Abstract
Over the past 20 years much attention has been given to characterizing the spatial accuracy of fMRI based signals and to techniques that improve on its co-localization with neuronal activity. While the vast majority of fMRI studies have always used the conventional positive BOLD signal, alternative contrast options have demonstrated superior spatial specificity. One of these options surfaced shortly after the initial BOLD fMRI demonstrations and was motivated by optical imaging studies which revealed an early signal change that was much smaller but spatially more specific than the delayed positive response. This early signal change was attributed to oxygenation changes prior to any subsequent blood flow increases. After observation of this biphasic hemodynamic response in fMRI, because this early response resulted in a small MR signal decrease prior to the onset of the large signal increase, it became known as the “initial dip”. While the initial dip in fMRI was subsequently reported by many studies, including those in humans, monkeys, and cats, there were conflicting views about the associated mechanisms and whether it could be generalized across brain regions or species, in addition to whether or not it would prove fruitful for neuroscience. These discrepancies, along with the implications that the initial dip might increase the spatial specificity of BOLD fMRI from 2-3 mm to something more closely associated with neural activity, resulted in lot of buzz and controversy in the community for many years. In this review, the authors provide an account of the story of the initial dip in MR based functional imaging from the Minnesota perspective, where the first demonstrations, characterizations, and applications of the initial dip commenced.
Keywords: initial dip, early response, fast response, BOLD, functional imaging, spatial specificity, fMRI
The Initial Dip
Since the first demonstrations of functional magnetic resonance imaging (fMRI) (Bandettini et al., 1992; Kwong et al., 1992; Ogawa et al., 1992) to map human and animal brain function non-invasively, the spatial specificity and limits of these hemodynamic based signals have been of a subject of investigation and controversy. Initial studies using the blood oxygen level dependent (BOLD) (Ogawa et al., 1990a; Ogawa et al., 1990b; Ogawa et al., 1993a; Ogawa et al., 1993b) signal, which is and was by far the most common fMRI technique, found that the dominant sources of signal changes in T2/T2* weighted images originated from large veins far removed from local neuronal activity (Bandettini et al., 1994; Duyn et al., 1994; Frahm et al., 1994; Haacke et al., 1994; Kim et al., 1994; Lai et al., 1993; Lee et al., 1995; Menon et al., 1993; Segebarth et al., 1994), resulting in a concern that the hyperemic response was not very specific. Further, due to the fact that the BOLD signal reflects changes in blood flow, blood volume, and oxygen consumption, interpretation of the physiological changes associated with the BOLD signal, although thoroughly investigated, continue to remain somewhat unclear. Despite these concerns, virtually all fMRI studies, even today, are based on the detection of the hyperemic hemodynamic (positive BOLD) signal secondary to brain activity. As such, there have been many attempts over the past 20 years to circumvent the apparent vascular limits of the conventional positive BOLD response in order to improve the spatial accuracy of fMRI based mapping signals. One such attempt was to capitalize on the spatial temporal dynamics of the hemodynamic response. These dynamics were first revealed by optical imaging of intrinsic signals which found that the deoxyhemoglobin concentration following the onset of neuronal stimulation/activation is biphasic, consisting of a small rise, peaking at 2 s and lasting approximately 4 s, and a subsequent decrease persisting several seconds after the cessation of the stimulation (Grinvald et al., 1991; Malonek and Grinvald, 1996). This initial phase was believed to originate from a rise in deoxyhemoglobin due to an increase in metabolism before the hemodynamic response kicks in. While the hyperemic phase is what most BOLD studies focus on, the initial phase was believed to more directly reflect the metabolic response and hence neural activity, and thus drew immediate interest in the MR community. Because a rise in deoxyhemoglobin would lead to a decrease in the MR signal, this initial phase in the fMRI signal is commonly referred to as the “initial dip” or the “early response” (see Fig.1).
Figure 1.
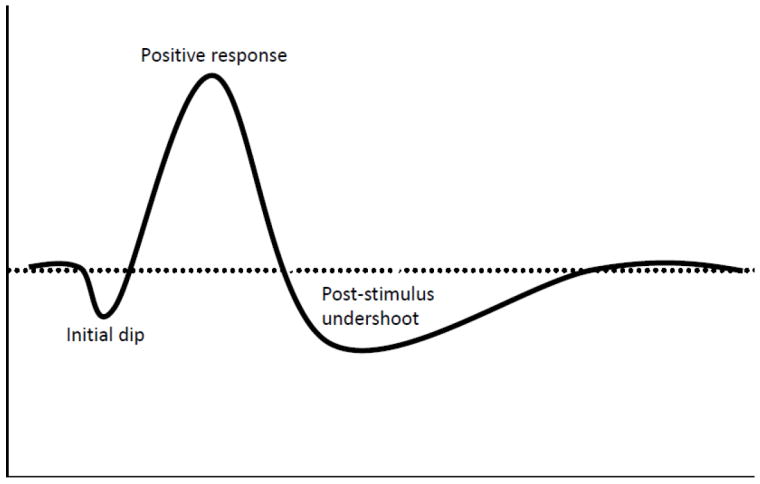
Temporal dynamics of the BOLD response resulting from the hemodynamic response to neuronal activation.
The initial MR studies
The first MR study to report a biphasic response was actually a single voxel functional spectroscopy experiment (Ernst and Hennig, 1994) (fMRS) which detected a very small but significant decrease shortly after stimulus onset. In a study performed at 2 Tesla by Ernst and Hennig, FIDs from a VOI (2×2×2 cm3) localized to the visual cortex were collected. FIDs were collected 0, 100, 500, 1500, or 5000 msec after visual stimulation and the subtraction of the baseline data from those acquired with different delays showed a -0.25% signal change at the 500 msec delay and a subsequent signal increase (0.59%) which occurred at 1.5-5 s, suggesting the existence of the initial dip in the MR signal.
The first MR imaging study of the initial response, which occurred shortly after the functional spectroscopy study, was performed on a 4 Tesla whole body (Varian) system using a head-gradient insert and a surface RF coil (Menon et al., 1995). At the time, the scanner did not have a product echo planar imaging (EPI) (Mansfield, 1977) sequence. In fact, that was the reason why the first fMRI study from Minnesota was conducted with a gradient-echo sequence, unlike the reports from Massachusetts General Hospital and Medical College of Wisconsin. In 1994, one of the authors of this review (XH) implemented an EPI sequence on the 4 T scanner, facilitating the study of the initial dip which required high temporal resolution.
Armed with this sequence, Ravi Menon carried out an experiment using a TR of 100 msec. He acquired 500 images during each 50 sec run. Within a run (stimulation period), 100 images were acquired during a 10 second baseline period, followed by 100 images during the visual stimulation (10 s) and finally 300 images during the recovery (30 s). Activation maps were made by subtracting the average of the control EPI images from average EPI images acquired during the first phase. The first phase consisted of images acquired between 0.5 and 2.5 s after the onset of visual stimulation and the second consisted of images acquired between 5 s and 15 s after the beginning of the visual stimulation. Maps were generated from a single trial on a single subject for both the initial signal decrease phase and the subsequent hyperemic response phase. The map of pixels from the first phase exhibited an initial negative response and contained areas more localized to the cortical gray matter identified above and below the calcarine fissure. In contrast, the map corresponding to the second phase was more widespread spatially, including some large veins. The time course of pixels exhibiting the early negative response showed that the negative response reached a maximum at 2s after stimulus onset and had a peak amplitude change of approximately 1% from the baseline signal intensity. Along with the optical imaging data, this initial MR imaging result lent support to the following view of the BOLD phenomena. The activity of the neurons draws oxygen out of the local capillary network, resulting in a local increase in paramagnetic deoxyhemoglobin and hence a decrease in MR image intensity. The hemodynamic response eventually overcompensates for this initial oxygen demand, resulting in a net decrease in local deoxyhemoglobin concentration, and thereby an increase in the MR signal. The fMRS study (Ernst and Hennig, 1994) appeared in print at the galley stage of this first fMRI paper. The fMRS study reported a smaller, shorter duration response and suggested different mechanisms. Menon et al (Menon et al., 1995) suggested the differences may be due to differences in stimuli (brief vs long durations) and/or partial voluming effects. A subsequent fMRS study performed by the same German group examined the echo time and stimulus duration dependence of the early fast response with fMRS (Hennig et al., 1995). It was reported that the amplitude of the early response decreased (although not significantly) with increasing TE as well as with stimulus duration. This observation seemed to contradict the BOLD nature of the response and did not fully agree with the optical imaging studies, raising some doubts about the detectability of deoxyhemoglobin based initial dip with MR, suggesting mechanisms related to T1 or proton density rather than oxygenation. The discrepancies between the fMRI and fMRS data set the stage for a series of subsequent fMRI studies (primarily by our group) (Hu et al., 1997; Menon et al., 1995; Yacoub and Hu, 1999, 2001; Yacoub et al., 1999; Yacoub et al., 2001) and commenced the infamous controversy surrounding the initial dip.
The Mechanism behind the fMRI Initial Dip
The first MR study of the initial dip (Menon et al., 1995) relied on subject breath-hold, prohibiting the use of multiple epochs in each run. In addition, with a single stimulus duration of 10 s, that study did not examine the early response’s dependence on stimulus duration. In view of a subsequent optical imaging study which examined visual stimuli using different durations (2-4 s) and showed that the early response is independent of the stimulus duration, we sought out to investigate the early response more extensively. We wanted to: 1) demonstrate the early response in individual subjects and 2) to examine the stimulus duration dependence of the early response. This extensive study was made possible by taking advantage of a technique for removing physiological fluctuations (Hu et al., 1995; Le and Hu, 1996) so that experiments could be performed without the need for breath-holding. In fact, when the physiological fluctuation removing technique was developed in 1995, we were searching for applications for the technique and the study of the initial dip became an ideal application for it. Data from this study showed that the initial dip was reliably detectable for a range of stimulus durations (1.5-6 s). More interestingly, the amplitude and duration of the initial dip did not change significantly with the stimulus duration except for short stimuli (1.5 s and 2.4 s). Despite the large disparity in spatial resolution, the fMRI data mirrored the findings in optical imaging (Frostig et al., 1990; Grinvald et al., 1991; Malonek and Grinvald, 1996) in several aspects. First, the duration and peak location of the early response are identical to those revealed by optical imaging. Second, the stimulus duration dependence is consistent with optical imaging data in that the early response in the MR signal is independent of the stimulus duration above 2 s. Third, the ratio of the peak positive signal change to the peak negative signal change is also consistent with the optical imaging observation, revealing that the initial increase in deoxyhemoglobin is about 1/3 of the subsequent decrease in deoxyhemoglobin arising from the over compensation. Finally, as expected from the optical imaging result (Frostig et al., 1990; Grinvald et al., 1991; Malonek and Grinvald, 1996), the maps from the early response were more localized when compared with the maps obtained from the hyperemic positive response. This fMRI study not only confirmed the existence of this initial negative change in the MR signal, but also provided independent confirmation of the results of the optical imaging studies. Interestingly, the fMRI data was a main topic at a dinner between XH, Amiram Grinvald and Kamil Ugurbil during the Human Brain Mapping meeting in Copenhagen in 1997.
However, the interest in the initial dip and the controversy surrounding it continued to grow primarily because it was not widely detected by other groups. The discrepancy in the TE dependence between fMRI and fMRS data, and whether or not the initial dip was related to oxygenation, led to suggestions that the response might be due to inflow effects, because of short TRs used, or that it was an artifact due to insufficient inter-stimulus intervals (Fransson et al., 1998; Lai et al., 1998), resulting in contamination with the post-stimulus undershoot after time locked averaging. A preliminary fMRI study, conducted on a 4.1 T system using a 6-segment spiral imaging sequence (McIntosh et al., 1996), sought to investigate the TE dependence of the initial dip with 3 echo times. The data showed that there is a log-linear dependence on the echo-time, consistent with a T2* dependence. However, due to the use of a brief inter-stimulus delay and short echo times, the result of that study was somewhat inconclusive. We then conducted a follow-up study evaluating both the echo time and inter-stimulus interval dependence of the initial dip (Yacoub et al., 1999). This paper established that the initial dip was indeed a BOLD (or T2/T2*) related phenomenon as the response increased with increasing TE and was not an artifact of short inter-stimulus intervals. The difference between the fMRI and fMRS results was attributed to a partial voluming effect.
The Initial Dip in fMRI at other Field Strengths
While successfully addressing many potential confounds and concerns in our initial papers, getting to the root of the mechanism was a more difficult problem given the vascular complexity of the BOLD signal. For example, modeling studies (i.e. balloon model (Buxton et al., 1998) suggested an alternative explanation to oxygenation effects –such as a fast initial change in blood volume, which was unlikely to originate from tissue regions (i.e. capillary recruitment). Being in Minneapolis, we were in the unique position of having access to not only high magnetic field strengths (4T), but also a conventional clinical 1.5T and, at the peak of the initial dip controversy, the first ever human 7 Tesla magnet. Further, given the field strength predictions of the vascular contributions to the BOLD signal (Kennan et al., 1994a; Kennan et al., 1994b; Ogawa et al., 1993b; Weisskoff et al., 1994), the investigation of the initial dip at different field strengths could provide valuable insight into its mechanism. Namely, if the microvascular component of the BOLD response was increasing faster than the macrovascular, as predicted by BOLD signal theory, and if the initial dip arose from primarily tissue oxygenation effects (i.e microvasculature), as opposed to the delayed positive response which contained a significant large vessel component, then the ratio of the 2 responses should change with field strength. Our lab proceeded to carry out 2 additional studies on the initial dip, one at 1.5T (Yacoub and Hu, 1999) and one at 7T (Yacoub et al., 2001), providing data on the field strength dependence. We found that the ratio of the initial response to positive response decreased at 1.5 T (0.1) and increased at 7T (0.6) compared to 4 T data (0.3). While the study at 1.5T provided information on the mechanism of the initial dip, due to how small the response was, it was going to be difficult to make it useful for mapping at lower field strengths, and it remained to be seen whether even high field strengths could make use of it. An independent study performed by a different group at 3 Tesla with a spiral imaging sequence also reported the initial dip (Vazquez et al., 1997). Spatial characteristics of the maps were comparable to those observed at 4 T. Interestingly, the amplitude of the early response relative to the amplitude of the late response was about 0.2, falling right between our results at 1.5T (0.1) and 4T (0.3). Further, the Hennig group, in addition to their fMRS studies, conducted fMRI studies at 2T, reporting again the initial dip in human visual cortrex (Janz et al., 2000; Janz et al., 1997), providing more evidence of the existence and detectability of the initial dip at different field strengths. Ultimately, the field strength dependence data was concordant with the view that the early response may predominantly reflect changes in the microvascular circulation, whose BOLD contrast is expected to scale quadratically with the field, while the positive response may scale less than quadratically.
Extension to other Models and Sensory Areas
In addition to the controversial mechanism of the initial dip (Ances, 2004; Buxton, 2001), it was also becoming peculiar and somewhat concerning that the detection of the initial dip was limited primarily to selected labs doing visual stimulation (Ances, 2004), primarily in humans. It was argued by us and others that the response was small and short that one needed extremely high SNR with short TRs, high magnetic fields, and sophisticated physiological noise removal in order to reliably see the response. However, animal studies, which should have been in a much better position with controlled physiology and even higher SNR, did not always observe the response. fMRI studies in the rat somatosensory cortex (Mandeville et al., 1999; Marota et al., 1999; Silva et al., 2000; Silva et al., 1999), some from Minnesota, with extremely high spatial and temporal resolution and sensitivity, were unable to detect the initial dip. Further, it was suggested that CBF and oxygen metabolism were tightly coupled even immediately after stimulation, casting doubt on the proposed delayed CBF delivery theory. Some hypothesized that the lack of initial dip observed in the rat may be due to vascular differences across species or possibly even region specific differences (i.e. an effect only in visual cortex). There were early conflicting results in the cat visual cortex, where a couple of fMRI studies reported the initial dip (Duong et al., 2000; Kim et al., 2000a), while another did not (Jezzard et al., 1997). A monkey fMRI study (Logothetis et al., 1999) did detect the early response in visual areas. In addition to discrepancies in MR data, to fuel the debate even more, there were conflicting reports in optical imaging of the rat where several studies demonstrated the initial dip (Jones et al., 2001; Mayhew et al., 2001; Nemoto et al., 1999; Vanzetta and Grinvald, 2001), while another did not (Lindauer et al., 2001). Questions also began to surface about the optical models and that artifactual increases in the deoxyhemoglobin level can be observed because of the optical path length analysis techniques. Vanzetta et al (Vanzetta and Grinvald, 1999) did follow up this criticism with a different technique (phosphorescence quenching by oxygen of an agent confined to blood) and again showed the there is an early change in the average PO2. However, the technique inaccurately assumes a well-mixed blood compartment with uniform pressure. The differences between the optical studies in the rat were suggestive of possible differences in physiological conditions, including anesthesia. It was also proposed that the initial dip may be smaller in the rat than in the cat or monkey (Vanzetta and Grinvald, 2001). Amidst these controversies within and between animals and humans in both MR and optical imaging, we conducted another human fMRI study to ascertain whether or not the initial dip could be detected in another (non-visual) area. In this study, we used a visual-motor (finger-tapping) paradigm, simultaneously monitoring BOLD signals in visual and motor cortex. We found that the initial dip was present in motor areas and behaved remarkably similar to what we had found in the visual cortex. The response peaked around 2 sec after stimulation and was more prominent in tissue areas.
Physiological Variations affecting the Initial Dip
The initial suggestion that the dip was due to an early increase in oxygen consumption provided insight into the governing mechanisms of metabolism and cerebral blood flow. Namely, it challenged the idea that blood flow and metabolism were tightly coupled as this early increase in oxygen consumption preceded changes in blood flow. Further, this lag in CBF may explain the variance in the observation of the initial dip. The degree and type of anesthesia, for example, might resolve some of the discrepancies. Studies investigating this showed that the dip was larger in the awake versus anesthetized animal and the degree of anesthesia influenced the coupling between metabolism and blood flow (Jones et al., 2001; Shtoyerman et al., 2000; Vanzetta and Grinvald, 2001). Another study showed that the oxygen levels in the blood could also alter the amplitude of the initial dip (Mayhew et al., 2001). They showed that when higher concentrations of oxygen were inhaled the amplitude of the initial dip was reduced. Levels of hypercapnia, which may increase oxygen consumption (Hyder et al., 1996; Hyder et al., 2000), were also suggested as a possible explanation of the variance in the initial dip (Ances, 2004). This in turn has implications for anesthetized animal studies and the initial dip. Initial animal studies using isoflurane, a vasodilator which slows the blood flow response, had typically detected the initial dip (Duong et al., 2000; Kim et al., 2000a; Logothetis et al., 1999), while studies using alpha-cholralose, which reduces the baseline blood flow, had generally not reported the initial dip (Mandeville et al., 1999; Marota et al., 1999; Silva et al., 2000). One group took this idea even further by intentionally suppressing the CBF response in order to more clearly visualize this potential early deoxygenation state (Fukuda et al., 2006). While they were able to do this using sodium nitroprusside to vasodilate, they found that only the first 2 seconds following stimulation exhibited the increased spatial specificity needed to map cortical columns. The corollary to this in the human has also been postulated with subject differences in caffeine (Behzadi and Liu, 2006), a vasoconstrictor which affects the blood flow response. That study, which gave doses of caffeine to subjects, hypothesized that the vasoconstriction induced by the caffeine caused the blood flow response to be quicker, thereby reducing the magnitude of the initial dip. While there does appear to be trends in the published data that may explain the different findings regarding the initial dip and a possible unifying mechanism (Ances, 2004), controversy never seemed to vanish as there were still studies that suggested other things. A study which used isoflurane did not detect the initial dip (Lindauer et al., 2001), while a study which used alpha-chloralose did (Ances et al., 2001). Ultimately, there are likely many factors which come into play and affect whether or not the initial dip will be (or was) observed, including physiological conditions, experimental methodologies, species, regions, data analysis, etc. Because of the many of the above referenced studies, it is unlikely that the observed signal is/was an artifact and more likely that the many physiological parameters affect the dip, leading to the discrepancies and the controversy that followed.
The Utility of the Initial Dip
In the end even if the mystery behind and the elusiveness of the initial dip or the mechanism from which it arises can be solved, whether or not it could be useful for fMRI was a completely different story. Optical imaging studies examining cortical columns in the visual cortex in cats (Malonek and Grinvald, 1996), revealed a delayed response that extended beyond the true area of activation, thereby suggesting that a 2-3 mm fundamental spatial resolution limitation of fMRI. On the other hand, they also demonstrated that when the initial deoxyhemoglobin increase is selectively mapped, the optical imaging signal provided column specific changes, indicating that the mapping of the early response can potentially overcome the spatial limitation of fMRI methodology.
Despite the many technical experiments and subsequent papers on the initial dip coming from Minnesota, albeit done primarily by one group (the authors of this review: EY under the advisement of XH), there were still doubts by many investigators even in Minnesota about the existence and utility of the fMRI initial dip in humans or animals. One reluctant believer was Seong-Gi Kim, who viewed the results we obtained with a cool reception, despite the authors being close friends with him and he being on EY’s PhD committee. Further, EY’s thesis work, under the supervision of XH, was on the initial dip in fMRI. At the time, his group was studying orientation columns in the cat visual cortex with multiple fMRI contrasts, including T2* weighted BOLD, CBV weighted imaging with the use of MION, and CBF. Dae-Shik Kim, a post-doctoral fellow in Seong-Gi’s lab, without having sought them out, observed pixels with the initial dip when he was looking at the T2*-weighted BOLD fMRI data of the cat visual cortex following the presentation of orientation gratings. The data excited us and became big news in our center as it represented an independent confirmation. Furthermore, they went on with further analysis and showed that the initial dip was able to, using single condition mapping, provide exquisite maps of orientation columns in the cat visual cortex while the hyperemic response did not exhibit the expected columnar structures. This was published in Nature Neuroscience and provided perhaps the most well-known demonstration of the initial dip (Duong et al., 2000; Kim et al., 2000a), while in turn unintentionally helping make the case for EY’s thesis defense.
The Years following the Initial Studies
While this study had enormous implications for the initial dip and the potential of fMRI mapping, it also, like everything else surrounding the initial dip, generated a lot of controversy. Logothetis et al (Kim et al., 2000b; Logothetis, 2000) raised doubt on the reliability, reproducibility, and interpretation of the results in a direct commentary on the paper. To date, since the study in the cat, there have been no additional studies in animals or in humans that have demonstrated the neuroscience utility of the initial dip to improve the spatial specificity of fMRI based mapping signals. A very recent study (Tian et al., 2010) did evaluate the initial dip at the level of cortical layers and demonstrated that the initial dip was most significant in the superficial layers and coincided with areas that experienced the most delayed onset of increased blood flow. These results were consistent with an early increase in oxygenation while the laminar dependence provided some explanation of differences between the optical data and fMRI – as fMRI can be sensitive to different layers while optical imaging is strongly sensitive to the superficial layers. Ultimately, while studies have continued to report the initial dip (Yesilyurt et al., 2008), because of the inability to make wide use of it, likely due to is short duration and low amplitude in addition to its aforementioned elusiveness, the controversy and interest in it, although still present (Sirotin et al., 2009; Uludag, 2010; Vanzetta and Grinvald, 2008) have faded significantly over the years. Alternatively, many fMRI studies have used differential mapping techniques (Cheng et al., 2001; Menon et al., 1997) and spin echo based BOLD (Yacoub et al., 2008; Yacoub et al., 2007) to suppress non-specific signals for increased spatial specificity at high resolution. While this has proven successful for mapping of cortical columns in humans, it requires a priori knowledge of orthogonal conditions. Although animal studies have been able to map columns with single condition methods using CBF (Duong et al., 2001) or CBV-based methods with contrast enhancement (Duong et al., 2001; Moon et al., 2007; Zhao et al., 2005), the translation to human studies has not been achieved.
Conclusions
Over the past 2 decades, while the fMRI technique has revolutionized the study of human brain function, the spatial accuracy and interpretation of the associated signals have always been a subject of debate. The BOLD technique reflects changes in blood oxygenation, cerebral blood flow, and blood volume, which are all dynamic in time and can be highly variable in space depending on experimental parameters. This complexity or “richness” has allowed the spatial accuracy and functional information garnered to match and remarkably in some cases possibly even surpass what can be learned from invasive animal techniques (Maier et al., 2008). Ironically, this has also been the reason why there has been so much controversy regarding the associated signals, such as the initial dip. While the initial dip is unlikely to be used extensively in fMRI mapping, it has and may continue to provide significant insight into our understanding of neurovascular coupling associated with brain activation. The understanding of the physiological signals and ultimate limits of fMRI will continue to grow with higher magnetic fields with advancements in MR hardware and pulse sequence design leading to higher spatial specificities and contrast to noise ratios.
Acknowledgments
Work supported in part by National Institutes of Health (grants, R01 EB000331, P41 RR08079, RO1EB002009), the W.M. Keck Foundation, Georgia Research Alliance, and MIND institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ances BM. Coupling of changes in cerebral blood flow with neural activity: what must initially dip must come back up. J Cereb Blood Flow Metab. 2004;24:1–6. doi: 10.1097/01.WCB.0000103920.96801.12. [DOI] [PubMed] [Google Scholar]
- Ances BM, Buerk DG, Greenberg JH, Detre JA. Temporal dynamics of the partial pressure of brain tissue oxygen during functional forepaw stimulation in rats. Neurosci Lett. 2001;306:106–110. doi: 10.1016/s0304-3940(01)01868-7. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med. 1992;25:390–398. doi: 10.1002/mrm.1910250220. [DOI] [PubMed] [Google Scholar]
- Bandettini PA, Wong EC, Jesmanowicz A, Hinks RS, Hyde JS. Spin-echo and gradient-echo EPI of human brain activation using BOLD contrast: a comparative study at 1.5 T. NMR Biomed. 1994;7:12–20. doi: 10.1002/nbm.1940070104. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Liu TT. Caffeine reduces the initial dip in the visual BOLD response at 3 T. Neuroimage. 2006;32:9–15. doi: 10.1016/j.neuroimage.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Buxton RB. The elusive initial dip. Neuroimage. 2001;13:953–958. doi: 10.1006/nimg.2001.0814. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: The balloon model. Magn Reson Med. 1998;39:855–864. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- Cheng K, Waggoner RA, Tanaka K. Human Ocular Dominance Columns as Revealed by High-Field Functional Magnetic Resonance Imaging. Neuron. 2001;32:359–374. doi: 10.1016/s0896-6273(01)00477-9. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Kim DS, Ugurbil K, Kim SG. Spatio-temporal dynamics of the BOLD fMRI signals: toward mapping submillimeter cortical columns using the early negative response. Magn Reson Med. 2000;44:231–242. doi: 10.1002/1522-2594(200008)44:2<231::aid-mrm10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Kim DS, Ugurbil K, Kim SG. Localized cerebral blood flow response at submillimeter columnar resolution. Proc Natl Acad Sci U S A. 2001;98:10904–10909. doi: 10.1073/pnas.191101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyn JH, Moonen CTW, Yperen GH, Boer RW, Luyten PR. Inflow versus deoxyhemoglobin effects in BOLD functional MRI using gradient echoes at 1.5T. NMR in Biomed. 1994;7:83–88. doi: 10.1002/nbm.1940070113. [DOI] [PubMed] [Google Scholar]
- Ernst T, Hennig J. Observation of a fast response in functional MR. Magn Reson Med. 1994;32:146–149. doi: 10.1002/mrm.1910320122. [DOI] [PubMed] [Google Scholar]
- Frahm J, Merboldt KD, Hanicke W, Kleinschmidt A, Boecker H. Brain or vein-oxygenation or flow? On signal physiology in functional MRI of human brain activation. NMR in Biomed. 1994;7:45–53. doi: 10.1002/nbm.1940070108. [DOI] [PubMed] [Google Scholar]
- Fransson P, Kruger G, Merboldt KD, Frahm J. The temporal evolution of oxygen-sensitive MRI responses to visual activations in humnas. Magn Reson Med. 1998;39:912–919. doi: 10.1002/mrm.1910390608. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Ts’o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci USA. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Wang P, Moon CH, Tanifuji M, Kim SG. Spatial specificity of the enhanced dip inherently induced by prolonged oxygen consumption in cat visual cortex: implication for columnar resolution functional MRI. Neuroimage. 2006;30:70–87. doi: 10.1016/j.neuroimage.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Grinvald A, Frostig RD, Siegel RM, Bartfeld RM. High-resolution optical imaging of functional brain architecture in the awake monkey. Proc Natl Acad Sci USA. 1991;88:11559–11563. doi: 10.1073/pnas.88.24.11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Hopkins A, Lai S, Buckley P, Friedman L, Meltzer H, Hedera P, Friedland R, Klein S, Thompson L, et al. 2D and 3D high resolution gradient echo functional imaging of the brain: venous contributions to signal in motor cortex studies. NMR Biomed. 1994;7:54–62. doi: 10.1002/nbm.1940070109. [DOI] [PubMed] [Google Scholar]
- Hennig J, Janz C, Speck O, Ernst T. Functional spectroscopy of brain activation following a single light pulse: examination of the mechanism of the fast initial response. Int J Imag Sys Tech. 1995;6:203–208. [Google Scholar]
- Hu X, Le TH, Parrish T, Erhard P. Retrospective estimation and correction of physiological fluctuation in functional MRI. Magn Reson Med. 1995;34:201–212. doi: 10.1002/mrm.1910340211. [DOI] [PubMed] [Google Scholar]
- Hu X, Le TH, Ugurbil K. Evaluation of the early response in fMRI in individual subjects using short stimulus duration. Magn Reson Med. 1997;37:877–884. doi: 10.1002/mrm.1910370612. [DOI] [PubMed] [Google Scholar]
- Hyder F, Chase JR, Behar KL, Mason GF, Siddeek M, Rothman DL, Shulman RG. Increased tricarboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C]NMR. Proc Natl Acad Sci U S A. 1996;93:7612–7617. doi: 10.1073/pnas.93.15.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Kennan RP, Kida I, Mason GF, Behar KL, Rothman D. Dependence of oxygen delivery on blood flow in rat brain: a 7 tesla nuclear magnetic resonance study. J Cereb Blood Flow Metab. 2000;20:485–498. doi: 10.1097/00004647-200003000-00007. [DOI] [PubMed] [Google Scholar]
- Janz C, Schmitt C, Speck O, Hennig J. Comparison of the hemodynamic response to different visual stimuli in single-event and block stimulation fMRI experiments. J Magn Reson Imaging. 2000;12:708–714. doi: 10.1002/1522-2586(200011)12:5<708::aid-jmri7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Janz C, Speck O, Hennig J. Time-resolved measurements of brain activation after a short visual stimulus: new results on the physiological mechanisms of the cortical response. NMR Biomed. 1997;10:222–229. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<222::aid-nbm462>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Rauschecker JP, Malonek D. An in vivo model for functional MRI in cat visual cortex. Magn Reson Med. 1997;38:699–705. doi: 10.1002/mrm.1910380504. [DOI] [PubMed] [Google Scholar]
- Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. Neuroimage. 2001;13:1002–1015. doi: 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- Kennan RP, Gao J-H, Zhong J, Gore JC. A general model of microcirculatory blood flow effects in gradient sensitized MRI. Med Phys. 1994a;21:539–545. doi: 10.1118/1.597170. [DOI] [PubMed] [Google Scholar]
- Kennan RP, Zhong J, Gore JC. Intravascular susceptibility contrast mechanisms in tissues. Magn Reson Med. 1994b;31:9–21. doi: 10.1002/mrm.1910310103. [DOI] [PubMed] [Google Scholar]
- Kim DS, Duong T, Kim S-G. High-resolution mapping of iso-orientation columns by fMRI. Nature Neuroscience. 2000a;3:164–169. doi: 10.1038/72109. [DOI] [PubMed] [Google Scholar]
- Kim DS, Duong TQ, Kim SG. Reply to “Can current fMRI techniques reveal the micro-architecture of cortex?”. Nat Neurosci. 2000b;3:414. doi: 10.1038/74771. [DOI] [PubMed] [Google Scholar]
- Kim S-G, Hendrich K, Hu X, Merkle H, Ugurbil K. Potential pitfalls of functional MRI using conventional gradient-recalled echo techniques. NMR in Biomed. 1994;7:69–74. doi: 10.1002/nbm.1940070111. [DOI] [PubMed] [Google Scholar]
- Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng H-M, Brady TJ, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Glover GH, Baseler HA. Pseudo fast response in functional MRI. Sixth Annual Meeting of the International Society of Magnetic Resonance in Medicine; Sydney, Australia. 1998. p. 1425. [Google Scholar]
- Lai S, Hopkins AL, Haacke EM, Li D, Wasserman BA, Buckley P, Friedman L, Meltzer H, Hedera P, Friedland R. Identification of vascular structures as a major source of signal contrast in high resolution 2D and 3D functional activation imaging of the motor cortex at 1.5 T: Preliminary results. Magn Reson Med. 1993;30:387–392. doi: 10.1002/mrm.1910300318. [DOI] [PubMed] [Google Scholar]
- Le TH, Hu X. Retrospective estimation and correction of physiological artifacts in fMRI by direct extraction of physiological activity from MR data. Magn Reson Med. 1996;35:290–298. doi: 10.1002/mrm.1910350305. [DOI] [PubMed] [Google Scholar]
- Lee AT, Glover GH, Meyer GH. Discrimination of large venous vessels in time-course spiral blood-oxygen-level-dependent magnetic resonance functional neuroimaging. Magn Reson Med. 1995;33:745–754. doi: 10.1002/mrm.1910330602. [DOI] [PubMed] [Google Scholar]
- Lindauer U, Royl G, Leithner C, Kuhl M, Gold L, Gethmann J, Kohl-Bareis M, Villringer A, Dirnagl U. No evidence for early decrease in blood oxygenation in rat whisker cortex in response to functional activation. Neuroimage. 2001;13:988–1001. doi: 10.1006/nimg.2000.0709. [DOI] [PubMed] [Google Scholar]
- Logothetis N. Can current fMRI techniques reveal the micro-architecture of cortex? Nat Neurosci. 2000;3:413–414. doi: 10.1038/74768. [DOI] [PubMed] [Google Scholar]
- Logothetis N, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nature Neuroscience. 1999;2:552–562. doi: 10.1038/9210. [DOI] [PubMed] [Google Scholar]
- Maier A, Wilke M, Aura C, Zhu C, Ye FQ, Leopold DA. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat Neurosci. 2008;11:1193–1200. doi: 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- Mandeville JB, Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR. MRI measurement of the temporal evolution of relative CMRO(2) during rat forepaw stimulation. Magn Reson Med. 1999;42:944–951. doi: 10.1002/(sici)1522-2594(199911)42:5<944::aid-mrm15>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Mansfield P. Multi-planar image formation using NMR spin echoes. J phys C: Solid State Phys. 1977;10:L55–L58. [Google Scholar]
- Marota JJ, Ayata C, Moskowitz MA, Weisskoff RM, Rosen BR, Mandeville JB. Investigation of the early response to rat forepaw stimulation. Magn Reson Med. 1999;41:247–252. doi: 10.1002/(sici)1522-2594(199902)41:2<247::aid-mrm6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Mayhew J, Johnston D, Martindale J, Jones M, Berwick J, Zheng Y. Increased oxygen consumption following activation of brain: theoretical footnotes using spectroscopic data from barrel cortex. Neuroimage. 2001;13:975–987. doi: 10.1006/nimg.2001.0807. [DOI] [PubMed] [Google Scholar]
- McIntosh J, Zhang Y, Kidambi S, Harshbarger T, Mason G, Prohost GM, Twieg D. Echo-time dependence of the functional MRI ‘fast response’. Soc Magn Reson Med 4th Scientific Meeting; New York, NY. 1996. p. 284. [Google Scholar]
- Menon R, Ogawa S, Strupp JP, Ugurbil K. Ocular dominance in human V1 demonstrated by functional magnetic resonance imaging. J Neurophysiol. 1997;77:2780–2787. doi: 10.1152/jn.1997.77.5.2780. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Hu X, Strupp JS, Andersen P, Ugurbil K. BOLD based functional MRI at 4 tesla includes a capillary bed contribution: Echo-planar imaging mirrors previous optical imaging using intrinsic signals. Magn Reson Med. 1995;33:453–459. doi: 10.1002/mrm.1910330323. [DOI] [PubMed] [Google Scholar]
- Menon RS, Ogawa S, Tank DW, Ugurbil K. 4 Tesla gradient recalled echo characteristics of photic stimulation-induced signal changes in the human primary visual cortex. Magn Reson Med. 1993;30:380–386. doi: 10.1002/mrm.1910300317. [DOI] [PubMed] [Google Scholar]
- Moon CH, Fukuda M, Park SH, Kim SG. Neural interpretation of blood oxygenation level-dependent fMRI maps at submillimeter columnar resolution. J Neurosci. 2007;27:6892–6902. doi: 10.1523/JNEUROSCI.0445-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto M, Nomura Y, Sato C, Tamura M, Houkin K, Koyanagi I, Abe H. Analysis of optical signals evoked by peripheral nerve stimulation in rat somatosensory cortex: dynamic changes in hemoglobin concentration and oxygenation. J Cereb Blood Flow Metab. 1999;19:246–259. doi: 10.1097/00004647-199903000-00002. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee T-M, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990a;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Lee T-M, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990b;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Barrere B. Sensitivity of magnetic resonance image signals of a rat brain to changes in the cerebral venous blood oxygenation. Magn Reson Med. 1993a;29:205–210. doi: 10.1002/mrm.1910290208. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim S-G, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. Biophys J. 1993b;64:800–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim S-G, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segebarth C, Belle V, Delon C, Massarelli R, Decety J, Le Bas J-F, Decorpts M, Benabied AL. Functional MRI of the human brain: Predominance of signals from extracerebral veins. Neuroreport. 1994;5:813–816. doi: 10.1097/00001756-199403000-00019. [DOI] [PubMed] [Google Scholar]
- Shtoyerman E, Arieli A, Slovin H, Vanzetta I, Grinvald A. Long-term optical imaging and spectroscopy reveal mechanisms underlying the intrinsic signal and stability of cortical maps in V1 of behaving monkeys. J Neurosci. 2000;20:8111–8121. doi: 10.1523/JNEUROSCI.20-21-08111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Iadecola C, Kim SG. Early temporal characteristics of cerebral blood flow and deoxyhemoglobin changes during somatosensory stimulation. J Cereb Blood Flow Metab. 2000;20:201–206. doi: 10.1097/00004647-200001000-00025. [DOI] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J Cereb Blood Flow Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Sirotin YB, Hillman EM, Bordier C, Das A. Spatiotemporal precision and hemodynamic mechanism of optical point spreads in alert primates. Proc Natl Acad Sci U S A. 2009;106:18390–18395. doi: 10.1073/pnas.0905509106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian P, Teng IC, May LD, Kurz R, Lu K, Scadeng M, Hillman EM, De Crespigny AJ, D’Arceuil HE, Mandeville JB, Marota JJ, Rosen BR, Liu TT, Boas DA, Buxton RB, Dale AM, Devor A. Cortical depth-specific microvascular dilation underlies laminar differences in blood oxygenation level-dependent functional MRI signal. Proc Natl Acad Sci U S A. 2010;107:15246–15251. doi: 10.1073/pnas.1006735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uludag K. To dip or not to dip: reconciling optical imaging and fMRI data. Proc Natl Acad Sci U S A. 2010;107:E23. doi: 10.1073/pnas.0914194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A. Increased Cortical Oxidative Metabolism Due to Sensory Stimulation: Implications for Functional Brain Imaging. Science. 1999;286:1555–1558. doi: 10.1126/science.286.5444.1555. [DOI] [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A. Evidence and lack of evidence for the initial dip in the anesthetized rat: implications for human functional brain imaging. Neuroimage. 2001;13:959–967. doi: 10.1006/nimg.2001.0843. [DOI] [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A. Coupling between neuronal activity and microcirculation: implications for functional brain imaging. HFSP J. 2008;2:79–98. doi: 10.2976/1.2889618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez A, Peltier S, Davis D, Noll D. Evidence of the fast response at 3.0 T. Fifth Annual Meeting of the International Society of Magnetic Resonance in Medicine; Vancouer, Canada. 1997. p. 726. [Google Scholar]
- Weisskoff RM, Zuo CS, Boxerman JL, Rosen BR. Microscopic susceptibility variation and transverse relaxation: theory and experiment. Magn Reson Med. 1994;31:601–610. doi: 10.1002/mrm.1910310605. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Ugurbil K. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci U S A. 2008;105:10607–10612. doi: 10.1073/pnas.0804110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Hu X. Detection of the early negative response in fMRI at 1.5 Tesla. Magn Reson Med. 1999;41:1088–1092. doi: 10.1002/(sici)1522-2594(199906)41:6<1088::aid-mrm3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Hu X. Detection of the early decrease in fMRI signal in the motor area. Magn Reson Med. 2001;45:184–190. doi: 10.1002/1522-2594(200102)45:2<184::aid-mrm1024>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Le TH, Ugurbil K, Hu X. Further evaluation of the initial negative response in functional magnetic resonance imaging. Magn Reson Med. 1999;41:436–441. doi: 10.1002/(sici)1522-2594(199903)41:3<436::aid-mrm2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Logothetis N, Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. Neuroimage. 2007;37:1161–1177. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Pfeuffer J, Van De Moortele PF, Adriany G, Ugurbil K, Hu X. Investigation of the initial dip in fMRI at 7 Tesla. NMR Biomed. 2001;14:408–412. doi: 10.1002/nbm.715. [DOI] [PubMed] [Google Scholar]
- Yesilyurt B, Ugurbil K, Uludag K. Dynamics and nonlinearities of the BOLD response at very short stimulus durations. Magn Reson Imaging. 2008;26:853–862. doi: 10.1016/j.mri.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Zhao F, Wang P, Hendrich K, Kim SG. Spatial specificity of cerebral blood volume-weighted fMRI responses at columnar resolution. Neuroimage. 2005;27:416–424. doi: 10.1016/j.neuroimage.2005.04.011. [DOI] [PubMed] [Google Scholar]


