Abstract
Verbal memory deficits attributed to late life depression (LLD) may result from executive dysfunction that is more detrimental to list-learning than story-based recall when compared to healthy aging. Despite these behavioral dissociations, little work has been done investigating related neuroanatomical dissociations across types of verbal memory performance in LLD. We compared list-learning to story-based memory performance in 24 non-demented individuals with LLD (age~66.1±7.8) and 41 non-demented/non-depressed healthy controls (HC; age~67.6±5.3). We correlated significant results of between-group analyses across memory performance variables with brain volumes of frontal, temporal and parietal regions known to be involved with verbal learning and memory. When compared to the HC group, the LLD group showed significantly lower verbal memory performance for spontaneous recall after repeated exposure and after a long-delay but only for the list-learning task; groups did not differ on story-based memory performance. Despite equivalent brain volumes across regions, only the LLD group showed brain associations with verbal memory performance and only for the list-learning task. Specifically, frontal volumes important for subjective organization and response monitoring correlated with list-learning performance in the LLD group. This study is the first to demonstrate neuroanatomical dissociations across types of verbal memory performance in individuals with LLD. Results provide structural evidence for the behavioral dissociations between list-learning and story-based recall in LLD when compared to healthy aging. More specifically, it points toward a network of predominantly anterior brain regions that may underlie the executive contribution to list-learning in older adults with depression.
Keywords: episodic memory, late life depression, prefrontal cortex, executive functioning, healthy ageing
1. Introduction
Late-life depression (LLD) is the most commonly diagnosed psychiatric disorder in adults over 60 years of age, affecting upwards of 16% of this aging population. LLD has significant implications for quality of life and independent living (Blazer, 2003) given that it may increase vulnerability to and exacerbation of existing age-related cognitive deficits. Although there is some debate over the profile and significance of the cognitive deficits, lower performance across episodic memory, information processing, executive functioning and visuospatial abilities have been reported when compared to healthy controls (see McClintock, Husain, Greer, & Cullum, 2010 for review). After treatment, some of these deficits resolve; however, deficits in executive functioning have been shown to persist following both treatment and remission of LLD (Alexopoulos et al., 2005; Alexopoulos et al., 2000; Kalayam & Alexopoulos, 1999) suggesting that there are residual executive deficits associated with LLD that may negatively impact other cognitive processes.
Verbal memory deficits attributed to LLD may result from these residual executive deficits, negatively impacting specific forms of recall performance. For example, impaired semantic organization – a skill often associated with executive functioning (Delis, Kramer, Kaplan, & Ober, 2000) – mediated performance on verbal list-learning from the California Verbal Learning Test (CVLT) in LLD but not healthy aging (Elderkin-Thompson, Mintz, Haroon, Lavretsky, & Kumar, 2006). The contribution of executive functioning to verbal memory performance was not replicated when tested via story-based recall as measured by the Logical Memory subtest (LM) of the WMS-III across aging populations with depression (Keiski, Shore, & Hamilton, 2007). In fact, successful performance on LM predicts successful treatment response in LLD (Story, Potter, Attix, Welsh-Bohmer, & Steffens, 2008) whereas executive dysfunction negatively impacts treatment response and remission rates in LLD (Alexopoulos et al., 2000). Taken together this would suggest that story-based verbal recall is not contingent upon executive function or dysfunction in LLD. Thus, executive deficits in LLD appear more detrimental to list-learning given the heavier (executive) burden placed on the individuals during list-learning tasks (e.g., the subjective organization of individually presented items heavily reliant executive processes; (Moscovitch & Winocur, 2002) when compared to story-based recall that provides contextually-based information (Rabin et al., 2009).
While this behavioral dissociation may have implications for predicting treatment response and facilitating learning and memory in older adults with LLD, identifying individuals for treatment or determining the best time to implement compensation strategies remains difficult. Investigating the underlying neuroanatomical dissociations of previously determined behavioral dissociations in verbal memory performance in LLD may assist clinicians in timing and targeting specific remediation techniques in this vulnerable population. To our knowledge, little to no work has been done examining the neuroanatomical dissociations associated with verbal memory performance in LLD compared to healthy aging despite the literature exploring neuroanatomical alterations within and between these groups of older adults.
Cerebral gray and white matter of individuals with LLD as well as healthy older adults shows age-related change; however, individuals with LLD also show disease-specific differences when compared to their healthy aging counterparts. Briefly, individuals with LLD show volumetric changes in gray matter structures including the orbitofrontal and dorsolateral prefrontal regions (Ballmaier et al., 2004; Chang et al., 2011; Taylor et al., 2007) as well as in temporal and subcortical structures including the hippocampus and anterior cingulate (Ballmaier et al., 2008; Ballmaier et al., 2004; Dotson, Davatzikos, Kraut, & Resnick, 2009; Lavretsky, Ballmaier, Pham, Toga, & Kumar, 2007) when compared to healthy controls. In addition, individuals with LLD show added white matter burden when compared to healthy controls (O’Brien et al., 2006) and regional vulnerability within prefrontal white matter regions (Bae et al., 2006) when compared to healthy controls. The degree to which alterations in brain volume – particularly gray matter structures known to play an important role in learning and memory such as the hippocampus, orbitofrontal and dorsolateral prefrontal regions (see Blumenfeld & Ranganath, 2007; Squire, Stark, & Clark, 2004 for review) – contribute to list-learning versus story-based recall performance in LLD when compared to healthy controls (HC) is unknown.
The aim of the current research was to combine behavioral measures of verbal memory with neuroanatomical measures of gray matter volumes to investigate the dissociations between structure and function of verbal memory performance in LLD and HC groups. Thus, we combined previously applied and well known clinical measures of list-learning (i.e., the CVLT) and story-based (i.e., LM) verbal recall performance with MRI-derived volumes of cerebral gray matter regions chosen based on their documented involvement in learning and memory (see Blumenfeld & Ranganath, 2007; Squire et al., 2004 for review). In addition to temporal regions targeted for their role in encoding and consolidation of information for long term memory stores (e.g., hippocampal regions including the entorhinal cortex), we included more anterior regions of prefrontal and cingulate cortices associated not only with memory processes (see Blumenfeld & Ranganath, 2007; Squire et al., 2004 for review) but also LLD (e.g., Ballmaier et al., 2004). This study is one of, if not the first of its kind to investigate the neuroanatomical dissociations associated with verbal memory performance dissociations in LLD.
Previous work in healthy older adults (Van Petten et al., 2004) has shown patterns of negative correlations between episodic memory performance (i.e., list-learning and story-based recall) and gray matter volumes (i.e., middle frontal and most temporal regions); however, we hypothesize that specific patterns of associations will exist for list-learning compared to story-based recall of verbally mediated information within the LLD group when compared to the HC group. More specifically, the LLD group will show lower levels of performance when compared to the HC group on the list-learning task only. Additionally, regions of the prefrontal cortex will correlate more strongly with list-learning than story-based recall in the LLD group when compared to the HC group.
2. Methods
2.1 Participants
Data were collected as part of a larger research study investigating late life depression (LLD) at the University of Illinois at Chicago (UIC). Individuals age 60 and older were recruited via community outreach (e.g., newspaper, radio, and television advertisements) and relevant outpatient clinics within the School of Medicine (e.g., mood and anxiety, geriatrics). The study was approved by the UIC Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
All participants underwent a preliminary telephone screen. Exclusion criteria consisted of current or past history of brain disorders (i.e., dementia, stroke, seizure, etc.), a history of head injury or loss of consciousness, a present or past history of substance abuse or dependence, an Axis I disorder other than major depression (i.e., bipolar disorder), psychotropic medication use including anti-depressant medication and the presence of metallic implant(s) that would preclude magnetic resonance imaging (MRI). Thus, all study participants, including those diagnosed with major depression (see below) were free of anti-depressant medication for at least two weeks in order to study depressed mood in an untreated state.
After passing the telephone screen, participants were scheduled for a more detailed evaluation which included cognitive, i.e., Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) and affective, i.e., Structured Clinical Interview for DSM-IV (SCID; Spitzer, Williams, Gibbon, & First, 1992) screens for final inclusion and exclusion determination. Screening measures were administered by a trained research assistant and followed by an evaluation by a board certified (AK) or board eligible (OA) psychiatrist who completed the Hamilton Depression Rating Scale (HDRS; Hamilton, 1960). All raters were blind to telephone screen information.
Final inclusion criteria for adults with LLD included a diagnosis of major depressive disorder based on the SCID and a score ≥15 on the 17-item HDRS. Thus, at the time of the study, individuals with LLD were in a major depressive episode and at the moderately depressed level or higher given the average HDRS score for this group (18.6±3.1). Inclusion criteria for healthy control (HC) participants included an absence of symptoms of depression based on the SCID and a score ≤8 on the HDRS. All subjects, regardless of group, had an MMSE score ≥ 24 and were native English speakers. All study participants completed the Center for Epidemiological Studies Depression scale (CESD; Radloff, 1977; Radloff & Teri, 1986) for a more subjective measure of depressive symtomatology independent of diagnostic criteria.
Participants also received an assessment of vascular risk using the criteria provided by the Framingham Heart Study’s Stroke Risk Profile (Wolf, D’Agostino, Belanger, & Kannel, 1991) given the impact of vascular risk on aging, cognition and depressive symptomatology (Alexopoulos et al., 1997; Au et al., 2006). The Framingham Stroke Risk Profile (FSRP) determines stroke risk based on data from the Framingham Heart Study using age, systolic blood pressure, antihypertensive therapy, diabetes mellitus, current cigarette smoking, cardiovascular disease, atrial fibrillation, and left ventricular hypertrophy. Laboratory testing documented levels of health related variables (i.e., hypercholesterolemia and glucose levels) and an electrocardiogram assessed for atrial fibrillation and left ventricular hypertrophy. History of stable (e.g., diabetes) or remitted medical illness (e.g., cancer) was not an exclusionary factor.
Ninety-one individuals attended for initial screening with informed consent obtained from all 91 individuals. Twenty-six individuals were excluded from analysis: 11 had past substance abuse or dependence (HC=4, LLD=7); three had English as a second language (all HC); two were on contra-indicative medication (both LLD); three had contra-indicative comorbidities (all LLD); one had neuropsychological testing one week prior to enrolment in the current study (HC); one individual suffered a sustained loss of consciousness (HC), five individuals had scores on depression measures (CESD or HAM-D) that did not match their SCID diagnosis (HC=2, LLD=3).
The final sample (n=65) included 24 adults with LLD and 41 HC. Clinical characteristics of the LLD group including duration of current symptoms (i.e., the amount of time the participant has met criteria for their current major depressive episode) and total lifetime episodes of depression are outlined in Table 1. It should be noted that a number of participants with LLD (n=7) reported a lifetime history of depression, that is ‘chronic’ depression lasting across their lifetime. We chose not to give these individuals a ‘1’ for total lifetime episodes of depression as this would be a different ‘1’ from someone reporting ‘1’ episode that was less than chronic; therefore we excluded chronic depression from this calculation. On average, participants reported the onset of their first (not their current) depressive episode in midlife (see Table 1 for details).
Table 1.
Group Demographics
| Healthy Controls n=41 | Late Life Depression n=24 | |
|---|---|---|
| Age (years) | 67.6±5.3 | 66.2±7.9 |
| Sex (M:F) | 16:25 | 9:15 |
| Educationa (degree years) | 16.2±3.2 | 14.2±2.7 |
| MMSE | 29.2±1.10 | 29.0±1.3 |
| WTAR Predicted FSIQ | 112.2±10.5 | 108.8±10.5 |
| CES-Db | 4.9±4.4 | 31.8±8.9 |
| Framingham Stroke Risk Profile | 10.4±4.3 | 10.1±4.3 |
| HDRS | 1.5±1.6 | 18.6±3.1 |
| Duration of Current Symptoms (mo) | n/a | 23.6±36.6 |
| Total # Depressive Episodes without ‘chronic’ reports (n=17) | n/a | 5.2±6.5 |
| Age of Onset of 1st Episode | n/a | 42.3±20.4 |
p<.05,
p<.001
NOTE: all entries reflect mean (total score)±standard deviation unless otherwise noted.
M:F=male to female ratio; MMSE=Mini-Mental State Examination; WTAR= Wechsler Test of Adult Reading; FSIQ=full scale intelligence quotient; CES-D=Center for Epidemiologic Studies Depression Scale; HDRS=Hamilton Depression Rating Scale; Duration of Current Symptoms (mo)=the amount of time the participant has met criteria for their current major depressive episode in months; #=number; n/a=not applicable.
2.2 Procedures
After eligibility was confirmed by the first visit, qualified subjects were scheduled for a second visit during which they were administered a comprehensive neuropsychological assessment by a trained research assistant blind to participant group. A third visit involved comprehensive neuroimaging data acquisition.
2.2.1 Neuropsychological Assessment
Participants completed a battery of neuropsychological tests including standardized measures of intelligence, episodic memory, language, visuospatial and executive functioning as well as information processing speed. Of interest for the current research were measures of verbal episodic memory assessed using the California Verbal Learning Test-second edition (CVLT-II; Delis et al., 2000) for list-learning and the Logical Memory (LM) subtest from the Wechsler Memory Scale III (WMS-III; Wechsler et al., 1998) for story-based recall.
As much as possible, comparable measures from each task were used to reflect immediate recall after initial exposure to test stimuli, immediate recall after repeat exposure to stimuli, learning, delayed recall and recognition memory. In order to ensure that measures used were clinically relevant, variables that had available normative data were used wherever possible. Immediate recall after initial exposure to test stimuli was measured using Trial 1 total recall from the CVLT-II (max=16) and Story A total recall from LM on the WMS-III (max=25). Immediate recall after repeat exposure to test stimuli was measured using total recall summed across Trials 1–5 of the CVLT-II (Trials1–5; max=80) and the summed total of items remember across Story A and the two presentations of Story B of LM (LMI; max=75). Learning was measured by calculating a slope of total recall performance across repeated presentation of stimuli from each task (i.e., CVLT-II: Learning Slope, Trials 1–5; LM: Learning Slope=Story B 2nd recall minus Story A 1st recall). Delayed Recall was measured by determining total recall after a 30 minute delay for both the CVLT-II (i.e., Long Delay Free Recall; max=16) and LM (i.e., Delayed Recall or LM-II; max=50). Recognition memory was measured after long delay free recall – and cued recall for the CVLT-II – via Yes/No accuracy to direct questioning across both measures resulting in a Recognition Discriminability Index on the CVLT-II [i.e., 1-(false positive errors+misses)/48)*100; max=100) and a Recognition Total memory score on LM (max=30).
2.2.2 Neuroimaging Protocol
Brain MRI were acquired on a Philips 3.0T Acheiva scanner (Philips Medical Systems, Best, The Netherlands) using an 8-channel SENSE (Sensitivity Encoding) head coil. Participants were positioned comfortably on the scanner bed and fitted with soft ear plugs; foam pads were used to minimize head movement. Participants were instructed to remain still throughout the scan. High resolution three-dimensional T1-weighted images were acquired with a MPRAGE (Magnetization Prepared Rapid Acquisition Gradient Echo) sequence (FOV=240mm; 134 contiguous axial slices; TR/TE=8.4/3.9ms; flip angle=8o; voxel size=1.1X1.1X1.1mm). The acquisition of these images was part of a larger protocol. Fifty-one individuals (LLL=18; HC=33) completed the MRI scan and had data suitable for analysis. There were no differences between this small sample and the overall sample in terms of demographic characteristics as seen in Table 1 with the exception that education was no longer significantly different between the groups (p=.14).
2.2.3 Image Analysis
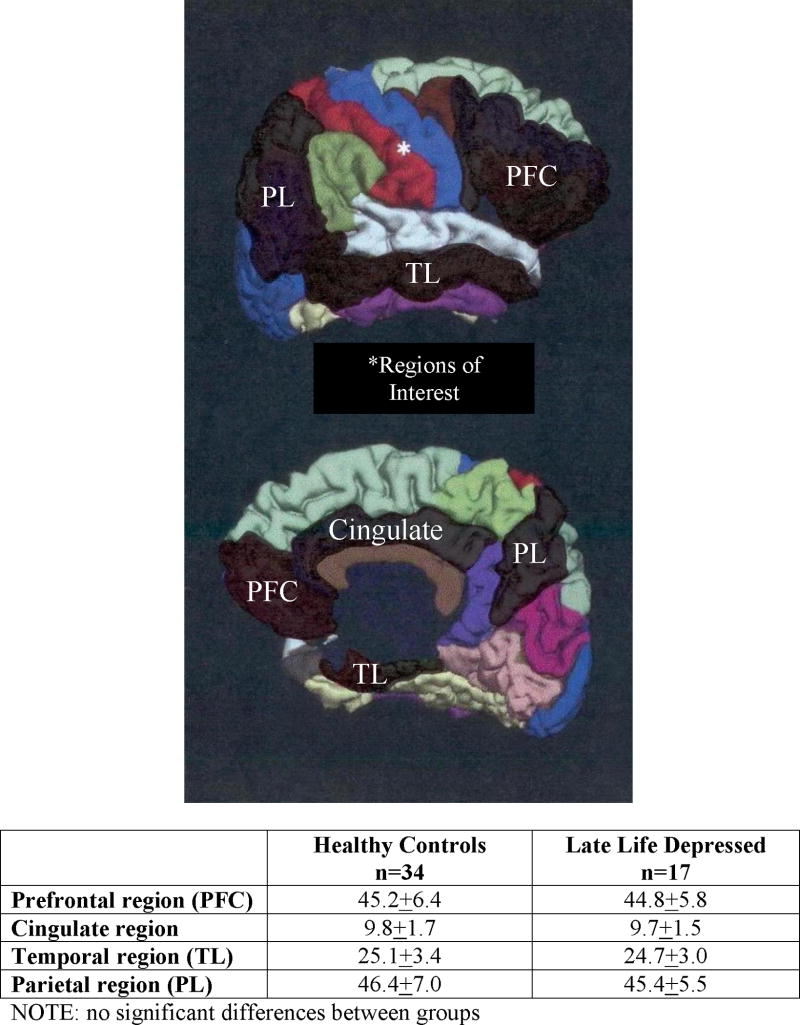
T1-weighted images were analyzed using FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) for volumetric segmentation. Processing includes motion correction, removal of non-brain tissue, transformation into Talairach space, registration of image to an atlas and parcellation of the cerebral cortex into units based on gyral and sulcal structures (Destrieux, Fischl, Dale, & Halgren, 2010; Fischl, Salat, et al., 2004; Fischl, van der Kouwe, et al., 2004). Regions of interest (ROIs) shown to be important for verbal memory based on animal and human memory studies (see (Blumenfeld & Ranganath, 2007; Squire et al., 2004) for review) were identified according to the FreeSurfer criteria of Desikan and colleagues (Desikan et al., 2006). Composite ROIs seen in Figure 1 were calculated by summing individual FreeSurfer areas of interest as follows: Prefrontal Cortex (PFC) – orbitofrontal cortex (medial and lateral), inferior frontal gyrus (pars opercularis, pars triangular is and pars orbitalis) and rostral division of the middle frontal gyrus; Cingulate Cortex – rostral anterior, caudal anterior and posterior divisions; Temporal – entorhinal cortex, parahippocampal gyrus and middle temporal gyrus; Parietal – superior parietal, inferior parietal and precuneus cortices. Composite brain variables were used as this is the first investigation into neuroanatomical dissociations of verbal memory performance dissociations in LLD; thus, we hoped to limit the number of regions considered and the number of analyses conducted as well as provide an overview of the role of the PFC in the verbal memory performance of individuals with LLD.
Figure 1.
Regions of interest derived for structure/function correlations with between-group mean volumes ± standard deviation in mm3. Figure adapted from Desikan et al., 2006. Neuroimage 31: 968–980 and published with permission.
2.3 Statistical Analyses
Group differences on demographic variables were assessed using individual analysis of variance (ANOVA) and Chi-square testing where appropriate. Differences in verbal memory performance and brain volume measures were also assessed using separate ANOVAs controlling for any demographic differences between the groups resulting from our initial analyses (see 3.1 Demographic Data below). Two-tailed Pearson’s partial correlational analyses were performed for verbal memory test variables where group differences were observed (see 3.4 Verbal Memory Performance and Brain Volume below). We conducted Fischer’s r-to-z transformation analyses on significant correlations to determine if correlations for the group in question were significant stronger than for the other group.
3. Results
3.1 Demographic Data
There were no significant differences between LLD patients and HC on age, sex, MMSE scores, predicted full scale IQ or stroke risk (all p-values≥.21; Table 1). The HC group reported more years of education compared to with LLD group, F(1,64)=6.6, p=.01. As expected, subjective reports of depressive symptomatology were higher in the LLD group when compared to the HC group, F(1,64)=262.0, p<.001.
3.2 Verbal Memory Performance
Significant group differences were observed on measures of Immediate Recall after repeat exposure [F(1,63)=8.5, p=.005] and Delayed Recall [F(1,63)=4.7, p=.03] but for the CVLT task only with the LLD group showing poorer recall than the HC group. No group differences were observed on any of the LM measures including comparable measures of Immediate Recall after repeat exposure and Delayed Recall (all p-values≥.12; Table 2). After controlling for years of education, only the results for Trials 1–5, i.e., Immediate Recall after repeat exposure on the CVLT, remained significant, F(1,63)=4.9, p=.03; however we subjected both Immediate and Delayed Recall measures and their LM counterparts to subsequent correlational analyses with brain volume data given that these analyses were conducted for each group separately.
Table 2.
Verbal Memory Performance
| Healthy Controls | Late Life Depression | |
|---|---|---|
| CVLT-II for list-learning | ||
| Trial 1 total recall | 6.1±1.8 | 5.5±2.1 |
| Trials 1–5 total recalla | 51.2±8.0 | 44.3±10.7 |
| Learning Slope | 1.5±0.5 | 1.4±0.6 |
| Long Delay Free Recallb | 11.3±2.6 | 9.4±4.2 |
| Recognition Discriminability | 90.8±8.2 | 86.8±10.1 |
|
| ||
| LM for story-based recall | ||
| Story A total recall | 14.6±3.7 | 13.7±4.0 |
| LMI | 41.7±9.7 | 37.8±9.9 |
| Learning Slope | 4.0±2.4 | 3.4±2.9 |
| LMII | 25.1±8.0 | 23.6±9.2 |
| Recognition total score | 26.3±2.1 | 25.8±2.6 |
p=.005, p=.03 after controlling for education;
p=.03, ns after controlling for education
NOTE: All values represent mean±standard deviation with significant results bolded.
ANCOVA=analysis of covariance controlling for years of education; CVLT=California Verbal Learning Test; LM=Logical Memory.
Immediate recall after initial exposure=Trial 1 total recall and Story A total recall; Immediate recall after repeat exposure=Trials1–5 total recall and LMI (i.e., Story A+Story B recall); Learning=Learning Slopes for both measures; Delayed recall= Correct Responses after a 30-minute delay for Long Delay Free Recall and LMII (i.e., Story A+Story B recall); Recognition memory=Recognition Discriminability Index and Recognition total score.
3.3 Brain Volume Data
There were no significant between-group differences on any of the four composite measures of regional brain volumes before or after controlling for years of education (all p-values≥.60; Figure 1).
3.4 Verbal Memory Performance and Brain Volume
Partial correlational analyses controlling for education were performed between select verbal memory performance scores of Immediate Recall after repeat exposure (i.e., CVLT Trials 1–5 and LM I) as well as Delayed Recall (i.e., CVLT long delay free recall and LMII) and brain volumes for the LLD and HC groups separately. Significance was set at p≤.01 to correct for multiple comparisons. For the HC group, neither performance measure from the CVLT nor LM correlated with any brain volumes (Table 3).
Table 3.
r-values for brain-behavior correlations by group.
| HEALTHY CONTROLS | Prefrontal Cortex | Cingulate | Temporal | Parietal | |||
|---|---|---|---|---|---|---|---|
| Left | Right | Right | Left | Right | Left | Right | |
| Immediate Recall | |||||||
| CVLT Trials 1–5 | −.22 | −.21 | −.01 | .08 | .04 | .09 | .09 |
| LM I | .03 | .01 | .20 | .07 | .12 | −.05 | .18 |
| Delayed Recall | |||||||
| CVLT LDF | −.21 | −.17 | −.09 | −.06 | −.15 | −.08 | −.07 |
| LM II | .03 | .02 | .23 | .15 | .15 | .00 | .25 |
| LATE LIFE DEPRESSED | Prefrontal Cortex | Cingulate | Temporal | Parietal | |||
|---|---|---|---|---|---|---|---|
| Left | Right | Right | Left | Right | Left | Right | |
| Immediate Recall | |||||||
| CVLT Trials 1–5 | .14 | .30 | .27 | .25 | .56 | .38 | .39 |
| LM I | .16 | .07 | .22 | .01 | .16 | .30 | .21 |
| Delayed Recall | |||||||
| CVLT LDF | .69 | .63 | .66 | .50 | .58 | .50 | .53 |
| LM II | .32 | .13 | .36 | .10 | .26 | .31 | .24 |
NOTE: All r-values represent partial correlations after controlling for education with significant results at p≤.01 bolded and comparisons for Fischer’s r-to-z transformation analyses highlighted in grey. CVLT=California Verbal Learning Test; LM=Logical Memory; LDF=long delay free recall. Immediate recall after repeat exposure=Trials1–5 total recall and LMI (i.e., Story A+Story B recall); Delayed recall= Correct Responses after a 30-minute delay for CVLT LDF and LMII (i.e., Story A+Story B recall).
For the LLD group, only CVLT long delay free recall correlated significantly with brain volumes including the PFC region bilaterally [left: r(14)=.69, p=.003; right: r(14)=.63, p=.009] and the right cingulate cortex [r(14)=.66, p=.005]. The correlation between left PFC volume and Delayed Recall performance was driven by a significant orbitofrontal cortex correlation, r(14)=.76, p=.001. The correlation with the right PFC volume was driven by the orbitofrontal cortex, r(14)=.72, p=.002, and the rostral division of the middle frontal gyrus, r(14)=.67, p=.005. For the right cingulate volume, the correlation with CVLT delayed free recall performance was driven by a significant association with the right caudal anterior cingulate, r(14)=.61, p=.01. Across all analyses reported, correlations were significantly stronger in the LLD group when compared to the HC group using Fischer’s r-to-z transformation analyses (z-scores ranging from 1.9 to 3.3; all p-values ≤ .04).
Although there were association trends in our LLD group between CVLT long delay free recall and temporal [left: r(14)=.50, p=.05; right: r(14)=.58, p=.02] as well as parietal [left: r(14)=.50, p=.05; right: r(14)=.53, p=.03] regions, they did not reach our a priori threshold of p≤.01 to be considered significant. Across all analyses reported, correlations were significantly stronger in the LLD group when compared to the HC group using Fischer’s r-to-z transformation analyses (z-scores ranging from 1.8 to 2.5; all p-values ≤ .05).
4. Discussion
To our knowledge, this is the first investigation combining structural and functional integrity across list-learning and story-based recall tasks in LLD. Our results not only verified previously known behavioral dissociates of episodic memory performance in LLD, they provided novel information about accompanying neuroanatomical dissociates underpinning list-learning versus story-based recall in this vulnerable population. Thus, individuals with LLD showed greater memory deficits when compared to their HC counterparts for list-learning but not story-based recall. Further, despite equivalent brain volumes across all regions assessed, individuals with LLD showed that the integrity of bilateral prefrontal and right cingulate cortices contributed to the integrity of list-learning performance. This structure/function association was neither present in the HC group nor relevant to story-based recall performance in either group.
While there is no denying the significant impact prefrontal regions have on memory retrieval processes across the lifespan and across types of episodic memory performance (see Lee, Robbins, & Owen, 2000 for review), our results suggest that declines in prefrontal volume are differentially associated with declines in list-learning performance when compared to story-based recall in LLD. This may suggest a more specific mechanism associated with PFC structural vulnerability is negatively impacting list-learning performance in LLD. Previous research would suggest this mechanism is related to subjective organization of information (Schacter, Savage, Alpert, Rauch, & Albert, 1996; Zahodne et al., 2011) and negatively impacted by the executive dysfunction long documented in LLD (Elderkin-Thompson et al., 2006).
The role of prefrontal brain regions in episodic memory has long been seen as one involving the enhanced encoding of to-be-remembered information through contextually rich associations (Squire & Alvarez, 1995); furthermore, individuals with prefrontal lesions do not spontaneously organize information during list-learning and recall (see Blumenfeld & Ranganath, 2007 for review). The orbitofrontal cortex, the PFC region driving the associations between PFC volumes and list-learning recall in our LLD sample, is integral in promoting distinctive retrieval strategies or cues to facilitate the transfer and recall of specific information from long term memory stores (Fletcher & Henson, 2001; Owen, 2000). Taken together, this would suggest that structural vulnerability within prefrontal, in particular orbitofrontal cortices may underlie deficits in list-learning for individuals with LLD due in part to this regions role in strategic encoding and subsequent recall of information; recall that is unaffected when contextual information is provided in the form of a narrative during encoding as is the case for story-based recall.
In addition to the role of the orbitofrontal cortices on list-learning performance in LLD, the right cingulate cortex, specifically the caudal anterior portion of the cingulate cortex (cACC) was also implicated in this population. The cACC is connected with the hippocampal formation which is integral for memory encoding and consolidation (Pandya, Van Hoesen, & Mesulam, 1981). Furthermore, the cACC is involved in error detection and response monitoring during memory processing across normal and pathological aging (Braskie, Small, & Bookheimer, 2009; Rushworth & Behrens, 2008). This suggests that, in conjunction with the OFC, cACC regions may contribute not only to the organizational strategies that contribute to successful list-learning performance but to the ability to filter previously encountered items or hits from erroneous yet seemingly related false positives items in LLD.
Both the orbitofrontal and anterior cingulate cortices have been implicated as structurally vulnerable regions of brain in the presence of LLD (Bae et al., 2006; Ballmaier et al., 2004; Dotson et al., 2009; Lavretsky et al., 2007; Taylor et al., 2007) with this vulnerability leading to blunted positive affect and decreased motivation/ arousal (Lavretsky et al., 2007). In contrast to reports in the literature, neither region was significantly reduced in our LLD group when compared to our HC group. These cortical gray matter volumes were equivalent across LLD and HC groups but nonetheless formed a network of associations with impaired recall performance on the list-learning task negatively contributed to behavioral performance in LLD. In addition to the cognitive contributions of these regions described above, affective components ascribed to the orbitofrontal and anterior cingulate may have negatively affected motivation to perform study tasks that negatively impacted performance. Similarly, several factors including affective and neuroanatomical components may be contributing to the lack of neuroanatomical differentiation in our sample.
From an affective standpoint, the state of depression in our late life group may have influenced results. For example, the LLD group was not medicated and lacked comorbid psychiatric diagnoses so that we could investigate the impact of LLD in its purest untreated form. Further, although duration of current depressive episode was approximately two years in our sample and first episode of depression was reported most often in midlife, the number of depressive episodes varied between one and 20 (with some participants reporting a life-long depressive episode) suggesting a heterogeneous group. Individuals having their first depressive episode in late life may not have the typical volumetric abnormalities associated with depression across the lifespan or even with depression beginning in midlife (Ballmaier et al., 2008). This may have impacted our neuroanatomical results.
From a neuroanatomical standpoint, MRI techniques and downstream white matter alterations may have influenced results. Microstructural changes may be present in this vulnerable clinical population that have yet to impact overall gross morphometry as measured by our current techniques. The lack of volume loss in LLD may also suggest a role for more downstream effects within the cerebral white matter as may be the case in healthy aging (Salat et al., 2010). Thus, the integrity of the connections between fronto-cingulo-striatal regions to other memory structures key to list-learning (e.g., connections between the cACC, middle frontal gyrus and the entorhinal cortex; Barbas & Pandya, 1989; Munoz & Insausti, 2005) may be mediating the gray matter associations found in the LLD group. Tract-based spatial statistics either in isolation (Smith et al., 2006) or combined within a comprehensive network analyses assessing the integrity of interactions between gray matter measurements and measures of white matter connectivity (Sporns, 2011) would facilitate our understanding of the role of these seemingly intact gray matter regions in list-learning when compared to story-based recall in our LLD group.
Our lack of association between temporal regions, particularly hippocampal volumes, and verbal memory performance regardless of task type or group is not inconsistent with previous findings across aging studies (see Raz & Rodrigue, 2006 for review). For example, a longitudinal study of normal aging (Rodrigue & Raz, 2004) assessed hippocampal volume at baseline and after five years in conjunction with episodic memory performance across list-learning and story-based recall and found no association between verbal memory performance and changes in hippocampal volume. Another study demonstrated no significant correlations between hippocampal volume and verbal memory performance in healthy older men (MacLullich et al., 2002). As a result of such findings Van Petten (Van Petten, 2004) has suggested that volume loss within the hippocampus does not contribute to memory decline in normal aging. Instead, it has been hypothesized that hippocampal volume may affect episodic memory abilities only when there is specific disease-related neuropathology, such as the atrophy observed in Alzheimer’s disease (Van Petten, 2004) or the prefrontal vulnerability to age-related volume loss (Raz et al., 1997; Resnick et al., 2000). Thus, in normal aging – and as this study has shown in LLD – the cause of verbal memory decline may be due to changes elsewhere in the memory network that have a different pathogenesis.
This study demonstrated neuroanatomical dissociations in verbal memory performance in individuals with LLD and provided structural evidence for the executive dysfunction known to be detrimental to list-learning performance in this group. The clinical applicability of these findings include decreasing executive demands during learning and memory in LLD either objectively by increasing the narrative structure of to-be-remembered information at presentation or subjectively by encouraging contextually-based mnemonic strategies. Furthermore, it suggests that when memory impairment in LLD begins to involve aspects of encoding and recall not heavily reliant on prefrontally mediated executive skills, this may suggest more posterior neuropathology and/or ‘purer’ memory impairment indicative of cognitive decline and dementia. Early detection of such changes in ‘pre-clinical’ phases of LLD could identify individuals to target for more aggressive interventions.
Highlights.
Late life depression-LLD-group had lower list-learning compared to healthy controls.
Groups did not differ on story-based recall on brain volumes associated with memory.
Frontal volumes (subjective organization) correlated with list-learning in LLD only.
First to demonstrate neuroanatomical dissociations across types of memory in LLD.
Anterior brain network may underlie the executive contribution to LLD list-learning.
Acknowledgments
Funding for this study was provided by NIMH 7RO1 MH073989-04 (AK). A portion of this data was presented at the 50th anniversary meeting of the American College of Neuropharmacology in Waikaloa Beach, Hawaii. We would like to thank Piotr Daranowski, Mai Lynn Grajewski, Laura Korthauer, Monya Meinel, and Emma Rhodes for their work with recruitment, screening, and testing of participants as well as the participants who volunteered their time for this research. We would also like to thank Dr. Leah Rubin for her statistical consultation during the revision of this manuscript. The authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biological Psychiatry. 2005;58(3):204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Archives of General Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, et al. Executive dysfunction and long-term outcomes of geriatric depression. Archives of General Psychiatry. 2000;57(3):285–290. doi: 10.1001/archpsyc.57.3.285. [DOI] [PubMed] [Google Scholar]
- Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Archives of Neurology. 2006;63(2):246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD. Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biological Psychiatry. 2006;60(12):1356–1363. doi: 10.1016/j.biopsych.2006.03.052. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, et al. Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. American Journal of Psychiatry. 2008;165(2):229–237. doi: 10.1176/appi.ajp.2007.07030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson J, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. American Journal of Psychiatry. 2004;161(1):99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. Journal of Computational Neurology. 1989;286(3):353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: review and commentary. The Journals of Gerontology: Biological Sciences. 2003;58(3):249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13(3):280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Small GW, Bookheimer SY. Entorhinal cortex structure and functional MRI response during an associative verbal memory task. Human Brain Mapping. 2009;30(12):3981–3992. doi: 10.1002/hbm.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Yu SC, McQuoid DR, Messer DF, Taylor WD, Singh K, et al. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Research. 2011;193(1):1–6. doi: 10.1016/j.pscychresns.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test. San Antonio, TX: 2000. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Davatzikos C, Kraut MA, Resnick SM. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. Journal of Psychiatry and Neuroscience. 2009;34(5):367–375. [PMC free article] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Mintz J, Haroon E, Lavretsky H, Kumar A. Executive dysfunction and memory in older patients with major and minor depression. Archives of Clinical Neuropsychology. 2006 doi: 10.1016/j.acn.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124(Pt 5):849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Folstein MR, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Archives of General Psychiatry. 1999;56(8):713–718. doi: 10.1001/archpsyc.56.8.713. [DOI] [PubMed] [Google Scholar]
- Keiski MA, Shore DL, Hamilton JM. The role of depression in verbal memory following traumatic brain injury. The Clinical Neuropsychologist. 2007;21(5):744–761. doi: 10.1080/13854040600775346. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Ballmaier M, Pham D, Toga A, Kumar A. Neuroanatomical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. America Journal of Geriatric Psychiatry. 2007;15(5):386–394. doi: 10.1097/JGP.0b013e3180325a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Robbins TW, Owen AM. Episodic memory meets working memory in the frontal lobe: functional neuroimaging studies of encoding and retrieval. Critical Reviews: Neurobiology. 2000;14(3–4):165–197. [PubMed] [Google Scholar]
- MacLullich AM, Ferguson KJ, Deary IJ, Seckl JR, Starr JM, Wardlaw JM. Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology. 2002;59(2):169–174. doi: 10.1212/wnl.59.2.169. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. 2010;24(1):9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The frontal cortex and working with memory. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 188–209. [Google Scholar]
- Munoz M, Insausti R. Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis) European Journal of Neuroscience. 2005;22(6):1368–1388. doi: 10.1111/j.1460-9568.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Firbank MJ, Krishnan MS, van Straaten EC, van der Flier WM, Petrovic K, et al. White matter hyperintensities rather than lacunar infarcts are associated with depressive symptoms in older people: the LADIS study. American Journal of Geriatric Psychiatry. 2006;14(10):834–841. doi: 10.1097/01.JGP.0000214558.63358.94. [DOI] [PubMed] [Google Scholar]
- Owen AM. The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. Experimental Brain Research. 2000;133(1):33–43. doi: 10.1007/s002210000398. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Van Hoesen GW, Mesulam MM. Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Research. 1981;42(3–4):319–330. doi: 10.1007/BF00237497. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Pare N, Saykin AJ, Brown MJ, Wishart HA, Flashman LA, et al. Differential memory test sensitivity for diagnosing amnestic mild cognitive impairment and predicting conversion to Alzheimer’s disease. Neuropsychology, Development and Cognition B Aging, Neuropsychology and Cognition. 2009;16(3):357–376. doi: 10.1080/13825580902825220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401. [Google Scholar]
- Radloff LS, Teri L. Use of the Center for Epidemiological Studies Depression Scale with older adults. Clinical Gerontology. 1986;5:119–136. [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Review. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, et al. One-year age changes in MRI brain volumes in older adults. Cerebral Cortex. 2000;10(5):464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. Journal of Neuroscience. 2004;24(4):956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience. 2008;11(4):389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, et al. White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiology of Aging. 2010;31(2):244–256. doi: 10.1016/j.neurobiolaging.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Savage CR, Alpert NM, Rauch SL, Albert MS. The role of hippocampus and frontal cortex in age-related memory changes: a PET study. Neuroreport. 1996;7(6):1165–1169. doi: 10.1097/00001756-199604260-00014. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID) I: History, Rationale, and Description. Archives of General Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Sporns O. The human connectome: a complex network. Annals of the New York Academy of Sciences. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5(2):169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Story TJ, Potter GG, Attix DK, Welsh-Bohmer KA, Steffens DC. Neurocognitive correlates of response to treatment in late-life depression. American Journal of Geriatric Psychiatry. 2008;16(9):752–759. doi: 10.1097/JGP.0b013e31817e739a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Macfall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, et al. Orbitofrontal cortex volume in late life depression: influence of hyperintense lesions and genetic polymorphisms. Psychological Medicine. 2007;37(12):1763–1773. doi: 10.1017/S0033291707000128. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42(10):1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42(10):1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Wycherley RJ, Benjamin L, Callanan M, Lavender T, JRC, et al. Wechsler Memory Scale-III. 3. London, UK: The Psychological Corporation; 1998. [Google Scholar]
- Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22(3):312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- Zahodne LB, Bowers D, Price CC, Bauer RM, Nisenzon A, Foote KD, et al. The case for testing memory with both stories and word lists prior to dbs surgery for Parkinson’s Disease. The Clinical Neuropsychologist. 2011;25(3):348–358. doi: 10.1080/13854046.2011.562869. [DOI] [PMC free article] [PubMed] [Google Scholar]