Abstract
Predictable sensorimotor perturbations can lead to cerebellum-dependent adaptation—i.e., recalibration of the relationship between sensory input and motor output. Here we asked if the cerebellum is also needed to recalibrate the relationship between two sensory modalities, vision and proprioception. We studied how people with and without cerebellar damage use visual and proprioceptive signals to estimate their hand’s position when the sensory estimates disagree. Theoretically, the brain may resolve the discrepancy by recalibrating the relationship between estimates (sensory realignment). Alternatively, the misalignment may be dealt with by relying less on one sensory estimate and more on the other (a weighting strategy). To address this question, we studied subjects with cerebellar damage and healthy controls as they performed a series of tasks. The first was a prism adaptation task that involves motor adaptation to compensate for a visual perturbation and is known to require the cerebellum. As expected, people with cerebellar damage were impaired relative to controls. The same subjects then performed two experiments in which they reached to visual and proprioceptive targets while a visuoproprioceptive misalignment was gradually imposed. Surprisingly, cerebellar patients performed as well as controls when the task invoked only sensory realignment, but were impaired relative to controls when motor adaptation was also possible. Additionally, individuals with cerebellar damage were able to use a weighting strategy similarly to controls. These results demonstrate that, unlike motor adaptation, sensory realignment and weighting are not cerebellum-dependent.
Keywords: motor adaptation, sensory adaptation, sensorimotor integration, reaching, cerebellum
1. Introduction
The cerebellum has long been considered important in motor control (e.g., Holmes, 1917; Thach et al., 1992) and has been implicated in a form of motor learning referred to here as motor adaptation. Cerebellum-dependent motor adaptation has been found in a variety of behaviors, including prism adaptation (Weiner et al., 1983; Martin et al., 1996a; Baizer et al., 1999). When healthy individuals throw a ball at a visual target while wearing prism goggles, they initially make errors in the direction of prismatic displacement. The difference between the brain’s prediction of the result of the throw and the actual result of the throw constitutes an error signal; these sensory prediction errors drive the brain to adapt its internal model of how the throwing movement is related to the sensory consequence (Wolpert et al., 1998). Subjects thus gradually adapt to the prisms throw by throw to eventually hit the target, and then display a negative aftereffect (errors opposite the direction of prismatic displacement) when the prisms are removed. The aftereffect indicates that a new calibration between gaze direction and throw direction has been learned and stored (Figure 1A and Martin et al., 1996b). Subjects with cerebellar lesions are impaired at this type of motor adaptation (Martin et al., 1996a).
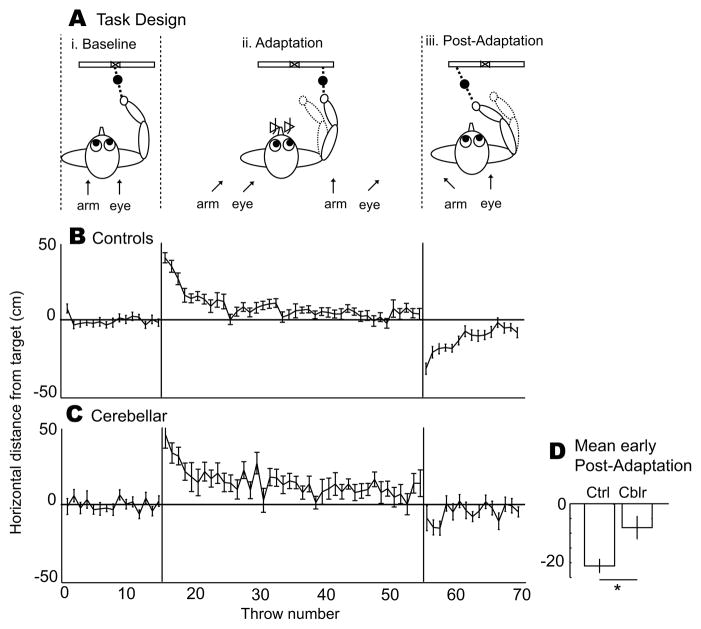
Figure 1. Experiment 1 (throwing with prisms) group data.
A. i. When throwing objects at a target without prisms (Baseline), arm direction and eye direction (arrows) are aligned. ii. While wearing laterally-displacing prism goggles (Adaptation), the subject initi ally makes errors in the direction of prism displacement, which are gradually reduced as the alignment between where the subject sees the target and where he throws changes. iii. This is manifested by a negative aftereffect (errors in the opposite direction) when the prisms are removed (Post-Adaptation), indicating that motor adaptation has occurred (Martin et al. 1996). B–C. The positive y axis reflects errors to the right (direction of prismatic shift), while the negative y axis reflects errors to the left (direction of negative aftereffect). Trial-by-trial throw error averaged across healthy control subjects (N = 12, B) and cerebellar patients (N=12, C). i. Controls and patients threw accurately during baseline. ii. When rightward-displacing prism goggles were worn during adaptation, controls initially made large rightward (positive axis) errors but gradually returned to baseline levels of accuracy. Patients were able to strategically reduce their throwing bias during adaptation, but did not reach baseline levels of error. iii. When prism goggles were removed, controls initially made large leftward (negative axis) errors. Patients had a smaller negative aftereffect. D. In controls (left), this negative aftereffect (mean −21.1 cm; significantly different from zero; t(11) = −8.99, p < 0.00001) reflects motor adaptation, i.e., a new calibration between gaze direction and throw direction was learned and stored. Negative aftereffect in patients (right) was significantly diminished relative to controls (mean −8.1 cm; two-sample t(22) = 2.81, p = 0.010) but not different from zero (t(11) = −2.03, p = 0.07), indicating that motor adaptation was impaired. All error bars represent standard error.
In addition to motor adaptation, a number of studies support a possible role for the cerebellum in sensory processing (e.g., Gao et al., 1996; Parsons et al., 1997; Hagura et al., 2009). Clower and colleagues (2001) showed that the cerebellar dentate nucleus projects to the posterior parietal cortex area 7b in monkeys, and explicitly suggested that “that the cerebellar projection to posterior parietal cortex may provide signals that contribute to (or initiate) the sensory recalibration that occurs during the adaptive process.” Here we ask if the cerebellum is important for sensory realignment, a process where the spatial relationship between different sensory estimates is changed (e.g., the proprioceptive estimate of hand position may be realigned to more closely match the visual estimate or vice versa; van Beers et al., 2002; Block and Bastian, 2011).
Sensory realignment may be computationally analogous to cerebellum-dependent motor adaptation, requiring recalibration of the relationship between sensory inputs rather than between gaze and throw direction as occurs in prism adaptation (Martin et al., 1996b). The cerebellum receives information from both vision (Snider and Stowell, 1944; Glickstein, 2000) and proprioception (Bauswein et al., 1983; Donga and Dessem, 1993), so it is conceivable that different sensory estimates could be compared by the cerebellum. Further, the complex spikes carried to cerebellar cortex by olivary climbing fibers (Ito, 2001) could convey an error signal arising from any mismatch between sensory inputs, similar to the mechanism proposed for motor adaptation (Marr 1969; Albus 1971; Bays and Wolpert, 2007).
We studied patients with bilateral cerebellar atrophy and healthy controls in three experiments. In Experiment 1, we tested subjects throwing at a visual target, using prism goggles to induce adaptation of the relationship between throw direction and gaze direction. In Experiments 2 and 3, we used a reaching task to create a misalignment between visual and proprioceptive estimates of hand position. Endpoint visual feedback in Experiment 2 created the potential for motor adaptation as well. We confirmed that cerebellar patients are impaired at prism adaptation, but found no impairment in sensory realignment, suggesting that unlike motor adaptation, sensory realignment is not a cerebellum-dependent process. These results have implications for our understanding of multisensory processing as well as the capacity for adaptation of individuals with cerebellar damage.
2. Materials and Methods
We conducted three experiments with healthy controls and cerebellar patients to explore the role of the cerebellum in sensory realignment. In the Motor experiment, we tested motor adaptation by changing the gaze-throw calibration; subjects threw balls at a visual target while wearing laterally-displacing prism goggles. In the Sensorimotor experiment, we tested visuoproprioceptive realignment and motor adaptation; subjects reached with their dominant hand to a series of visual (V), proprioceptive (P), and combined (VP) targets in a virtual reality setup with no vision of either arm, with the non-dominant hand serving as proprioceptive target. A misalignment between visual and proprioceptive estimates of target hand position was imposed by gradually shifting the V component away from the P component of VP targets. Subjects received endpoint visual feedback indicating a hit or a miss after reaches to VP targets only. The Sensory experiment was identical to the Sensorimotor experiment, except no endpoint visual feedback was given, to test whether cerebellar patients were impaired at sensory realignment in the absence of a cue to drive motor adaptation. Please note that we assigned names to the experiments based on the type of adaptation (Motor, Sensorimotor or Sensory) each one was intended to elicit. Thus, the nomenclature reflects our interpretation of the paradigm rather than the explicit design of each experiment.
2.1 Subjects
We studied 13 patients with bilateral cerebellar atrophy (9 women; Table I) and 17 neurologically healthy control subjects matched for age and handedness (9 women). Most of the patients and controls did the Motor experiment plus one or both of the Sensorimotor and Sensory experiments. As such, the Motor experiment had 12 patients (mean 57 years) and 12 controls (mean 55 years); the Sensorimotor experiment had 10 patients (mean 55 years) and 10 controls (mean 54 years); and the Sensory experiment had 7 patients (mean 59 years) and 7 controls (mean 58 years). All patients had normal or corrected vision, normal proprioception, and normal fine touch sensation. Subjects were screened to ensure that any eye movement deficits were minimal (e.g. nystagmus) and none had double vision. All subjects gave written informed consent, and protocols were approved by the Johns Hopkins Institutional Review Board.
Table I. Cerebellar patient characteristics and results.
The ICARS is a 100 point semi-quantitative scale of cerebellar ataxia, with sub-scores for postural, gait, speech, and oculomotor disorders. Larger numbers reflect greater impairment (Trouillas et al., 1997). Motor expt.: number reflects negative aftereffect in cm (positive indicates motor adaptation occurred and was stored). Sensorimotor and Sensory expt.: number reflects ΔP, the change in P endpoints (in mm) during the adaptation block. Blank spaces are left where a patient did not participate in the experiment. Mean value for control subjects is given in brackets at the top of the column. Mean values for patients are given in last row.
| Subject | Age | Dominant Hand | Diagnosis | ICARS1 | Motor expt. (21.1 cm) | Sensori-motor expt. (28 mm) | Sensory expt. (13 mm) |
|---|---|---|---|---|---|---|---|
| CB1 | 56 | R | SCA62 | 33 | 25.2 | 3.53 | |
| CB2 | 35 | L | SCA83 | 45 | −2.4 | 7.2 | 22.9 |
| CB3 | 49 | R | SCA6 | 16 | 32.0 | ||
| CB4 | 74 | L* | SCA6 | 54 | −18 | 11.6 | 12.4 |
| CB5 | 54 | R** | SCA6/SCA8 | 65 | 1.2 | 18.3 | −2.4 |
| CB6 | 69 | R | SCA6 | 67 | 13.2 | 1.8 | |
| CB7 | 70 | R | ADCA III4 | 18 | 13.2 | 11.1 | |
| CB8 | 40 | R | SCA6 | 47 | −9.6 | 15.7 | |
| CB9 | 30 | R | ADCA III | 3 | 20.4 | 22.4 | |
| CB10 | 67 | R | ADCA III | 33 | 22.8 | 7.1 | |
| CB11 | 67 | R | Sporadic cblr atrophy | 56 | 1.2 | 34.2 | 9.2 |
| CB12 | 56 | R | Sporadic cblr atrophy | 24 | 8.4 | 21.0 | |
| CB13 | 67 | R | SCA6 | 12 | 21.6 | 35.3 | |
| Mean | 56.5 | 36 | 8.1 | 16.1 | 14.6 |
Patient reached/threw with non-dominant hand in all experiments, as did matched control
Patient reached with non-dominant hand in Sensory expt., as did matched control
International Cooperative Ataxia Rating Scale (Trouillas et al., 1997)
Spinocerebellar Ataxia type 6
Spinocerebellar Ataxia type 8
Autosomal Dominant Cerebellar Ataxia type III, indicates an inherited disorder with pure cerebellar signs but no definite genetic testing.
2.2 Motor experiment – Throwing with prisms
The purpose of this experiment was to confirm that the cerebellum is important in motor adaptation. We refer to this experiment as “Motor” because we have previously shown that this particular throwing paradigm leads to adaptation of the motor commands rather than visual or proprioceptive perception (Martin et al., 1996b). Subjects threw 3 cm-diameter balls of clay at an 8 cm square target displayed at shoulder height on a wall 1.83 m away. Subjects were instructed to throw overhand with the dominant hand as quickly as they could, try to hit the target, and to not look at their hands. The experimenter recorded the place each throw landed using a numbered grid on the wall with a margin for error of ±3 cm. A second experimenter placed a clay ball in the subject’s throwing hand before every throw. For the adaptation block, safety goggles containing 30 diopter prisms (Fresnel Prism and Lens, Eden Prairie, MN) were placed on the subject. The prisms were oriented base left so as to shift the visual field about 173 to the right.
The task was as follows: a baseline block of 15 throws was completed before the prismatic perturbation was introduced. Subjects then performed 40 throws while wearing the prisms, after which prisms were removed and 15 more throws (post-adaptation block) were recorded (Figure 1A). To quantify the motor adaptation that was learned and stored (i.e., how much the relationship between gaze direction and throw direction was recalibrated by wearing prisms), we calculated the aftereffect: the error subjects made immediately after prisms were removed, in the direction opposite the prismatic offset. This was the mean horizontal error of the first five post-adaptation throws with the mean horizontal error of the last five baseline throws subtracted to eliminate any bias.
2.3 Sensorimotor and Sensory experiments
The purpose of these experiments was to assess whether the cerebellum is important in sensory realignment. The Sensorimotor experiment retained the possibility of motor adaptation in addition to sensory realignment, but the Sensory experiment involved only sensory realignment. Subjects sat at a reflected rear projection apparatus (Figure 2A) and reached with their dominant hand index finger (exceptions noted in Table 1) to visual (V), proprioceptive (P), or combined (VP) targets with no vision of either arm (Figure 2B). The index fingertip of the non-dominant hand, positioned on one of two tactile markers beneath the reaching surface (Figure 2B, Screen 1), served as the proprioceptive target. Infrared-emitting markers were placed on each index fingertip, and an Optotrak 3020 (Northern Digital) was used to record 3D position data at 100 Hz. The three target types repeated in the order VP-P-VP-V throughout the baseline (28 reaches) and adaptation (84 reaches) blocks. The particular start and target positions (5 possible start positions and 2 possible target positions; Figure 2B, Screen 1) for a given reach were randomized such that it was unlikely a subject could memorize a reach direction or extent. For a participant of average arm length, reach distance (from start box to target location) averaged 14 cm. To reduce any effect of motor impairment in the cerebellar patients, all subjects were instructed to move at whatever speed they were comfortable with and to indicate the perceived target location as accurately as they could.
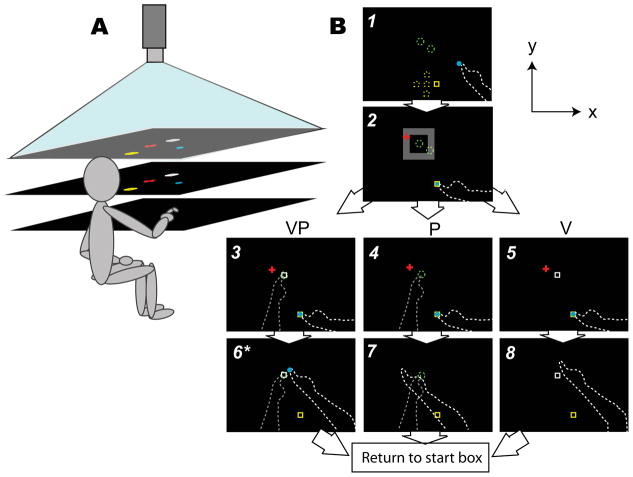
Figure 2. Experimental setup for Sensorimotor and Sensory experiments (reaching with and without endpoint visual feedback).
A. Subject looks down into a horizontal mirror (middle) and sees targets and cursors indicating hand position reflected from a horizontal rear projection screen (top). The dominant reaching hand rests on a hard acrylic reaching surface (bottom) below the mirror. The non-dominant target hand remains below the reaching surface at all times. Mirror is positioned midway between screen and reaching surface such that objects in the mirror appear to be in the plane of the reaching surface. Not pictured: black drapes obscuring the subject’s vision of his arms and the room outside the apparatus.
B. Timeline of a single reach in the baseline block, and bird’s eye view of the display. Solid objects were visible to the subject; dashed lines were not. The total display area was 75×100 cm. Schematic not to scale.
Screen 1: Subjects placed their reaching finger (white dashed line) in the yellow start box, which appeared in one of five possible positions (yellow dashed squares; the central square was 20 mm from each of the others), with the aid of an 8 mm-diameter blue cursor indicating reaching finger position (veridical in order to minimize proprioceptive drift between reaches; Wann and Ibrahim, 1992).
Screen 2: A red fixation cross appeared in a random location within an invisible 10×10 cm zone (grey), and subjects were instructed to fixate on it for the duration of the reach.
Screens 3–5: Subjects positioned their target finger (dashed grey line) as instructed: on one of the two tactile markers stuck to the bottom of the reaching surface about 40 mm apart (green dashed circles) for a P or VP target, or down in their lap for a V target, which appeared as a 12×12 mm white box in one of the two possible target locations. For VP reaches, the V target was projected on the P target during the baseline block, but was gradually offset in the y direction during the adaptation block. Once both hands were correctly positioned, subjects reached toward the target, with the cursor disappearing at movement initiation. Movement speed was not restricted, and subjects were permitted to make adjustments.
Screens 6–8: When the reaching finger had not moved more than 2 mm for 2 seconds, the reach endpoint was recorded and the trial ended. In the Sensorimotor experiment only, endpoint visual feedback (blue dot) was displayed for 2 seconds at the movement endpoint location for VP targets. In both experiments, subjects were instructed to lower their target hand to their lap (for P or VP targets) and move their reaching finger to the next yellow start box to begin the next trial.
*In Sensory experiment, no endpoint visual feedback was given.
2.3.1. Perturbation
VP targets, in which a V target was initially projected on top of a P target, represented the situation of interest (i.e., the subject was estimating the position of his target hand with both visual and proprioceptive estimates available). VP targets were used to impose a misalignment between these estimates: after a baseline in which the V component of VP targets was projected directly over the P component, the V component gradually shifted away from the P component in the positive y direction such that, by the end of the adaptation block, the V component was 70 mm farther away from the subject than the P component. The unimodal V and P targets were used to assess realignment and sensory weights as discussed below. During the adaptation block, the unimodal V target shifted away from the subject at the same rate as the V component of VP targets (1.67 mm shift added every VP reach). Every subject was questioned at the end of the experiment about whether they felt the V component of VP targets was always on top of the P component. If a subject felt that the V component was displaced from the P component in the direction of the 70 mm perturbation (straight ahead), we discarded that subject’s data. This occurred in 6 experimental sessions (4 control, 2 patient), in addition to the 34 (20 Sensorimotor, 14 Sensory) analyzed here.
2.3.2. Fixation and Visual Feedback
Before every reach, a red fixation cross appeared within an invisible 10 cm-zone around the target (Figure 2B, Screen 2). Subjects were instructed to look at it for the duration of the reach. The purpose of the fixation cross was to maintain some consistency in gaze direction across subjects during P trials; i.e., to avoid a situation where some subjects look at where they think the P target is and others stare off into space. To prevent subjects from noticing that the V targets were shifting during the adaptation block, the fixation cross zone shifted with the V target. Reaching endpoints did not appear to be influenced by the position of the fixation cross (Appendix).
During the task, subjects saw a blue cursor indicating reaching finger position while placing their reaching finger in the yellow start box (Figure 2B, Screen 1). The cursor disappeared as soon as the reach began, thus no online visual feedback about the reaching hand was given. In the Sensorimotor experiment, subjects received endpoint visual feedback on reaches to VP targets only (Figure 2B, Screen 6–8); after the end of the movement, the cursor reappeared at the location of the reaching finger so subjects could see where they had landed in relation to the V component of the VP target. If the reaching finger landed within 10 mm of the center of the V component, it appeared to explode, and a point was added to the subject’s score Subjects were told that the goal was to earn as many points as possible. In the Sensory experiment, no online or endpoint visual feedback was given once the reaching hand left the starting position; subjects thus never found out whether they had hit or missed the targets.
2.3.3. Proprioceptive realignment (ΔP)
We measured P reach endpoint shifts (ΔP) to quantify proprioceptive realignment. Note that reaches to misaligned VP targets provided two potential sources of error information in the Sensorimotor experiment: explicit endpoint visual feedback relative to the V component of the target, which could drive motor adaptation of the reaching hand; and the misalignment of proprioceptive and visual estimates of target hand position, which could drive sensory realignment of target hand position estimates. We have shown previously (Block and Bastian, 2011) that subjects respond to both types of error, leading to motor adaptation of the reaching hand (i.e., the brain observes the reaching hand undershooting the target, so the motor command to the reaching hand is modified) in addition to sensory realignment of the target hand (a shift in the proprioceptive estimate of target hand position to be closer to the visual estimate).
In the Sensory experiment, however, the absence of endpoint visual feedback means the brain will observe no reaching errors at all. Thus, there is no reason to modify the motor command to the reaching hand, and any shift in P reaching endpoints (ΔP) can be attributed to proprioceptive realignment of the target hand in response to the visuoproprioceptive misalignment. Thus, in the Sensorimotor experiment, ΔP represents some combination of motor adaptation and sensory realignment of proprioception (e.g., Figure 3A.i). In the Sensory experiment, however, ΔP represents only proprioceptive realignment (e.g., Figure 3C.i). If yPα is the mean of the first four P endpoint y-coordinates in the adaptation block, and yPβ is the mean of the last four, then
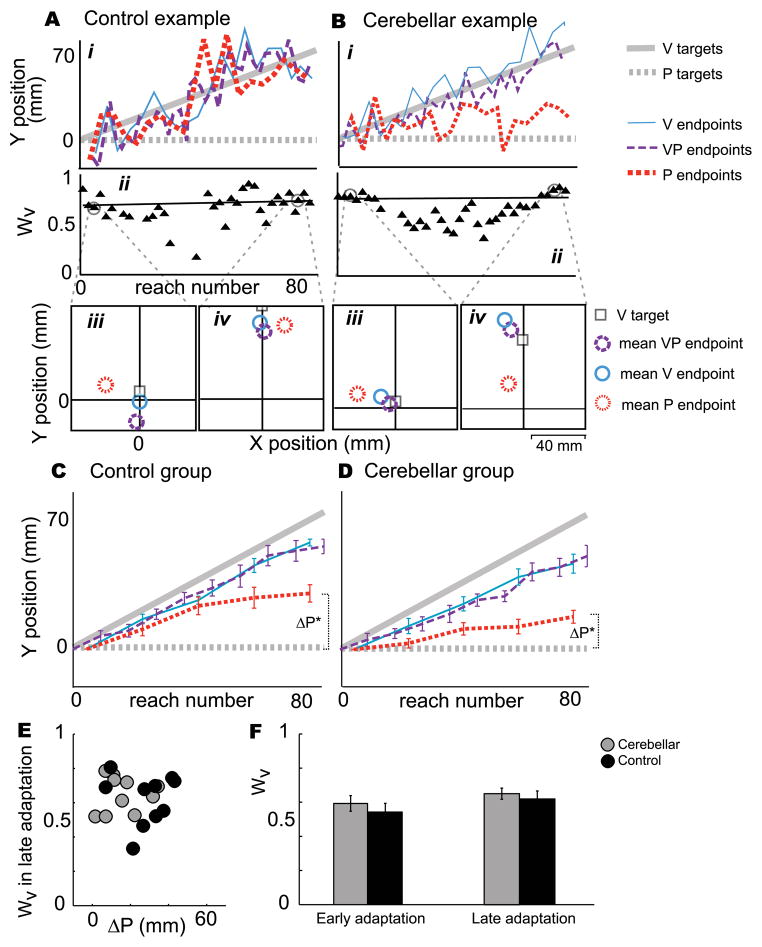
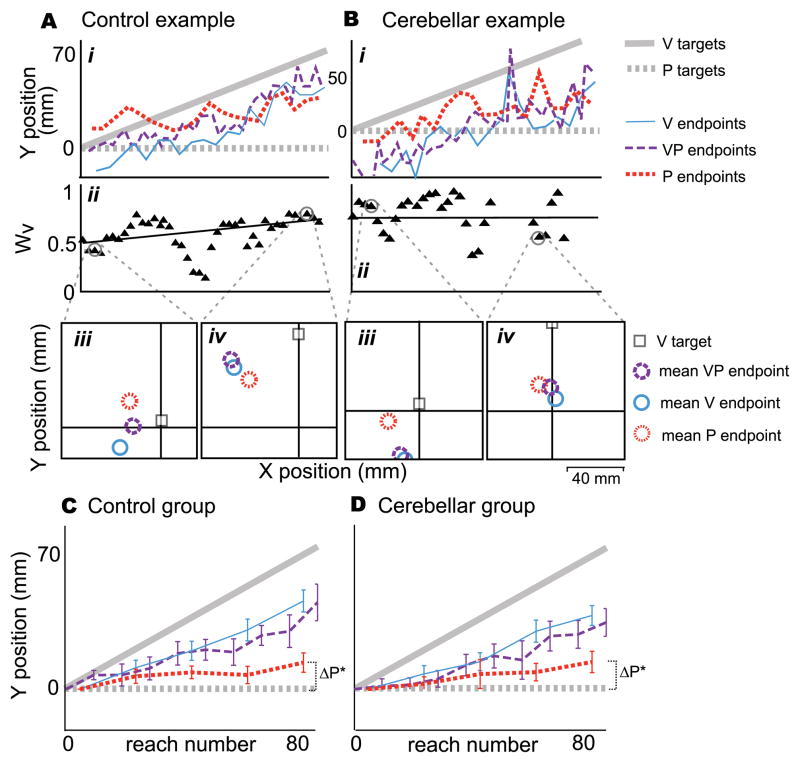
Figure 3. Sensorimotor experiment results.
A. A healthy control subject in the Sensorimotor experiment (reaching with endpoint visual feedback), age 65. i. VP endpoints (purple dashed line) followed the V component of VP targets (solid grey line), reflecting that the subject succeeded in hitting or nearly hitting the target. P endpoints (red dotted line) shifted substantially (ΔP = 42.1 mm), reflecting motor adaptation and/or proprioceptive realignment (Block & Bastian 2011).
ii. This subject relied heavily on vision, such that the weight of vision (Wv) at the end of the adaptation block was 0.69. iii. Early in the adaptation block, VP endpoints (purple circle) fell closer to V endpoints (blue circle) than P endpoints (red circle), reflecting a high weight of vision. P targets were at the origin.
iv. VP endpoints continue to be closer to V endpoints in late adaptation. The shift of P endpoints (red circle) in the positive y direction reflects proprioceptive realignment or motor adaptation.
B. Patient CB7 in the Sensorimotor experiment.
i. VP endpoints (purple dashed line) followed the V component of VP targets (solid grey line), reflecting that the subject succeeded in hitting or nearly hitting the target. P endpoints changed less than the control example from early to late adaptation (ΔP = 11.1 mm).
ii. The subject relied heavily on vision, with Wv = 0.76 late in the adaptation block; VP endpoints (purple circle) fell closer to V endpoints (blue circle) than to P endpoints (red circle), both early (iii.) and late (iv.) in adaptation.
C. Significant realignment occurred in controls (ΔP = 28.0 mm, t = 7.1, p < 0.0001, N = 10), probably reflecting both sensory realignment of the proprioceptive estimate of target hand position and motor adaptation of the reaching hand (Block & Bastian, 2011).
D. Significant realignment occurred in cerebellar patients as well (ΔP = 16.1 mm, t = 4.8, p = 0.001, N = 10), but it was significantly less than controls (t = −2.3, p = 0.035). We speculated that the realignment exhibited by patients represents sensory realignment of proprioception and not motor adaptation of the reaching hand, since cerebellar patients are impaired at motor adaptation (Motor experiment). *significantly different from zero.
E. Weight of vision in late adaptation (Wv) versus realignment of P endpoints (ΔP) for cerebellar patients (grey circles) and age-matched controls (black circles). Cerebellar patients tend to have a smaller ΔP, but (F) mean Wv in early and late adaptation is similar for patients and controls. Two-way ANOVA showed no difference across groups (p=0.36) or time (p=0.13). This suggests weighting of vision vs. proprioception may not be a cerebellum-dependent process.
| (2) |
The realignment calculation was not unduly influenced by the shifting visual target, fixation cross position, or subjects’ memory of reaching distance (Appendix). In the Sensory experiment, with no endpoint visual feedback to encourage subjects to regard vision as more correct than proprioception, there is no reason that vision would not realign as much as or more than proprioception. Because we only evaluate ΔP, we can compare ΔP across groups within each experiment but not across experiments.
2.3.4. Weighting of vision vs. proprioception (Wv)
We also evaluated how much subjects relied upon vision versus proprioception (Block and Bastian, 2010; Block and Bastian, 2011). We relied on the fact that reaches to targets of different modalities are biased in different directions (e.g., Foley and Held, 1972; Crowe et al., 1987; Haggard et al., 2000; Smeets et al., 2006). We reasoned that on VP reaches, subjects would point closer to their mean V endpoint position if they were assigning more weight to vision, and closer to their mean P endpoint position if they were assigning more weight to proprioception (e.g., Figure 3A.iii). If Wv is experimental weight of vision and Wp is experimental weight of proprioception:
| (3) |
| (4) |
For simplicity, we will refer to weights only in terms of vision (Wv). We computed a separate Wv for every VP reach: For the Wv associated with the ith VP reach (VPi), we used the mean position of the four V and four P endpoints occurring closest in time, and compared these two positions to the mean position of VPi, VPi−1 and VPi+1. Thus, we could estimate the weight of vision on a trial-by-trial basis, a time scale at which the change in VP misalignment is very small and any realignment is likely to be very small as well. Subjects’ weights were not unduly affected by the endpoint error feedback in Experiment 2 or the modality of the previous target (Appendix).
2.4 Statistical analysis
To determine whether patients and controls learned differently in the Motor experiment, we used a two-sample t-test to determine whether aftereffect was different in controls vs. patients, and one-sample t-tests to determine if each group aftereffect (early post-adaptation) was different from zero. To test whether controls and patients weighted vision comparably in the Sensorimotor experiment, we compared Wv in controls versus patients with a two–sample t-test. To determine if patients and controls shifted their P endpoints comparably in the Sensorimotor and Sensory tasks, we used t-tests in each experiment to determine if group mean ΔP was different from zero, and if ΔP was different for patients vs. controls. To assess whether the patients who shifted their P endpoints most in the Sensorimotor experiment were the least impaired at prism adaptation in the Motor experiment, we calculated a correlation coefficient for prism aftereffect versus Sensorimotor ΔP in patients. To determine if the variance of reaching endpoints is similar in patients and controls in the Sensorimotor experiment, we performed a 2-way ANOVA, with group and target type as factors. Finally, to find out if cerebellar patients maintain the relationship between sensory weighting and realignment we have seen previously (Block and Bastian, 2011), we calculated correlation coefficients for Wv early in Sensory experiment baseline and throughout adaptation versus ΔP in patients. All tests were performed two-sided.
3. Results
3.1 Motor experiment (throwing with prisms)
Cerebellar patients are impaired at prism adaptation
After a baseline (Figure 1A.i and 1B.i), subjects wore prism goggles that shifted the visual field to the right. This caused large rightward errors at the beginning of the adaptation block (Figure 1A.ii and 1B.ii). Healthy control subjects were able to correct their errors and return to baseline levels by the end of adaptation (Figure 1B.ii), and when the prism goggles were removed, they made large leftward errors (i.e. negative after-effects, Figure 1B.iii). This average negative aftereffect of 21.1 cm was significantly different from zero (t(11) = −8.99, p < 0.00001, Figure 1C), indicating that a new relationship between gaze direction and throw direction had been learned and stored. The cerebellar patients also reduced their rightward errors somewhat during the adaptation block (Figure 1C.ii), but error reduction can have a conscious, cognitive component (Weiner et al., 1983). A negative aftereffect is regarded as a sign of true adaptation, as the initial error after prisms are removed is not subject to conscious control (Weiner et al., 1983). In this regard, cerebellar patients were impaired; negative aftereffect was present (mean 8.1 cm; Figure 1D), but it was not statistically significant (t(11) = −2.03, p = 0.07). Negative aftereffect in cerebellar patients was, however, different from negative aftereffect in healthy controls (two-sample t(22) = 2.81, p = 0.01). Thus, cerebellar patients show abnormal prism adaptation.
3.2 Sensorimotor experiment (reaching with endpoint visual feedback)
Cerebellar patients adapt less than controls when both sensory and motor adaptation are possible
When subjects received endpoint visual feedback after reaches to misaligned VP targets, two potential sources of error information were available: explicit endpoint visual feedback relative to the V component of the target, which could drive motor adaptation of the reaching hand, and the misalignment of proprioceptive and visual estimates of target hand position, which could drive proprioceptive realignment.
A typical healthy control subject responded to the misalignment by shifting proprioceptive endpoints (red dashed line deviates from grey dashed line, Figure 3Ai) and relied heavily on vision (Figure 3Aii, weighting calculation illustrated in iii–iv). Cerebellar patients were able to perform the task without difficulty—indeed their endpoints were not more variable than controls when reaching to any of the targets (ANOVA group effect p > 0.7). However, their performance differed from controls in some respects. For example, patient CB7 succeeded in pointing at the V component of VP targets (purple dashed line follows solid grey line, Figure 3B.i) similarly to controls (e.g., Figure 3Ai). Patient CB7 also relied more on vision than proprioception (high Wv, Figure 3B.ii–iv), but shifted his P endpoints less than most controls (ΔP as shown by red dotted line, Figure 3B.i).
As a group, cerebellar patients shifted their P endpoints significantly (Figure 3D; ΔP = 16.1 mm, t(9) = 4.8, p = 0.001), but markedly less than controls did (Figure 3C; ΔP = 28.0 mm, two-sample t(18) = −2.3, p = 0.035). In a plot of ΔP vs. Wv in late adaptation (Figure 3E), cerebellar patients (grey circles) and controls (black circles) overlap substantially. While the two groups differ in ΔP, reliance on vision versus proprioception is similar for patients and controls both early and late in adaptation (Figure 3F), suggesting that sensory weighting may not be a cerebellum-dependent process.
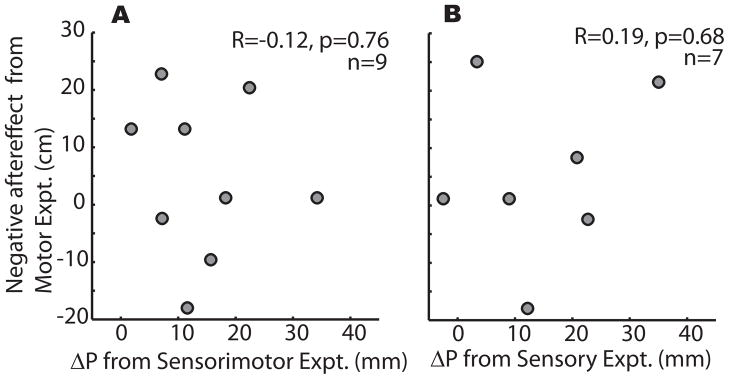
It is apparent from Figure 3E that ΔP has a substantial range (e.g., 1.8 – 34.2 mm in patients). We wondered if perhaps the patients who had a high ΔP were the ones who were least impaired at prism adaptation in the Motor experiment. However, there was no relationship between prism negative aftereffect and ΔP in cerebellar patients (correlation R = −0.12, p > 0.75; Figure 4A). This suggests that cerebellar patients had a smaller ΔP than controls in the Sensorimotor experiment because they did not rely on motor adaptation as much as controls did. We hypothesized that if cerebellar patients are impaired at the motor adaptation component of ΔP, then perhaps the sensory realignment component of ΔP is intact. This would account for the significant ΔP in cerebellar patients in Experiment 2, as well as the lack of correlation between ΔP (sensory realignment) and prism negative aftereffect (motor adaptation) in cerebellar patients.
Figure 4.
Cerebellar patients who were least impaired at prism adaptation were not the ones who exhibited the greatest shift in proprioceptive target endpoints during reaching in either the Sensorimotor (A) or Sensory (B) experiment. Patients had significantly impaired motor adaptation, as seen in the Motor experiment; the lack of correlation here suggests that patients relied on a different, non-cerebellum dependent process in the Sensorimotor and Sensory experiments.
3.3 Sensory experiment (reaching without endpoint visual feedback)
In a pure sensory realignment task, cerebellar patients are unimpaired
To determine if cerebellar patients are impaired at sensory realignment as well as motor adaptation, no endpoint visual feedback was given in the Sensory experiment. Thus, no signal to drive motor adaptation was present, and the only available error signal was the visuoproprioceptive misalignment of target hand position estimates, which would lead to sensory realignment. Much to our surprise, cerebellar patients were unimpaired in this situation: Cerebellar patients shifted their P endpoints as much as controls (e.g., red dashed line in Figure 5A and 5B). This is reflected in group data, with ΔP = 13.0 mm for controls (Figure 5C) and 14.6 mm for patients (Figure 5D). Both values were significantly different from zero (t(6) = 2.7, p = 0.034 for controls; t(6) = 3.0, p = 0.024 for patients), but not from each other (t(12) = 0.22, p = 0.83). This suggests that sensory realignment is not a cerebellum-dependent process. Indeed, when we did a power analysis to determine how many subjects we would need to detect a difference in the Sensory experiment, we found that it would require groups of 990 patients and controls (assumptions: mean difference is 1.6 mm, probability (power) of 0.8 and Type 1 error probability 0.05). In addition, there was no relationship between ΔP (sensory realignment) and prism negative aftereffect (motor adaptation) for cerebellar patients (correlation R = 0.19, p > 0.68; Figure 4B), further supporting the idea that sensory realignment is neurologically independent of motor adaptation.
Figure 5. Sensory experiment results (reaching without endpoint visual feedback).
A. A healthy control subject in the Sensory experiment, age 53.
i. P endpoints shifted during the adaptation block (ΔP = 14.0 mm), reflecting proprioceptive realignment. ii. This subject relied heavily on vision by late adaptation (Wv = 0.68). iii. Early in adaptation, VP endpoints (purple circle) were about equally distant from V (blue circle) and P endpoints (red circle), resulting in a Wv near 0.5. iv. By late adaptation, VP endpoints were closer to V endpoints, resulting in a higher Wv.
B. Patient CB02 in the Sensory experiment.
i. P endpoints shifted during the adaptation block (ΔP = 22.9 mm), reflecting proprioceptive realignment. ii. This subject relied heavily on vision by late adaptation (Wv = 0.70). iii–iv. VP endpoints (purple circle) varied throughout adaptation in their proximity to V and P endpoints, sometimes being closer to V endpoints (iii) and other times equidistant between them (iv).
C. Significant sensory realignment of proprioception occurred in controls (ΔP = 13.0 mm, t = 2.7, p = 0.033, N = 7).
D. Significant sensory realignment of proprioception occurred in cerebellar patients as well (ΔP = 14.6 mm, t = 3.0, p = 0.024, N = 7) and was not significantly different from controls (t = 0.22, p = 0.83), suggesting that sensory realignment is not a cerebellum-dependent process.
*significantly different from zero.
Finally, we wanted to know if cerebellar patients had the relationship between Wv and ΔP we have previously observed: in a similar experiment with 19 healthy controls, there was a significant correlation between Wv and ΔP (r = 0.55, p = 0.01), such that subjects who weighted vision higher tended to realign proprioception more (Block and Bastian, 2011). This is consistent with the idea that the lower-weighted modality will realign more (Ghahramani et al., 1997). Cerebellar patients in the present study appear to have a similar relationship between weight and realignment, whether ΔP is compared to Wv early in baseline (r = 0.62, p = 0.14), to Wv early in adaptation (r=0.74, p = 0.056), or to Wv averaged across adaptation (r = 0.84, p = 0.017). This suggests that the cerebellum is not required for the weight-realignment relationship we previously observed in healthy controls (Block and Bastian, 2011).
4. Discussion
We and others have shown that individuals with cerebellar damage are impaired at recalibrating the relationship between gaze and throw direction when a prismatic perturbation is introduced. While prism adaptation can affect vision and proprioception as well as motor commands, we used the task paradigm described by Martin et al. (1996b), who showed that the pattern of generalization from this task is inconsistent with visual or proprioceptive recalibration. The authors concluded that the adaptation was mostly motor (Martin et al. 1996b), thus we interpret the cerebellar impairment in prism adaptation in the present study as a deficit in motor adaptation. Within the same subjects, we find intact ability to perform visuoproprioceptive realignment similarly to healthy controls in a reaching task: impairment was seen only when motor adaptation was possible in addition to sensory realignment. This suggests that unlike motor adaptation, sensory realignment is not a cerebellum-dependent process.
Motor adaptation is a well-studied phenomenon. When a mismatch is detected between the predicted and actual sensory outcome of a motor command, the command is adjusted accordingly (e.g., Tseng et al., 2007). Storage of a new relationship between motor command and sensory outcome is assessed by looking for an aftereffect, and is the only robust test of whether motor adaptation has occurred. This is because cerebellar patients are known to be able to use a conscious aiming strategy to reduce error during adaptation, which does not lead to storage of a new motor command (Taylor and Ivry, 2011). Cerebellum-dependent motor adaptation has been found in reaching (e.g., Weiner et al., 1983; Baizer et al., 1999; Tseng et al., 2007), walking (Morton and Bastian, 2006), and throwing (Martin et al., 1996a). When a visuomotor perturbation is introduced in a throwing task by placing prisms over the subject’s eyes, for example, cerebellar patients do not recalibrate gaze direction and throw direction as much as controls do (Martin et al., 1996a), a finding we confirmed in Experiment 1 of the present study.
However, the cerebellum has been linked to sensory as well as motor processing. Gao et al. (1996) found, by imaging of the dentate nucleus, that the lateral cerebellum is activated by the acquisition and discrimination of sensory information. In a study of illusory hand flexion with congruent or incongruent visual feedback, Hagura et al. (2008) determined that activity in the posterolateral cerebellum was correlated with the subjective sensory perception of hand movement, suggesting that this region of the cerebellum is involved in multisensory processing. Indeed, it has been proposed that the lateral cerebellar zones are involved exclusively in sensory processing and not motor control (Parsons et al., 1997). Synofzik et al. (2008) found that patients with cerebellar lesions were impaired at predicting visual consequences of movement and suggest that the cerebellum is important for perceptual learning.
We therefore wondered if the cerebellum might be important for sensory realignment, when the spatial relationship between different sensory estimates is changed (e.g., the proprioceptive estimate of hand position may be realigned to more closely match the visual estimate or vice versa; van Beers et al., 2002). The cerebellum receives information from virtually every sensory modality (Brodal, 1978), including vision (Snider and Stowell, 1944; Glickstein, 2000) and proprioception (Bauswein et al., 1983; Donga and Dessem, 1993), so it is conceivable that different sensory estimates could be compared by the cerebellum. Further, characteristics of the complex spikes carried to cerebellar cortex by olivary climbing fibers (reviewed by Ito, 2001) make them possible candidates for an error signal arising from any mismatch between sensory modalities. The cerebellum also receives projections from premotor and association areas of cortex, such as posterior parietal cortex (Brodal, 1978) and projects to motor and premotor areas. Thus, other brain regions that are concerned with the spatial relationships among sensory modalities could theoretically have access to information processed by cerebellar circuits.
In the Sensorimotor experiment (reaching with endpoint visual feedback), we found that patients with bilateral cerebellar ataxia weighted vision and proprioception similarly to controls, suggesting that sensory weighting, at least, is not a cerebellum-dependent process. As a misalignment between visual and proprioceptive estimates of target hand position was gradually imposed, patients and controls both changed the extent of their reaches to unimodal proprioceptive targets in the direction of the perturbation, suggesting some adaptive process was at work. However, the effect was significantly smaller in patients than controls. We have previously found that in controls, the change in reach endpoints for P targets in the Sensorimotor experiment reflects both motor adaptation of the reaching hand and sensory realignment affecting the target hand. The former was presumably driven by the explicit error signal provided by endpoint visual feedback, and the latter by the mismatch imposed between visual and proprioceptive estimates of target hand position (Block and Bastian, 2011). Given that cerebellar patients are impaired at motor adaptation (Motor experiment, throwing with prisms), we speculated that the change in P endpoints for patients in the Sensorimotor experiment reflects mostly sensory realignment. In other words, the patients had a smaller change in P endpoints than controls because they had the sensory realignment component but largely lacked the cerebellum-dependent motor adaptation component exhibited by controls.
A reasonable question is whether the difference could instead be due to the dissimilarity of the two tasks: the perturbation was gradual in the Sensorimotor experiment (reaching with endpoint visual feedback), but sudden in the Motor experiment (throwing with prisms). This raises the possibility that cerebellar patients shifted their P endpoints in the Sensorimotor experiment because the gradual perturbation was less difficult or less cerebellum-dependent, and not because they were using sensory realignment rather than motor adaptation. Criscimagna-Hemminger et al. (2010) found that cerebellar patients were less impaired at force field learning when the perturbation was gradual as opposed to abrupt. It is possible that the adaptation exhibited by cerebellar patients in the Sensorimotor experiment had both sensory and motor components, but regardless, they adapted significantly less than healthy controls, and the Sensory experiment suggests their deficit is not in sensory realignment. Also, Robertson and Miall (1999) have shown by reversibly inactivating monkey dentate that the cerebellum is just as crucial for gradual visuomotor adaptation, a task more similar to the present study, as for sudden, if not more so. It therefore seems unlikely that the difference in perturbation can account for the differences in patient performance between the Motor and Sensorimotor experiments.
Another question to consider is whether patients were impaired in the Sensorimotor experiment because they had difficulty using the visual error feedback, which was not present in the Sensory experiment, rather than because they were impaired at motor adaptation. Data from the Motor experiment suggests that the patients in this study did not have difficulty responding to visual error feedback, however. The patients changed their behavior to throw closer to the target when wearing prisms. The prism literature (e.g., Welch 1986) indicates that when people adapt to prisms, some amount of cognitive correction for visual errors can occur on top of the visuomotor adaptation. This is why negative aftereffects are the only “proof” that visuomotor adaptation (and not just a cognitive correction strategy) has occurred. Given that their negative aftereffects were small, the cerebellar patients in the Motor experiment appear to have been relying heavily on such cognitive strategies during adaptation (i.e., using the visual feedback to make corrections), consistent with a recent report by Taylor and Ivry (2011). The fact that patients could use this type cognitive strategy with prisms, even though they did not learn and store the new calibration, suggests that they do not have difficulty responding to visual error feedback.
The results of the Sensory experiment (reaching without endpoint visual feedback) demonstrate that, in the context tested here, cerebellar patients are unimpaired at sensory realignment. Recall that the Sensory experiment was identical to the Sensorimotor experiment, except no endpoint visual feedback was given, so the explicit error signal needed to drive motor adaptation was absent, and any shift in P endpoints can be interpreted as a change in the proprioceptive estimate of target hand position, i.e., sensory realignment (Block and Bastian, 2011). In this situation, patients shifted P endpoints just as much as controls did, suggesting that sensory realignment is not dependent on the cerebellum.
Further support of this comes from the lack of correlation between prism adaptation negative aftereffect (Motor experiment) and the shifting of proprioceptive target endpoints (Sensorimotor and Sensory experiments). i.e., the patients who were least impaired at prism adaptation were not the ones who exhibited the greatest shift in proprioceptive target endpoints during reaching. The lack of correlation suggests that in the Sensorimotor and Sensory experiments, cerebellar patients relied primarily on a process that is not cerebellum-dependent; i.e., sensory realignment.
Given the lesions of the patients we tested, we can speculate that sensory realignment requires neither the cerebellar cortex nor the deep cerebellar nuclei, nucleo-olivary pathway, nor dentate-to-thalamocortical pathways. We propose that sensory realignment may depend instead on regions within posterior parietal cortex (PPC). Many regions within PPC are multimodal; that is, they respond to more than one sensory modality. This multimodal quality is widely thought to be the basis for sensory integration (e.g., Grefkes and Fink 2005). Additionally, a neuroimaging study by Clower et al. (1996) suggested that activity in a region of PPC could represent proprioceptive realignment in response to continual prism perturbations. This activity was located in an area within PPC along the intraparietal sulcus in the angular gyrus.
In sum, we have shown that cerebellar patients with motor adaptation impairments do not have sensory realignment deficits. This suggests that unlike motor adaptation, sensory realignment is not cerebellum-dependent. These results have implications for our understanding of sensorimotor processing as well as the capacity for adaptation of individuals with cerebellar damage. That sensory realignment and motor adaptation rely on different neural substrates raises interesting questions for future study. It seems likely that the two processes may operate at the same time in any sensorimotor task where a sensory misalignment occurs and explicit error feedback is available, as in the Sensorimotor experiment. Sensory weightings also appear to operate in support of hitting the target in this situation (Block and Bastian, 2011). If the neural bases of all three processes can be found, the mechanisms and relationships among them may be better clarified, which could help answer fundamental questions about how sensory and motor processes interact when the brain is confronted with a sensory perturbation, and also provide direction in rehabilitation of patient groups with sensorimotor deficits.
Here we asked if the cerebellum is important for sensory as well as motor adaptation.
We studied cerebellar patients reaching to misaligned visuoproprioceptive targets.
Our cerebellar patients adapted sensory alignment as much as controls.
The same patients were impaired at motor adaptation.
Sensory adaptation is not cerebellum-dependent, unlike motor adaptation.
Acknowledgments
Funding
This work was supported by National Institutes of Health Grants R01-HD- 040289 and 1-F31-NS-061547-01.
Appendix
We assessed several possible extraneous influences on our determination of weighting and realignment in the Sensorimotor and Sensory experiments (reaching with and without endpoint visual feedback).
A.1. Sensory weights were not affected by previous target modality or reaching error
We wondered whether weight of vision (Wv) calculated for a given VP reach could be affected by the modality of the previous target, i.e., if Wv was higher on VP reaches that followed V reaches, and lower on VP reaches that followed P reaches. To find out, we divided VP endpoints into those that occurred after a V reach and those that occurred after a P reach. For both sets, we did an alternate Wv calculation using only one VP endpoint instead of the mean of three (see Methods section 2.3.4). For each subject, we tested to see if the two populations of Wv were significantly different (2-sample t-tests with n1 = n2 = 22 VP reaches per subject). Wv was not significantly higher after V reaches for any patient or control in either the Sensorimotor or the Sensory experiments, suggesting that subjects’ weights were not substantially affected by the modality of the previous target.
With regard to the Sensorimotor experiment only, there is a question of whether the errors subjects saw on VP reaches (endpoint visual feedback in relation to V component of the target) substantially influenced the subsequent V or P reach. The random changes in start position, target position, and fixation cross position between every reach were intended to minimize this possibility. But to make sure, we divided VP reaches into those that occurred before a V reach and those that occurred before a P reach. We used the VP endpoint error seen by subjects (distance from reach endpoint to the V component of the target) and calculated the correlation with the reach error on the subsequent V or P reach (actual reach endpoint, not seen by subjects). We calculated separate correlations for errors in x and y. Two patients and two controls had a significant negative correlation between P and VP errors in y (i.e. the error observed on a VP reach may have caused the subject to reach in the opposite direction on the subsequent P reach). Results were not substantially altered by the removal of these 4 subjects, however. We therefore do not think the VP reaching error subjects observed was an important influence on subsequent reaching endpoints to unimodal targets, or, by extension, on our calculation of weighting and realignment.
A.2. Shifting of P endpoints (ΔP) was not influenced by fixation cross or remembered V target position or distance
Since the fixation cross, on average, was shifting position in the positive y direction along with the V target, any influence of fixation cross position on subjects’ reaching endpoints could cause a shift in P endpoints (ΔP) independent of sensory realignment of the proprioceptive estimate of target hand position. To rule out this possibility, we calculated the regression coefficient between fixation cross x coordinates and P endpoint x coordinates (R2fixation cross) as a way to estimate the influence of fixation cross position on P reaching endpoints. We considered only the x coordinates because the y coordinates should be related whether or not ΔP is influenced by the fixation cross, since both shift in the positive y direction during the adaptation block. Mean R2fixation cross was 0.08 and 0.11 for patients and controls in the Sensorimotor experiment, and 0.14 and 0.06 for patients and controls in the Sensory experiment. We also checked to see if R2fixation cross was correlated with ΔP (i.e., do subjects with higher R2fixation cross have larger ΔP). There was no correlation in the Sensorimotor experiment (R = 0.36, p = 0.31 for patients; R = 0.05, p = 0.90 for controls) or the Sensory experiment (R = 0.21, p = 0.65 for patients; R = 0.29, p = 0.52 for controls). This indicates that the realignment we measured as ΔP was not an artifact of the shifting fixation cross zone.
A second possible explanation for ΔP is that on reaches to P targets, subjects were simply reaching the remembered distance to the most recent (shifted) V target. We do not think this could account for ΔP, however, because (a) no subject was consciously aware of any shift in V targets in the direction of the misalignment, and (b) the arrangement of start and target positions was such that the direction and distance between start position and target was highly variable throughout the experiment, and did not change significantly from early to late adaptation (rank sum test p > 0.1).
A third explanation might be that on P reaches, subjects were pointing to the remembered location of the most recent V target (or V component of VP target), causing P endpoints to shift along with the V target. We thought this possibility unlikely because of the random changes in target position, starting position, and fixation position between every reach, but to be certain we examined the relationship between P endpoint x coordinates and the x coordinate of the V component of the VP target immediately preceding, throughout baseline and adaptation blocks (recall that P targets are always preceded by VP targets). The mean regression coefficient for this relationship (with P endpoint x coordinates as the dependent variable) was very small in all four groups (0.09 and 0.02 for patients and controls in the Sensorimotor experiment; 0.08 and 0.06 for patients and controls in the Sensory experiment) No subject had a significant positive correlation between the two variables, signifying that subjects did not point to the remembered location of V targets on P reaches, so this cannot account for the ΔP we measured.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Albus JS. A theory of cerebellar function. Mathematical Biosciences. 1971;10:25–61. [Google Scholar]
- Baizer JS, Kralj-Hans I, Glickstein M. Cerebellar lesions and prism adaptation in macaque monkeys. Journal of Neurophysiology. 1999;81:1960–1965. doi: 10.1152/jn.1999.81.4.1960. [DOI] [PubMed] [Google Scholar]
- Bauswein E, Kolb FP, Leimbeck B, Rubia FJ. Simple and complex spike activity of cerebellar Purkinje cells during active and passive movements in the awake monkey. Journal of Physiology. 1983;339:379–394. doi: 10.1113/jphysiol.1983.sp014722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays PM, Wolpert DM. Computational principles of sensorimotor control that minimize uncertainty and variability. Journal of Physiology. 2007;578:387–396. doi: 10.1113/jphysiol.2006.120121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block HJ, Bastian AJ. Sensory reweighting in targeted reaching: effects of conscious effort, error history, and target salience. Journal of Neurophysiology. 2010;103:206–217. doi: 10.1152/jn.90961.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block HJ, Bastian AJ. Sensory weighting and realignment: Independent compensatory processes. Journal of Neurophysiology. 2011;106:59–70. doi: 10.1152/jn.00641.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978;101:251–283. doi: 10.1093/brain/101.2.251. [DOI] [PubMed] [Google Scholar]
- Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP, Alexander GE. Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature. 1996;383(6601):618–621. doi: 10.1038/383618a0. [DOI] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. Journal of Neuroscience. 2001 Aug 15;21(16):6283–91. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe A, Keessen W, Kuus W, van VR, Zegeling A. Proprioceptive accuracy in two dimensions. Perceptual and Motor Skills. 1987;64:831–846. doi: 10.2466/pms.1987.64.3.831. [DOI] [PubMed] [Google Scholar]
- Donga R, Dessem D. An unrelayed projection of jaw-muscle spindle afferents to the cerebellum. Brain Research. 1993;626:347–350. doi: 10.1016/0006-8993(93)90601-i. [DOI] [PubMed] [Google Scholar]
- Foley JM, Held R. Visually directed pointing as a function of target distance, direction, and available cues. Perception & Psychophysics. 1972;12:263–268. [Google Scholar]
- Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–547. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- Ghahramani Z, Wolpert DM, Jordan MI. Computational models for sensorimotor integration. In: Morasso PG, Sanguineti V, editors. Self-Organization, Computational Maps and Motor Control. Amsterdam: North-Holland; 1997. pp. 117–147. [Google Scholar]
- Glickstein M. How are visual areas of the brain connected to motor areas for the sensory guidance of movement? Trends in Neurosciences. 2000;23:613–617. doi: 10.1016/s0166-2236(00)01681-7. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. Journal of Anatomy. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P, Newman C, Blundell J, Andrew H. The perceived position of the hand in space. Perception & Psychophysics. 2000;62:363–377. doi: 10.3758/bf03205556. [DOI] [PubMed] [Google Scholar]
- Hagura N, Oouchida Y, Aramaki Y, Okada T, Matsumura M, Sadato N, Naito E. Visuokinesthetic Perception of Hand Movement Is Mediated by Cerebro-Cerebellar Interaction between the Left Cerebellum and Right Parietal Cortex. Cerebral Cortex. 2009;19:176–86. doi: 10.1093/cercor/bhn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. The symptoms of acute cerebellar injuries. Brain. 1917;40:461–535. [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiological Reviews. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. Journal of Physiology. 1969;202(2):437–70. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996a;119 ( Pt 4):1183–1198. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996b;119 ( Pt 4):1199–1211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. Journal of Neuroscience. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LM, Bower JM, Gao JH, Xiong J, Li J, Fox PT. Lateral cerebellar hemispheres actively support sensory acquisition and discrimination rather than motor control. Learning and Memory. 1997;4:49–62. doi: 10.1101/lm.4.1.49. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Miall RC. Visuomotor adaptation during inactivation of the dentate nucleus. Neuroreport. 1999;10:1029–1034. doi: 10.1097/00001756-199904060-00025. [DOI] [PubMed] [Google Scholar]
- Smeets JB, van den Dobbelsteen JJ, de Grave DD, van Beers RJ, Brenner E. Sensory integration does not lead to sensory calibration. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18781–18786. doi: 10.1073/pnas.0607687103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider RS, Stowell A. Receiving areas of the tactile, auditory, and visual systems in the cerebellum. Journal of Neurophysiology. 1944;7:331–357. [Google Scholar]
- Synofzik M, Lindner A, Their P. The cerebellum updates predictions about the visual consequences of one’s behavior. Current Biology. 2008;18:814–818. doi: 10.1016/j.cub.2008.04.071. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Flexible cognitive strategies during motor learning. Public Library of Science Computational Biology. 2011;7(3):e1001096. doi: 10.1371/journal.pcbi.1001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annual Reviews Neuroscience. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben HM, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. Journal of the Neurological Sciences. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. Journal of Neurophysiology. 2007;98:54–62. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Sittig AC, Gon JJ. Integration of proprioceptive and visual position-information: An experimentally supported model. Journal of Neurophysiology. 1999;81:1355–1364. doi: 10.1152/jn.1999.81.3.1355. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Wolpert DM, Haggard P. When feeling is more important than seeing in sensorimotor adaptation. Current Biology. 2002;12:834–837. doi: 10.1016/s0960-9822(02)00836-9. [DOI] [PubMed] [Google Scholar]
- Wann JP, Ibrahim SF. Does limb proprioception drift? Experimental Brain Research. 1992;91:162–166. doi: 10.1007/BF00230024. [DOI] [PubMed] [Google Scholar]
- Weiner MJ, Hallett M, Funkenstein HH. Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology. 1983;33:766–772. doi: 10.1212/wnl.33.6.766. [DOI] [PubMed] [Google Scholar]
- Welch RB. Perceptual Modification. New York: Academic Press; 1978. [Google Scholar]