be influential in terms of treatment decisions, and increasing CCOP exposure may be associated with greater CCOP influence (Table 4). As it pertains to interpreting these models, we must point out that our study design and multiple sensitivity analyses led us to focus on models which produce odds ratios, which may be an overestimate of risk ratios under certain conditions.35 Future research may examine additional measures of health status, greater characterization of providers’ roles, and other factors in treatment decision-making than are allowed by these data.
Appendix Table 2.
Regression: Probability of receipt of oxaliplatin-containing therapy among those initiating oxaliplatin-containing therapy or other 5-FU therapy in 2003–2006; CCOP exposure measured as binary measure (Any CCOP claims vs. No CCOP claims).
| Patient Characteristics | β | (SE) | p | OR | 95% CI |
|---|---|---|---|---|---|
| Gender (ref = Male) | |||||
| Female | −0.201 ** | 0.099 | 0.043 | 0.818 | (0.674–0.993) |
| Race (ref = Caucasian) | |||||
| African American | 0.235 | 0.211 | 0.265 | 1.265 | (0.837–1.912) |
| Other/Unknown | −0.022 | 0.235 | 0.924 | 0.978 | (0.617–1.549) |
| Age (ref = 65–69) | |||||
| 70–74 | −0.555 *** | 0.124 | <.001 | 0.574 | (0.450–0.732) |
| 75–79 | −0.939 *** | 0.121 | <.001 | 0.391 | (0.309–0.496) |
| 80–84 | −1.555 *** | 0.179 | <.001 | 0.211 | (0.149–0.300) |
| 85+ | −2.675 *** | 0.376 | <.001 | 0.069 | (0.033–0.144) |
| Education1 (ref=Quartile 1) | |||||
| Quartile 2: low–med education | 0.057 | 0.131 | 0.665 | 1.058 | (0.818–1.369) |
| Quartile 3: med–high education | −0.020 | 0.142 | 0.889 | 0.980 | (0.742–1.295) |
| Quartile 4: highest education | 0.339 ** | 0.151 | 0.025 | 1.403 | (1.044–1.887) |
| Primary site of tumor (ref=Cecum, ascending) | |||||
| Flexures, Transverse/Descending | −0.137 | 0.133 | 0.306 | 0.872 | (0.672–1.133) |
| Large intestine/NOS | −0.443 | 0.331 | 0.181 | 0.642 | (0.335–1.229) |
| Sigmoid | −0.088 | 0.120 | 0.466 | 0.916 | (0.724–1.160) |
| Tumor grade (ref=poorly differentiated) | |||||
| Well-differentiated | −0.420 | 0.270 | 0.120 | 0.657 | (0.387–1.116) |
| Moderately differentiated | −0.241 ** | 0.114 | 0.034 | 0.786 | (0.628–0.982) |
| Unknown | −0.102 | 0.279 | 0.716 | 0.903 | (0.523–1.560) |
| Year of Chemo (ref=2004) | |||||
| 2003 | −1.033 *** | 0.125 | <.001 | 0.356 | (0.279–0.454) |
| 2005 | 0.771 *** | 0.118 | <.001 | 2.163 | (1.716–2.725) |
| 2006 | 0.995 *** | 0.216 | <.001 | 2.704 | (1.770–4.131) |
| Dual eligibility (ref=no) | |||||
| Yes | −0.180 | 0.170 | 0.291 | 0.835 | (0.598–1.166) |
| Comorbidity conditions (ref=no, for each) | |||||
| Liver disease | 0.935 | 0.822 | 0.255 | 2.547 | (0.509–12.752) |
| Rheumatoid disease or AIDs | −0.129 | 0.558 | 0.818 | 0.879 | (0.295–2.626) |
| Renal disease | −1.935 ** | 0.756 | 0.011 | 0.144 | (0.033–0.636) |
| Dementia or paralysis | −0.400 | 0.665 | 0.547 | 0.670 | (0.182–2.467) |
| CHF or acute MI or CVD or COPD | −0.258 | 0.130 | 0.047 | 0.773 | (0.599–0.997) |
| Neuropathy | −0.239 | 0.373 | 0.521 | 0.787 | (0.379–1.636) |
| Diabetes | −0.287 ** | 0.136 | 0.035 | 0.750 | (0.574–0.980) |
|
| |||||
| Organization characteristics (of treating hospital) | |||||
|
| |||||
| CCOP-affiliated MD or Hospital | 0.319 ** | 0.149 | 0.032 | 1.375 | (1.028–1.840) |
| Any Direct Cooperative group affiliation (ref=no) | 0.106 | 0.131 | 0.421 | 1.111 | (0.859–1.438) |
| Any Medical School Affiliation (ref=unaffiliated) | −0.144 | 0.148 | 0.329 | 0.866 | (0.648–1.156) |
| NCI-center affiliated (ref=no) | 0.653 | 0.895 | 0.465 | 1.922 | (0.333–11.097) |
|
| |||||
| Intercept | 0.557 *** | 0.210 | 0.008 | 1.745 | (1.157–2.631) |
|
| |||||
| Estimates of Model Fit | |||||
| QIC | 2,595.47 | ||||
| QICu | 2,588.16 | ||||
| Sample Size | 2,253 | ||||
Significance:
.10;
.05;
.01
Proportion of patient’s census tract population with high school education or greater.
This study demonstrates an association between CCOP membership and accelerated innovation adoption, though it is not possible to definitively ascertain whether there is a direct causal relationship between the two. It is quite plausible that physicians who join the CCOP network are predisposed to follow the clinical research literature, engage with academic centers, and thus more rapidly learn of and adopt state-of-the-art innovations in their practice. It may be more simply that the pre-FDA approval, clinical trial era protocol-guided experience and familiarity with the innovation contribute a short-term advantage which, with momentum, remains persistent across time. The arguably greater rate of oxaliplatin adoption among non-CCOP providers following FDA approval suggests CCOP experience momentum is relevant; however, Figure 1 suggests the CCOP and non-CCOP patient population proportions are plateauing without converging, and thus other factors uniquely characterizing CCOP practitioners or their PBRNs are also relevant. Empirically proving causality is difficult in any study, and employing a study design that would allow a definitive causal determination is virtually impossible given the organizational setting, the tremendous size of the network, and the depth of intervention that is necessary. This being said, the CCOP Program is nearly 30 years old, as are several of the CCOP sites in this study. As such, this study suggests that once the local CCOP practices are funded, their infrastructure and systems can successfully support and perpetuate the prioritization of research and the corresponding translation of research into practice through multiple generations of physicians at these practices.
Study data did not include a patient-level measure of trial participation and were deidentified, precluding the ability to differentiate those enrolled in a trial from those not enrolled. Inability to identify trial participants may contribute bias to our interpretation of the association between CCOP and oxaliplatin use among non-trial participants. This being said, not all CCOPs activated oxaliplatin trials; trial participants represent a small minority of community practice patients, even in CCOPs; and through randomization, only half of participants would have been in the trials’ experimental arm (receiving oxaliplatin); suggesting this risk of bias is somewhat limited. Moreover, CCOP patients were more likely to receive oxaliplatin than were non-CCOP community patients in all years – even after trial closure and FDA approval –suggesting robustness of the study’s primary findings.
The apparent multi-focal benefit of PBRNs compels us to revisit our national policies regarding paying for cancer clinical trials. Several studies have shown only a nominal increase in costs associated with NCI trial participation, yet hospital and insurance plan executives have consistently protested the additional cost and inefficiency incurred with offering trials to their patients, which they are loathe to subsidize directly or indirectly through clinical operations.36,37 This study suggests that the two worlds of clinical research and clinical practice are tightly intertwined, and efforts to separate and manage them independently may in-fact be counterproductive to the goals of each. There have been myriad calls to improve reimbursement for clinical trials and establish federal and state policies to lower barriers to insurance coverage for trial participants.38,39 While such policies have had mixed evidence of effectiveness,40,41 “pay-for-performance” and other emerging plans are gaining momentum, with a central premise of reimbursement incentives for higher quality care.42,43 It may be that a pay-for-performance reimbursement premium for improved quality will underwrite clinical research inefficiencies, and in a circular fashion the concomitant greater quality will help justify that premium.
We cannot disregard the current economic environment and the additional challenges facing CMS as the baby boomer generation ages into Medicare; however, we may simultaneously have an unprecedented opportunity for positive change as the nation works to refine and implement historic health care reform, while the NCI retools its clinical research enterprise following recent evaluations by the IOM and the NCI Operational Efficiency Work Group,32,44 and the NCI CCOP program continues its long history of self-evaluation and continuous improvement.22
Acknowledgments
Funding/Support: This work was funded by NCI grant 5R01CA124402, and directly informed by work performed in support of NCI Contract HHSN261200800726P. The work was supported in part by the Integrated Cancer Information and Surveillance System (ICISS), UNC Lineberger Comprehensive Cancer Center, through the University Cancer Research Fund via the State of North Carolina. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; NCI contracts N01-PC-35136, N01-PC-35139, and N02-PC-15105; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02. This work was conducted following IRB review and approval at the University of North Carolina (IRB 05-2761).
Role of the Sponsors: The NCI had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript. ICISS members (Carpenter, Meyer, and Wu) were involved in the design and conduct of the study, collection, management, analysis and interpretation of the data, and preparation, review, and approval of the manuscript.
References
- 1.Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine National Roundtable on Health Care Quality. JAMA. 1998 Sep 16;280(11):1000–1005. doi: 10.1001/jama.280.11.1000. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Ensuring Quality Cancer Care. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 3.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 4.Institute of Medicine. Bridging the Gap Between Practice and Research. Washington DC: National Academies Press; 1998. [PubMed] [Google Scholar]
- 5.Balas E. Information technology and physician decision support. Program and abstracts of Accelerating Quality Improvement in Health Care: Strategies to Speed the Diffusion of Evidence-based Innovations, sponsored by National Committee for Quality Health Care; Washington DC. January 27–28, 2003. [Google Scholar]
- 6.Westfall JM, Mold J, Fagnan L. Practice-based research--“Blue Highways” on the NIH roadmap. JAMA. 2007 Jan 24;297(4):403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 7.Mariotto A, Feuer EJ, Harlan LC, Wun LM, Johnson KA, Abrams J. Trends in use of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States: 1975–1999. J Natl Cancer Inst. 2002 Nov 6;94(21):1626–1634. doi: 10.1093/jnci/94.21.1626. [DOI] [PubMed] [Google Scholar]
- 8.Zerhouni E. The NIH Roadmap. Science. 2003;302:63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]
- 9.Green LA, Fryer GE, Jr, Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited. N Engl J Med. 2001 Jun 28;344(26):2021–2025. doi: 10.1056/NEJM200106283442611. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter WR, Weiner BJ. Encyclopedia of Health Services Research. SAGE Publications; 2009. Provider-Based Research Networks (PBRNs) [Google Scholar]
- 11.Lanier D. Practice-based research networks: laboratories for improving colorectal cancer screening in primary care practice. Med Care. 2008 Sep;46(9 Suppl 1):S147–152. doi: 10.1097/MLR.0b013e31817f0d00. [DOI] [PubMed] [Google Scholar]
- 12.Sales A, Smith J, Curran G, Kochevar L. Models, strategies, and tools. Theory in implementing evidence-based findings into health care practice. J Gen Intern Med. 2006 Feb;21( Suppl 2):S43–49. doi: 10.1111/j.1525-1497.2006.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warnecke RB, Johnson TP, Kaluzny AD, Ford LG. The community clinical oncology program: its effect on clinical practice. Jt Comm J Qual Improv. 1995 Jul;21(7):336–339. doi: 10.1016/s1070-3241(16)30158-4. [DOI] [PubMed] [Google Scholar]
- 14.Laliberte L, Fennell ML, Papandonatos G. The relationship of membership in research networks to compliance with treatment guidelines for early-stage breast cancer. Medical care. 2005 May;43(5):471–479. doi: 10.1097/01.mlr.0000160416.66188.f5. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter WR, Reeder-Hayes K, Bainbridge J, et al. The role of organizational affiliations and research networks in the diffusion of breast cancer treatment innovation. Med Care. 2011 Feb;49(2):172–179. doi: 10.1097/MLR.0b013e3182028ff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Cancer Society. [Accessed January 15, 2011];Cancer Facts and Figures. 2010 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf.
- 17.NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. Jama. 1990 Sep 19;264(11):1444–1450. [PubMed] [Google Scholar]
- 18.Sanoff HK, Carpenter WR, Martin CF, et al. Comparative Effectiveness of Oxaliplatin vs Non-Oxaliplatin-containing Adjuvant Chemotherapy for Stage III Colon Cancer. J Natl Cancer Inst. 2012 Jan 20; doi: 10.1093/jnci/djr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004 May 15;22(10):1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 20.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004 Jun 3;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 21.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007 Jun 1;25(16):2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 22.Minasian LM, Carpenter WR, Weiner BJ, et al. Translating research into evidence-based practice: the National Cancer Institute Community Clinical Oncology Program. Cancer. 2010 Jun 22; doi: 10.1002/cncr.25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute. The NCI Strategic Plan for Leading the Nation To Eliminate the Suffering and Death Due to Cancer. Rockville, MD: National Cancer Institute; 2007. http://strategicplan.nci.nih.gov/pdf/nci_2007_strategic_plan.pdf. [Google Scholar]
- 24.Hewitt M, Simone J. Enhancing Data Systems to Improve the Quality of Care. Washington DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 25.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 26.Altekruse S, Kosary C, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 27.Mack CD, Carpenter W, Meyer AM, Sanoff H, Sturmer T. Racial disparities in receipt and comparative effectiveness of oxaliplatin for stage III colon cancer in older adults. Cancer. 2011 Nov 9; doi: 10.1002/cncr.26622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999 Jan;10(1):37–48. [PubMed] [Google Scholar]
- 29.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007 Aug;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 30.American College of Surgeons Commission on Cancer. [Accessed June 1, 2010];Commission on Cancer: About the CoC. 2010 http://www.facs.org/cancer/coc/whatis.html.
- 31.Reeder-Hayes KE, Bainbridge J, Meyer AM, et al. Race and age disparities in receipt of sentinel lymph node biopsy for early-stage breast cancer. Breast Cancer Res Treat. 2011 Aug;128(3):863–871. doi: 10.1007/s10549-011-1398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Cancer Institute. Report of the Operational Efficiency Working Group Clinical Trials of the and Translational Research Advisory Committee. Rockville, MD: NCI; 2010. [Accessed July 15, 2011]. http://ccct.cancer.gov/files/OEWG-Report.pdf. [Google Scholar]
- 33.American Society of Clinical Oncology. [Accessed August 28, 2011];QOPI: The Quality Oncology Practice Initiative. http://qopi.asco.org/
- 34.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. The New England journal of medicine. 2001 Oct 11;345(15):1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 35.Rothman K, Greenland S, Lash T. Modern Epidemiology. Philadelphia: Lippencott Williams & Wilkens; 2008. [Google Scholar]
- 36.Goldman DP, Berry SH, McCabe MS, et al. Incremental treatment costs in national cancer institute-sponsored clinical trials. JAMA: the journal of the American Medical Association. 2003 Jun 11;289(22):2970–2977. doi: 10.1001/jama.289.22.2970. [DOI] [PubMed] [Google Scholar]
- 37.Ault A. Who should subsidize NCI clinical trials? Nat Med. 1998 Sep;4(9):992. doi: 10.1038/1975. [DOI] [PubMed] [Google Scholar]
- 38.Emanuel EJ, Schnipper LE, Kamin DY, Levinson J, Lichter AS. The costs of conducting clinical research. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003 Nov 15;21(22):4145–4150. doi: 10.1200/JCO.2003.08.156. [DOI] [PubMed] [Google Scholar]
- 39.McBride G. More states mandate coverage of clinical trial costs, but does it make a difference? Journal of the National Cancer Institute. 2003 Sep 3;95(17):1268–1269. doi: 10.1093/jnci/95.17.1268. [DOI] [PubMed] [Google Scholar]
- 40.Gross CP, Wong N, Dubin JA, Mayne ST, Krumholz HM. Enrollment of older persons in cancer trials after the medicare reimbursement policy change. Arch Intern Med. 2005 Jul 11;165(13):1514–1520. doi: 10.1001/archinte.165.13.1514. [DOI] [PubMed] [Google Scholar]
- 41.Martel CL, Li Y, Beckett L, et al. An evaluation of barriers to accrual in the era of legislation requiring insurance coverage of cancer clinical trial costs in California. Cancer J. 2004 Sep-Oct;10(5):294–300. doi: 10.1097/00130404-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Berwick DM. Measuring physicians’ quality and performance: adrift on Lake Wobegon. JAMA. 2009 Dec 9;302(22):2485–2486. doi: 10.1001/jama.2009.1801. [DOI] [PubMed] [Google Scholar]
- 43.Rosenthal MB. Beyond pay for performance--emerging models of provider-payment reform. N Engl J Med. 2008 Sep 18;359(12):1197–1200. doi: 10.1056/NEJMp0804658. [DOI] [PubMed] [Google Scholar]
- 44.Institute of Medicine. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program. Washington DC: National Academies Press; 2010. [PubMed] [Google Scholar]