Abstract
Objective
To define altered gene expression networks in endometriosis.
Design
Experiments using endometriotic tissues and primary cells.
Setting
Division of Reproductive Biology Research, Northwestern University
Patients
Premenopausal women.
Interventions
Matched samples of eutopic endometrium and ovarian endometriosis (n=8 patients) were analyzed by microarray and verified in a separate set of tissues (n=6 patients). Experiments to define signaling pathways were performed in primary endometriotic stromal cells (n=12 patients).
Main Outcomes Measures
Using a genome-wide in vivo approach, we identified 1,366 differentially expressed genes and a new gene network favoring increased glucocorticoid levels and action in endometriosis.
Results
Transcript and protein levels of 11β-hydroxysteroid dehydrogenase (HSD11B1), which produce cortisol, the biologically active glucocorticoid, were strikingly higher, whereas mRNA levels of the cortisol-degrading HSD11B2 enzyme were significantly lower in endometriotic tissue. Glucocorticoid receptor (GR) mRNA and protein levels were significantly higher in endometriosis. The inflammatory cytokine tumor necrosis factor (TNF) robustly induced mRNA and protein levels of HSD11B1 and GR, but suppressed HSD11B2 mRNA in primary endometriotic stromal cells, suggesting that TNF stimulates cortisol production and action. We also uncovered a subset of genes critical for prostaglandin synthesis and degradation, which favor high eicosanoid levels and activity in endometriosis.
Conclusion
The pro-inflammatory milieu of the endometriotic lesion stimulates cortisol synthesis and action in endometriotic lesions.
Keywords: HSD11B1, Glucocorticoid Receptor, Endometriosis, PLA2G2, HPGD
INTRODUCTION
Endometriosis is an estrogen-dependent disease that is characterized by the presence of endometrial tissue outside of the uterine cavity. It affects 5–10% of women of reproductive age, and is characterized by inflammation, pelvic pain and infertility (1–3). The pain associated with endometriosis is most commonly treated with non-steroidal anti-inflammatory drugs or with hormonal therapies, such as gonadotropin releasing hormone analogs, oral contraceptives, or anti-progestins. Some women undergo surgical removal of the endometriotic implants, although evidence suggests that this is far from curative and subsequent surgeries are frequently necessary (2).
Sampson proposed the most accepted theory for the development of endometriosis whereby endometriosis develops as a result of refluxed menstrual endometrium that is passed through the fallopian tubes becomes implanted and persists on peritoneal surfaces (4). Certain genetic abnormalities predispose women to developing endometriosis (1, 5–7), as the incidence of endometriosis is higher among women with a family history of the disease (8). Genome wide association studies (GWAS) identified chromosomal regions that are associated with endometriosis, however the specific contribution of genetics to the development of endometriosis has not been fully delineated (9–11).
In endometriosis, abnormalities in steroid hormone synthesis, degradation, and binding have been well characterized. Relative to normal endometrium, the elevated expression of P450aromatase (CYP19A1), steroidogenic acute regulatory protein (StAR), and 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1) in endometriosis facilitate de novo steroid hormone synthesis (7). In addition, decreased levels of 17β-hydroxysteroid dehydrogenase type 2 (HSD17B2) in endometriotic tissues contribute to the deficient inactivation of the potent estradiol to the less potent estrone (12). Nuclear receptor abnormalities are also prevalent, such as the decreased expression of the progesterone receptor and the increased estrogen receptor β (5, 13, 14).
We performed a microarray analysis on matched samples of ovarian endometriosis (endometrioma walls) and eutopic endometrium and we focused our analysis by identifying the overlapping genes between this microarray and two published differentially expressed gene profiles in endometriosis vs. endometrial tissues. We also interrogated the expression levels of the genes encoding all the nuclear receptors, the oxidoreductase enzymes, and the alpha-ketoreductase enzymes, which are frequently involved in steroid hormone synthesis and/or inactivation. Using this approach we identified abnormalities in the pathways regulating the metabolism and action of prostaglandins and glucocorticoids in endometriosis. We also demonstrated the role of a critical cytokine, TNF, in regulating the expression of the newly identified glucocorticoid pathway in endometriotic stromal cells.
MATERIALS AND METHODS
Tissue acquisition and primary cell culture
The study was approved by the Northwestern University Institutional Review Board (1375-005), and informed consent was obtained from all participants. Matched eutopic endometrium and ovarian endometrioma samples were collected from 14 patients (ages 24–46 and not on hormonal therapy) with confirmed endometriosis at the time of laparoscopic surgery and placed in RNAlater (Ambion, Austin, TX), then snap frozen on dry ice. Eleven of the twelve tissue samples used for microarray and validation were histologically determined to be in the follicular phase of the menstrual cycle. The tissues were used for microarray analysis (n=8) or subsequent Real-Time RT-PCR target validation (n=6).
Unmatched normal endometrium and ovarian endometrioma cyst walls from an additional 12 cases were used for primary stromal cell cultures. The normal stromal cells were from hysterectomies performed for benign reasons other than endometriosis. Endometriotic stromal cells were isolated from the cyst walls of ovarian endometriomas. Tissues were obtained during the follicular phase from women not receiving hormonal therapy. Disease was confirmed by subsequent pathological evaluation.
Microarray Expression Analysis
For the microarray experiment, eight matched eutopic endometrial and ovarian endometriosis samples were used. Tissue was homogenized and purified using RNeasy columns (Qiagen, Valencia, CA). cDNA was synthesized, converted to biotinylated cRNA, fragmented and hybridized onto U133A Human Affymetrix Gene Chips (Affymetrix, Palo Alto, CA). The image files and .cel files were generated using Affymetrix GCOS1.3. These files were loaded into Array Studio expression data analysis system (version 1.1.180) and expression intensities were generated using MAS5 normalization with target intensity of 150. The within group replicates were combined in Array Studio and a MADScore was calculated to identify outliers. MAS 5 data was filtered at an intensity level of 100 and ratios were built using the group replicates above an intensity of 100 between the matched tissues to identify the genes differentially expressed by 2-fold (P<0.05). A classic dendrogram with hierarchical clustering was produced using the normalized data and Array Studio.
Gene Expression Analysis Using Real Time Reverse Transcriptase PCR
Real Time RT-PCR was performed as previously described (7). Analysis of PCR data was performed using the ΔΔCt method, Graphpad Prism v. 5 was used for a Student’s t-test and/or Mann-Whitney tests with a Tukey post-test.
TNF treatment of endometriotic stromal cells
Stromal cells were treated with 10 ng/ml of TNF (Sigma-Aldrich) for 6 and 24 hours. mRNA was isolated using TriZol (Sigma-Aldrich) and 1μg was used to generate cDNA with qScript cDNA mix (Quanta Biosciences). cDNA was amplified with primers for HSD11B1, HSD11B2, GR, MR and GapDH with SYBR Green Reagent (Applied Biosystems) with an ABI7900 cycler. Primer sequences are available upon request.
Antibodies
Proteins were detected with antibodies for the Glucocorticoid Receptor (Santa Cruz sc-1002), HSD11B1 (Cayman, #4599) and HSD11B2 (Cayman, #4303). B-actin antibody (Sigma) was used as a loading control.
RESULTS
Differential Gene Expression Between Endometriosis and Endometrium
We analyzed the gene expression profiles of eight simultaneously biopsied tissues of normal endometrium and ovarian endometrioma cyst walls. The expression profiles were obtained using the U133A Human Affymetrix Gene Chips, which analyzes the expression of 47,000 transcripts. Intensity data were used to identify genes that were differentially expressed by greater than 2-fold with p<0.05. The normalized data was used to generate a classic dendrogram with hierarchical clustering.
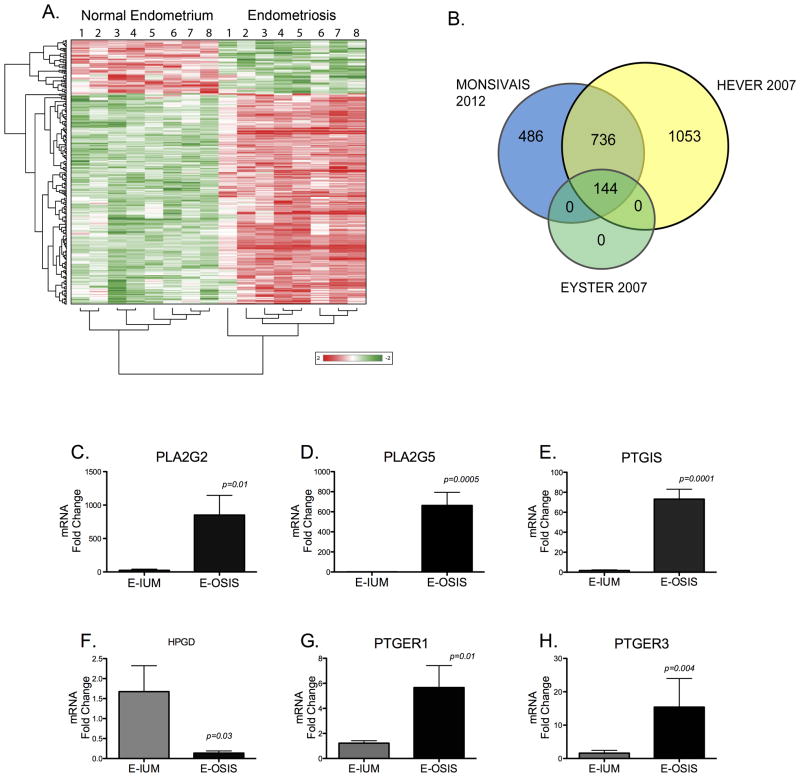
Figure 1A demonstrates that there are a large number of differentially regulated genes between endometrial and endometriotic tissues. In the hierarchical cluster, endometriotic tissue has a distinct gene expression profile from eutopic endometrial tissue. The dendrogram also shows that a higher number of differentially expressed genes are upregulated in endometriotic tissue, whereas more genes are downregulated in endometrial tissue.
Figure 1.
A, Hierarchical Clustering Demonstrates a Unique Gene Expression Profile for Ovarian Endometriosis when Compared to Matched Eutopic Tissues. Fold change values ≥ 2-fold for significantly regulated genes were subjected to hierarchical clustering. Red represents the upregulation of a gene, while green denotes a decrease relative to the mean intensity value for each probe. Each row represents a single probe set and the columns represent the individual tissue samples. B, Numbers in the overlapping regions indicate similarly regulated genes according to three microarray studies of ovarian endometriosis, the current study, Hever et al (2007), and Eyster et al (2007). The selected genes used to construct the Venn diagram from each study had a ≥ 2-fold change and P < 0.05 for endometriosis compared to the eutopic endometrium. C–H, Genes in the Prostaglandin Pathway are Altered in Endometriosis (E-OSIS) Compared to the Eutopic Endometrium (E-IUM). RNA was isolated from 6 matched endometriotic and eutopic tissues and subjected to Real Time-PCR as described in Materials and Methods. C–D, Phospholipase Enzymes; C, PLA2G2; D, PLA2G5; E, Prostacyclin synthase (PTGIS); F, 15-hydroxyprostaglandin dehydrogenase (HPGD); G–H, prostaglandin E2 receptors; G, PTGER1; H, PTGER3. The dataare represented as Fold Change ± SEM.
We demonstrated that the genes identified in this microarray correlate well with the findings from other genome-wide expression studies of ovarian endometriosis. Figure 1B shows that 64% of the 1,366 differentially expressed genes in this study were also significantly regulated in the study published by Hever et al (15). Furthermore, the 144 significantly altered genes in ovarian endometriosis from the microarray study by Eyster et al, were also differentially regulated in our microarray study (16). Thus, a unique set of genes is consistently deregulated in ovarian endometriosis, indicating that endometriosis may have a characteristic gene expression signature that is inherently different than the eutopic endometrial tissue, from which it is postulated to originate.
Overactive Prostaglandin Pathway in Endometriosis
Ingenuity Pathway Analysis (IPA) indicated that a number of genes in the prostaglandin synthesis pathway and prostaglandin receptors were up- or down-regulated in endometriosis relative to the eutopic endometrium. For example, phospholipase A2 group 2 (PLA2G2) and phospholipase A2 group 5 (PLA2G5), which encode the enzyme responsible for the production of the prostaglandin precursor arachidonic acid, were strikingly upregulated in endometriosis relative to the normal endometrium by 59.3-fold, (p=0.0002), and by 6.9-fold, (p =0.001), respectively (Summarized in Table 1). On the other hand, 15-hydroxy-prostaglandin-dehydrogenase (HPGD), the enzyme that metabolizes prostaglandins, was decreased by 2-fold in endometriosis. PTGIS was one of the most highly overexpressed genes in endometriotic tissue (179-fold, p<0.0001). In reference to prostaglandin action, the prostaglandin E receptor genes, PTGER2, PTGER3 and PTGER4, were significantly upregulated in endometriosis by 2.8-, 2.3-, and 3-fold, respectively (see Table 1). Aberrations in this pathway suggest that the overproduction or enhanced action of prostaglandins, such as the inflammatory PGE2 (Table 1), may result from a differentially expressed group of genes in the endometriotic lesion. Overall, critical genes that regulate prostaglandin synthesis, metabolism or action, are abnormally regulated in endometriosis.
Table 1. Monsivais et al.
Microarray gene expression values for the genes involved in prostaglandin synthesis that show abnormal expression in endometriosis relative to the normal endometrium. Also listed are the genes encoding abnormally expressed members of the nuclear receptor superfamily of proteins, the oxidoreductase enzymes, and the hydroxysteroid dehydrogenases. Genes involved in cortisol synthesis, metabolism and action, are altered in endometriosis relative to the normal endometrium.
| PHOSPHOLIPASES | Gene Symbol | E-Osis vs. E-IUM Fold Change | p-value |
|---|---|---|---|
| phospholipase A2, group IIA (platelets, synovial fluid) | PLA2G2A | 59.3 | 2.03E-04 |
| phospholipase A2, group XII | PLA2G12A | 3.78 | 1.21E-02 |
| phospholipase A2, group V | PLA2G5 | 6.92 | 1.23E-03 |
| PROSTAGLANDIN SYNTHASES | |||
| hydroxyprostaglandin dehydrogenase 15-(NAD) | HPGD | −5.28 | 3.03E-03 |
| PROSTAGLANDIN RECEPTORS | |||
| prostaglandin E receptor 2 (subtype EP2) | PTGER2 | 2.81 | 2.76E-02 |
| prostaglandin E receptor 3 (subtype EP3) | PTGER3 | 2.39 | 2.12E-03 |
| prostaglandin E receptor 4 (subtype EP4) | PTGER4 | 3.01 | 4.80E-02 |
| NUCLEAR RECEPTORS | |||
| Thyroid hormone receptor-β | THRB (NR1A2) | 1.77 | 1.09E-02 |
| RAR-related orphan receptor-β | RORB (NR1F2) | −49.18 | 1.70E-06 |
| Farnesoid X receptor | NR1H4 | 3.68 | 2.66E-03 |
| COUP-TF2 | NR2F2 | 2.19 | 3.79E-02 |
| V-erbA-related gene | NR2F6 | −3.89 | 9.13E-05 |
| Estrogen receptor-α | ESR1 (NR3A1) | −4.63 | 1.44E-02 |
| Estrogen receptor-β | ESR2 (NR3A2) | 3.89 | 2.43E-03 |
| Glucocorticoid receptor | NR3C1 | 3.32 | 5.78E-04 |
| Mineralocorticoid receptor | NR3C2 | 10.13 | 6.10E-05 |
| Progesterone receptor | NR3C3 | −13.83 | 6.85E-04 |
| Nerve Growth factor IB | NR4A1 | 4.76 | 4.66E-03 |
| Nuclear receptor related 1 | NR4A2 | 10.34 | 1.17E-04 |
| Neuron-derived orphan receptor 1 | NR4A3 | 3.86 | 1.51E-02 |
| DAX1 | NR0B1 | 2.89 | 4.11E-03 |
| peroxisome proliferative activated receptor, gamma | PPARG | 1.97 | 2.91E-02 |
| OXIDOREDUCTASES | |||
| hydroxysteroid (11-beta) dehydrogenase 1 | HSD11B1 | 7.78 | 3.09E-02 |
| hydroxysteroid (11-beta) dehydrogenase 2 | HSD11B2 | −11.79 | 6.27E-03 |
| ALPHA-KETO REDUCTASES | |||
| Aldo-keto reductase family 1, member A1 (aldehyde reductase) | AKR1A1 | −1.62 | 3.30E-04 |
| aldo-keto reductase family 1, member C1 (dihydrodiol dehydrogenase 1; 20-alpha (3- alpha)-hydroxysteroid dehydrogenase) | AKR1C1 | 3.20 | 1.33E-03 |
| aldo-keto reductase family 1, member C2 (dihydrodiol dehydrogenase 2; bile acid binding protein; 3-alpha hydroxysteroid dehydrogenase, type III) | AKR1C2 | 2.81 | 4.79E-03 |
| aldo-keto reductase family 1, member C3 (3-alpha hydroxysteroid dehydrogenase, type II) | AKR1C3 | 3.48 | 3.39E-02 |
| aldo-keto reductase family 7, member A2 (aflatoxin aldehyde reductase) | AKR7A2 | −1.54 | 2.81E-02 |
To validate the results obtained from the microarray analysis, we used Real Time RT-PCR with 6 matched eutopic and ectopic ovarian endometriotic tissues from a new set of patients. We verified that PLA2G2 and PLA2G5 were significantly upregulated by 900-fold (p<0.05) and 600-fold (p<0.001) in endometriosis, respectively (Figure 1C, D). The levels for PTGIS were elevated significantly by 70-fold (P<0.0001) in endometriosis relative to the eutopic endometrium (Figure 1E). Figure 1G–H shows that PTGER1 is 5-fold higher in endometriosis relative to eutopic endometrium; similarly, PTGER3 was higher by 15-fold in endometriosis. Although PTGER2 and PTGER4 were slightly higher in endometriosis, the differences were not significant (data not shown). The mRNA levels of HPGD were, on the other hand, significantly lower in endometriosis relative to the eutopic endometrium (Figure 1F). Overall, these results indicate that severely altered gene expression of critical enzymes in prostaglandin synthesis and metabolism favor elevated levels of prostaglandins in endometriotic tissue. Moreover, PGE2 action may be enhanced by the elevated expression of its receptors.
Altered Cortisol Production, Metabolism and Action in Endometriosis
We interrogated the expression levels of all the members of the nuclear receptor superfamily, along with the oxidoreductase and alpha-keto reductase family of enzymes in our microarray results (Table 1). We identified that key genes involved in glucocorticoid production, metabolism and action were altered in endometriosis. Microarray expression values showed that the mRNA levels of HSD11B1 were 7.8-fold higher and those of HSD11B2 were 11.8-fold lower in endometriosis relative to the ectopic endometrium (Table 1). The HSD11B1 gene encodes an enzyme that catalyzes the conversion of inactive cortisone to cortisol, the biologically potent glucocorticoid, in peripheral tissues. In contrast, HSD11B2 inactivates cortisol via catalyzing its conversion to cortisone. Thus, the differential expression pattern favors higher cortisol levels in endometriosis.
The glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) transcripts were increased by 3.5-fold and 5.2-fold, respectively (Table 1). Higher cortisol levels combined with elevated levels of its receptors in endometriosis are suggestive of a previously unknown, but possibly important role of glucocorticoid and mineralocorticoid action in this tissue.
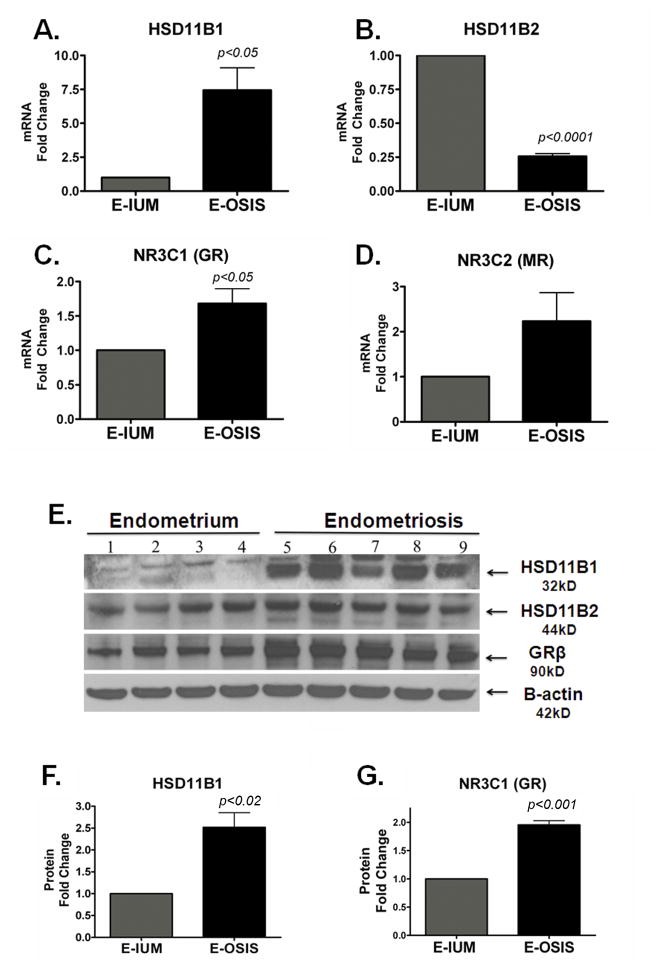
To validate these results, we used Real Time PCR analysis in normal (n=6) and endometriotic (n=6) stromal cells. Figure 2A and 2C show that HSD11B1 and GR were significantly higher in endometriotic stromal cells, while HSD11B2 was significantly lower in endometriosis (Figure 2B). MR mRNA levels appeared higher in endometriotic stromal cells, but this trend was not significant (Figure 2D). To confirm the mRNA levels, we performed western blots for HSD11B1, -B2, and GR. The GR antibody recognizes both the GRα (95kD) and GRβ (90kD) isoforms, and we observed that the major difference occurs in the GRβ isoform. The protein levels of HSD11B1, HSD11B2, and GRβ showed differences comparable to those in mRNA levels between primary endometrial and endometriotic stromal cells (Figure 2E). Densitometric analysis showed that the differences were statistically significant for HSD11B1 and GRβ, but not HSD11B2 (Figure 2F–G).
Figure 2. Genes Involved in Cortisol Synthesis and Action are Altered in Endometriosis.
A–D, Real Time-PCR validation of gene expression in stromal cells from 6 eutopic (E-IUM)and 6 endometriosis (E-OSIS) samples. A, HSD11B1 was significantly upregulated by 7.4-fold and, B, HSD11B2 was significantly downregulated by 0.46-fold in E-OSIS relative to the E-IUM; C, Glucocorticoid Receptor was increased by 2.4-fold in E-OSIS relative to E-IUM; D, Mineralocorticoid Receptor was increased but not significantly. E, Protein expression for HSD11B1, HSD11B2, GR, and β-actin in stromal cells from four normal patients (1–4) and five endometriosis patients (5–9); F–G, Densitometric quantification of HSD11B1 (F), and GR (G) protein levels in eutopic (E-OSIS) versus endometriotic (E-OSIS). Fold Change ± SEM.
TNF Enhances Glucocorticoid Action in Endometriosis
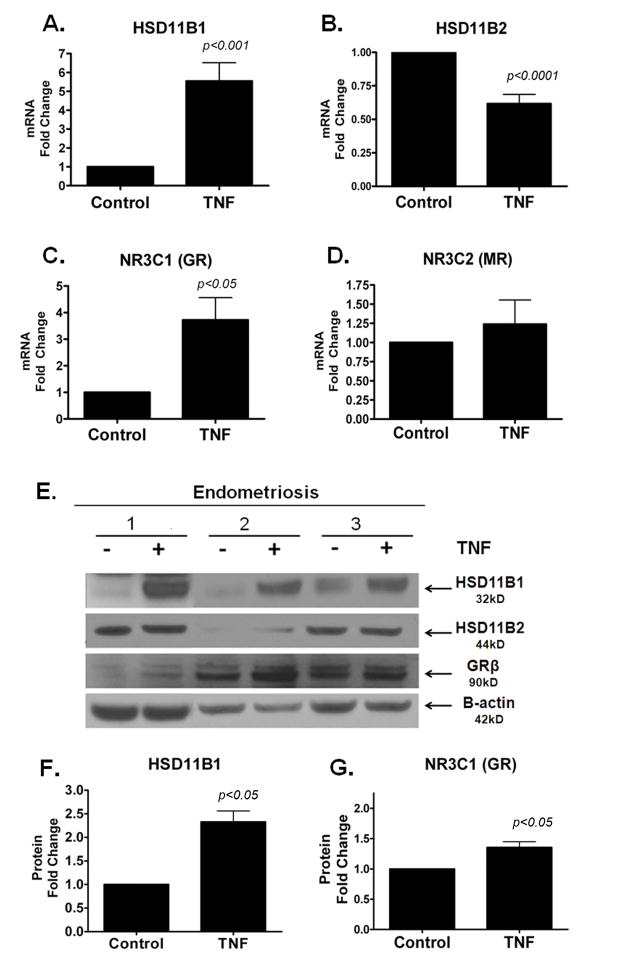
Many groups reported that TNF is overproduced in endometriosis, and in other systems it has been demonstrated that TNF’s pro-inflammatory signal elicits increased local cortisol synthesis (17, 18). We asked whether the inflammatory milieu of the ectopic endometrial tissues, characterized by increased TNF levels, contributes to the altered gene expression of the enzymes involved in cortisol production, metabolism or action. Following the incubation of cultured primary stromal cells from ovarian endometriomas in TNF for 24 hours, mRNA levels of HSD11B1 and GR significantly increased by 5-fold and 3-fold, respectively (Figure 3A, C). TNF did not affect the gene expression of MR and while TNF significantly decreased the gene expression of HSD11B2 (Figure 3B) the effect was not observed at the protein level (Figure 3E). Comparable to the increases in mRNA expression, elevated HSD11B1 and GR protein levels were also observed in endometriotic stromal cells incubated with TNF. After a 24-hour exposure to TNF, the protein levels of HSD11B1 and GR significantly increased relative to the control, whereas the expression of HSD11B2 remained unchanged (Figure 3E–G). Although a modest but significant suppression of HSD11B2 mRNA levels was observed, TNF treatment did not alter HSD11B2 protein levels.
Figure 3. The Pro-Inflammatory Cytokine TNF Increases HSD11B1 and Glucocorticoid Receptor Expression in Endometriotic Stromal Cells.
A–D, Endometriotic Stromal Cells were cultured and treated for 24 hrs with TNF (10 ng/ml). Gene and protein expression analysis was conducted for HSD11B1, HSD11B2, GR and MR. A, HSD11B1 gene expression was increased by 4.9-fold; B, HSD11B2 was significantly decreased by 0.68-fold after exposure to TNF; C, GR is significantly increased by 3-fold after treatment; D, MR gene expression was unchanged after TNF treatment; E, Protein levels in 3 endometriosis patients treated +/− TNF for 24 hours; F–G, Densitometric quantification of HSD11B1 (F) and GR (G) protein shows that HSD11B1 and GR protein levels increase significantly after TNF treatment by 2.5-fold and 1.5-fold, respectively. HSD11B2 was unchanged (Densitometric analysis not shown) and β-actin was used as a loading control. Fold Change ± SEM.
DISCUSSION
As a strategy to identify in vivo molecular abnormalities that are characteristic of endometriosis, we performed gene expression profiling of matched eutopic endometrium and ovarian endometriosis tissue. We determined that a large number of transcripts from this array overlapped with two previously published transcriptomes comparing eutopic endometrial and ovarian endometriotic tissues (15, 16). The current study, however, revealed a pathway not previously described in ovarian endometriosis: the glucocorticoid synthesis and signaling pathway. While the prostaglandin pathway in endometriosis has been studied, this study highlights alterations in a complete circuitry of genes that regulate the production, metabolism and binding of prostaglandins in endometriosis. Intriguingly, we also uncovered a previously unrecognized gene signature of glucocorticoid synthesis, metabolism and action. Moreover, we find that the components of the pro-inflammatory environment surrounding the endometriotic lesion perturb the genes involved in cortisol conversion and cortisol binding.
Altered regulation of genes important for prostaglandin synthesis have been characterized in endometriosis and demonstrate the detrimental effects of excess PGE2 on the pain and inflammation associated with endometriosis (19). Our study identified previously unrecognized genes in the prostaglandin cascade that affect prostaglandin availability and metabolism in endometriotic tissue. Phospholipase (PLA2) is the enzyme that catalyzes the initiating step in the prostaglandin synthetic pathway; it liberates arachidonic acid (AA) from membrane stores by catalyzing the hydrolysis of the sn-2 bond in the glycerol phospholipids. COX-1 or COX-2 then process AA into the substrate for the terminal prostaglandin synthases. Thus, the 600- to 900-fold increase in PLA2G5 and PLA2G2 expression correlates with the elevated prostaglandin levels observed in the peritoneal fluid of women with endometriosis (20).
Although previously reported to be elevated in endometriosis, we found no significant difference in the gene expression levels COX-1 or COX-2 between normal and endometriotic tissues; this however, does not preclude the possibility that COX-1 and COX-2 are upregulated at the translational level (21, 22). In addition, other studies have demonstrated the contribution of COX-2 activity by the peritoneal macrophages in women with endometriosis, suggesting that COX-2 overexpression may not be an inherent defect of the endometriotic stromal cells (20). Our approach, which analyzed whole endometriotic tissues instead of pure stromal cell cultures, does not discriminate between the two possible sources.
Gene expression levels of PTGES1 and PTGES2, which convert PGH2 into PGE2, were unchanged (PTGES1) or decreased (PTGES2) in endometriosis relative to the eutopic endometrium. PTGIS, which was elevated by 70-fold in endometriosis, converts PGH2 to prostacyclin (PGI), which promotes vasodilation by preventing platelet aggregation. PTGIS has anti-mitogenic effects and in the cardiovascular system, COX-2 and PTGIS promote angiogenesis and regulate apoptosis in the endothelium (23). PGI is also a PPARδ ligand, and the PPARδ/PGI interaction is important for embryo implantation and decidualization (24, 25). Further investigation on the role of increased PTGIS is warranted to understand its role in endometriosis.
PGE2 receptors are G-protein-coupled membrane receptors that activate intracellular signaling cascades upon binding of the extracellular PGE2 ligand. Previous studies showed that pharmacological inhibition of certain prostaglandin receptors activated apoptotic signaling pathways in an endometriotic stromal cell line (26). Thus, the elevated PTGER1 and PTGER3 expression identified in this study may contribute to the viability and survival of ectopic endometrial tissue and could be potential pharmacological targets.
HPGD is a tumor suppressor that metabolizes and inactivates prostanoids by reducing their 15S-hydroxyl group (27). The decrease in HPGD expression in endometriosis may contribute to abnormal prostaglandin metabolism and possibly to other altered molecular pathways in the disease. The prostaglandin pathway is a clinically important target in endometriosis since COX-2 inhibitors are beneficial for endometriosis and primary dysmenorrhea (28, 29). It would be helpful to conduct additional studies to explore new targets in the prostaglandin pathway.
Glucocorticoids control inflammatory processes in the body by negatively regulating the expression of pro-inflammatory gene products (30). Glucocorticoids exert this effect via the glucocorticoid receptor (GR), which upon binding of its ligand, represses DNA-binding factors that are involved in pro-inflammatory responses (30). Systemically, pro-inflammatory cytokines activate the hypothalamic pituitary axis by inducing a surge of glucocorticoids in the bloodstream and eliciting an anti-inflammatory response (31). At specific tissue sites, the pro-inflammatory cytokines IL1β and TNF, also exert the local synthesis of glucocorticoids by increasing HSD11B2 transcription (32–37).
Using the differentially expressed values from the microarray, we compiled a list of the genes encoding the hydroxysteroid dehydrogenases, oxidoreductases, and nuclear hormone receptors. While several aberrations in these genes were demonstrated elsewhere, here we summarize those genes that displayed differentially expressed values. Here we found that endometriotic stromal cells locally synthesize high levels of the biologically active hormone, cortisol, via the activity of HSD11B1. Combined with a reduced inactivation of cortisol due to its deficient metabolism by HSD11B2, this possibly leads to remarkably high local cortisol concentrations in endometriotic tissue.
Cortisol, but not cortisone, binds with high affinity to GR or MR and activates them physiologically or pathophysiologically in a number of tissues. The significantly elevated GR and HSD11B1 levels that we found in endometriosis suggest that the increased conversion of cortisol by HSD11B1 may have a pathological consequence, which is mediated primarily by GRβ. The two GR isoforms are generated via alternative splicing of exon 9, and each isoform is thought to have overlapping but unique functions (38). We speculate that increased cortisol biosynthesis and GR levels develop as a response to the pro-inflammatory milieu of the lesion, which is characterized by increased macrophage infiltration and elevated cytokine levels (20). This in turn may enhance cell survival in endometriosis.
The expression patterns of the cortisol converting enzymes are similar between endometriosis and other diseases characterized by inflammation, such as rheumatoid arthritis, ulcerative colitis, Crohn’s disease, and in the microglia of a mouse model of neurodegeneration (32–36). This cortisone to cortisol shuttle is known to be affected by the activity of pro-inflammatory cytokines, such as IL1β and TNF in glomerular mesangial cells (37). In primary endometriotic stromal cells, we observed a similar increase in glucocorticoid synthesis, metabolism and action in response to TNF, indicating that the endometriotic inflammatory microenvironment perturbs cortisol biosynthesis and metabolism. Currently, HSD11B1 inhibitors are being investigated for the treatment of metabolic abnormalities such as insulin resistance, type 2 diabetes, hypertension and visceral obesity (32).
While glucocorticoids can induce apoptosis of certain cell types, such as lymphocytes, they can also induce pro-survival signaling cascades resulting in cell survival and contributing to tumorigenesis (39, 40). Thus, it is likely that in endometriosis, elevated cortisol synthesis and activity contribute to endometriotic cell survival. Future studies are required to determine the implications of the altered cortisol pathway in endometriosis.
Acknowledgments
We thank Ryan Boyle and Robin Mason for their technical expertise in the analysis of the microarray data.
This work was supported by funding from NIH R37HD038691 MTD was supported by Award Number T32DK007169
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Diana Monsivais, Division of Reproductive Biology Research, Northwestern University, Chicago, IL
Jeffrey D. Bray, Department of Urogenital Biology, Cardiovascular and Urogenital Center for Excellence in Drug Discovery, GlaxoSmithKline Pharmaceuticals, King of Prussia, PA
Emily Su, Division of Reproductive Biology Research, Northwestern University, Chicago, IL
Mary Ellen Pavone, Division of Reproductive Biology Research, Northwestern University, Chicago, IL
Matthew T. Dyson, Division of Reproductive Biology Research, Northwestern University, Chicago, IL
Antonia Navarro, Division of Reproductive Biology Research, Northwestern University, Chicago, IL
Toshiyuki Kakinuma, Division of Reproductive Biology Research, Northwestern University, Chicago, IL
Serdar E. Bulun, Division of Reproductive Biology Research, Northwestern University, Chicago, IL.
References
- 1.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 2.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 3.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. 1927;3:93–110. 143. [PMC free article] [PubMed] [Google Scholar]
- 5.Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77:681–687. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- 6.Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab. 2007;92:3261–3267. doi: 10.1210/jc.2007-0494. [DOI] [PubMed] [Google Scholar]
- 7.Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, Gurates B, Attar R, Yaegashi N, Hales DB, Bulun SE. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94:623–631. doi: 10.1210/jc.2008-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson JL, Elias S, Malinak LR, Buttram VC., Jr Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137:327–331. doi: 10.1016/0002-9378(80)90917-5. [DOI] [PubMed] [Google Scholar]
- 9.Uno S, Zembutsu H, Hirasawa A, Takahashi A, Kubo M, Akahane T, Aoki D, Kamatani N, Hirata K, Nakamura Y. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet. 42:707–710. doi: 10.1038/ng.612. [DOI] [PubMed] [Google Scholar]
- 10.Adachi S, Tajima A, Quan J, Haino K, Yoshihara K, Masuzaki H, Katabuchi H, Ikuma K, Suginami H, Nishida N, Kuwano R, Okazaki Y, Kawamura Y, Sasaki T, Tokunaga K, Inoue I, Tanaka K. Meta-analysis of genome-wide association scans for genetic susceptibility to endometriosis in Japanese population. J Hum Genet. 55:816–821. doi: 10.1038/jhg.2010.118. [DOI] [PubMed] [Google Scholar]
- 11.Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, Lambert A, Zhao ZZ, Roseman F, Guo Q, Gordon SD, Wallace L, Henders AK, Visscher PM, Kraft P, Martin NG, Morris AP, Treloar SA, Kennedy SH, Missmer SA, Montgomery GW, Zondervan KT. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. doi: 10.1038/ng.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delvoux B, Groothuis P, D′Hooghe T, Kyama C, Dunselman G, Romano A. Increased production of 17beta-estradiol in endometriosis lesions is the result of impaired metabolism. J Clin Endocrinol Metab. 2009;94:876–883. doi: 10.1210/jc.2008-2218. [DOI] [PubMed] [Google Scholar]
- 13.Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, Tokunaga H, Utsunomiya H, Yin P, Luo X, Lin Z, Imir G, Thung S, Su EJ, Kim JJ. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28:36–43. doi: 10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulun SE, Cheng YH, Pavone ME, Yin P, Imir G, Utsunomiya H, Thung S, Xue Q, Marsh EE, Tokunaga H, Ishikawa H, Kurita T, Su EJ. 17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28:44–50. doi: 10.1055/s-0029-1242992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hever A, Roth RB, Hevezi P, Marin ME, Acosta JA, Acosta H, Rojas J, Herrera R, Grigoriadis D, White E, Conlon PJ, Maki RA, Zlotnik A. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc Natl Acad Sci USA. 2007;104:12451–12456. doi: 10.1073/pnas.0703451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyster KM, Klinkova O, Kennedy V, Hansen KA. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril. 2007;88:1505–1533. doi: 10.1016/j.fertnstert.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 17.Scholl B, Bersinger NA, Kuhn A, Mueller MD. Correlation between symptoms of pain and peritoneal fluid inflammatory cytokine concentrations in endometriosis. Gynecol Endocrinol. 2009;25:701–706. doi: 10.3109/09513590903159680. [DOI] [PubMed] [Google Scholar]
- 18.Escher G, Galli I, Vishwanath BS, Frey BM, Frey FJ. Tumor necrosis factor alpha and interleukin 1beta enhance the cortisone/cortisol shuttle. J Exp Med. 1997;186:189–198. doi: 10.1084/jem.186.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu MH, Lu CW, Chuang PC, Tsai SJ. Prostaglandin E2: the master of endometriosis? Exp Biol Med (Maywood) 2010;235:668–677. doi: 10.1258/ebm.2010.009321. [DOI] [PubMed] [Google Scholar]
- 20.Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, Tsai SJ. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod. 2002;8:1103–1110. doi: 10.1093/molehr/8.12.1103. [DOI] [PubMed] [Google Scholar]
- 21.Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2008;149:1190–1204. doi: 10.1210/en.2007-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho S, Park SH, Choi YS, Seo SK, Kim HY, Park KH, Cho DJ, Lee BS. Expression of cyclooxygenase-2 in eutopic endometrium and ovarian endometriotic tissue in women with severe endometriosis. Gynecol Obstet Invest. 69:93–100. doi: 10.1159/000261017. [DOI] [PubMed] [Google Scholar]
- 23.Cathcart MC, Reynolds JV, O’Byrne KJ, Pidgeon GP. The role of prostacyclin synthase and thromboxane synthase signaling in the development and progression of cancer. Biochim Biophys Acta. 1805:153–166. doi: 10.1016/j.bbcan.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARdelta. Genes Dev. 1999;13:1561–1574. doi: 10.1101/gad.13.12.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim H, Dey SK. A novel pathway of prostacyclin signaling-hanging out with nuclear receptors. Endocrinology. 2002;143:3207–3210. doi: 10.1210/en.2002-220159. [DOI] [PubMed] [Google Scholar]
- 26.Banu SK, Starzinski-Powitz A, Speights VO, Burghardt RC, Arosh JA. Induction of peritoneal endometriosis in nude mice with use of human immortalized endometriosis epithelial and stromal cells: a potential experimental tool to study molecular pathogenesis of endometriosis in humans. Fertil Steril. 2009;91:2199–2209. doi: 10.1016/j.fertnstert.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 27.Frank B, Hoeft B, Hoffmeister M, Linseisen J, Breitling LP, Chang-Claude J, Brenner H, Nieters A. Association of hydroxyprostaglandin dehydrogenase 15-(NAD) (HPGD) variants and colorectal cancer risk. Carcinogenesis. doi: 10.1093/carcin/bgq231. [DOI] [PubMed] [Google Scholar]
- 28.Alsalameh S, Burian M, Mahr G, Woodcock BG, Geisslinger G. Review article: The pharmacological properties and clinical use of valdecoxib, a new cyclo-oxygenase-2-selective inhibitor. Alimentary pharmacology & therapeutics. 2003;17:489–501. doi: 10.1046/j.1365-2036.2003.01460.x. [DOI] [PubMed] [Google Scholar]
- 29.Cobellis L, Razzi S, De Simone S, Sartini A, Fava A, Danero S, Gioffre W, Mazzini M, Petraglia F. The treatment with a COX-2 specific inhibitor is effective in the management of pain related to endometriosis. Eur J Obstet Gynecol Reprod Biol. 2004;116:100–102. doi: 10.1016/j.ejogrb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 30.De Bosscher K, Haegeman G. Minireview: latest perspectives on antiinflammatory actions of glucocorticoids. Mol Endocrinol. 2009;23:281–291. doi: 10.1210/me.2008-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 32.Chapman KE, Coutinho AE, Gray M, Gilmour JS, Savill JS, Seckl JR. The role and regulation of 11beta-hydroxysteroid dehydrogenase type 1 in the inflammatory response. Mol Cell Endocrinol. 2009;301:123–131. doi: 10.1016/j.mce.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 33.Gottfried-Blackmore A, Sierra A, McEwen BS, Ge R, Bulloch K. Microglia express functional 11 beta-hydroxysteroid dehydrogenase type 1. Glia. 58:1257–1266. doi: 10.1002/glia.21007. [DOI] [PubMed] [Google Scholar]
- 34.Neeck G, Renkawitz R, Eggert M. Molecular aspects of glucocorticoid hormone action in rheumatoid arthritis. Cytokines Cell Mol Ther. 2002;7:61–69. doi: 10.1080/13684730412331302081. [DOI] [PubMed] [Google Scholar]
- 35.Neeck G, Kluter A, Dotzlaw H, Eggert M. Involvement of the glucocorticoid receptor in the pathogenesis of rheumatoid arthritis. Ann N Y Acad Sci. 2002;966:491–495. doi: 10.1111/j.1749-6632.2002.tb04252.x. [DOI] [PubMed] [Google Scholar]
- 36.Stegk JP, Ebert B, Martin HJ, Maser E. Expression profiles of human 11beta-hydroxysteroid dehydrogenases type 1 and type 2 in inflammatory bowel diseases. Mol Cell Endocrinol. 2009;301:104–108. doi: 10.1016/j.mce.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Escher G, Galli I, Vishwanath BS, Frey BM, Frey FJ. Tumor necrosis factor alpha and interleukin 1beta enhance the cortisone/cortisol shuttle. J Exp Med. 1997;186:189–198. doi: 10.1084/jem.186.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform beta: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci. 2009;66:3435–3448. doi: 10.1007/s00018-009-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 40.Wu W, Pew T, Zou M, Pang D, Conzen SD. Glucocorticoid receptor-induced MAPK phosphatase-1 (MPK-1) expression inhibits paclitaxel-associated MAPK activation and contributes to breast cancer cell survival. J Biol Chem. 2005;280:4117–4124. doi: 10.1074/jbc.M411200200. [DOI] [PubMed] [Google Scholar]