Abstract
Human malignant mesothelioma (MM) is an aggressive and highly lethal cancer that is believed to be caused by chronic exposure to asbestos and erionite. Prognosis for this cancer is generally poor due to late-stage diagnosis and resistance to current conventional therapies. The damage-associated molecular pattern (DAMP) protein HMGB1 has been implicated previously in transformation of mesothelial cells. Here we show that HMGB1 establishes an autocrine circuit in MM cells that influences their proliferation and survival. MM cells strongly expressed HMGB1 and secreted it at high levels in vitro. Accordingly, HMGB1 levels in MM patient sera were higher than that found in healthy individuals. The motility, survival and anchorage-independent growth of HMGB1-secreting MM cells was inhibited in vitro by treatment with monoclonal antibodies directed against HMGB1 or against the receptor for advanced glycation end products (RAGE), a putative HMGB1 receptor. HMGB1 inhibition in vivo reduced the growth of MM xenografts in SCID mice and extended host survival. Taken together, our findings indicate that MM cells rely on HMGB1 and they offer a preclinical proof of principle that antibody-mediated ablation of HMBG1 is sufficient to elicit therapeutic activity, suggesting a novel therapeutic approach for MM treatment.
Keywords: HMGB1, mesothelioma, inflammation, biomarker, therapy
Introduction
Human malignant mesothelioma (MM) arises from the neoplastic transformation of mesothelial cells lining the pleural, peritoneal and pericardial cavities. MM has been linked to occupational and environmental exposure to asbestos, causing over 100,000 deaths per year worldwide (1). Moreover, in rapidly industrializing countries, such as India and China where the use of asbestos is unrestricted, the incidence of MM is expected to rise dramatically (2). Erionite, a natural mineral fiber that can be dispersed in the environment by human activities also causes MM (3). We have recently discovered extensive erionite exposure in the US (4). It has been estimated that over 25 million people have been exposed to asbestos in the US, while the number of those exposed to erionite is still unknown (1, 4).
MM is a very aggressive cancer, usually diagnosed at late stages, when it is refractory to most therapeutic modalities, leading to poor prognosis with a patients’ median survival of 8–12 months from diagnosis. MM is considerably resistant to all current treatments, and survival may only be extended by about 11 weeks in patients treated with Cisplatin/Alimta as the standard of care (5–7). However, in the 5% of patients diagnosed at an early stage (Stage Ia) survivals of 5 or more years are not uncommon (5–7). Therefore, the development of new biomarkers for early detection and of novel targets for preventive and therapeutic approaches to MM are most needed. Moreover, recently we discovered a novel cancer syndrome caused by BAP1 germline mutations, characterized by the development of uveal melanoma and mesothelioma and possibly other cancers (8). When individuals with BAP1 mutations are exposed to asbestos or erionite, mesothelioma predominates. Thus, it has become possible to identify within asbestos and erionite exposed cohorts those individuals at the highest risk of mesothelioma for early diagnosis.
We recently showed that asbestos- and erionite-exposed primary human mesothelial cells (HM) release High Mobility Group Box 1 protein (HMGB1), which plays a critical role in the carcinogenesis of these mineral fibers (4, 9). HMGB1 is a Damage Associated Molecular Pattern (DAMP) and a key mediator of inflammation (10). Although HMGB1 is a nuclear protein, it is detected in the cytoplasm of cells undergoing necrosis and in some cell types that can actively secrete it, such as macrophages. Once in the extracellular space, HMGB1 binds to the Receptor for Advanced Glycation Endproducts (RAGE) (11) and to the Toll-like Receptors (TLRs 2 and 4) (12) starting the inflammatory process (13–17). HMGB1 induces the secretion of tumor necrosis factor-alpha (TNF-α) by macrophages, and activation of nuclear factor-kappa B (NF-κB), a key regulator of oncogenesis (9). Activation of NF-κB promotes cell proliferation and inhibits cell death, leading to enhanced survival of HM that have accumulated DNA alterations following asbestos exposure, thus facilitating their malignant transformation (18).
MM biopsies often show a marked inflammatory infiltrate that contains a large number of tumor-associated macrophages. Here we show that HMGB1 is highly expressed and secreted by MM cells, establishing an autocrine circuit. Consistently, MM patients have elevated HMGB1 serum levels, suggesting that HMGB1 may be a novel MM biomarker. In addition, inhibition of HMGB1 impaired the motility, survival and anchorage-independent growth of HMGB1-secreting MM cells in vitro. Finally, a monoclonal antibody against HMGB1 reduced tumor growth in xenografted SCID mice, extending their survival.
Our data indicate that the sustained release of HMGB1 by MM cells, along with its secretion by surrounding inflammatory cells, supports the MM malignant phenotype. These findings provide the rationale for inhibiting HMGB1 as a novel molecular targeted therapy of MM.
Materials and Methods
Cell cultures
Primary HM were obtained from pleural effusions of eight different patients, pathologically diagnosed free of malignancy. HM were characterized and cultured as previously described (19). MM cell lines were established from surgically resected human MM specimens. REN cells were provided by Dr. Steven Albelda (University of Pennsylvania), while all other cell lines used in this study were provided by Dr. Harvey I. Pass (NYU). REN/luc luciferase-were generated as previously described (20).
Reagents and materials
Full-length, LPS-free purified HMGB1 and BoxA were obtained from HMGBiotech, the neutralizing monoclonal anti-HMGB1 (DPH1.1) was from Dia.Pro Diagnostics; the neutralizing monoclonal anti-RAGE (Clone #176902) was from R&D Systems, normal mouse IgG was from Sigma-Aldrich. Crocidolite asbestos was obtained from the Union Internationale Contre le Cancer (Switzerland) and processed as previously described (9, 19).
Immunohistochemistry
Immunohistochemistry was performed on human MM and normal pleura paraffin-embedded tissues with rabbit polyclonal anti-HMGB1 (Abcam). Goat anti-rabbit secondary antibody and Vectastain Elite ABC kit (Rabbit IgG) (Vector Labs) were used according to the manufacturer’s instructions. HMGB1 immunostaining was analyzed blindly by two board certified pathologists [A.P. and M.C.].
Immunocytochemistry
Immunocytochemistry on MM and HM cells was performed using the Vectastain ABC kit (Vector Labs) according to the manufacturer’s instructions. Mouse monoclonal anti-HMGB1 (Abcam) was used for the detection of intracellular HMGB1 protein.
HMGB1 ELISA
The human HMGB1 ELISA kit (IBL International) was used to measure the levels of HMGB1 in patients’ sera and in HM and MM conditioned media. Samples were tested in duplicate. Sera were obtained from 20 untreated (pre-chemotherapy and pre-surgery) mesothelioma patients and 20 age- and gender-matched healthy individuals. All participants provided informed consent, and procedures and protocols were approved by the institutional review board (IRB). For the detection of extracellular HMGB1 released by MM and HM cell lines, 2 × 106 cells were cultured in DMEM with 1% FBS for 24 hours. The culture media were then collected and concentrated by ultrafiltration using Amicon Ultra Centrifugal Filters (Millipore) and 10 μl aliquots were assayed in duplicate by ELISA. All culture media were collected under identical condition.
Quantitative Real-Time PCR
Total RNA from MM and HM cells was isolated using RNeasy kit (Qiagen) and treated with RNase-free DNase. The following primers from Qiagen were used: Hs_AGER_1_SG (QT00000119), Hs_HMGB1_1_SG (QT01002190), Hs_TLR4_2_SG (QT01670123), and Hs_TLR2_1_SG (200) (QT00236131) to amplify the respective cDNAs as previously described (18).
Western blotting
HM and MM cells were lysed and the cytoplasmic and nuclear fractions separated using the protein extraction kit from Active Motif, according to the manufacturer’s instructions. A total of 50 μg of protein lysates were used for Western blotting performed as previously described (18), using mouse monoclonal anti-HMGB1, rabbit polyclonal anti-RAGE, mouse monoclonal anti-TLR2 and goat polyclonal anti-TLR4 (Abcam). Anti-α-Tubulin (Calbiochem) and anti-Lamin B (Abcam) were used as loading controls for the cytoplasmic and nuclear fractions, respectively.
Viability and cytotoxicity assays
MM cells (1 × 104 per well) were incubated for 24 hours in DMEM with 1% FBS containing one of the HMGB1 antagonists: BoxA (100 ng/ml); anti-HMGB1 (1.0 μg/ml); or anti-RAGE (1.7 μg/ml). Mouse IgG (1.7 μg/ml) was used as control. The CellTiter 96 Aqueous Cell Proliferation Assay-MTS (Promega) was used to evaluate cell viability, and the LDH cytotoxicity detection kit (Roche) was used to evaluate cytotoxicity.
Migration and invasion assays
The in vitro cell migration and invasion assays were performed using Costar Transwell permeable polycarbonate supports (8.0 μm pores) in 24-well plates (Corning Inc.). For the migration assays, 1 × 105 MM cells in serum-free DMEM were used. For the invasion assays, 2 × 105 MM cells in serum-free DMEM were seeded in the upper compartment coated with Matrigel. The lower compartment contained serum-free DMEM (negative control) or DMEM plus 10% FBS (positive control), purified recombinant HMGB1 (100 ng/ml) or concentrated medium (200 μl) from REN or PPM-Mill cells.
Wound healing assay
MM cells were seeded in 6-well plates and grown to 80–90% confluence in DMEM plus 1% FBS. One hour prior to wounding, the cells were treated with either BoxA (100 ng/ml), anti-HMGB1 (1.0 μg/ml), anti-RAGE (1.7 μg/ml) or IgG control (1.7 μg/ml). The cell monolayer was wounded with a P200 pipette tip, and wound closure was observed after 48 hours.
Soft agar assay
Anchorage-independent cell proliferation (REN 4 × 103 cells) was determined by the soft agar assay. After 23 days of culture, the number and size of the colonies formed in each treatment (BoxA (100 ng/ml), anti-HMGB1 (1.0 μg/ml), anti-RAGE (1.7 μg/ml) or irrelevant IgG control (1.7 μg/ml)) were evaluated.
SCID human MM xenografts
Female SCID mice aged 6 to 8 weeks (Jackson Laboratories) were housed and handled under aseptic conditions, in accordance with our institution’s Institutional Animal Care and Use Committee (IACUC) guidelines. Twenty-one SCID mice were injected intra peritoneum (i.p.) with 5 × 105 REN/luc cells suspended in 500 μl of PBS, as described (20, 21). Xenografts were visualized by luminescence after D-luciferin injection (150 mg/kg) using the In Vivo Imaging System (IVIS, Xenogen Corp., Alameda, CA), with regions of interest (ROI) quantified as total photon counts by Living Image software (Xenogen Corp.). Four days were required for the formation of detectable tumor nodules by IVIS imaging. Mice were then weighed and randomly assigned to control (IgG isotype control or PBS) and treatment (anti-HMGB1 mAb) groups of seven animals each. The “treatment” group received 200 μg i.p. every two days for the first week, then every week until day 38, for a total of 1.8 mg/mouse of anti-HMGB1 mAb. Control groups received either i.p. injections of matched isotype control IgG (200 μg/injection) or PBS with the same schedule as the anti-HMGB1-treated group. Tumor dimension was measured every 7th day as average radiance (photons/s/cm2/sr). The majority of the animals died spontaneously, except (i) one mouse in the anti-HMGB1 group died accidently from a misplaced injection and was not counted towards the survival analysis; (ii) two mice, one each from vehicle and anti-HMGB1 groups, were euthanized and necropsied when tumor development caused severe ascites limiting the animal’s mobility, according to IACUC regulations.
Statistical Analysis
Where not otherwise indicated, statistical significance between two groups of interest was evaluated by unpaired Student’s t test. Differences were considered significant at P < 0.05. Differences in the HMGB1 levels in human sera were analyzed by paired t test. The association between HMGB1 and RAGE mRNA levels in MM cell lines and the association between tumor stage and HMGB1 cytoplasmic staining in MM tissues were assessed by calculating the Pearson’s correlation coefficient (r). For the SCID MM xenografts experiment, a two-way ANOVA assessed the effects of treatment, time, and the treatment by time interaction on weight. Bonferroni-corrected post-tests compared HMGB1 mAb to the control groups (PBS or IgG controls) at each time point. Differences of survival across groups were assessed by fitting a parametric model to the survival time data; a Weibull distribution was assumed; the LIFEREG procedure in SAS 9.2 performed the analysis.
Results
HMGB1 inhibitors hinder asbestos-induced HM transformation
In vivo, macrophages are recruited to the sites of asbestos deposition (22) where they are known to release pro-inflammatory cytokines into the microenvironment (9, 18). In order to mimic the cross-talk between HM and macrophages, we developed a co-culture system in which HM form tridimensional foci about 1–2 months after asbestos exposure (4). Using this assay, we tested two different HMGB1 inhibitors, BoxA (23) and an anti-HMGB1 neutralizing monoclonal antibody (24). The number of foci (mean ± SEM) formed in the HM-macrophages co-cultures treated with either BoxA (53.5 ± 6.4) or HMGB1 mAb (70.0 ± 9.9) was significantly lower than in the untreated co-cultures (136.5 ± 7.8; P < 0.05, Supplementary Fig. S1). Moreover, a two-week delay in the initial development of foci was observed in HMGB1-neutralized co-cultures. These results showed that these HMGB1 inhibitors interfere with asbestos-induced HM transformation.
HMGB1 is highly expressed in MM tissues and sera of MM patients
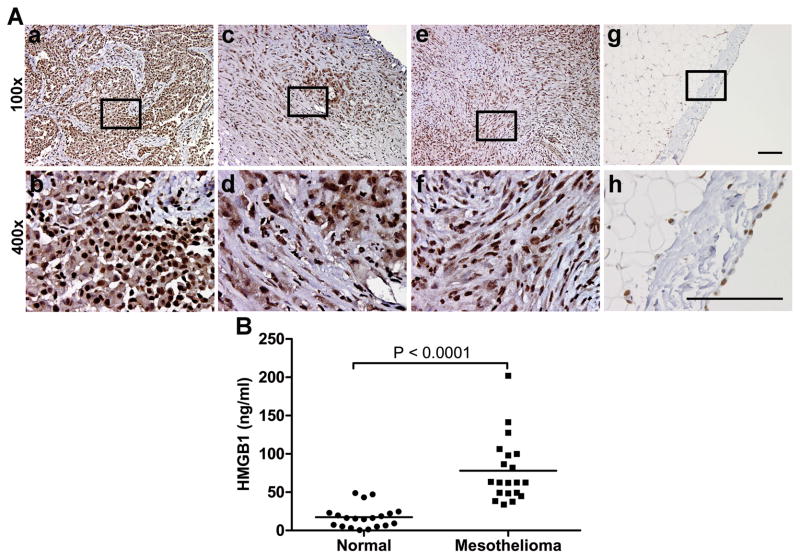
Since MM biopsies often show a marked inflammatory infiltrate, we tested whether HMGB1 might be also involved in maintaining chronic inflammation in the MM microenvironment, after the establishment of cell transformation. We analyzed HMGB1 in 31 MM biopsies representing all 3 main histological subtypes of MM (21 epithelioid, 6 biphasic and 4 sarcomatoid). All the MM biopsies showed uniform strong nuclear staining (Fig. 1A). Most MM specimens also showed a variable degree of cytoplasmic staining (epithelioid: 17/21; biphasic: 5/6; sarcomatoid: 4/4) (Fig. 1A; Table 1). In those specimens that were scored negative, there are focal areas of cytoplasmic positivity, usually corresponding to clusters of invading tumor cells. Moreover, statistical significance (r = 0.61, P = 0.002) was found in the correlation between tumor stage and HMGB1 cytoplasmic staining in the tissues. The higher tumor stage was associated with stronger HMGB1 staining; however, further research using a larger sample size may be needed to validate this correlation. In normal pleura, HMGB1 staining was fainter and was localized only in the nucleus (Fig. 1A).
Figure 1. HMGB1 is highly expressed in MM tissues and sera of MM patients.
(A) Strong expression of HMGB1 was detected in the nuclei of 31/31 MM biopsies representing all 3 main histological subtypes of MM: epithelial (a, b), biphasic (c, d) and sarcomatoid (e, f). In 26/31 MM biopsies HMGB1 is detected in both nucleus and cytoplasm. In the single-cell mesothelial layer of normal pleura (g, h), HMGB1 is only detected in nucleus. Rectangles in x100 magnification pictures (top) indicate the area shown in x400 magnification (bottom). Scale bar, 100 μm. (B) HMGB1 levels in sera of 20 mesothelioma patients are significantly higher (P < 0.0001) than in 20 healthy individuals. Bars show mean of HMGB1 levels.
Table 1.
Stages and cytoplasmic HMGB1 expression of MM cases.
| Specimen ID | Subtype | Stage | Score |
|---|---|---|---|
| SP-001 | E | III | 3 + |
| SP-009 | E | II | 0 |
| SP-011 | E | II | 1 + |
| SP-012 | E | III | 2 + |
| SP-013 | B | III | 1 + |
| SP-014 | E | III | 1 + |
| SP-015 | E | IV | 1 + |
| SP-016 | E | unknown | 1 + |
| SP-018 | E | III | 1 + |
| SP-019 | S | III | 1 + |
| SP-020 | E | unknown | 1 + |
| SP-021 | E | I | 0 |
| SP-022 | E | unknown | 1 + |
| SP-023 | B | unknown | 1 + |
| SP-023 | B | unknown | 1 + |
| SP-024 | E | III | 1 + |
| SP-025 | E | III | 1 + |
| SP-026 | E | unknown | 1 + |
| SP-027 | B | IV | 3 + |
| SP-028 | S | III | 3 + |
| SP-029 | S | IV | 2 + |
| SP-030 | E | III | 1 + |
| SP-031 | E | III | 0 |
| SP-032 | B | I | 0 |
| SP-033 | E | unknown | 1 + |
| SP-034 | E | II or III | 1 + |
| SP-035 | B | III | 2 + |
| SP-036 | S | IV | 2 + |
| SP-037 | E | IV | 1 + |
| SP-038 | E | IV | 2 + |
| SP-039 | E | II | 0 |
E = Epithelioid; B = Biphasic; S = Sarcomatoid
Correlation between tumor stage and cytoplasmic HMGB1: r = 0.61; P = 0.002
Since cytoplasmic HMGB1 is usually associated with HMGB1 secretion or release, these data suggested that HMGB1 could be secreted or released into the extra-cellular space, making its way into the patient’s blood. We tested HMGB1 levels in serum samples from 20 MM patients and 20 age- and gender-matched healthy individuals. HMGB1 concentration (mean ± SEM) in MM patients’ sera was 77.9 ± 9.4 ng/ml, significantly higher than that in sera from healthy controls (17.5 ± 3.2 ng/ml, P < 0.0001; Fig. 1B).
The high levels of HMGB1 expression in the majority of MM biopsies tested, its cytoplasmic localization as well as its high levels in sera of MM patients suggested that extracellular HMGB1 might be relevant to the biology of MM cells.
HMGB1 and RAGE are up-regulated in MM cells
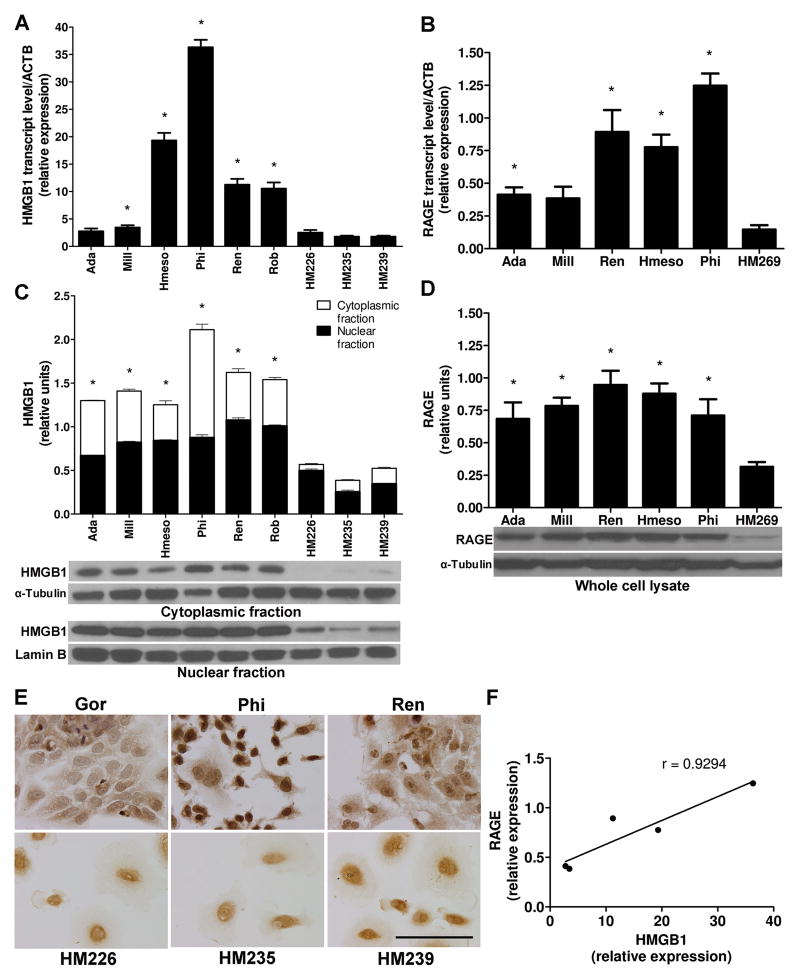
We investigated the expression of HMGB1 and its receptors RAGE, TLR2 and TLR4 in a panel of 6 MM cell lines and 6 distinct primary HM cultures. qRT-PCR revealed that in 5 out of 6 MM cell lines the amount of HMGB1 transcripts was significantly higher compared to HM. A large degree of variability was observed: PPM-Ada and PPM-Mill cells expressed relatively low levels of HMGB1 transcripts, while in REN, PPM-Hmeso and PPM-Phi cells the amount of HMGB1 transcripts exceeded that found in primary HM cells by 6, 10 and 20 times respectively (Fig. 2A).
Figure 2. HMGB1 and RAGE expression are both upregulated in MM cells.
(A) HMGB1 mRNA levels are higher in MM than in HM. Different primary HM cell cultures have similar low HMGB1 levels (three representatives HM cells are shown). *P < 0.05; MM versus HM. (B) RAGE mRNA levels are higher in MM than in HM. Experiments were performed as in (A). *P < 0.05; MM versus HM. (C) Cell compartmentalization. Total HMGB1 protein levels are higher in MM than in HM, and HMGB1 is localized in both nucleus and cytoplasm of MM but mainly in the nucleus of HM. α-Tubulin and Lamin B, loading controls for the cytoplasmic and nuclear fractions, respectively. HMGB1 relative densitometry units were calculated. *P < 0.05; MM versus HM. (D) Western blotting shows higher RAGE expression in MM than in HM. α-Tubulin, loading control. RAGE relative densitometry units were calculated. *P < 0.05; MM versus HM. All the experiments were performed three times; error bars represent SEM. (E) Immunocytochemistry. HMGB1 is detected in both nucleus and cytoplasm of MM but mainly in the nucleus of HM (three representatives MM and three representatives HM cells are shown). Original magnification, x400. Scale bar, 100 μm. (F) HMGB1 and RAGE transcript levels show significant positive correlation in five different MM cell lines tested (r = 0.93, P = 0.022).
Cells with abundant HMGB1 transcripts (PPM-Phi, REN, and PPM-Hmeso) also had higher amounts of RAGE transcripts, while cells with low HMGB1 mRNA (PPM-Ada and PPM-Mill) had also low levels of RAGE mRNA (Fig. 2B). We found that the correlation between HMGB1 and RAGE mRNA levels in the five different MM cell lines tested was statistically significant (r = 0.93, P = 0.022) (Fig. 2F). TLR2 and TLR4 transcripts were also higher in MM than in HM cells, although their overall levels were much lower than for RAGE (Supplementary Fig. S2A). The sub-cellular compartmentalization of HMGB1 protein was determined by cell fractionation and Western blotting. In HM cells, HMGB1 was almost exclusively detected in the nuclear fraction; instead, MM cells contained high amounts of HMGB1 in both the nucleus and the cytoplasm (Fig. 2C). These results were confirmed by immunostaining: MM cells had both nuclear and cytoplasmic HMGB1 positive staining, while HM cells had exclusively nuclear staining (Fig. 2E), a result that was in accordance with the findings in MM biopsies (Fig. 1A). Consistently with the results on RAGE, TLR2 and TLR4 transcripts, the corresponding proteins were expressed at higher levels in MM cells (Fig. 2D and Supplementary Fig. S2B).
These results indicated that most MM cell lines express high levels of both HMGB1 and its main receptors RAGE, TLR2 and TLR4, suggesting that HMGB1 signaling may influence the MM tumor phenotype.
HMGB1 induces migration and proliferation of MM cells
HMGB1 induces migration and proliferation in certain cell types (25, 26). Therefore, we tested its activity also on MM cells. Both REN and PPM-Phi cells migrated towards purified recombinant human HMGB1 (100 ng/ml), while the incubation with anti-RAGE monoclonal antibody abrogated cell migration (Supplementary Fig. S3A and B). Recombinant HMGB1 also significantly increased the proliferation rate of REN and PPM-Phi cells MM (Supplementary Fig. S3C). Down-regulation of HMGB1 with two different gene-specific shRNA constructs significantly inhibited cell proliferation of REN cells compared to cells transfected with a scrambled control non-effective shRNA (Supplementary Fig. S3D).
These experiments indicated that HMGB1 is a chemotactic and mitogenic factor for MM cells.
HMGB1 is an autocrine motility factor for MM cells
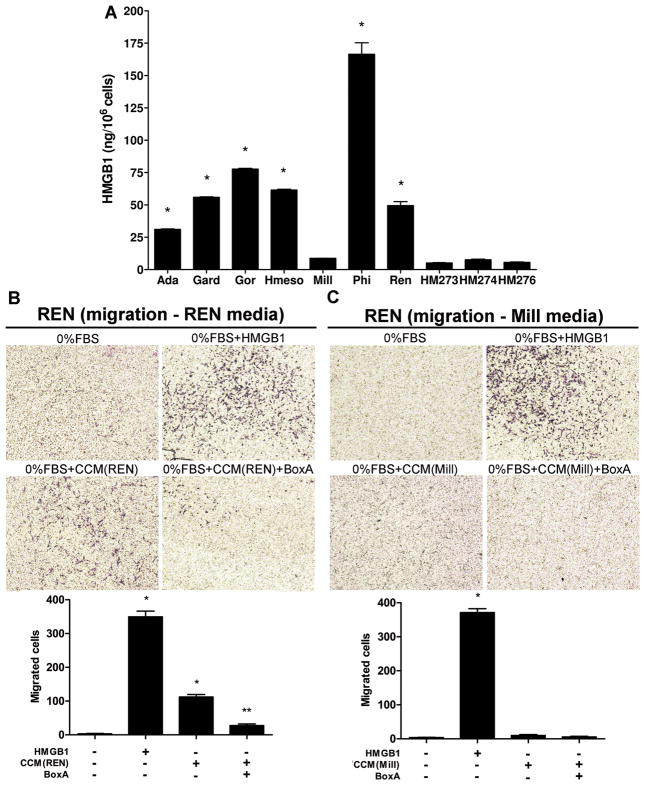
We found that significantly higher levels of HMGB1 were present in the 24 hours conditioned media of 6 out of 7 MM cell lines, compared to HM cells (both HM and MM were at 80–85% confluence). Consistently, PPM-Mill and HM cells, with low HMGB1 expression, released barely detectable amounts of HMGB1 (Fig. 3A).
Figure 3. HMGB1 secreted by MM cells is biologically active.
(A) MM cells release larger amounts of HMGB1 in the culture media than HM, as measured by ELISA. Culture media for the different cells were collected and concentrated under identical condition. Experiments were done in duplicate and performed twice. *P < 0.05; MM versus HM. (B) Concentrated conditioned media (CCM) from REN cells induce the migration of REN cells themselves. Migration is blocked by BoxA. (C) CCM collected from PPM-Mill cells does not induce a significant chemotactic response in REN cells. All CCM were collected under identical conditions. Migrated cells were counted using ImageJ software and represent mean values per field from at least three fields. Experiments were done in duplicate. *P < 0.05; HMGB1 and CCM from REN/PPM-Mill versus 0% FBS. **P < 0.05; CCM alone versus CCM plus BoxA. In all panels, error bars represent SEM.
To verify the possible occurrence of an autocrine loop, we collected and concentrated the conditioned medium from REN cells (high HMGB1 producers) and from PPM-Mill (low HMGB1 producers) and tested their chemoattractant activity. Concentrated conditioned media (CCM) from REN cells induced the migration of REN cells themselves (Fig. 3B) and PPM-Phi cells (Supplementary Fig. S4A). In both cell lines, cell motility was blocked by BoxA. Instead, CCM collected from PPM-Mill cells did not induce a chemotactic response in any of the two MM cell lines tested (Fig. 3C and Supplementary Fig. S4B).
These results demonstrated that HMGB1 secreted by MM cells is biologically active and induces migration of the same cells in an autocrine fashion.
MM cells require HMGB1 for viability
Since recombinant HMGB1 enhances the growth rate of MM cells, we tested whether the HMGB1 secreted by MM cells plays a role in their survival.
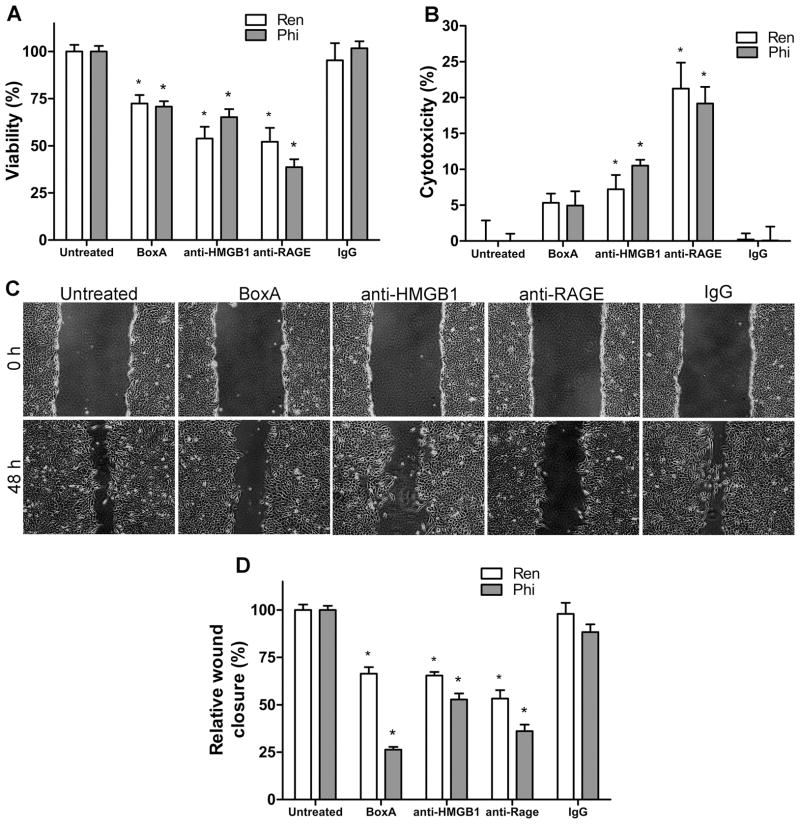
The viability of REN and PPM-Phi cells and PPM-Mill cells was tested following HMGB1 inhibition. BoxA, anti-HMGB1 and anti-RAGE antibodies significantly reduced the viability of REN and PPM-Phi cells (Fig. 4A) but had only mild or no effects on PPM-Mill cells (Supplementary Fig. S5A).
Figure 4. MM cells require HMGB1 for survival and migration.
(A) Viability, determined by MTS assay. Inhibition of HMGB1 by BoxA or anti-HMGB1 or anti-RAGE antibodies substantially decreases the viability of REN and PPM-Phi cells. *P < 0.05; treated versus untreated. (B) Cytotoxicity, determined by LDH assay. Both anti-HMGB1 and anti-RAGE antibodies induce substantial cytotoxicity in REN and PPM-Phi cells. Experiments were done in quadruplicate and performed twice. *P < 0.05; treated versus untreated. (C and D) Wound healing assay. One hour prior to scratching the monolayer, the cells were treated with either BoxA (100 ng/ml), anti-HMGB1 (1.0 μg/ml), anti-RAGE (1.7 μg/ml), or IgG control (1.7 μg/ml). (D) For quantification of wound closure, the scratched area covered by the cells after 48 hours was measured using ImageJ software and normalized to control. All HMGB1 antagonists reduced wound healing by REN (C and D) and PPM-Phi cells (D). Original magnification, x40. Figure shows representative images of wound healing (REN) from three experiments done in duplicate. *P < 0.05; treated versus untreated. In all panels, error bars represent SEM.
Both anti-HMGB1 and anti-RAGE antibodies induced marked cytotoxicity in REN and PPM-Phi cells (Fig. 4B), while a mild cytotoxic effect was observed in PPM-Mill cells only when treated with anti-RAGE antibody (Supplementary Fig. S5B), compared with untreated controls (P < 0.05). In primary HM cells, HMGB1 inhibition did not cause any change in cell viability or proliferation, or cytotoxicity (Supplementary Fig. S6A, B and C).
We further analyzed cell death induced by HMGB1 antagonists. Flow cytometry revealed that BoxA, anti-HMGB1 and anti-RAGE antibodies significantly induced apoptosis in REN cells (Supplementary Fig. S7A and B), but not in PPM-Mill cells (Supplementary Fig. S7C and D). These results strongly suggest that MM cells secreting high levels of HMGB1 are “addicted” to HMGB1 for their viability.
HMGB1 is required for MM cell motility
All HMGB1 inhibitors significantly reduced wound closure of REN (Fig. 4C and D) and PPM-Phi (Fig. 4D) cells (high HMGB1 producers), but there was no effect on low HMGB1 producers PPM-Mill cells (Supplementary Fig. S5C and D). The results showed that HMGB1 is critical for the motility of MM cells that secrete it.
HMGB1 inhibition disrupts MM cells invasiveness and anchorage-independent growth
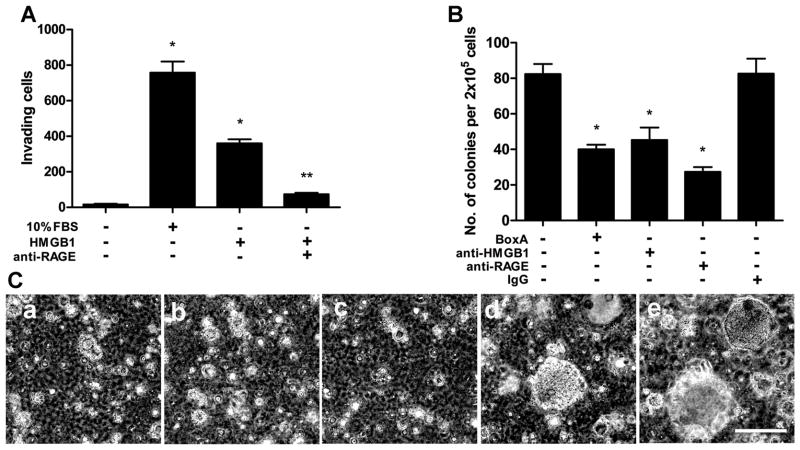
We performed a Matrigel invasion assay with REN (Fig. 5A) and PPM-Phi cells (Supplementary Fig. S8). Recombinant HMGB1 significantly enhanced the invasion of Matrigel by both cell lines, and the anti-RAGE monoclonal antibody significantly reduced MM cell invasion induced by HMGB1.
Figure 5. HMGB1 supports the malignant phenotype of MM cells.
(A) Matrigel invasion assay. Exogenous recombinant HMGB1 induces REN cells invasion. Anti-RAGE monoclonal antibodies significantly inhibited HMGB1-induced cell invasion. Invading cells were counted using ImageJ software and represent mean values per field from at least three fields. *P < 0.05; HMGB1 and 10% FBS versus 0% FBS. **P < 0.05; HMGB1 alone versus HMGB1 plus anti-RAGE. (B and C) Anchorage-independent growth. REN cells treated with HMGB1 antagonists BoxA (a), anti-HMGB1 (b), and anti-RAGE (c) formed fewer (B) and smaller (C) colonies in soft agar than IgG (d) and untreated (e) controls. Original magnification, x40. Figure shows representative images from two experiments done in duplicate. Colonies larger than 0.1 mm in diameter were counted using ImageJ software. *P < 0.05; treated versus IgG control. In all panels, error bars represent SEM. Scale bar, 0.5 mm.
To verify anchorage-independent growth, we performed soft agar assays. All HMGB1 inhibitors (BoxA, anti-HMGB1, and anti-RAGE) caused a significant decrease in REN anchorage-independent growth, as indicated by a marked reduction in the number (Fig. 5B) and size (Fig. 5C) of colonies.
Expression analysis, performed with the Affymetrix HumanGene 1.0 ST array, revealed that stimulation with HMGB1 enhanced the transcription of multiple genes controlled by the activation of NF-κB, and downstream genes. Genes such as TNF-α and IL-1α were up-regulated and genes downstream of TNFR1 and TNFR2 signaling were activated (Supplementary Fig. S9). Activation of these genes has been linked to MM growth and invasion (1, 27).
These results indicated that HMGB1 sustains the main properties of the malignant phenotype (invasiveness and anchorage-independent growth) of MM cells.
Inhibition of HMGB1 in vivo reduces the growth of MM xenografts and extends the mice survival
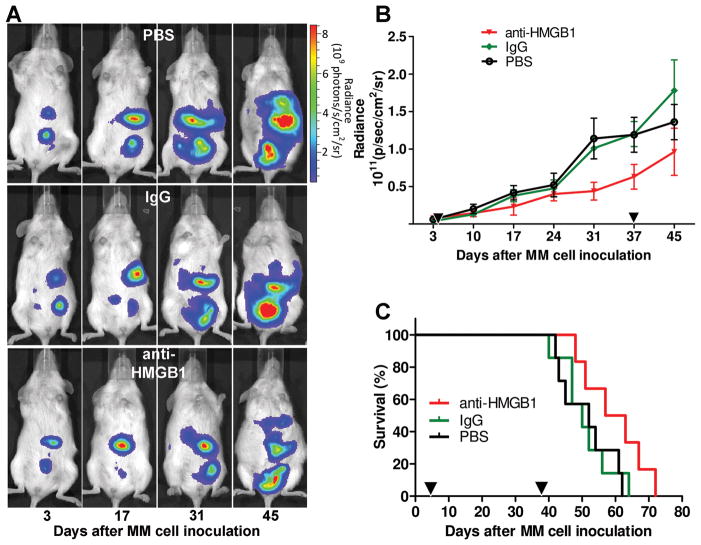
Our in vitro results suggested that HMGB1 supports the maintenance of the MM cancer phenotype, at least in cells expressing high levels of HMGB1 that appear addicted to it. We validated this hypothesis in MM xenografts in SCID mice (20, 21). Treatment with anti-HMGB1 mAb did not significantly inhibit tumor growth in mice inoculated with PPM-Mill cells, which are not “addicted” to HMGB1 (Supplementary Fig. S10A and B); however, anti-HMGB1 mAb significantly reduced tumor growth (P < 0.05; Fig. 6A and B) in mice inoculated with “HMGB1-addicted” REN cells, and significantly extended animal survival by about 15% (P < 0.05; Fig. 6C).
Figure 6. Anti-HMGB1 monoclonal antibody reduces tumor growth and extends survival in a mouse model of MM.
(A) Bioluminescence imaging of mice xenografted i.p. with 5 × 105 REN/luc shows tumor development. One representative mouse from each group is shown. (B) Tumor growth rate is reduced in mice injected with anti-HMGB1 mAb in comparison with controls. Mean ± SEM of each group is shown (n = 7 per group). P < 0.05; two-way ANOVA analysis. (C) Survival curve. Anti-HMGB1 mAb enhances animal survival in comparison with PBS and IgG control groups. Differences across groups (P < 0.05) were assessed by fitting a parametric model to the survival time data. Black triangles on (B) and (C) graphs represent first (day 4) and last (day 38) injections.
Discussion
In previous studies we demonstrated that, following asbestos and erionite exposure, HMGB1 is released by mesothelial cells undergoing programmed cell necrosis (9). HMGB1 starts a chronic inflammatory process that contributes to HM malignant transformation and mesothelioma development (5). Here we show that: 1) HMGB1 is secreted by MM cells in the serum of MM patients, making HMGB1 a potential MM biomarker; 2) HMGB1 supports MM cell viability and hallmarks of malignant phenotype, such as tumor invasion and tumor cell proliferation; and 3) treatment with HMGB1 inhibitors extended the survival of mice xenografted with MM cells. Strong expression of HMGB1 was detected in the nuclei of the tumor cells of all MM biopsies (31/31) tested. HMGB1 was also detected in the cytoplasm of the tumor cells in 26/31 MM biopsies, a finding not observed in nearby normal stromal cells or in non-malignant mesothelial cells.
HMGB1 is a biologically active protein, released by some immune cells (monocytes, macrophages, and dendritic cells) and other cells (pituicytes, enterocytes, and hepatocytes), in response to specific stimuli, including lipopolysaccharide (LPS), TNF-α, interleukin-1 (IL-1), and interferon-gamma (IFN-γ) (28–33). The presence of cytoplasmic HMGB1 staining suggested that MM tumor cells might also secrete HMGB1. Indeed, our results show that MM cells secrete HMGB1 into the extracellular space, as clearly demonstrated by the detection of high concentrations of HMGB1 in the cytoplasm and in the tissue culture media of 6/7 MM cell lines. The same 6 cell lines expressed high levels of RAGE, one of the main HMGB1 receptors. Moreover, we detected high levels of HMGB1 in the sera of all 20 MM patients tested. The latter finding suggests that HMGB1 may be a biomarker of MM, a hypothesis that we plan to test in a clinical trial in an area of Cappadocia (Turkey) where a very high incidence of MM is observed (3). The finding that HMGB1 secretion in the medium by MM cells parallels the expression of RAGE receptors, suggests the occurrence of an autocrine mechanism of growth.
Other tumor cells, such as erythroleukemia, neuroblastoma, and colon cancer cells, have also been shown to secrete HMGB1 (34, 35). Once extracellular, HMGB1 triggers inflammation (26) and when secreted by tumor cells promotes their proliferation, migration, invasion and neo-angiogenesis (36–41). We show here that HMGB1 supports the proliferation, viability, motility and invasiveness of MM cell lines.
To test whether the withdrawal of HMGB1 would affect MM cells, we used different HMGB1 inhibitors: 1) BoxA; 2) anti-HMGB1 mAb and 3) anti-RAGE mAb. Inhibition of the binding of HMGB1 to RAGE significantly diminished the viability of MM cell lines expressing high levels of HMGB1.
We found that HMGB1 inhibitors impaired the anchorage-independent growth of MM cells, a hallmark of malignant transformation (42). Next, we tested whether HMGB1 inhibition could reduce the growth of tumors in MM xenografted mice. Indeed, treatment with the anti-HMGB1 mAb in mice injected with human MM cells that secrete high levels of HMGB1, such as REN, led to a significant decrease in MM tumor growth and resulted in a significant extension in the survival of the xenografted mice. Notably, the only MM cell line that secretes low levels of HMGB1 was much less sensitive to HMGB1 inhibitors for its viability, motility and invasiveness, consistent with our hypothesis that only tumor cells secreting HMGB1 constitutively become “addicted” to it. We cannot exclude the possibility that all MM cells are primarily dependent on HMGB1, but some clones lose their dependence and their secretion ability as a secondary event.
In summary, we report that MM cells become addicted to HMGB1 when it is upregulated together with its main receptor RAGE, and we propose that blockade of the HMGB1-RAGE interaction may represent a novel approach for MM therapy.
Supplementary Material
Acknowledgments
Financial support: This work was supported by the NCI R01, the Mesothelioma Applied Research Foundation, the Riviera United-4 a CURE (to H.Y.), the Hawai’i Community Foundation (to H.Y. and to G.G.), the NCI R01 and P01 (to M.C.), the UH Foundation (to M.C.) and Associazione Italiana per la Ricerca sul Cancro (to M.E.B.).
The authors are grateful to HMGBiotech for providing BoxA and to Dia.Pro for providing the neutralizing anti-HMGB1 mAb DPH1.1. We thank Drs. Michael T. Lotze, Franco M. Marincola and David Ward for their discussion and advice regarding this work.
Footnotes
Disclosure of Potential Conflicts of Interest
The University of Hawai’i has filed for patents on HMGB1 and mesothelioma, on which H.Y., M.C., M.E.B and H.I.P are named as inventors. M.E.B. is founder and part owner of HMGBiotech.
References
- 1.Carbone M, Ly BH, Dodson RF, Pagano I, Morris PT, Dogan UA, et al. Malignant mesothelioma: facts, myths, and hypotheses. J Cell Physiol. 2012;227:44–58. doi: 10.1002/jcp.22724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burki T. Health experts concerned over India’s asbestos industry. Lancet. 2010;375:626–7. doi: 10.1016/s0140-6736(10)60251-6. [DOI] [PubMed] [Google Scholar]
- 3.Carbone M, Emri S, Dogan AU, Steele I, Tuncer M, Pass HI, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. 2007;7:147–54. doi: 10.1038/nrc2068. [DOI] [PubMed] [Google Scholar]
- 4.Carbone M, Baris YI, Bertino P, Brass B, Comertpay S, Dogan AU, et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc Natl Acad Sci U S A. 2011;108:13618–23. doi: 10.1073/pnas.1105887108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin Cancer Res. 2012;18:598–604. doi: 10.1158/1078-0432.CCR-11-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fennell DA, Gaudino G, O’Byrne KJ, Mutti L, van Meerbeeck J. Advances in the systemic therapy of malignant pleural mesothelioma. Nat Clin Pract Oncol. 2008;5:136–47. doi: 10.1038/ncponc1039. [DOI] [PubMed] [Google Scholar]
- 7.Pass HI, Vogelzang N, Hahn SM, Carbone M. Benign and Malignant Mesothelioma. In: De Vita VT, Hellmann S, Rosemberg SA, editors. Cancer, Principles & Practice of Oncology. 9. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer business; 2011. pp. 2052–80. [Google Scholar]
- 8.Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–5. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H, Rivera Z, Jube S, Nasu M, Bertino P, Goparaju C, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A. 2010;107:12611–6. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 11.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–61. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 12.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, et al. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 13.Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–60. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 14.Palumbo R, Bianchi ME. High mobility group box 1 protein, a cue for stem cell recruitment. Biochem Pharmacol. 2004;68:1165–70. doi: 10.1016/j.bcp.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164:441–9. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messmer D, Yang H, Telusma G, Knoll F, Li J, Messmer B, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–13. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 17.Mitola S, Belleri M, Urbinati C, Coltrini D, Sparatore B, Pedrazzi M, et al. Cutting edge: extracellular high mobility group box-1 protein is a proangiogenic cytokine. J Immunol. 2006;176:12–5. doi: 10.4049/jimmunol.176.1.12. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Bocchetta M, Kroczynska B, Elmishad AG, Chen Y, Liu Z, et al. TNF-alpha inhibits asbestos-induced cytotoxicity via a NF-kappaB-dependent pathway, a possible mechanism for asbestos-induced oncogenesis. Proc Natl Acad Sci U S A. 2006;103:10397–402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bocchetta M, Di Resta I, Powers A, Fresco R, Tosolini A, Testa JR, et al. Human mesothelial cells are unusually susceptible to simian virus 40-mediated transformation and asbestos cocarcinogenicity. Proc Natl Acad Sci U S A. 2000;97:10214–9. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertino P, Piccardi F, Porta C, Favoni R, Cilli M, Mutti L, et al. Imatinib mesylate enhances therapeutic effects of gemcitabine in human malignant mesothelioma xenografts. Clin Cancer Res. 2008;14:541–8. doi: 10.1158/1078-0432.CCR-07-1388. [DOI] [PubMed] [Google Scholar]
- 21.Nasu M, Carbone M, Gaudino G, Ly BH, Bertino P, Shimizu D, et al. Ranpirnase Interferes with NF-kappaB Pathway and MMP9 Activity, Inhibiting Malignant Mesothelioma Cell Invasiveness and Xenograft Growth. Genes Cancer. 2011;2:576–84. doi: 10.1177/1947601911412375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka S, Choe N, Iwagaki A, Hemenway DR, Kagan E. Asbestos exposure induces MCP-1 secretion by pleural mesothelial cells. Exp Lung Res. 2000;26:241–55. doi: 10.1080/019021400404528. [DOI] [PubMed] [Google Scholar]
- 23.Sitia G, Iannacone M, Muller S, Bianchi ME, Guidotti LG. Treatment with HMGB1 inhibitors diminishes CTL-induced liver disease in HBV transgenic mice. J Leukoc Biol. 2007;81:100–7. doi: 10.1189/jlb.0306173. [DOI] [PubMed] [Google Scholar]
- 24.Sitia G, Iannacone M, Aiolfi R, Isogawa M, van Rooijen N, Scozzesi C, et al. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog. 2011;7:e1002061. doi: 10.1371/journal.ppat.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muller S, Scaffidi P, Degryse B, Bonaldi T, Ronfani L, Agresti A, et al. New EMBO members’ review: the double life of HMGB1 chromatin protein: architectural factor and extracellular signal. EMBO J. 2001;20:4337–40. doi: 10.1093/emboj/20.16.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol. 2008;9:147–57. doi: 10.1007/s11864-008-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–70. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 30.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–4. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Vishnubhakat JM, Bloom O, Zhang M, Ombrellino M, Sama A, et al. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126:389–92. [PubMed] [Google Scholar]
- 32.Liu S, Stolz DB, Sappington PL, Macias CA, Killeen ME, Tenhunen JJ, et al. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol. 2006;290:C990–9. doi: 10.1152/ajpcell.00308.2005. [DOI] [PubMed] [Google Scholar]
- 33.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–23. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Passalacqua M, Zicca A, Sparatore B, Patrone M, Melloni E, Pontremoli S. Secretion and binding of HMG1 protein to the external surface of the membrane are required for murine erythroleukemia cell differentiation. FEBS Lett. 1997;400:275–9. doi: 10.1016/s0014-5793(96)01402-0. [DOI] [PubMed] [Google Scholar]
- 35.Wahamaa H, Vallerskog T, Qin S, Lunderius C, LaRosa G, Andersson U, et al. HMGB1-secreting capacity of multiple cell lineages revealed by a novel HMGB1 ELISPOT assay. J Leukoc Biol. 2007;81:129–36. doi: 10.1189/jlb.0506349. [DOI] [PubMed] [Google Scholar]
- 36.Sparatore B, Patrone M, Passalacqua M, Pedrazzi M, Ledda S, Pontremoli S, et al. Activation of A431 human carcinoma cell motility by extracellular high-mobility group box 1 protein and epidermal growth factor stimuli. Biochem J. 2005;389:215–21. doi: 10.1042/BJ20050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 38.Kuniyasu H, Oue N, Wakikawa A, Shigeishi H, Matsutani N, Kuraoka K, et al. Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol. 2002;196:163–70. doi: 10.1002/path.1031. [DOI] [PubMed] [Google Scholar]
- 39.Kuniyasu H, Chihara Y, Takahashi T. Co-expression of receptor for advanced glycation end products and the ligand amphoterin associates closely with metastasis of colorectal cancer. Oncol Rep. 2003;10:445–8. [PubMed] [Google Scholar]
- 40.Kuniyasu H, Chihara Y, Kondo H, Ohmori H, Ukai R. Amphoterin induction in prostatic stromal cells by androgen deprivation is associated with metastatic prostate cancer. Oncol Rep. 2003;10:1863–8. [PubMed] [Google Scholar]
- 41.van Beijnum JR, Dings RP, van der Linden E, Zwaans BM, Ramaekers FC, Mayo KH, et al. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339–48. doi: 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- 42.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.