Abstract
Background
Cardiovascular dysfunction, characterized by reduced cardiac contractility and depressed endothelium-dependent vascular relaxation, is common in severe sepsis. Although it is known that ghrelin produces beneficial effects following various adverse circulatory conditions, it remains unknown whether ghrelin increases cardiac contractility and improves vascular responsiveness to vasoactive agents in severe sepsis.
Methods
Male adult rats were subjected to sepsis by cecal ligation and puncture (CLP). At 5 h after CLP, a bolus intravenous injection of 2 nmol ghrelin was followed by a continuous infusion of 12 nmol ghrelin via a primed mini-pump over 15 h. At 20 h after CLP (i.e., severe sepsis), the maximal rates of ventricular pressure increase (+dP/dtmax) and decrease (−dP/dtmax) were determined in vivo. In additional groups of animals, the thoracic aortae were isolated at 20 h after CLP. The aortae were cut into rings, and placed in organ chambers. Norepinephrine (NE) was used to induce vascular contraction. Dose responses for an endothelium-dependent vasodilator, acetylcholine (ACh), and an endothelium-independent vasodilator, nitroglycerine (NTG) were carried out.
Results
+dP/dtmax and −dP/dtmax decreased significantly at 20 h after CLP. Treatment with ghrelin significantly increased +dP/dtmax and −dP/dtmax by 36% (P<0.05) and 35% (P<0.05), respectively. Moreover, NE-induced vascular contraction and endothelium-dependent (ACh-induced) vascular relaxation decreased significantly at 20 h after CLP. Administration of ghrelin, however, increased NE-induced vascular contraction and ACh-induced vascular relaxation. In contrast, no significant reduction in NTG-induced vascular relaxation was seen in rats with severe sepsis irrespective of ghrelin treatment.
Conclusions
Ghrelin may be further developed as a useful agent for maintaining cardiovascular stability in severe sepsis.
Keywords: Ghrelin, Sepsis, Heart performance, Vascular endothelial cell function
INTRODUCTION
Despite advances in our understanding of the underlying mechanisms of sepsis, severe sepsis continues to account for an increasing number of deaths in critically ill patients. It is the most common cause of death in the non-cardiac intensive care units [1–5]. The incidence of severe sepsis in the United States is estimated at 3.0 cases per 1000 population per year, resulting in approximately 750,000 cases per year in the United States, with a mortality rate approaching 30% [6]. As the American population ages, the incidence of sepsis is projected to increase since the incidence and mortality rate of sepsis rise steadily with age [2,6]. Over the past 20 years, tens of thousands of patients have been enrolled in sepsis clinical trials with little success. An analysis of hospital records indicates that the total number of patients who have died of sepsis is actually increasing [2]. The resources required to care for critically ill patients with severe sepsis create an enormous burden on the health care system. As such, there is an urgent unmet medical need for an effective therapy for septic patients.
Cardiovascular dysfunction, characterized by reduced cardiac contractility and depressed endothelium-dependent vascular relaxation, is common in severe sepsis [7–9]. If left untreated, these derangements result in tissue hypoperfusion and contribute to the development of multiple organ failure and ultimately, death. Ghrelin, a novel gastrointestinal hormone, was first identified in 1999 as an endogenous ligand for the growth hormone secretagogue receptor type 1a (GHSR-1a, i.e., ghrelin receptor) [10]. Although ghrelin was initially recognized for its growth hormone releasing property, recent studies have indicated that the cardiovascular system is also an important target for ghrelin [11]. Our recent studies have shown that ghrelin levels decrease significantly during the late stage of sepsis. Decreased ghrelin production heralds the hypodynamic response of severe sepsis [12]. Administration of ghrelin mitigates organ injury, increases tissue perfusion, and improves survival in a rat model of polymicrobial sepsis induced by cecal ligation and puncture (CLP) [13,14]. However, it remains unknown whether ghrelin maintains cardiovascular stability in severe sepsis. The aim of this study, therefore, was to determine whether or not administration of ghrelin after the onset of sepsis improves heart performance and attenuates the impaired endothelium-dependent vascular relaxation.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats (275–325g) purchased from Charles River Laboratories (Wilmington, MA) were housed in a light-controlled room with a 12-h light/dark cycle and allowed free access to water and standard rat chow. Rats were acclimatized for at least 1 week before experimentation. All animals received humane care in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of The Feinstein Institute for Medical Research.
Animal model of polymicrobial sepsis
Prior to the induction of sepsis, rats were fasted overnight, but allowed water ad libitum. Rats were anesthetized with isoflurane inhalation and the ventral neck, abdomen and groin were shaved and washed with 10% povidone iodine. Cecal ligation and puncture (CLP) was performed as we previously described [14–16]. Briefly, a 2-cm midline abdominal incision was performed. The cecum was exposed, ligated just distal to the ileocecal valve to avoid intestinal obstruction, punctured twice with an 18-gauge needle, squeezed slightly to allow a small amount of fecal matter to flow from the holes, and then returned to the abdominal cavity, following which the abdominal incision was closed in layers. Sham-operated animals (i.e., control animals) underwent the same procedure with the exception that the cecum was neither ligated nor punctured. The animals were resuscitated with 3 ml/100g BW normal saline subcutaneously immediately after surgery. The animals were then returned to their cages with free access to food and water. Clinically, the cardiovascular response to sepsis is characterized by an early, hyperdynamic phase followed by a late, hypodynamic phase [17]. If fluid resuscitation is not provided following CLP, the animals will not demonstrate a hyperdynamic phase circulatory state [18]. The volume of resuscitation fluid in this study was chosen based on our previous experience, which showed that with this amount of resuscitation fluid the animals had an early, hyperdynamic response and a late, hypodynamic response [12].
Administration of ghrelin
Rat ghrelin (Phoenix Pharmaceuticals, Belmont, CA) was dissolved in normal saline to a final concentration of 100 μM. 200-μl mini-pumps (Alzet, infusion rate 8 μl/h) were primed with ghrelin solution or vehicle (normal saline) for 3 h prior to implantation. At 5 h after CLP, the animals were anesthetized again with isoflurane inhalation for ghrelin or vehicle administration. Briefly, after a slow intravenous bolus injection of 2 nmol ghrelin (or 200 μl vehicle), the mini-pump was then connected to a jugular venous catheter and implanted subcutaneously. Various measurements were performed at 20 h after CLP (i.e., 15 h after implantation of the mini-pump). The total dose of ghrelin each rat received was 14 nmol (i.e., ~45 nmol/kg BW). The dose of ghrelin was determined based on our previous experience [13,16]. Our previous study had shown that plasma levels of ghrelin were 47 ng/ml at 15 min after the bolus injection and maintain above 1 ng/ml even at15 h after the bolus injection, which was more than 25 fold higher than non-treated animals (i.e., 0.04 ng/ml) [13].
In vivo heart performance measurement
At 20 h after CLP or sham-operation, the animals were anesthetized with isoflurane inhalation. A steady state of sedation was maintained with a subsequent intravenous injection of sodium pentobarbital (~30 mg/kg BW). The right carotid artery was cannulated with PE-50 tubing. The catheter was then connected to a heart performance analyzer (HPA; Model 410, Digi-Med, Louisville, KY) and advanced into the left ventricle. The correct placement of the catheter was verified by the end diastolic pressure reading on the HPA and confirmed using a pressure transducer and polygraph. The exact position of the catheter tip in the left ventricle was verified at autopsy. The catheter was secured in place using 5-0 silk. After catheter placement in the left ventricle, the animal was allowed to stabilize for 5 min before any measurements were conducted. After stabilization, left ventricular performance parameters including the maximal rates of ventricular pressure increase (+dP/dtmax, mmHg/sec), decrease (−dP/dtmax), ventricular peak systolic pressure (VPSP), and ventricular end diastolic pressure (VEDP) were determined using HPA.
Preparation of blood vessel rings
In additional groups of animals, the thoracic cavity was opened and the thoracic aorta was rapidly removed at 20 h after CLP or sham-operation. The blood vessel was then placed in ice-cold Krebs-Ringer bicarbonate solution (composition: NaCl, 118.3 mM; KCl, 4.7 mM; CaCl2, 2.5 mM; MgSO4, 1.2 mM; KH2 PO4, 1.2 mM; NaHCO3, 25.0 mM; Ca-EDTA, 0.026 mM; glucose, 11.1 mM), which was aerated with 95% oxygen:5% CO2. The thoracic aorta was dissected with care to prevent any damage to vascular endothelial cells and cut into rings of 2.5 mm in length with similar ring weight (2.0 mg each). The vessel rings were mounted on two specimen holders and placed in glass organ chambers containing 20 ml of aerated Krebs-Ringer bicarbonate solution at 37 °C. One holder was stationary and the other was connected to a Digi-Med® Tissue Force Analyzer (Model 410, Micro-Med, Louisville, KY). The vascular rings were incubated for 45 min at a tension of ~1 g, during which time the organ chamber was rinsed every 15 min with the aerated Krebs-Ringer bicarbonate solution. When basal tension was stable, the vascular rings were contracted with 2 × 10−7 M norepinephrine (NE, Sigma Chemical, St. Louis, MO). An endothelium-dependent vasodilator, acetylcholine (ACh) (Sigma Chemical; concentration range from 10−8 to 5 × 10−5 M) or an endothelium-independent vasodilator, nitroglycerin (NTG) (American Regent Laboratories, Shirley, NY; concentration range from 10−8 to 5 × 10−5 M) was applied cumulatively thereafter, respectively. Following each series of agent additions, the ring preparations were washed with aerated Krebs-Ringer bicarbonate solution and allowed to reequilibrate for at least 30 min. At the end of the experiment, the contractive responses of the vessel ring preparations were checked by 5 × 10−6 M NE and no significant decrease in vascular tension induced by this agent in both sham and septic animals was found. The doses of NE, ACh and NTG used in this study were based on our previous experience [19].
Measurement of blood gas values
Arterial blood samples (0.4 ml in a heparinized glass syringe) were collected for pH and blood gas measurement at 20 h after CLP or sham-operation. Blood gas values were analyzed with a NPT7 Blood Gas Analyzer (Radiometer Copenhagen, DK 2700 Brønshøj). The blood gas values were adjusted to standard atmospheric pressure and temperature.
Statistical analysis
All data were expressed as means ± SE and compared by one-way or two-way ANOVA and the Student-Newman-Keuls test. Differences in values were considered significant if P < 0.05.
RESULTS
Effects of ghrelin administration on the left ventricular contractility
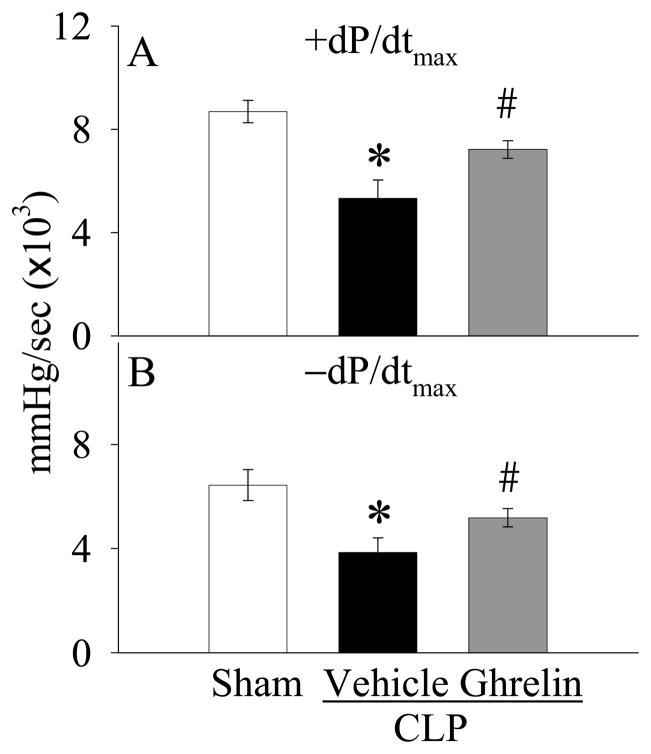
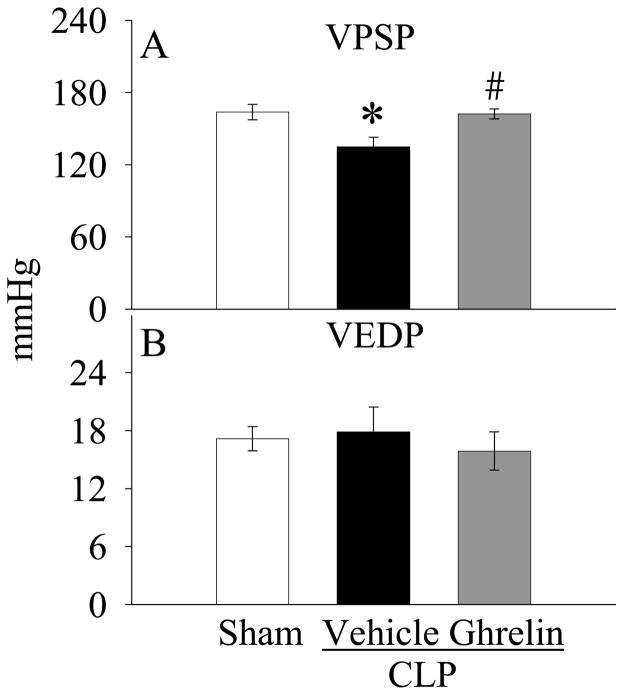
As shown in Figure 1A, the values of +dP/dtmax decreased by 39% at 20 h after CLP as compared with those in sham-operated animals (P<0.05). Ghrelin administration increased the depressed +dP/dtmax by 36% (P<0.05, Fig. 1A) and there was no significant difference in +dP/dtmax values between sham-operated and ghrelin-treated septic animals at 20 h after CLP (Fig. 1B). Similarly, −dP/dtmax values in vehicle-treated septic animals were significantly lower than those in the sham-operated animals (P<0.05, Fig. 1B). Treatment with ghrelin significantly improved the depressed −dP/dtmax (P<0.05, Fig. 1B). Moreover, the significantly decreased levels of VPSP in vehicle-treated septic animals were improved by ghrelin treatment (P<0.05, Fig. 2A). On the other hand, the levels of VEDP were not altered at 20 h after CLP irrespective of ghrelin treatment as compared with those in sham-operated animals (Fig. 2B). No differences were observed in other cardiac parameters including heart rate, duration of relaxation and Tau (data not shown).
Figure 1.
Alterations in the maximal rates of ventricular pressure increase (+dP/dtmax, A) and decrease (−dP/dtmax, B) in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Data are expressed as means ± SE (n=6–7/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method: *P<0.05 versus sham-operated animals; #P<0.05 versus CLP animals treated with vehicle.
Figure 2.
Alterations in the ventricular peak systolic pressure (VPSP, A) and ventricular end diastolic pressure (VEDP, B) in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Data are expressed as means ± SE (n=6–7/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method: *P<0.05 versus sham-operated animals; #P<0.05 versus CLP animals treated with vehicle.
Effect of ghrelin administration on NE-induced vascular contraction
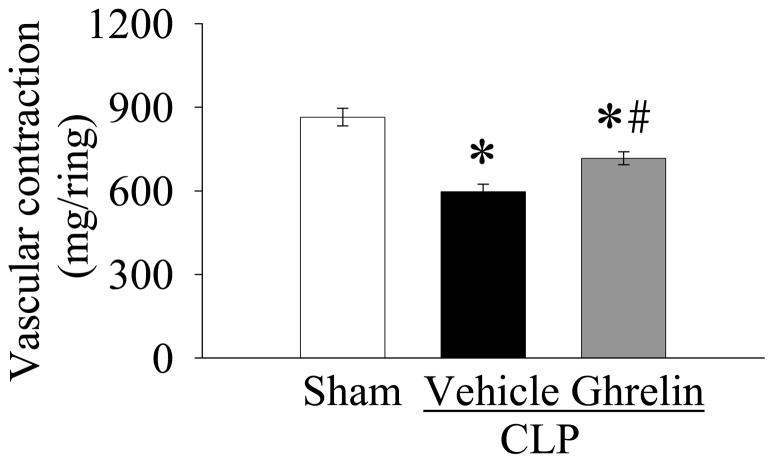
NE is a potent α1-adrenergic agonist with a weaker but still significant β-adrenergic agonist effect. It increases blood pressure mainly by increasing systemic vascular resistance as a consequence of its vasoconstrictive effects. It is a common vasopressor agent used in patients in septic shock who do not respond to fluid resuscitation [20]. As shown in Figure 3, the vascular contraction induced by 2 × 10−7 M NE was 864 ± 31.8 mg/ring in sham-operated animals. NE-induced vascular contraction decreased by 31% at 20 h after CLP in vehicle-treated animals (P<0.05, Fig. 3). Ghrelin treatment markedly increased NE-induced vascular contraction in septic animals, however, it was still significantly lower than that in sham-operated animals (P<0.05, Fig. 3).
Figure 3.
Alterations in NE (2 × 10−7 M)-induced vascular contraction in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Data are expressed as means ± SE (n=8/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method: *P<0.05 versus sham-operated animals; #P<0.05 versus CLP animals treated with vehicle.
Effect of ghrelin administration on ACh-induced vascular relaxation
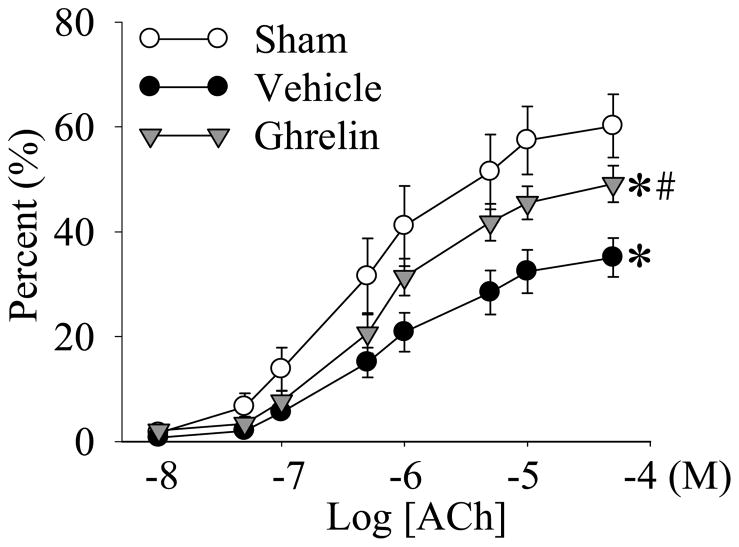
ACh induces vascular relaxation via the stimulation of the release of endothelium-derived nitric oxide (NO) [21]; therefore, it was used to measure endothelium-dependent vasodilation. Nitroglycerine (NTG), on the other hand, directly induces vascular relaxation [21]; therefore, it was used to measure endothelium-independent vasodilation. As shown in Figure 4, there was almost no vascular relaxation in response to 10−8 M ACh in both sham and septic animals. However, the vascular relaxation increased when the concentration of ACh was increased. In sham operated animals, ACh-induced relaxation in the aortic rings was 57.4 ± 6.5% and 60.2 ± 6.0% at concentrations of 1 × 10−5 and 5 × 10−5 M ACh, respectively. ACh-induced vascular relaxation was significantly depressed at 20 h after the onset of sepsis (P<0.05, Fig. 4). Ghrelin administration significantly improved ACh-induced vascular relaxation at 20 h after CLP (P<0.05, Fig. 4).
Figure 4.
Cumulative dose responses to various concentrations of acetylcholine (ACh) in the aortic rings from sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). The data are presented as percent (%) vascular relaxation, expressed as means ± SE (n=8/group), and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method: *P<0.05 versus sham-operated animals; #P<0.05 versus CLP animals treated with vehicle.
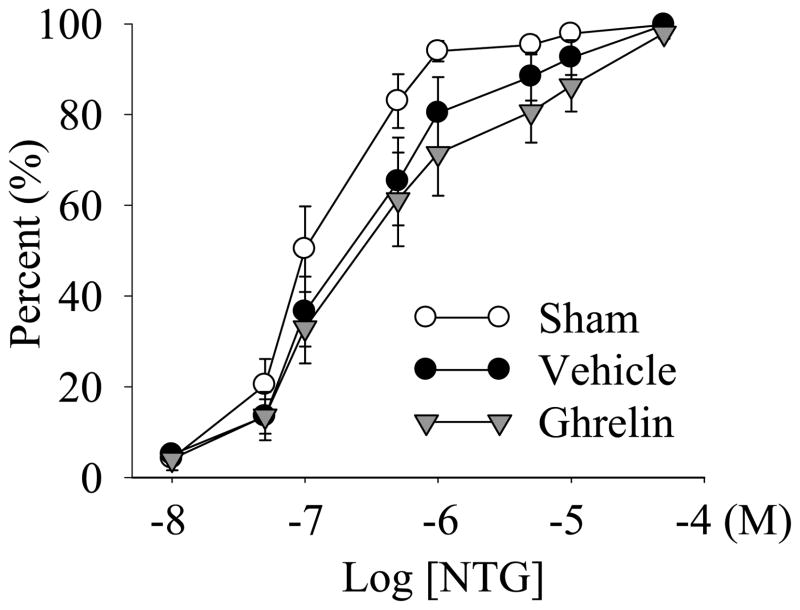
Effect of ghrelin administration on NTG-induced vascular relaxation
As shown in Figure 5, 100% vascular relaxation in the aortic rings was observed at 5 × 10−5 M NTG in sham-operated as well as septic animals. There was no significant difference in NTG-induced vascular relaxation rings in sham animals and CLP animals with saline or ghrelin treatment at 20 h after the onset of sepsis (Fig. 5).
Figure 5.
Cumulative dose responses to various concentrations of nitroglycerin (NTG) in the aortic rings from sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). The data are presented as percent (%) vascular relaxation, expressed as means ± SE (n=8/group), and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method: *P<0.05 versus sham-operated animals; #P<0.05 versus CLP animals treated with vehicle.
Effects of ghrelin administration on arterial blood gases
As shown in Table 1, arterial blood pH, actual bicarbonate (HCO−3), and base excess (ABE and SBE) increased significantly at 20 h after CLP. Ghrelin treatment reduced arterial blood pH from 7.48 ± 0.005 to 7.46 ± 0.008 at 20 h after CLP, however, the decrease was not statistically significant (P=0.129). And Arterial blood pH in ghrelin treated septic animals was still significantly higher than that in sham-operated animals (P<0.05). Moreover, actual bicarbonate (HCO−3) and base excess (ABE and SBE) were significantly reduced by ghrelin treatment (P<0.05). On the other hand, there are no differences in PaCO2 and PaO2 among the groups (Table 1).
Table 1.
Alterations in arterial pH, actual bicarbonate (HCO−3), actual base excess (ABE), standard base excess (SBE), PaCO2 and PaO2 in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP).
| Sham | CLP-Vehicle | CLP-Ghrelin | |
|---|---|---|---|
| pH | 7.40±0.008 | 7.48±0.005* | 7.46±0.008* |
| HCO3− (mmol/L) | 23.6±1.15 | 28.5±0.96* | 25.1±0.97# |
| ABE (mmol/L) | 0.03±0.79 | 4.79±0.71* | 2.08±0.73# |
| SBE (mmol/L) | −0.46±1.04 | 4.78±0.85* | 1.61±0.86# |
| PaCO2 (mmHg) | 38.8±1.96 | 40.4±2.14 | 35.9±1.72 |
| PaO2 (mmHg) | 87.8±2.99 | 83.9±2.89 | 87.1±3.29 |
Data are expressed as means ± SE (n=8/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls method:
P<0.05 versus sham-operated animals;
P<0.05 versus CLP animals treated with vehicle.
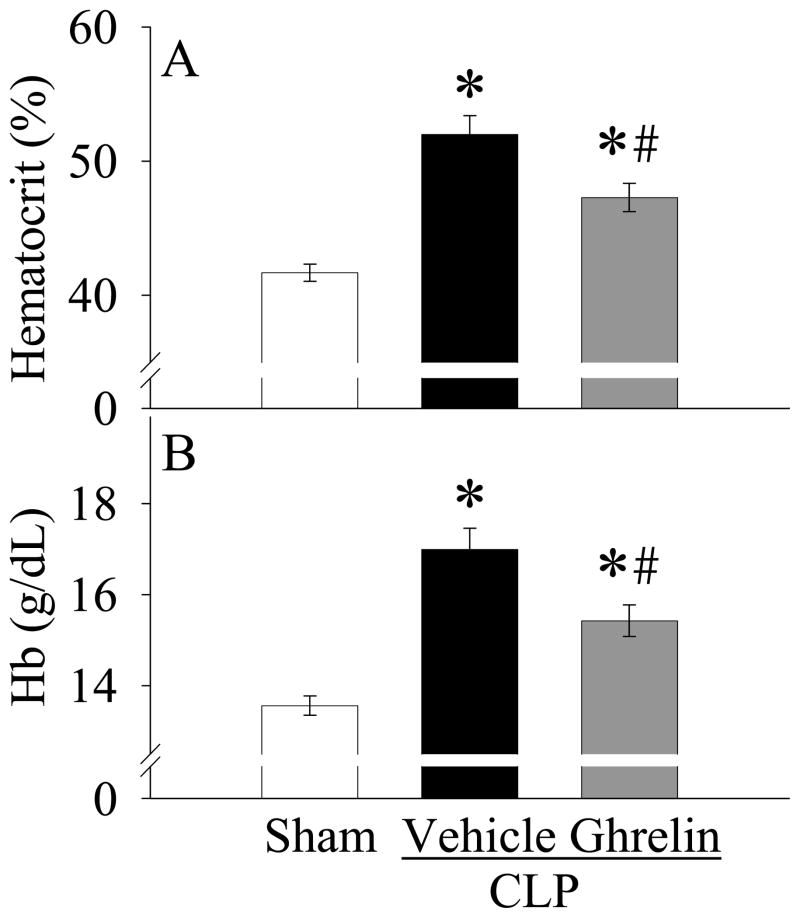
Effects of ghrelin administration on systemic hematocrit and hemoglobin levels
As shown in Figures 6A–B, rat subjected to CLP had 25% increases in both systemic hematocrit and hemoglobin levels as compared with sham-operated animals, indicating dehydration at 20 h after the onset of sepsis. When CLP animals were treated with ghrelin, the levels of systemic hematocrit and hemoglobin were markedly decreased (P<0.05, Figs. 6A–B).
Figure 6.
Alterations in systemic levels of hematocrit (A) and hemoglobin (Hb, B) in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Data are expressed as means ± SE (n=8/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman- Keuls method: *P<0.05 versus sham-operated animals; #P<0.05 versus CLP animals treated with vehicle.
DISCUSSION
Despite decades of research and development efforts and significant advances in antimicrobial therapy and overall medical care, the continued high mortality rate in severe sepsis/septic shock is a sobering reflection of current therapeutic approaches. One difficulty in the management of septic patients is to maintain their cardiovascular stability. In the present study, we found that treatment with ghrelin significantly improved heart performance and increased NE-induced vascular contraction and endothelium-dependent (ACh-induced) vascular relaxation, which lead to the improved blood gas status in a rat model of polymicrobial sepsis. Thus, ghrelin may be further developed as a useful agent for maintaining cardiovascular stability in severe sepsis.
Myocardial depression is a common accompaniment of sepsis [22]. It puts septic patients at high risk to develop multiple organ failure, which is associated with a very high mortality. In this regard, cardiac dysfunction plays a pivotal and sometimes decisive role in determining survival or death of septic patients. A characteristic pattern of ventricular dilation and decreased ejection fraction is seen in patients with severe sepsis. Although initially associated with regulation of growth hormone release and appetite, the cardiovascular system has been recently recognized as an important target for ghrelin [10,11]. Chronic administration of ghrelin has been shown to improve left ventricular dysfunction in a rat model of chronic heart failure [23]. A limited number of clinical studies also suggest a role for ghrelin in the treatment of congestive heart failure [24,25]. In this study, we found that the maximal rates of ventricular pressure increase (+dP/dtmax), decrease (−dP/dtmax) and ventricular peak systolic pressure (VPSP) decreased significantly at 20 h after CLP. Ghrelin administration increased ±dP/dt by >30% and restored VPSP to sham levels. Therefore, ghrelin may be effective in the treatment of septic cardiomyopathy.
The possible mechanisms responsible for ghrelin’s effects on cardiac contractility may be related, at least in part, with the anti-inflammation property (i.e., downregulation of proinflammatory cytokines) of this agent. Circulating levels of TNF-α are significantly elevated in sepsis[16,26,27]. Studies by Walley et al. indicate that administration of TNF-α decreased left ventricular contractility by 23 to 52% at 1 to 5 h after its infusion [28]. Similarly, studies by Murray and Freeman demonstrate that left ventricular performance decreases significantly after TNF-α administration in conscious dogs [29]. We have recently shown that ghrelin effectively decreases TNF-α production in animal models of both polymicrobial sepsis and endotoxemia [14,16,30]. Thus, ghrelin may exert its cardioprotective effect by downregulating TNF-α. Moreover, cardiac tissue expresses high levels of ghrelin receptors. In isolated perfused rat hearts subjected to ischemia reperfusion injury, Chang et al. have shown that administration of ghrelin during reperfusion results in improvement in left ventricular systolic pressure, enhanced rates of left ventricular contraction and relaxation, and reduced myocardial release of lactate dehydrogenase and myoglobin [31]. Therefore, ghrelin’s direct effects on the heart may also contribute to its cardioprotection in sepsis.
Clinically, the cardiovascular response to sepsis is characterized by an early, hyperdynamic phase followed by a late, hypodynamic phase [17]. Early correction of hypotension and tissue hypoperfusion provides significant benefits in patients with severe sepsis and septic shock [32]. However, numerous studies have shown decreased response to fluid resuscitation and catecholamine stimulation during the hypodynamic phase of sepsis [22,33]. The severity of shock and a requirement for high-dose catecholamines are both important prognostic indicators. Impaired vascular reactivity with an abnormal balance between vasoconstrictor and vasodilator tone results in an inability to regulate blood flow distribution between and within tissues, which can alter blood flow to vital organs and eventually lead to organ failure. Our previous study has shown that mean arterial pressure (MAP) in CLP vehicle-treated animals was 14% lower than that in sham-operated animals at 20 h after CLP [13]. Therefore, these septic animals were at the early stage of hypodynamic sepsis. The data presented here demonstrate that both NE-induced vascular contraction and ACh-induced vascular relaxation in the aorta decreased significantly at 20 h after the onset of sepsis. In contrast, NTG-induced vascular relaxation was not significantly altered under such conditions. Since ACh induces vascular relaxation via the stimulation of the release of endothelium-derived nitric oxide (NO), and NTG directly induces vascular relaxation [21], the depressed ACh-induced vascular relaxation reflects a decreased productive capacity of endothelium-derived NO. Administration of ghrelin, however, improved both NE-induced vascular contraction and ACh-induced vascular relaxation at 20 h after CLP. A number of studies [34–36] have suggested that up-regulation of proinflammatory cytokine such as TNF-α production is responsible for producing the impaired vascular function in sepsis. We have also shown previously that TNF-α induces vascular dysfunction under both in vivo and in vitro conditions [37]. Ghrelin’s anti-inflammatory property may also contribute to the improved vascular function after its administration in sepsis. In addition, increasing evidence has shown that ghrelin can directly protect endothelial cells from injury [38–42]. Administration of exogenous ghrelin acutely improves endothelial function by increasing NO bioavailability and normalizing the altered balance between endothelin-1/NO within the vasculature [40,41]. In endothelial cell cultures, ghrelin has been shown to directly stimulate NO production [43] and inhibit endothelial cell apoptosis [44]. Thus, administration of ghrelin also directly improves vascular function at the vascular level in sepsis.
One difficulty in the management of septic patients is to prevent the transition from the early, hyperdynamic to the late, hypodynamic phase. The rodent model of CLP mimics many features of clinical peritonitis-sepsis. This model of sepsis is associated with an early, hyperdynamic phase (i.e., 2–10 h after CLP), which is followed by a late, hypodynamic phase (16 h after CLP and later) [18,45,46]. In order to determine whether ghrelin prevents the transition from the early, hyperdynamic to the late, hypodynamic phase, we chose to treat septic animals at the hyperdynamic phase (i.e., 5 h after CLP) and measure cardiovascular responses at the hypodynamic phase (i.e., 20 h after CLP). Our previous study has shown that mean arterial blood pressure decreased by 14% at 20 h after CLP, indicating the early stage of hypodynamic phase. Ghrelin treatment at 5 h after CLP restored the significantly decreased blood pressure to almost sham levels [13]. Our current result also demonstrated that administration of ghrelin at 5 h after CLP increased heart contractility and improved vascular responsiveness to vasoactive agents at 20 h after CLP.
Ghrelin is an appetite stimulant. Although we did not measure water and/or food intake in this study, our previous study has shown that there were no significant differences in body weight changes between septic animals treated with vehicle and ghrelin 10 days after CLP [14]. Therefore it is unlikely that ghrelin treatment caused significant differences in water and/or food intake within 20 h after CLP in this experiment.
In summary, this study demonstrates for the first time that ghrelin treatment maintains cardiovascular stability as indicated by increased ±dP/dt, restored VPSP, and improved vascular responsiveness to NE and ACh in a rat model of severe sepsis. Since a major difficulty in the management of septic patients is to maintain their cardiovascular stability, this agent may be further developed as a useful adjunct for the treatment of patients with severe sepsis. In our future studies, we will determine the therapeutic window of ghrelin and examine the effect of ghrelin on water and/or food intake after CLP.
Acknowledgments
This study was supported by NIH grants R01 GM057468, R01 GM053008, and R01 AG028352 (P. Wang).
Footnotes
Presented at the 34th Annual Conference on Shock, June 11 – 14, 2011, Norfolk, VA
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Esper AM, Moss M, Lewis CA, et al. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit Care Med. 2006;34:2576. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danai PA, Sinha S, Moss M, et al. Seasonal variation in the epidemiology of sepsis. Crit Care Med. 2007;35:410. doi: 10.1097/01.CCM.0000253405.17038.43. [DOI] [PubMed] [Google Scholar]
- 5.Strehlow MC, Emond SD, Shapiro NI, et al. National study of emergency department visits for sepsis, 1992 to 2001. Ann Emerg Med. 2006;48:326. doi: 10.1016/j.annemergmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Wang P, Zhou M, Rana MW, et al. Differential alterations in microvascular perfusion in various organs during early and late sepsis. Am J Physiol. 1992;263:G38–G43. doi: 10.1152/ajpgi.1992.263.1.G38. [DOI] [PubMed] [Google Scholar]
- 8.Wang P, Ba ZF, Chaudry IH. Endothelium-dependent relaxation is depressed at the macro- and microcirculatory levels during sepsis. Am J Physiol. 1995;269:R988–R994. doi: 10.1152/ajpregu.1995.269.5.R988. [DOI] [PubMed] [Google Scholar]
- 9.Wang P. Adrenomedullin and cardiovascular responses in sepsis. Peptides. 2001;22:1835. doi: 10.1016/s0196-9781(01)00534-4. [DOI] [PubMed] [Google Scholar]
- 10.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 11.Isgaard J, Granata R. Ghrelin in cardiovascular disease and atherogenesis. Mol Cell Endocrinol. 2011 doi: 10.1016/j.mce.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Wu R, Zhou M, Cui X, et al. Upregulation of cardiovascular ghrelin receptor occurs in the hyperdynamic phase of sepsis. Am J Physiol Heart Circ Physiol. 2004;287:H1296–H1302. doi: 10.1152/ajpheart.00852.2003. [DOI] [PubMed] [Google Scholar]
- 13.Wu R, Dong W, Zhou M, et al. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc Res. 2005;68:318. doi: 10.1016/j.cardiores.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Wu R, Dong W, Zhou M, et al. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176:805. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui X, Wu R, Zhou M, et al. Differential expression of cytochrome P450 isoforms in the lungs of septic animals. Crit Care Med. 2004;32:1186. doi: 10.1097/01.ccm.0000124877.86743.37. [DOI] [PubMed] [Google Scholar]
- 16.Wu R, Dong W, Cui X, et al. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245:480. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnagopalan S, Kumar A, Parrillo JE, et al. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care. 2002;8:376. doi: 10.1097/00075198-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock: a review of laboratory models and a proposal. J Surg Res. 1980;29:189. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Ba ZF, Cioffi WG, et al. Salutary effects of ATP-MgCl2 on the depressed endothelium-dependent relaxation during hyperdynamic sepsis. Crit Care Med. 1999;27:959. doi: 10.1097/00003246-199905000-00035. [DOI] [PubMed] [Google Scholar]
- 20.Sharma VK, Dellinger RP. The International Sepsis Forum's controversies in sepsis: my initial vasopressor agent in septic shock is norepinephrine rather than dopamine. Crit Care. 2003;7:3. doi: 10.1186/cc1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer RMJ. The discovery of nitric oxide in the vessel wall: A unifying concept in the pathogenesis of sepsis. Arch Surg. 1993;128:396. doi: 10.1001/archsurg.1993.01420160034004. [DOI] [PubMed] [Google Scholar]
- 22.Levy RJ, Deutschman CS. Evaluating myocardial depression in sepsis. Shock. 2004;22:1. doi: 10.1097/01.shk.0000129198.53836.15. [DOI] [PubMed] [Google Scholar]
- 23.Nagaya N, Uematsu M, Kojima M, et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation. 2001;104:1430. doi: 10.1161/hc3601.095575. [DOI] [PubMed] [Google Scholar]
- 24.Nagaya N, Miyatake K, Uematsu M, et al. Hemodynamic, renal, and hormonal effects of ghrelin infusion in patients with chronic heart failure. J Clin Endocrinol Metab. 2001;86:5854. doi: 10.1210/jcem.86.12.8115. [DOI] [PubMed] [Google Scholar]
- 25.Nagaya N, Moriya J, Yasumura Y, et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- 26.Miksa M, Das P, Zhou M, et al. Pivotal role of the alpha(2A)-adrenoceptor in producing inflammation and organ injury in a rat model of sepsis. PLoS ONE. 2009;4:e5504. doi: 10.1371/journal.pone.0005504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu R, Higuchi S, Dong W, et al. Reversing established sepsis in rats with human vasoactive hormone adrenomedullin and its binding protein. Mol Med. 2009;15:28. doi: 10.2119/molmed.2008.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walley KR, Hebert PC, Wakai Y, et al. Decrease in left ventricular contractility after tumor necrosis factor-alpha infusion in dogs. J Appl Physiol. 1994;76:1060. doi: 10.1152/jappl.1994.76.3.1060. [DOI] [PubMed] [Google Scholar]
- 29.Murray DR, Freeman GL. Tumor necrosis factor-α induces a biphasic effect on myocardial contractility in conscious dogs. Circ Res. 1996;78:154. doi: 10.1161/01.res.78.1.154. [DOI] [PubMed] [Google Scholar]
- 30.Wu R, Zhou M, Dong W, et al. Ghrelin hyporesponsiveness contributes to age-related hyperinflammation in septic shock. Ann Surg. 2009;250:126. doi: 10.1097/SLA.0b013e3181ad85d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang L, Ren Y, Liu X, et al. Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart. J Cardiovasc Pharmacol. 2004;43:165. doi: 10.1097/00005344-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 33.Chernow B, Rainey TG, Lake CR. Endogenous and Exogenous catecholamines in critical care medicine. Crit Care Med. 1982;10:409. doi: 10.1097/00003246-198206000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg S, Xie J, Wang Y, et al. Tumor necrosis factor-alpha inhibits endothelium- dependent relaxation. J Appl Physiol. 1993;74:2394. doi: 10.1152/jappl.1993.74.5.2394. [DOI] [PubMed] [Google Scholar]
- 35.Xie J, Wang Y, Lippton H, et al. Tumor necrosis factor inhibits stimulated but not basal release of nitric oxide. Am Rev Respir Dis. 1993;148:627. doi: 10.1164/ajrccm/148.3.627. [DOI] [PubMed] [Google Scholar]
- 36.Aoki N, Siegfried M, Lefer AM. Anti-EDRF effect of tumor necrosis factor in isolated, perfused cat carotid arteries. Am J Physiol. 1989;256:H1509–H1512. doi: 10.1152/ajpheart.1989.256.5.H1509. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Ba ZF, Chaudry IH. Administration of tumor necrosis factor-α in vivo depresses endothelium-dependent relaxation. Am J Physiol. 1994;266:H2535–H2541. doi: 10.1152/ajpheart.1994.266.6.H2535. [DOI] [PubMed] [Google Scholar]
- 38.Tesauro M, Schinzari F, Caramanti M, et al. Cardiovascular and metabolic effects of ghrelin. Curr Diabetes Rev. 2010;6:228. doi: 10.2174/157339910791658871. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Zhu JJ, Cheng F, et al. Ghrelin ameliorates hypoxia-induced pulmonary hypertension via phospho-GSK3{beta}/{beta}-catenin signaling in neonatal rats. J Mol Endocrinol. 2011 doi: 10.1530/JME-10-0143. [DOI] [PubMed] [Google Scholar]
- 40.Tesauro M, Schinzari F, Rovella V, et al. Ghrelin restores the endothelin 1/nitric oxide balance in patients with obesity-related metabolic syndrome. Hypertension. 2009;54:995. doi: 10.1161/HYPERTENSIONAHA.109.137729. [DOI] [PubMed] [Google Scholar]
- 41.Taddei S, Virdis A. Exogenous ghrelin on nitric oxide-endothelin 1 imbalance in metabolic syndrome: can we kill 2 birds with 1 stone? Hypertension. 2009;54:960. doi: 10.1161/HYPERTENSIONAHA.109.141176. [DOI] [PubMed] [Google Scholar]
- 42.Chow KB, Cheng CH, Wise H. Anti-inflammatory activity of ghrelin in human carotid artery cells. Inflammation. 2009;32:402. doi: 10.1007/s10753-009-9149-8. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Narsinh K, Zhao L, et al. Effects and mechanisms of ghrelin on cardiac microvascular endothelial cells in rats. Cell Biol Int. 2011;35:135. doi: 10.1042/CBI20100139. [DOI] [PubMed] [Google Scholar]
- 44.Baldanzi G, Filigheddu N, Cutrupi S, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. J Cell Biol. 2002;159:1029. doi: 10.1083/jcb.200207165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang P, Chaudry IH. Mechanism of hepatocellular dysfunction during hyperdynamic sepsis. Am J Physiol. 1996;270:R927–R938. doi: 10.1152/ajpregu.1996.270.5.R927. [DOI] [PubMed] [Google Scholar]
- 46.Yang S, Cioffi WG, Bland KI, et al. Differential alterations in systemic and regional oxygen delivery and consumption during the early and late stages of sepsis. J Trauma. 1999;47:706. doi: 10.1097/00005373-199910000-00015. [DOI] [PubMed] [Google Scholar]