Abstract
Exercise, specifically voluntary wheel running, is a potent stimulator of hippocampal neurogenesis in adult mice. In addition, exercise induces behavioral changes in numerous measures of anxiety in rodents. However, the physiological underpinnings of these changes are poorly understood. To investigate the role of neurogenesis in exercise-mediated anxiety, we examined the cellular and behavioral effects of voluntary wheel running in mice with a reduction in hippocampal neurogenesis, achieved through conditional deletion of ataxia telangeictasia-mutated and rad-3 related protein (ATR), a cell cycle checkpoint kinase necessary for normal levels of neurogenesis. Following hippocampal microinjection of an adeno-associated virus expressing Cre recombinase to delete ATR, mice were exposed to four weeks of voluntary wheel running and subsequently evaluated for anxiety-like behavior. Wheel running resulted in increased cell proliferation and neurogenesis, as measured by bromodeoxyuridine and doublecortin, respectively. Wheel running also resulted in heightened anxiety in the novelty-induced hypophagia, open field, and light-dark box tests. However, both the neurogenic and anxiogenic effects of wheel running were attenuated following hippocampal ATR deletion, suggesting increased neurogenesis is an important mediator of exercise-induced anxiety.
Keywords: neurogenesis, anxiety, exercise, wheel running, hyponeophagia
Introduction
Exercise is associated with a wide array of health benefits (van Praag, 2008, for review). In addition to the physical benefits, exercise can improve cognition (van Praag et al., 1999; Van der Borght et al., 2007; Winter et al., 2007; Nichol et al., 2009), alleviate symptoms of neurodegenerative diseases (Tillerson et al., 2003; Nichol et al., 2009), and aid in recovery from mood disorders (Blumenthal et al., 1999; Babyak et al., 2000; Duman et al., 2008).
Rodents exhibit a strong penchant for voluntary wheel running, which activates brain reward pathways (Brene et al., 2007) and is used as a model to investigate the mechanisms underlying the benefits of exercise in humans. Voluntary running results in a robust increase in hippocampal neurogenesis in adult mice. Indeed, running has been shown to increase proliferation of neural progenitor cells (Kronenberg et al., 2003), and increase both the number and percentage of newborn cells which become mature neurons (van Praag et al., 1999). Running and neurogenesis have been directly correlated, further highlighting the potent influence of exercise on hippocampal neurogenesis (Allen et al., 2001; Rhodes et al., 2003; Clark et al., 2011). A multitude of changes are implicated in the therapeutic effects of exercise, including heightened synaptic plasticity (van Praag et al., 1999; Farmer et al., 2004), angiogenesis (Van der Borght et al., 2009) and growth factor expression (Neeper et al., 1996; Kitamura et al., 2003). As evidenced by the importance of newborn neurons in cognition and mood regulation (Sahay et al., 2011; Surget et al., 2011), neurogenesis is an additional mechanism which may contribute to the therapeutic effects of exercise.
In light of clinical evidence for the mood-improving effects of exercise (Deslandes et al., 2009, for review), we set out to investigate the underlying mechanisms associated with these effects. A number of studies have investigated the effects of voluntary wheel running on mood and anxiety-related behaviors in rodents, the results of which are inconsistent. While some studies find decreased anxiety (Dishman et al., 1996; Salam et al., 2009), others demonstrate increased anxiety (Burghardt et al., 2004; Fuss et al., 2010). Yet others demonstrate either increased or decreased anxiety depending on the specific anxiety measure used and the timing of the measurement (Binder et al., 2004; Duman et al., 2008). A recent study demonstrated that running-induced anxiety was prevented by x-ray irradiation, a technique to ablate neurogenesis (Fuss et al., 2010), supporting a direct role for neurogenesis in the development of exercise-induced anxiety. Alternatively, Revest and colleagues (Revest et al., 2009) find increased anxiety following a partial knockdown of neurogenesis. These conflicting observations regarding the relationship between rate of neurogenesis and anxiety state support the need for additional research in this arena.
We previously showed partial suppression of hippocampal neurogenesis following hippocampal deletion of ATR, a cell cycle checkpoint kinase (Onksen et al., 2011). Here, we explore the possibility that deletion of ATR from the hippocampus results in resistance to the neurogenic effects of voluntary exercise and examine the behavioral implications of that resistance.
Methods
Animals
Mice homozygous for the Cre/lox-conditional allele of ATR (ATRf/f) on a 129S2/SvPas/C57BL/6 mixed background were generated as previously described (Ruzankina et al., 2007). Briefly, mice were generated from a D3 ES cell line originally derived from 129Sv blastocysts. Homologously recombined ES cells were injected into C57BL/6 blastocysts and chimeric offspring were mated with mice from the C57Bl/6 strain to produce mice of all three geneotypes; ATRf/f, ATRf/-, ATR-/-. ATRf/f were obtained from intercrosses and ultimately maintained as ATRf/f from approximately 4 generations of homozygous crosses of ATRf/f × ATRf/f. ATRf/f mice receiving hippocampal microinjection of AAV.Cre and having ATR deleted throughout the hippocampus are subsequently referred to as ATRΔHipp, while control mice injected with AAV.eGFP retain the ATRf/f designation.
Stereotaxic surgery was performed at 7-8 weeks of age and all behavioral experiments were conducted on adult males and females at least 6 weeks following stereotaxic surgery. Mice were housed in groups of 3-4 following surgery and subsequently single-housed at least one week prior to being placed in to cages containing running wheels. Cages with running wheels (Mini Mitter, Bend OR) measured 20×36 cm with an 11.5 cm diameter wheel mounted to the cage top. Wheel rotations were monitored continuously via VitalView (Mini Mitter). Sedentary control animals were housed in identical cages with immobilized wheels. Animals were maintained on a 12 hr light/dark cycle (lights on 7:00 AM to 7:00 PM) with food and water available ad libitum in accordance with the University of Pennsylvania Institutional Animal Care and Use Committee.
Experimental Design
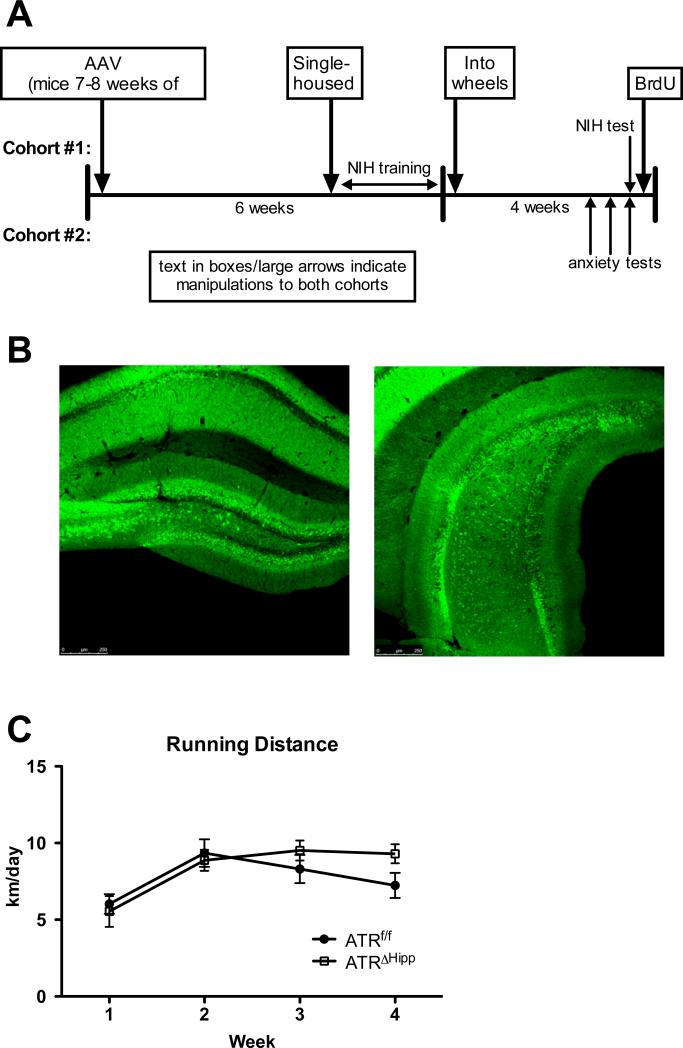
Two cohorts of ATRf/f mice were used in this study. Mice in both cohorts received hippocampal micro-injections at 8 weeks of age. For a schematic of the experimental design see Figure 1a. Mice in cohort #1 were used for the novelty-induced hypophagia (NIH) test and were therefore single housed and trained to consume peanut butter chips in their home cages prior to being placed in cages containing running wheels. Cohort #2 was used to evaluate behavior in the open field, marble-burying and light-dark box tests (separated by 3 days). All behavioral testing was performed during the 4th week of wheel running. All mice were injected with 200 mg/kg bromodeoxyuridine (BrdU, Sigma) 24 hours following the last behavioral test and subsequently perfused 24 hours following BrdU injection. Mice had ad libitum access to running wheels until they were perfused (except when removed from the cages for behavioral testing). Data from both cohorts was pooled to examine effects of AAV injection and voluntary running on neurogenesis.
Figure 1.
Experimental design. A Two cohorts of mice were used in this study, the first for novelty-induced hypophagia and the second for open field and light-dark box tests. Both cohorts were injected with AAV at 7-8 weeks of age, single-housed approximately 5 weeks later, and then placed in running wheels for 4 weeks. Behavioral testing occurred in the final week of running. BrdU (200 mg/kg, IP) was injected 24 hours following the last behavioral test and mice were perfused 24 hours following BrdU injections. B,C Photomicrographs depicting eGFP expression in the dorsal (B) and ventral (C) hippocampus 8 weeks following AAV.eGFP injection. D Average weekly running distance. No significant differences were observed in running distance between ATRf/f and ATRΔHipp mice. Error bars represent SEM.
Adeno-associated virus
Adeno-associated viruses (AAV) expressing Cre recombinase (AAV2/9.CMV.PI.CRE, titer 2.84*1013 gc/ml) and eGFP (AAV2/9.CMV.eGFP, titer 3.74*1013 gc/ml) were generated by the University of Pennsylvania Vector Core. The expression cassette consists of the AAV2 terminal repeats flanking the CMV promoter-PI-Cre recombinase/eGFP sequences packaged into AAV9. The vectors were purified using CsCl sedimentation method. Quantification of vector genome copies (gc) was performed by Q-PCR. AAVs were diluted in sterile PBS for microinjections.
Hippocampal Injections
ATRf/f mice (6-8 weeks) were anesthetized with isoflurane and secured in a stereotaxic frame (Kopf, Tujuna, CA). Holes were drilled bilaterally in the skull at sites of injection. Stereotaxic coordinates to target the dorsal and the ventral hippocampus are (from Bregma) anterior-posterior -2.1, lateral +/-1.4, dorso-ventral -2.0, and anterior-posterior -2.9, lateral +/-3.0, dorso-ventral -3.8. 0.5 ul of 1*1010 gc/ul AAV was injected at each site through a 33 gauge needle on a 5 ul Hamilton syringe using a KDS310 Nano Pump (KD Scientific, Holliston, MA) mounted to the stereotaxic frame, at a rate of 0.1 ul/min. The needle remained in place for 4 additional minutes at each injection site. The skin was sutured and the animal recovered on a heating pad before returning to the home cage.
Immunohistochemistry
Mice were anesthetized with nembutol (10 mg/kg) and transcardially perfused with cold 0.1 M phosphate-buffered saline (PBS) for 5 minutes, followed by cold 4% paraformaldehyde (PFA) in PBS for 10 minutes. Brains were postfixed overnight in PFA at 4°C and subsequently stored at 4°C in 30% sucrose. Brains were frozen on dry ice, sectioned coronally at a thickness of 40 μm, and transferred to PBS + 0.5% Sodium Azide at 4°C prior to processing for immunohistochemistry.
For BrdU analysis, sections were wet-mounted on Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA) for stereological analysis using a modified version of the optical fractionator method (West et al., 1991). Mounted sections were incubated in 0.1 M citric acid, pH 6.0, for antigen retrieval. Subsequently, slides were incubated in trypsin, 2N HCl, then primary antibody (mouse anti-BrdU, 1:200, Becton Dickinson) with 0.5% Tween 20 overnight. On the second day, slides were incubated for 1 hour in secondary antibody (biotinylated anti-mouse IgG, Vector Laboratories), 1 hour in avidin-biotin-HRP (1:100, Vector Laboratories), and labeled cells were visualized using diaminobenzidine (Sigma). PBS-washed slides were then counterstained and coverslipped with Permount. Systematic sampling using a predetermined periodicity (every ninth slice) and a random starting point was employed for quantification. All BrdU-IR cells in every 9th section of the hippocampus, along the complete extent of its rostralcaudal axis, were counted with a 100x oil immersion lens, omitting cells in the upper-most focal plane to avoid double-counting. Every 9th coronal slice between -0.9 mm and -4.0 mm from bregma were analyzed to ensure complete sampling. Within each section, all immuno-reactive cells within the granule cell layer (GCL) and the subgranular zone (SGZ) were counted. The SGZ was defined as the layer within two cells of the GCL. Cells within clusters were counted by distinguishing nuclear borders while focusing down through the tissue using an objective with a narrow depth of focus.
For doublecortin (DCX) analysis, free-floating sections of a 1/9 series through the hippocampus were blocked for 1 hour with 3% Normal Horse Serum, 0.5% Tween 20 and 0.2% Triton in PBS (blocking solution). Sections were then incubated for 72 hours at 4°C in primary antibody (Goat anti-DCX, Santa Cruz #8066) diluted 1:500 in blocking solution. Sections were washed and incubated for 1 hour in secondary antibody (Horse anti-goat, Vector Laboratories) diluted 1:200 in blocking solution. Sections were then washed and treated with 0.75% H2O2 for 20 minutes prior to incubation in avidin-biotin-HRP (1:200, Vector Laboratories). Labeled cells were visualized using nickel-enhanced diaminobenzidine. Free-floating sections were mounted onto slides and dried overnight before dehydrating and coverslipping with Permount. DCX-IR cells in every 9th section were counted with a 100x oil immersion lens, omitting cells in the upper-most focal plane. All cells located within the GCL and SGZ were counted.
Novelty-induced Hypophagia
For 1 week prior to the training period, and through the experiment, mice were single housed. On 11 consecutive training days, mice were exposed to highly palatable food (peanut butter chips, Nestle, Glendale CA) in a plastic dish in their home cage. On each training day mice were acclimated to the presence of a plastic cage divider for 1 hour, consistent with previous NIH studies (Gur et al., 2007; Onksen et al., 2011). Peanut butter chips were then placed in the cage for 15 minutes and latency to initiate feeding was measured. Subsequent to training, mice were placed in cages containing running wheels and left for 28 days. On days 25 and 26, a home cage test was performed in which mice were removed from wheel-containing cages and placed into a standard mouse cage, allowed to acclimate to the cage and divider for 1 hour, and latency to feed was subsequently measured. On day 27, latency to feed in a novel environment was measured. Mice were removed from wheel-containing cages, placed for 1 hour in the same standard mouse cage with divider that was used for home cage tests, and subsequently placed in a novel, anxiety provoking environment where latency to feed was measured. The novel environment consisted of an empty cage with no bedding, set inside a white box with a bright light and a novel odor (pine sol). Following each test session, mice were returned to their running wheel-containing cages.
Open Field
Open field behavior was monitored in a white box measuring 41×35 cm in dim light conditions (40 lux). Activity was recorded by an overhead camera and analyzed with TopScanLite 2.0 (Clever Sys, Inc., Reston, VA) Parameters assessed were time spent and distance travelled in the center and outer zones. The center zone was defined as 25% of the total area.
Light-Dark Box
The light-dark box consisted of two adjacent chambers, connected by a 5×5 cm opening. Each chamber measured 17×20 cm. The dark chamber was black and covered. The light chamber was white and illuminated with bright light (750 lux). Each test session lasted 5 minutes and was initiated by placing the mouse in the corner of the light chamber. Behavior was recorded by an overhead camera and analyzed with TopScanLite 2.0. Parameters assessed were transitions between the two chambers, time spent in each chamber, and distance travelled in the light chamber.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 5 and JMP software. Data are reported as mean ± SEM. Studies involving two variables were analyzed by two-way ANOVA, with time as a repeated measure where appropriate. Bonferroni post hoc tests were performed to compare individual treatment groups. Data from the NIH test was analyzed by three-factor ANOVA to account for gene, exercise, and test environment.
Results
ATR deletion attenuates the neurogenic effect of voluntary wheel running
To determine if ATR deletion confers resistance to the neurogenic effects of wheel running, we microinjected AAV.Cre or AAV.eGFP into the hippocampus of ATRf/f mice and assigned them to either the running or the sedentary condition. The pattern of AAV spread and subsequent eGFP expression is depicted in photomicrographs of the dorsal (Fig. 1b) and ventral (Fig. 1c) hippocampus. We previously demonstrated a 78% reduction of ATR in the hippocampus following microinjection of AAV.Cre (Onksen et al., 2011). Six weeks following AAV injection, mice were single housed in running wheel-containing cages. Running distance plateaued in the 2nd week in both ATRf/f and ATRΔHipp mice (Fig. 1d), which is consistent with other studies involving wheel running (Clark et al., 2009). No differences were observed in wheel running distance between treatment groups (ATRf/f, 7.73±2.49 km/day; ATRΔHipp, 8.31±2.14 km/day; gene main effect, F(1,72)=0.404, p=0.53; time main effect, F(1,72)=16.57, p<0.001).
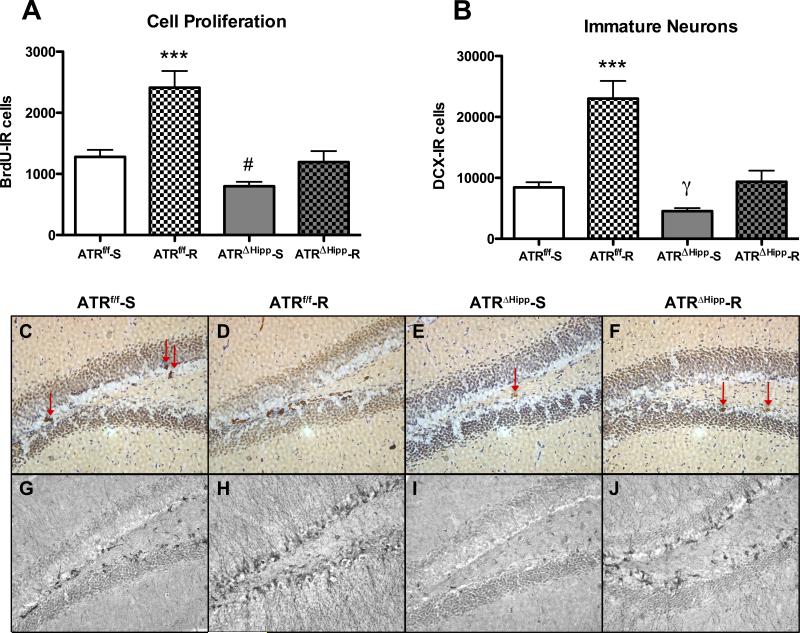
Following 28 days of running, immunohistochemical analysis was performed to measure cell proliferation and number of immature neurons. BrdU (200 mg/kg) was injected 24 hours prior to perfusing the animals. An average of 8.29±0.61 brain sections per mouse were analyzed for BrdU quantification, and 8.31±0.56 sections per mouse were analyzed for doublecortin (DCX) quantification. Primary statistical analysis revealed no significant effects of sex on BrdU or DCX counts. For BrdU, three-factor ANOVA (sex, gene, exercise as independent factors) revealed no main effect of sex (F(1,53)=0.636, p=0.43), no sex × gene interaction (F(1,53)=0.191, p=0.66) and no sex × exercise interaction (F(1,53)=0.0005, p=0.98). For DCX, three-factor ANOVA (sex, gene, exercise as independent factors) revealed no main effect of sex (F(1,54)=0.035, p=0.85), no sex × gene interaction (F(1,54)=0.196, p=0.66), and no sex × exercise interaction (F(1,54)=0.747, p=0.39). Based on these results, male and female mice were pooled for subsequent analyses. Two-way ANOVA of BrdU-positive cells in the hippocampus (Fig. 2a,b) revealed main effects of gene (F(1,50)=28.89, p<0.0001) and exercise (F(1,50)=23.33, p<0.0001), as well as a significant interaction (F(1,50)=5.26, p<0.05). Post tests indicate that wheel running resulted in a significant increase in cell proliferation in ATRf/f runners compared to all other treatment groups (+89% vs. ATRf/f sedentary, +206% vs. ATRΔHipp sedentary, +102% vs. ATRΔHipp runners, each p<0.001). Unlike ATRf/f runners, ATRΔHipp runners did not exhibit significantly increased cell proliferation compared to their sedentary counterparts. In addition, as previously observed, ATR deletion resulted in significantly reduced cell proliferation among sedentary mice (-38%, p<0.05). Two-way ANOVA of DCX-positive cells in the hippocampus (Fig. 2c,d) revealed significant main effects of gene (F(1,51)=32.65, p<0.0001) and exercise (F(1,51)=39.73, p<0.0001), as well as a significant interaction (F(1,51)=10.08, p<0.01). Similar to our BrdU observations, post tests indicated DCX expression was significantly increased in ATRf/f runners compared to all other groups (+172% vs. ATRf/f sedentary, +406% vs. ATRΔHipp sedentary, +146% vs. ATRΔHipp runners, each p<0.001). ATRΔHipp runners did not exhibit significantly increased DCX-expressing cells compared to their sedentary counterparts. Post tests did not show a significant effect of ATR deletion among sedentary mice. However, we previously demonstrated an effect of ATR deletion to reduce DCX-expressing cells, so we therefore performed a Student's t-test comparing sedentary ATRf/f and ATRΔHipp mice to verify this effect had again been achieved, and indeed it had (-46%, t(30)=4.00, p<0.001). The effects of ATR deletion and wheel running were similarly observed in both the dorsal and ventral hippocampus (Supp. Fig. S1). Taken together, BrdU and DCX data indicate that mice lacking hippocampal ATR exhibit a reduced neurogenic response to wheel running.
Figure 2.
Effects of ATR deletion and voluntary wheel running on neurogenesis. A Voluntary exercise resulted in a significant increase in BrdU-expressing cells in ATRf/f mice (***, p<0.001 vs. ATRf/f-S, ATRΔHipp-S and ATRΔHipp-R groups, n=11-16 per treatment group). Among sedentary controls, ATR deletion resulted in significantly reduced BrdU-expressing cells (#, p<0.05 vs. ATRf/f-S group). B Voluntary exercise resulted in a significant increase in DCX-expressing cells in ATRf/f mice (***, p<0.001 vs. ATRf/f-S, ATRΔHipp-S and ATRΔHipp-R groups, n=11-16 per treatment group). Among sedentary controls, ATR deletion resulted in reduced DCX-expressing cells (γ, p<0.001 vs. ATRf/f-S group by Student's t-test). C-F. Representative photomicrographs of BrdU immunohistochemistry in each of 4 treatment groups: ATRf/f-S (C), ATRf/f-R (D), ATRΔHipp-S (E) and ATRΔHipp-R (F). G-J. Representative photomicrographs of DCX immunohistochemistry in each of 4 treatment groups: ATRf/f-S (G), ATRf/f-R (H), ATRΔHipp-S (I) and ATRΔHipp-R (J). Error bars represent SEM.
Running-induced anxiety in the NIH test is attenuated in ATRΔHipp mice
We recently observed reduced feeding latency in a novel environment in mice with reduced levels of neurogenesis (Onksen et al., 2011). The NIH paradigm consists of training mice to consume a palatable food in their home cage, and subsequently measuring their latency to consume this same food in a novel, anxiety provoking environment. Thus, reduced neurogenesis may contribute to reduced anxiety in this particular paradigm. As others have demonstrated heightened anxiety as a result of voluntary exercise, we sought to determine whether our voluntary wheel running paradigm would result in increased anxiety in the NIH test, and whether this anxiogenic phenotype would be altered by reduced neurogenesis.
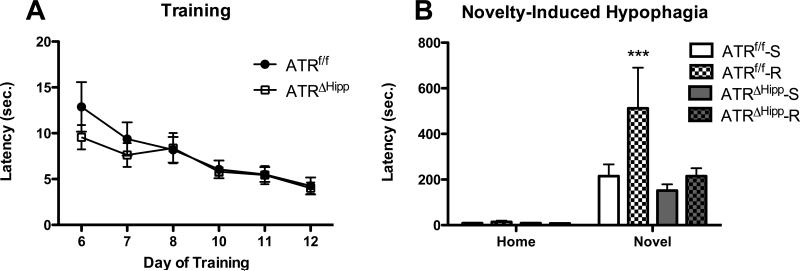
Six weeks following hippocampal microinjection of AAV to delete ATR, mice were single housed and subsequently trained to consume peanut butter chips in their home cage. No differences were observed in training performance between ATRf/f and ATRΔHipp mice (Fig. 3a), as measured by two-way ANOVA of feeding latency on days 6-11, with day as a repeated measure (day main effect, F(5,150)=15.91, p<0.0001; gene main effect, F(1,150)=0.339, p=0.565). Training was followed by 28 days of housing in running-wheel-containing cages.
Figure 3.
Effects of ATR deletion and voluntary wheel running on behavior in the NIH test. A Home cage feeding latency prior to placement in running wheels. No gene effect observed. B Home cage and novel environment feeding latencies following wheel running. In the home test all mice exhibited comparable feeding latencies. On the novel test day ATRf/f runners exhibited significantly greater latency compared to each of the other treatment groups (***, p<0.001 vs. ATRf/f-S, ATRΔHipp-S and ATRΔHipp-R, n=4-8 per group). Error bars represent SEM.
On the 25th and 26th days of running, mice were given access to peanut butter chips in a home cage environment. Latency was measured on the 2nd home environment exposure. Our unpublished observations indicated exercise to be anxiogenic when mice are initially removed from running wheels and tested for feeding latency in a home cage. Thus, we measured feeding latency during the second day of home cage feeding, during which all treatment groups performed similarly (Fig. 3b). Normalization of home cage behavior allows for clear interpretation of any anxiety phenotypes observed in the novel test. On the 27th day of running, feeding latency in a novel, anxiety-provoking environment was measured.
Three-factor ANOVA of feeding latency revealed significant main effects of gene (F(1,51)=7.64, p<0.01), exercise (F(1,51)=7.94, p<0.01) and environment (F(1,51)=23.31, p<0.001), in addition to a significant gene × exercise × environment interaction (F(1,51)=24.29, p<0.001). Post test comparisons indicated significantly greater latency in the ATRf/f runners compared to all other treatment groups in the novel environment (Fig. 3b, p<0.05). Among ATRΔHipp mice, there was no difference in latency between runners and their sedentary counterparts in the novel environment (Fig. 3b), indicating ATR deletion blocked the anxiogenic effects of running on feeding latency. As with cellular effects, no main effect of sex, no sex × gene interaction and no sex × exercise interactions were revealed, therefore male and female mice were pooled for these and subsequent behavioral analyses.
Running-induced anxiety in an open field is attenuated in ATRΔHipp mice
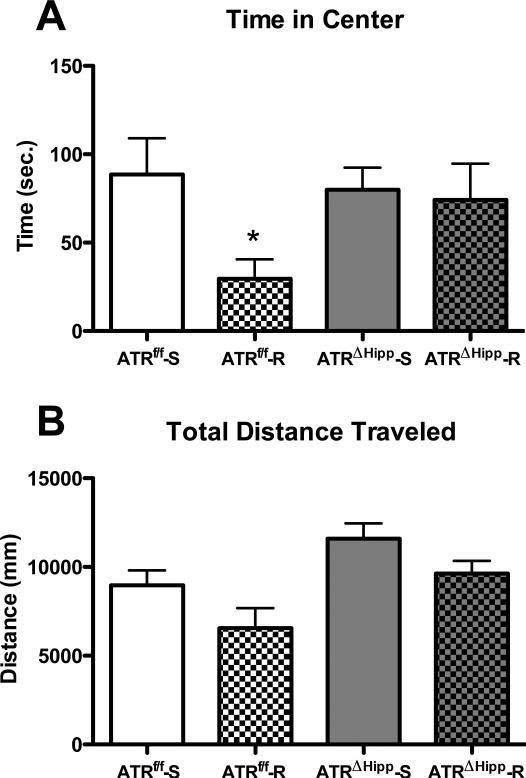
Voluntary wheel running results in increased anxiety in an open field test, as measured by reduced time spent in the center zone. This anxiogenic phenotype was attenuated in ATRΔHipp mice. Two-way ANOVA of time spent in the center zone (Fig. 4a) revealed a near-significant effect of exercise (F(1,24)=3.67, p=0.067). Bonferroni post tests to compare individual treatment groups indicated significantly reduced time spent in the center zone in ATRf/f runners compared to their sedentary counterparts (-67%, t(13)=2.445, p<0.05). ATRΔHipp runners did not differ from either sedentary control group. In addition to time spent in the center zone, total distance traveled in the open field was measured (Fig. 4b). We observed a significant main effect of exercise (F(1,24)=5.75, p<0.05) and of ATR deletion (F(1,24)=9.77, p<0.01), with no exercise × gene interaction (F(1,24)=0.06, p=0.81). This is indicative of an overall inhibitory effect of exercise and an overall stimulatory effect of ATR deletion on exploratory behavior in the open field.
Figure 4.
Effects of ATR deletion and voluntary wheel running on open field behavior. A Time in center zone. ATRf/f runners spent less time in the center zone compared to their sedentary counterparts (*, p<0.05 vs. ATRf/f-S). B Total distance traveled. Running exerted a main effect of reduced total distance traveled compared to sedentary mice. ATR deletion exerted a main effect of increased total distance traveled compared to control ATRf/f mice. There were no significant differences between individual groups. N=6-8 per group. Error bars represent SEM.
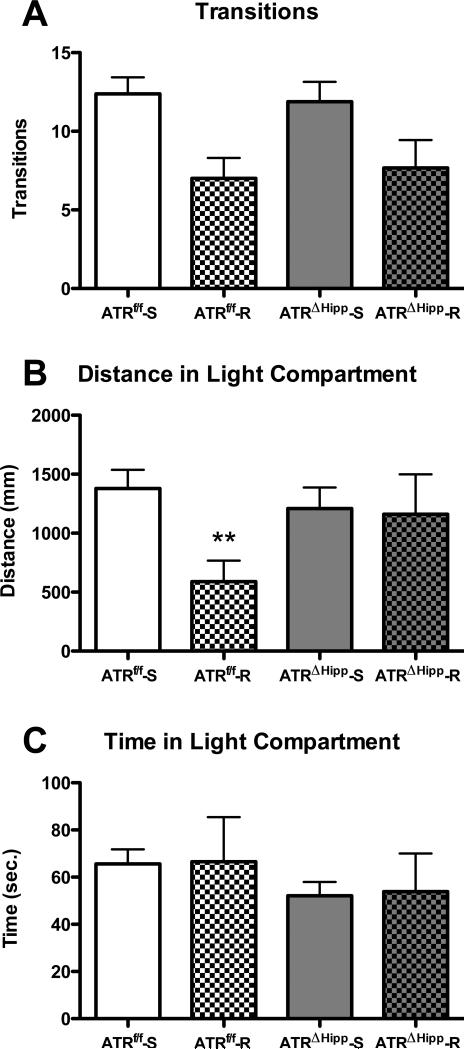
Running-induced anxiety in the light-dark box test is attenuated in ATRΔHipp mice
As an additional measure of anxiety, we evaluated behavior in the light-dark box following voluntary wheel running. Two-way ANOVA revealed a significant main effect of exercise (F(1,25)=12.82, p<0.01) on light-to-dark transitions (Fig. 5a). There was no effect of gene (F(1,25)=0.004, p<0.95) and no exercise × gene interaction (F(1,25)=0.19, p<0.67) on this outcome measure, suggesting the effect of exercise on transitions is not dependent on a robust stimulation of neurogenesis. Analysis of distance travelled in the light compartment revealed a trend towards an anxiogenic effect of running being blunted in ATRΔHipp mice (Fig. 5b). Two-way ANOVA revealed a near-significant main effect of exercise (F(1,25)=3.97, p=0.057) and a non-significant, but trending, exercise × gene interaction (F(1,25)=3.105, p=0.09). Post tests revealed a significant decrease in distance travelled in the light compartment in ATRf/f runners compared to their sedentary counterparts (p<0.05). This effect was not apparent in ATRΔHipp mice. No effects of running or ATR deletion were observed on time spent in the light compartment (exercise main effect F(1,25)=0.01, p<0.92; gene main effect F(1,25)=1.14, p=0.30; exercise × gene interaction F(1,25)=0.001, p=0.97). Reduced distance traveled in the light compartment in the absence of any changes in total time spent in the compartment may be indicative of reduced exploration.
Figure 5.
Effects of ATR deletion and voluntary wheel running on light-dark box behavior. A Running exerted a main effect of reduced transitions compared to sedentary mice. B ATRf/f runners traveled a shorter distance in the light compartment compared to their sedentary counterparts (**, p<0.01 vs. ATRf/f-S). This effect was not observed in ATRΔHipp mice. C No differences were observed in total time spent in the light compartment. N=6-8 per group. Error bars represent SEM.
Discussion
Exercise is associated with many health benefits in humans, ranging from the cognitive to the physiological (van Praag, 2008). Preclinical research has established exercise as a potent enhancer of hippocampal neurogenesis (van Praag et al., 1999), which may underlie its health benefits. Interestingly, exercise leads to changes in anxiety-like behavior in rodents. The potential role of neurogenesis in modulating anxiety-related behaviors following exercise is only now being explored. In this study, we utilized a transgenic mouse in which Cre recombinase-inducible deletion of ATR from the hippocampus of adult mice leads to reduced levels of hippocampal neurogenesis. Our previous characterization of hippocampal ATR deletion examined effects on basal neurogenesis only. Here, we found that ATR deletion attenuated the neurogenic effect of wheel running. Additionally, wheel running resulted in heightened anxiety in the novelty-induced hypophagia and trends in anxiolytic behavior in the open field, and light-dark box tests. Some of the anxiety phenotypes induced by wheel running were absent following ATR deletion (NIH), while others were attenuated (open field and light-dark box). While our findings are in agreement with work by others (Fuss et al., 2010), there are conflicting reports in the literature regarding effects of exercise on anxiety (Dishman et al., 1996; Binder et al., 2004; Burghardt et al., 2004; Duman et al., 2008; Salam et al., 2009). These discrepancies may arise from a multitude of factors including variation in housing conditions and exercise parameters. However, the most relevant factor is likely differences in running distance and neurogenic response to running across mouse strains (Clark et al., 2011), which will subsequently influence behavioral outcome measures.
We utilized a 4 week, ad libitum access wheel running paradigm, as we previously observed increased cell proliferation and BDNF mRNA in the hippocampus in naïve ATRf/f mice using this paradigm (J.L.O., unpublished observations). Wheel running exerted a potent stimulatory effect on neurogenesis in ATRf/f mice, as indicated by increased BrdU and DCX immunostaining to measure cell proliferation and immature neurons, respectively (Fig. 2). This effect of wheel running was attenuated in ATRΔHipp mice; levels of neurogenesis in ATRΔHipp runners were comparable to those of sedentary ATRf/f controls. Our neurogenesis data suggests ATRΔHipp mice retain some neurogenic capacity in response to a strong stimuli. However, the overall magnitude of the increase remains well below that which is observed in the control group, allowing for investigation of the causal role of excessive neurogenesis in the behavioral changes observed following exercise.
To identify behavioral implications of a blunted neurogenic response to exercise, we examined the anxiety state of the animals. We first examined behavior in the NIH paradigm following exercise. In the novel environment, we observed a large increase in latency to feed in ATRf/f runners compared to sedentary ATRf/f controls (Fig. 3b). This increase was not apparent in the ATRΔHipp runners, suggesting heightened neurogenesis may underlie the effect observed in ATRf/f mice. Because ATR deletion does not influence feeding latency in the home cage test, it is possible that the role of neurogenesis in exercise-induced anxiety is context-dependent and is most relevant in novel environments. Previously, we observed a statistically significant reduction in latency to feed in a novel environment following ATR deletion (Onksen et al., 2011), whereas here we observe only a trend in the NIH test (Fig. 3b) and ATR deletion alone does not reduce all measures of anxiety examined in the present study. We hypothesize that the single-housing conditions necessary for the wheel running studies result in alterations to anxiety state (Kwak et al., 2009) such that certain differences are less likely to be observed.
In addition to the NIH paradigm, we examined anxiety-like behavior in the open field and light-dark box tests. In the open field test (Fig. 4), ATRf/f runners spent less time in the center zone compared to their sedentary counterparts, indicative of increased anxiety. While there were no significant interactions between exercise and ATR deletion in the hippocampus, the ATRΔHipp runners spent similar amounts of time in the center of the open field as the sedentary group, suggesting that ATR and/or the increase in neurogenesis associated with running in wild-type mice is required for some anxiolytic properties of exercise.
In the light-dark box test (Fig. 5), running resulted in a trend for fewer transitions between the compartments. This effect was present in both ATRf/f and ATRΔHipp runners. However, the overall distance traveled in the light compartment was reduced in ATRf/f mice exposed to running, whereas no such reductions were observed between runners and sedentary mice in which ATR had been deleted in the hippocampus (ATRΔHipp). Among previous reports using the light-dark or dark-light tests, some report changes in both transitions and time spent in the light compartment as measures of anxiety (Chaouloff et al., 1997; Frye et al., 2008; Varadarajulu et al., 2011), while others report one or the other (Binder et al., 2004; Correa et al., 2008; Fuss et al., 2010; Pilhatsch et al., 2010). Variability may be dependent on differences in mouse strain or parameters of the testing environment, including size and lighting conditions. Thus, we analyzed transitions, distance traveled in the light compartment, and time in the light compartment in our mice.
Taken together, this data from multiple anxiety tests suggests mice with increased neurogenesis exhibit heightened anxiety. Because increased neurogenesis is associated with heightened cognition in rodent models (Sahay et al., 2011), the phenotype exhibited by ATRf/f runners may be due to increased awareness of novel surroundings and subsequent caution in exploring the environment, evidenced by general trends for reduced distance traveled in the open field and transitions in the light-dark box. An additional hypothesis to be considered is that reduced neurogenesis and the behavioral changes observed herein are parallel effects of ATR deletion. This, however, is an unlikely possibility, as previous research has demonstrated an essential role for ATR in cell proliferation, and no role for ATR within mature neurons on numerous behavioral and circadian outcome measures (Brown and Baltimore, 2003; Ruzankina et al., 2007), suggesting deletion of ATR from the neural stem/progenitor cells directly affects neurogenesis and the subsequent behavioral changes are a result of that altered neurogenesis. In addition, irradiation–induced deficits in neurogenesis and associated behavioral alterations in the open field test were ameliorated by running, further suggesting the link between neurogenesis and behavioral changes in anxiety paradigms (Naylor et al., 2008).
In conclusion, we have shown here that hippocampal neurogenesis is an important determinant of some anxiety-like behaviors in mice. Findings of heightened anxiety would seem at odds with clinical evidence for the beneficial effects of moderate exercise. However, the data presented herein, in addition to recent literature highlighting the prevention of running-induced anxiety by x-ray irradiation (Fuss et al., 2010), strengthens the hypothesis that neurogenesis directly influences the anxiety-like behavior induced by running. As further explanation of exercise effects which seem counterintuitive to the beneficial effects observed in humans, running distances achieved by rodents may not be representative of the exercise behavior that is beneficial to the treatment of mood and anxiety disorders in humans and may indicate that too much neurogenesis can be detrimental to certain behaviors (Saxe et al., 2007). Indeed, moderate exercise is recommended as therapy for depressed mood in humans (Greer and Trivedi, 2009), often in conjunction with pharmacotherapy, and excessive exercise can have negative consequences (Peluso and Guerra de Andrade, 2005; Purvis et al., 2010; Czepluch et al., 2011). Thus, while rodent models of exercise are a valuable tool for studying neurogenesis, caution must be exercised in applying the results of unlimited voluntary wheel running to the human population. Future work should focus on developing rodent models of exercise which accurately mirror moderate exercise in humans, and on understanding the mechanisms through which newborn neurons in the hippocampus influence anxiety-like behaviors. This is especially pertinent in the context of efforts to develop therapeutic compounds that stimulate hippocampal neurogenesis for the treatment of mood disorders.
Acknowledgements
This research was supported by NIH grant P50-CA-143187. J. L. Onksen was supported by a pre-doctoral NRSA (MH-087103).
Footnotes
Disclosure/Conflict of Interest
The authors have no biomedical financial interests or potential conflicts of interest to report.
References
- Allen DM, van Praag H, Ray J, Weaver Z, Winrow CJ, Carter TA, Braquet R, Harrington E, Ried T, Brown KD, Gage FH, Barlow C. Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev. 2001;15(5):554–566. doi: 10.1101/gad.869001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62(5):633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Binder E, Droste SK, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav Brain Res. 2004;155(2):197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, Waugh R, Napolitano MA, Forman LM, Appelbaum M, Doraiswamy PM, Krishnan KR. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;92(1-2):136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17(5):615–628. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019(1-2):84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Durand M, Mormede P. Anxiety- and activity-related effects of diazepam and chlordiazepoxide in the rat light/dark and dark/light tests. Behav Brain Res. 1997;85(1):27–35. doi: 10.1016/s0166-4328(96)00160-x. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Bhattacharya TK, Miller DS, Rhodes JS. Induction of c-Fos, Zif268, and Arc from acute bouts of voluntary wheel running in new and pre-existing adult mouse hippocampal granule neurons. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19(10):937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Kohman RA, Miller DS, Bhattacharya TK, Brzezinska WJ, Rhodes JS. Genetic influences on exercise-induced adult hippocampal neurogenesis across 12 divergent mouse strains. Genes Brain Behav. 2011;10(3):345–353. doi: 10.1111/j.1601-183X.2010.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa M, Manrique HM, Font L, Escrig MA, Aragon CM. Reduction in the anxiolytic effects of ethanol by centrally formed acetaldehyde: the role of catalase inhibitors and acetaldehyde-sequestering agents. Psychopharmacology (Berl) 2008;200(4):455–464. doi: 10.1007/s00213-008-1219-3. [DOI] [PubMed] [Google Scholar]
- Czepluch FS, Barres R, Caidahl K, Olieslagers S, Krook A, Rickenlund A, Zierath JR, Waltenberger J. Strenuous physical exercise adversely affects monocyte chemotaxis. Thromb Haemost. 2011;105(1):122–130. doi: 10.1160/TH10-06-0363. [DOI] [PubMed] [Google Scholar]
- Deslandes A, Moraes H, Ferreira C, Veiga H, Silveira H, Mouta R, Pompeu FA, Coutinho ES, Laks J. Exercise and mental health: many reasons to move. Neuropsychobiology. 2009;59(4):191–198. doi: 10.1159/000223730. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Dunn AL, Youngstedt SD, Davis JM, Burgess ML, Wilson SP, Wilson MA. Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiol Behav. 1996;60(3):699–705. doi: 10.1016/0031-9384(96)00102-3. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124(1):71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2008;33(5):1049–1061. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P. Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss J, Ben Abdallah NM, Vogt MA, Touma C, Pacifici PG, Palme R, Witzemann V, Hellweg R, Gass P. Voluntary exercise induces anxiety-like behavior in adult C57BL/6J mice correlating with hippocampal neurogenesis. Hippocampus. 2010;20(3):364–376. doi: 10.1002/hipo.20634. [DOI] [PubMed] [Google Scholar]
- Greer TL, Trivedi MH. Exercise in the treatment of depression. Curr Psychiatry Rep. 2009;11(6):466–472. doi: 10.1007/s11920-009-0071-4. [DOI] [PubMed] [Google Scholar]
- Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, Malberg JE, Blendy JA. cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci. 2007;27(29):7860–7868. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neurosci Res. 2003;47(1):55–63. doi: 10.1016/s0168-0102(03)00171-8. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467(4):455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kwak C, Lee SH, Kaang BK. Social Isolation Selectively Increases Anxiety in Mice without Affecting Depression-like Behavior. Korean J Physiol Pharmacol. 2009;13(5):357–360. doi: 10.4196/kjpp.2009.13.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor AS, Bull C, Nilsson MKL, Zhu C, Bjork-Eriksson T, Eriksson PS, Blomgren K, Kuhn HG. Voluntary running rescues adult hippocampla neurogenesis after irradiation of the young mouse brain. PNAS. 2008;105(38):14632–14637. doi: 10.1073/pnas.0711128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(1-2):49–56. [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5(4):287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onksen JL, Brown EJ, Blendy JA. Selective Deletion of a Cell Cycle Checkpoint Kinase (ATR) Reduces Neurogenesis and Alters Responses in Rodent Models of Behavioral Affect. Neuropsychopharmacology. 2011;36(5):960–969. doi: 10.1038/npp.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MA, Guerra de Andrade LH. Physical activity and mental health: the association between exercise and mood. Clinics (Sao Paulo) 2005;60(1):61–70. doi: 10.1590/s1807-59322005000100012. [DOI] [PubMed] [Google Scholar]
- Pilhatsch M, Winter C, Nordstrom K, Vennstrom B, Bauer M, Juckel G. Increased depressive behaviour in mice harboring the mutant thyroid hormone receptor alpha 1. Behav Brain Res. 2010;214(2):187–192. doi: 10.1016/j.bbr.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Purvis D, Gonsalves S, Deuster PA. Physiological and psychological fatigue in extreme conditions: overtraining and elite athletes. PM R. 2010;2(5):442–450. doi: 10.1016/j.pmrj.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14(10):959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, van Praag H, Jeffrey S, Girard I, Mitchell GS, Garland T, Jr., Gage FH. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117(5):1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1(1):113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav Brain Res. 2009;197(1):31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, Sofroniew MV, Kandel ER, Hen R. Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A. 2007;104(11):4642–4646. doi: 10.1073/pnas.0611718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003;119(3):899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Havekes R, Bos T, Eggen BJ, Van der Zee EA. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav Neurosci. 2007;121(2):324–334. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- Van der Borght K, Kobor-Nyakas DE, Klauke K, Eggen BJ, Nyakas C, Van der Zee EA, Meerlo P. Physical exercise leads to rapid adaptations in hippocampal vasculature: temporal dynamics and relationship to cell proliferation and neurogenesis. Hippocampus. 2009;19(10):928–936. doi: 10.1002/hipo.20545. [DOI] [PubMed] [Google Scholar]
- van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10(2):128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Varadarajulu J, Lebar M, Krishnamoorthy G, Habelt S, Lu J, Bernard Weinstein I, Li H, Holsboer F, Turck CW, Touma C. Increased anxiety-related behaviour in Hint1 knockout mice. Behav Brain Res. 2011;220(2):305–311. doi: 10.1016/j.bbr.2011.02.012. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A, Knecht S. High impact running improves learning. Neurobiol Learn Mem. 2007;87(4):597–609. doi: 10.1016/j.nlm.2006.11.003. [DOI] [PubMed] [Google Scholar]