Abstract
Duchenne muscular dystrophy (DMD) is a fatal genetic disease caused by the absence of the sarcolemmal protein dystrophin. Dilated cardiomyopathy leading to heart failure is a significant source of morbidity and mortality in DMD. We recently demonstrated amelioration of DMD heart disease in 16 to 20-m-old dystrophin-null mdx mice using adeno-associated virus (AAV) mediated micro-dystrophin gene therapy. DMD patients show severe heart disease near the end of their life expectancy. Similarly, mdx mice exhibit profoundly worsening heart disease when they reach beyond 21 months of age. To more rigorously test micro-dystrophin therapy, we treated mdx mice that were between 21.2 to 22.7-m-old (average, 22.1 ± 0.2 months; N=8). The ΔR4-23/ΔC micro-dystrophin gene was packaged in the cardiotropic AAV-9 virus. 5 × 1012 viral genome particles/mouse were delivered to mdx mice via the tail vein. AAV transduction, myocardial fibrosis and heart function were examined 1.7 ± 0.2 months after gene therapy. Efficient micro-dystrophin expression was observed in the myocardium of treated mice. Despite the robust dystrophin expression, myocardial fibrosis was not mitigated. Most hemodynamic parameters were not improved either. However, ECG abnormalities were partially corrected. Importantly, treated mice became more resistant to dobutamine-induced cardiac death. In summary, we have revealed for the first time the potential benefits and limitations of AAV micro-dystrophin therapy in end-stage Duchenne dilated cardiomyopathy. Our findings have important implications for the use of AAV gene therapy in dilated cardiomyopathy and heart failure.
Keywords: Dilated cardiomyopathy, heart failure, gene therapy, Duchenne muscular dystrophy, dystrophin, AAV, Duchenne cardiomyopathy
1. Introduction
The 427 kd dystrophin protein is essential for the survival of cardiomyocytes. In Duchenne muscular dystrophy (DMD) and certain X-linked dilated cardiomyopathy, dystrophin expression is abolished in the heart due to various mutations in the dystrophin gene and/or its promoter. The pathogenesis of dystrophin-deficient Duchenne cardiomyopathy is not completely understood [1, 2]. The current model suggests that the loss of dystrophin weakens sarcolemmal integrity. Further, dystrophin deficiency perturbs cellular signaling and channel activity in cardiomyocytes. The diseased heart shows myocardial necrosis, inflammation and fibrosis. At the advanced stage, patients develop dilated cardiomyopathy and many die from heart failure.
Mdx mice are naturally occurring dystrophin-null mice with an average life span of approximately 22.5 months [3, 4]. Despite dystrophin deficiency, the heart of young adult mdx mice is minimally affected. In contrast, terminally aged (>21-m-old) mdx mice show severe heart disease identical to that seen in end-stage Duchenne cardiomyopathy patients. At this time, the mdx heart becomes highly fibrotic and dilated [5-7]. Lethal heart failure at this age contributes substantially to the premature death.
An appealing therapeutic approach for Duchenne cardiomyopathy is to express a micro-dystrophin gene via an adeno-associated viral vector (AAV) [1, 2]. A micro-dystrophin protein is approximately one-third the size of the full-length protein. Despite significant truncation, AAV-mediated microgene therapy has yielded amazing cardiac protection in mdx mice that are less than 20 months of age [7-12]. A more challenging question is whether AAV-micro-dystrophin therapy can treat mdx cardiomyopathy at its most severe stage (i.e. at the terminal age).
In this study, we performed AAV micro-dystrophin therapy in eight mdx mice that were between 21.2 to 22.7-m-old (average 22.1 ± 0.2 months) (Table 1). 5 × 1012 viral genome (vg) particles/mouse of AAV-9 ΔR4-23/ΔC micro-dystrophin vectors were injected via the tail vein (Table 1). AAV transduction, myocardial fibrosis and heart weight ratio were examined. To determine functional outcome, we performed ECG, cardiac catheter and dobutamine stress assays. Robust micro-dystrophin expression was observed in non-fibrotic areas in the heart. However, we did not see an appreciable reduction of myocardial fibrosis in treated mice. ECG assay revealed some improvement but cardiac catheter assay showed minimal changes in hemodynamics. Nevertheless, AAV microgene therapy increased resistance to dobutamine-induced cardiac death. Our results reveal for the first time the potential benefits and limitations of AAV-mediated micro-dystrophin therapy in end-stage Duchenne dilated cardiomyopathy.
Table 1.
Experimental summary
| Subject | Age at AAV injection (m) |
Weight ratio |
ECG | Cardiac catheter |
Duration of therapy (m) |
|---|---|---|---|---|---|
| Mouse #1 |
21.2 | Yes | Yes | Yes | 2.1 |
| Mouse #2 |
21.2 | Yes | Yes | No | 2.1 |
| Mouse #3 |
21.7 | Yes | Yes | Yes | 2.5 |
| Mouse #4 |
22.3 | Yes | No | No | 1.2 |
| Mouse #5 |
22.4 | Yes | No | No | 1.3 |
| Mouse #6 |
22.6 | Yes | Yes | Yes | 1.3 |
| Mouse #7 |
22.6 | Yes | Yes | Yes | 1.3 |
| Mouse #8 |
22.7 | Yes | Yes | Yes | 2.1 |
|
| |||||
| Average | 22.1 | 1.7 | |||
| S.E.M. | 0.2 | 0.2 | |||
No, assay was not performed for technical reasons.
Yes, assay was performed.
2. Materials and Methods
2.1 Animal model development, recombinant viral vector production and treatment protocol
Animal experiments were approved by the University of Missouri Animal Care and Use Committee and in accordance with NIH guidelines. Experimental mice were generated in a barrier facility using founders from The Jackson Laboratory (Bar Harbor, ME). Only female mice were used in the study. At the time of AAV micro-dystrophin therapy, the average age of mdx mice was 22.1 ± 0.2 months (range, 21.2 to 22.7 months). A total of eight mdx mice received AAV microgene therapy. ECG assay was performed in six AAV treated mice. Left ventricular catheterization was performed in five AAV treated mice. All physiologic assays were performed in age-matched mice. All mice were maintained in a specific-pathogen-free animal care facility. Due to decreased mobility in aged mdx mice, moistened transgenic dough diet (BioServ, Frenchtown, NJ) was placed in a shallow dish on the floor of the cage. All experimental mice were carefully monitored daily.
The micro-dystrophin gene AAV vector has been reported before [7-9, 13, 14]. This microgene contains the N-terminal domain, hinge 1, 2 and 4, spectrin-like repeats 1, 2, 3 and 24 and the cysteine-rich domain. Expression was driven by the cytomegalovirus promoter. Recombinant AAV virus was packaged in AAV-9 capsid. A single dose of AAV microgene vector (5 × 1012 vg particles/mouse) was delivered to conscious mice via tail vein injection. Mice were evaluated 1.2 to 2.5 months (average 1.7 ± 0.2 months) after treatment.
2.2 Evaluation of micro-dystrophin expression and myocardial fibrosis
Morphology studies were performed on 8 μm cryo-sections. The micro-dystrophin gene expression was examined with a monoclonal antibody against the N-terminal domain of the dystrophin protein (Manex1A, 1:300, clone 4C7, IgG2b; a gift from Dr. Glenn Morris, The Robert Jones and Agnes Hunt Orthopaedic Hospital, UK) [15]. Immunofluorescence staining was performed according to our previously published protocol [8, 10, 16]. Cardiac fibrosis was evaluated by two different methods. For immunofluorescence detection of fibrosis, wheat germ agglutinin (WGA)-Oregon Green conjugate (Molecular Probes, W6748; 5 ug/mL PBS) was used. For histochemical detection, Masson trichrome staining was used as we reported before [6, 7, 16]. Photomicrographs were taken with a Qimage Retiga 1300 camera using a Nikon E800 fluorescence microscope.
2.3 Cardiac function assays
Heart function was evaluated by ECG and cardiac catheterization. ECG was performed using a system from AD Instruments (Colorado Springs, CO) as described elsewhere [17]. Heart hemodynamics were examined using a 1.4 F Millar catheter. The pressure/volume data were analyzed using the PVAN software (Millar Instruments, Houston, TX). Dobutamine survival assay was performed by intraperitoneal injection of dobutamine (5 μg/g body weight; Sigma, St. Louis, MO). Survival was monitored for 15 min. Cardiac death was diagnosed when heart rate was reduced to less than 250 bpm and systolic pressure dropped to less than 30 mmHg.
2.4 Statistical Analysis
Data are presented as mean ± standard error of mean. The SPSS software (SPSS, Chicago, IL) was used for statistical analysis of weight data, ECG and hemodynamic results. One-way ANOVA analysis and Bonferroni post hoc analysis were used for multiple group comparisons. Dobutamine stress survival was analyzed by the Prism 4 software (GraphPad, San Diego, CA) using the Kaplan-Meier method. The Mantel-Cox log-rank test was used for statistical analysis of survival with the Bonferroni corrected threshold used for determining significance. A p < 0.05 was considered as statistically significant.
3. Results
3.1 AAV-9 micro-dystrophin vector efficiently transduced cardiomyocytes in mdx mice older than 21 months
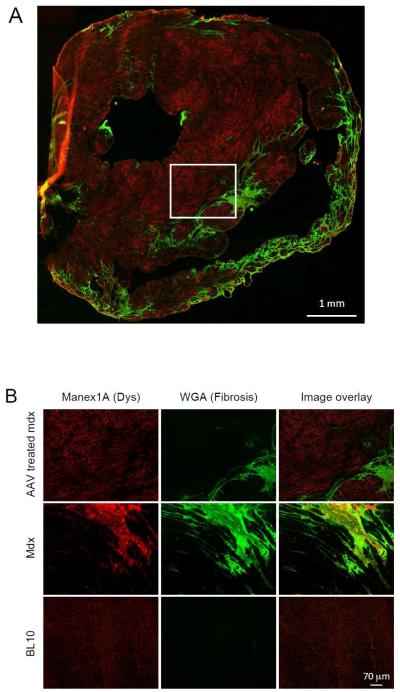
AAV delivery was successfully achieved in eight terminally aged (>21-m-old) mdx mice (Table 1). Each mouse received a single dose of 5 × 1012 vg particles of AAV-9 micro-dystrophin vector via the tail vein. The heart was collected from all experimental mice for immunofluorescence staining and histology study. Cardiomyocyte transduction was evaluated by immunofluorescence staining using Manex 1A, a dystrophin N-terminal specific antibody (Figure 1, Supplementary Figure 1). Fibrotic region was revealed with Oregon Green 488-conjugated wheat germ agglutinin on the same slide. Robust microgene expression was observed in the myocardium in every treated mouse. A representative image from the oldest treated mouse (AAV injection at the age of 22.7 months and tissue harvest at the age of 24.8 months) is shown in Figure 1. Except for areas that were highly fibrotic, strong micro-dystrophin expression was seen in cardiomyocytes throughout the heart (Figure 1).
Figure 1. Evaluation of AAV micro-dystrophin transduction in the heart of >21-m-old mdx mice.
Myofibers that were successfully transduced by the AAV microgene vector showed bright red sarcolemmal staining. Fibrotic collagen deposition was revealed with Oregon green 488-conjugated wheat germ agglutinin (green). A, Representative immunofluorescence staining photomicrograph of the whole heart from a micro-dystrophin treated mdx mouse. B, Representative high power immunofluorescence staining photomicrographs of the heart from treated and untreated mdx mice, and normal BL10 mice. The images of AAV treated mdx mice correspond to the boxed area in panel A.
3.2 Micro-dystrophin therapy did not reduce myocardial fibrosis in terminally aged mdx mice
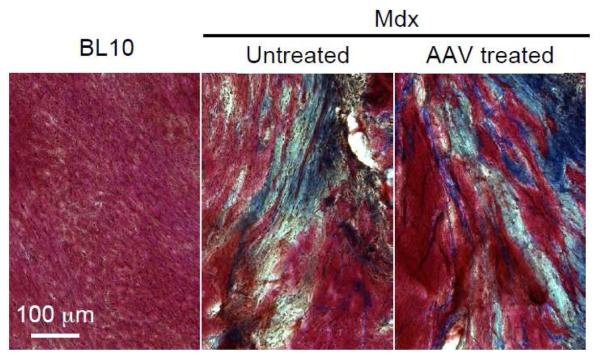
To further examine myocardial fibrosis, we performed Masson trichrome staining (Figure 2). The heart of age-matched normal BL10 mouse showed minimal fibrosis (Figures 1b, 2). In contrast, extensive fibrosis was detected in the heart of terminally aged mdx mice (Figures 1b, 2). The myocardium of AAV microgene injected mice resembled that of untreated mdx mice (Figures 1, 2). There was no appreciable reduction of myocardial fibrosis following micro-dystrophin therapy. Heart weight (HW) and ventricle weight (VW) were not different between treated and untreated mdx mice (Table 2). AAV microgene therapy also did not alter the HW to body weight (BW) ratio and the VW to BW ratio (Table 2).
Figure 2. Micro-dystrophin expressed from a therapeutic AAV vector did not alter myocardial fibrosis in >21-m-old mdx mice.
Representative heart photomicrographs of Masson trichrome staining from age and gender matched BL10, untreated mdx and AAV micro-dystrophin treated mdx mice. Fibrotic region is in blue color.
Table 2. Weights and weight ratios.
| BL10 | Mdx | AAV treated mdx |
|
|---|---|---|---|
| Sample Size (N) | 8 | 12 | 8 |
| Age (m) | 23.98 ± 0.68 | 23.97 ± 0.15 | 23.83 ± 0.17 |
| BW (g) | 29.79 ± 1.47 a | 19.17 ± 0.90 | 20.30 ± 0.51 |
| TW (mg) | 35.70 ± 2.14 | 28.60 ± 2.19 | 32.53 ± 2.85 |
| HW (mg) | 124.69 ± 5.56 a | 105.80 ± 2.80 | 109.64 ± 3.22 |
| VW (mg) | 117.03 ± 5.22 b | 97.70 ± 3.16 | 103.91 ± 3.09 |
| TW/BW (mg/g) | 1.20 ± 0.06 c | 1.49 ± 0.08 | 1.62 ± 0.17 |
| HW/BW (mg/g) | 4.23 ± 0.20 a | 5.64 ± 0.28 | 5.42 ± 0.18 |
| HW/TW (mg/g) | 3.59 ± 0.28 | 3.91 ± 0.27 | 3.73 ± 0.62 |
| VW/BW (mg/g) | 3.97 ± 0.18 a | 5.20 ± 0.27 | 5.14 ± 0.18 |
| VW/TW (mg/g) | 3.36 ± 0.24 | 3.70 ± 0.31 | 2.75 ± 0.83 |
BW: Body Weight.
HW: Heart Weight.
TW: Anterior Tibialis Muscle Weight.
VW: Ventricle Weight.
Significantly different from other two groups.
Significantly different from untreated mdx only.
Significantly different from AAV treated mdx only.
3.3 AAV micro-dystrophin therapy partially corrected dystrophic ECG abnormalities
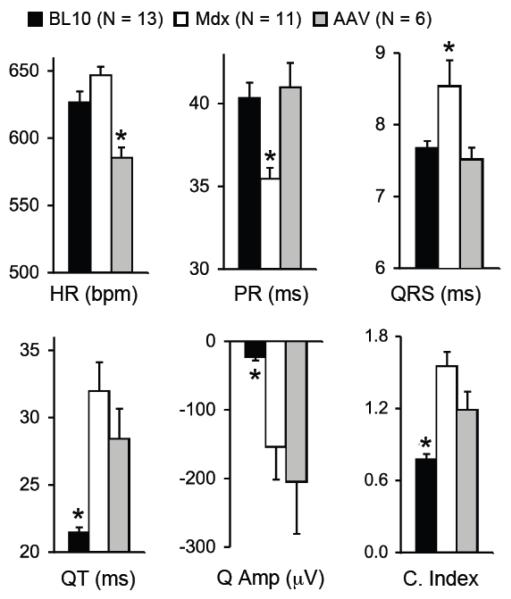
The post-treatment ECG was successfully recorded in six AAV micro-dystrophin treated mice (ECG recording failed in two treated mice) (Table 1). Compared to age and gender matched BL10, untreated mdx mice showed a higher heart rate and significantly reduced PR interval. QRS duration, QT interval, Q amplitude (absolute value) and the cardiomyopathy index were all significantly higher in untreated mdx mice (Figure 3). Interestingly, AAV treated mdx mice showed a heart rate significantly lower than that of normal and untreated mdx mice. AAV micro-dystrophin treatment significantly prolonged PR interval and shortened QRS duration (Figure 3). However, AAV microgene therapy did not alter QT interval, Q amplitude and the cardiomyopathy index (Figure 3).
Figure 3. AAV micro-dystrophin therapy partially improved ECG in >21-m-old mdx mice.
Quantitative evaluation of ECG profiles from BL10, mdx and AAV treated mdx mice. HR, heart rate; bpm, beat per min; Q Amp, Q wave amplitude; C. Index, cardiomyopathy index. Asterisk, significantly different from the other two groups.
3.4 AAV micro-dystrophin therapy failed to improve left ventricular hemodynamics but reduced dobutamine-induced acute cardiac death
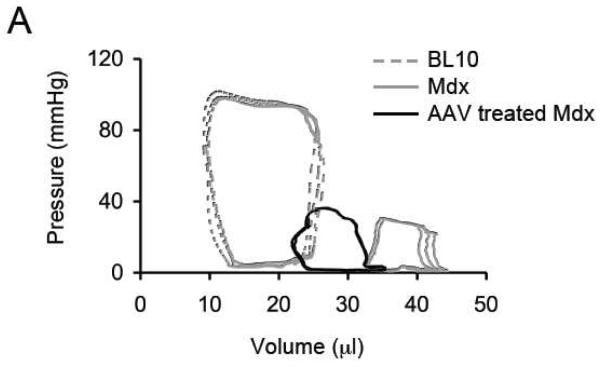
Left ventricular catheterization was successfully performed in five treated mice (Table 1). Representative pressure-volume (PV) loops from normal, untreated mdx and AAV treated mdx mice are shown in Figure 4A. Compared to that of normal controls, the PV loops of untreated mdx mice shifted rightward (chamber dilation) and downward (pressure reduction) (Figure 4A) [5, 6]. AAV micro-dystrophin treatment resulted in minimal change in the PV loops. The pressure curve of the treated mice was not different from that of untreated. The volume curve shifted slightly leftward in the treated mice, suggesting a reduction of chamber dilation (Figure 4A).
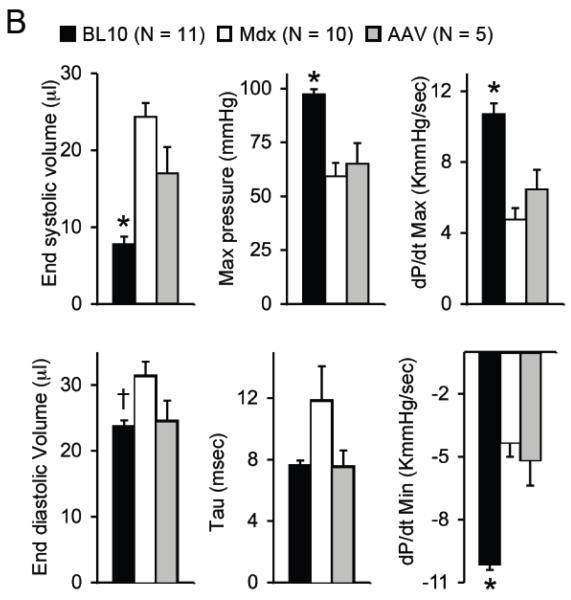
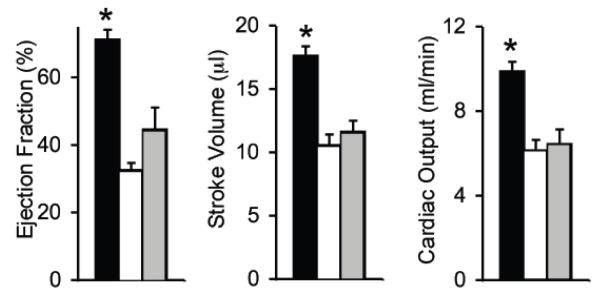
Figure 4. AAV micro-dystrophin therapy yielded minimal hemodynamic improvement in mdx mice that were older than 21 months at the time of therapy.
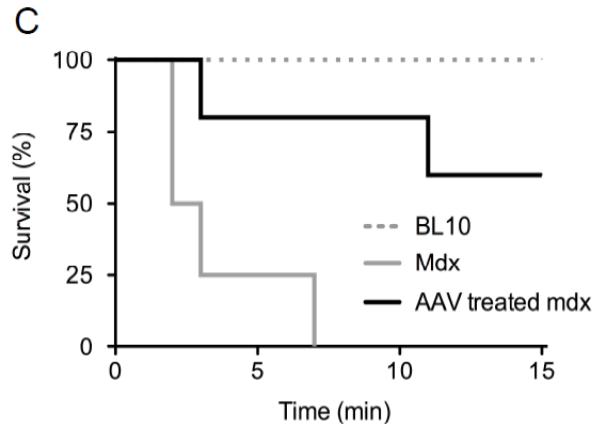
A, Representative pressure-volume loops from BL10, mdx and AAV treated mdx mice. B, Quantitative evaluation of hemodynamic profiles from BL10, mdx and AAV treated mdx mice. Asterisk, significantly different from the other two groups; Cross, significantly different from mdx only. C, Kaplan-Meier survival curve within 15 min of dobutamine administration. N=7 for BL10, N=4 for untreated mdx, and N=5 for AAV treated mdx. The P value between BL10 and mdx is 0.0011. The P value between BL10 and AAV treated mdx is 0.101. The P value between untreated and AAV treated mdx is 0.0154.
Statistical analysis was performed on hemodynamic data (Figure 4B). The vast majority of hemodynamic parameters were not altered by AAV micro-dystrophin therapy (Figure 4B). Specifically, the end-systolic volume, the maximal pressure, the maximal rate of left ventricular contraction (dP/dt max) and the minimal rate of left ventricular relaxation (dP/dt min) were identical between treated and untreated mdx mice. The end-diastolic volume and the time constant for heart relaxation (tau) showed a trend toward reduction but did not reach statistical significance. Indices of overall heart performance (ejection fraction, stroke volume and cardiac output) were not improved by AAV microgene therapy (Figure 4B).
To further evaluate the therapeutic efficacy, we applied dobutamine stress. Most untreated mdx mice died within the first 3 min and the longest survival was 7 min (Figure 4C). None of BL10 mice died within the first 15 min. One AAV treated mdx mouse died at 3 min and another died at 11 min. Remaining micro-dystrophin treated mice survived up to 15 min, the last time point of observation (Figure 4C). The difference between BL10 and AAV treated mdx mice was not statistically significant (p = 0.101).
To help determine the reason for improved survival under dobutamine stress, we examined left ventricular function at 5 minutes after dobutamine injection in surviving mice (Supplementary Figure 2). BL10 mice showed an appropriate inotropic response with significant increases in dP/dt max, stroke volume and cardiac output (Supplementary Figure 2, top panel). Importantly, the AAV treated mdx mice also showed tolerance of the adrenergic stimulation with a significant increase in dP/dt max. Stroke volume and cardiac output showed a trend towards improvement but did not reach statistical significance (Supplementary Figure 2, bottom panel). Untreated mdx mice developed decompensated left ventricular failure and we were unable to collect post dobutamine data.
As an additional effort to understand cardiac functional changes following AAV micro-dystrophin treatment, we examined several calcium handling proteins including calsequestrin and cardiac isoform of sarcoplasmic reticulum calcium ATPase (SERCA2a) [18, 19]. Interestingly, microgene therapy did not significantly altered calsequestrin and SERCA2a levels in the heart of terminally aged female mdx mice (data not shown).
4. Discussion
AAV-mediated micro-dystrophin gene therapy has been considered as a promising therapy for dystrophin-deficient cardiomyopathy. Until recently, most studies have focused on young adult mdx mice that lack typical clinical manifestation of dilated cardiomyopathy [8, 10-12]. Collectively, AAV microgene therapy significantly stabilized the sarcolemma, repressed myocardial fibrosis, and improved ECG performance and the hemodynamic profile in these young mdx mice. In other word, early intervention with micro-dystrophin may effectively prevent dystrophic heart disease. To test whether AAV microgene therapy ameliorates ongoing cardiomyopathy, Bostick et al treated 16 to 20-m-old mdx mice [7]. Encouraging results were seen on histologic examination and cardiac function assays. Specifically, myocardial fibrosis was significantly reduced as measured by Masson trichrome staining and hydroxyproline quantification. On ECG assay, Q wave amplitude and QT interval were rectified. On hemodynamic assay, the majority of systolic and diastolic parameters were corrected [7]. Despite the overall favorable changes, mice that were treated at 20 months of age seemed to show less improvement than those that were treated at 16 months [7]. This raises an important question. Will micro-dystrophin therapy benefit patients that are already at the advanced disease stage?
Mdx mice are the most widely used animal models for DMD. Interestingly, mdx heart disease displays a unique age-associated pattern [1, 2]. Evident dilated cardiomyopathy has only been confirmed in ≥ 21-m-old female (but not male) mdx mice [5, 6]. For this reason, treatments performed in >21-m-old mdx mice may more accurately predict the possible outcomes in Duchenne cardiomyopathy patients. However, studying end-stage mdx mice is not a trivial task. First, it is very difficult to get sufficient number of mice at this age group. Second, experimental subjects may die naturally at any moment because they are already near the end of their life span. Third, severe muscular dystrophy in aged mdx mice renders it extremely difficult to conduct experimental procedures (such as gene transfer, ECG and cardiac catheter assays) in these mice.
Though a daunting challenge, considering the potential implications to future clinical translation, we proceeded with the study in mdx mice that were near the end of their life span (>21-m-old). Among eight mice that were successfully injected with AAV, two died before any functional assay was performed. Another one died during cardiac catheterization. ECG from six treated mice and hemodynamic data from five treated mice were analyzed for statistical significance.
We initially thought that extensive myocardial fibrosis in terminally aged mdx mice might hinder AAV spreading and hence reduce transduction. Surprisingly, this did not occur. Robust micro-dystrophin expression was observed in cardiomyocytes throughout the entire heart despite prevailing collagen deposition in the myocardium (Figure 1, Supplementary Figure 1). Gene replacement may prevent further deterioration of a dystrophin-null myofiber but it cannot revert dead cells that have already been replaced by fibrotic tissue. Consistent with this notion, there was negligible change of myocardial fibrosis in treated mice (Figures 1 and 2). This is an important point since significant hydroxyproline reduction was noticed in mdx mice that were treated before 20-m-old [7]. Together, these data suggest a rapid progression of dystrophic cardiomyopathy in terminal aged mdx mice. Further, it highlights the importance of early intervention. Treatment started only a couple of months earlier may result in substantial histology improvement.
Several hypotheses have been proposed to explain abnormal ECG changes in DMD. These include myocardial fibrosis, fatty infiltration, vacuolar degeneration, abnormal nitric oxide synthase activity and imbalanced sympathetic/parasympathetic tone [20-23]. In terminally aged mdx mice, micro-dystrophin therapy did not affect QT interval and Q wave amplitude (Figure 3). However, in less than 20-m-old mdx mice, AAV-mediated micro-dystrophin expression normalized QT interval and Q wave defects. On the other hand, shortened PR interval and prolonged QRS duration were ameliorated in both age groups (whether treated before 20 or after 21 months of age). An interesting difference between two studies is myocardial fibrosis. In > 21-m-old mdx mice, the extent of myocardial fibrosis remained the same irrespective of microgene therapy (Figure 2). However, myocardial fibrosis was significantly reduced in mice that were treated before 20 months of age [7]. It appears that reducing fibrosis correlates with the improvement of QT interval and Q wave amplitude but not with the correction of PR interval and QRS duration. Future studies are warranted to further dissect these intriguing observations. In summary, the aberrant ECG seen in Duchenne cardiomyopathy may likely reflect multiple pathogenic mechanisms that could be either structural or non-structural.
Another peculiar ECG finding is the reduction of heart rate in AAV micro-dystrophin infected mdx mice (Figure 3). We currently do not have an explanation for this intriguing observation. However, this appears a consistent finding. Besides the current study, we have also observed the same phenomenon in 19-m-old mdx mice treated with AAV micro-dystrophin vector and in 12-m-old mdx mice treated AAV SERCA2a vector [7, 24]. Further studies are needed to clarify this mysterious finding.
Perhaps one of the most striking findings is the hemodynamic response to AAV micro-dystrophin therapy. Treatment started before 20 months of age resulted in clear-cut improvement in both systolic and diastolic function. Further, ejection fraction, stroke volume and cardiac output were all significantly increased following AAV microgene therapy [7]. In contrast, none of the systolic indices were corrected in mdx mice treated after 21 months of age (Figure 4B). For diastolic parameters, dP/dt min was not improved in mice treated at the terminal age. End diastolic volume and heart relaxation time constant (Tau) showed a trend of reduction but the difference was not statistically significant (Figure 4B). Overall markers of heart function (as reflected by ejection fraction, stroke volume and cardiac output) remained compromised when AAV microgene vector was delivered to >21-m-old mdx mice (Figure 4B).
Despite a lack of apparent influence on baseline hemodynamics, micro-dystrophin expression enhanced the tolerance of terminally aged mdx mice to β-adrenergic stress (Figure 4C). When stimulated with dobutamine, the survival rate of AAV treated mice was significantly higher than that of untreated mdx mice (Figure 4C). We evaluated left ventricular function after dobutamine stress to help elucidate the underlying mechanisms for the improved survival following adrenergic stimulation. Mdx mice treated with AAV micro-dystrophin showed the expected improvement in dP/dt max along with a trend towards improved stroke volume and cardiac output (Supplementary Figure 2). These findings suggest that AAV micro-dystrophin therapy strengthens the aged mdx heart, allowing appropriate inotropic improvements after β-adrenergic stimulation. This protection prevented decompensated left ventricular failure as was seen in the acute death of untreated mdx mice.
Taken together, our results in >21-m-old mdx mice have revealed for the first time the limitations of AAV micro-dystrophin gene therapy in severe Duchenne cardiomyopathy. Unlike what has been reported in younger mice, microgene replacement therapy alone may not provide significant hemodynamic benefit once dilated cardiomyopathy has reached the final stage. There is no doubt that early therapy is necessary for the best protection. However, this may not always be the case in a clinical scenario. For severe patients, one may need to develop supplementary gene therapy to directly target heart failure. In this regard, AAV-mediated SERCA2a expression may represent a promising strategy to boost micro-dystrophin therapy [24-27].
Supplementary Material
Supplementary Figure 1. Whole heart immunofluorescence staining photomicrograph of the untreated mdx mouse shown in Figure 1B. The high power images of the boxed regions are shown in Figure 1B.
Supplementary Figure 2. Quantitative comparison of hemodynamic changes in dobutamine-stressed mice. After assessment of baseline LV function during cardiac catheterization, mice were given an intraperitoneal injection of dobutamine (5 μg/g body weight; Sigma, St. Louis, MO). At 5 minutes post injection, left ventricular function data was again collected. The dosage of dobutamine and timing of collection were similar to a previously published protocol to achieve maximal adrenergic stimulation (Bostick et al 2008 Circ Res 102:121-130). The dobutamine stress data was compared to the pre-dobutamine reading using the paired student’s t-test. Top panels, pre and post-dobutamine comparison of heart rate, dP/dt max, stroke volume and cardiac output in BL10 mice. Bottom panels, pre- and post-dobutamine comparison of heart rate, dP/dt max, stroke volume and cardiac output in AAV treated mdx mice. Asterisk, significantly different from the other group. Filled box, pre-dobutamine; Open box, post-dobutamine.
Highlights.
AAV-9 efficiently transduced end-stage dystrophic myocardium.
Micro-dystrophin gene therapy in end-stage Duchenne cardiomyopathy mouse model.
Micro-dystrophin improved ECG but not heart fibrosis in terminal aged mdx mice.
AAV-9 Micro-dystrophin did not correct hemodynamic defects in >21-m-old mdx mice.
AAV-9 micro-dystrophin prevented death from dobutamine stress in end-stage mdx mice.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (HL-91883, DD) and the Muscular Dystrophy Association (DD).
Glossary
- Mdx
C57BL/10ScSn-Dmdmdx/J, dystrophin-deficient BL10 mouse
- AAV
adeno-associated virus, recombinant replication deficient parvovirus
- Microgene
truncated genetically engineered dystrophin gene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
References
- [1].Shin J-H, Bostick B, Yue Y, Duan D. Duchenne cardiomyopathy gene therapy. In: Duan D, editor. Muscle gene therapy. Springer Science + Business Media, LLC; New York: 2010. pp. 141–62. [Google Scholar]
- [2].Duan D. Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Hum Mol Genet. 2006 Oct 15;15(2):R253–61. doi: 10.1093/hmg/ddl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. Faseb J. 2007 Jul;21(9):2195–204. doi: 10.1096/fj.06-7353com. [DOI] [PubMed] [Google Scholar]
- [4].Li D, Long C, Yue Y, Duan D. Sub-physiological sarcoglycan expression contributes to compensatory muscle protection in mdx mice. Hum Mol Genet. 2009 Apr 1;18(7):1209–20. doi: 10.1093/hmg/ddp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bostick B, Yue Y, Duan D. Gender influences cardiac function in the mdx model of Duchenne cardiomyopathy. Muscle Nerve. 2010 Oct;42(4):600–3. doi: 10.1002/mus.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bostick B, Yue Y, Long C, Duan D. Prevention of Dystrophin-Deficient Cardiomyopathy in Twenty-One-Month-Old Carrier Mice by Mosaic Dystrophin Expression or Complementary Dystrophin/Utrophin Expression. Circ Res. 2008 Jan 4;102(1):121–30. doi: 10.1161/CIRCRESAHA.107.162982. [DOI] [PubMed] [Google Scholar]
- [7].Bostick B, Shin J-H, Yue Y, Duan D. AAV-microdystrophin therapy improves cardiac performance in aged female mdx mice. Mol Ther. 2011;19(10):1826–32. doi: 10.1038/mt.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bostick B, Yue Y, Lai Y, Long C, Li D, Duan D. Adeno-associated virus serotype-9 microdystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice. Hum Gene Ther. 2008 Aug;19(8):851–6. doi: 10.1089/hum.2008.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L, et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat Med. 2006 Jul;12(7):787–9. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS, Duan D. Microdystrophin Gene Therapy of Cardiomyopathy Restores Dystrophin-Glycoprotein Complex and Improves Sarcolemma Integrity in the Mdx Mouse Heart. Circulation. 2003 Sep 30;108(13):1626–32. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shin JH, Nitahara-Kasahara Y, Hayashita-Kinoh H, Ohshima-Hosoyama S, Kinoshita K, Chiyo T, et al. Improvement of cardiac fibrosis in dystrophic mice by rAAV9-mediated microdystrophin transduction. Gene Ther. 2011 Sep;18(9):910–9. doi: 10.1038/gt.2011.36. [DOI] [PubMed] [Google Scholar]
- [12].Townsend D, Blankinship MJ, Allen JM, Gregorevic P, Chamberlain JS, Metzger JM. Systemic Administration of Micro-dystrophin Restores Cardiac Geometry and Prevents Dobutamine-induced Cardiac Pump Failure. Mol Ther. 2007 Jun;15(6):1086–92. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- [13].Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 2002;8(3):253–61. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- [14].Yue Y, Liu M, Duan D. C-terminal truncated microdystrophin recruits dystrobrevin and syntrophin to the dystrophin-associated glycoprotein complex and reduces muscular dystrophy in symptomatic utrophin/dystrophin double knock-out mice. Mol Ther. 2006 Jul;14(1):79–87. doi: 10.1016/j.ymthe.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Morris G, Man NT, Sewry CA. Monitoring duchenne muscular dystrophy gene therapy with epitope-specific monoclonal antibodies. Methods Mol Biol. 2011;709:39–61. doi: 10.1007/978-1-61737-982-6_3. [DOI] [PubMed] [Google Scholar]
- [16].Bostick B, Yue Y, Long C, Marschalk N, Fine DM, Chen J, et al. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged Mdx mice. Mol Ther. 2009 Feb;17(2):253–61. doi: 10.1038/mt.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bostick B, Yue Y, Duan D. Phenotyping cardiac gene therapy in mice. Methods Mol Biol. 2011;709:91–104. doi: 10.1007/978-1-61737-982-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gyorke S, Stevens SC, Terentyev D. Cardiac calsequestrin: quest inside the SR. J Physiol. 2009 Jul 1;587(Pt 13):3091–4. doi: 10.1113/jphysiol.2009.172049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008 Jan 15;77(2):265–73. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- [20].Perloff JK. Cardiac rhythm and conduction in Duchenne’s muscular dystrophy: a prospective study of 20 patients. J Am Coll Cardiol. 1984;3(5):1263–8. doi: 10.1016/s0735-1097(84)80186-2. [DOI] [PubMed] [Google Scholar]
- [21].Urasawa N, Wada MR, Machida N, Yuasa K, Shimatsu Y, Wakao Y, et al. Selective vacuolar degeneration in dystrophin-deficient canine Purkinje fibers despite preservation of dystrophin-associated proteins with overexpression of Dp71. Circulation. 2008 May 13;117(19):2437–48. doi: 10.1161/CIRCULATIONAHA.107.739326. [DOI] [PubMed] [Google Scholar]
- [22].Bia BL, Cassidy PJ, Young ME, Rafael JA, Leighton B, Davies KE, et al. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol. 1999;31(10):1857–62. doi: 10.1006/jmcc.1999.1018. [DOI] [PubMed] [Google Scholar]
- [23].Chu V, Otero JM, Lopez O, Sullivan MF, Morgan JP, Amende I, et al. Electrocardiographic findings in mdx mice: a cardiac phenotype of Duchenne muscular dystrophy. Muscle Nerve. 2002 Oct;26(4):513–9. doi: 10.1002/mus.10223. [DOI] [PubMed] [Google Scholar]
- [24].Shin JH, Bostick B, Yue Y, Hajjar R, Duan D. SERCA2a gene transfer improves electrocardiographic performance in aged mdx mice. J Transl Med. 2011;9:132. doi: 10.1186/1479-5876-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gwathmey JK, Yerevanian AI, Hajjar RJ. Cardiac gene therapy with SERCA2a: from bench to bedside. J Mol Cell Cardiol. 2011 May;50(5):803–12. doi: 10.1016/j.yjmcc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B, et al. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A Phase 2 Trial of Intracoronary Gene Therapy of Sarcoplasmic Reticulum Ca2+-ATPase in Patients With Advanced Heart Failure. Circulation. 2011 Jun 27;124(3):304–13. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lai Y, Duan D. Progress in gene therapy of dystrophic heart disease. Gene Ther. 2012 Feb 9; doi: 10.1038/gt.2012.10. doi: 10.1038/gt.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Whole heart immunofluorescence staining photomicrograph of the untreated mdx mouse shown in Figure 1B. The high power images of the boxed regions are shown in Figure 1B.
Supplementary Figure 2. Quantitative comparison of hemodynamic changes in dobutamine-stressed mice. After assessment of baseline LV function during cardiac catheterization, mice were given an intraperitoneal injection of dobutamine (5 μg/g body weight; Sigma, St. Louis, MO). At 5 minutes post injection, left ventricular function data was again collected. The dosage of dobutamine and timing of collection were similar to a previously published protocol to achieve maximal adrenergic stimulation (Bostick et al 2008 Circ Res 102:121-130). The dobutamine stress data was compared to the pre-dobutamine reading using the paired student’s t-test. Top panels, pre and post-dobutamine comparison of heart rate, dP/dt max, stroke volume and cardiac output in BL10 mice. Bottom panels, pre- and post-dobutamine comparison of heart rate, dP/dt max, stroke volume and cardiac output in AAV treated mdx mice. Asterisk, significantly different from the other group. Filled box, pre-dobutamine; Open box, post-dobutamine.