Abstract
To identify selective high-affinity ligands for the vesicular acetylcholine transporter (VAChT), we have incorporated a carbonyl group into the structures of trozamicol and prezamicol scaffolds, and also converted the secondary amines of the piperidines of trozamicols and prezamicols into amides. Of 18 new racemic compounds, 4 compounds displayed high affinity for VAChT (Ki = 10 - 20 nM) and greater than 300-fold selectivity for VAChT over σ1 and σ2 receptors, namely (4-(4-fluorobenzoyl)-4'-hydroxy-[1,3'-bipiperidin]-1'-yl)(3-methylthiophen-2-yl)methanone oxalate (9g) (Ki-VAChT = 11.4 nM, VAChT/σ1 = 1063, VAChT/σ2 = 370), (1'-benzoyl-4'-hydroxy-[1,3'-bipiperidin]-4-yl)(4-methoxyphenyl)methanone oxalate (10c) (Ki-VAChT = 15.4 nM, VAChT/σ1 = 374, VAChT/ σ2 = 315), (4'-hydroxy-1'-(thiophene-2-carbonyl)-[1,3'-bipiperidin]-4-yl)(4-methoxyphenyl)methanone oxalate (10e) (Ki-VAChT = 19.0 nM, VAChT/σ1 = 1787, VAChT/ σ2 = 335), and (4'-hydroxy-1'-(3-methylthiophene-2-carbonyl)-[1,3'-bipiperidin]-4-yl)(4-methoxyphenyl)methanone oxalate (10g) (Ki-VAChT = 10.2 nM, VAChT/σ1 = 1500, VAChT/ σ2 = 2030). These 4 compounds can be radiosynthesized with C-11 or F-18 to validate their possibilities of serving as PET probes for quantifying the levels of VAChT in vivo.
Keywords: VAChT, σ receptor, Vesamciol, Parkinson Disease, CNS disorders
1. Introduction
The progressive loss of cognitive function is a characteristic feature of neurodegenerative disorders such as Alzheimer's disease, Down's syndrome, Parkinson disease, and schizophrenia. It is associated with the loss of cholinergic neurons and synapses in the brain.1-6 Alteration of cholinergic function also has been implicated in drug addiction, especially to nicotine, ethanol, and neurostimulants such as cocaine, amphetamine, and opiates.7, 8 Thus, the ability to quantitatively assess the status of cholinergic neurons in the brain will be very valuable for diagnosis of many neurological disorders and to monitor therapeutic efficacy. Positron emission tomography (PET) is a sensitive and non-invasive method to perform these tasks. When used with suitable radio-pharmaceuticals, PET imaging is able to provide biochemical and functional information on a living subject.
Vesicular acetylcholine transporter (VAChT), a 12 transmembrane domain protein discovered in the early 1980s, is a widely accepted biomarker for assessing the status of cholinergic neurons in the central nervous system.9-11 It is located in the membrane of synaptic vesicles in the cholinergic presynaptic terminal and is responsible for transporting ACh and choline into the vesicles using a proton electrochemical gradient to drive transport.12,13 Decreased VAChT expression strongly interferes with the loading of ACh into vesicles and further impairs the cognitive abilities of subjects.13, 14
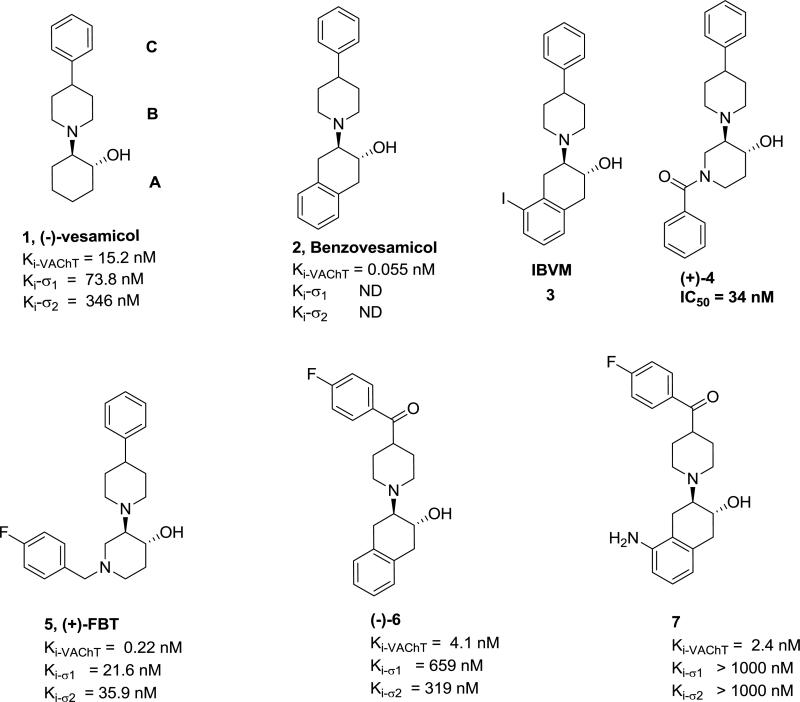
Earlier studies have shown that VAChT tightly binds to the prototypical compound 2-(4-phenylpiperidino)cyclohexanol (vesamicol, 1),15-17 which blocks the ACh binding site in VAChT. Although vesamicol has nanomolar affinity for VAChT, it also has high affinity for σ1 and σ2 receptors, which are expressed throughout the brain.18-20 As a result, the ability of vesamicol-like agents to accurately measure levels of VAChT in brain using PET is compromised. To solve this problem, investigators have tried to optimize the structure of vesamicol with the goals of retaining high VAChT affinity while reducing the σ receptor affinity, and improving the pharmacokinetics.11, 19-22 The most significant improvement has been to identify benzovesamicol (2) analogues having high potency for VAChT binding. As an example, (-)-5-[123I]iodobenzovesamicol ([123I]IBVM, 3) was used as a single photon emission computed tomography (SPECT) radio-tracer in patients with AD.23 However, patients have to wait an undesirable 6 hr post-injection to attain equilibration before the SPECT scan.21, 23 Until now, no clinically suitable PET tracer for quantifying VAChT in human brain has been reported. Recently, our group developed a new class of analogues, in which a carbonyl group is interposed between the phenyl and piperidine rings of the benzovesamicol structure. The in vitro data suggest that this new class of ligands can greatly reduce σ receptor binding and retain high VAChT binding.24, 25 In particular, the two leading compounds (-)-trans-2-hydroxy-3-(4-(4-fluorobenzoyl)piperidino)-tetralin (6)25 and 5-amino-3-[4-(4-fluorobenzoyl)piperidinyl]-2-hydroxy-1,2,3,4,-tetrahydro-naphthalene (7)24 display high affinity and selectivity for VAChT versus σ receptors. More importantly, our initial in vivo validation suggested that (-)-[18F]6 is a very promising PET probe of VAChT.25 In addition, (+)-benzoyltrozamicol ((+)-4) and (+)-4-fluorobenzyltrozamicol ((+)-[18F]FBT, 5)26 have high VAChT binding affinities. Particularly, (+)-[18F]FBT was reported as a very promising candidate to image VAChT in vivo,27, 28 although it was later found that this compound is not suitable due to its high σ binding affinity and slow washout rate.29
To identify a PET radioligand possessing high potency and selectivity for VAChT in vivo, our group has continually explored the new class of analogues containing one or more carbonyl groups. In current study, the following strategies were employed: 1) retaining the carbonyl group between the phenyl group and piperidyl group in the 4-phenylpiperidyl moiety of the trozamicol scaffold; 2) converting the secondary amines in the trozamicol scaffold to tertiary amides using aromatic/heteroaromatic carboxylic acids; and 3) optimizing the substituents in the aromatic rings to improve the VAChT binding affinity of the new trozamicol scaffolds. In this manuscript, we report our work on the synthesis and in vitro bioactivity evaluation of these new analogues.
2. Results and Discussion
2.1. Chemistry
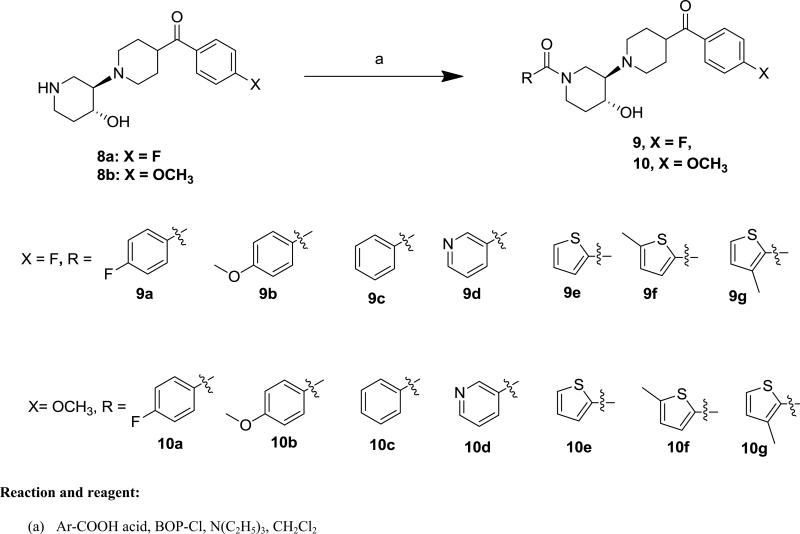
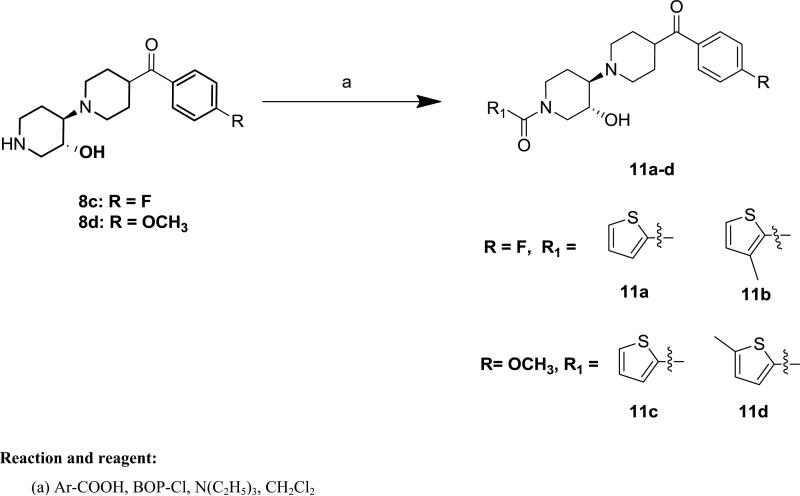
The target compounds were synthesized according to Schemes 1-2. The key trozamicol-like intermediates (1'-Benzoyl-4'-hydroxy-[1,3'-bipiperidin]-4-yl)(4-fluoro-phenyl)methanone (8a), and (1'-Benzoyl-4'-hydroxy-[1,3'-bipiperidin]-4-yl)(4-methoxy-phenyl)methanone (8b) as well as the key prezamicol-like intermediates(4-Fluorophenyl)(3'-hydroxy-[1,4'-bipiperidin]-4-yl)-methanone (8c), and (3'-Hydroxy-[1,4'-bipiperidin]-4-yl)(4-methoxyphenyl)-methanone (8d) were synthesized by following the procedure reported by our group.30 We have previously reported the structural configuration of prezamicol by obtaining the x-ray crystal structure,30 which allowed us to assign the structures of 8. The target compounds 9a-g and 10a-g were prepared via trozamicol like intermediates 8a or 8b by acylating with various substituted benzoyl or substituted heteroaromatic carboxylic acids by using bis(2-oxo-3-oxazolidinyl)phosphinic chloride (BOPCl) coupling agent as shown in Scheme 1. Compounds 11a-d were synthesized by following a similar synthetic procedure as for 9a-g or 10a-g, except the prezamicol-like intermediates 8c or 8d replacing trezamicol-like 8a or 8b as shown in Scheme 2. All the products were dissolved in acetone or acetone/ethanol solvent and treated with oxalic acid to convert into the oxalate salts of 9a-g, 10a-g and 11a-d for determining binding affinities.
Scheme 1.
Synthesis of Compounds 9a-g and 10a-g
Scheme 2.
Synthesis of Compounds 11a-d
2.2. Biological binding studies
Competitive inhibition of the binding of different radioligands under highly selective conditions was performed to estimate the σ1,σ2, and VAChT affinities (Ki, nM) of 9a-g, 10a-g and 11ad.24, 25, 30 The details are given in Section 4.5. Apparent dissociation constants for in vitro binding of the novel compounds are given in Table 1.
Table 1.
Affinities of new analogues for σ1 receptor, σ2 receptor, and VAChTa
| Compounds | Ki (nM) | Selectivity Ratio | LogPe | |||
|---|---|---|---|---|---|---|
| VAChTd | σ 1 b | σ 2 c | VAChT/σ1 | VAChT/σ2 | ||
| Vesamicol | 15.2 ± 1.05 | 73.8 | 346 | 4.86 | 22.8 | |
| 9a | 8700 ± 1620 | 3119 ± 360 | 1375 ± 135 | 0.36 | 0.16 | 2.00 |
| 9b | 13000 ± 1900 | 661 ± 72 | >10000 | 0.05 | ND | 2.25 |
| 9c | 62.7 ± 14.3 | 37900 ± 270 | 3950 ± 280 | 604 | 63 | 1.75 |
| 9d | 810 ± 140 | 13800 ± 2600 | 15500 ± 2200 | 17.1 | 19.2 | 0.66 |
| 9e | 123 ± 14.9 | 22500 ± 5880 | 4000 ± 240 | 183 | 32.9 | 2.07 |
| 9f | 1190 ± 140 | 2800 ± 210 | 16400±1480 | 2.35 | 13.8 | 2.53 |
| 9g | 11.4 ± 3.67 | 12100 ± 2400 | 4220 ± 200 | 1063 | 370 | 2.53 |
| 10a | 233 ± 36.8 | 11900 ± 5600 | 2590 ± 360 | 51.2 | 11.1 | 1.98 |
| 10b | 3020 ± 640 | 5260 ± 350 | 40400 ± 5490 | 1.7 | 7.68 | 2.23 |
| 10c | 15.4 ± 0.94 | 5760 ± 480 | 4850 ± 650 | 374 | 315 | 1.73 |
| 10d | 112 ± 12.9 | 8600 ± 380 | 24300 ± 1900 | 77 | 217 | 0.65 |
| 10e | 19.0 ± 2.12 | 34000 ± 6120 | 6380 ± 1000 | 1787 | 335 | 2.05 |
| 10f | 142 ± 16.4 | 39400 ± 4100 | 24400 ± 3600 | 277 | 172 | 2.51 |
| 10g | 10.2 ± 0.76 | 15300 ± 2870 | 20700 ± 2670 | 1500 | 2030 | 2.51 |
| 11a | 1300 ± 190 | 1660 ± 130 | 12300 ± 1200 | 1.27 | 1.02 | 1.99 |
| 11b | 2020 ± 480 | 5770 ± 160 | 7830 ± 90 | 2.85 | 3.88 | 2.45 |
| 11c | 206 ± 21.8 | 1334 ± 135 | 9709 ± 1443 | 6.47 | 47.1 | 1.97 |
| 11d | 1350 ± 433 | 2340 ± 320 | 20500 ± 7560 | 1.73 | 15.2 | 2.43 |
Ki values (mean ± SEM) were determined in at least three experiments.
The σ1 binding assay used membrane preparations of guinea pig brain.
The σ2 binding assay used homogenates of rat liver.
The VAChT binding assay used expressed human VAChT.
Calculated value at pH 7.4 by ACD/Labs, version 7.0 (Advanced Chemistry Development, Inc., Canada)
Compounds 9g, 10c, 10e and 10g displayed high affinity for VAChT (Ki < 20 nM) and much higher selectivity for VAChT relative to σ1 and σ2 receptors (at least > 300 fold). Moreover, a few interesting structure-activity trends were identified. Firstly, the strategy of converting the secondary amine of trozamicol-like structures (8a and 8b) to tertiary amides (9a-g and 10a-g) successfully reduced the binding affinities for σ receptors; the new amide analogues displayed very low affinity for σ1 (Ki > 1300 nM) and σ2 (Ki > 2500 nM) receptors with the exception of compound 9b (Ki = 661 nM for σ1). We previously reported that when the secondary amine of trozamicol is converted to a tertiary amine by benzylation, binding affinity toward σ1 receptor is high.30 Secondly, thiophene derivatives of the new amide analogues 9g (Ki = 11.4 ± 3.67 nM) and 10g (Ki = 10.2 ± 0.76 nM) have higher affinities for VAChT than vesamicol does. More importantly, both new compounds have very low σ affinities. For 9g, Ki = 12100 ± 2400 nM for σ1 and 4220 ± 200 nM for σ2. For 10g, Ki = 15300 ± 2870 nM for σ1 and 20700 ± 2670 nM for σ2. The selectivity of VAChT vs. σ receptors for 9g was greater than 370 fold and that for 10g was greater than 1500-fold, both of which are much higher than for vesamicol. As VAChT binds to the ligands enantioselectively,31 it is expected that one of the corresponding enantiomers of the new potent compounds 9g and 10g will have much higher binding affinity for VAChT than the racemic mixtures do.
Furthermore, in the para-position to the carbonyl group of the 4-fluorobenzoylpiperidinyl fragment, compounds 9g and 10g contain either a fluorine atom or a methoxy group, respectively, which is suitable for replacement with [18F] or [11C]CH3 that can be used in PET imaging studies. Compound [18F]9g can be made easily by the displacement of the nitro group of a corresponding precursor with K[18F]/fluoride25, and [11C]10g can be made easily by reacting corresponding desmethyl phenol substrate with [11C]CH3I in the presence of base. The Log P values of 9g and 10g are 2.53 and 2.51 respectively, suggesting that 9g and 10g have suitable lipophilicity for crossing the brain blood barrier (BBB) into the brain. If further in vitro studies of both 9g and 10g confirm they have high affinity and selectivity for VAChT, the potent isomer of [18F]9g or [11C]10g is worth exploring further in vivo.
With the exception of 9g and 10g, compounds in the 10 series that contain an electron-rich methoxy substitution para to the carbonyl of the ketone favored VAChT binding over compounds in the 9 series that contain an electron-withdraw fluoro substitution para to the carbonyl of the ketone. VAChT binding affinity was increased 37-fold from 9a (8700 ± 1620 nM) to 10a (233 ± 37 nM), 4.3-fold from 9b (13000 ± 1900 nM) to 10b (3020 ± 640 nM), 4-fold from 9c (62.7 ± 14.3 nM) to 10c (15.4 ± 0.94 nM), 7.2-fold from 9d (807 ± 139 nM) to 10d (112 ± 13 nM), 6.4-fold from 9e (123 ± 14 nM) to 10e (19.0 ± 2.12 nM), and 8.4-fold from 9f (1190 ± 137 nM) to 10f (142 ± 16.4 nM). This suggests that the electron-rich methoxy substitution is important for VAChT binding.
In ligands 9a, 9b and 9c, where a fluorine atom is para to the ketone carbonyl, both fluoro substituted 9a (Ki = 8700 ± 1620 nM) and methoxy substituted 9b (Ki = 13000 ± 1900 nM) para to the amide carbonyl displayed dramatic reductions in binding affinity compared to that of unsubstituted benzamide 9c (Ki = 62.7 ± 14.3 nM). The binding affinities of compounds 9a and 9b were reduced approximately 139- and 207-fold compared to 9c. A similar trend was observed for compounds 10a, 10b and 10c, for which the decrease in binding affinities were 15-fold and 196-fold, respectively, relative to compound 10c. Comparison of compounds 9d vs 9c, and of 10d vs 10c, shows that the 3-pyridyl amides exhibit decreases in affinity for VAChT compared to the benzamides.
Compounds 9e and 10e which contain the thiophene-2-carbonyl group showed decreased affinity for VAChT compared to 9c and 10c which contain the benzoyl group. The reduction in affinity was minor; 2-fold from 9c (Ki = 62.7 ± 14.3 nM) to 9e (Ki = 123 ± 14.9 nM) and 1.2-fold from 10c (Ki = 15.4 ± 0.94 nM) to 10e (Ki = 19.0 ± 2.12 nM). When a methyl group was introduced at the 3- or 5-position in thiophene-2-carbonyl containing compounds, 9f and 9g were obtained. The minor structural differences cause remarkable changes in the affinities. Compound 9e has a modest to low VAChT binding affinity (Ki = 123 ± 14.9 nM), and compound 9f has very low VAChT binding affinity (Ki = 1190 ± 137 nM). A similar trend was observed for compounds 10e and 10f, as the affinity reduced 7-fold from 10e (Ki = 19.0 ± 2.12 nM) to 10f (Ki = 142 ± 16.4 nM). However, when the position of the methyl group was changed from position 5 to position 3 (9g to 9f), the affinity increased by 104-fold from Ki = 1190 ± 137 nM for 9f to Ki = 11.4 ± 3.67 nM for 9g. The VAChT binding affinity of 9g was approximately 6-fold higher than the affinities of 9c and vesamicol (Ki = 15.2 ± 1.05 nM). Similar results were observed for compounds 10f (Ki = 142 ± 16.4 nM) and 10g (10.2 ± 0.76 nM). This result demonstrates that the position of the substituent on the thiophene in this new class of ligands is very critical to VAChT binding. Substitution at various positions may have different steric effects, which result in dramatic change in the affinity of these ligands.
This observation provides important information for further structure-activity relationship studies. To test the impact of thiophene and methyl substituted thiophenes in the prezamicol-like scaffold, compounds 11a-d were synthesized, and their binding affinities for VAChT were determined. For 11a, 11b, 11c and 11d, the Ki values are 1300 ± 193 nM, 2020 ± 482 nM, 206 ± 21.8 nM and 1350 ± 433 nM, respectively. This result shows that thiophene and substituted thiophenes in the prezamcol-like scaffold (11a-d) show lower binding affinities. The observation is consistent to the reported result that prezamicol analogues are less potent toward VAChT.30
In summary, our approaches to optimizing the structure of trozamicol analogue, fluorobenzoyltrozamicol (FBT) include: (1) introducing a carbonyl group between the aromatic and piperi-dine rings; (2) replacing the substituted benzyl group of tertiary amines with a substituted benzoyl group to make tertiary amides. These approaches not only retain high VAChT affinity, but also greatly decrease the σ binding affinities of trozamicol analogues and increase selectivity for VAChT vs. σ receptors. Electronic effects of substitution at the position para to the ketone carbonyl in trozamicol-like analogues have a predominant effect; ligands with electron-rich methoxy substitution (10) display higher potencies toward VAChT compared to ligands with fluoro substitution (9). When the amide part of trozamicol-like ligands is substituted with 3-methyl-thiophene-2-amide, two new racemic ligands 9g and 10g were obtained that display high affinity (Ki value ~10 nM) and selectivity for VAChT. In current work, the binding affinities were measured only for new synthesized racemic compounds. Racemates having high potency for VAChT and high selectivity for VAChT versus σ receptors will be further resolved in future to obtain corresponding enantiomers. Due to the VAChT stereoselective binding property, it is expected that the more potent enantiomers will have higher potency and higher selectivity than that of the corresponding racemates have. Nevertheless, these results reported here, provide important new information for structure-activity analysis, which could lead to identification of suitable PET tracers for imaging VAChT in vivo.
3. Conclusion
In this study, we reported our exploration on the development of new VAChT analogues based on a hybrid structure of fluorobenzyltrozamicol 5 and 6. Our structural modifications led us to four potent ligands, namely 9g (Ki = 11.4 nM), 10c (Ki = 15.4 nM), 10e (Ki = 19.0 nM), and 10g (Ki = 10.2 nM) having similar or higher affinity for VAChT compared to that of vesamicol. However, the selectivity for VAChT vs. σ receptors is much higher than for prior VAChT ligands. Particularly, two of the most potent ligands, 9g and 10g displayed high selectivity over σ receptors. For 9g, the selectivity reached 1063-fold, and 370-fold of VAChT versus σ1 and σ2 receptor; for 10g, it reached 1500-fold, 2030-fold of VAChT versus σ1 and σ2 respectively.
4. Experimental
4.1. General
All reagents and chemicals were purchased from commercial suppliers and used without further purification unless otherwise stated. All anhydrous reactions were carried out in oven-dried glassware under an inert nitrogen atmosphere unless otherwise stated. Flash column chromatography was conducted on silica gel 60A “40 Micron Flash” [32-63μm](Scientific absorbents, INC); the mobile phase used is reported in the experimental procedure for each compound. Melting points were determined using the MEL-TEMP 3.0 apparatus and left uncorrected. 1HNMR spectra were recorded at 400 MHz on a Varian Mercury-VX spectrometer with CDCl3 as solvent and tetramethylsilane (TMS) as the internal standard unless otherwise stated. All chemical shift values are reported in parts per million (ppm) (δ). Peak multiplicities are singlet, s; doublet, d; triplet t; multiplet, m; broad, br. Elemental analyses (C, H, N) were determined by Atlantic Microlab, Inc. Elemental analysis was used to determine the purity of the target compounds that were assessed in vitro. All the compounds reported in the manuscript have a purity of ≥95%.
4.2. Procedure C. General method of preparing 9a-g,10a-g and 11a-b by aroylation of piperidines 8a-d and conversion to the corresponding oxalates
4.2.1. (4'-Hydroxy-[1,3'-bipiperidine]-1',4-diyl)bis(4-fluorophenyl)methanone oxalate (9a)
In a solution of 8a (56 mg, 0.183 mmol), BOPCl (100 mg, 0.40 mmol), triethylamine (1 mL) in methylene chloride (30 mL), 4-fluorobenzoic acid (40 mg, 0.285 mmol) was added. The reaction mixture was stirred overnight at room temperature. The reaction mixture was washed with saturated aqueous Na2CO3 (20 mL × 5) and brine solution (20 mL). The organic phase was dried over Na2SO4 and concentrated under vacuum. The crude product was purified by silica gel column chromatography using ethyl acetate followed by ethyl acetate/triethylamine (98/2, v/v) to afford 9a as a colorless oil (76 mg, 97%). 1H NMR (400 MHz, CDCl3) δ 7.96 (t, J = 6.9 Hz, 2H), 7.50 – 7.36 (m, 2H), 7.13 (dt, J = 12.2, 8.5 Hz, 4H), 4.87 (br s, 1H), 3.98 – 3.46 (m, 3H), 3.35 – 3.16 (m, 1H), 3.16 – 2.58 (m, 5H), 2.52 – 2.27 (m, 2H), 2.22 – 2.00 (m, 1H), 2.01 – 1.67 (m, 4H), 1.56 – 1.34 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.6, 169.7, 164.7, 164.6, 132.2, 131.7, 130.9, 129.2, 116.0, 115.7, 67.8, 67.4, 52.3, 45.2, 43.6, 32.8, 29.5, 29.1. The free base was converted to the oxalate by dissolving in acetone and mixing with 1 equivalent of oxalic acid to afford the corresponding oxalate salt as white solid. mp: 151 °C (decomposed). Anal. (C24H26F2N2O3 •H2C2O4•0.5H2O) C, H, N.
4.2.2. (4-(4-Fluorobenzoyl)-4'-hydroxy-[1,3'-bipiperidin]-1'-yl)(4-methoxyphenyl)methanone oxalate (9b)
Free base 9b (105 mg, 55%) was obtained as a light yellow solid.1H NMR (400 MHz, CDCl3) δ 7.96 (dd, J = 8.6, 5.4 Hz, 2H), 7.38 (d, J = 8.7 Hz, 2H), 7.14 (t, J = 8.6 Hz, 2H), 6.93 (d, J = 8.7 Hz, 2H), 4.99 – 4.58 (br s, 1H), 3.84 (s, 3H), 3.74 – 3.51 (m, 2H), 3.29 – 3.14 (m, 1H), 3.14 – 3.03 (m, 1H), 3.01 – 2.59 (m, 4H), 2.51 – 2.22 (m, 2H), 2.17 – 2.05 (m, 1H), 1.97 – 1.81 (m, 3H), 1.81 – 1.67 (m, 1H), 1.59 – 1.43 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.6, 170.5, 166.9, 160.8, 132.2, 130.8, 128.8, 127.7, 115.9, 113.7, 68.0, 67.4, 55.3, 52.1, 45.1, 43.5, 32.7, 29.4, 29.0. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 217 °C (decompose). Anal. (C25H29FN2O4•H2C2O4) C, H, N.
4.2.3. (1'-Benzoyl-4'-hydroxy-[1,3'-bipiperidin]-4-yl)(4-fluoro-phenyl)methanone oxalate (9c)
Free base 9c (0.20 g, 65%) was obtained as a pale solid .1H NMR (400 MHz, CDCl3) δ 8.06 – 7.77 (br s, 2H), 7.49 – 7.29 (m, 5H), 7.13 (t, J = 8.4 Hz, 2H), 5.01 – 4.59 (m, 1H), 3.97 – 3.58 (m, 3H), 3.33 – 3.17 (m, 1H), 3.16 – 2.57 (m, 5H), 2.54 – 2.30 (m, 2H), 2.11 – 1.98 (m, 1H), 1.98 – 1.65 (m, 4H), 1.62 – 1.35 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.6, 170.6, 166.9, 163.4, 130.9, 129.8, 128.5, 126.7, 115.9, 113.8, 67.6, 67.4, 52.3, 45.2, 43.5, 32.9, 29.4, 29.0. The free base was converted to the oxalate salt as white solid. mp: 193 °C (decomposed). Anal. (C24H27FN2O3•H2C2O4• H2O) C, H, N.
4.2.4. (4-(4-Fluorobenzoyl)-4'-hydroxy-[1,3'-bipiperidin]-1'-yl)(pyridin-3-yl)methanone oxalate (9d)
Free base 9d (33 mg, 81%) was obtained as a grease oil. 1H NMR (400 MHz, CDCl3) δ 8.75 – 8.60 (m, 2H), 8.03 – 7.84 (m, 2H), 7.76 (d, J = 7.7 Hz, 1H), 7.43 – 7.31 (m, 1H), 7.14 (t, J = 8.5 Hz, 2H), 4.99 – 4.67 (m, 1H), 3.91 – 3.49 (m, 3H), 3.32 – 3.18 (m, 1H), 3.17 – 2.61 (m, 5H), 2.51 – 2.33 (m, 2H), 2.15 – 2.03 (m, 1H), 1.99 – 1.68 (m, 4H), 1.54 – 1.39 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.6, 167.9, 164.5, 151.0, 147.8, 134.9, 132.3, 131.6, 130.8, 123.6, 115.8, 67.5, 67.3, 45.3, 43.6, 39.7, 33.0, 29.5, 29.1. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 139 °C (decomposed). Anal. (C23H26FN3O3•H2C2O4• H2O) C, H, N.
4.2.5. (4-(4-Fluorobenzoyl)-4'-hydroxy-[1,3'-bipiperidin]-1'-yl)-(thiophen-2-yl)methanone oxalate (9e)
Free base 9e (26 mg, 64%) was obtained as a grease oil. 1H NMR (400 MHz, CDCl3)δ 7.96 (dd, J = 8.7, 5.4 Hz, 2H), 7.46 (d, J = 5.8 Hz, 1H), 7.30 (d, J = 3.6 Hz, 1H), 7.14 (t, J = 8.6 Hz, 2H), 7.10 – 7.01 (m, 1H), 4.83 – 4.58 (br s, 1H), 4.50 – 4.27 (br s, 1H), 3.71 (td, J = 10.3, 4.8 Hz, 1H), 3.65 – 3.50 (br s, 1H), 3.29 – 3.17 (m, 1H), 3.14 – 3.04 (m, 1H), 3.04 – 2.65 (m, 4H), 2.52 – 2.29 (m, 2H), 2.21 – 2.08 (m, 1H), 1.97 – 1.68 (m, 4H), 1.61 – 1.47 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.6, 166.9, 163.8, 136.8, 132.3, 130.9, 130.8, 128.8, 126.7, 115.7, 67.9, 67.4, 52.2, 45.2, 43.6, 32.7, 29.5, 29.1. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 160 °C (decomposed). Anal. (C22H25FN2O3S•H2C2O4• H2O) C, H, N.
4.2.6. (4-(4-Fluorobenzoyl)-4'-hydroxy-[1,3'-bipiperidin]-1'-yl)(5-methylthiophen-2-yl)methanone oxalate (9f)
Free base 9f (33 mg, 77%) was obtained as a greasy oil. 1H NMR (400 MHz, CDCl3) δ 8.00 – 7.91 (m, 2H), 7.18 – 7.08 (m, 3H), 6.75 – 6.67 (m, 1H), 4.77 – 4.60 (m, 1H), 4.51 – 4.35 (m, 1H), 3.70 (td, J = 10.3, 4.7 Hz, 1H), 3.62 – 3.52 (br s, 1H), 3.30 – 3.17 (m, 1H), 3.13 – 3.04 (m, 1H), 3.02 – 2.82 (m, 3H), 2.80 – 2.68 (m, 1H), 2.51 (s, 3H), 2.48 – 2.31 (m, 2H), 2.20 – 2.10 (m, 1H), 1.97 – 1.67 (m, 4H), 1.61 – 1.45 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.6, 167.0, 169.8, 144.0, 134.4, 132.3, 130.9, 129.3, 125.1, 115.7, 67.9, 67.5, 52.2, 45.2, 43.6, 32.7, 29.5, 29.2, 15.3. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 140 °C (decomposed). Anal. (C23H27FN2O3S•H2C2O4) C, H, N.
4.2.7. (4-(4-Fluorobenzoyl)-4'-hydroxy-[1,3'-bipiperidin]-1'-yl)(3-methylthiophen-2-yl)methanone oxalate (9g)
Free base 9g (53 mg, 76%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 8.00 – 7.90 (m, 2H), 7.29 (d, J = 5.0 Hz, 1H), 7.14 (t, J = 8.6 Hz, 2H), 6.86 (d, J = 5.0 Hz, 1H), 4.69 – 4.38 (br s, 1H), 4.23 – 3.99 (br s, 1H), 3.75 – 3.52 (m, 2H), 3.28 – 3.16 (m, 1H), 3.13 – 3.00 (m, 1H), 2.98 – 2.68 (m, 4H), 2.51 – 2.30 (m, 2H), 2.28 (s, 3H), 2.16 – 2.07 (m, 1H), 1.96 – 1.66 (m, 4H), 1.60 – 1.42 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.6, 166.9, 164.8, 137.5, 132.2, 130.7, 129.9, 129.7, 125.8, 115.9, 67.9, 67.3, 52.1, 45.2, 43.5, 32.6, 29.4, 29.1, 14.6. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 146 °C (decomposed). Anal. (C23H27FN2O3S•H2C2O4• 0.25H2O) C, H, N.
4.2.8. (1'-(4-Fluorobenzoyl)-4'-hydroxy-[1,3'-bipiperidin]-4-yl)(4-methoxyphenyl)methanone oxalate (10a)
Free base 10a (90 mg, 82%) was obtained as a white solid. 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 8.4 Hz, 2H), 7.45 – 7.35 (m, 2H), 7.11 (t, J = 8.5 Hz, 2H), 6.94 (d, J = 8.5 Hz, 2H), 4.98 – 4.72 (br s, 1H), 3.87 (s, 3H), 3.76 – 3.50 (m, 3H), 3.32 – 3.15 (m, 1H), 3.11 – 2.58 (m, 5H), 2.49 – 2.29 (m, 2H), 2.18 – 1.99 (m, 1H), 1.96 – 1.61 (m, 4H), 1.57 – 1.36 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.7, 169.7, 164.7, 163.5, 131.7, 130.5, 129.0, 128.8, 115.5, 113.8, 67.7, 67.4, 55.5, 52.4, 45.2, 43.3, 32.8, 29.7, 29.2. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 192 °C (decomposed). Anal. (C25H29FN2O4•H2C2O4• 1.5H2O) C, H, N.
4.2.9. (1'-(4-Methoxybenzoyl)-4'-hydroxy-[1,3'-bipiperidine]-4-yl)(4-methoxyphenyl)methanone oxalate (10b)
Free base 10b (234 mg, 62%) was obtained as a light yellow solid.1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.5 Hz, 2H), 7.35 (d, J = 8.6 Hz, 2H), 6.97 – 6.82 (m, 4H), 4.95 – 4.60 (br s, 1H), 3.84 (s, 3H), 3.82 (s, 3H), 3.71 – 3.51 (m, 2H), 3.27 – 3.12 (m, 1H), 3.12 – 2.98 (m, 1H), 2.98 – 2.55 (m, 4H), 2.47 – 2.19 (m, 2H), 2.16 – 2.03 (m, 1H), 1.91 – 1.64 (m, 4H), 1.57 – 1.39 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.7, 170.5, 163.4, 160.7, 130.4, 128.8, 128.7, 127.7, 113.8, 113.7, 67.8, 67.4, 55.4, 55.3, 52.3, 45.2, 43.2, 32.7, 29.6, 29.2. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 164 °C (decomposed). Anal. (C26H32N2O5.C2H2O4) C, H, N.
4.2.10. (1'-Benzoyl-4'-hydroxy-[1,3'-bipiperidin]-4-yl)(4-methoxy-phenyl)methanone oxalate (10c)
Free base 10c (0.134 g, 74%) was obtained as a white solid. 1H NMR (400 MHz, CDCl3) δ 8.01 – 7.78 (m, 2H), 7.48 – 7.31 (m, 5H), 6.94 (d, J = 8.0 Hz, 2H), 4.97-4.60 (m, 1H), 3.84 (s, 3H), 3.81 – 3.56 (m, 3H), 3.35 – 2.60 (m, 6H), 2.56 – 2.31 (m, 2H), 2.30 – 2.13 (m, 1H), 2.00 – 1.70 (m, 4H), 1.63 – 1.40 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.8, 170.7, 163.5, 135.8, 130.5, 130.0, 128.8, 128.6, 126.8, 113.9, 67.8, 67.4, 55.5, 52.2, 45.2, 43.2, 32.9, 29.7, 29.3. The free base was treated with oxalic acid to afford the corresponding oxa-late salt as white solid. mp: 180 °C (decomposed). Anal. (C25H30N2O4•H2C2O4• H2O) C, H, N.
4.2.11. (4'-Hydroxy-1'-nicotinoyl-[1,3'-bipiperidin]-4-yl)(4-methoxy-phenyl)methanone oxalate (10d)
Free base 10d (36 mg, 90%) was obtained as a colorless grease. 1H NMR (400 MHz, CDCl3) δ 8.72 – 8.64 (m, 2H), 7.96 – 7.85 (m, 2H), 7.76 (d, J = 7.7 Hz, 1H), 7.38 (t, J = 6.3 Hz, 1H), 6.94 (d, J = 8.4 Hz, 2H), 4.99 – 4.68 (m, 1H), 3.87 (s, 3H), 3.79 – 3.59 (m, 2H), 3.34 – 3.19 (m, 1H), 3.12 – 2.62 (m, 5H), 2.51 – 2.33 (m, 2H), 2.28 – 2.05 (m, 1H), 1.98 – 1.71 (m, 4H), 1.49 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 200.7, 168.0, 163.5, 151.0, 147.8, 134.8, 131.6, 130.5, 128.8, 123.6, 113.9, 67.6, 67.3, 55.5, 52.5, 45.3, 43.3, 33.0, 29.7, 29.2. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 148 °C (decomposed). Anal. (C24H29N3O4•H2C2O4• 1.5H2O) C, H, N.
4.2.12. (4'-Hydroxy-1'-(thiophene-2-carbonyl)-[1,3'-bipiperidin]-4-yl)(4-methoxyphenyl)methanone oxalate (10e)
Free base 10e (22 mg, 55%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.92 (d, J = 8.9 Hz, 2H), 7.46 (dd, J = 5.1, 1.2 Hz, 1H), 7.31 (dd, J = 3.7, 1.3 Hz, 1H), 7.06 (dd, J = 5.0, 3.6 Hz, 1H), 6.94 (d, J = 8.9 Hz, 2H), 4.78 – 4.54 (br s, 1H), 4.50 – 4.30 (br s, 1H), 3.86 (s, 3H), 3.77 – 3.59 (m, 2H), 3.30 – 3.17 (m, 1H), 3.15 – 3.03 (m, 1H), 3.03 – 2.71 (m, 4H), 2.52 – 2.31 (m, 2H), 2.22 – 2.09 (m, 1H), 1.93 – 1.66 (m, 4H), 1.61 – 1.46 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.6, 163.5, 163.2, 136.6, 130.3, 128.6, 128.5, 128.4, 126.6, 113.6, 67.7, 67.2, 55.3, 52.0, 45.1, 43.0, 32.5, 29.4, 29.1. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 137 °C (decomposed). Anal. (C23H28N2O4S•H2C2O4• 0.5H2O) C, H, N.
4.2.13. (4'-Hydroxy-1'-(5-methylthiophene-2-carbonyl)-[1,3'-bipiperidin]-4-yl)(4-methoxyphenyl)methanone oxalate (10f)
Free base 10f (35 mg, 84%) was obtained as a light yellow solid. 1H NMR (400 MHz, CDCl3) δ 7.92 (d, J = 8.7 Hz, 2H), 7.11 (d, J = 3.6 Hz, 1H), 6.94 (d, J = 8.9 Hz, 2H), 6.71 (d, J = 3.6 Hz, 1H), 4.77 – 4.61 (m, 1H), 4.52 – 4.36 (m, 1H), 3.87 (s, 3H), 3.75 – 3.55 (m, 2H), 3.30 – 3.17 (m, 1H), 3.13 – 3.04 (m, 1H), 3.00 – 2.69 (m, 4H), 2.51 (s, 3H), 2.48 – 2.31 (m, 2H), 2.19 – 2.09 (m, 1H), 1.96 – 1.69 (m, 4H), 1.61 – 1.48 (m, 1H). 13C NMR (101 MHz, CDCl 3) δ 200.8, 163.8, 163.5, 144.0, 134.4, 130.5, 129.3, 128.9, 125.1, 113.9, 67.9, 67.8, 55.5, 52.4, 45.3, 43.3, 32.7, 29.7, 29.3, 15.3. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 137 °C (decomposed). Anal. (C24H30N2O4S•H2C2O4• 0.5H2O) C, H, N.
4.2.14. (4'-Hydroxy-1'-(3-methylthiophene-2-carbonyl)-[1,3'-bi-piperidin]-4-yl)(4-methoxyphenyl)methanone oxalate (10g)
Free base 10g (88.6 mg, 60%) was obtained as a pale solid. 1H NMR (400 MHz, Chloroform-d) δ 7.93 – 7.74 (m, 2H), 7.27 – 7.15 (m, 1H), 6.94 – 6.83 (m, 2H), 6.83 – 6.71 (m, 1H), 4.76 – 4.27 (m, 1H), 4.27 – 3.93 (m, 1H), 3.80 (s, 3H), 3.71 – 3.49 (m, 2H), 3.25 – 3.07 (m, 1H), 3.07 – 2.93 (m, 1H), 2.93 – 2.57 (m, 4H), 2.46 – 2.23 (m, 2H), 2.21 (s, 3H), 2.13 – 2.00 (m, 1H), 1.88 – 1.58 (m, 4H), 1.53 – 1.34 (m, 1H). 13C NMR (101 MHz, cdcl3) δ 200.6, 164.5, 163.2, 137.2, 130.3, 129.8, 129.6, 128.6, 125.7, 113.6, 67.8, 67.1, 55.3, 52.0, 45.1, 43.0, 32.5, 29.4, 29.1, 14.5. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 134 °C (decompose). Anal. (C24H30N2O4S•C2H2O4•1.5H2O) C, H, N.
4.2.15. (4-(4-Fluorobenzoyl)-3'-hydroxy-[1,4'-bipiperidin]-1'-yl)-(thiophen-2-yl)methanone oxalate (11a)
Free base 11a (28 mg, 68%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 8.03 – 7.87 (m, 2H), 7.45 (d, J = 5.1 Hz, 1H), 7.31 (d, J = 3.9 Hz, 1H), 7.14 (t, J = 8.6 Hz, 2H), 7.04 (dd, J = 5.0, 3.6 Hz, 1H), 4.89 – 4.47 (br s, 2H), 3.73 – 3.56 (br s, 1H), 3.56 – 3.43 (m, 1H), 3.31 – 3.16 (m, 1H), 3.02 – 2.91 (m, 1H), 2.91 – 2.68 (m, 4H), 2.56 – 2.41 (m, 1H), 2.35 – 2.23 (m, 1H), 1.98 – 1.82 (m, 4H), 1.82 – 1.66 (m, 1H), 1.58 – 1.46 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.7, 167.0, 163.9, 136.8, 132.3, 130.8, 128.9, 128.7, 126.7, 115.7, 69.6, 65.9, 51.9, 45.2, 43.6, 29.7, 29.4, 29.2. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 187 °C (decomposed). Anal. (C22H25FN2O3S•H2C2O4) C, H, N.
4.2.16. (4-(4-Fluorobenzoyl)-3'-hydroxy-[1,4'-bipiperidin]-1'-yl)(3-methylthiophen-2-yl)methanone oxalate (11b)
Free base 11b (23 mg, 54%) was obtained as a color less oil. 1H NMR (400 MHz, CDCl3) δ 8.00 – 7.93 (m, 2H), 7.29 – 7.26 (m, 1H), 7.14 (t, J = 8.6 Hz, 2H), 6.83 (d, J = 4.9 Hz, 1H), 4.80 – 4.25 (m, 2H), 3.73 – 3.53 (br s, 1H), 3.50 – 3.39 (m, 1H), 3.30 – 3.17 (m, 1H), 3.00 – 2.90 (m, 1H), 2.86 – 2.69 (m, 4H), 2.52 – 2.40 (m, 1H), 2.34 – 2.27 (m, 1H), 2.26 (s, 3H), 1.96 – 1.81 (m, 4H), 1.80 – 1.67 (m, 1H), 1.56 – 1.45 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.7, 167.0, 165.0, 137.5, 132.3, 130.8, 130.0, 129.8, 126.0, 115.7, 69.6, 65.9, 51.9, 45.2, 43.6, 29.7, 29.4, 29.1, 14.7. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 130 °C (decomposed). Anal. (C23H27FN2O3S•H2C2O4• 0.75H2O) C, H, N.
4.2.17. (3'-Hydroxy-1'-(thiophene-2-carbonyl)-[1,4'-bipiperidin]-4-yl)(4-methoxyphenyl)methanone oxalate (11c)
Free base 11c (35 mg, 87%) was obtained as a pale solid. 1H NMR (400 MHz, CDCl3) δ 7.92 (d, J = 8.9 Hz, 2H), 7.44 (d, J = 4.8 Hz, 1H), 7.30 (d, J = 3.7 Hz, 1H), 7.04 (dd, J = 5.0, 3.7 Hz, 1H), 6.94 (d, J = 8.9 Hz, 2H), 4.82 – 4.51 (br s, 2H), 3.87 (s, 3H), 3.73 – 3.58 (br s, 1H), 3.56 – 3.44 (m, 1H), 3.30 – 3.17 (m, 1H), 3.00 – 2.90 (m, 1H), 2.90 – 2.70 (m, 4H), 2.54 – 2.42 (m, 1H), 2.35 – 2.23 (m, 1H), 1.95 – 1.83 (m, 4H), 1.81 – 1.67 (m, 1H), 1.62 – 1.47 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.9, 163.9, 163.4, 136.8, 130.5, 128.9, 128.7, 126.7, 113.8, 69.6, 65.9, 55.5, 52.0, 45.3, 43.3, 29.5, 29.3, 22.0. CIMS: Calcd, 429.1843 (M+); Found, 429.1843 (M+). The purity of 11c was greater than 95%. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 203 °C (decomposed). Anal. (C23H28N2O4S•H2C2O4) C, H, N.
4.2.18. (3'-Hydroxy-1'-(5-methylthiophene-2-carbonyl)-[1,4'-bipiperidin]-4-yl)(4-methoxyphenyl)methanone oxalate (11d)
Free base 11d (29 mg, 70%) was obtained as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 8.8 Hz, 2H), 7.12 (d, J = 3.6 Hz, 1H), 6.94 (d, J = 8.9 Hz, 2H), 6.69 (d, J = 3.6 Hz, 1H), 4.83 – 4.58 (m, 2H), 3.87 (s, 3H), 3.72 – 3.58 (br s, 1H), 3.55 – 3.45 (m, 1H), 3.29 – 3.18 (m, 1H), 2.99 – 2.90 (m, 1H), 2.89 – 2.70 (m, 4H), 2.49 (s, 3H), 2.48 – 2.42 (m, 1H), 2.34 – 2.23 (m, 1H), 1.95 – 1.81 (m, 4H), 1.81 – 1.67 (m, 1H), 1.59 – 1.46 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 200.9, 163.9, 163.5, 144.0, 134.3, 130.5, 129.5, 128.9, 125.1, 113.8, 69.7, 65.9, 55.5, 52.0, 45.3, 43.3, 29.5, 29.3, 22.0, 15.3. The free base was treated with oxalic acid to afford the corresponding oxalate salt as white solid. mp: 137 °C (decomposed). Anal. (C23H28N2O4S•H2C2O4•0.25H2O) C, H, N.
4.5. In vitro Biological Evaluation
Sigma Receptor Binding affinity
The compounds were dissolved in DMF, DMSO, or ethanol, and then diluted in 50 mM Tris-HCl buffer containing 150 mM NaCl and 100 mM EDTA at pH 7.4 prior to performing σ1 and σ2 receptor binding measurement. The detail procedures for performing the σ1 and σ2 receptor binding measurement have been described.24, 32 To measure the σ1 receptor binding affinity, guinea pig brain membrane homogenates (~300 μg protein) tissues were the receptor resource and ~5 nM [3H](+)-pentazocine (34.9 Ci/mmol, Perkin-Elmer, Boston, MA). Incubation was performed in 96-well plates for 90 min at room temperature. Nonspecific binding was determined from samples temperature. Nonspecific binding was determined from samples that contained 10 μM of nonradioactive haloperidol. After 90 min, the incubation was stopped by addition of 150 μL of ice-cold wash buffer (10 mM Tris-HCl, 150 mM NaCl, pH 7.4). The harvested samples were filtered rapidly through a 96-well fiberglass filter plate (Millipore, Billerica, MA) that had been presoaked with 100 μL of 50mM Tris-HCl buffer at pH 8.0 for 60 min. Each filter was washed with 3 × 200 μL portinos of ice-cold wash buffer, and the filter was placed in a Wallac 1450 MicroBeta liquid scintillation counter (Perkin-Elmer, Boston, MA) to count the σ2 receptor binding affinity was determined using rat liver membrane homogenates (~300 μg protein) and ~5 nM [3H]DTG (58.1 Ci/mmol, Perkin-Elmer, Bostan, MA) in the presence of 1 μ of(+)- pentazocine to block σ1 sites. The incubation time was 120 min at room temperature. Nonspecific binding was determined from samples that contained 10 μM of nonradioactive haloperidol. All other procedures were same to those describe for the σ1 receptor binding assay above.
The concentration that inhibits 50% of the specific binding of the radioligand (IC50 value) was determined based on the data from competitive inhibition experiment by using nonlinear regression analysis. Competitive curves were best fit to a one-site model and gave pseudo-Hill coefficients of 0.6-1.0. Ki values were calculated using the method of Cheng and Prusoff33 and are presented as the mean (± 1 SEM). For these calculations, we used a Kd value of 7.89 nM for [3H](+)-pentazocine binding to σ1 receptor in guinea pig brain and a Kd value of 30.7 nM for [3H]DTG binding to σ2 receptor in rat liver.
Vesicular acetylcholine transporter binding affinity
In vitro binding affinities to VAChT were conducted with human VAChT permanently expressed in PC12 cells at about 50 pmol/mg of crude extract. No significant amounts of σ1 or σ2 receptors were present.
The radioligand used was 5 nM (-)-[3H]vesamicol, and the assay was conducted as described at final concentrations of 10-11 M to 10-5 M novel compounds.24, 25 Unlabeled (-)-vesamicol was used as an external standard, for which Ki = 15 nM, and the mixture was allowed to equilibrate at 23 ° for 20 hours. Duplicate data were averaged and fitted by regression with a rectangular hyperbola to estimate the Ki value of the novel compound. All compounds were independently assayed at least two times.
Supplementary Material
Figure 1.
Vesamicol, Benzovesamicol, Trozamicol and Related Structures
Acknowledgements
Financial support for these studies was provided by the National Institutes of Health under NS061025, NS075527 MH092797, and Mcdonnell Center for Systems Neuroscience.
Abbreviation List
- AD
Alzhemer's disease
- ACh
acetylcholine
- Anal.
Analysis
- BBB
brain blood barrier
- BOP-C1
bis(2-oxo-3-oxazolidinyl)phosphonic acid
- C
Celsius
- Calcd.
calculated
- CIM.
chemical ionization mass spectrometry
- DTG
1,3-ditolylguanidine
- m-CPBA
meta-chloroperoxybenzoic acid
- ND
not determined
- PD
Parkison's disease
- PET
positron emission tomography
- SPECT
single-photon emission computed tomography
- VAChT
vesicular acetylcholine transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Elemental analytical data of new analogues is available free of charge via internet.
References and notes
- 1.Coyle JT, Price DL, DeLong MR. Science. 1983;219:1184. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 2.Bohnen NI, Frey KA. Mol. Imag. Biol. 2007;9:243. doi: 10.1007/s11307-007-0083-6. [DOI] [PubMed] [Google Scholar]
- 3.Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Constantine GM, Mathis Ch A, Davis JG, Moore RY, Dekosky ST. J. Neurol. 2006;253:242. doi: 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- 4.Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, Mathis CA, Moore RY, DeKosky ST. Arch. Neurol. 2003;60:1745. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- 5.DeKosky ST, Scheff SW. Ann. Neurol. 1990;27:457. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 6.Marlatt MW, Webber KM, Moreira PI, Lee H-G, Casadesus G, Honda K, Zhu X, Perry G, Smith MA. Curr. Med. Chem. 2005;12:1137. doi: 10.2174/0929867053764644. [DOI] [PubMed] [Google Scholar]
- 7.Siegal D, Erickson J, Varoqui H, Ang L, Kalasinsky KS, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Synapse. 2004;52:223. doi: 10.1002/syn.20020. [DOI] [PubMed] [Google Scholar]
- 8.Terry AV, Jr., Mahadik SP. J. Pharmacol. Exp. Ther. 2007;320:961. doi: 10.1124/jpet.106.106047. [DOI] [PubMed] [Google Scholar]
- 9.Marien MR, Parsons SM, Altar CA. Proc. Natl. Acad. Sci. U. S. A. 1987;84:876. doi: 10.1073/pnas.84.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roghani A, Feldman J, Kohan SA, Shirzadi A, Gundersen CB, Brecha N, Edwards RH. Proc. Natl. Acad. Sci. U. S. A. 1994;91:10620. doi: 10.1073/pnas.91.22.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giboureau N, Som IM, Boucher-Arnold A, Guilloteau D, Kassiou M. Curr. Top. Med. Chem. 2010;10:1569. doi: 10.2174/156802610793176846. [DOI] [PubMed] [Google Scholar]
- 12.Elwary SM, Chavan B, Schallreuter KU. J. Invest. Dermatol. 2006;126:1879. doi: 10.1038/sj.jid.5700268. [DOI] [PubMed] [Google Scholar]
- 13.de Castro BM, De Jaeger X, Martins-Silva C, Lima RD, Amaral E, Menezes C, Lima P, Neves CM, Pires RG, Gould TW, Welch I, Kushmerick C, Guatimosim C, Izquierdo I, Cammarota M, Rylett RJ, Gomez MV, Caron MG, Oppenheim RW, Prado MA, Prado VF. Mol. Cell. Biol. 2009;29:5238. doi: 10.1128/MCB.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prado VF, Martins-Silva C, de Castro BM, Lima RF, Barros DM, Amaral E, Ramsey AJ, Sotnikova TD, Ramirez MR, Kim HG, Rossato JI, Koenen J, Quan H, Cota VR, Moraes MF, Gomez MV, Guatimosim C, Wetsel WC, Kushmerick C, Pereira GS, Gainetdinov RR, Izquierdo I, Caron MG, Prado MA. Neuron. 2006;51:601. doi: 10.1016/j.neuron.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DC, King SC, Parsons SM. Mol. Pharmacol. 1983;24:48. [PubMed] [Google Scholar]
- 16.Carroll PT. Brain Res. 1985;358:200. doi: 10.1016/0006-8993(85)90964-3. [DOI] [PubMed] [Google Scholar]
- 17.Michaelson DM, Burstein M. FEBS Lett. 1985;188:389. doi: 10.1016/0014-5793(85)80408-7. [DOI] [PubMed] [Google Scholar]
- 18.Efange SM, Khare AB, Langason RB. Nucl. Med. Biol. 1995;22:437. doi: 10.1016/0969-8051(94)00135-7. [DOI] [PubMed] [Google Scholar]
- 19.Coffeen PR, Efange SM, Haidet GC, McKnite S, Langason RB, Khare AB, Pennington J, Lurie KG. Nucl. Med. Biol. 1996;23:923. doi: 10.1016/s0969-8051(96)00132-1. [DOI] [PubMed] [Google Scholar]
- 20.Shiba K, Ogawa K, Ishiwata K, Yajima K, Mori H. Biorg. Med. Chem. 2006;14:2620. doi: 10.1016/j.bmc.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Sorger D, Scheunemann M, Vercouillie J, Großmann U, Fischer S, Hiller A, Wenzel B, Roghani A, Schliebs R, Steinbach J, Brust P, Sabri O. Nucl. Med. Biol. 2009;36:17. doi: 10.1016/j.nucmedbio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Scheunemann M, Sorger D, Wenzel B, Heinitz K, Schliebs R, Klingner M, Sabri O, Steinbach J. Biorg. Med. Chem. 2004;12:1459. doi: 10.1016/j.bmc.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 23.Dickey CA, Petrucelli L. Expert Opin. Ther. Tar. 2006;10:665. doi: 10.1517/14728222.10.5.665. [DOI] [PubMed] [Google Scholar]
- 24.Efange SM, Khare AB, von Hohenberg K, Mach RH, Parsons SM, Tu Z. J. Med. Chem. 2010;53:2825. doi: 10.1021/jm9017916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu Z, Efange SM, Xu J, Li S, Jones LA, Parsons SM, Mach RH. J. Med. Chem. 2009;52:1358. doi: 10.1021/jm8012344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efange SM, Khare AB, Foulon C, Akella SK, Parsons SM. J. Med. Chem. 1994;37:2574. doi: 10.1021/jm00042a010. [DOI] [PubMed] [Google Scholar]
- 27.Efange SM, Mach RH, Khare A, Michelson RH, Nowak PA, Evora PH. Appl. Radiat. Isot. 1994;45:465. doi: 10.1016/0969-8043(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 28.Mach RH, Voytko ML, Ehrenkaufer RL, Nader MA, Tobin JR, Efange SM, Parsons SM, Gage HD, Smith CR, Morton TE. Synapse. 1997;25:368. doi: 10.1002/(SICI)1098-2396(199704)25:4<368::AID-SYN8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Alz-org Alzheimers and Dement. 2008;4:110. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Cui J, Lu X, Padakanti PK, Xu J, Parsons SM, Luedtke RR, Rath NP, Tu Z. J. Med. Chem. 2011;54:5362. doi: 10.1021/jm200203f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahr BA, Parsons SM. Proc. Natl. Acad. Sci. U. S. A. 1986;83:2267. doi: 10.1073/pnas.83.7.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tu Z, Xu J, Jones LA, Li S, Dumstorff C, Vangveravong S, Chen DL, Wheeler KT, Welch MJ, Mach RH. J. Med. Chem. 2007;50:3194. doi: 10.1021/jm0614883. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Y, Prusoff WH. Biochem. Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.