Abstract
Performance of a motor task is improved by practicing a specific task with added “challenges” to a training regimen. We tested the hypothesis that in the absence of brain control the performance of a motor task is enhanced by training using specific variations of that task. We utilized modifications of step performance training to improve the ability of spinal rats to forward step. After a complete thoracic spinal cord transection, 20 adult rats were divided randomly to bipedally step on a treadmill in the forward, sideward, or backward direction for 28 sessions (20 min, 5d/week) and subsequently tested for their ability to step in the forward direction. Although the animals from all trained groups showed improvement, the rats in the sideward trained and backward trained groups had greater step consistency and coordination along with higher peak amplitudes and total integrated activity of the rectified electromyography signals from selected hindlimb muscles per step during forward stepping than the rats in the forward trained group. Our results demonstrate that by retaining the fundamental features of a motor task (bipedal stepping) the ability to perform that motor task can be enhanced by the addition of specific contextual variations to the task (direction of stepping). Our data suggest that the forward stepping neuronal locomotor networks are partially complemented by synchronous activation of interneuronal/motoneuronal populations that are also a part of the sideward or backward stepping locomotor networks. Accordingly, the overlap and interaction of neuronal elements may play a critical role in positive task transference.
Keywords: spinal transection, rats, step training, variability, electromyography
Introduction
Animals with a complete spinal cord transection (spinal) can learn to step (Barbeau et al., 1993; Ichiyama et al., 2005) and stand (De Leon et al., 1998; de Leon et al., 1999) with practice. A characteristic of such chronically learned motor behaviors, in general, seems to be that the greater the similarity of the trained task the greater the transfer of learning. Spinal cats trained to step perform better hindlimb stepping than cats trained to stand (hindlimb weight bearing) (De Leon et al., 1998). Similarly, spinal cats trained to stand, learn to support their body weight for longer durations than cats trained to step or not trained for either task (Hodgson et al., 1994; De Leon et al., 1998).
In light of the above examples, it seems reasonable to hypothesize that the more similar the muscles and the neural networks that execute a motor task, the more effective will be the transfer of the motor skill learned. Neuromuscular activation patterns differ during standing, walking, and swimming, suggesting that these behaviors may involve, at least in part, different neuronal but overlapping circuitries (Bigbee et al., 2007; Magnuson et al., 2009; Kuerzi et al., 2010). In contrast to the idea of distinct circuitries for individual tasks, studies on multifunctional networks from invertebrates have established that, given the appropriate sensory modulation, many motorbehaviors are driven by the coordinated activity of a common interneuronal pool (Briggman and Kristan, 2008; Berkowitz, 2010). These observations raise the issue as to whether the degree of commonality among the mammalian neural networks that are involved in different motor tasks could be a factor that could either facilitate or hinder the performance of one task when training for another task.
In task-specific neurorehabilitation training paradigms attempts have been made to improve motor performance by introducing complexities to the specific motor task being trained (Behrman and Harkema, 2000). Musselman et al. (2009) report that training in a variety of relevant walking skills in varying situations and environments is more optimal than simply training forward walking on a treadmill for improving the over-ground locomotor capability of incomplete SCI subjects. Moreover, we recently showed that spinal transected mice and rats are able to bipedally step more effectively when the mode of forward bipedal step training allows for some critical level of variability during stepping (Cai et al., 2006; Ziegler et al., 2010). This strategy allows for variation in interneuron and motor unit activation patterns while also retaining the common neuronal elements underlying bipedal stepping.
In the present study, we used qualitative variants of bipedal step training, i.e., sideward and backward stepping, to determine the effects of these “challenges” on the recovery of forward stepping ability. Assuming that there are some common neural elements underlying different stepping behaviors (Courtine et al., 2009), we hypothesized that the net ensemble of the neural networks that control forward stepping will be more extensive if spinal rats are trained with sideward or backward stepping than with forward stepping.
Materials and Methods
Animals
Twenty adult female Sprague–Dawley rats (200–250 g body weight) underwent EMG and epidural stimulating electrode implantations and complete spinal cord transection surgeries. The rats were assigned randomly to one of three groups: 1) forward step trained (FT, n = 6), sideward step trained (ST, n = 7), or backward step trained (BT, n = 7) for ~5 weeks (28 sessions). In addition, five non-injured and non-trained control (Con) rats were used to obtain control data for EMG and kinematics analyses. All surgeries were performed under aseptic conditions with the animals deeply anesthetized with isoflurane gas (1.0 to 2.5% via facemask as needed). Surgery was performed with the animals on a water-circulating heating pad maintained at 37°C to prevent hypothermia. All incisions were closed in layers using 4.0 Dexon for the muscle and fascia and 4.0 Vicryl for the skin. After surgery, the rats were placed in an incubator maintained at 37°C until fully recovered and administered antibiotics and analgesics once or twice per day as needed for 3–4 days. Thereafter, the rats were housed in a room maintained at 26±1°C and 40% humidity and on a 12:12 hours light:dark cycle with access to food and water ad libitum. The cage floors were covered with CareFresh bedding. Pieces of fruit were given once daily. All experimental procedures were approved by the University of California Los Angeles Chancellor’s Animal Research Committee and complied with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
EMG implantation procedures
A small incision was made at the midline of the skull. The muscles and fascia were retracted laterally, small grooves were made in the skull with a scalpel, and the skull was dried thoroughly. Two Omnetics connectors with Teflon-coated stainless steel wires (AS632, Cooner Wire, Chatsworth, CA, US) were attached securely to the skull with screws and dental cement as previously described (Roy et al., 1991; Ichiyama et al., 2005). A skin incision was made in the mid-dorsal region of the back and 24 wires from the connector were routed subcutaneously to the most distal end of the opening. Two wires were coiled subcutaneously for later implantation on the spinal cord (see below). Skin and fascial incisions were made to expose the belly of the adductor brevis (Add), vastus lateralis (VL), semitendinosus (St), medial gastrocnemius (MG), and tibialis anterior (TA) muscles bilaterally. All EMG data reported are from the muscles in the right limb, which was the lead leg for ST. Two wires were routed subcutaneously from the back incision to each muscle site. Bipolar intramuscular EMG electrodes were formed and secured into the mid-belly of each muscle as described previously (Roy et al., 1991). The EMG wires were coiled near each implant site to provide stress relief. Stimulation through the connector implanted on the skull was used to verify the proper placement of the electrodes in each muscle. In addition, proper placement of the electrodes was verified post-mortem via dissection.
Spinal cord transection procedures
Spinal cord transections were performed 14 days after the implantation of the EMG electrodes. A dorsal midline skin incision was made from ~T6 to T10 and the paravertebral muscles and fascia from ~T7 to T9 were reflected laterally to expose the vertebrae. To expose the spinal cord, a partial laminectomy was performed via removal of the spinous processes and a portion of the lateral bodies of the T7 and T8 vertebrae. The dura was picked up using fine forceps and microscissors were used to completely transect the spinal cord(including the entire extent of the dura). Small cotton balls were used to separate the cut ends of the spinal cord and to clean the transection site. Two surgeons independently verified a complete transection by gently passing a fine glass probe through the transection site and then lifting the cut ends of the spinal cord. Gelfoam was inserted in the transection site to minimize bleeding and to separate (~2 to 3 mm) the cut ends of the spinal cord. Post-surgery, the bladders of all animals were expressed manually three times daily for the first two weeks and two times thereafter throughout the study.
Epidural stimulation electrode implant procedures
Epidural electrodes were implanted at the same time as the spinal cord transection surgery. A partial laminectomy was performed over spinal cord segments L2 (between vertebral levels T12 and T13) and S1 (vertebral level L2). Two Teflon-coated stainless-steel wires were inserted through an opening made between the T10 and T11 vertebrae and one wire was passed epidurally to each partial laminectomy site. A small region (~1 mm notch) of the Teflon coating was removed from each wire to form the stimulating electrodes: the exposed surface was positioned towards the spinal cord and the wire was secured to the dura at the midline of the spinal cord at each site with 9.0 silk sutures.
Locomotor training procedures
Rats spinalized as adults do not recover any stepping ability spontaneously (Ichiyama et al., 2008b; Kubasak et al., 2008). To facilitate stepping, all rats received an injection of the a 5-HT2 agonist quipazine (0.3 mg/kg, i.p.) 10–15 min prior to each training session as described previously (Gerasimenko et al., 2007; Ichiyama et al., 2008a). A treadmill was mounted over and secured with clamps to a rotating turntable such that the direction of the treadmill could be changed easily to enable stepping in one of three directions: forward, sideward, or backward. Evidence from our laboratory highlight the importance of using a combination of pharmacological facilitation and epidural stimulation to evoke a good bipedal stepping response in spinal rats (Ichiyama et al., 2005; Gerasimenko et al., 2007; Ichiyama et al., 2008a). Accordingly, epidural electrical stimulation at L2 and S1 was delivered continuously during the training and testing sessions at 40 Hz with 200 μs duration rectangular pulses as described previously (Ichiyama et al., 2005). An upper body harness was used to position the rats over a treadmill belt and to partially support their body weight during bipedal locomotion. Rats were trained 5 days/week, 20 min/session for ~5 weeks (28 training sessions) starting 7 days after the spinal cord transection surgery. The treadmill belt speed was increased progressively from 6 to 13.5 cm/s. By 8 training sessions, all FT and ST rats were able to step forward and sideward, respectively, at 13.5 cm/s for 20 min. BT rats, however, stepped only at slower treadmill speeds (9 cm/s or slower) for as long as the 18th session and were dependent on tail pinching to step backward. For the last ten sessions (18th session onwards) of backward step training, the treadmill speed was increased to 13.5 cm/s, but most rats continued to require tail pinching to step backward. Tail pinching was not used during any testing session.
Behavioral testing procedures
All kinematics data were collected after 28 sessions of locomotor training. Throughout the testing session, both EMG and kinematics data were collected from each rat as the rats (irrespective of training status) stepped in the forward direction. The connector implanted on the rat’s head was connected to a Grass S88 Stimulator(Grass Instruments) through a stimulus isolation unit (Grass SIU5; Grass Instruments). EMG signals (2 kHz) were amplified and filtered(10–1000 Hz bandpass). Rats in all groups stepped bipedally on a moving treadmill (13.5 cm/s) and three-dimensional video recordings (Basler Vision Technologies)were made using four cameras (2 cameras on each side at 100 fps) oriented at 45° and 135° bilaterally with respect to the forward direction of locomotion. Reflective markers were attached bilaterally to the shaved skin overlying the anterior superior iliac spine of the iliac crest, greater trochanter, lateral condyle, lateral malleolus, the distal end of the fifth metatarsal, and the lateral surface of the fourth metatarsal. SIMI motion capture software (SIMI Reality Motion Systems, Unterschleissheim, Germany) was used to obtain three-dimensional coordinates of the markers. All rats were tested in the presence of quipazine (0.3 mg/kg, i.p. administered ~15 min prior to the testing) as well as epidural electrical stimulation (at spinal segments L2 and S1, a frequency of 40Hz, and an intensity of 2.5 to 3.5 V) (Ichiyama et al., 2008b; Courtine et al., 2009).
Data analysis
The body was modeled as an interconnected chain of rigid segments, and joint trajectories and angles were generated accordingly. A range of kinematics gait parameters including cycle period, stance phase, swing phase, drag (defined as the time that the foot lags behind the ankle just before toe-off during the initial swing phase in a step cycle) normalized to the step cycle, step trajectories including step length, step height, and joint angle measurements were computed during forward stepping behavior in all rats. Six to ten step cycles when the rats were stepping consistently were analyzed for each rat. The quality of forward stepping was compared among the three groups by quantifying the following: i) percentage of the average number of plantar steps taken, where a plantar step is defined as foot placement on the treadmill with extended as opposed to curled toes; ii) variability in step length between adjacent steps (step length consistency), as measured by the first order principal component analysis; iii) horizontal mid-swing stepping velocity, as defined as the mean horizontal velocity of the foot during the period representing 30 to 80% of the completion of the swing phase of a normalized step cycle. This portion of the swing phase was chosen because we observed an obvious slower foot velocity in the mid-swing phase of the step cycle in some rats; and iv) gait timing variability, as assessed by the coefficient of variation of the lag-time between the onset of the right and left leg stance phases within a step cycle.
Activation patterns (muscle waveforms and timing) for each muscle were obtained by taking an average of 6 to 10 filtered, rectified, and normalized (to the step cycle) EMG bursts from each muscle of each rat during consistent stepping. The threshold for inducing locomotor activity with epidural stimulation (40 Hz) was assessed for all rats after 10 days of training and at the end of the last testing session. These data were used to determine any change in threshold voltage required to induce locomotion (see below) at the end of 28 training sessions. Mean peak amplitudes, durations, and integrals of identified EMG bursts from each muscle were computed for each rat during the testing at the end of the 28 training sessions (Ichiyama et al., 2008b; Courtine et al., 2009).
Statistical analyses
All data are reported as mean ± SEM or median values. Six to ten consecutive weight-bearing steps from each rat in each group were included in the analyses of all measures. Overall significant differences in trajectory characteristics (trajectory length, step length, and step height), kinematics measures (joint angles, velocity characteristics) and stepping quality (step length consistency, the lag time between the onset of the right and left hindlimbs, percentage of plantar steps) between the Con, FT, ST, and BT were obtained using univariate analysis of variance measures (ANOVA). For all ANOVA measures, animal groups were treated as the independent variable and the levels (sub categories) of outcome variables (example trajectory characteristics) as the dependent variable. Two-way ANOVA was run to test the difference in EMG burst characteristics (cycle period, burst durations, amplitudes and integrals) between animal groups for all muscles tested (with animal groups and muscle groups defined as the two factors). Change in stimulation threshold over time was calculated using a repeated measures one way ANOVA. There was homogeneity of variance between groups as assessed by Levene’s test for equality of error variances. Normality of distribution was assessed by the Shapiro Wilk test. For data sets that were not distributed normally and with differences in variances - step cycle parameters (stance, swing, and drag durations) and consistency in the interlimb phase differences (coefficient of variation of the lag time), the nonparametric Kruskal-Wallis rank test was utilized. Bonferroni post-hoc adjusted tests (and Dunn’s Test for non-parametric measures) were used to identify significant differences among individual groups and to reduce Type I errors. Differences between groups were considered statistically significant at P<0.05. All statistical analyses were performed using MATLAB (Mathworks) and GraphPad (GraphPad Software, Inc.) for Windows Software.
Results
Our results demonstrate that irrespective of the training regimen, all spinal rats were able to step in a forward direction in the presence of quipazine treatment and epidural stimulation. Detailed analyses, however, revealed distinct differences in the kinematics and EMG characteristics of the forward stepping behavior among the rats in the three groups. Overall, our results demonstrate that the rats trained to step sideward or backward showed greater stepping consistency, a higher percentage of plantar steps, faster horizontal velocities during the swing phase, and more highly coordinated interlimb coordination during forward stepping than rats trained to step forward. These behavioral distinctions between groups were reflected in the hindlimb kinematics and EMG activation patterns.
Step kinematics
Qualitatively, the stepping consistency between the right and left hindlimbs (interlimb coordination) was more similar to Con in most rats in ST and BT than in FT (Figure. 1). In addition, more rats in ST and BT than in FT displayed a relatively normal alternating pattern, i.e., similar to Con, between the right and left hindlimbs across step cycles. This more consistent interlimb coordination in the BT and ST is reflected in the L-shaped pattern in the joint probability plots of vertical step heights between the two hindlimbs, whereas a more variable pattern is observed in the FT.
Figure 1.
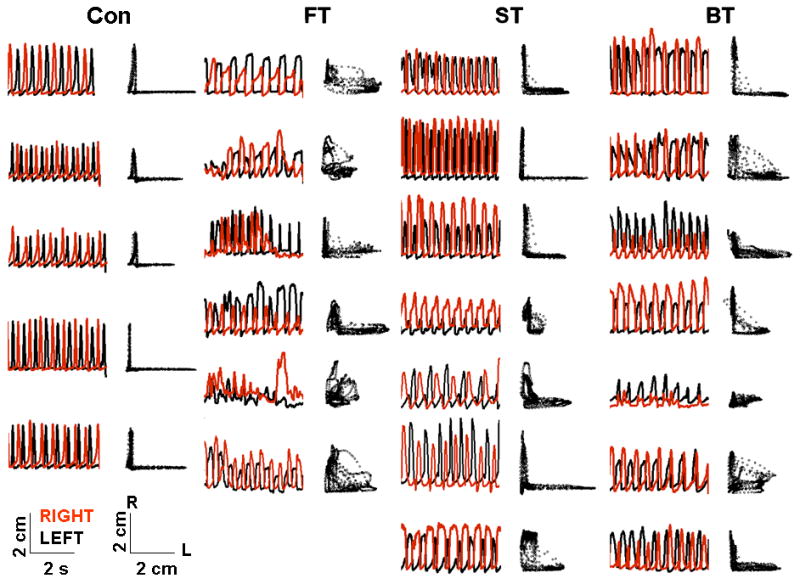
Interlimb coordination during forward stepping. Vertical step height trajectories of the metatarsophalangeal joint during consecutive left (black) and right (red) hindlimb forward stepping in individual rats in the control (Con, n=5), forward trained (FT, n=6), sideward trained (ST, n=7), and backward trained (BT, n=7) groups. Adjacent traces to the right (black) are joint probability distributions of the left and right MTP joint movements: an L-shaped pattern indicates an alternating motion of the two hindlimbs, whereas a D-shaped pattern indicates less alternation between the two hindlimbs.
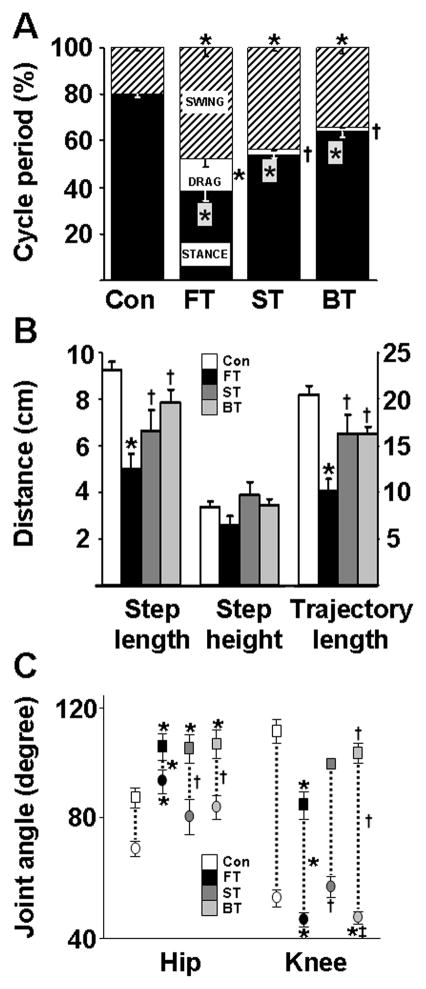
The kinematics of a typical forward step cycle in ST and BT more closely resembled the step cycle of Con than did the step cycle of FT (Figure. 2). The percentage of the swing phase duration in a step cycle was longer (Kruskal Wallis Statistic = 13.17, P= 0.0043) and the stance phase duration was shorter (Kruskal Wallis Statistic = 14.43, P = 0.0024)in all SCI groups than in Con (Figure. 2A). FT had a longer period of foot drag during a step cycle than Con, ST, and BT (Kruskal Wallis Statistic = 13.10, P = 0.004).
Figure 2.
Mean (±SEM) kinematics measures for 6–10 step cycles for all rats in the Control (Con), forward trained (FT), sideward trained (ST), and backward trained (BT) groups during forward stepping after five weeks of training in a specific direction. (A) Percentage of stance duration (black), swing duration (hashed lines), and foot drag at the beginning of the swing phase (white) normalized to the step cycle duration. (B) Step lengths, step heights, and trajectory lengths of the step cycles for each group. (C) Minimum (circles), maximum (squares), and the range of hip and knee joint angles during forward stepping for each group. Abbreviations and number of animals are the same as in Figure 1. *, †, and ‡: significantly different from the Con, FT, and ST groups, respectively, at P< 0.05.
Mean step length (F3, 21 = 6.602, P =0.002) and trajectory lengths (F3, 21 = 8.424, P =0.0007) were shorter in FT than in Con, ST, and BT (Figure. 2B). Mean step height was similar among all four groups. The mean maximum hip joint angle was smaller in all SCI groups (F3, 21 = 4.196, P =0.017) than Con and the mean minimum hip joint angle was smaller in FT (t= 3.358, P =0.026) than Con (Figure. 2C). The excursion (range) of the hip joint was shorter in FT than Con, ST and BT (F3, 21 = 7.803, P =0.001). The mean maximum knee joint angle was smaller in FT than in Con and BT (F3, 21 = 8.448, P =0.0006), whereas the minimum knee joint angle was larger (F3, 21 =4.218, P =0.021) in ST and Con than both FT and BT. The knee excursion (range) was larger in Con and BT than in FT (F3, 21 =5.604, P =005).
Stepping ability
The percentage of plantar steps was higher in Con than in FT and BT (F3, 21 = 9.230, P = 0.0004) and higher in the ST (~60%) than in FT (~10%) (t = 2.472) (Figure. 3A). Based on PCA analysis, mean step length consistency was greater in Con and ST than in FT and BT (F3, 21 = 13.78; P =0.002, Figure. 3B). The mean horizontal velocity of the foot during the mid-swing phase was slower in all SCI groups than in Con (F3, 21=36.19; P <0.0001) and in FT than in ST and BT (F2, 17 = 6.174; P = 0.020) (Figure. 3C). The coefficient of variation of the lag time between the onset of the right and left hindlimbs for consecutive steps (reflecting consistency in the interlimb phase differences for each step cycle) was lower in Con and ST than in BT and FT (Kruskal Wallis Statistic = 13.90; P =0.003, Figure. 3D).
Figure 3.
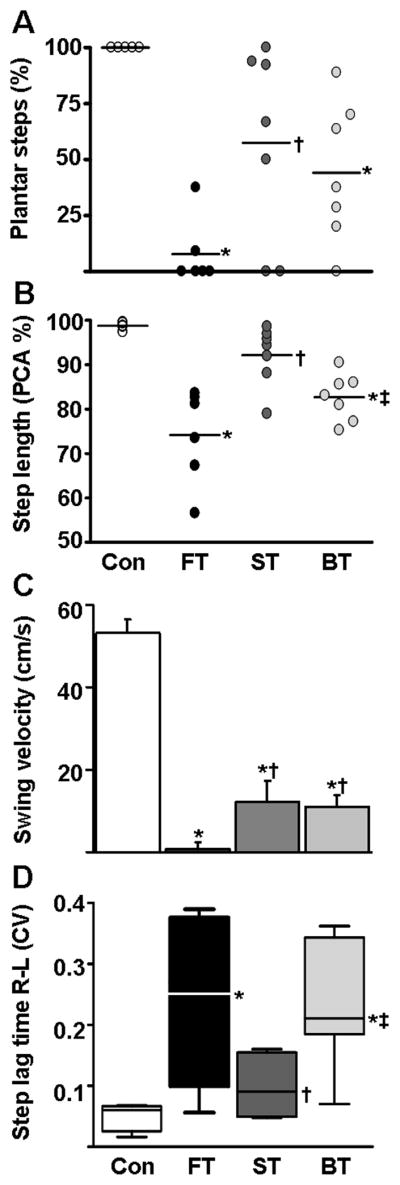
Phase-dependent measures of stepping. (A) Percentage of plantar steps performed by the rats in each group during forward stepping after five weeks of forward, sideward, or backward training. (B) Consistency of the hindlimb horizontal trajectory as measured by the first principal component (PCA %): higher PCA values indicate greater consistency in stepping. Values for individual rats (circles) and the mean values (horizontal line) are shown for each group in both (A) and (B). (C) Mean (± SEM) horizontal velocity during the mid-swing phase for each group. (D) Coefficient of variation of the lag-time between the onset of the right and left leg movements within a step cycle. Lower values indicate less variation between the right and left hindlimbs during forward stepping. Horizontal lines on the box plot demonstrate quartile and median values in each group. Abbreviations and number of animals and steps analyzed in each group are the same as in Figure 1 and 2. *, †, and ‡: significantly different from the Con, FT, and ST groups, respectively, at P< 0.05.
Hindlimb muscle EMG burst characteristics
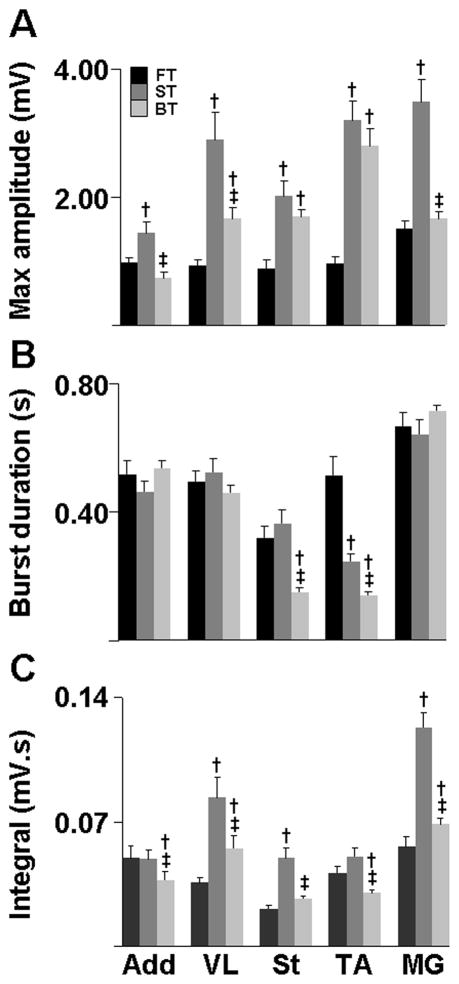
The threshold of epidural stimulation required to produce reliable locomotion in the forward direction was lower after 28 than 10 days of training in the ST (2.2± 0.1 vs. 1.7±0.1 V, t = 3.771, P = 0.0093), but not in FT (2.4±0.3 vs. 2.2±0.2 V) or BT (1.7±0.1 vs. 1.4±0.2 V). There was a significant interaction between the effects of animal groups and muscle peak amplitude values (F2, 4, 8 = 5.071, P <0.0001). Simple main effects analysis showed that the EMG burst peak amplitudes were different between groups for all muscles tested (F2, 4, 8 = 23.51, P < 0.0001). Post-hoc analysis showed significant higher EMG burst peak amplitudes in the ST and BT compared to the FT for all muscles (P < 0.001) except for the ADD and MG in BT (Figure. 4A). In addition, the maximum amplitudes of the adductor (t=3.858, P <0.001) and extensor muscles (t=2.947, P <0.01 and MG t=5.211, P <0.001) were higher in ST than BT. There was a significant interaction between the effects of animal groups and burst durations (F2, 4, 8 = 7.21, P <0.0001). Simple main effects analysis showed that the EMG burst durations were different between groups (F2, 4, 8 = 2.28, P <0.0001) with post-hoc analysis demonstrating shorter mean EMG burst durations for the TA and St in BT than in FT and ST and shorter for the TA in ST than FT (P < 0.001, Figure. 4B). There was a significant interaction between the effects of animal groups and burst integrals (F2, 4, 8 = 4.29, P <0.0001). Simple main effects analysis showed that the EMG burst integrals were different between groups (F2, 4, 8 = 3.98, P <0.0001). Post-hoc analysis demonstrated that the mean EMG burst integrals for both extensors (VL and MG) were smaller (P <0.01) in FT than in ST and BT, and smaller in all muscles (P <0.01) in BT than in ST (Figure. 4C). In addition, the mean burst integral of the St (t=9.970, P <0.001) was smaller in FT than in ST.
Figure 4.
EMG characteristics from select hindlimb muscles during forward stepping averaged for all rats in each group while forward stepping (n=10 steps). (A) Mean (± SEM) maximum amplitudes, (B) burst durations, and (C) burst integrals normalized to the step cycle period for each muscle in each group during forward stepping. Abbreviations: Add, adductor; VL, vastus lateralis; St, semitendinosus; TA, tibialis anterior; MG, medial gastrocnemius. Group abbreviations, number of rats and number of steps analyzed in each group are the same as in Figure 1. † and ‡: significantly different from the FT and ST groups, respectively, at P< 0.05.
Muscle activation patterns
The cycle period measured using the TA EMG burst was longer in FT than Con, ST, and BT (F3, 21=8.233, P <0.0001, Figure. 5A). The relatively long cycle period in FT appears to reflect the slow horizontal velocities (and consequent longer time) in the mid-swing phase of the step cycle (Figure. 3C) and not to other step characteristics such as trajectory length and step height (Figure. 3B). Flexor-extensor muscle synergies were similar in all three groups such that the flexors (TA, St) were active primarily during the swing phase and the extensors (MG, VL) were active primarily during the stance phase of each normalized step cycle (Figure. 5C and D, only TA and MG shown). This alternating antagonistic muscle activity pattern is similar to that seen in Con (Figure. 5B). The extensor, but not the flexor or adductor, muscles showed different temporal patterns across groups (data not shown for Add, VL, and St). Qualitatively, MG activity was initiated at ~40% of the normalized step cycle in FT, but much earlier, i.e., at ~17% of the step cycle, in ST and BT (Figure. 5D). Similarly, the VL activity was initiated at ~50% of the normalized step cycle in FT, but much earlier, i.e., at ~30% of the step cycle, in ST and BT (data not shown).
Figure 5.
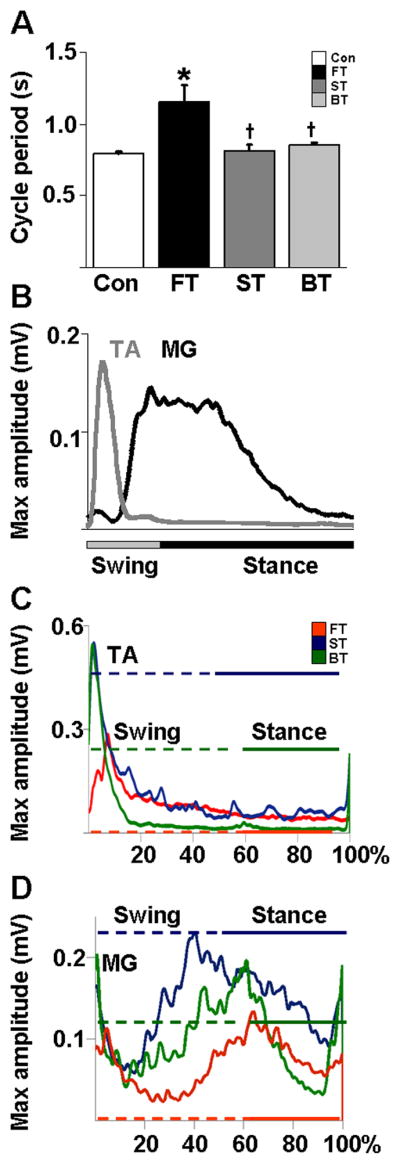
Phase-dependent EMG characteristics. (A) Mean (±SEM) cycle period obtained from the MG EMG burst activity during forward stepping in each group. *, and †: significantly different from the Con and FT group at P< 0.05. (B) Mean integrated EMG activity of the TA and MG muscles from non-injured control rats (n=5) showing a typical flexor-extensor activation pattern for a normalized step cycle during forward stepping. (C) Mean rectified EMG activity of the TA and (D) MG normalized to a step cycle for all rats in each group. The horizontal lines in the TA and MG muscle trace depict the swing (dotted) and stance (solid) phase durations for each group. Group abbreviations, number of rats and number of steps analyzed in each group are the same as in Figure 1.
Discussion
We compared three qualitatively different task-specific bipedal step training strategies, i.e., forward, sideward, and backward step training, for their ability to improve forward bipedal stepping in rats receiving a complete spinal cord transection as adults. We found that the best forward stepping, as described by similarity to non-injured control rats, was observed in spinal rats that were trained to step sideward. The better performance in the forward stepping behavior of the ST group was associated with higher peak EMG amplitudes and muscle activation patterns that were more similar to that observed in intact rats than either the FT or BT groups. In addition, the horizontal velocity during swing, foot trajectories, and the quality and coordination of stepping were more similar to stepping in control rats in ST and BT than in FT.
Imposing variations to task-specific training improves the performance of that task
Given the many examples that task-specific training enhances motor performance after a SCI (Hodgson et al., 1994; Behrman et al., 2005; Bigbee et al., 2007; Harkema, 2008; Magnuson et al., 2009), the present results were unexpected. For instance, spinal cats trained to step can regain more effective hindlimb stepping than cats trained to stand (De Leon et al., 1998). Similarly, spinal cats trained to stand, learn to support their body weight for longer durations than cats trained to step or not trained for either task (Hodgson et al., 1994; De Leon et al., 1998). Rats trained to reach and grasp perform better in this task, but worse in skilled running across a ladder, and vice versa, rats trained to run across a ladder performed better in this task but worse in reaching and grasping (Girgis et al., 2007; Garcia-Alias et al., 2009). An essential difference among the different modes of training in these studies compared to the present study is that the fundamental feature of the different training modes in the present study involved bipedal stepping in different directions. In contrast, standing versus stepping entails qualitatively different patterns a static postural task as opposed to a dynamic repetitive task. Likewise, reaching is a fundamentally different task than stepping on a ladder.
Physiologically, the implication from the combination of all of these studies is that there must be some critical level of qualitative similarity of the spinal circuits engaged within the trained tasks and the task that is being tested. As a consequence, if the neuronal circuits being trained and those being tested partially overlap, then this could reinforce the synaptic efficacy of a more expansive neural circuit. This, in turn, could result in engagement of a larger circuitry that can become more responsive to the intrinsic variability that appears to be important to the spinal locomotor circuitry (Cai et al., 2006; Ziegler et al., 2010). Our results are consistent with studies showing that practicing variations of a task along with the specific task can be much more beneficial than training in a single task (Shea and Kohl, 1990). After SCI, rats that were trained to stair climb (Singh et al., 2010)or injured mice that were trained on a running wheel (Engesser-Cesar et al., 2005)showed improvement in open-field locomotion. Similarly, Starkey and colleagues (Starkey et al., 2011) observed enhanced performance in a novel untrained staircase grasping test after training incomplete SCI rats in a specific single pellet reaching task. In the clinical setting, imposing a more “challenging” training task, such as negotiating obstacles during step-training, walking on different terrains, and walking while reaching/carrying objects, can improve the walking behavior of patients with a SCI (Behrman et al., 2005; Musselman et al., 2009; Manella et al., 2010). Some of the ways to “challenge” a walking task is to increase the speed and/or incline of the treadmill belt and/or add weights to the ankle during locomotion each of these parameters alter the magnitude of the same behavior in a graded, continuous manner. In the present work, we selected the direction of stepping as a specific contextual variation to the task of forward stepping, and define sideward and backward stepping as “challenging” tasks for the forward stepping task.
Based on the present results, we propose that by adding variations of a qualitatively similar task to a specific motor task a wider ensemble of afferent feedback could enhance the performance of the specific motor task. Consequently, because the cutaneous receptor fields are densely distributed on the foot pad of a rat (Leem et al., 1993), the inherent nature of sideward step training (sideward brushing of the foot against the treadmill) may have activated a relatively larger ensemble of afferent feedback from cutaneous as well as mechanoreceptors of the lower limb. As a result, sideward step training might have engaged and thereby trained a broader spinal neural circuitry than forward step training leading to greater neuronal activation (and hence the resultant elevated EMG activity) in ST as compared to FT during forward locomotion. Our observation is supported by the evidence that the threshold for stimulation that evoked forward locomotion was significantly lower in the ST than the other two SCI groups, perhaps due to a compensatory facilitating input from peripheral afferents and a corresponding greater excitability of specific neuronal populations that drove the motor output in the ST group.
Role of multifunctional spinal networks in “challenging” training
Neuromuscular activation patterns of fundamentally discrete motor behaviors such as standing, reaching, and swimming suggest that these may involve, to a large extent, distinct neuronal circuitries (Bigbee et al., 2007; Girgis et al., 2007; Magnuson et al., 2009; Kuerzi et al., 2010). One could hypothesize that distinct spinal circuits control forward, backward, and sideward stepping behaviors that, in turn, would facilitate stepping only in a given direction. In addition, it also would be reasonable to expect that training the potential for learning a novel motor task is less if only that task is practiced (Bigbee et al., 2007). Our results, however, show that independent from the nature of the stepping regimen all animals were able to forward step, albeit the best forward stepping capability was observed in sideward trained animals.
Alternatively, we hypothesize that stepping in different directions is likely controlled by a multifunctional network of neurons, thereby implying that qualitatively different, but fundamentally similar motor tasks would engage a broader and more dissimilar neural circuitry than when stepping without these contextual variations. Multifunctional networks have been widely studied in invertebrates and it is well established that given the appropriate sensory modulation, many motor behaviors are driven by the coordinated activity of a common neuronal pool (Briggman and Kristan, 2008; Berkowitz, 2010). Swimming and crawling behaviors in leeches, for example, are mutually exclusive motor behaviors that are controlled by a common network of neurons (Briggman and Kristan, 2006). Similarly, swimming (a rostrocaudal movement) involves all, plus a larger population of, neurons that organize the struggling (a more caudorostral pattern) behavior in tadpoles (Soffe 1996). At a more cellular level, studies have shown that in the somatogastric nervous system of the crab at least twenty different neuromodulator patterns elicit comparable motor responses of the gastric mill (chewing behavior) while each of these patterns also share the same core central pattern generator (Saideman et al., 2007). Based on the similarities among muscle synergies in swimming, kicking, and walking behaviors in the frog, D’Avella et al. (D’Avella et al., 2003) suggest that there is a sharing of neural circuitries across different motor tasks. Similarly, based on differences in some key kinematics features and muscle activation patterns, it has been suggested that there is involvement of partially overlapping neuronal circuits among different behaviors such as forward (Buford and Smith, 1990), backward (Buford and Smith, 1990), upslope (Carlson-Kuhta et al., 1998), and down slope (Trank and Smith, 1996)stepping in cats and forward and backward stepping in dogs (Vilensky and Cook, 2000), and in non-injured human adults (Grasso et al., 1998)and infants (Lamb and Yang, 2000). Interestingly, within 10 days of daily training to walk backward in human subjects, the modulation of the soleus H-reflex is similar to that seen during forward walking (Schneider and Capaday, 2003). This finding further suggests a seemingly interactive feature of the neuronal components underlying similar motor behaviors.
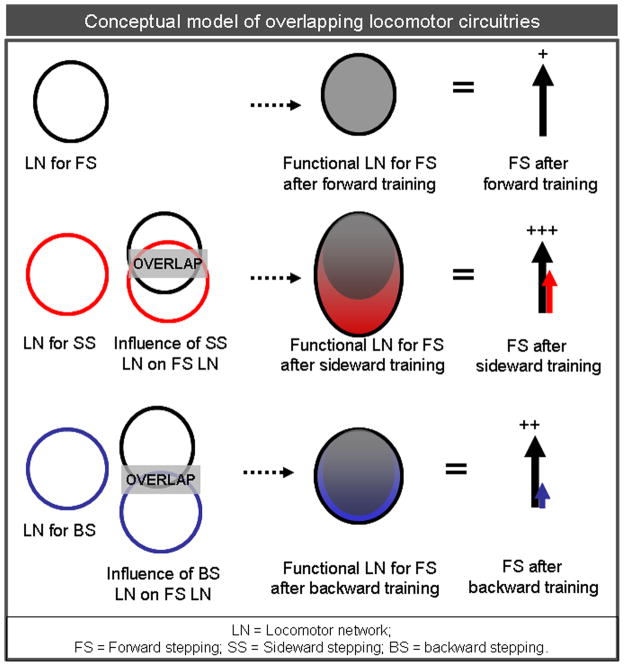
In our present work, as far as we could observe, the same muscles were activated during sideward and forward stepping, although the coordination patterns of the involved motor pools were different. For the TA, but not the MG, during forward stepping there were some similarities in the patterns of bursts of activity among FT, BT, and ST, demonstrating that the neuronal circuitries for the three directions of stepping might be composed of at least partially overlapping circuits. While theoretically these overlapping neural circuits could facilitate or interfere with one another in a training paradigm (Carew et al., 1971; Byrne, 1987), the present results show a clear beneficial effect of the recovery of forward stepping as a result of training with sideward, and to a lesser degree backward, stepping. The observation that backward training did not transfer as effectively to forward stepping as did sideward training leads us to surmise that there is a rather large overlap of circuitries with synergistic potential between forward and sideward stepping compared to forward and backward stepping (Fig. 6). To what extent the findings in the present study can be generalized to different motor tasks associated with weight-bearing locomotion remains uncertain. We observed improved forward stepping following both backward and sideward training. The degree to which the motor task being trained varies relative to the task being tested is a key unresolved issue. For example, Grasso et al report that forward step trained individuals with a spinal cord injury were not able to step in a backward direction unless trained to step in a backward direction; but backward trained subjects were able to step forward(Grasso et al., 2004). Overall, our data suggest that, at least after SCI, there are additional advantages in repetitively activating a broader network of neurons associated with stepping rather than activating a more restricted population of neurons involved with stepping in a single direction. A similar potentiation of motor activity has been well described for the aplysia (Antzoulatos et al., 2006). A brief electrical training stimulus that is not sufficient to induce sensitization of the gill withdrawal reflex (generate a greater response to repetitive stimuli) can do so if it is preceded by 4 days of training on the contra lateral side. The authors suggested that the sensitization of the gill response on the ipsilateral side was a consequence of a sensitizing, but sub-threshold, response mediated via contralateral neurons projecting ipsilaterally. In view of the fact that muscle activation levels during forward stepping were greater in ST and BT than in FT (Figs. 4 and 5), we postulate that training the locomotor circuitry associated with sideward or backward stepping could have potentiated the activity of the circuitry associated with forward stepping, thereby providing a more expansive circuitry that could serve as a broader source of control of the sensory input that can contribute to improved forward stepping (Fig. 6).
Figure 6.
Conceptual model explaining our study hypothesis. Theoretically, sideward and backward training engaged not only the circuitry necessary for sideward and backward stepping but also involved those circuits that generate forward stepping, suggesting the contribution of a multifunctional network of neurons in evoking stepping in different directions. The circles indicate locomotor networks (LN) for forward (FS, black) and sideward (SS, red) and backward (BS, blue) stepping. The LN for FS is partially complemented by activity from the LN for SS and BS after sideward and backward training respectively. Additionally, the extent of overlap of circles indicate that sideward stepping circuitry overlaps to a larger extent with the forward stepping circuitry than backward stepping circuitry overlaps with the forward stepping circuitry. Accordingly, neuronal activation for FS is greater after sideward training(vertical arrows with +++) than after backward training (vertical arrow with ++) and forward training (vertical arrow with +).
Conclusion
In summary, our data demonstrate a positive transfer of one motor task to another by imposing qualitatively altering features of a specific oscillatory motor task. This implies that there are overlapping neuronal elements that can be used to provide more precise control of another qualitatively similar motor task. Therefore, training a fundamentally similar but more expansive circuitry appears to enable better motor performance than training a specific task that engages a more limited circuitry. Testing this hypothesis will require extensive neurophysiological and kinematics data that can be tightly linked to the activation of specific interneurons and motoneurons during the motor tasks of interest. The clinical implication of this work directly impacts motor rehabilitation after CNS injury. Motor recovery following a spinal cord injury, for example, is often limited to the scarce availability of physical rehabilitation strategies. Our data clearly highlight the importance of adding variations to a task for better performance and suggests that one motor task can facilitate the performance of another task as long as there is some degree of neural network sharing between the different motor tasks; thereby expanding upon the existing physical therapies available to enhance motor recovery in patients with a neurological injury. Additionally, this work raises questions that necessitate a much deeper understanding of the neurological mechanisms underlying the addition of “challenges” to task-specific physical rehabilitation strategies.
Acknowledgments
This work was supported in part by grants from the Christopher and Dana Reeve Foundation [VEC2010]; Roman Reed Foundation [RR10-274]; Craig Neilsen Foundation [187914]; the National Institutes of Health [NSR01062009 to V.R.E, BRP5R01EB007615 to V.R.E]; and the Russian Foundation for Basic Research Grant [10-04-01172a, 11-04-1274-OFI-M-2011].
Abbreviations
- Add
adductor
- BT
backward trained
- Con
control
- EMG
electromyography
- FT
forward trained
- MG
medial gastrocnemius
- SCI
spinal cord injury
- ST
sideward trained
- St
semitendinosus
- TA
tibialis anterior
- VL
vastus lateralis
- LN
locomotor network
- FS
forward stepping
- SS
sideward stepping
- BS
backward stepping
References
- Antzoulatos EG, Wainwright ML, Cleary LJ, Byrne JH. Long-term sensitization training primes Aplysia for further learning. Learn Mem. 2006;13:422–425. doi: 10.1101/lm.230306. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Chau C, Rossignol S. Noradrenergic agonists and locomotor training affect locomotor recovery after cord transection in adult cats. Brain Res Bull. 1993;30:387–393. doi: 10.1016/0361-9230(93)90270-l. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther. 2000;80:688–700. [PubMed] [Google Scholar]
- Behrman AL, Lawless-Dixon AR, Davis SB, Bowden MG, Nair P, Phadke C, Hannold EM, Plummer P, Harkema SJ. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys Ther. 2005;85:1356–1371. [PubMed] [Google Scholar]
- Berkowitz A. Multifunctional and specialized spinal interneurons for turtle limb movements. Ann N Y Acad Sci. 2010;1198:119–132. doi: 10.1111/j.1749-6632.2009.05428.x. [DOI] [PubMed] [Google Scholar]
- Bigbee AJ, Crown ED, Ferguson AR, Roy RR, Tillakaratne NJ, Grau JW, Edgerton VR. Two chronic motor training paradigms differentially influence acute instrumental learning in spinally transected rats. Behav Brain Res. 2007;180:95–101. doi: 10.1016/j.bbr.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Kristan WB., Jr Imaging dedicated and multifunctional neural circuits generating distinct behaviors. J Neurosci. 2006;26:10925–10933. doi: 10.1523/JNEUROSCI.3265-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Kristan WB. Multifunctional pattern-generating circuits. Annu Rev Neurosci. 2008;31:271–294. doi: 10.1146/annurev.neuro.31.060407.125552. [DOI] [PubMed] [Google Scholar]
- Buford JA, Smith JL. Adaptive control for backward quadrupedal walking. II. Hindlimb muscle synergies. J Neurophysiol. 1990;64:756–766. doi: 10.1152/jn.1990.64.3.756. [DOI] [PubMed] [Google Scholar]
- Byrne JH. Cellular analysis of associative learning. Physiol Rev. 1987;67:329–439. doi: 10.1152/physrev.1987.67.2.329. [DOI] [PubMed] [Google Scholar]
- Cai LL, Fong AJ, Otoshi CK, Liang Y, Burdick JW, Roy RR, Edgerton VR. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J Neurosci. 2006;26:10564–10568. doi: 10.1523/JNEUROSCI.2266-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew TJ, Castellucci VF, Kandel ER. An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int J Neurosci. 1971;2:79–98. doi: 10.3109/00207457109146995. [DOI] [PubMed] [Google Scholar]
- Carlson-Kuhta P, Trank TV, Smith JL. Forms of forward quadrupedal locomotion. II. A comparison of posture, hindlimb kinematics, and motor patterns for upslope and level walking. J Neurophysiol. 1998;79:1687–1701. doi: 10.1152/jn.1998.79.4.1687. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci. 2003;6:300–308. doi: 10.1038/nn1010. [DOI] [PubMed] [Google Scholar]
- De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol. 1998;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. J Neurophysiol. 1999;82:359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol. 2007;98:2525–2536. doi: 10.1152/jn.00836.2007. [DOI] [PubMed] [Google Scholar]
- Girgis J, Merrett D, Kirkland S, Metz GA, Verge V, Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130:2993–3003. doi: 10.1093/brain/awm245. [DOI] [PubMed] [Google Scholar]
- Grasso R, Bianchi L, Lacquaniti F. Motor patterns for human gait: backward versus forward locomotion. J Neurophysiol. 1998;80:1868–1885. doi: 10.1152/jn.1998.80.4.1868. [DOI] [PubMed] [Google Scholar]
- Grasso R, Ivanenko YP, Zago M, Molinari M, Scivoletto G, Lacquaniti F. Recovery of forward stepping in spinal cord injured patients does not transfer to untrained backward stepping. Exp Brain Res. 2004;157:377–382. doi: 10.1007/s00221-004-1973-3. [DOI] [PubMed] [Google Scholar]
- Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res Rev. 2008;57:255–264. doi: 10.1016/j.brainresrev.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task? Med Sci Sports Exerc. 1994;26:1491–1497. [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett. 2005;383:339–344. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko Y, Jindrich DL, Zhong H, Roy RR, Edgerton VR. Dose dependence of the 5-HT agonist quipazine in facilitating spinal stepping in the rat with epidural stimulation. Neurosci Lett. 2008a;438:281–285. doi: 10.1016/j.neulet.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008b;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasak MD, Jindrich DL, Zhong H, Takeoka A, McFarland KC, Munoz-Quiles C, Roy RR, Edgerton VR, Ramon-Cueto A, Phelps PE. OEG implantation and step training enhance hindlimb-stepping ability in adult spinal transected rats. Brain. 2008;131:264–276. doi: 10.1093/brain/awm267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerzi J, Brown EH, Shum-Siu A, Siu A, Burke D, Morehouse J, Smith RR, Magnuson DS. Task-specificity vs. ceiling effect: step-training in shallow water after spinal cord injury. Exp Neurol. 2010;224:178–187. doi: 10.1016/j.expneurol.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T, Yang JF. Could different directions of infant stepping be controlled by the same locomotor central pattern generator? J Neurophysiol. 2000;83:2814–2824. doi: 10.1152/jn.2000.83.5.2814. [DOI] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Smith RR, Brown EH, Enzmann G, Angeli C, Quesada PM, Burke D. Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehabil Neural Repair. 2009;23:535–545. doi: 10.1177/1545968308331147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella KJ, Torres J, Field-Fote EC. Restoration of walking function in an individual with chronic complete (AIS A) spinal cord injury. J Rehabil Med. 2010;42:795–798. doi: 10.2340/16501977-0593. [DOI] [PubMed] [Google Scholar]
- Musselman KE, Fouad K, Misiaszek JE, Yang JF. Training of walking skills over ground and on the treadmill: case series on individuals with incomplete spinal cord injury. Phys Ther. 2009;89:601–611. doi: 10.2522/ptj.20080257. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals, Eighth Edition Edition. Washington, DC: National Academy Press; 2011. [Google Scholar]
- Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol. 1991;70:2522–2529. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- Saideman SR, Ma M, Kutz-Naber KK, Cook A, Torfs P, Schoofs L, Li L, Nusbaum MP. Modulation of rhythmic motor activity by pyrokinin peptides. J Neurophysiol. 2007;97:579–595. doi: 10.1152/jn.00772.2006. [DOI] [PubMed] [Google Scholar]
- Schneider C, Capaday C. Progressive adaptation of the soleus H-reflex with daily training at walking backward. J Neurophysiol. 2003;89:648–656. doi: 10.1152/jn.00403.2002. [DOI] [PubMed] [Google Scholar]
- Shea CH, Kohl RM. Specificity and variability of practice. Res Q Exerc Sport. 1990;61:169–177. doi: 10.1080/02701367.1990.10608671. [DOI] [PubMed] [Google Scholar]
- Singh A, Murray M, Houle JD. A training paradigm to enhance motor recovery in contused rats: effects of staircase training. Neurorehabil Neural Repair. 2010;25:24–34. doi: 10.1177/1545968310378510. [DOI] [PubMed] [Google Scholar]
- Starkey ML, Bleul C, Maier IC, Schwab ME. Rehabilitative training following unilateral pyramidotomy in adult rats improves forelimb function in a non-task specific way. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Trank TV, Smith JL. Adaptive control for backward quadrupedal walking VI. metatarsophalangeal joint dynamics and motor patterns of digit muscles. J Neurophysiol. 1996;75:678–679. doi: 10.1152/jn.1996.75.2.678. [DOI] [PubMed] [Google Scholar]
- Vilensky JA, Cook JA. Do quadrupeds require a change in trunk posture to walk backward? J Biomech. 2000;33:911–916. doi: 10.1016/s0021-9290(00)00071-3. [DOI] [PubMed] [Google Scholar]
- Ziegler MD, Zhong H, Roy RR, Edgerton VR. Why variability facilitates spinal learning. J Neurosci. 2010;30:10720–10726. doi: 10.1523/JNEUROSCI.1938-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]