Abstract
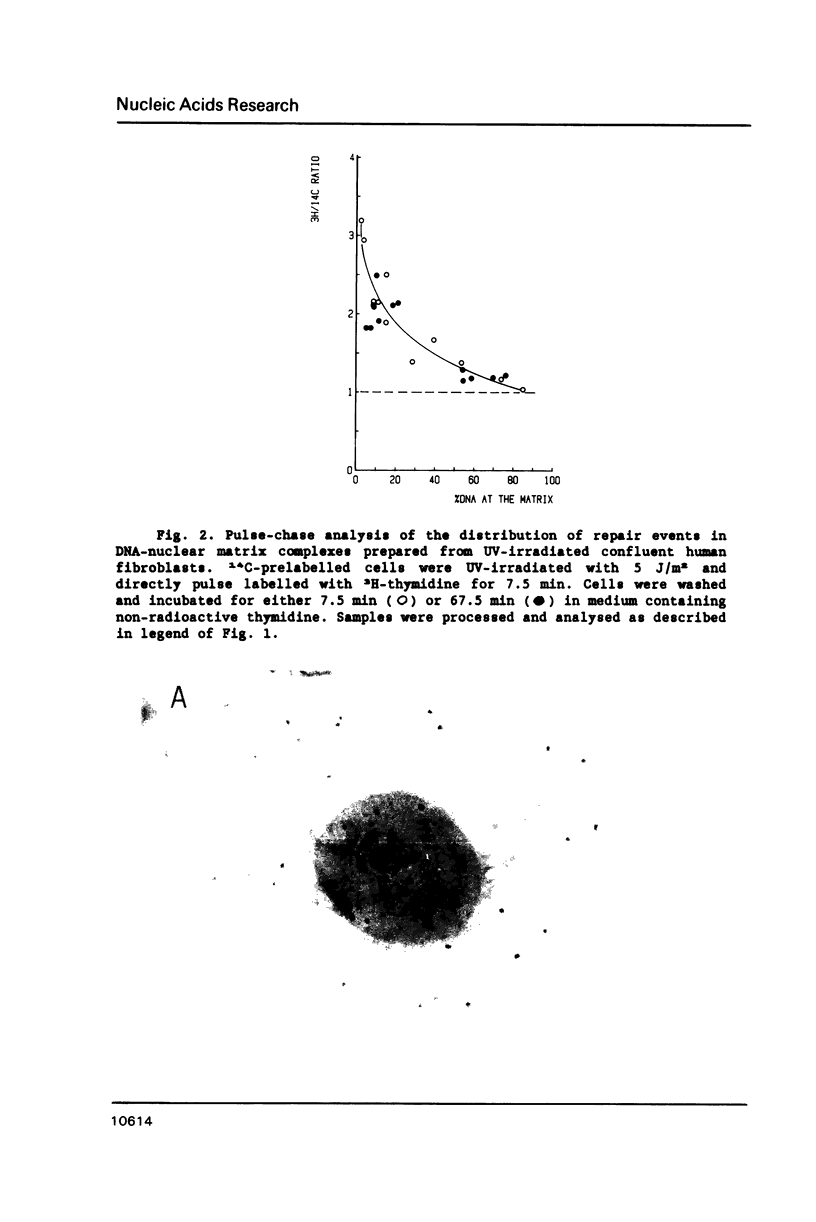
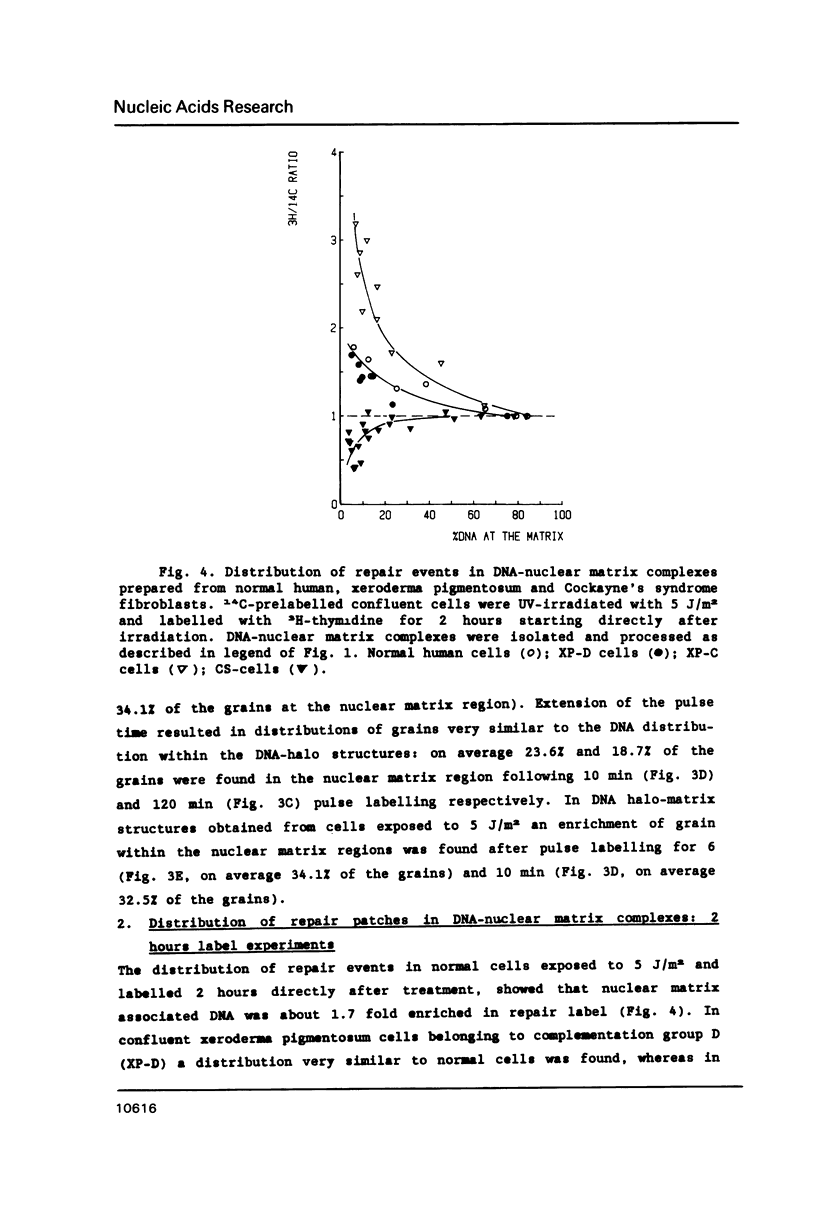
In this study we addressed the questions as to whether repair is confined to the nuclear matrix compartment, analogous to replication and transcription and how repair events are distributed in DNA loops associated with the nuclear matrix. Pulse labelling of ultraviolet (254 nm) irradiated confluent human fibroblasts revealed that repair was preferentially located in nuclear matrix associated DNA in cells exposed to 5 J/m2. However, in cells exposed to 30 J/m2 repair approached a random distribution. The non-random distribution of repair label at 5 J/m2 was most pronounced directly after irradiation and gradually changed to a more random distribution within two hours after treatment. The results of pulse-chase experiments exclude the possibility of transient binding of repair sites to the matrix and favour the model of preferential repair of DNA sequences permanently associated with the nuclear matrix. Pronounced differences in distribution pattern of repair events in DNA loops were found among normal and UV-sensitive cell lines exposed to 5 J/m2. Repair in nuclear matrix associated DNA was 1.7 fold more efficient than in loop DNA in normal and xeroderma pigmentosum group D cells and over 3 fold in xeroderma pigmentosum group C cells. In Cockayne's syndrome fibroblasts repair in nuclear matrix DNA was found to be 2 fold less efficient than in loop DNA. This heterogeneity in distribution of repair correlates well with preferential removal of pyrimidine dimers from transcriptionally active DNA in normal and xeroderma pigmentosum group C cells and its absence in Cockayne's syndrome cells as recently reported by Mayne et al., 1988. The results suggest that Cockayne's syndrome cells have a defect in excision of UV-damage from transcriptionally active genes located proximal to the nuclear matrix. Xeroderma pigmentosum group C cells may possess a defect in DNA repair associated with chromatin regions outside transcriptionally active DNA.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkvens T. M., Gerritsen E. J., Oldenburg M., Breukel C., Wijnen J. T., van Ormondt H., Vossen J. M., van der Eb A. J., Meera Khan P. Severe combined immune deficiency due to a homozygous 3.2-kb deletion spanning the promoter and first exon of the adenosine deaminase gene. Nucleic Acids Res. 1987 Nov 25;15(22):9365–9378. doi: 10.1093/nar/15.22.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Okumoto D. S., Hanawalt P. C. Survival of UV-irradiated mammalian cells correlates with efficient DNA repair in an essential gene. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3830–3833. doi: 10.1073/pnas.83.11.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Cockerill P. N., Garrard W. T. Chromosomal loop anchorage of the kappa immunoglobulin gene occurs next to the enhancer in a region containing topoisomerase II sites. Cell. 1986 Jan 31;44(2):273–282. doi: 10.1016/0092-8674(86)90761-0. [DOI] [PubMed] [Google Scholar]
- Dalton S., Younghusband H. B., Wells J. R. Chicken histone genes retain nuclear matrix association throughout the cell cycle. Nucleic Acids Res. 1986 Aug 26;14(16):6507–6523. doi: 10.1093/nar/14.16.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel P. A., Mullenders L. H., Wanka F. Analysis of the attachment of replicating DNA to a nuclear matrix in mammalian interphase nuclei. Nucleic Acids Res. 1979 Jan;6(1):219–230. doi: 10.1093/nar/6.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkwel P. A., Wenink P. W., Poddighe J. Permanent attachment of replication origins to the nuclear matrix in BHK-cells. Nucleic Acids Res. 1986 Apr 25;14(8):3241–3249. doi: 10.1093/nar/14.8.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. S., Collins A. R. Effects of DNA replication inhibitors on UV excision repair in synchronised human cells. Nucleic Acids Res. 1982 Sep 11;10(17):5357–5368. doi: 10.1093/nar/10.17.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Hanawalt P. C. The timecourse of DNA repair replication in ultraviolet-irradiated HeLa cells. Biochim Biophys Acta. 1973 Oct 12;324(2):206–217. doi: 10.1016/0005-2787(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Erixon K., Ahnström G. Single-strand breaks in DNA during repair of UV-induced damage in normal human and xeroderma pigmentosum cells as determined by alkaline DNA unwinding and hydroxylapatite chromatography: effects of hydroxyurea, 5-fluorodeoxyuridine and 1-beta-D-arabinofuranosylcytosine on the kinetics of repair. Mutat Res. 1979 Feb;59(2):257–271. doi: 10.1016/0027-5107(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Harless J., Hewitt R. R. Intranuclear localization of UV-induced DNA repair in human VA13 cells. Mutat Res. 1987 Mar;183(2):177–184. doi: 10.1016/0167-8817(87)90060-5. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. Transcription occurs at a nucleoskeleton. EMBO J. 1985 Apr;4(4):919–925. doi: 10.1002/j.1460-2075.1985.tb03719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel F. Transcribed human ribosomal RNA genes are attached to the nuclear matrix. J Mol Biol. 1986 Jan 5;187(1):15–21. doi: 10.1016/0022-2836(86)90402-x. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Rapidly occurring DNA excision repair events determine the biological expression of u.v.-induced damage in human cells. Carcinogenesis. 1987 Sep;8(9):1251–1256. doi: 10.1093/carcin/8.9.1251. [DOI] [PubMed] [Google Scholar]
- Mayne L. V., Lehmann A. R. Failure of RNA synthesis to recover after UV irradiation: an early defect in cells from individuals with Cockayne's syndrome and xeroderma pigmentosum. Cancer Res. 1982 Apr;42(4):1473–1478. [PubMed] [Google Scholar]
- McCready S. J., Cook P. R. Lesions induced in DNA by ultraviolet light are repaired at the nuclear cage. J Cell Sci. 1984 Aug;70:189–196. doi: 10.1242/jcs.70.1.189. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Mullenders L. H., Van Zeeland A. A., Natarajan A. T. Analysis of the distribution of DNA repair patches in the DNA-nuclear matrix complex from human cells. Biochim Biophys Acta. 1983 Sep 9;740(4):428–435. doi: 10.1016/0167-4781(83)90091-x. [DOI] [PubMed] [Google Scholar]
- Mullenders L. H., van Kesteren A. C., Bussmann C. J., van Zeeland A. A., Natarajan A. T. Distribution of u.v.-induced repair events in higher-order chromatin loops in human and hamster fibroblasts. Carcinogenesis. 1986 Jun;7(6):995–1002. doi: 10.1093/carcin/7.6.995. [DOI] [PubMed] [Google Scholar]
- Mullenders L. H., van Zeeland A. A., Natarajan A. T. Comparison of DNA loop size and super-coiled domain size in human cells. Mutat Res. 1983 Aug;112(4):245–252. doi: 10.1016/0167-8817(83)90010-x. [DOI] [PubMed] [Google Scholar]
- Mullenders L. H., van Zeeland A. A., Natarajan A. T. The localization of ultraviolet-induced excision repair in the nucleus and the distribution of repair events in higher order chromatin loops in mammalian cells. J Cell Sci Suppl. 1987;6:243–262. doi: 10.1242/jcs.1984.supplement_6.17. [DOI] [PubMed] [Google Scholar]
- Player A. N., Kantor G. J. The endogenous nuclease sensitivity of repaired DNA in human fibroblasts. Mutat Res. 1987 Sep;184(2):169–178. doi: 10.1016/0167-8817(87)90074-5. [DOI] [PubMed] [Google Scholar]
- Razin S. V., Kekelidze M. G., Lukanidin E. M., Scherrer K., Georgiev G. P. Replication origins are attached to the nuclear skeleton. Nucleic Acids Res. 1986 Oct 24;14(20):8189–8207. doi: 10.1093/nar/14.20.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D., Nelkin B., Vogelstein B. The association of transcribed genes with the nuclear matrix of Drosophila cells during heat shock. Nucleic Acids Res. 1985 Apr 11;13(7):2413–2431. doi: 10.1093/nar/13.7.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen C. A., van Venrooij W. J. hnRNA and its attachment to a nuclear protein matrix. J Cell Biol. 1981 Mar;88(3):554–563. doi: 10.1083/jcb.88.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]