Abstract
Objective
Intraoperative imaging of intravascular thrombi is limited by the inability of visible light to penetrate thick-walled vessels. Near-infrared (NIR) light has relatively high tissue penetration, and low autofluorescence and scatter, offering significant advantages. We hypothesized that the development of 700-nm NIR fluorophores for platelet labeling, in conjunction with existing 800-nm NIR fluorophores, would permit simultaneous and separable quantitation of intravascular thrombi, and measurement of the antiplatelet effect of drugs.
Methods
We synthesized a series of lipophilic, cationic, polymethine indocyanine dyes (MHI-86, 94, 106, 114) that emit at approximately 700 nm. Platelet uptake was optimized in vitro and the bioactivity and blood half-life of labeled platelets was characterized in vitro and in vivo. FeCl3-induced injury of the femoral arteries and intravascular thrombus formation was performed in 35-kg Yorkshire pigs. A combination of 700-nm and 800-nm NIR fluorophore-labeled platelets was used in conjunction with the FLARE™ imaging system to image and quantify the antiplatelet effect of cilostazol and acetylsalicylic acid.
Results
MHI-114 was incorporated at nearly 4.1 ×106 molecules per platelet without affecting platelet function. When infused into pigs, the signal-to-background ratio (SBR) of MHI-114-labeled platelets exhibited a blood half-life of 16.4 ± 2.2 (mean ± SEM; n = 3) min and generated a SBR of 2.5 ± 0.5 (mean ± SEM; n = 3) at the site of thrombi. Utilizing dual-NIR-labeled platelet populations, cilostazol and acetylsalicylic acid were found to cause a reduction in platelet incorporation into thrombi of 51 ± 2% and 10 ± 1% (mean ± SEM; n = 3), respectively, relative to vehicle-only treated control thrombi.
Conclusions
New platelet-avid 700-nm NIR fluorophores permit simultaneous 2-wavelength NIR fluorescence imaging and quantitation of intravascular thrombi in intact vessels approaching the size of humans, and can be used to study the antiplatelet effect of drugs.
INTRODUCTION
Real-time visualization of thrombus formation in intact vessels is an important goal in both research and clinical settings. Although probes such as fluorophore-conjugated antibodies or platelets labeled with fluorescent dyes have been used to visualize thrombi,1–4 visible wavelengths cannot penetrate large vessels due to high absorption and scatter; therefore, this approach has been largely limited to rodents.1–4 Nonoptical probes such as radiotracers and ultrasound enable deep penetration of tissue in large animal models of thrombus formation.5–8 Compared to nonoptical probes, optical imaging enables simultaneous localization of multiple probes emitting at different wavelengths, without the need for ionizing radiation. The use of near-infrared (700 nm to 900 nm) fluorescence emission enables optical imaging of thick tissues. Our group has previously demonstrated the use of a commercially available 800-nm, platelet-avid NIR fluorophore for thrombus imaging in large vessels of pig model systems.9
The enabling technology for our study is the Fluorescence-Assisted Resection and Exploration (FLARE™) optical imaging system, which provides simultaneous acquisition of color video and 2 independent channels of invisible NIR fluorescence, one centered at 700 nm and the other at 800 nm. The potential use of this imaging system is quantitative comparison of 2 cellular subpopulations within a single location. This approach enables direct comparison of a population that has been modified (e.g., by a drug or genetic manipulation) with a control population. For example, a useful technique in assessing mechanisms of thrombus formation and testing antithrombotic compounds has been the use of videomicroscopy for near-simultaneous imaging to measure 2 platelet populations stained with different visible fluorophores.10
In this study, we hypothesized that synthesis of 700-nm platelet-avid NIR fluorophores would permit 2 independent populations of platelets to be studied in large vessels approaching the size of human vessels.
MATERIALS AND METHODS
Preparation of IR-786 or MHI-Dye-Labeled Washed Platelets
For in vitro studies, washed human platelets were prepared from fresh blood obtained from aspirin-free donors and processed using differential centrifugation.4,11 For in vivo studies, autologous washed pig platelets were isolated as reported previously9 from the blood of anesthetized Yorkshire pigs. Briefly, platelet-rich plasma (PRP) was prepared by centrifugation at 200 × g for 20 min. Platelets were then isolated from PRP by centrifugation at 1,400 × g for 10 min in the presence of 50-ng/mL PGE1 and 10% (v/v) acid citrate/dextrose, pH 4.6, and resuspended at a concentration of 4 × 108 cells/mL in Tyrode’s-HEPES buffer. The perchlorate salt of IR-786 (CAS #102185-03-5) was purchased from Sigma-Aldrich (St. Louis, MO). The platelets isolated from one pig were used only for that pig.
Quantification of NIR Dye Uptake into Platelets
Platelet counts were measured using the HEMAVET Multispecies Hematology Analyzer (Drew Scientific, Oxford, CT). To determine which MHI (MHI-84, 96, 106, 114) showed the highest uptake into platelets, 1 mL samples of washed human platelets (approximately 4 × 108 total) were incubated for 0, 15, 30, 60, 90, or 120 min at room temperature (RT) with 4 µM of each MHI dye or 2-µM IR-786 (n = 3 for each dye). To determine the optimal loading concentration, washed human platelets were incubated with 0.5, 1, 2, 4, 8 µM of the NIR fluorophore under study (n = 3 for each dye).
Spectral Measurements
The optical properties of all agents were measured in fetal bovine serum (FBS). The quantum yield (QY) of contrast agents in FBS was calculated using ICG in dimethyl sulfoxide (DMSO) (QY 13%12) as the calibration standard. For in vitro optical property measurements, online fiberoptic HR2000 absorbance (200–1100 nm) and USB2000FL fluorescence (350–1000 nm) spectrometers (Ocean Optics, Dunedin, FL) were used. NIR excitation was provided by a 770-nm NIR laser diode light source (Electro Optical Components, Santa Rosa, CA) set to 8 mW and coupled through a 300-µM core diameter, 0.22-NA fiber (Fiberguide Industries, Stirling, NJ).
NIR Fluorescence Microscopy
NIR fluorescence microscopy was performed on a 4 filter set Nikon Eclipse TE300 epifluorescence microscope as previously described.13 The microscope was equipped with a 100-W mercury light source, NIR-compatible optics, and a NIR-compatible 10× Plan Fluor objective lens and a 100X Plan Apo oil immersion objective lens (Nikon, Melville, NY). Images were acquired on an Orca-AG (Hamamatsu, Bridgewater, NJ). Image acquisition and analysis was performed using IPLab software (Scanalytics, Fairfax, VA). A custom filter set (Chroma Technology Corporation, Brattleboro, VT) composed of a 750 ± 25 nm excitation filter, a 785-nm dichroic mirror, and an 810 ± 20-nm emission filter were used to detect IR-786. For MHI detection, we used 650 ± 22-nm and 710 ± 25-nm excitation and emission filters.
Platelet Aggregation Studies
Control and experimental platelets labeled with MHI-114 or IR-786 were resuspended at a density of 4 × 108 cells/mL in modified Tyrode's-HEPES buffer, and stimulated with agonists in siliconized glass tubes in an optical aggregometer (Chrono-log, Havertown, PA). Assays were performed at 37°C with constant stirring. Aggregation was monitored by measuring the optical density of the platelet suspension.14
Animal Studies
Sixteen female Yorkshire pigs (E.M. Parsons and Sons, Hadley, MA) averaging 35.4 kg were used under the supervision of an approved institutional protocol. Because the standard error of NIR fluorescence measurements was low, and we are mandated by the institutional protocol to use the smallest number of animals needed to assess statistical significance, we used n = 3 pigs to meet the predefined statistical criteria. Consequently, we employed n = 3 pigs for each of the following studies: blood half-life measurement of MHI-114 (n = 3) and IR-786 (n = 3), SBR measurement of MHI-114-labeled thrombi (n = 3), and measurement of the antiplatelet effects of cilostazol (n = 3) and acetylsalicylic acid (n = 3). Additionally, 1 pig was used for in vivo microscopic imaging. All 16 pigs were induced with 4.4-mg/kg intramuscular Telazol™, intubated, and maintained with 2% isoflurane. ECG, heart rate, oxygen saturation, and body temperature were monitored throughout the experiment. A 14G central venous catheter was inserted into the external jugular vein. After preparation, the indicated vessels were exposed using standard surgical techniques and samples processed for hematoxylin and eosin (H&E) staining. Cilostazol, a phosphodiesterase 3A inhibitor was purchased from Tocris Bioscience (Ellisville, MO). Acetylsalicylic acid (aspirin) was purchased from Bayer Healthcare (Morristown, NJ).
Blood Clearance of the NIR Signal
Washed autologous pig platelets (3.9 × 1010) were labeled with MHI-114 or IR-786 and infused through a central venous catheter inserted into the external jugular vein (n = 3 for each dye). Blood samples were obtained at 2, 5, 10, 15, 30, 45, 60, 90, 120, 180 min, and the platelet-rich plasma (PRP) was analyzed. The fluorescence intensity (FI) of PRP was measured. The signal-to-background ratio (SBR) was assessed by quantifying the 12-bit fluorescence signal. For blood clearance, the SBR was defined as the NIR fluorescence intensity of the platelet-rich plasma at the indicated time divided by that of the preinjection platelet-rich plasma.
Vessel Injury Models
Platelets (1.3–1.9 × 1010 total) in Tyrode's-HEPES were labeled with MHI-114 dye or IR-786 and infused intravenously prior to induction of thrombi using FeCl3. For induction of thrombus formation, a 0.5 × 1 cm Whatman Grade 413 Filter Paper was saturated with a 50% solution of FeCl3 (Sigma-Aldrich) and applied beneath the vessel. Parafilm (Pechiney, Chicago, IL) was used to protect the surrounding tissue from oxidative injury induced by FeCl3. Thrombus formation was then imaged continuously until the fluorescence signal was stabilized.
Intraoperative NIR Fluorescence Imaging System and Quantitation
The dual-NIR channel FLARE™ imaging system has been described in detail previously.15,16 In this study, 670-nm excitation and 760-nm excitation fluence rates used were 4.0 and 11.0 mW/cm2, respectively, with white light (400 to 650 nm) at 40,000 lx. Color video and 2 independent channels (700 nm and 800 nm) of NIR fluorescence images were acquired simultaneously with custom software at rates up to 15 Hz over a 15-cm diameter field of view (FOV). In the color-NIR merged images, 700-nm NIR fluorescence (MHI dyes) and 800-nm fluorescence (IR-786) were pseudo-colored red and green, respectively. The imaging system was positioned at a distance of 18 inches from the surgical field. Regions of interest (ROI) of a defined shape and pixel number were drawn to quantify the fluorescence intensity (FI) of intravascular thrombi. SBR was defined as the FI of the ROI over the thrombus divided by that of the same size and shape ROI over the vessel proximal to the thrombus.
Statistical Analysis
Results were presented as mean ± SEM. A one-way ANOVA followed by Tukey’s multiple comparisons test were used to assess the statistical differences between multiple groups. A P value of less than 0.05 was considered significant.
RESULTS
Synthesis and Optical Properties of 700 nm Platelet-Avid NIR Fluorophores
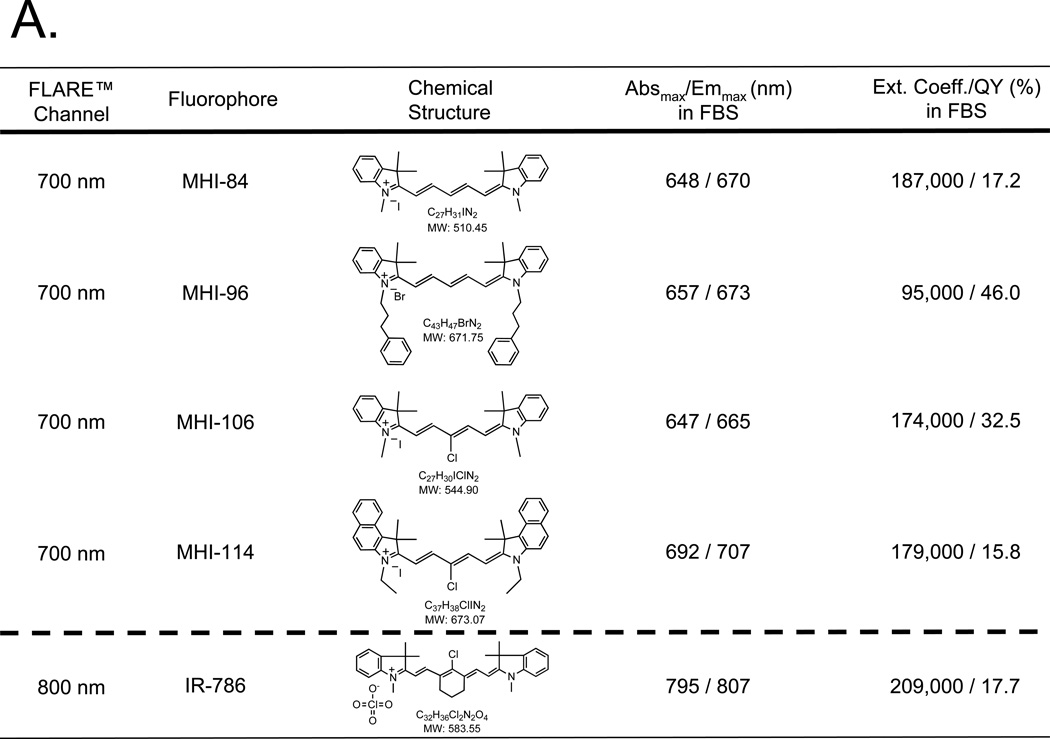
To simultaneously measure 2 different platelet populations in intact blood vessels, we synthesized a novel class of 700-nm emitting, platelet-avid NIR fluorophores that complemented the 800-nm fluorescence of the previously described platelet-labeling agent IR-786. Four structural analogs of IR-786 and their optical properties are shown in Figure 1A. Their detailed chemical synthesis will be described elsewhere (Henary et al., manuscript in preparation). Peak excitation and emission of all 4 NIR fluorophores were compatible with the 700-nm channel of the FLARE™ imaging system.
Figure 1. Optical properties of NIR fluorophores and their incorporation into platelets.
A. Chemical structures and optical properties of novel 700-nm NIR fluorophores (above dashed line) and commercially available 800-nm fluorophore IR-786 (below dashed line).
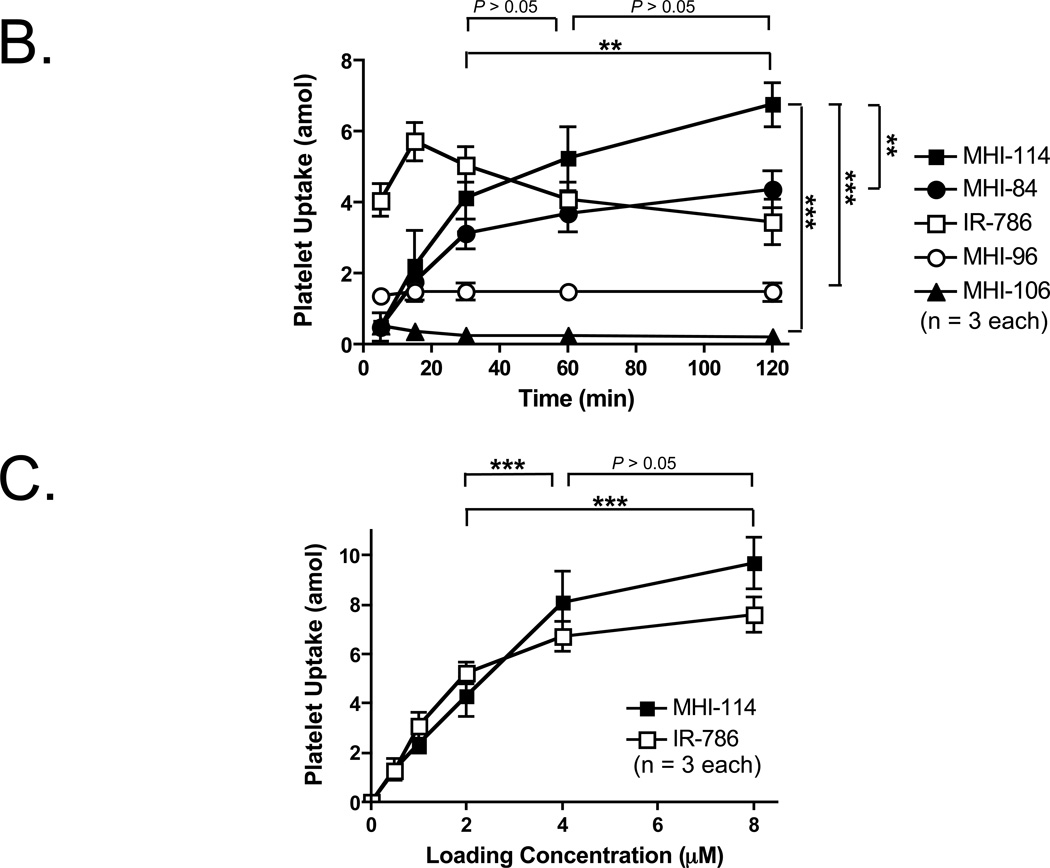
B. NIR fluorophore incorporation into platelets as a function of time at RT. Shown are mean ± SEM for n = 3 independent experiments. Statistical assessments of MHI-114 vs. MHI-106, MHI-114 vs. MHI-96, and MHI-114 vs. MHI-84 (right) are shown. Statistical assessments of the various incubation times of MHI-114 at 30 min vs. 60 min, 60 min vs. 120 min, and 30 min vs. 120 min are also shown. **P < 0.01, and ***P < 0.001, using a one-way ANOVA followed by Tukey’s multiple comparison test.
C. Relationship between extracellular NIR fluorophore concentration and final uptake into platelets for MHI-114 and IR-786. Shown are mean ± SEM for n = 3 independent experiments. Statistical assessments of the various loading concentrations of MHI-114 at 2 µM vs. 4 µM, 2 µM vs. 8 µM, and 4 µM vs. 8 µM are shown. ***P < 0.001, using a one-way ANOVA followed by Tukey’s multiple comparison test.
Optimization of Platelet Loading Parameters
Although all 4 molecules were lipophilic cations, uptake into platelets varied markedly over time (Figure 1B). Because agent MHI-114 showed a significantly higher level of uptake than other MHI dyes (P < 0.01; (Figure 1B), it was chosen for subsequent studies. In terms of the incubation time of MHI-114, the statistical assessment of 30 min vs. 60 min, 60 min vs. 120 min, and 30 min vs. 120 min are show in Figure 1B. The uptake at 120 min is significantly higher than at 30 min (P < 0.01). On the other hand, the uptake at 60 min is not significantly higher than at 30 min. Therefore, 120 min was employed for further studies. In terms of the loading concentration of MHI-114, the statistical assessment of 2 µM vs. 4 µM, 4 µM vs. 8 µM, and 2 µM vs. 8 µM are shown in Figure 1B. This assessment shows there is no significant difference between 4 µM and 8 µM, which suggests that a plateau occurs at ≈ 4 µM. Therefore, 4 µM of MHI-114 was used for subsequent studies. For IR-786, the labeling was performed with 2-µM IR-786 at 30 min in our previous study. 9 The incubation time and loading concentration for IR-786 was also used in the current study.
Bioactivity and Blood Half-Life of MHI-114- and IR-786-Labeled Platelets
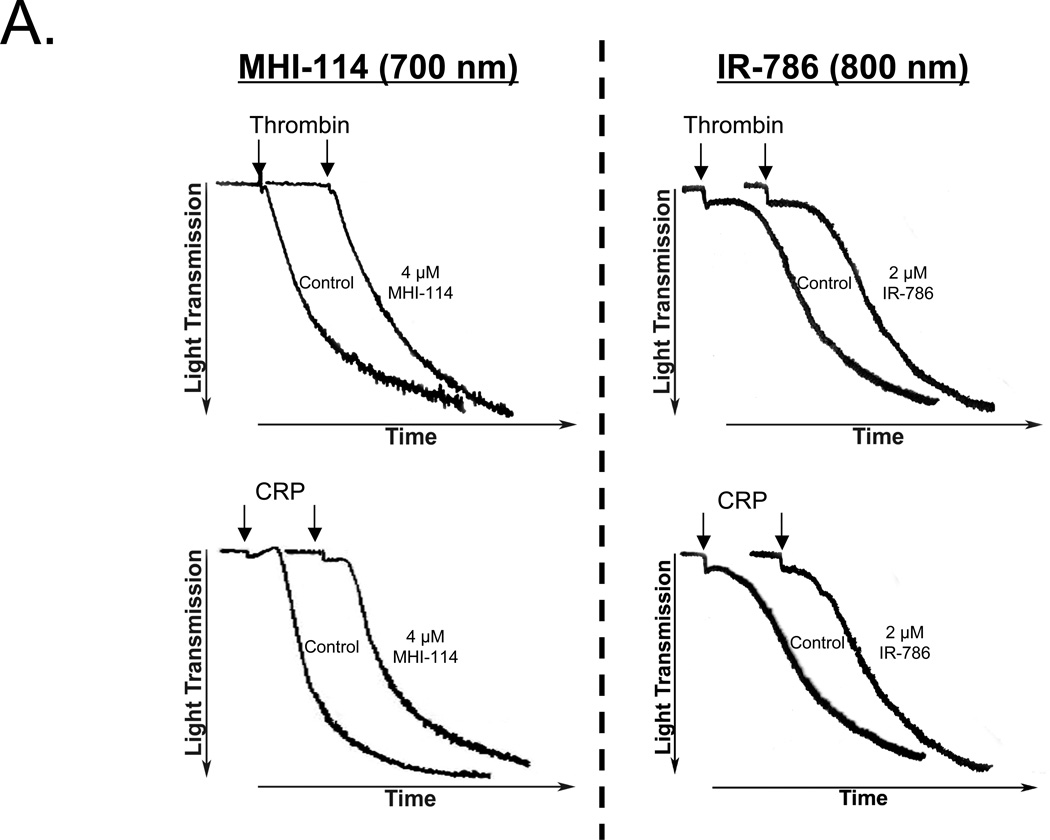
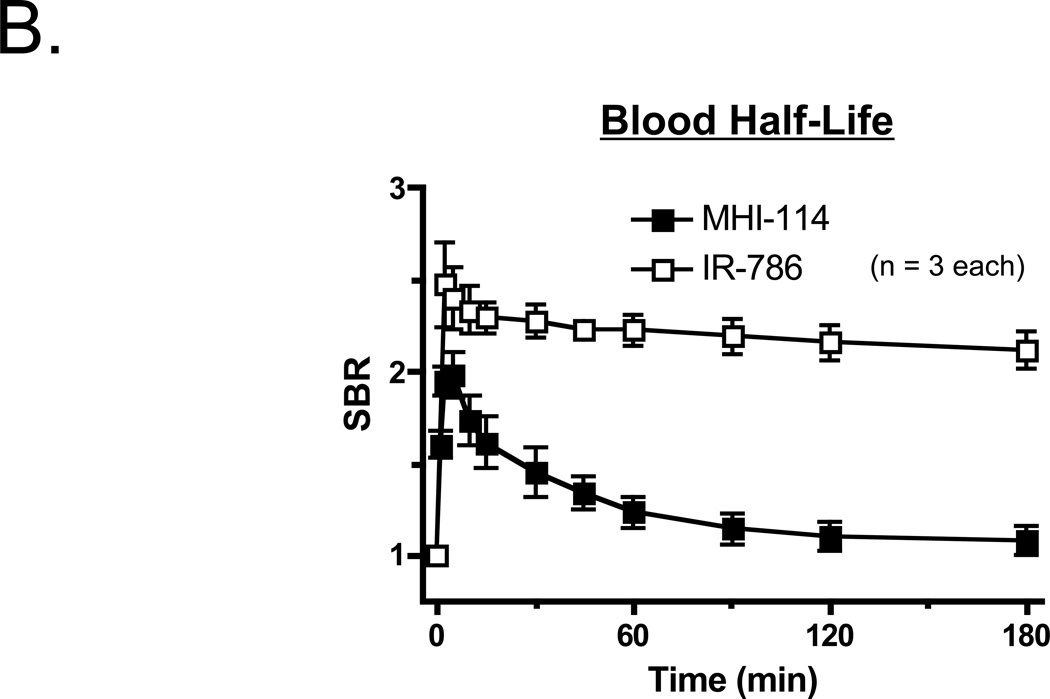
To determine whether NIR fluorophore-loaded platelets remained functional, we assessed their ability to aggregate in response to agonists. The responses of labeled platelets to either thrombin or collagen related protein (CRP) are shown in Figure 2A. Platelet aggregometry tracings from platelets labeled with either MHI-114 or IR-786 were nearly superimposable onto aggregometry tracings from vehicle-only (DMSO treated) control platelets. This observation indicates that there were no measurable differences in response to thrombin and CRP in these studies. The behavior of the SBR of MHI-114- and IR-786-labeled platelets in vivo, though, differed markedly. Whereas the SBR of IR-786 labeled platelets had a long beta-phase blood half-life that could not be calculated accurately over the duration of the experiment, the SBR of MHI-114-labeled platelets were more short-lived, resulting in a blood half-life of 16.4 ± 2.2 (mean ± SEM) min (n = 3) (Figure 2B). The mechanism of the SBR of MHI-labeled platelet clearance from the bloodstream is presently not known.
Figure 2. Bioactivity and blood half-life of MHI-114-labeled platelets.
A. Washed platelets incubated with either DMSO (vehicle control) or NIR fluorophores (4 µM MHI-114 for 120 min at RT; left and 2 µM IR-786 for 30 min at RT; right), then stimulated with either 1 U/ml thrombin (top) or 2 µg/ml collagen-related protein (CRP; bottom). Representative aggregometry tracings are from n = 4 independent experiments.
B. Blood half-life of 1.9 × 1010 autologous washed platelets labeled with 4 µM MHI-114 (black squares) or 2 µM IR-786 (white squares) for 30 min at RT, measured after intravenous infusion into Yorkshire pigs. Platelet rich plasma isolated from blood samples taken at the indicated times were quantified using the FLARE™ imaging system. Shown are mean ± SEM for n = 3 independent experiments.
MHI-114-Labeled Platelets and Intravascular Thrombus Detection
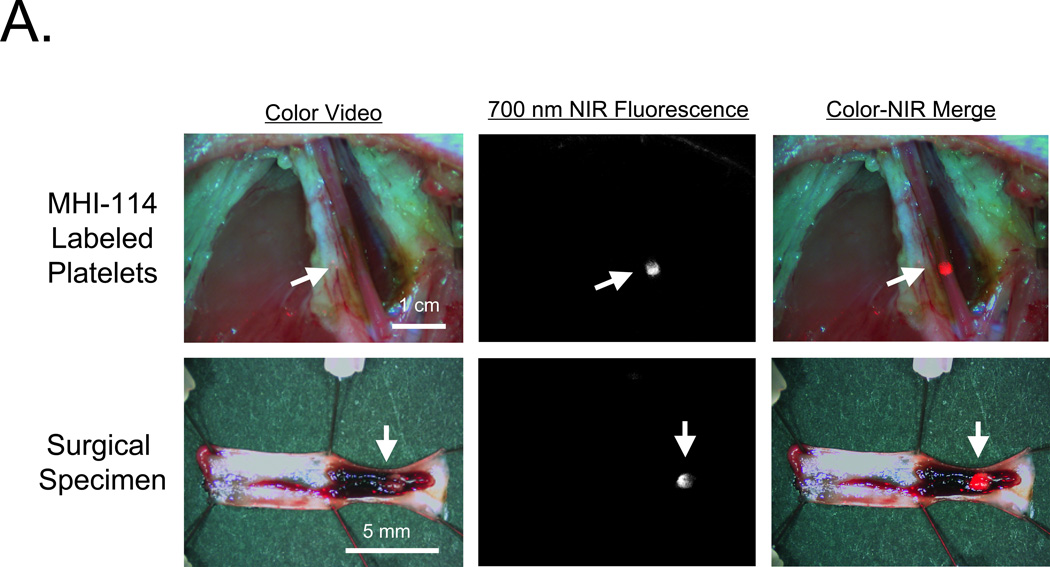
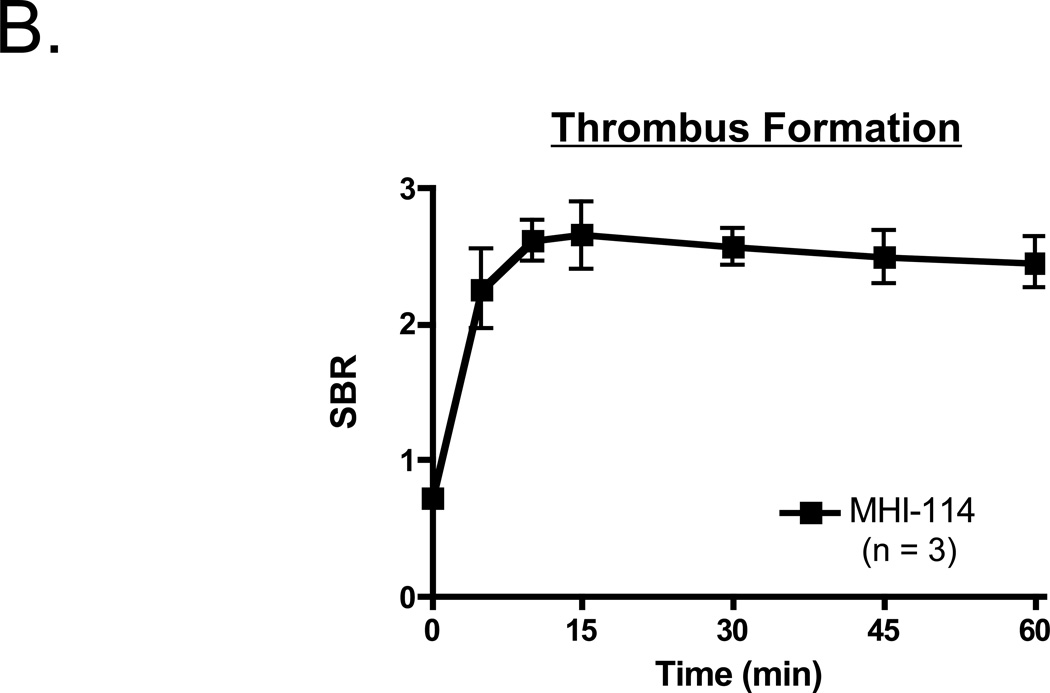
To determine whether MHI-114-labeled platelets remained bioactive and were able to detect preexisting thrombi, 3.9 × 1010 autologous porcine platelets were infused concurrently with application of FeCl3 to a femoral artery (n = 3). As shown in Figure 3A, the growing thrombus could be visualized in the intact vessel using 700-nm fluorescence. No signal could be detected in the 800-nm channel (data not shown), suggesting that MHI-114 could be combined with IR-786 to follow 2 independent platelet populations. Quantitation of thrombus formation revealed that MHI-114-labeled platelets could be detected at the site within 5 min of FeCl3 application, with peak fluorescence occurring at ≈ 15 min (Figure 3B).
Figure 3. Real-time detection of thrombus formation in intact porcine femoral arteries using MHI-114-labeled platelets.
A. MHI-114-labeled autologous pig platelets (3.9 × 1010) were infused 20 min following exposure of femoral artery to FeCl3. Shown are color video (left), 700-nm NIR fluorescence (middle), and a pseudo-colored (red) merge of the 2 (right). The femoral artery was subsequently dissected (bottom row) and the thrombus exposed and imaged. Arrows indicate location of intravascular thrombus. Representative images from n = 3 independent femoral artery thrombi are shown.
B. Quantitation of NIR fluorescence signal at the site of FeCl3-induced femoral artery injury following infusion of MHI-114-labeled platelets. Shown are mean ± SEM for n = 3 independent experiments.
Simultaneous Detection of 700-nm MHI-114-Labeled Platelets and 800-nm IR-786-Labeled Platelets In Vitro and In Vivo
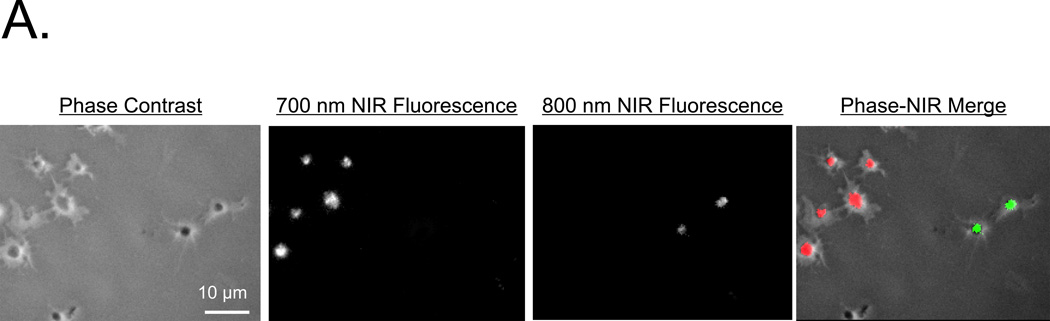
Because lipophilic cations could theoretically translocate passively among cells, we split a platelet preparation in half and labeled each half separately with MHI-114 or IR-786. Then, platelets were mixed together and examined microscopically at the single platelet level for evidence of dye transfer. As shown in Figure 4A, no such transfer was detected, and no cross-contamination of the 2 NIR fluorescence channels was found (i.e., yellow color in the merged image).
Figure 4. Two-wavelength detection of thrombus following FeCl3-induced injury using IR-786-labeled and MHI-114-labeled platelets.
A. Platelets separately loaded with 4 µM MHI-114 for 120 min at RT or 2 µM IR-786 for 30 min at RT and settled onto glass slides. Shown are phase contrast, 700-nm NIR fluorescence, 800-nm NIR fluorescence, and a pseudo-colored merge of the 3, with red used as the pseudo-color for 700 nm and green for 800 nm. A representative image from n = 3 independent femoral artery thrombi is shown.
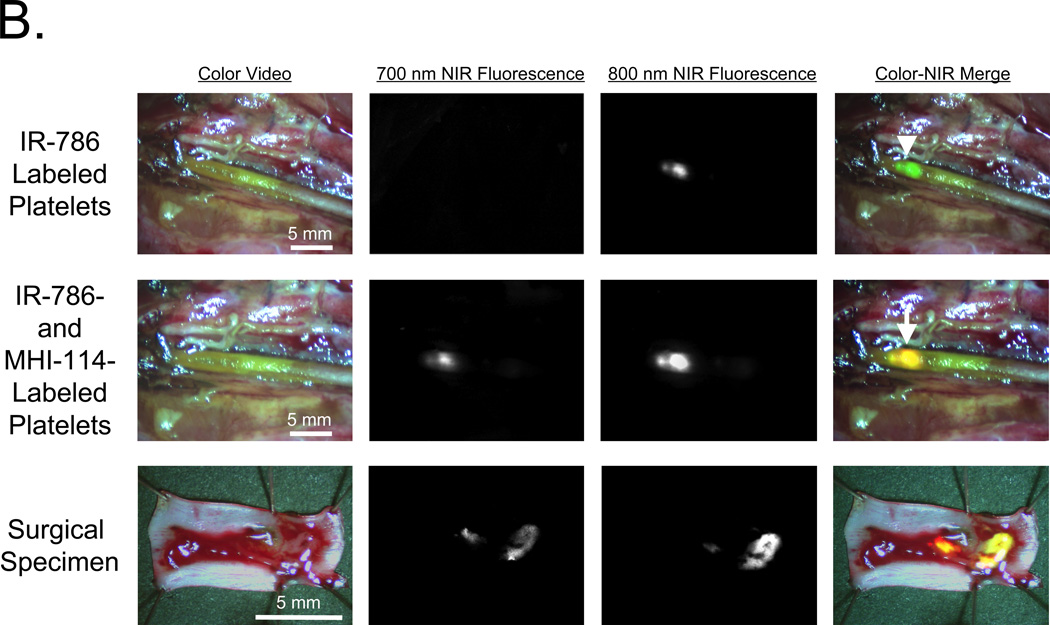
B. Imaging of independent platelet populations. Porcine femoral artery exposed to FeCl3 was imaged first with 1.8 × 1010 IR-786-labeled platelets (top), then 40 min later using 1.8 × 1010 MHI-114-labeled platelets (middle row). The transected surgical specimen was also imaged (bottom row). Shown are color video, 700-nm NIR fluorescence, 800-nm NIR fluorescence, and a pseudo-colored merge of the 3 as described in (A). Representative images from n = 3 independent femoral artery thrombi are shown.
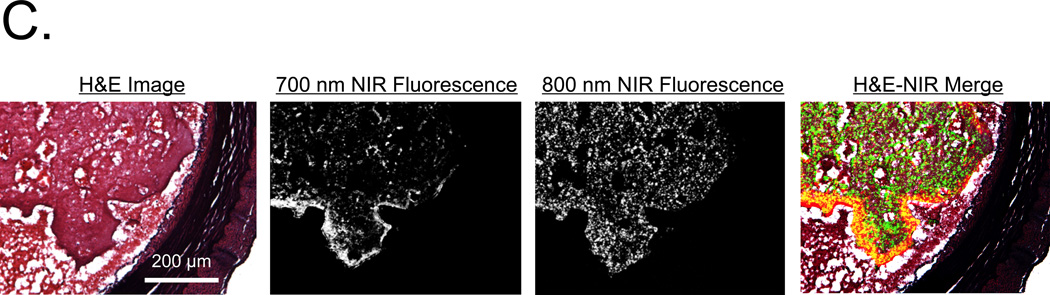
C. H&E histology and consecutive NIR fluorescence images from the surgical specimen described in (B). Note localization of MHI-114-labeled platelets at the luminal surface of the thrombus and IR-786-labeled platelets dispersed throughout the entire thrombus. Shown are H&E histology, 700 nm NIR fluorescence, 800 nm NIR fluorescence and a pseudo-colored merge of the 3 as described in (A). Red color was used for 700-nm NIR, green for 800-nm NIR, and yellow for colocalization in the merged image. A representative image from n = 3 independent femoral artery thrombi is shown.
A similar experiment was performed in vivo (n = 1) (Figure 4B), where 1.9 × 1010 IR-786-labeled platelets were infused into pigs 20 min before thrombus formation in the femoral artery using FeCl3. Imaging at 700 nm and 800 nm confirmed no cross-contamination. Yet, when 1.9 × 1010 MHI-114-labeled platelets were infused, 700-nm fluorescence became visible as a subset of the 800 nm fluorescence signal, which was more clearly visible in the surgical specimen (Figure 4B). H&E staining of these vessels demonstrated large thrombi oriented towards the portion of the vessel exposed to FeCl3 (Figure 4C). NIR microscopy at 800 nm showed that IR-786-labeled platelets were diffusely incorporated throughout the body of the thrombus. In contrast, 700-nm fluorescence revealed MHI-114-labeled platelets localized exclusively to the luminal rim of the thrombus. These studies confirmed our ability to follow 2 independent platelet populations during thrombus formation in thick-walled vessels in vivo.
Quantitation of Antiplatelet Drug Effects using 2-Wavelength In Vivo NIR Fluorescence Imaging
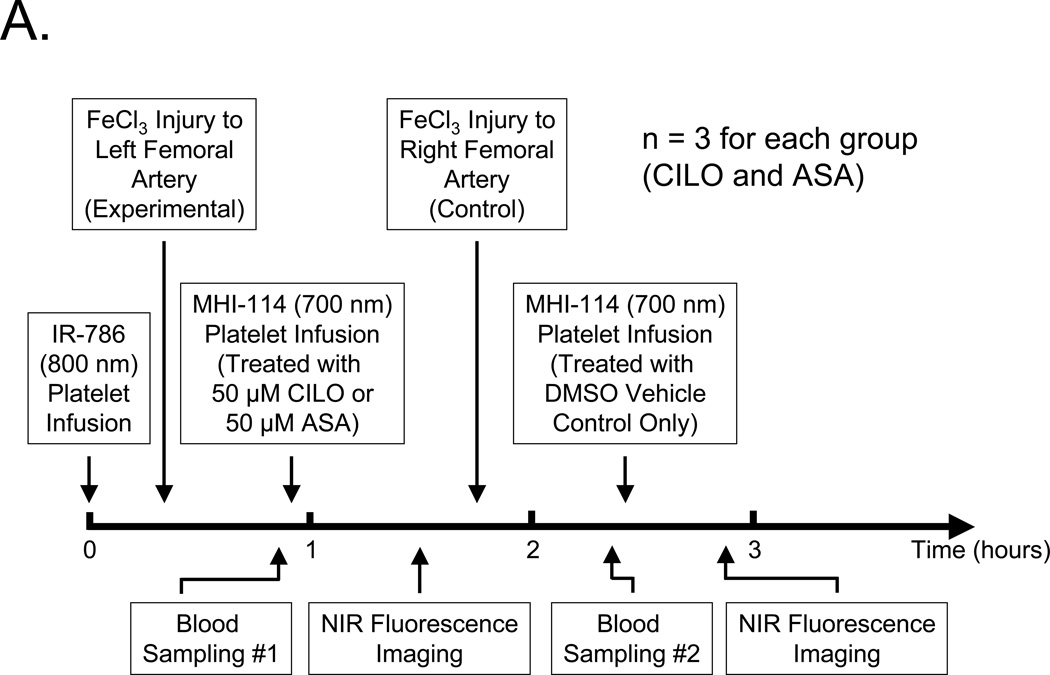
Because the SBR of MHI-114- and IR-786-labeled platelets could be quantified independently in vivo, and the former had a much shorter blood half-life, we designed an experimental protocol (Figure 5A) to measure the antiplatelet effect of cilostazol (CILO) and aspirin (ASA) on thrombus formation in vivo (n = 3 for each drug). In this protocol, a single infusion of 1.3 × 1010, long-acting, 800-nm IR-786-labeled platelets served as an internal control for the fluorescence intensity of the initial thrombus induced by FeCl3 treatment of the left femoral artery. Then, a second population of autologous platelets was loaded with 700-nm MHI-114 and treated with either 50-µM cilostazol (CILO) or 50-µM aspirin (ASA) prior to infusion. The fluorescence intensity of the left femoral artery thrombus was measured over time, and once blood sampling confirmed complete clearance of MHI-114-labeled platelets from the circulation, a control thrombus was formed in the contralateral (right) femoral artery using FeCl3 and measured in the same animal using MHI-114-labeled platelets pretreated with vehicle (DMSO) only.
Figure 5. Quantitation of the effect of cilostazol (CILO) and aspirin (ASA) on platelet incorporation into preexisting thrombi.
A. Protocol for femoral artery injury, drug-treated platelet infusion, blood sampling, and intraoperative NIR fluorescence imaging.
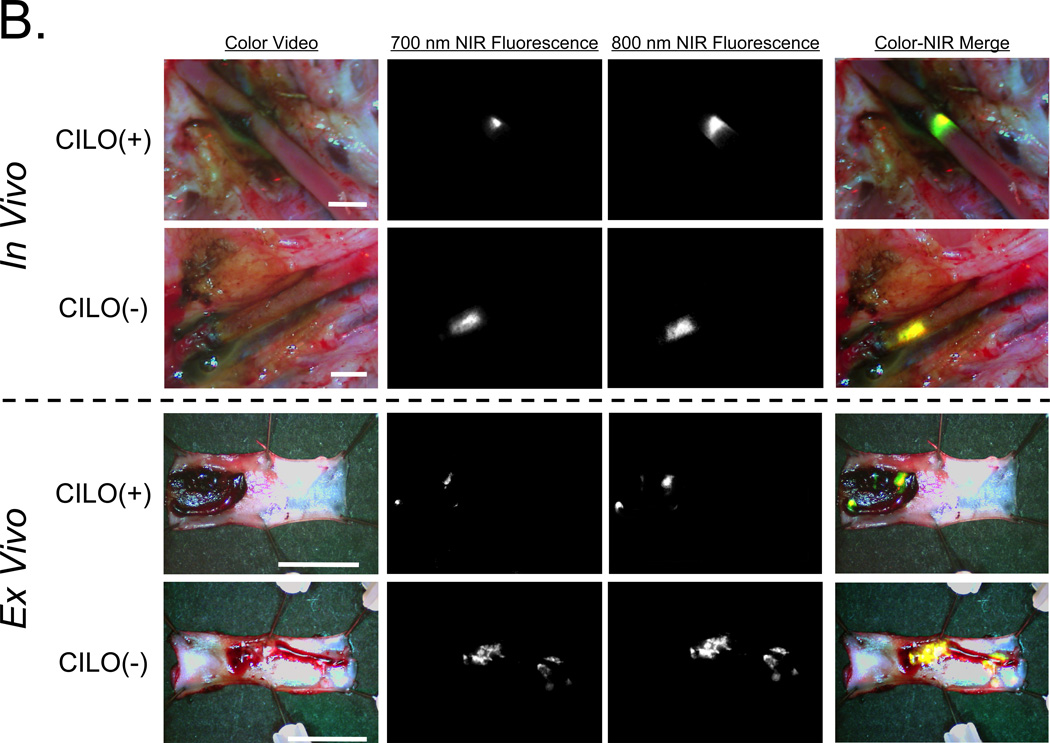
B. Simultaneous 700-nm and 800-nm NIR fluorescence imaging of the intact femoral artery as described in detail in (A) after pretreatment with 50 µM CILO. Shown are color video, 700-nm NIR fluorescence, 800-nm NIR fluorescence, and a pseudo-colored merge of the 3, with red used as the pseudo-color for 700 nm, green for 800 nm, and yellow for co-localization in the merged image. Ex vivo surgical specimens are shown below the dashed line. Representative images are from n = 3 independent femoral artery thrombi using CILO-treated platelets. ASA results are not shown.
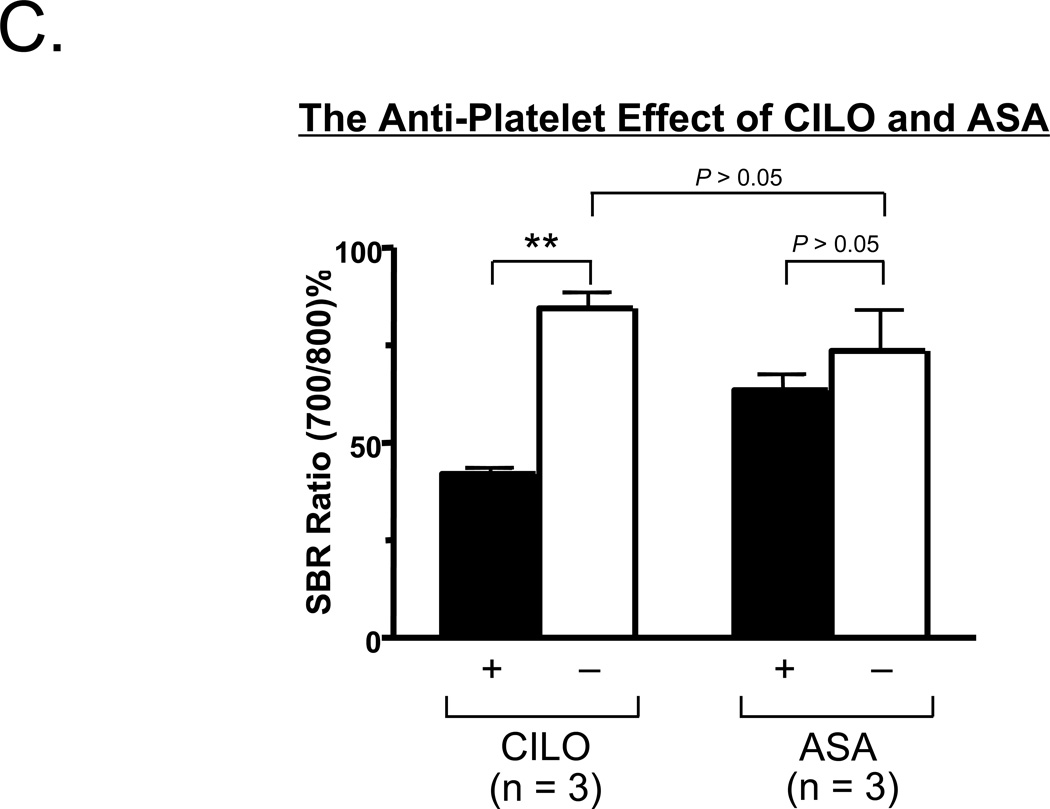
C. Comparison of the SBR ratios (700 nm/800 nm %; mean ± SEM) between CLIO (+) and CILO (−), or between ASA (+) and ASA (−) are shown. Shown are the P values for the indicated statistical comparisons by a one-way ANOVA followed by Tukey’s multiple comparisons test. ** = P < 0.01.
As shown in Figure 5B, intraoperative imaging and analysis of the surgical specimen revealed that cilostazol pretreatment markedly reduced the fluorescence intensity of the growing thrombus. Quantitation of the ratio of 700-nm to 800-nm fluorescence (Figure 5C) confirmed a significant antiplatelet effect of cilostazol as compared to aspirin in this large animal model system. In the setting of cilostazol pretreatment, thrombus size was reduced by 51 ± 2 (mean ± SEM; n = 3) % relative to the control thrombus, but in the setting of aspirin was only reduced by 10 ± 1 (mean ± SEM; n = 3) % relative to the control thrombus.
DISCUSSION
We describe the synthesis and characterization of 700-nm platelet-avid indocyanine derivatives that incorporate at relatively high levels after simple addition to the extracellular buffer. MHI-114 concentrated to ≈ 4.1 × 106 molecules of fluorophore per platelet, which is similar to the ≈ 3.5 × 106 molecules of fluorophore per platelet reported previously for IR-786. As long as quenching is avoided, and bioactivity is maintained, these high levels of fluorophore loading enable sensitive detection of thrombi (SBR ≈ 2.5), even when only ≈ 3% of circulating platelets are labeled.
Because the SBR of 700-nm MHI-114-labeled platelets are relatively short-lived in vivo, we used this as an advantage to study multiple thrombi formation in the same animal (Figure 5). This short blood half-life also complements that of 800-nm IR-786-labeled-platelets, which are more useful for longer duration experiments. The difference in half-lives also permitted us to study the antiplatelet effects of cilostazol and acetylsalicylic acid on platelet accumulation in intact vessels approaching the size of humans.
The use of NIR fluorescent platelets as molecular probes offers new opportunities for studying thrombus formation in animal models of myocardial infarction, stroke, and peripheral vascular disease. They also enable real-time intraoperative interrogation of intact vessels; for example, intraoperative graft occlusion occurs as a complication of cardiac bypass surgery in approximately 1.5% to 5.0% of procedures.17,18 It should now be possible to use the technology we describe to test drugs that prevent thrombus formation, as well as to test drugs that disaggregate established thrombi. MHI-114 and IR-786 could also be used to label leukocytes, stem cells, or other cell types thought to contribute to thrombus formation, and to quantify the contribution of any 2 cell populations to thrombogenesis.
Clinical Relevance Paragraph.
Occult thrombus formation during surgery leads to significant morbidity and mortality. We describe the synthesis of a novel family of 700-nm near-infrared (NIR) fluorophores that concentrate into platelets and render them highly fluorescent while maintaining bioactivity. Using these platelets and a NIR imaging system, we can detect thrombi in thick-walled vessels intraoperatively with high signal-to-background ratio (SBR ≈ 2.5). The optical properties and blood half-life of these 700-nm fluorophores complement a previously described 800-nm platelet-avid fluorophore and enable the assessment of antiplatelet interventions intraoperatively.
ACKNOWLEDGEMENTS
Funding: NIH grants R01-CA-115296 (JVF), R21-CA-110185 (JVF), and P01-HL-87203 (RF), a Center for Integration of Medicine and Innovative Technology (Department of Defense) Application Development Award (JVF), an Established Investigator Award from the American Heart Association (RF), and a Special Programs Award from Bayer Healthcare (RF).
We thank Rita G. Laurence for assistance with animal surgery, Lindsey Gendall for editing, and Eugenia Trabucchi and Keys Linda for administrative assistance. SHK was supported by WCU Program (R31-20029) from Korea Ministry of Education, Science and Technology (KMEST).
Footnotes
AUTHOR STATEMENTS OF FINANCIAL INTEREST
Yoshitomo Ashitate: None
Soon Hee Kim: None
Eiichi Tanaka: None
Robert Flaumenhaft: None
Maged Henary: None
Hak Soo Choi: None
John V. Frangioni, M.D., Ph.D.: All FLARE™ technology is owned by Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. As inventor, Dr. Frangioni may someday receive royalties if products are commercialized. Dr. Frangioni is the founder and unpaid director of The FLARE™ Foundation, a non-profit organization focused on promoting the dissemination of medical imaging technology for research and clinical use.
REFERENCES
- 1.Frenette PS, Johnson RC, Hynes RO, Wagner DD. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc Natl Acad Sci U S A. 1995;92(16):7450–7454. doi: 10.1073/pnas.92.16.7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massberg S, Enders G, Matos FC, Tomic LI, Leiderer R, Eisenmenger S, et al. Fibrinogen deposition at the postischemic vessel wall promotes platelet adhesion during ischemia-reperfusion in vivo. Blood. 1999;94(11):3829–3838. [PubMed] [Google Scholar]
- 3.Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B. Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat Med. 2002;8(10):1175–1181. doi: 10.1038/nm782. [DOI] [PubMed] [Google Scholar]
- 4.Sim DS, Merrill-Skoloff G, Furie BC, Furie B, Flaumenhaft R. Initial accumulation of platelets during arterial thrombus formation in vivo is inhibited by elevation of basal cAMP levels. Blood. 2004;103(6):2127–2134. doi: 10.1182/blood-2003-04-1133. [DOI] [PubMed] [Google Scholar]
- 5.Rubin BG, Toursarkissian B, Petrinec D, Yang LY, Eisenberg PR, Abendschein DR. Preincubation of Dacron grafts with recombinant tissue factor pathway inhibitor decreases their thrombogenicity in vivo. J Vasc Surg. 1996;24(5):865–870. doi: 10.1016/s0741-5214(96)70024-1. [DOI] [PubMed] [Google Scholar]
- 6.Heijmen RH, Grundeman PF, Borst C. Intima-adventitia apposition in end-to-side arterial anastomosis: an experimental study in the pig. Ann Thorac Surg. 1998;65(3):705–711. doi: 10.1016/s0003-4975(97)01310-6. [DOI] [PubMed] [Google Scholar]
- 7.Badimon JJ, Lettino M, Toschi V, Fuster V, Berrozpe M, Chesebro JH, et al. Local inhibition of tissue factor reduces the thrombogenicity of disrupted human atherosclerotic plaques: effects of tissue factor pathway inhibitor on plaque thrombogenicity under flow conditions. Circulation. 1999;99(14):1780–1787. doi: 10.1161/01.cir.99.14.1780. [DOI] [PubMed] [Google Scholar]
- 8.St Pierre J, Yang LY, Tamirisa K, Scherrer D, De Ciechi P, Eisenberg P, et al. Tissue factor pathway inhibitor attenuates procoagulant activity and upregulation of tissue factor at the site of balloon-induced arterial injury in pigs. Arterioscler Thromb Vasc Biol. 1999;19(9):2263–2268. doi: 10.1161/01.atv.19.9.2263. [DOI] [PubMed] [Google Scholar]
- 9.Flaumenhaft R, Tanaka E, Graham GJ, De Grand AM, Laurence RG, Hoshino K, et al. Localization and quantification of platelet-rich thrombi in large blood vessels with near-infrared fluorescence imaging. Circulation. 2007;115(1):84–93. doi: 10.1161/CIRCULATIONAHA.106.643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sim DS, Dilks JR, Flaumenhaft R. Platelets possess and require an active protein palmitoylation pathway for agonist-mediated activation and in vivo thrombus formation. Arterioscler Thromb Vasc Biol. 2007;27(6):1478–1485. doi: 10.1161/ATVBAHA.106.139287. [DOI] [PubMed] [Google Scholar]
- 11.Rozenvayn N, Flaumenhaft R. Protein kinase C mediates translocation of type II phosphatidylinositol 5-phosphate 4-kinase required for platelet alpha-granule secretion. J Biol Chem. 2003;278(10):8126–8134. doi: 10.1074/jbc.M206493200. [DOI] [PubMed] [Google Scholar]
- 12.Benson C, Kues HA. Absorption and fluorescence properties of cyanine dyes. J. Chem. Eng. Data. 1977;22(4):379–383. [Google Scholar]
- 13.Nakayama A, Bianco AC, Zhang CY, Lowell BB, Frangioni JV. Quantitation of brown adipose tissue perfusion in transgenic mice using near-infrared fluorescence imaging. Mol Imaging. 2003;2(1):37–49. doi: 10.1162/15353500200303103. [DOI] [PubMed] [Google Scholar]
- 14.Croce K, Flaumenhaft R, Rivers M, Furie B, Furie BC, Herman IM, et al. Inhibition of calpain blocks platelet secretion, aggregation, and spreading. J Biol Chem. 1999;274(51):36321–36327. doi: 10.1074/jbc.274.51.36321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gioux S, Kianzad V, Ciocan R, Gupta S, Oketokoun R, Frangioni JV. High-power, computer-controlled, light-emitting diode-based light sources for fluorescence imaging and image-guided surgery. Mol Imaging. 2009;8(3):156–165. [PMC free article] [PubMed] [Google Scholar]
- 16.Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16(10):2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falk V, Walther T, Philippi A, Autschbach R, Krieger H, Dalichau H, et al. Thermal coronary angiography for intraoperative patency control of arterial and saphenous vein coronary artery bypass grafts: results in 370 patients. J Card Surg. 1995;10(2):147–160. doi: 10.1111/j.1540-8191.1995.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 18.Hol PK, Lingaas PS, Lundblad R, Rein KA, Vatne K, Smith HJ, et al. Intraoperative angiography leads to graft revision in coronary artery bypass surgery. Ann Thorac Surg. 2004;78(2):502–505. doi: 10.1016/j.athoracsur.2004.03.004. discussion 5. [DOI] [PubMed] [Google Scholar]