Abstract
The Rhes/RASD2 GTPase complex is involved in dopamine D1/D2 receptor-mediated signaling and behavior. This GTP binding protein belongs to the RAS superfamily, along with Dexras1/RASD1, and is primarily expressed in the striatum. RASDs differ from typical small GTPases as they have an extended C-terminal tail of roughly 7 kDa. Previously, it has been shown that dopamine depletion reduces Rhes mRNA expression in the brain. Here we show that Rhes interacts with p85, the regulatory subunit of PI3K. Specifically, the C-terminal unique tail region of Rhes is responsible for this interaction. The interaction between p85 and the C-terminal region of Rhes is enhanced upon growth factor treatment in vitro, while AKT translocation to the membrane is facilitated in the presence of Rhes or the Rhes-p85 complex. These findings suggest that Rhes is a novel striatal regulator of the AKT-mediated pathway in the striatum.
Keywords: AKT signal transduction, Rhes/RASD2, protein-protein interaction
Introduction
Dopamine is a critical neurotransmitter for the normal functioning of the central nervous system. Components of the dopamine signaling pathway are expressed during mammalian development [1; 16], and modulation of dopamine signaling alters the proliferation of embryonic neural progenitor cells [17]. Dopamine regulates the migration of GABAergic neurons in the telencephalon [6]. The D1 dopamine receptor (D1) is coupled to the stimulatory G protein (Gs), activating adenylyl cyclase, leading to an activation of protein kinase A; while the D2 dopamine receptor (D2) is coupled to the inhibitory G protein (Gi), which inhibits the activation of adenylyl cyclase [13]. Signaling through the D2 modulates AKT signaling in the adult rodent and primate striatum, which is associated with regulating motor control [2; 3]. Abnormal dopamine signal transmission in the brain has been implicated in diseases such as Parkinson’s disease and schizophrenia, as well as in various types of drug addiction [17; 23].
Rhes, a Ras homolog, is highly expressed in the striatum where dopamine plays a major role in psychomotor behavior [8;18;19]. Though it shares homology with Dexras1, it is not transcriptionally regulated by dexamethasone, but rather, thyroid hormone [8;21]. The cDNA encoding the protein was found to be roughly 900 bases or about 30 kDa. Like all G-proteins, Rhes is inactive when bound to GDP. Upon binding to GTP, it undergoes a conformational change, at which point it can bind to downstream effector molecules. While Dexras1 and Rhes contain all of the conserved G-protein regions, like the magnesium binding loop, the C-terminal CAAX box, and the prenylation site, they differ from the typical small GTPases as they have an extended C-terminal tail of about 7 kDa in length [12;22]. Even with this conformational knowledge, it is still unclear how the Rhes GTPase is regulated in the striatum.
Genetic deletion of Rhes in mice increases D1 signaling via adenylyl cyclase, D1 agonist-initiated locomotor activation, and D2 antagonist initiated catalepsy. This suggests that depletion of Rhes leads to an increase in dopamine receptor activation [7;11;15]. Also, it has been shown that Rhes mRNA, as well as protein expression are decreased by lesion of the nigrostriatal pathway or dopaminergic denervation of striatum with neurotoxin 6-hydroxydopamine [10]. Rhes also interacts with the mTOR pathway, mediating L-DOPA-induced dyskinesia [20]. The detailed mechanism by which Rhes regulates the mTOR pathway is not clearly understood.
Numerous studies illustrate a link between Rhes and dopamine-mediated behavior. However, the molecular mechanism by which Rhes modulates dopaminergic signaling in the striatum is not clearly defined. It has been known that the dopamine receptor agonists inactivate AKT [2] by recruiting β-arrestin 2 and phosphatase 2A to the dopamine receptor complex [3]. Here we report that Rhes interacts with p85 of PI3Kinase, and this interaction enhances AKT activation. Growth factor-treatment augments the interaction of p85/PI3K and the C-terminal unique region of Rhes and promotes the translocation of AKT to the membrane. These observations illustrate a link between dopaminergic signaling and the AKT pathway, suggesting new potential therapeutic targets to treat dopamine-related disorders.
Materials and Methods
Cells
HEK293T cells and rat phaeochromocytoma (PC12) cells were cultured and transfected as previously described [5; 19].
GST pull-down assay and Immunoblot analysis
For GST pull-down assays, cells were lysed in lysis buffer (100 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 15% Glycerol and 1mM PMSF) supplemented with a complete protease inhibitor tablet (Roche) and phosphatase inhibitors (Sigma). 0.3 mg of total protein will be incubated with glutathione-Sepharose beads for 3hrs at 4°C and washed 3 times in wash buffer (100 mM Tris pH 7.4, 500 mM NaCl, 1% Triton X-100, 15% Glycerol). Beads were quenched in sample buffer (100 mM Tris, pH 6.8, 10% glycerol, 250 mM β-mercaptoethanol, 2% sodium dodecyl sulfate and bromophenol blue). For immunoblot analysis, the cell lysates or the immunoprecipitates were subjected to SDS-PAGE. The separated proteins were transferred to the membrane by using the Bio-Rad gel transfer system (Bio-Rad). Blots were blocked for 1 h at room temperature with 5% skim milk in TBS-T (0.1% Tween-20) and then incubated overnight with specific primary antibody at 4 °C. Secondary antibody incubations were for 1 h at room temperature. For phospho- and total AKT blots, HRP-conjugated secondary antibodies were used for detection with SuperSignal West Pico chemiluminescence reagent (Thermo Scientific).
Immunofluorescence
Stable cell-line of HEK293 expressing AKT-PH domain-EGFP was seeded on glass cover-slips and transfected. After 24 hours, cells were serum-starved 20h, and subsequently stimulated with 10 nM Insulin for 15 min. Cells were then fixed with 4% PFA, washed, permeabilized, and blocked for 30 min with 1% BSA, 2% normal goat serum in PBS. Rhes and AKT-PH translocation in EGFP-positive cells were detected with a mouse anti-Myc epitope primary antibody. DAPI was used as a nuclear stain. Bound antibodies were visualized using Alexa Fluor 594 goat anti-mouse IgGs (Invitrogen). Confocal microscopy was performed under oil immersion on Leica DMI6000 (Leica Microsystems) with a 63X objective.
Subcellular fractionation
For determination of serum effect on protein translocation, empty control vector (Myc) or Myc-Rhes plasmid was transfected into HEK293T cells. After 24 h, the cells were incubated serum-free media for 20 h, and then treated for 15 min with 10nM insulin. Cells were fractionated into cytoplasmic, membrane, and nuclear fractions using a Fraction PREP fractionation Kit (BioVision), as described by the manufacturer. Each fraction was isolated for western blot analysis probing for anti-phospho-AKT Thr308, anti-phospho-AKT Ser473, AKT, Myc (for Rhes), CREB (nuclear marker), EGFR (membrane marker) and GAPDH (cytoplasmic marker) antibodies.
Results
Upon initial sequencing and analysis of Rhes, we found the protein contains PtdIns (3,4,5)and a P3 (PIP3) binding site (www.scansite.mit.edu). Growth factors activate PI3 kinase generating PIP3. Because it is well established that AKT is the primary target of PI3K [4], we first investigated whether Rhes has any effect on PI3K/AKT pathway. We overexpressed either Myc-Rhes or Myc plasmid, for control, in HEK293T cells and stimulated cells with serum. We found that Rhes augments serum-mediated activation of AKT, as determined by phosphorylation of AKT (Fig. 1A). In order to examine the specificity of the apparent growth factor effect, we treated HEK293T cells overexpressed with Myc-Rhes or Myc control with individual growth factors. We observed that all growth factors tested: IGF-1, EGF, PDGF and FGF, enhanced AKT activation in the presence of Rhes overexpression more so than in the Myc control condition. This suggests that the effect of Rhes is not growth factor specific (Fig. 1B and 1C). We also constructed a stable Rhes-inducible cell line to eliminate the heterogeneity of transient transfection. Inducible T-REx293 cells were treated with tetracycline for 6h to induce Rhes expression and incubated with IGF-1 for 10 min (Fig. 1D). We observed that acutely induced Rhes enhanced phosphorylation of AKT. To examine the physiologic relevance of Rhes function, we compared the influence of Rhes overexpression on EGF-induced AKT activation in differentiated rat phaeochromocytoma (PC12) cells. We found that overexpression of Rhes enhanced phosphorylation of Akt (T308) in differentiated PC 12 cells (Fig. 1E).
Fig. 1. Rhes-mediated AKT activation by growth factors.

(A–C) Immunoblot analysis of phosphorylation of AKT after growth factor treatment in HEK293T and PC12 cells over-expressing Rhes. Cells were serum-starved for 20 h, and treated with 5% serum (A), 50 ng/ml IGF-1 (B), other growth factors (25 ng/ml EGF, 50ng/ml PDGF, or 100ng/ml FGF) for indicated time point (C).
(D) Inducible T-REx HEK293 stable cell lines that express Myc or Myc-Rhes were either left untreated or induced by 1μg/ml tetracycline. After 6 h, cells were incubated serum-free media in the absence or presence of tetracycline for 16h followed by 25ng/ml IGF-1 treatment for 10 min. Cell lysates were analyzed for P-AKT.
(E) Differentiated rat phaeochromocytoma (PC12) cells that express Myc or Myc-Rhes were serum-starved for 20 h, and treated with 25ng/ml EGF for 5 min.
To investigate the potential mechanism by which Rhes activates the AKT pathway, we first examined whether Rhes interacts with AKT. We overexpressed GST-Rhes into HEK293T cells and performed GST-pull down assay. We found that endogenous AKT physically interacts with GST-Rhes (Supplementary Fig. 1A). Rhes contains a PIP3 binding site which can interact with the Pleckstrin homology (PH) domain. We then questioned whether the PH-domain of AKT is involved in the interaction between Rhes and AKT. We overexpressed GST-Rhes and either a fragment of AKT PH domain, or a mutant fragment of AKT PH domain (R25C) which does not bind to PIP3 nor translocate to the membrane upon serum stimulation. We found that Rhes only interacts with wild type AKT PH domain (Supplementary Fig. 1B).
To further understand how Rhes participates in PI3K/AKT pathway, we tested whether Rhes modulates PI3K activity to influence AKT signaling. An essential process for PI3K activation is its recruitment to the plasma membrane, whereby PI3K can access its substrate such as PIP2 [4]. To examine this process, we measured levels of PIP3 in a competitive ELISA but no change was detected in PIP3 levels in the presence of overexpressed Rhes. This lack of change suggests that Rhes does not affect PI3K activity. We next examined whether Rhes can physically interact with PI3K. At the basal state, p85/PI3K is required to stabilize the catalytic p110 subunit of PI3K [24]. By overexpressing GST-Rhes and Flag-p85/PI3K plasmids, we found that p85/PI3K interacts with Rhes in HEK293T cells in a GST pull down assay (Fig. 2A).
Fig. 2. Rhes interacts with p85 regulatory subunit of PI3K.

(A) HEK293T cells were co-transfected with GST or GST-Rhes and Flag-p85/PI3K for 48 h, followed by pull-down and immunoblotting for p85.
(B) GST or GST-Rhes (1–266, 210–266 or 1–209) was transfected in HEK293T cells for 48 h, followed by pull-down and immunoblotting for endogenous p-85/PI3K.
(C) GST, GST-Rhes or GST-Rhes (1–209) was co-transfected in HEK293T cells with Flag- p85/PI3K for 24 h, cells were serum-starved for 20 h, and treated with IGF-1 (25ng/ml) for 7 or 15 min. Cell lysates were GST pull-downed and immunoblotted for Flag.
(D) GST or GST-Rhes (1–266, 210–266 or 1–209) was transfected in HEK293T cells for 24 h Cells were serum-starved for 20 h, and treated with IGF-1 (25 ng/ml) for 15 min, followed by pull-down and immunoblotting for endogenous p-85/PI3K.
In order to identify where Rhes binds p85/PI3K, we generated fragments of Rhes fused with GST and probed against p85/PI3K in a GST pull-down assay. We found that C-terminal tail (210–266), the unique region for RASD1 family, contains the PIP3 binding domain and is responsible for the interaction between Rhes and p85/PI3K (Fig. 2B). Moreover, we found that the interaction between p85/PI3K and Rhes gradually increases upon IGF-1 treatment, while cells overexpressing the Rhes mutant, without the binding C-terminal tail, does not interact with p85 at all (Fig 2C). Interestingly, we found that IGF-1-mediated association between p85/PI3K and Rhes is solely mediated by C-terminal end (210–266) (Fig. 2D).
Upon growth factor treatment, AKT is translocated to the membrane from cytosol and is phosphorylated at Thr308 and Ser473[4]. We then examined whether Rhes has an effect on AKT translocation to membrane. To elucidate this type of interaction, HEK293T cells overexpressed with Myc-Rhes or Myc control were treated with 10 nM insulin for 15 min, followed by subcellular fractionation. By examining each of the fractions through western blotting, we found that AKT translocation to membrane is enhanced in cells overexpressed with Rhes (Fig. 3A). To further examine the effect Rhes has on AKT translocalization, we transfected HEK293 cells containing AKT-PH-EGFP with either Myc-Rhes or a Myc control plasmids, and treated with low dose of insulin (10 nM for 15 min). We observed that AKT is completely translocated to the membrane in cells overexpressing Rhes while cells expressing the Myc control plasmid showed only partial translocation of AKT to membrane after insulin treatment (Fig. 3B).
Fig. 3. Growth factor stimulation induces Rhes-mediated AKT activation and subcellular localization.
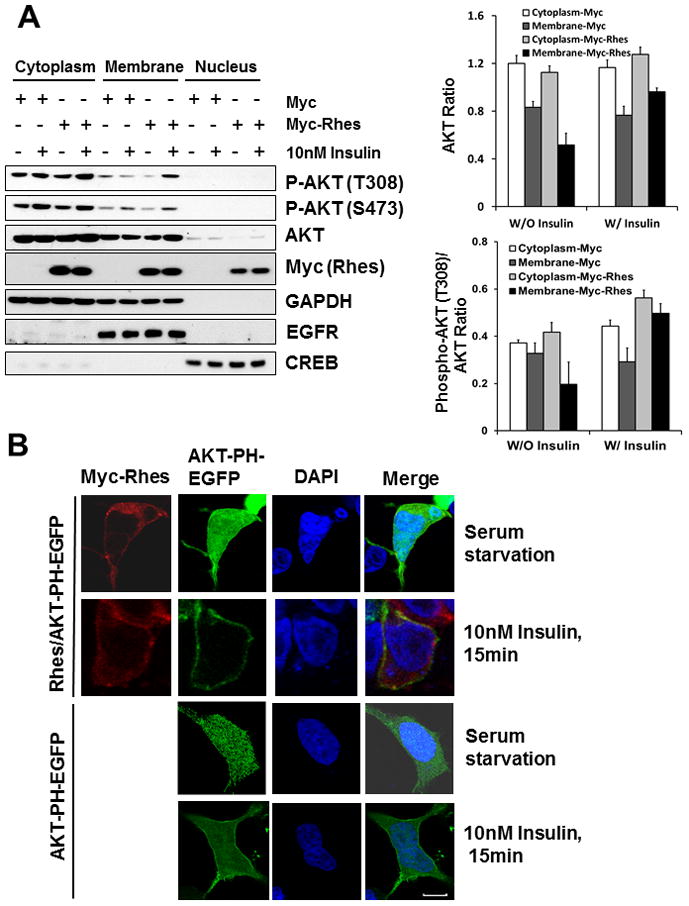
(A) HEK293T cells were transfected with Myc or Myc-Rhes for 24 h. Cells were serum-starved for 20 h, and treated with 10nM Insulin for 15 min. Each fraction was isolated for western blot analysis as described in Material and Methods. (Top) Relative AKT levels in cytoplasmic and membrane fractions normalized by GAPDH and EGFR, respectively. (Bottom) Relative P-AKT levels are normalized by total AKT. Bars represent mean ± SD, n=3.
(B) Stable cell line of HEK293 expressing AKT-PH domain-EGFP were transfected with Myc or Myc-Rhes. 24 h later, cells were serum-starved for 20h, and stimulated with 10nM Insulin for 15 min. Cells were fixed, and stained with anti-myc and DAPI for confocal microscopy, as described in Materials and Methods. Merge represents an overlay of Rhes in red and AKT-PH in green. The blue indicates the nuclei stained with DAPI. Scale bar, 5 μm.
Discussion
Rhes, as well as Dexras1 belong to the Ras superfamily of small GTPase proteins, but differ slightly by having a unique 7 kDa C-terminal extended tail. Vargiu et al [22] proposed that Rhes may modulate PI3K/Akt pathway but its detailed mechanism is still unknown. We report here that Rhes plays a critical role in AKT signaling pathway through PI3K and AKT interaction. Rhes binds to p85, the regulatory subunit of PI3K, via the unique 7 kDa C-terminal tail, and their interaction is enhanced upon growth factor treatment. Moreover, Rhes interacts with the PH domain of AKT and facilitates the localization of AKT to the membrane upon growth factor treatment (Fig. 4). Our data strongly implicates that Rhes may function as a critical bridge between PI3K and the AKT pathway.
Fig. 4. Model for Rhes regulation of AKT membrane localization.
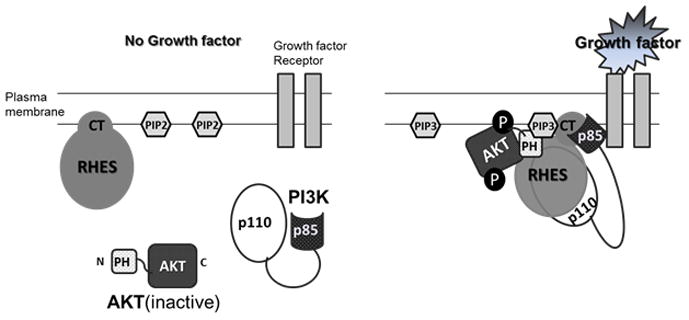
(Left) In unstimulated cells, Rhes is present in the plasma membrane, whereas PI3K and AKT are located in the cytosol. (Right) Upon growth factor stimulation, PI3K localizes to the growth factor receptor, p85 subunit of PI3K binds to c-terminal (CT) of Rhes and p110 subunit converting PIP2 to PIP3. Concomitant with this, PI3K generated PIP3 binding to PH domain of AKT and Rhes results in translocation of AKT to the plasma membrane, where AKT become phosphorylated and activated.
Rhes is also highly expressed in the striatum where dopaminergic input is prevalent. Many laboratories have demonstrated that expression of Rhes is modulated by dopaminergic input, and Rhes plays a role in D1/D2-mediated behavior [7]. A recent study showed that Rhes is responsible for L-DOPA-mediated dyskinesia [20]. However, the mechanism by which Rhes interacts with the dopaminergic pathway is still elusive. Souza et al. showed that the dopamine receptor pathway is inversely related to AKT activation levels [17]. Rhes mRNA expression is decreased by denervation in which dopamine supersensitivity occurs [10]. Hence, it is tempting to speculate that the data presented here may provide a potential mechanism by which dopamine supersensitivity leads to a decrease in Rhes expression and reduced AKT activity [9;14]. Additionally, our study may have novel therapeutic relevance. Since Rhes is highly enriched in the striatum and an interaction between p85 and Rhes is mediated by unique C-terminal tail of Rhes, a small peptide or drug that can blocks such interactions may be an alternative approach to modulate dopamine pathway in the striatum.
Supplementary Material
Highlights.
Rhes interacts with p85 regulatory subunit of PI3K, via C-terminal unique tail
Interaction between p85 and Rhes is enhanced upon growth factor treatment in vitro
AKT translocation to the membrane is facilitated in the presence of Rhes
Rhes is a novel striatal regulator of the AKT-mediated pathway in the striatum.
Acknowledgments
This work was supported by HD026979 and MH079614 (SFK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Araki KY, Sims JR, Bhide PG. Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain Res. 2007;1156:31–45. doi: 10.1016/j.brainres.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 5.Cheah JH, Kim SF, Hester LD, Clancy KW, Patterson SE, III, Papadopoulos V, Snyder SH. NMDA receptor-nitric oxide transmission mediates neuronal iron homeostasis via the GTPase Dexras1. Neuron. 2006;51:431–440. doi: 10.1016/j.neuron.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crandall JE, McCarthy DM, Araki KY, Sims JR, Ren JQ, Bhide PG. Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J Neurosci. 2007;27:3813–3822. doi: 10.1523/JNEUROSCI.5124-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Errico F, Santini E, Migliarini S, Borgkvist A, Centonze D, Nasti V, Carta M, De C, Prosperetti VC, Spano D, Herve D, Pasqualetti M, Di LR, Fisone G, Usiello A. The GTP-binding protein Rhes modulates dopamine signalling in striatal medium spiny neurons. Mol Cell Neurosci. 2008;37:335–345. doi: 10.1016/j.mcn.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Falk JD, Vargiu P, Foye PE, Usui H, Perez J, Danielson PE, Lerner DL, Bernal J, Sutcliffe JG. Rhes: A striatal-specific Ras homolog related to Dexras1. J Neurosci Res. 1999;57:782–788. [PubMed] [Google Scholar]
- 9.Fujita H, Ogino T, Kobuchi H, Fujiwara T, Yano H, Akiyama J, Utsumi K, Sasaki J. Cell-permeable cAMP analog suppresses 6-hydroxydopamine-induced apoptosis in PC12 cells through the activation of the Akt pathway. Brain Res. 2006;1113:10–23. doi: 10.1016/j.brainres.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 10.Harrison LM, Lahoste GJ. Rhes, the Ras homolog enriched in striatum, is reduced under conditions of dopamine supersensitivity. Neuroscience. 2006;137:483–492. doi: 10.1016/j.neuroscience.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Harrison LM, He Y. Rhes and AGS1/Dexras1 affect signaling by dopamine D1 receptors through adenylyl cyclase. J Neurosci Res. 2011;89:874–882. doi: 10.1002/jnr.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemppainen RJ, Behrend EN. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J Biol Chem. 1998;273:3129–3131. doi: 10.1074/jbc.273.6.3129. [DOI] [PubMed] [Google Scholar]
- 13.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 14.Morissette M, Samadi P, Hadj TA, Belanger N, Di PT. Striatal Akt/GSK3 signaling pathway in the development of L-Dopa-induced dyskinesias in MPTP monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:446–454. doi: 10.1016/j.pnpbp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Quintero GC, Spano D, Lahoste GJ, Harrison LM. The Ras homolog Rhes affects dopamine D1 and D2 receptor-mediated behavior in mice. Neuroreport. 2008;19:1563–1566. doi: 10.1097/WNR.0b013e3283118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shearman LP, Zeitzer J, Weaver DR. Widespread expression of functional D1-dopamine receptors in fetal rat brain. Brain Res Dev Brain Res. 1997;102:105–115. doi: 10.1016/s0165-3806(97)00091-6. [DOI] [PubMed] [Google Scholar]
- 17.Souza BR, Romano-Silva MA, Tropepe V. Dopamine D2 receptor activity modulates Akt signaling and alters GABAergic neuron development and motor behavior in zebrafish larvae. J Neurosci. 2011;31:5512–5525. doi: 10.1523/JNEUROSCI.5548-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spano D, Branchi I, Rosica A, Pirro MT, Riccio A, Mithbaokar P, Affuso A, Arra C, Campolongo P, Terracciano D, Macchia V, Bernal J, Alleva E, Di LR. Rhes is involved in striatal function. Mol Cell Biol. 2004;24:5788–5796. doi: 10.1128/MCB.24.13.5788-5796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramaniam S, Napolitano F, Mealer RG, Kim S, Errico F, Barrow R, Shahani N, Tyagi R, Snyder SH, Usiello A. Rhes, a striatal-enriched small G protein, mediates mTOR signaling and L-DOPA-induced dyskinesia. Nat Neurosci. 2011 doi: 10.1038/nn.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vargiu P, Morte B, Manzano J, Perez J, de AR, Gregor SJ, Bernal J. Thyroid hormone regulation of rhes, a novel Ras homolog gene expressed in the striatum. Brain Res Mol Brain Res. 2001;94:1–8. doi: 10.1016/s0169-328x(01)00140-1. [DOI] [PubMed] [Google Scholar]
- 22.Vargiu P, de AR, Garcia-Ranea JA, Valencia A, Santisteban P, Crespo P, Bernal J. The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene. 2004;23:559–568. doi: 10.1038/sj.onc.1207161. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Qi Z, Voit EO. Impact of delays and noise on dopamine signal transduction. Stud Health Technol Inform. 2011;162:222–235. [PubMed] [Google Scholar]
- 24.Yu J, Zhang Y, McIlroy J, Rordorf-Nikolic T, Orr GA, Backer JM. Regulation of the p85/p110 phosphatidylinositol 3′-kinase: stabilization and inhibition of the p110alpha catalytic subunit by the p85 regulatory subunit. Mol Cell Biol. 1998;18:1379–1387. doi: 10.1128/mcb.18.3.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


