Abstract
In the early days of BOLD fMRI, the acquisition of T2* weighted data was greatly facilitated by rapid scan techniques such as EPI. The latter, however, was only available on a few MRI systems that were equipped with specialized hardware that allowed rapid switching of the imaging gradients. For this reason, soon after the invention of fMRI, the scan technique PRESTO was developed to make rapid T2* weighted scanning available on standard clinical scanning. This method combined echo shifting, which allows for echo times longer than the sequence repetition time, with acquisition of multiple k-space lines per excitation. These two concepts were combined in order to achieve a method fast enough for fMRI, while maintaining a sufficiently long echo time for optimal contrast. PRESTO has been primarily used for 3D scanning, which minimized the contribution of large vessels due to inflow effects. Although PRESTO is still being used today, its appeal has lessened somewhat due to increased gradient performance of modern MRI scanners. Compared to 2D EPI, PRESTO may have somewhat reduced temporal stability, which is a disadvantage for fMRI that may not outweigh the advantage of reduced inflow effects provided by 3D scanning. In this overview, the history of the development of the PRESTO is presented, followed by a qualitative comparison with EPI.
Keywords: Functional magnetic resonance imaging (fMRI), Blood oxygenation level dependent (BOLD) contrast, PRESTO (Principles of Echo-Shifting with a Train of Observation), Echo shifting
History and Background
The PRESTO sequence was developed as a functional imaging technique in the early days of fMRI, starting in 1991, at the In-Vivo NMR Center at the National Institutes of Health (NIH). The name stands for ‘Principles of Echo-Shifting with a Train of Observations’, referring to two of the most significant features of the sequence, echo-shifting and a multi-gradient echo type acquisition, as is used in interleaved echo planar imaging (EPI). At the time, the term ‘functional imaging’ was used rather broadly and understood to include most techniques that provided information other than structural (Moonen et al. 1990), as the method of studying brain activity with BOLD fMRI was not yet developed. Rather, blood volume measurements using exogenous contrast agents such as Gd-DTPA appeared to be the most promising MRI technique for this purpose. (Belliveau et al. 1991). Another circumstance relevant to the development of PRESTO was the state of the hardware available on a standard (clinical) scanner at the time. ‘High field’ then meant 1.5T, the gradients had low switching rates and strength, and RF receive coils had sub-optimal sensitivity. Just about all head imaging was performed with a birdcage volume coil well larger than the average human head, and accelerated (parallel) imaging with multiple receive elements was still several years into the future. On the MRI system at NIH, the available gradient amplitude was limited to 10mT/m, the slew rate to 17T/m/s. This, together with duty-cycle limits and residual eddy-currents, meant that EPI was not yet practical on clinical scanners; it was only available with the use of dedicated fast gradient coils (e.g. (Bandettini et al. 1992, Belliveau et al. 1991, Turner et al. 1993)). On clinical scanners, the fast imaging method of choice was gradient echo imaging (FLASH, GRASS etc.) using a short repetition time (TR), for both bolus tracking applications based on Gd-DTPA as well as BOLD contrast based fMRI (e.g. (Kim et al. 1993)).
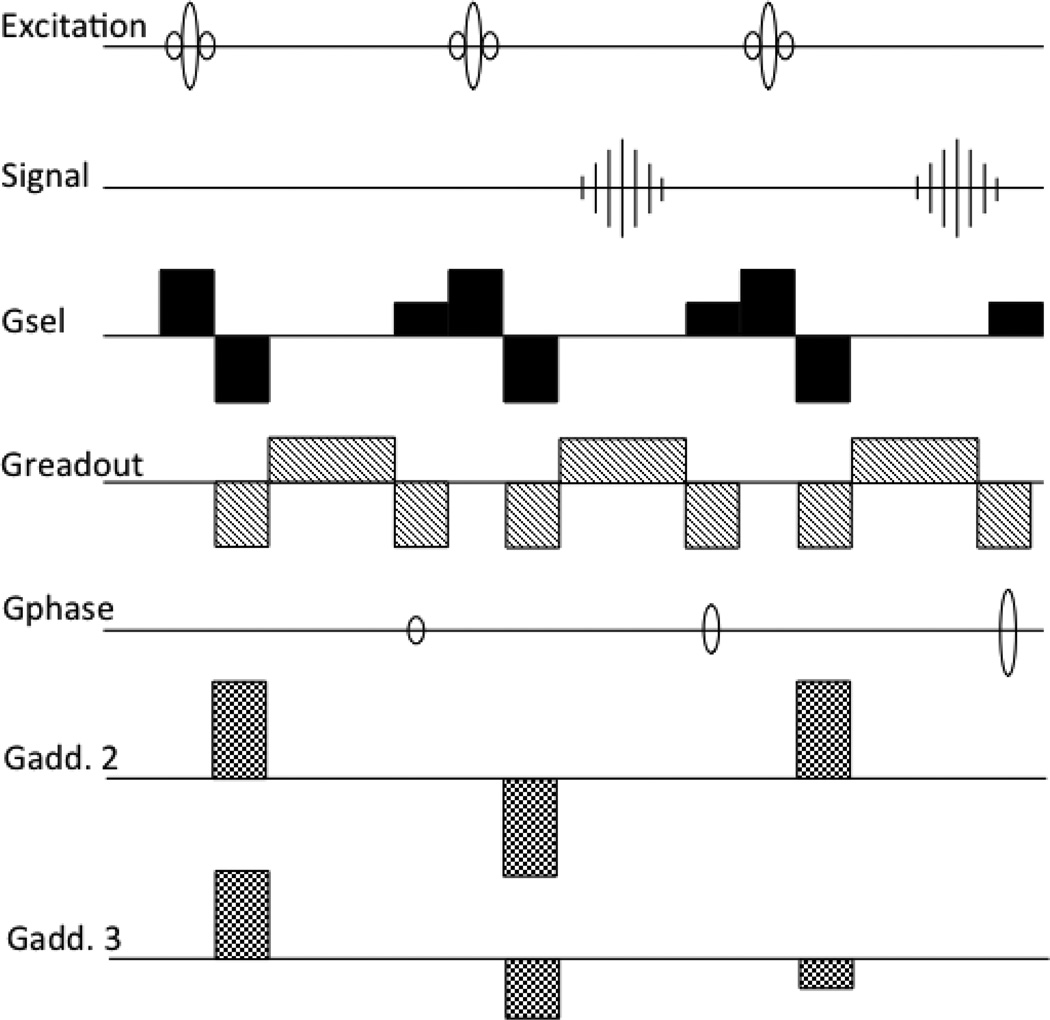
In experiments at NIH using bolus tracking (Zigun et al. 1993), it was realized that accelerating the scan by shortening the TR severely restricted the range of available echo times (TE’s), which could be detrimental for optimal contrast for the T2* effect of the bolus; this problem was minimized by shifting the echo towards the end of the TR interval. As it is written in that paper: ”MR images were obtained with an unmodified 1.5T unit (GE Signa) by means of a fast GRASS sequence, with the echo shifted towards the end of the repetition period by means of an increase in the readout dephasing gradient, this increase produced a repetition time of 16ms and an echo time of 12ms……” (the length of the dephaser effectively controlled the delay between excitation and acquisition). It was essentially this push towards longer TEs, together with the restriction on the repetition time of the sequence related to the desired spatial resolution and image-to-image time resolution, that led to the idea of delaying the echo into a subsequent TR period, resulting in a TE longer than the TR of the sequence (Moonen et al. 1992). This idea was called ‘Echo Shifting’ (ES). The first implementation is shown in Fig.1, with the gradient waveforms modified and added to refocus the signal from each RF pulse after the subsequent one. The modifications entailed: refocusing of the readout gradient in each TR, moving the phase encoding to the end of the TR period, and changing the slice select gradient to a +1/2, +1, −1 shape, where ‘+1’ refers to the surface area (time integral) of the slice select gradient used during the RF pulse. Crushers were added (the line ‘Gadd2’ in Fig. 1) to further suppress the signal directly after the RF pulse. This additional gradient was reversed in the next TR to refocus the desired signal. This scheme was generalized to an arbitrary number of TR shifts (Liu et al. 1993b) by changing the slice select gradient to a 1/n,+1,−1 shape and the additional gradient to step through a cycle of n-values; an example for a three cycle scheme for a 2 TR shift is shown in Fig. 1 in line “Gadd3”. While first implementations were done on a 4.7T animal scanner and demonstrated with bolus tracking on a cat, subsequently the echo shifting method was ported to a clinical scanner for fMRI with bolus tracking in humans (Moonen et al. 1994).
Figure 1.
First two implementations of the Echo Shifted gradient echo imaging. ‘Gsel’ is the slice selection gradient,’Gadd’ the additional gradient used for shifting the echoes, shown in two versions (2 and 3), see text for more details.
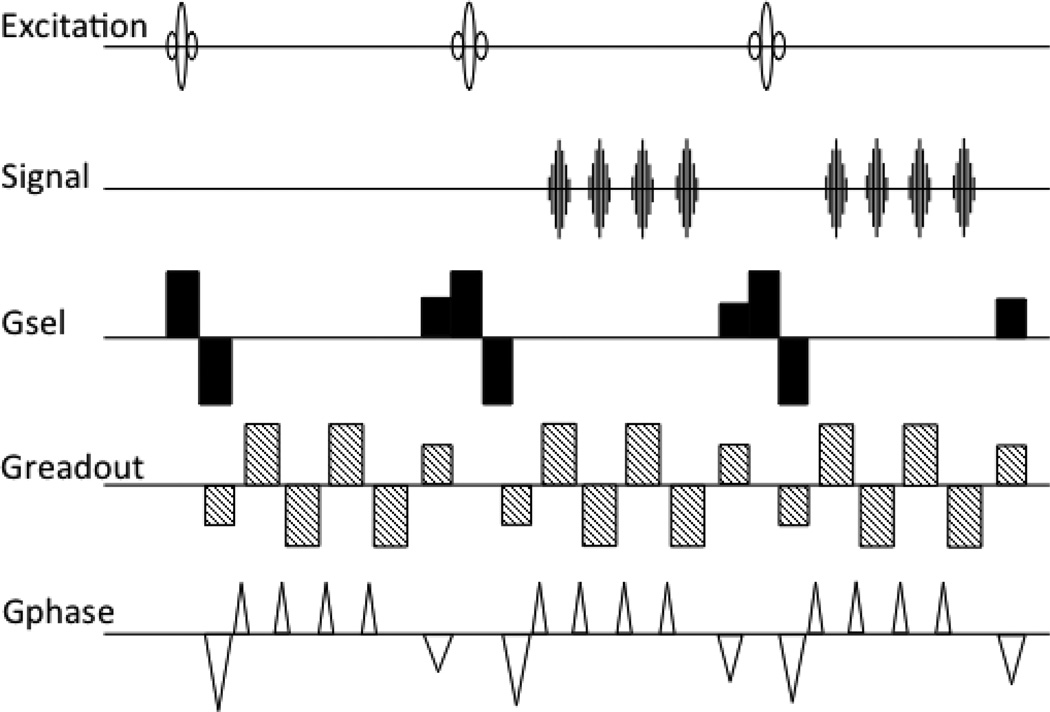
The ES-FLASH acquisition was then accelerated by using multiple readout echoes within every TR in an interleaved EPI fashion, creating the PRESTO sequence (Liu et al. 1993a), see Fig. 2. The first implementation used just the slice select waveform to de- and rephase the desired signals. It was a 2D, single slice sequence, again demonstrated with bolus tracking in a cat brain. With a TR of 9ms, a TE of 13.5ms, a 64×64 image was acquired every 153ms.
Figure 2.
The first PRESTO sequence, combining the echo shifting from Fig. 1 with multiple readouts per TR.
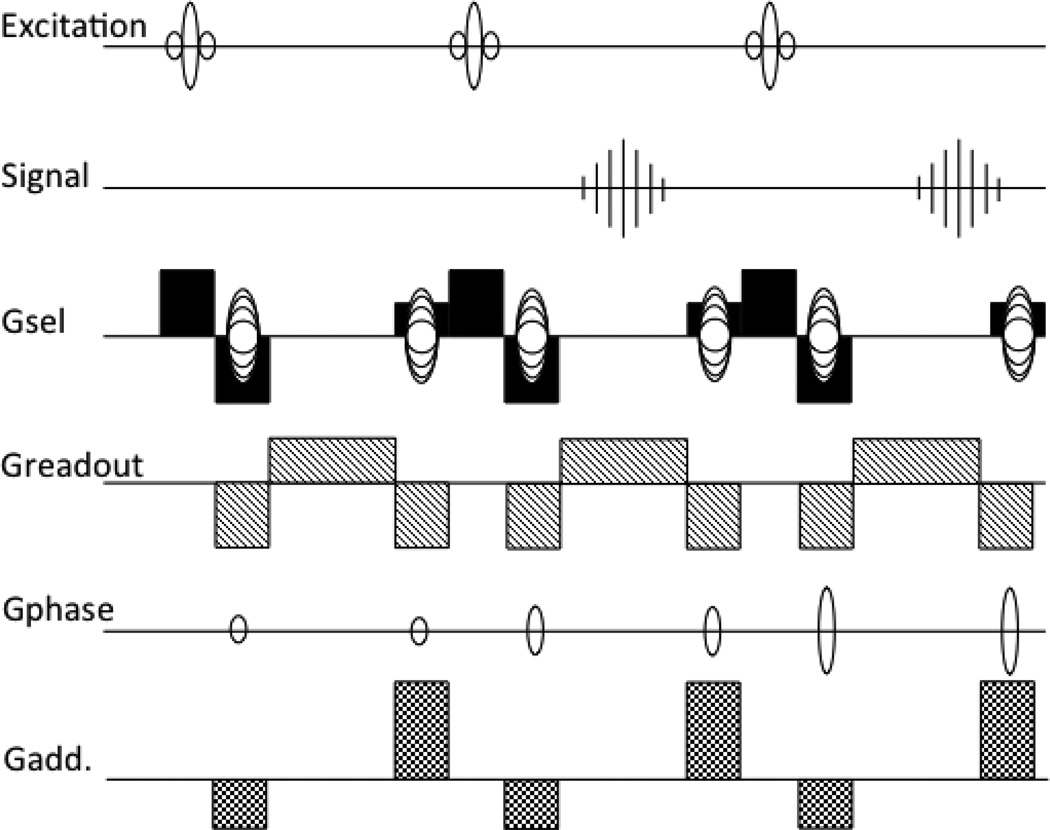
The next step came with an improved scheme for the additional gradients in an ES-FLASH implementation on a clinical scanner (Duyn et al. 1994), see Fig. 3. This was the first time echo shifting was applied to fMRI, requiring a change in the gradient scheme to improve the temporal stability. The new scheme allowed for an arbitrary number of TR shifts while keeping the same waveforms for every TR period. This was achieved by using two crushers in every TR, one before and one after the acquisition, with a ratio of 1:2 for a shift of one TR. It can be generalized to a ratio of n:(n+1) to shift over n TR periods. The slice select gradient was still used as well for the echo shifting, similar to the previous implementations. Also the phase encoding was rewound in every TR to keep the gradient moments constant, improving the stability. Two other additions brought this closer to the final PRESTO version: the extension to 3D encoding and the use of phase scrambling to improve suppression of stimulated (RF) echoes. This suppression again was essential to improve the stability for the fMRI application (see e.g. (Duyn 1997)). The method was used this time for BOLD contrast fMRI with a visual stimulus. A 30ms TE was used, with a 20ms TR, resulting in a 20s 64×64×16 volume acquisition time.
Figure 3.
Echo Shifted FLASH with an improved gradient design and 3D encoding.
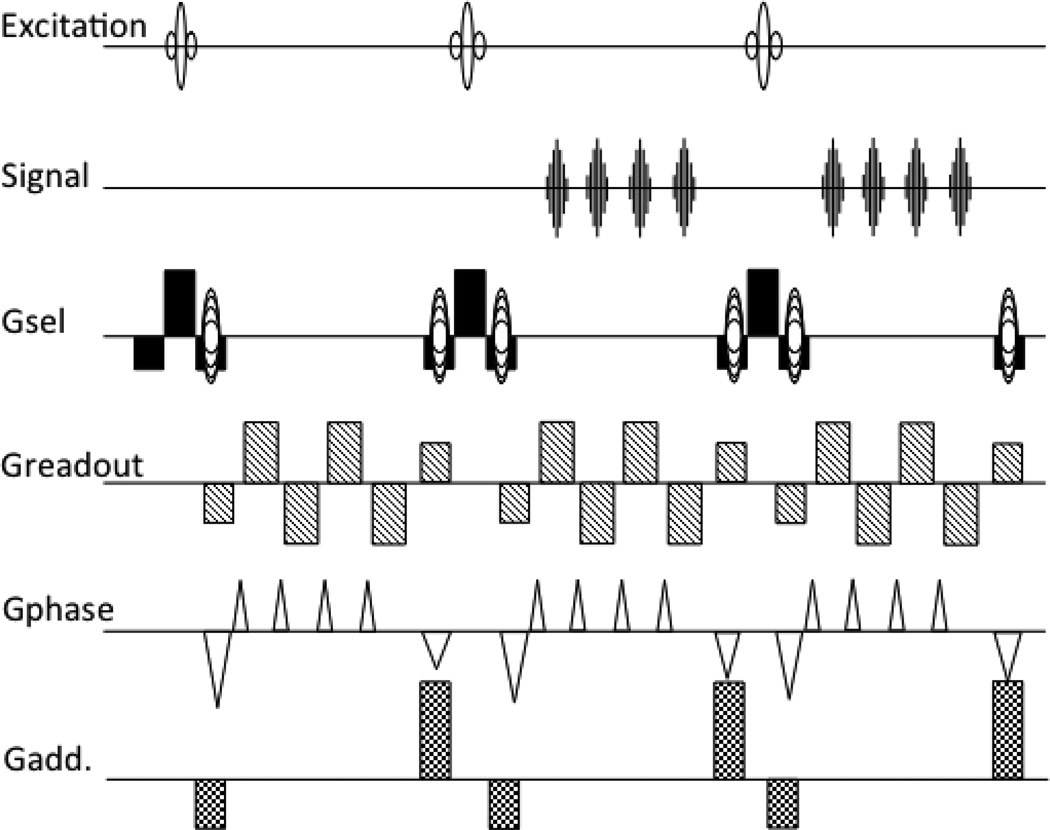
The final version came out a year later (van Gelderen et al. 1995), see Fig. 4, final at least in the sense that this is more or less the sequence that we now understand to be PRESTO. It combined the 3D phase encoding with the multiple echo acquisition and echo shifting from the previous version. It also had the additional gradient design from the previous 3D ES-FLASH method, as well as its phase scrambling. One small difference is that now the slice select gradient is finally completely separated in function from the echo shifting additional gradients, the −1:2:−1 design will refocus both the signal from the current excitation as well as the preceding ones. This implementation acquired 5 echoes per 24ms TR, a TE from 30 to 40ms, for a 64×50×24 voxel volume every 5.8s. It was demonstrated as a BOLD based fMRI method to detect finger-tapping induced activation in the motor and sensory brain areas.
Figure 4.
The PRESTO sequence in its final form: 3D encoding, with multiple echoes per TR period and additional gradients to shift the echo time beyond the TR time.
One more technical detail of interest for PRESTO is the choice of the phase encoding order over the echoes within one TR and over subsequent TR periods. This choice affects the nature of the EPI type artifacts (ghosting and distortion) stemming from the alternating sign of the readout gradient and the different T2* weighting and phase accumulation for the different echoes within one TR. Our choice in general has been to make the steps between the echoes in one TR (the ‘blips’) large, and fill in the intermediate lines in subsequent TRs, see Fig. 5, similar to that proposed for the GRASE sequence (Oshio and Feinberg 1991). This pattern results in N-ghosts for an N-interleave acquisition, but less distortion than a single shot acquisition. Alternative schemes with for example small blips, covering adjacent k-space lines with one echo train, can offer a different trade off between ghosting and distortion.
Figure 5.
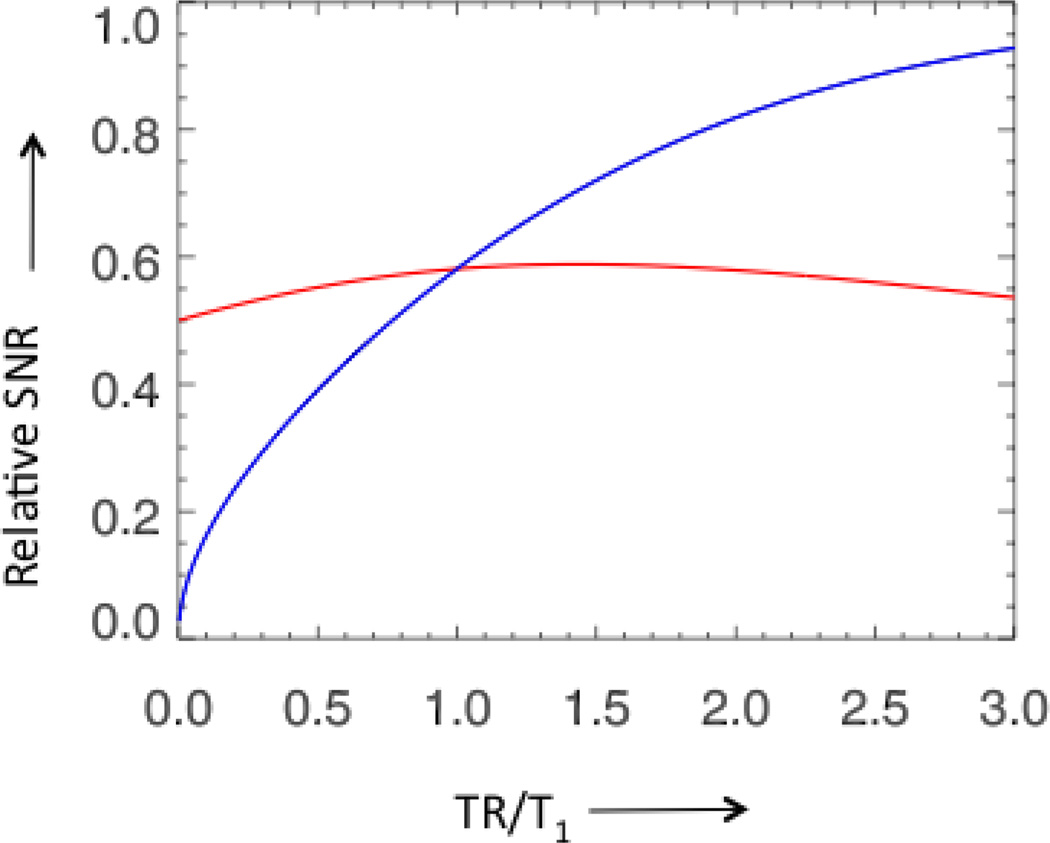
The relative SNR (blue line) and the relative SNR per unit time (red line) of a gradient echo acquisition with constant bandwidth and echo time, and optimal flip angle as function of the ratio of TR and T1. The blue curve reflects the signal in a single shot of a gradient echo acquisition for a range of TR times, the red curve reflects the time averaged signal, showing that over a large range the increase in signal is compensated by the decrease in the number of averages.
Further development and Applications
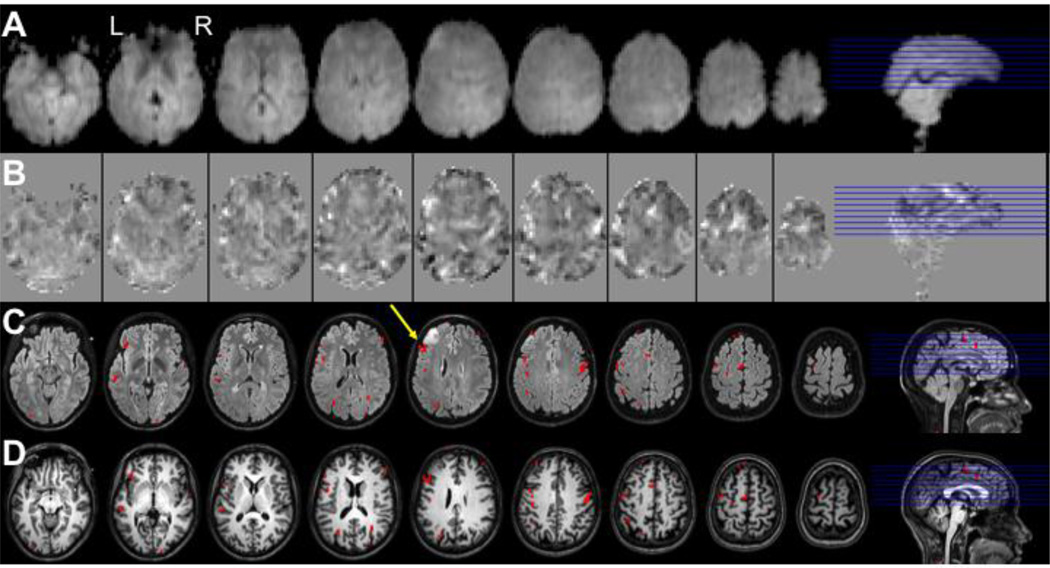
Although the PRESTO method has been applied to fMRI by a number of groups, its use has been much less widespread than EPI based methods. Examples of applications include studies of the motor system (Hanakawa et al. 2008), age related changes in motor activity (Hesselmann et al. 2001), time course analysis of a visual motor task (Hanakawa et al. 2008), cross-modal integration (Bushara et al. 2003), working memory (Jansma et al. 2001, Ramsey et al. 2004), resting-state connectivity (van den Heuvel et al. 2008), language (Rutten et al. 2002b, Sommer et al. 2002) and schizophrenia (Callicott et al. 1998, Raemaekers et al. 2002, Weinberger et al. 1996). Validations studies in our own group looked at reproducibility and correlation with O15-PET activation data (Ramsey et al. 1996a, Ramsey et al. 1996b). Validations of the spatial accuracy have come from comparison to cortical electro-stimulation (Rutten et al. 2002a, Rutten et al. 1999) and electro-corticographic recordings (Hermes et al. 2011, Vansteensel et al. 2010). An example of a PRESTO application is shown in Fig. 6, where a patient with a left-frontal glioma was scanned for to localize the language areas to inform the neurosurgeon. Activity exceeding a statistical threshold are projected onto a FLAIR scan which shows the tumor, and on a T1-weighted anatomical scan. In this case the frontal language area (Broca’s area) was immediately posterior to the tumor (yellow arrow in figure), prompting the surgeon to perform awake surgery involving electrical stimulation to avoid damaging that region.
Figure 6.
Demonstration of the localization of Broca’s language area in a candidate for surgical resection of a brain tumor using PRESTO fMRI. Shown are 9 slices of a PRESTO experiment with 2D SENSE and 8-channel headcoil on a Philips 3T. Images are of language task (verb generation) in a patient with a left frontal glioma, used for surgical planning. Rows display: A - average PRESTO scan, B – T-maps of language activity (range −10 to 6), C – activity exceeding a threshold of t=5 (p<0.05 Bonferroni-corrected) projected onto a FLAIR scan, D – same on T1-weighted anatomical scan. The yellow arrow shows the location of Broca’s area in this subject (with the tumor in front). Scan parameters PRESTO: TR 22.5ms; TE 33.2ms; echo-shifting of 1 TR; flip angle = 10°; FOV 224×256×160mm3; matrix 56×64×40; voxel size 4.0mm isotropic; 0.6075 seconds per volume; 40 slices; sagittal orientation. Courtesy of Department of Neurosurgery, UMC Utrecht, The Netherlands.
Improvements in gradient performance on our clinical GE scanners to 22mT/m amplitude and 115T/m/s slew rate allowed for a speed up of the PRESTO sequence to make 3D bolus tracking feasible in 2000 (with 64×52×32 resolution in a 2s volume TR) (Flacke et al. 2000, van Gelderen et al. 2000) and similarly on a clinical Philips scanner (Flacke et al. 2000). Some applications of PRESTO bolus tracking followed (Manka et al. 2005, Pedersen et al. 2004, Sobesky et al. 2004), but again in much smaller numbers than EPI-based studies. Apart from bolus tracking, PRESTO has also been applied to T2* contrast based anatomical imaging (Sakurai et al. 2010) and venography (Tsuboyama et al. 2008).
The addition of a navigator echo improved the stability and with it the detection sensitivity (Ramsey et al. 1998), as some of the signal fluctuation inherent to multi-shot acquisition can be corrected for by compensating the shot-to-shot amplitude and phase variations. Another technical development that further increased the available speed was the application of accelerated imaging (Pruessmann et al. 1999), resulting in the PRESTO SENSE (sensitivity encoding) combination (Golay et al. 2000) and later a PRESTO-SENSE with partial k-space scanning (Klarhofer et al. 2003). The acceleration allows for a reduction in phase encoding steps, resulting in a 1s volume TR for acceleration in one dimension, or 500ms for a 2D acceleration (Neggers et al. 2008) for a 4mm isotropic resolution in a whole brain acquisition. In a way, the development of parallel imaging may have reduced the demand for PRESTO, as even high-resolution (close to 1mm) single-shot EPI images can now be acquired with manageable levels of geometric distortions. Combining multiple acquisitions to achieve the desired resolution within a reasonable acquisition window is no longer that important.
The various successful applications and on the other hand lack of general acceptance naturally leads to the question: Why use PRESTO instead of multi-slice EPI for fMRI? Some papers have concluded EPI is better (Hesselmann et al. 2004, Ragnehed et al. 2010), while others (Barry et al. 2011, Neggers et al. 2008) found better results with PRESTO. Based on theory, a couple of arguments can be made, considering the signal-to-noise-ratio (SNR), sensitivity, time and spatial resolution, and artifact levels. For the basic image SNR, one has to consider the SNR per unit time, as normally the sequence is repeated many times and the signal effectively averaged in further processing steps. The relative SNR per unit time as function of TR time for a gradient echo sequence with an optimal (Ernst) flip angle is shown in Fig. 5. As can been seen in the plot, the relative SNR per unit time is not very sensitive to the choice to TR, at least up to 3 times the T1, in other words the time averaged image SNR is nearly constant up to a TR time of several seconds. This means two PRESTO sequences with the same acquisition parameters and TE, but different TR times and number of echo shifts, will have approximately the same image SNR. Note that if the acquisition window is reduced to accommodate echo-shifting, by for example increasing the bandwidth, the SNR will be lower. In an EPI sequence optimized for SNR the acquisition window would be extended to acquire during all available time without a delay between for example the excitation and acquisition by either lowering the bandwidth or by acquiring images at multiple echo times (Gowland and Bowtell 2007); if echo shifting is applied in this condition, the SNR will be reduced.
For fMRI however, detection sensitivity in not determined by image SNR alone, but is dependent on temporal signal fluctuations as well, the latter being a combination of signal stability and SNR. Three factors influencing the PRESTO stability are the additional crushers, the multi-shot acquisition mode and the volume TR time. The extra crushers make any ES-sequence more motion sensitive than a non-ES counterpart. Combining multiple excitations into one image also incurs sensitivity to shot-to-shot phase instability, potentially adding to signal fluctuations not present in a single shot approach. In a 2D version of PRESTO-SENSE, i.e. echo shifted EPI (Gibson et al. 2006), this penalty can be avoided, as one slice can be acquired in a single acquisition. The shorter TR time may have several advantages, discussed in the following paragraphs.
The stability and therefore the BOLD signal detection sensitivity is partly determined by the physiological noise. Signal fluctuations originating from physiological rather than instrumental sources generally scale with the signal amplitude (Kruger and Glover 2001), and at high SNR can limit the overall fMRI detection sensitivity. In a simple model of broad-band physiological noise, this favors the lower TR and increased averaging offered by echo shifting, but for band-limited fluctuations this benefit stops when the noise is sufficiently sampled (Gibson et al. 2006). If one considers cardiac-cycle induced fluctuations, which occur at a frequency of about 1 Hz, this means the TR should be as short as 500 ms, achievable with PRESTO (Neggers et al. 2008). With the advance of receive technology and availability of high field scanners (7 T and up), the baseline SNR increases and the influence of the physiological noise becomes more important. Under high SNR conditions, the PRESTO may have the advantage, as suggested in (Barry et al. 2011).
The gain in speed may also have some advantage if one needs the time resolution to for example look at small timing differences for different tasks or different brain areas. A 1s TR would in general be expected to be sufficient to fully sample the BOLD contrast signal changes, as they are band-limited by the BOLD impulse response, however, multi slice EPI may not achieve this time resolution for the desired coverage (number of slices).
The volume acquisition as used in PRESTO, also results in a different sensitivity to subject motion compared to a multi slice approach. As mentioned above, PRESTO, like any multi-shot acquisition, is sensitive to shot-to-shot phase variations resulting from motion. For this reason, motion artifacts appear differently in PRESTO than single shot 2D EPI. Abrupt motion may lead to ghosting and blurring throughout the entire volume in PRESTO, the severity of which would depend on which part of k-space gets affected. In EPI, the induced artifacts would be concentrated in a single slice, possibly making that one slice unusable, although the rest of the volume would be unaffected. Subject motion can also change the spin history in a multi slice excitation resulting in signal fluctuations, as the excited slices are not in the same position in the subject after motion (Friston et al. 1996). Volume excitations are much less sensitive to this effect, as the motion is almost always small compared to the size of the excited volume. In addition, the contrast in the PRESTO images (e.g. Fig. 6a) tends to be lower than in EPI, which helps to reduce motion sensitivity.
The additional motion sensitivity introduced by the echo-shifting gradients in PRESTO can by minimized by keeping their area small. However, to suppress the unwanted echoes, the crusher should be large enough to induce at least 2 cycles of dephasing over the voxel size, preferably in all three directions. With a typical voxel size in the order of 3mm, this would suggest a crusher of about 20mT/m amplitude and 1ms duration (combined amplitude of three axis for first dephasing gradient). This would result in velocity sensitivity (first order moment) of about 100rad s/m for a typical TE and TR, and a b-value of 0.5 s/mm2.
Subject motion in fMRI can to some extent be corrected by spatial registration routines. As this is generally done on a volume-by-volume basis, using a volume acquisition may have an advantage in this respect. Subject motion during the acquisition of a set of slices results in a slice stack that does not form a continuous volume, resulting in a compromise registration averaging the position before and after the motion. In a volume acquisition, the motion may induce some blurring, but unless the center of k-space is severely affected, the registration can still work. A short volume TR time, as offered by PRESTO, also facilitates the registration as the higher temporal sampling rate will tend to reduce the amount of motion per volume. Lastly, the slice timing correction commonly performed for EPI-based fMRI, is not necessary with PRESTO, as all slices are acquired simultaneously. This correction can be problematic in combination with registration, as the registration can result in a blurring in time when neighboring slices acquired far apart in time in a slice-interleaved EPI are mixed in the registration process.
Another factor that could influence the choice for (or against) PRESTO is the reduced sensitivity to inflow and BOLD contrast changes in larger veins. As PRESTO has a volume excitation, it is less sensitive to inflow effects that can amplify flow changes in EPI data. Also the additional crusher, while increasing motion sensitivity, effectively suppresses intravascular signal. This may reduce the number of activated voxels, but could increase spatial fidelity. Based on this rationale, PRESTO has been applied to neurosurgical planning (Kho et al. 2005, Rutten et al. 2002a).
Finally, geometric distortions are less severe in PRESTO than in EPI, as the effective bandwidth in the phase encoding direction is higher. Although small distortions may be correctable given sufficient knowledge of the underlying magnetic fields, large distortions where a voxel is mixed with its neighbor may not be, and even when corrected it can result in a loss of SNR due to the change in effective voxel size.
In conclusion, PRESTO allows rapid 3D fMRI with flexible T2* weighting. This additional flexibility allows one to better optimize sensitivity, however this comes at the price of a somewhat reduced temporal stability. The amount of stability reduction is dependent on measurement parameters and conditions, and may or may not outweigh the sensitivity benefit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–397. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Barry RL, Strother SC, Gatenby JC, Gore JC. Data-driven optimization and evaluation of 2D EPI and 3D PRESTO for BOLD fMRI at 7 Tesla: I. ocal coverage. Neuroimage. 2011;55:1034–1043. doi: 10.1016/j.neuroimage.2010.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau JW, Kennedy DN, Jr, McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS, Vevea JM, Brady TJ, Rosen BR. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Hanakawa T, Immisch I, Toma K, Kansaku K, Hallett M. Neural correlates of cross-modal binding. Nat Neurosci. 2003;6:190–195. doi: 10.1038/nn993. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Ramsey NF, Tallent K, Bertolino A, Knable MB, Coppola R, Goldberg T, van Gelderen P, Mattay VS, Frank JA, Moonen CT, Weinberger DR. Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology. 1998;18:186–196. doi: 10.1016/S0893-133X(97)00096-1. [DOI] [PubMed] [Google Scholar]
- Duyn JH. Steady state effects in fast gradient echo magnetic resonance imaging. Magn Reson Med. 1997;37:559–568. doi: 10.1002/mrm.1910370414. [DOI] [PubMed] [Google Scholar]
- Duyn JH, Mattay VS, Sexton RH, Sobering GS, Barrios FA, Liu G, Frank JA, Weinberger DR, Moonen CT. 3-dimensional functional imaging of human brain using echo-shifted FLASH MRI. Magn Reson Med. 1994;32:150–155. doi: 10.1002/mrm.1910320123. [DOI] [PubMed] [Google Scholar]
- Flacke S, Urbach H, Folkers PJ, Keller E, van den Brink JS, Traber F, Block W, Gieseke J, Schild HH. Ultra-fast three-dimensional MR perfusion imaging of the entire brain in acute stroke assessment. J Magn Reson Imaging. 2000;11:250–259. doi: 10.1002/(sici)1522-2586(200003)11:3<250::aid-jmri3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gibson A, Peters AM, Bowtell R. Echo-shifted multislice EPI for high-speed fMRI. Magn Reson Imaging. 2006;24:433–442. doi: 10.1016/j.mri.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Golay X, Pruessmann KP, Weiger M, Crelier GR, Folkers PJ, Kollias SS, Boesiger P. PRESTO-SENSE: an ultrafast whole-brain fMRI technique. Magn Reson Med. 2000;43:779–786. doi: 10.1002/1522-2594(200006)43:6<779::aid-mrm1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Gowland PA, Bowtell R. Theoretical optimization of multi-echo fMRI data acquisition. Phys Med Biol. 2007;52:1801–1813. doi: 10.1088/0031-9155/52/7/003. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Dimyan MA, Hallett M. Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI. Cereb Cortex. 2008;18:2775–2788. doi: 10.1093/cercor/bhn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes D, Miller KJ, Vansteensel MJ, Aarnoutse EJ, Leijten FS, Ramsey NF. Neurophysiologic correlates of fMRI in human motor cortex. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21314. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselmann V, Girnus R, Wedekind C, Hunsche S, Bunke J, Schulte O, Sorger B, Lasek K, Krug B, Sturm V, Lackner K. Functional MRI using multiple receiver coils: BOLD signal changes and signal-to-noise ratio for three-dimensional-PRESTO vs. single shot EPI in comparison to a standard quadrature head coil. J Magn Reson Imaging. 2004;20:321–326. doi: 10.1002/jmri.20101. [DOI] [PubMed] [Google Scholar]
- Hesselmann V, Zaro Weber O, Wedekind C, Krings T, Schulte O, Kugel H, Krug B, Klug N, Lackner KJ. Age related signal decrease in functional magnetic resonance imaging during motor stimulation in humans. Neurosci Lett. 2001;308:141–144. doi: 10.1016/s0304-3940(01)01920-6. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Slagter HA, Kahn RS. Functional anatomical correlates of controlled and automatic processing. J Cogn Neurosci. 2001;13:730–743. doi: 10.1162/08989290152541403. [DOI] [PubMed] [Google Scholar]
- Kho KH, Leijten FS, Rutten GJ, Vermeulen J, Van Rijen P, Ramsey NF. Discrepant findings for Wada test and functional magnetic resonance imaging with regard to language function: use of electrocortical stimulation mapping to confirm results. Case report. J Neurosurg. 2005;102:169–173. doi: 10.3171/jns.2005.102.1.0169. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Klarhofer M, Dilharreguy B, van Gelderen P, Moonen CT. A PRESTO-SENSE sequence with alternating partial-Fourier encoding for rapid susceptibility-weighted 3D MRI time series. Magn Reson Med. 2003;50:830–838. doi: 10.1002/mrm.10599. [DOI] [PubMed] [Google Scholar]
- Kruger G, Glover GH. Physiological noise in oxygenation-sensitive magnetic resonance imaging. Magn Reson Med. 2001;46:631–637. doi: 10.1002/mrm.1240. [DOI] [PubMed] [Google Scholar]
- Liu G, Sobering G, Duyn J, Moonen CT. A functional MRI technique combining principles of echo-shifting with a train of observations (PRESTO) Magn Reson Med. 1993a;30:764–768. doi: 10.1002/mrm.1910300617. [DOI] [PubMed] [Google Scholar]
- Liu G, Sobering G, Olson AW, van Gelderen P, Moonen CT. Fast echo-shifted gradient-recalled MRI: combining a short repetition time with variable T2* weighting. Magn Reson Med. 1993b;30:68–75. doi: 10.1002/mrm.1910300111. [DOI] [PubMed] [Google Scholar]
- Manka C, Traber F, Gieseke J, Schild HH, Kuhl CK. Three-dimensional dynamic susceptibility-weighted perfusion MR imaging at 3.0 T: feasibility and contrast agent dose. Radiology. 2005;234:869–877. doi: 10.1148/radiol.2343040359. [DOI] [PubMed] [Google Scholar]
- Moonen CT, Barrios FA, Zigun JR, Gillen J, Liu G, Sobering G, Sexton R, Woo J, Frank J, Weinberger DR. Functional brain MR imaging based on bolus tracking with a fast T2*-sensitized gradient-echo method. Magn Reson Imaging. 1994;12:379–385. doi: 10.1016/0730-725x(94)92530-5. [DOI] [PubMed] [Google Scholar]
- Moonen CT, Liu G, van Gelderen P, Sobering G. A fast gradient-recalled MRI technique with increased sensitivity to dynamic susceptibility effects. Magn Reson Med. 1992;26:184–189. doi: 10.1002/mrm.1910260118. [DOI] [PubMed] [Google Scholar]
- Moonen CT, van Zijl PC, Frank JA, Le Bihan D, Becker ED. Functional magnetic resonance imaging in medicine and physiology. Science. 1990;250:53–61. doi: 10.1126/science.2218514. [DOI] [PubMed] [Google Scholar]
- Neggers SF, Hermans EJ, Ramsey NF. Enhanced sensitivity with fast three-dimensional blood-oxygen-level-dependent functional MRI: comparison of SENSE-PRESTO and 2D-EPI at 3 T. NMR Biomed. 2008;21:663–676. doi: 10.1002/nbm.1235. [DOI] [PubMed] [Google Scholar]
- Oshio K, Feinberg DA. GRASE (Gradient- and spin-echo) imaging: a novel fast MRI technique. Magn Reson Med. 1991;20:344–349. doi: 10.1002/mrm.1910200219. [DOI] [PubMed] [Google Scholar]
- Pedersen M, Klarhofer M, Christensen S, Ouallet JC, Ostergaard L, Dousset V, Moonen C. Quantitative cerebral perfusion using the PRESTO acquisition scheme. J Magn Reson Imaging. 2004;20:930–940. doi: 10.1002/jmri.20206. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- Raemaekers M, Jansma JM, Cahn W, Van der Geest JN, van der Linden JA, Kahn RS, Ramsey NF. Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Arch Gen Psychiatry. 2002;59:313–320. doi: 10.1001/archpsyc.59.4.313. [DOI] [PubMed] [Google Scholar]
- Ragnehed M, Leinhard OD, Pihlsgard J, Wirell S, Sokjer H, Fagerstam P, Jiang B, Smedby O, Engstrom M, Lundberg P. Visual grading of 2D and 3D functional MRI compared with image-based descriptive measures. Eur Radiol. 2010;20:714–724. doi: 10.1007/s00330-009-1578-0. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Jansma JM, Jager G, Van Raalten T, Kahn RS. Neurophysiological factors in human information processing capacity. Brain. 2004;127:517–525. doi: 10.1093/brain/awh060. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Kirkby BS, Van Gelderen P, Berman KF, Duyn JH, Frank JA, Mattay VS, Van Horn JD, Esposito G, Moonen CT, Weinberger DR. Functional mapping of human sensorimotor cortex with 3D BOLD fMRI correlates highly with H2(15)O PET rCBF. J Cereb Blood Flow Metab. 1996a;16:755–764. doi: 10.1097/00004647-199609000-00001. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Tallent K, van Gelderen P, Frank JA, Moonen CT, Weinberger DR. Reproducibility of human 3D fMRI brain maps acquired during a motor task. Hum Brain Mapp. 1996b;4:113–121. doi: 10.1002/(SICI)1097-0193(1996)4:2<113::AID-HBM3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, van den Brink JS, van Muiswinkel AM, Folkers PJ, Moonen CT, Jansma JM, Kahn RS. Phase navigator correction in 3D fMRI improves detection of brain activation: quantitative assessment with a graded motor activation procedure. Neuroimage. 1998;8:240–248. doi: 10.1006/nimg.1998.0358. [DOI] [PubMed] [Google Scholar]
- Rutten GJ, Ramsey NF, van Rijen PC, Noordmans HJ, van Veelen CW. Development of a functional magnetic resonance imaging protocol for intraoperative localization of critical temporoparietal language areas. Ann Neurol. 2002a;51:350–360. doi: 10.1002/ana.10117. [DOI] [PubMed] [Google Scholar]
- Rutten GJ, Ramsey NF, van Rijen PC, van Veelen CW. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang. 2002b;80:421–437. doi: 10.1006/brln.2001.2600. [DOI] [PubMed] [Google Scholar]
- Rutten GJ, van Rijen PC, van Veelen CW, Ramsey NF. Language area localization with three-dimensional functional magnetic resonance imaging matches intrasulcal electrostimulation in Broca's area. Ann Neurol. 1999;46:405–408. doi: 10.1002/1531-8249(199909)46:3<405::aid-ana17>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Sakurai K, Kawaguchi T, Kawai T, Ogino H, Hara M, Okita K, Yamawaki T, Shibamoto Y. Usefulness of 3D-PRESTO imaging in evaluating putaminal abnormality in parkinsonian variant of multiple system atrophy. Neuroradiology. 2010;52:809–814. doi: 10.1007/s00234-009-0621-9. [DOI] [PubMed] [Google Scholar]
- Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Thiel A, Dohmen C, Jacobs A, Neveling M, Heiss WD. Which time-to-peak threshold best identifies penumbral flow? A comparison of perfusion-weighted magnetic resonance imaging and positron emission tomography in acute ischemic stroke. Stroke. 2004;35:2843–2847. doi: 10.1161/01.STR.0000147043.29399.f6. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandl RC, Kahn RS. Language lateralization in monozygotic twin pairs concordant and discordant for handedness. Brain. 2002;125:2710–2718. doi: 10.1093/brain/awf284. [DOI] [PubMed] [Google Scholar]
- Tsuboyama T, Imaoka I, Shimono T, Nakatsuka T, Ashikaga R, Okuaki T, Koyama N, Murakami T. T2*-sensitized high-resolution magnetic resonance venography using 3D-PRESTO technique. Magn Reson Med Sci. 2008;7:73–77. doi: 10.2463/mrms.7.73. [DOI] [PubMed] [Google Scholar]
- Turner R, Jezzard P, Wen H, Kwong KK, Le Bihan D, Zeffiro T, Balaban RS. Functional mapping of the human visual cortex at 4 and 1.5 tesla using deoxygenation contrast EPI. Magn Reson Med. 1993;29:277–279. doi: 10.1002/mrm.1910290221. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Boersma M, Hulshoff Pol HE. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage. 2008;43:528–539. doi: 10.1016/j.neuroimage.2008.08.010. [DOI] [PubMed] [Google Scholar]
- van Gelderen P, Grandin C, Petrella JR, Moonen CT. Rapid three-dimensional MR imaging method for tracking a bolus of contrast agent through the brain. Radiology. 2000;216:603–608. doi: 10.1148/radiology.216.2.r00au27603. [DOI] [PubMed] [Google Scholar]
- van Gelderen P, Ramsey NF, Liu G, Duyn JH, Frank JA, Weinberger DR, Moonen CT. Three-dimensional functional magnetic resonance imaging of human brain on a clinical 1.5-T scanner. Proc Natl Acad Sci U S A. 1995;92:6906–6910. doi: 10.1073/pnas.92.15.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteensel MJ, Hermes D, Aarnoutse EJ, Bleichner MG, Schalk G, van Rijen PC, Leijten FS, Ramsey NF. Brain-computer interfacing based on cognitive control. Ann Neurol. 2010;67:809–816. doi: 10.1002/ana.21985. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Mattay V, Callicott J, Kotrla K, Santha A, van Gelderen P, Duyn J, Moonen C, Frank J. fMRI applications in schizophrenia research. Neuroimage. 1996;4:S118–S126. doi: 10.1006/nimg.1996.0062. [DOI] [PubMed] [Google Scholar]
- Zigun JR, Frank JA, Barrios FA, Jones DW, Foo TK, Moonen CT, Press DZ, Weinberger DR. Measurement of brain activity with bolus administration of contrast agent and gradient-echo MR imaging. Radiology. 1993;186:353–356. doi: 10.1148/radiology.186.2.8421733. [DOI] [PubMed] [Google Scholar]