Abstract
The Asian tiger mosquito, Aedes albopictus, is a medically important invasive species whose geographic distribution has expanded dramatically during the past 20 years, and one of the key elements of its success is its capacity to survive long distance transport as a diapausing pharate first instar larva, encased within the chorion of the egg. We report that pharate larvae entering diapause are larger and contain 30% more lipid than their nondiapausing counterparts. To improve our understanding of the molecular regulation of lipid metabolism during diapause, we assessed the relative mRNA abundance of 21 genes using qRT-PCR. Elevated expression of lipid storage droplet protein 2 during embryonic development likely contributes to the higher amounts of lipid we noted in diapausing individuals. The conservation of lipids during diapause is reflected in downregulation of genes involved in lipid catabolism, including lipase 2, lipase 3, lipase 4, acyl-CoA dehydrogenase 4, and isovaleryl-CoA dehydrogenase. Two genes involved in fatty acid synthesis and modification, Δ(9)-desaturase, and fatty acyl-CoA elongase, were both upregulated in diapausing pharate larvae, suggesting roles for their gene products in generating unsaturated fatty acids to enhance membrane fluidity at low temperatures and generating precursors to the surface hydrocarbons needed to resist desiccation, respectively. Together, the results point to substantial distinctions in lipid metabolism within the embryo as a consequence of the diapause program, and these differences occur both before the actual onset of diapause as well as during the diapause state.
Keywords: phenotypic plasticity, β-oxidation, lipid metabolism, invasive species, embryogenesis, pharate first instar larvae
1. Introduction
The Asian tiger mosquito, Aedes albopictus (Skuse), is among the most invasive animal species on the planet. Its habitat range has significantly increased over the past 20 years, expanding from Asia to every continent except Antarctica (Hawley et al. 1987; Benedict et al. 2007; Lounibos, 2002; Lounibos et al. 2003). The rapid range expansion of this vector of dengue fever and other viruses is, at least partially, due to its ability to enter diapause, a specific type of dormancy that significantly improves survival during winter in temperate regions (Wang, 1966; Mori et al. 1981; Sota and Mogi, 1992; Hanson et al. 1994). Entering diapause extends life span and provides insects, including Ae. albopictus, a means to “escape” from seasonally predictable periods of unfavorable conditions. Entering diapause also allows populations to synchronize periods of growth and reproduction with predictable periods of favorable conditions. While the adaptive significance of diapause is clear, the molecular regulation of diapause in this, or any other, species remains poorly understood.
Diapause is an alternative developmental pathway that is characterized by developmental arrest and metabolic restructuring. Diapause entry includes coordinated downregulation of oxidative processes that produce energy, suppression of processes that consume energy (e.g., transcription, translation, cell growth and division), and upregulation of processes (e.g. production of heat shock proteins) that enhance stress resistance (Lees, 1955; Tauber and Tauber, 1976; Denlinger 2002, MacRae 2010; Hand et al., 2011). Metabolic restructuring is a key component of diapause because it maintains cellular homeostasis and allows for the conservation of metabolic fuels (e.g. carbohydrate, lipids, and proteins) that are critical for maintaining basic housekeeping functions during diapause and for resuming development post-diapause. Failure to maintain adequate fuel stores through a lengthy period of dormancy can lead to premature diapause termination, inhibit post-diapause development or reduce fecundity (Hahn and Denlinger, 2007 and 2010; Hand et al. 2011). Fuel conservation is especially critical for embryos and pharate larvae in diapause because insect eggs are a closed system. Only water and gases are exchanged between enclosed embryos and their environment, and there is no opportunity to obtain additional fuels.
Metabolic downregulation during diapause has been documented for a variety of insect species that enter diapause (e.g., Rakshpal, 1962, Denlinger et al., 1972, Braune, 1976). However, there are few studies that have examined the proximal mechanisms that promote metabolic downregulation and the conservation of metabolic fuels before and during diapause. The goal of the present research is to identify transcriptional changes that are the basis for metabolic restructuring during diapause in Ae. albopictus. We specifically focus on lipid metabolism pathways because lipids, which make up 30–40 % of the dry weight of insect oocytes, are the primary fuel consumed during embryogenesis in mosquitoes (Van Handel, 1993). In addition, lipids have a key role protecting against desiccation (Blomquist et al. 1987; Gibbs et al. 1991) and cold stress (Hazel et al., 1995; Tiku et al. 1996; Cossins et al. 2006; Hochachka and Somero, 2002). We predict there will be significant changes in transcript abundance of genes whose products are involved in lipid storage, lipid catabolism, and unsaturated fatty acid synthesis; and that these changes promote lipid conservation and enhance survival in this species. Together these results will provide a clearer picture of the physiological basis for diapause in this invasive species.
2. Methods
2.1 Insect Rearing
Laboratory colonies were established with larvae collected from water in used tires in Manassas VA in 2008, and they were maintained as previously described (Urbanski et al. 2010a, b). Briefly, larvae were reared to adulthood at a density of ~ 30 larvae per 90 ml of deionized water and were fed a slurry of dog food (Nutro Brand Large Breed Adult, Nutro Products Inc. City of Industry, CA, USA) and brine shrimp (Sally’s Frozen Brine Shrimp, San Francisco Bay Brand Newark, CA, USA). Male and female pupae were transferred into adult cages and provided moist paper towels and organic raisins. Pupae and adults were maintained under either diapause-inducing, short day (SD) conditions (21°C, 8:16 L: D hours) or diapause-averting, long day (LD) conditions (21 °C, 16:8 L: D hours). Females were allowed to blood feed to repletion on a human host 10–13 d post-adult eclosion. Oviposition cups, made from 200 ml black cups lined with unbleached seed germination paper and filled with distilled H2O, were placed into cages to stimulate oviposition beginning 3 or 4 d after blood-feeding. Females were allowed to oviposit for 6 hours starting 4 h after “lights on”. Eggs were maintained under SD conditions at 80% relative humidity until they were sampled to measure egg size, total lipid content, or transcript abundance at the times shown in Fig. 1. Eggs sampled from a single cage were considered to be a replicate.
Figure 1.
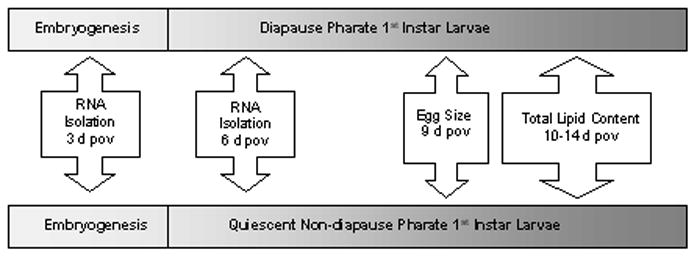
The developmental and sampling timeline for diapause (top) and non-diapause (bottom) Ae. albopictus embryos post-oviposition (pov). To determine whether there are physiological differences between these two types of embryos, egg size was measured 9 d pov and total lipid content was measured 10–14 d pov. Transcriptional basis for physiological differences was assessed by comparing mRNA expression of select genes during pre-diapause (3 d pov) and in diapause embryos (6 d pov) to transcript abundance in RNA isolated from non-diapause embryos 3 d and 6 d pov.
2.2 Embryo Staging and Imaging
Eggs were chemically cleared using the method described by Trpis (1970). Briefly, a piece of seed paper containing eggs was placed inside a 1 dram shell vial (Fisher Scientific, Houston, TX USA) with enough clearing solution (sodium chlorite + acetic acid) so that the paper was completely immersed. Vials were capped and placed at 4 °C to prevent larval emergence of 6 d, non-diapause pharate larvae. Embryos and pharate larvae were observed with an Olympus SZH Dissecting Microscope and images were digitally recorded with an Olympus Magnafire low light color digital camera (Olympus Corporation, Tokyo, Japan). Embryonic development was monitored for 7 d post-oviposition (pov) to determine the amount of time required to complete embryogenesis at 21°C. Developmental stages 3 and 6 d pov were determined by comparing the morphology of embryos we observed to published images of Aedes aegypti embryos (Vital et a., 2010; Farnesi et al., 2009; Rezende et al. 2008). The morphology of randomly selected embryos from SD-reared females was compared to that of embryos from LD females to estimate whether diapause-destined embryos develop at the same rate as non-diapause embryos.
2.3 Physical and Physiological Characteristics of Eggs
Egg volume was calculated nine days post-oviposition. Eggs were randomly selected from SD or LD adults; each replicate was from a single cage. Egg length and width were measured for 30–45 randomly selected eggs; these measurements were used to calculate egg volume (V) as that of a prolate spheroid from:
where L is egg length and W is egg width (Armbruster et al. 2001). Samples were taken from two oviposition dates for each egg type. Student’s t-test was used to compare egg volumes of SD and LD eggs.
2.4 Total Lipid Analysis
Total lipid content was measured for 5 replicates of diapause and non-diapause embryos 10–14 d post-oviposition. For each replicate, 17.3–39.6 mg of eggs were crushed and mixed with 0.5 mL chloroform and methanol (1:1). After centrifugation the supernatant was transferred to a clean tube and the solvent was evaporated; 0.2 mL of sulfuric acid was added and the mixture was heated for 10 min. After cooling, 0.5 mL of vanillin extract was added and a reddish brown color was allowed to develop for 5 min. Lipid content was estimated based on absorbance at 525 nm compared to a standard curve (Van Handel, 1985) and was normalized per mg wet weight. Student’s t-test was used to compare total lipids of diapause and non-diapause embryos.
2.5 Transcript Profiling
A comparative genomics approach was used to identify 20 candidate genes whose products are predicted to have roles in lipid metabolism (e.g. lipid storage, lipid transport, lipid catabolism, and fatty acid synthesis) or are known to be regulators of lipid metabolism. Nucleotide sequences of Aedes albopictus homologs (Table 1) were identified by using annotated Culex pipiens nucleotide sequences (Sim and Denlinger, 2009) to perform blastn searches against an Ae. albopictus EST database (http://www.albopictusexpression.org/; Poelchau et al., 2011).
Table 1.
Putative lipid metabolism genes in Aedes albopictus.
| Putative ID | Predicted Physiological Process | Genbank ID | Species with closest homology | E | % ID |
|---|---|---|---|---|---|
| acc | Acetyl-CoAcarboxylase_acc | JO861035 | Ae. aegypti | 3e-90 | 100% |
| lsd2 | Lipid storage droplet protein | JO907205 | Ae. aegypti | 2e-164 | 90% |
| fabp | Fatty acid binding protein | JO853034 | Ae. aegypti | 1e-72 | 97% |
| apoLp-III | Apolipophorin-III | JO863154 | Ae. aegypti | 2e-116 | 93% |
| lip2 | Lipase* | Ae. aegypti AAEL001057 |
0.00 | 89% | |
| lip3 | Lipase* | Ae. aegypti AAEL007044 |
0.00 | 93% | |
| lip4 | Lipase* | Ae. aegypti AAEL012343 |
7e-163 | 89% | |
| acs | Acetyl-CoA synthase | Ae. aegypti | 4e-62 | 92% | |
| cpt2 | Carnitine O-palmitoyltransferase | JO882235 | Ae. aegypti | 0.00 | 94% |
| ech2 | 3,2-Trans-enoyl-CoA isomerase | JO850771 | Ae. aegypti | 3e-170 | 95% |
| ech3 | Cyclo-hex-1-ene-1-carboxyl CoA hydratase | JO879395 | Ae. aegypti | 9e-153 | 91% |
| acd2 | Acyl-CoA dehydrogenase | JO888990 | Ae. aegypti | 0.00 | 97% |
| acd4 | Acyl-CoA dehdrogenase | JO893690 | Ae. aegypti | 0.00 | 98% |
| acd5 | Isovaleryl-CoA dehydrogenase | JO891909 | Ae. aegypti | 0.00 | 97% |
| hcdh | 3-hydroxyacyl-CoAdehydrogenase | JO884349 | Ae. aegypti | 0.00 | 97% |
| ampk-alpha | AMP activated protein kinase-alpha | JO905089 | Ae. aegypti | 0.00 | 99% |
| ampk-beta | AMP activated protein kinase beta | JO867565 | Ae. aegypti | 9e-105 | 99% |
| ampk-gamma | AMP activated protein kinase gamma | JO855296 | Ae. aegypti | 0.00 | 98% |
| desat | Δ(9) desaturase | JO908589 | Ae. aegypti | 0.00 | 98% |
| fad-3 | Acyl-CoA Δ(11) desaturase | JO854414 | Ae. aegypti | 5e-81 | 94% |
| face | Fatty Acyl-CoA Elongase | GQ168593 | Ae. albopictus | 0.00 | 100% |
Sequences accessible in NCBI’s short read archive (SRA under submission numbers SRA044835 and SRA051478.
Relative transcript abundance was assessed at 3 d and 6 d post-oviposition in embryos (3 d pov) and pharate larvae (6 d pov) from short-day or long-day reared females. For clarity, 3 d embryos from short-day females will henceforth be referred to as pre-diapause embryos and 6 d embryos from short-day females will be referred to as diapause pharate larvae. Embryos from long-day females will be referred to as 3 d or 6 d non-diapause embryos or pharate larvae, respectively.
Total RNA was isolated from 3–4 replicates of embryos from each of the four groups described above using Tri® Reagent (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturers directions. Following isopropanol precipitation, resuspended RNA was treated with Turbo-DNAfree (Life Technologies, Carlsbad, CA, USA) to remove genomic DNA contamination. The concentration of each RNA sample was assessed using a Nano Drop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). cDNA was synthesized using the iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Inc. Hercules, CA, USA) according to the manufacturer’s directions; equal amounts of total RNA were used in each reaction. For each replicate sample, two independent reactions were carried out and then pooled.
Relative mRNA abundance of selected genes of interest was measured using an iQ5™ Multicolor Real-Time PCR Detection System (Bio-Rad) and iQ™ SYBR Green Supermix (Bio-Rad). Primers were designed with PrimerQuest software (IDT DNA, Coralville, IA, USA) and conformed to MIQE standards (Bustin et al. 2009) as shown in Table S1. Cycling parameters were 95 °C for 3 min followed by 40–50 cycles of 95 ° for 10 s, 58 °C for 30 s and 72 °C for 30 s. Melt curve analysis and 1 % agarose gel electrophoresis of PCR products verified that only one product was amplified in each reaction.
mRNA abundance was evaluated for 3–4 replicate samples for each group with three technical replicates for each primer pair. A modified 2−ΔCt method was used to calculate relative mRNA abundance for each gene of interest. After averaging the threshold cycles (Ct) of the technical replicates for each replicate sample, the geometric mean Ct for three reference genes, rpl34, histone h3, and nucleosome assembly protein (nap), was subtracted from the mean Ct for each gene of interest (ΔCt). This value was then log transformed to give relative mRNA abundance (2−ΔCt). One-way ANOVA was used to compare the relative mRNA abundance of each gene of interest (GOI) in the two types of embryos (diapause or non-diapause) at two developmental times (3 d and 6 d) and Fishers LSD was used post hoc for pair-wise comparisons. For illustration purposes, the relative mRNA abundances are shown as normalized values in Figures 3–6. Values were normalized for each gene by dividing the mean value for each embryo type by the mean value for non-diapause 3 d embryos. All statistical tests were performed using raw, non-normalized data.
Figure 3.
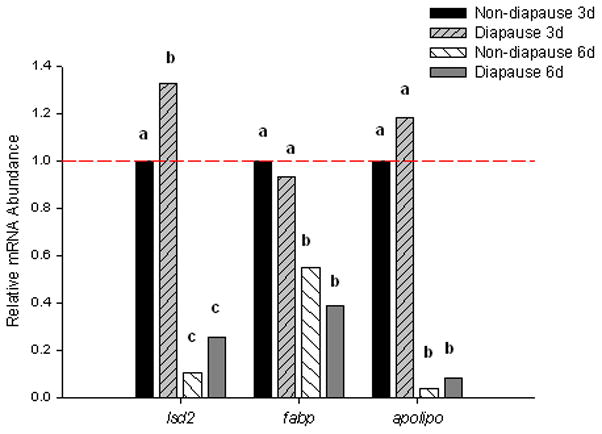
Relative mRNA abundance of genes encoding proteins involved in lipid storage and transport. lsd2 mRNA was 25% higher in pre-diapause embryos than in 3 d non-diapause embryos, but there was not a difference 6 d pov (One-way ANOVA, P < 0.001; Fishers LSD, α = 0.05). fabp mRNA expression decreased 50% from 3 to 6 d pov, but there was no difference between diapause and non-diapause embryos (One-way ANOVA, P < 0.00; Fishers LDS, α = 0.05). apoLp-III abundance decreased between 3 and 6 d pov, but there was no difference between diapause and non-diapause types (One-way ANOVA, P < 0.001; Fishers LSD α = 0.05). Bars show normalized mRNA abundances relative to the mean abundance of non-diapause, 3 d embryos; values above the dashed line are upregulated while values below the dashed line are downregulated. N=3–4 replicates for each embryo type and developmental stage. Ct values for genes of interest were corrected for the geometric mean of reference genes rpl34, histone h3, and nap. Bars marked with different letters were significantly different from other bars for the same gene.
Figure 6.
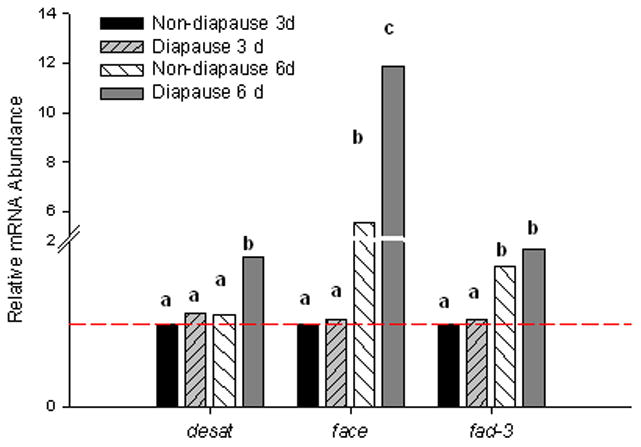
mRNA profiles for genes encoding proteins involved in fatty acid synthesis. desat was higher in diapause embryos at 6 d pov compared to non-diapause 6 d embryos. There was no difference between diapause and non-diapause embryos at 3 d pov (One-way ANOVA, P < 0.001, Fisher’s LSD α = 0.05). fad-3 increased from 3 d to 6 d pov in both diapause and non-diapause embryos, but there was no difference between the two embryo types for either age (One-way ANOVA, P = 0.03; Fishers LSD α= 0.05). face mRNA expression was low in 3 d embryos and increased in both non-diapause and diapause embryos 6 d pov; expression was higher in diapause embryos than non-diapause embryos (One-way ANOVA P < 0.001, Fishers LDS α = 0.05). Bars show normalized mRNA abundances relative to the mean abundance of non-diapause, 3 d embryos; values above the dashed line are upregulated while values below the dashed line are downregulated. N=3–4 replicates for each embryo type and developmental stage. Ct values for genes of interest were corrected for the geometric mean of reference genes rpl34, histone h3, and nap. Bars marked with different letters are significantly different from other bars for the same gene. Break in the y-axis occurs between 2 and 5.
3. Results and Discussion
3.1 Morphology and Physical Characteristics of Diapause and Non-diapause Embryos and Pharate Larvae
Ae. albopictus reared at 21°C completed embryogenesis and became pharate first instar larvae approximately 5 d post-oviposition. Diapause and non-diapause embryos were morphologically similar at 3 d and 6 d pov, which suggested that development proceeded at the same rate regardless of the embryo’s future diapause status. Although they are ready to emerge as free-swimming larvae ~ 5 d post-oviposition, diapause larvae remain enclosed within the chorion, even if they are in an environment (i.e., in water) that supports continued development. Non-diapause, pharate larvae, which have the capacity to emerge, may remain quiescent within the chorion for several months if conditions are inadequate to support larval development (Perez and Noriega, 2012; Clements, 1992). Together, these features of Ae. albopictus embryonic development allowed us to compare morphologically and chronologically matched embryos and allowed us to separate ontogenetic changes from those that are a component of the diapause program.
Although embryonic development was morphologically similar for diapause- destined and diapause-averting (i.e., non-diapause) embryos, there were some significant physical and physiological differences between diapause and non-diapause embryos and pharate larvae. Eggs containing diapause pharate larvae were larger than those containing non-diapause pharate larvae (Fig. 2A). In addition, diapause pharate larvae contained ~30% more total lipid at 10–14 d than non-diapause pharate larvae (Fig. 2B). Together, the increased size and lipid content suggest that diapause pharate larvae have more lipid stores than non-diapause pharate larvae either because of differences in lipid storage and transport pathways, decreased lipid catabolism, increased lipid biosynthesis, or a combination of the above.
Figure 2.
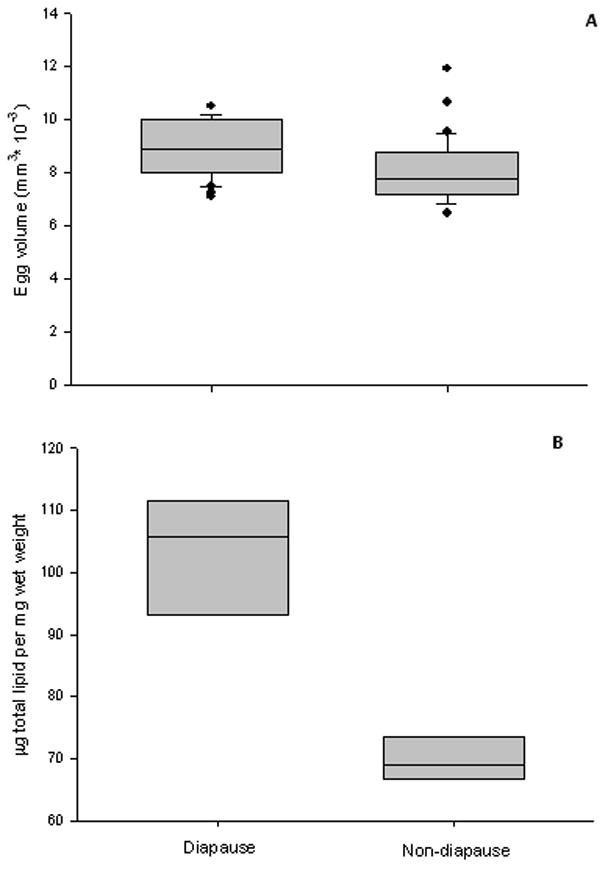
Physical and physiological characteristics of diapause and non-diapause Ae. albopictus eggs. A) Eggs with diapause embryos were larger than non-diapause eggs 9 d post-oviposition (Student’s t-test, P < 0.001) and B) had ~30% more total lipid than non-diapause embryos 10–14 d post-oviposition (Students t-test, P < 0.001).
3.2 Transcriptional Differences between Diapause and Non-diapause Embryos and Pharate Larvae
We used qRT-PCR to measure transcript abundance of 21 candidate genes with known roles in lipid metabolism (Table 1) in pre-diapause and non-diapause embryos (3 d pov) and in diapause and non-diapause pharate first instar larvae (6 d pov). Of the genes analyzed, ten have expression profiles that suggest they contribute to changes in lipid abundance in diapause pharate first instar larvae. To facilitate further analysis and discussion, the genes analyzed were divided into four functional groups: lipid storage, lipid catabolism, unsaturated fatty acid synthesis, and regulators of lipid metabolism.
3.2.1 Lipid Storage
Maternally provided lipids are stored in droplets consisting of a triglyceride core surrounded by a layer of phospholipids and embedded proteins (for review see Arrese and Soulages, 2010). Lipid storage droplet protein 2 (LSD2) is commonly found on the surface of lipid droplets and is required for lipid storage in Drosophila (Teixeira, 2003); increased expression of lsd2 correlates with increased triglyceride levels (Gronke et al. 2003). The Ae. albopictus gene identified as a putative lsd2 was significantly upregulated, approximately 35%, in pre-diapause embryos compared to same age non-diapause embryos (Fig. 3), suggesting that increased lipid storage contributes to higher levels of lipids in diapause pharate larvae. Transcript levels of two genes that encode putative lipid transport proteins, fatty acid binding protein (fabp) and Apolipophorin III (apoLp-III) decreased as a function of developmental time (Figs. 3), an observation consistent with the results of studies on other insect species (Tsuchida et al., 2010). However, these genes do not appear to have a role in diapause.
3.2.2 Lipid Catabolism
Lipid stores are mobilized through combined action of lipases and enzymes of the β-oxidation pathway. Lipases hydrolyze triglycerides to yield glycerol and free fatty acids (FFAs). We identified three putative lipases in Ae. albopictus, designated lip2, lip3, and lip4. There were remarkable ontogenetic changes in the mRNA expression of all three of these genes (Fig. 4), with an 85–90% increase in transcript abundance occurring between 3 d and 6 d in non-diapause embryos. There was also an ontogenetic increase in mRNA expression for all three genes in pre-diapause embryos compared to diapause pharate first instar larvae, but mRNA abundance was at least 50% lower in diapause pharate larvae compared to non-diapause pharate larvae, 6 d pov. Thus the ontogenetic increase observed in non-diapause embryos was at least partly blocked in diapause embryos. Reduced amounts of lipases likely restrict lipolysis in diapause embryos. It is notable that transcription of these genes was not blocked entirely during diapause. It is likely that some lipid catabolism is needed to provide energy required to maintain ion gradients and support other “house-keeping” processes that are needed during diapause to prevent mortality even though pharate larvae are dormant.
Figure 4.
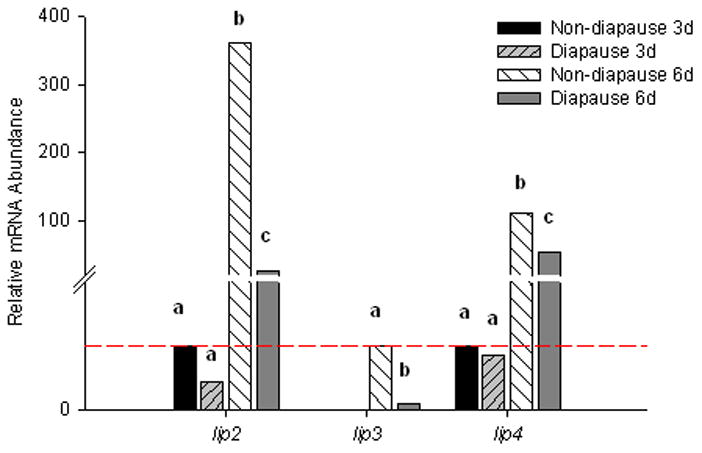
Relative mRNA abundance for three genes encoding proteins involved in lipolysis. Minimal amounts of lip2 and lip4 were present 3 d pov in both diapause and non-diapause embryos; the amount of lip3 was below the limit of detection in 3 d pov and was considered to be zero in statistical tests. mRNA expression of all 3 genes increased by 6 d pov, but there was significantly more mRNA in non-diapause than diapause embryos (One-way ANOVA P < 0.001, Fishers LSD α = 0.05 for all three genes). Bars show normalized mRNA abundances relative to the mean abundance of non-diapause, 3 d embryos; values above the dashed line are upregulated while values below the dashed line are downregulated. N=3–4 replicates for each embryo type and developmental stage. Ct values for genes of interest were corrected for the geometric mean of reference genes rpl34, histone h3, and nap. Bars marked with different letters are significantly different from other bars for the same gene. The break in the Y-axis occurs between 2 and 20
β-oxidation is the primary fatty acid degradation pathway in eukaryotes. We analyzed eight genes predicted to encode enzymes in this pathway: Acetyl-coA synthetase (acs); Carnitine O-palmitoyltransferase (cpt); 3, 2-Trans-enoyl-CoA-isomerase (ech2); Cyclohex-1-ene-1-carboxyl-CoA hydratase (ech3), Acyl-CoA dehydrogenase (acd2); Acyl-CoA dehydrogenase (acd4); Isovaleryl-CoA dehydrogenase (acd5); and 3-Hydroxyacyl-CoA-dehydrogenase (hcdh1). Seven of these genes were differentially expressed, and four had expression patterns that are consistent with regulation of lipid metabolism during diapause. acs catalyzes cytosolic activation of FFAs, which is the first irreversible step of the β-oxidation pathway. mRNA expression of this gene was 30% lower in pre-diapause embryos compared to 3 d non-diapause embryos (Fig. 5A). A similar expression profile was observed for cpt (Fig. 5A), a gene that encodes the enzyme that transports activated FFAs into the mitochondrial matrix, which is the second rate limiting step of the β-oxidation pathway. Together, the downregulation of these two rate-limiting enzymes in pre-diapause embryos suggests that lipid catabolism is reduced in diapause embryos and that it is downregulated well before embryos actually become dormant.
Figure 5.
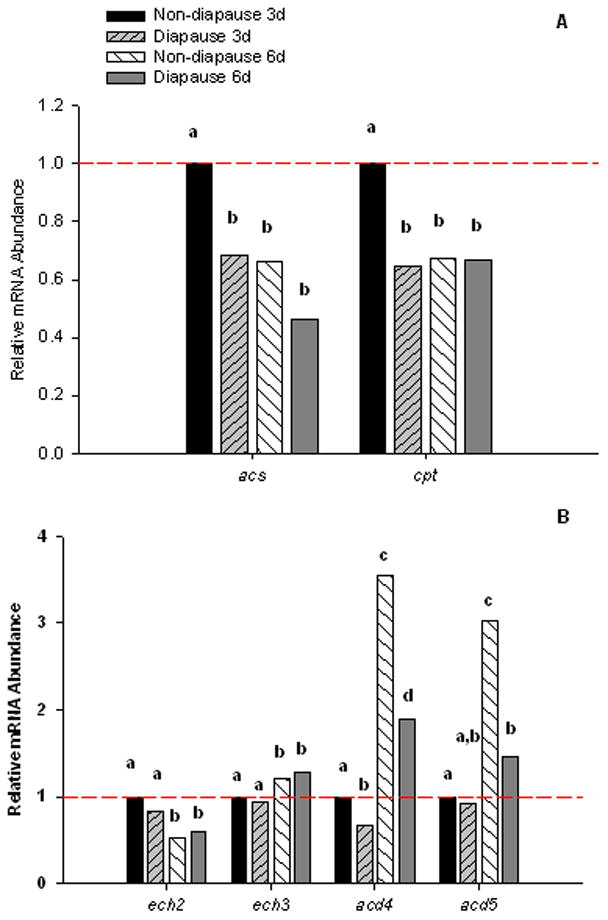
mRNA profiles for genes encoding proteins in the β-oxidation pathway. A) acs and cpt were 30%–35% lower in diapause embryos compared to non-diapause embryos at 3 d pov. There was a decrease in mRNA from 3 to 6 d pov, but no difference between diapause and non-diapause at this age (One-way ANOVA, P = 0.003 and P = 0.015 for acs and cpt, respectively; Fishers LDS α = 0.05). B) ech2 and ech3 were differentially expressed at 3 d compared to 6 d, but there were no differences between diapause and non-diapause at either age (One-way ANOVA, P < 0.001 for both genes; Fishers LSD α = 0.05). acd4 and acd5 were differentially expressed as described in the text (One-way ANOVA, P < 0.001 for both genes; Fisher’s LSD α = 0.05. Bars show normalized mRNA abundances relative to the mean abundance of non-diapause, 3 d embryos; values above the dashed line are upregulated while values below the dashed line are downregulated. N=3–4 replicates for each embryo type and developmental stage. Ct values for genes of interest were corrected for the geometric mean of reference genes rpl34, histone h3, and nap. Bars marked with different letters are significantly different from other bars for the same gene.
In the mitochondrial matrix, acetyl-CoA moieties are removed one at a time by enzymes encoded by genes such as ech2, ech3, acd2, acd4, acd5, and hcdh1. Among these, there were no significant differences in transcript abundance of acd2 or hcdh1 (One-way ANOVA, P = 0.53 and P = 0.15, respectively), and the mRNA abundance of ech2 and ech3 changed only as a function of developmental time (Fig. 5B). Only acd4 and acd5 had expression patterns that suggest a role in regulating lipid catabolism during diapause. mRNA expression of acd4 increases between 3 d and 6 d pov in both diapause and non-diapause embryos; transcript abundance of acd5 increased in non-diapause embryos between 3 and 6 d pov but did not change in diapause embryos. As seen with lipase genes, the ontogenetic increase in the transcription of these genes that occurred in non-diapause embryos was blocked in embryos that entered diapause. These data further support the hypothesis that lipid catabolism is reduced during diapause and likely contributes to the additional lipid stores observed in diapause pharate larvae.
3.2.3 Lipid Biosynthesis
Lipid synthesis, in general, is limited within insect embryos; most lipid content is maternally provided during oogenesis (Canavoso et al., 2001). Nevertheless, we measured transcript abundance of several genes that encode proteins that are involved in fatty acid synthesis, including a putative Acetyl-CoA carboxylase (acc), Δ (9)-desaturase (desat), Acyl-CoA Δ (11) desaturase (fad-3), and Fatty Acyl-CoA elongase (face; Fig. 6). These four genes are differentially expressed during diapause in other species (Sim and Denlinger, 2009; Reynolds and Hand, 2009b), thus we predicted they would have a role in regulating lipid metabolism during diapause in Ae. albopictus embryos. Two of the four genes analyzed, desat and face, were differentially expressed in diapause embryos and may contribute to the increase in total lipids during diapause (Fig. 6). fad-3 mRNA abundance changes over time, but there is no difference between diapause and non-diapause embryos or pharate larvae (Fig. 6). There was not a significant change in transcript abundance of acc (One-way ANOVA, P = 0.07).
Δ(9) desaturases (desat) are rate-limiting enzymes that catalyze the synthesis of monounsaturated fatty acids (MUFAs) from saturated fatty acids. The presence of MUFAs in cell membranes is important for maintaining fluidity, a feature critical for cold resistance during winter (Tiku et al. 1996; Cossins et al. 2006; Hochachka and Somero, 2002), and they are critical for efficient lipid storage in species ranging from worms to humans (Castro et al., 2012 and Hulver et al. 2005). Moreover, the transcript abundance of desat genes and enzyme activity of Δ (9) desaturases correlate with the amount of fatty-acids and the rate of lipid catabolism (Castor et al. 2012). Given the role of Δ (9) desaturases as key regulators of lipid metabolism, we predict the increased expression of desat is critical for lipid conservation during diapause in Ae. albopictus. In addition, upregulated transcription of a Δ (9) desaturase in diapause cricket embryos (Reynolds and Hand, 2009b) suggests changes in the amount of these enzymes may be a common feature of insect diapause.
Fatty Acyl-CoA Elongase (FACE) mRNA increased between 3 and 6 d post-oviposition; at 6 d pov it was significantly upregulated in diapause pharate larvae compared to non-diapause pharate larvae (Fig. 6). In insects, FACE catalyzes the synthesis of very long chain fatty acids that contribute to desiccation resistance in flies and cockroaches (Blomquist et al. 1987; Vaz et al. 1988; Juarez, 1994 and 2004; Yoder et al. 1995). mRNA expression of face is known to be upregulated in oocytes produced by Ae. albopictus females reared in diapause-inducing conditions compared to those from long-day reared females (Urbanski et al. 2010b). We predict that the increased mRNA expression of face in oocytes and pharate larvae contributes to an increase in surface hydrocarbons and a decrease in water loss from Ae. albopictus embryos during diapause (Urbanski et al. 2010b). Thus, increased face likely contributes to the increase in total lipid content of diapause pharate larvae. However, its function is perhaps more closely tied to desiccation resistance rather than to the storage of fuel lipids.
3.2.4 Regulators of Lipid Metabolism
In addition to analyzing transcription abundance of key lipid metabolism genes, we measured transcript abundance of genes that encode subunits of the AMP-activated protein kinase (AMPK). This kinase is an upstream regulator of many metabolic enzymes including acc and cpt, and it is essential for regulation of lipid catabolism in C. elegans dauers (Narbonne and Roy, 2009). There were no significant differences in transcript abundance for any of the three subunits of the AMPK heterotrimer (ampka-alpha, P = 0.90; ampk-beta, P = 0.481; ampk-gamma, P = 0.087). However, we cannot rule out a regulatory role for AMPK during diapause in Ae. albopictus because this enzyme is regulated via phosphorylation; there may be differences in the activity of this enzyme in diapause pharate larvae compared to non-diapause pharate larvae that are beyond the scope of this study that focuses on transcripts.
4. Conclusions and Significance
Understanding the physiological and molecular basis for diapause in Ae. albopictus will allow us to construct a more accurate model to predict seasonal outbreaks of this medically-important pest (Gong, et al., 2011; Cailly et al., 2012), and will also enable us to improve our ability to identify the thermal limits of this species and to predict its potential habitat range (Travis et al., 1999; Feder et al., 2000). Among the 21 genes we examined in relation to lipid metabolism, 10 were differentially expressed in a manner that suggests they have a role in diapause. Specifically, we found significant changes in the transcript abundance of genes that regulate lipid storage (lsd2), lipolysis (lip2, lip3, and lip4), β-oxidation (acs, cpt, acd4, and acd5), and unsaturated fatty acid synthesis (desat, and face). Together these data suggest that transcriptional changes in multiple processes contribute to increased amounts of lipids in diapause embryos.
Several studies have examined the transcriptional basis for diapause in insect embryos (Blitvich, et al., 2001; Reynolds and Hand 2009b; Sashibhushan et al. 2012). In addition, there is a large body of literature exploring physiological changes, including the conservation of lipids, that occur for a variety of species in different diapause stages (e.g., Visscher, 1976; Lee and Denlinger, 1996; Lee et al., 1997; Reynolds and Hand, 2009a). However, there are few studies that show a clear link between transcriptional and physiological changes. Based on what we know about lipid storage during diapause and the well-established functional role of homologs of the genes considered in this study, these data provide new insight into the molecular pathways affecting lipid metabolism during diapause in Ae. albopictus.
Prior research on Ae. albopictus diapause has focused on maternal regulation of this dormant state by comparing oocytes produced by females reared under diapause inducing, short-day conditions to oocytes from females reared under diapause-averting, long-day conditions (Urbanski et al. 2010a, b; Poelchau et al. 2011). The current research on Ae. albopictus builds on that foundation by focusing on diapause-destined embryos and on the diapausing stage, the pharate first-instar larvae. Some differences in total lipid content are likely due to increased maternal provisioning, but other aspects appear to be under the control of the embryos and pharate larvae themselves. Differences in transcript abundance of key genes were found in embryos 3 d pov; at this point, germ-band extension is complete and dorsal closure is progressing. At 3 d, embryos are well past the point of mid-blastula transition, the point in embryogenesis when zygotes stop utilizing maternally provided transcripts and begin to make their own mRNA (Schier, 2007); thus, it is unlikely that the observed difference in transcript abundance are due to differences in maternally provided transcripts.
Supplementary Material
Highlights.
Pre-diapause development is morphologically similar to non-diapause embryogenesis.
Diapause-destined Ae. albopictus embryos are 30% larger than non-diapause embryos.
Diapause pharate larvae contain 30% more lipids than non-diapause pharate larvae.
Transcription of lsd2, a gene for lipid storage protein, increases before diapause.
Genes for enzymes lipid catabolism pathways are suppressed in early diapause.
Acknowledgments
We thank Dr. Lauren McIntyre of the University of Florida for many helpful comments and suggestions during the writing of this manuscript. This research was supported in party by NIH grant R21 AI081041.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armbruster P, Bradshaw WE, Ruegg K, Holzapfel CM. Geographic variation and the evolution of reproductive allocationin the pitcher-plant mosquito, Wyeomyia smithii. Evolution. 2001;55:439–444. doi: 10.1111/j.0014-3820.2001.tb01306.x. [DOI] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annual Review of Entomology. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict M, Levine R, Hawley W, Lounibos L. Spread of the tiger: global risk of invasion by the mosquito Aedes albopictus. Vector-Borne and Zoonotic Diseases. 2007;7:76–87. doi: 10.1089/vbz.2006.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitvich BJ, Rayms-Keller A, Blair CD, Beaty BJ. Identification and sequence determination of mRNAs detected in dormant (diapausing) Aedes triseriatus mosquito embryos. DNA Sequence. 2001;12:197–202. doi: 10.3109/10425170109080775. [DOI] [PubMed] [Google Scholar]
- Blomquist GJ, Nelson DR, de Renobales M. Chemistry, biochemistry and physiology of insect cuticular lipids. Archives of Insect Biochemistry and Physiology. 1987;6:227–265. [Google Scholar]
- Braune HJ. Effects of temperature on rates of oxygen consumption during morphogenesis and diapause in the egg stage of Leptoterna dolobrata (Heteropters, Miridae) Oecologia. 1976;25:77–87. doi: 10.1007/BF00345035. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Noan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cailly P, Tran A, Balenghien T, L’Ambert G, Toty C, Ezanno P. A climate-driven abundance model to assess mosquito control strategies. Ecological Modelling. 2012;227:7–17. [Google Scholar]
- Canavoso LE, Jouni AE, Karnas KJ, Pennington JE, Wells MA. Fat metabolism in insects. Annual Review of Nutrition. 2001;21:23–46. doi: 10.1146/annurev.nutr.21.1.23. [DOI] [PubMed] [Google Scholar]
- Castro C, Sar F, Shaw WR, Mishima M, Miska EA, Griffin JL. A metabolomic strategy defines the regulation of lipid content and global metabolism by Delta-9 desaturases in Caenorhabditis elegans. BMC Genomics. 2012;13:36. doi: 10.1186/1471-2164-13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN. The Biology of Mosquitoes. 1. Chapman and Hall; London: 1992. [Google Scholar]
- Cossins A, Fraser J, Hughes M, Gracey A. Post-genomic approaches to understanding the mechanisms of environmentally induced phenotypic plasticity. Journal of Experimental Biology. 2006;209:2328–2336. doi: 10.1242/jeb.02256. [DOI] [PubMed] [Google Scholar]
- Denlinger DL. Regulation of diapause. Annual Review of Entomology. 2002;47:93–122. doi: 10.1146/annurev.ento.47.091201.145137. [DOI] [PubMed] [Google Scholar]
- Denlinger DL, Willis JH, Fraenkel G. Rates and cycles of oxygen consumption during pupal diapause in Sarcophaga flesh flies. Journal of Insect Physiology. 1972;18:871–882. doi: 10.1016/0022-1910(72)90026-1. [DOI] [PubMed] [Google Scholar]
- Farnesi LC, Martins AJ, Valle D, Rezende GL. Embryonic development of Aedes aegypti (Diptera: Cullicidae): influence of different constant temperatures. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2009;104:124–126. doi: 10.1590/s0074-02762009000100020. [DOI] [PubMed] [Google Scholar]
- Feder ME, Bennett AF, Huey RB. Evolutionary Physiology. Annual Review of Ecology and Systematics. 2000;31:315–341. [Google Scholar]
- Gibbs A, Mousseau TA, Crowe JH. Genetic and acclamatory variation in biophysical properties of insect cuticle lipids. Proceedings of the National Academy of Science, USA. 1991;88:7257–7260. doi: 10.1073/pnas.88.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, DeGaetano AT, Harrington LC. Climate-based models for West Nile Culex mosquito vectors in the Northeastern US. International Journal of Biometerology. 2011;55:435–446. doi: 10.1007/s00484-010-0354-9. [DOI] [PubMed] [Google Scholar]
- Gronke S, Beller M, Fellert S, Ramakrishnan H, Jackle H, Kuhnlein RP. Control of fat storage by a Drosophila PAT domain protein. Current Biology. 2003;13:603–606. doi: 10.1016/s0960-9822(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Hahn DA, Denlinger DL. Energetics of insect diapause. Annual Review of Entomology. 2011;56:103–21. doi: 10.1146/annurev-ento-112408-085436. [DOI] [PubMed] [Google Scholar]
- Hahn DA, Denlinger DL. Meeting the energetic demands of insect diapause: nutrient storage and utilization. Journal of Insect Physiology. 2007;53:760–773. doi: 10.1016/j.jinsphys.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Hand SC, Menze MA, Borcar A, Patil Y, Covi JA, Reynolds JA, Toner M. Metabolic restructuring during energy-limited states: insights from Artemia fransicsana and other animals. Journal of Insect Physiology. 2011;57:584–594. doi: 10.1016/j.jinsphys.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Craig GB. Cold acclimation, diapause, and geographic origin affect cold hardiness in eggs of Aedes albopictus (Diptera: Culicidae) Journal of Medical Entomology. 1994;31:192–201. doi: 10.1093/jmedent/31.2.192. [DOI] [PubMed] [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr Aedes albopictus in North America: Probably introduction in used tires from northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Hazel JR. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annual Review of Physiology. 1995;57:19–42. doi: 10.1146/annurev.ph.57.030195.000315. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Somero GN. Biochemical Adaptation. New York: Oxford University Press; 2002. [Google Scholar]
- Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated steroyl-CoA desaturase-1 expression inskeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metabolism. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Denlinger DL. A role for edcysteroids in the induction and maintenance of the pharate first instar diapause of the gypsy moth, Lymantria dispar. Journal of Insect Physiology. 1997;43:289–296. doi: 10.1016/s0022-1910(96)00082-0. [DOI] [PubMed] [Google Scholar]
- Lee KY, Valaitis AP, Denlinger DL. Further evidence that diapause in the gypsy moth, Lymantria dispar, is regulated by ecdysteroids: a comparison of diapause and nondiapause strains. Journal of Insect Physiology. 1997;43:897–803. doi: 10.1016/s0022-1910(97)00054-1. [DOI] [PubMed] [Google Scholar]
- Lees AD. Physiology of diapause in Arthropods. Cambridge University Press; Cambridge: 1955. [Google Scholar]
- Lounibos L. Invasions by insect vectors of human disease. Annual Review of Entomology. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Escher RL, Lourenço-de-oliveira R. Asymmetric Evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Annals of Entomological Society of America. 2003;96:512–518. [Google Scholar]
- MacRae TH. Gene expression, metabolic regulation, and stress tolerance during diapause. Cellular and Molecular Life Sciences. 2010;67:2405–2424. doi: 10.1007/s00018-010-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A, Oda T, Wada Y. Studies on the egg diapause and overwintering of Aedes albopictus in Nagasaki. Tropical Medicine. 1981;23:79–90. [Google Scholar]
- Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–214. doi: 10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- Perez MH, Noriega FG. Aedes aegypti pharate 1st instar quiescence affects larval fitness and metal tolerance. Journal of Insect Physiology. 2012 doi: 10.1016/j.jinsphys.2012.03.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchau MF, Reynolds JA, Denlinger DL, Elsik CG, Armbruster PA. A high confidence, de novo, transcriptome of the Asian tiger mosquito, Aedes albopictus, to identify candidate transcripts for diapause preparation. BMC Genomics. 2011;12:619. doi: 10.1186/1471-2164-12-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakshpal R. Respiratory metabolism during embryogenesis of a diapause species of field cricket, Gryllus pennsylvanicus Burmeister (Orthoptera, Gryllidae) Journal of Insect Physiology. 1962;8:217–221. [Google Scholar]
- Reynolds JA, Hand SC. Decoupling development and energy flow during embryonic diapause in the cricket, Allonemobius socius. Journal of Experimental Biology. 2009a;212:2065–2074. doi: 10.1242/jeb.027359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JA, Hand SC. Embryonic diapause highlighted by differential expression of mRNAs for ecdysteroidogenesis, transcription and lipid sparing in the cricket Allonemobius socius. Journal of Experimental Biology. 2009b;212:2075–2084. doi: 10.1242/jeb.027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende GL, Martins AJ, Gentile C, Farnesi LC, Pelajo-Machado M, Peixoto AA, Vale D. Embryonic desiccation resistance in Aedes aegypti: presumptive role of the chitinized serosal cuticle. BMC Developmental Biology. 2008;8:182. doi: 10.1186/1471-213X-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashibhushan S, Ponnuvel KM, Vijayaprakash NB. Diapause specific gene expression in the eggs of multivoltine silkworm Bombyx mori, identified by suppressive subtractive hybridization. Comparative Biochemistry and Physiology B. 2012 doi: 10.1016/j.cbpb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Schier AF. The maternal-zygotic transition: death and birth of RNAs. Science. 2007;316:406–407. doi: 10.1126/science.1140693. [DOI] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. Transcription profiling and regulation of fat metabolism in genes in diapausing adults of the mosquito Culex pipiens. Physiological Genomics. 2009;39:202–209. doi: 10.1152/physiolgenomics.00095.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sota T, Mogi M. Survival time and resistance to dessication of diapause and non-diapause eggs of temperate Aedes albopictus. Entomol Exp Appl. 1992;63:155–161. [Google Scholar]
- Tauber M, Tauber CA. Insect seasonality – diapause maintenance, termination, and post-diapause development. Annual Review of Entomology. 1976;21:81–107. [Google Scholar]
- Teixeira L, Rabouille C, Rorth P, Ephrussi A, Vanzo NF. Drosophila perilipin/ADRP homologue Lsd2 regulates lipid metabolism. Mechisms of Development. 2003;120:1071–81. doi: 10.1016/s0925-4773(03)00158-8. [DOI] [PubMed] [Google Scholar]
- Tiku PE, Gracey AY, Macartney AI, Beynon RJ, Cossins AR. Cold-induced expression of delta 9-desaturase in carp by transcriptional and posttranslational mechanisms. Science. 1996;271:815–818. doi: 10.1126/science.271.5250.815. [DOI] [PubMed] [Google Scholar]
- Trpis M. A new bleaching and decalcifying method for general use in zoology. Canadian Journal of Zoology. 1970;48:892–893. [Google Scholar]
- Tsuchida K, Yokoyama T, Sakudoh T, Katagiri C, Tsurumaru S, Takada N, Fujimoto H, Zeigler R, Iwano H, Hamano K, Yaginuma T. Apolipophorin-III expression and low density lipophorin formation during embryonic development of the silkwormBombyx mori. Comparative Biochemistry and Physiology B. 2010;155:363–370. doi: 10.1016/j.cbpb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Urbanski JM, Aruda A, Armbruster P. A transcriptional element of the diapause program in the Asian tiger mosquito, Aedes albopictus, identified by suppressive subtractive hybridization. Journal of Insect Physiology. 2010a doi: 10.1016/j.jinphys.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Urbanski JM, Benoit JB, Michaud MR, Denlinger DL, Armbruster P. The molecular physiology of increased egg dessication resistance during diapause in the invasive mosquito, Aedes albopictus. Proceedings of the Royal Society, B. 2010b;277:2683–2692. doi: 10.1098/rspb.2010.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel E. Fuel metabolism of the mosquito (Culex quinquefasciatus) embryo. Journal of Insect Physiology. 1993;39:831–833. [Google Scholar]
- Van Handel E. Rapid determination of total lipids in mosquitoes. Journal of the American Mosquito Control Association. 1985;1:302–304. [PubMed] [Google Scholar]
- Visscher SN. The embryonic diapause of Aulocara elliotti (Orthoptera, Acrididiae) Cell and Tissue Research. 1976;174:433–452. doi: 10.1007/BF00232831. [DOI] [PubMed] [Google Scholar]
- Vital W, Rezende GL, Abreu L, Moraes J, Lemos FJA, da Silva Vaz I, Jr, Logullo C. Germ band retraction as a landmark in glucose metabolism during Aedes aegypti embryogenesis. BMC Developmental Biology. 2010;10:25. doi: 10.1186/1471-213X-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RL. Observations on the influence of photoperiod on egg diapause in Aedes albopictus Skuse. Acta Entomologica Sinica. 1966;15:75–77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


