Abstract
Psychological stress is a risk factor to develop musculoskeletal pain of the head and neck; however, the basis for this relationship remains uncertain. This study tested the hypothesis that psychophysical stress alone was sufficient to alter the encoding properties of spinomedullary dorsal horn neurons and masseter muscle activity in male rats. Repeated forced swim conditioning increased markedly both the background firing rate and temporomandibular joint (TMJ)-evoked activity of neurons in deep dorsal horn, while neurons in superficial laminae were less affected. Stress also increased the responses to stimulation of facial skin overlying the TMJ of neurons in deep and superficial dorsal horn. TMJ-evoked masseter muscle activity was enhanced significantly in stressed rats, an effect that was reduced by prior blockade of the spinomedullary junction region. These data indicated that repeated psychophysical stress induced widespread effects on the properties of medullary dorsal horn neurons and masseter muscle activity. The effects of stress were seen preferentially on neurons in deep dorsal horn and included enhanced responses to chemosensory input from the TMJ and mechanical input from overlying facial skin. The stress-induced elevation in TMJ-evoked masseter muscle activity matched well the changes seen in dorsal horn neurons. It is concluded that the spinomedullary junction region plays a critical role in the integration of psychophysical stress and sensory information relevant for nociception involving deep craniofacial tissues.
Keywords: pain, forced swim stress, trigeminal subnucleus caudalis, temporomandibular joint, masseter muscle
Introduction
A link between psychological state and chronic pain in deep tissues has long been appreciated; however, the mechanisms that underlie this relationship remain uncertain (Merskey et al., 1996: Yunus, 2007; Keefe & Somers, 2010). Psychological factors influence orofacial pain expression (Aggarwal et al., 2010; Benoliel et al., 2011) and appear to have a prominent effect on temporomandibular joint/muscle disorders (TMJD), a family of conditions involving the temporomandibular joint (TMJ) and masticatory muscles (Huang et al., 2002; Korszun, 2002, Slade et al., 2007; Kanehira et al., 2008; Maixner, 2009). Several aspects of TMJD pain suggest a central neural origin: TMJD pain patients often present with no overt signs of injury or inflammation, pain expression is often described as fluctuating and non-progressive (Ohrbach & Dworkin, 1998; Rammelsberg et al., 2003) and behavioral therapies rather than pharmacological treatments often provide the most successful approaches to pain management (List & Axelsson, 2010). Although chronic pain and psychological distress share features consistent with a central origin such as altered stress hormone secretion and autonomic nervous system dysfunction (Yunus, 2007; Maixner, 2009; McEwen & Kalia 2010), the neural substrate that underlies the integration of psychological stress with pain processing remains poorly defined.
Several models have been used to demonstrate that repeated stress modifies nociceptive processing and aversive behavior in animals (see Imbe, 2006); however, few studies have tested the effects of stress against measures of TMJ nociception and jaw-specific function (Huang et al., 2011). The present study used an established model for psychophysical stress, repeated forced swim conditioning (FS) that produces persistent muscle and cutaneous hyperalgesia at spinal levels (Quintero et al., 2000, 2011, Suarez-Roca et al., 2006a,b). The effects of FS were assessed on the encoding properties of spinomedullary dorsal horn neurons excited by TMJ stimulation and on masseter muscle electrical activity in the rat. The spinomedullary region, the junction of the trigeminal subnucleus caudalis (Vc) and the upper cervical cord junction (Vc/C1-2), is the main termination site for sensory fibers supplying the lower jaw (Hathaway et al., 1995; Takeshita et al., 2001; Shigenaga et al., 1986, 1988; Dessem et al., 2007). Particular emphasis was directed at the effects of FS on spinomedullary neurons in superficial versus deep laminae since previous studies have reported distinct differences suggesting that neurons in each region contribute to different aspects of nociceptive processing in trigeminal (Tashiro et al., 2007) and spinal systems (Suzuki et al., 2002; Eckert et al., 2006). Masseter muscle electromyographic (EMG) activity was monitored as an index of peripheral jaw muscle reflex behavior. Altered levels of EMG activity have been linked to orofacial pain conditions in humans (Wang et al., 2004; Bodere et al., 2005; Peck et al. 2008), while jaw muscle EMG has been used extensively as an index of jaw-related behavior in animal models of craniofacial pain (Yu et al. 1995; Cairns et al. 1998).
Materials and methods
The protocols were approved by the Institutional Animal Care and Use Committee of University of Minnesota and conformed to established guidelines set by The National Institutes of Health guide for the care and use of laboratory animals (PHS Law 99-158, revised 2002). All efforts were made to minimize the number of animals used for experiments and their suffering.
General design and conditioning protocol-neurophysiology
Male rats (250-400 g, Sprague-Dawley, Harlan, Indianapolis) were maintained on a 12:12 h light/dark cycle with lights on at 08:00 and given free access to food and water. Rats were exposed to repeated forced swim conditioning (FS) by placement in a plastic cylinder (diameter 30 cm, height 50 cm) containing 20 cm water (24-26°C) for 10 min between 09:00 and 11:00 for three days (Days -3, -2 and -1) as described previously (Quintero et al., 2000, 2003; Duenes et al., 2010). Sham rats served as controls and were placed in an empty swim chamber using the same schedule. During the swim session “immobility” and “struggling” times were recorded (Porsolt et al., 1977). Immobility was defined as time spent in minimal bodily movement to maintain its head above water, whereas struggling was defined as active diving, jumping, or vigorously moving its limbs to break the surface of the water and attempting to escape the container. Rats were dried in a warm environment (30-33°C) after each swim session and fresh water was used for each rat. On Day 0, rats were anesthetized and prepared for neural or muscle recording.
Measurement of grip force-neurophysiology
Forelimb grip force was measured each day before the swim session and on the day of recording (Kehl et al., 2000). Briefly, the rat was held by the tail and passed gently (~10 cm/s) three times over a wire mesh grid attached to a strain gauge. The maximum forelimb grip force (GF) observed during the 3 trials was determined for each of the 4 days and averaged daily for each rat.
Extracellular recordings
Rats were anesthetized initially with pentobarbital sodium (70 mg/kg, i.p.) and respired artificially with isoflurane (1.5~2.0%) and oxygen-enriched room air after tracheotomy. Catheters were placed in the right femoral artery (blood pressure monitor) and right jugular vein (infusion of gallamine triethiodide). Anesthesia was maintained after surgery with isoflurane (1.2-2.0%) and a paralytic agent, gallamine triethiodide (15-20 mg/kg/h) was infused after completion of all surgical procedures and immediately prior to the recording session. Adequate depth of anesthesia was confirmed by the absence of corneal and hind limb withdrawal reflexes prior to gallamine, fully constricted pupils and constant arterial blood pressure and heart rate. Expiratory end-tidal CO2 (3.5-4.5%) and mean arterial pressure (MAP, 90-120mmHg) were monitored throughout the experiment and body temperature was maintained 38°C with a heating blanket and thermal probe.
Animals were placed in a stereotaxic frame and portions of the C1 and C2 vertebrae were removed to expose the spinomedullary junction Vc/C1-2 region and bathed in warm mineral oil. The Vc/C1-2 region, located approximately 5 to 7 mm caudal to the obex and ipsilateral to the exposed mandibular condyle, was explored for TMJ-responsive units using the entrance of the C2 rootlet as a landmark. Extracellular unit activity was recorded with tungsten microelectrodes (5~9 Mohm, Frederick Haer Inc., Bowdoinham, ME) that penetrated the brainstem tangential (~43° off vertical, 60° off midline for laminae I-II, ~36° off vertical, 45° off midline for lamina V) to the surface. Unit activity was amplified, discriminated, stored and analyzed offline on a computer (Apple G4) using a PowerLab interface board and LabChart software (AD Instruments, Colorado Springs, CO). Spike amplitude and shape were monitored continuously and stored on digital tape to confirm unit isolation in off-line analyses. All units included in this study were identified by a vigorous response to gentle mechanical probing of the exposed dorsal surface of the posterior mandibular condyle (see Okamoto et al., 2003, Fig 1). Units were further classified on the basis of cutaneous RF properties as either wide dynamic range (WDR) and activated by brush, press and pinch stimulation of facial skin or as nociceptive specific (NS) and activated only by press or pinch of the skin.
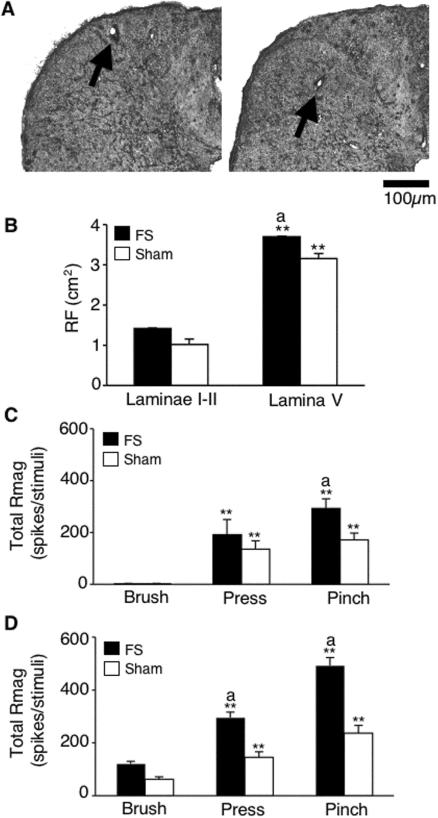
Figure 1.
A. Examples of recording locations of TMJ units in superficial (left) and deep (right) laminae for FS rats. B. Convergent cutaneous RF sizes of TMJ units in superficial and deep laminae. * * p < 0.01 vs. superficial laminae units. a = P <0.05 vs. sham group. C. Response magnitude of TMJ units in superficial laminae to mechanical stimulation of facial skin for 10sec. D. Response magnitude of TMJ units in deep laminae to mechanical stimulation of facial skin for 10sec. ** P < 0.01 vs. response to innocuous brush. a = P < 0.05 vs. sham rats.
Experimental design-neural recording
A single TMJ-responsive unit was recorded in each animal preparation. Units were recorded in superficial laminae (I-II, see Fig 1A, left) or deeper lamina (V, Fig 1A, right) and within 1.5 mm rostral to the level of entrance of the C2 rootlets. After confirming the response to posterior condyle stimulation, the face and neck were explored for cutaneous input. The cutaneous receptive field (RF) was then tested for responses to “brushing” (camel hair), “press” (arterial clip, ~20 mm2) and “pinch” (stiff arterial clip, ~15 mm2) stimuli applied for 10s. The high threshold RF area was mapped using a small blunt forceps (~3 mm2) onto a standardized series of rat face drawings. After cutaneous RF testing, a guide cannula (26 gauge) was positioned in the TMJ joint space (~3 mm deep) by a dorsal approach directed at the posterior aspect of the mandibular condyle to allow delivery of chemical stimuli. TMJ units were activated by injection of adenosine triphosphate (ATP) into the joint space (Tashiro et al., 2007, 2008). Test solutions were delivered from a microsyringe attached by polyethylene tubing to an inner cannula (33 gauge) that protruded ~0.5 mm from the end of the guide cannula. Test solutions consisted of phosphate buffered saline (PBS) and ATP and were delivered slowly over 30 s (total volume = 20 μl) with an inter-injection interval of 20 min to reduce the likelihood of tachyphylaxis. Previously we demonstrated that five consecutive injections of 1 mM ATP at 20 min intervals produced consistent responses (Tashiro et al., 2008). The protocol for chemical injections into the TMJ was: PBS (pH 7.4) followed by four successive doses of ATP (0.001, 0.01, 0.1, and 1.0 mM, pH 7.4, disodium salt, Sigma, St Louis, MO).
Data analysis-neural recording
Neural data were acquired and displayed as peristimulus time histograms (PSTHs) of spikes per 1 s bins, exported to a spreadsheet and analyzed off-line. Spontaneous activity (spikes/s) was calculated as the average spike count over a 1 min epoch immediately preceding each stimulus. The evoked responses were assessed by calculating the response magnitude (Rmag), determined by subtracting the mean plus 2 times the standard deviation (SD) of background activity from the total spike count for each bin. The total Rmag for each stimulus was defined as the cumulative sum of spikes over contiguous bins in which the spike count minus the background was a positive value. The total Rmag is equivalent to the “area under the curve” for each stimulus period. The response duration was defined as the time interval after stimulus onset until three consecutive bins with a positive spike count occurred above background (initial latency) and until the value of three consecutive bins no longer exceeded the mean + 2 SD above background activity as described previously (Hirata et al. 1999). Units that failed to show three consecutive bins with positive Rmag values within 100 s after stimulus onset were considered unresponsive to that condition. Units were defined as ATP-responsive if the total Rmag exceeded the response to PBS by >50%, independent of ATP dose. The threshold dose of ATP was defined as the lowest concentration that produced a total Rmag exceeding that to PBS by >50%. The total Rmag to mechanical stimulation of the skin overlying the TMJ (e.g., brush, press, pinch) was determined over a 10 s stimulus period. Total Rmag, response duration and latency to chemical and mechanical stimuli were assessed statistically by analysis of variance (ANOVA) corrected for repeated measures and individual comparisons were made by Newman-Keuls after ANOVA. Fisher's Exact Probability test determined if the number of units responding to the lowest concentration of ATP (> 50% versus Rmag response to intra-TMJ injection of PBS) was different for FS and Sham groups. The cutaneous high threshold RF areas for units were digitized and quantified by a planimetric method using NIH ImageJ software and compared by ANOVA. Values in the text were expressed as mean ± SEM and p < 0.05 was regarded as significant.
Electromyographic (EMG) recording
Animals were anesthetized with urethane (1.2 g/kg ip) and a catheter was positioned in the right femoral artery for monitoring MAP. After tracheotomy, animals were respired artificially with oxygen-enriched room air. End-tidal CO2, MAP and body temperature were monitored throughout the experiment and kept within normal range. Animals were placed in a stereotaxic frame and the TMJ region was exposed for cannula implantation and ATP injections as described above. A small skin incision was made to expose the surface of the left masseter muscle and paired wire electrodes (0.12 mm diameter, 5 mm interpolar distance) were implanted ~1 mm into the central portion of the muscle. EMG activity was sampled at 1000 Hz, amplified (x10k), filtered (bandwidth 300-3000 Hz), displayed and stored online for analyses.
EMG activity and the role of the Vc/C1-2 region
EMG activity was assessed in two separate series of experiments. In the first series EMG activity was measured in FS and sham groups in response to PBS followed by cumulative doses of ATP (0.01, 0.1, 1 mM, 20 μl, pH = 7.4) delivered to the left TMJ region at 20 min intervals. In a second group of rats, lidocaine (2%, 100 nl, pH 7) was injected into the dorsal horn at the Vc/C1-2 region via a glass micropipette to assess the influence of the Vc/C1-2 region on TMJ-evoked EMG activity. In this series EMG activity was sampled in response to an intra-TMJ injection of ATP (1 mM) followed 10 min later by lidocaine injection and then after 10 min a second ATP (1 mM) stimulus was delivered. Additional naive male rats served as controls to assess if repeated ATP injection to the TMJ region evoked consistent EMG responses in 20 min intervals (n = 11).
Data analysis
EMG activity was sampled continuously for 4 min, beginning 2 min before each TMJ stimulus and for 2 min after stimulation. EMG activity was rectified and stored as 1 s bins for off-line analyses. Baseline activity was quantified as the area under the curve (AUC) for the 2 min epoch (μV per 2 min) sampled immediately prior to stimulation. TMJ-evoked EMG activity was calculated as AUC post-ATP injection minus baseline AUC. The response latency (onset) was defined as the first time point when AUC for 1s exceeded the average baseline activity/s. Results were assessed statistically by ANOVA, corrected for repeated measures and individual comparisons were made by Newman-Keuls after ANOVA. The threshold dose of ATP was defined as the lowest concentration that produced an AUC exceeding that to PBS by > 50%. Fisher's Exact Probability test determined if the number of rats responding to the lowest concentration of ATP (> 50% versus AUC to intra-TMJ injection of PBS) was different for FST and Sham groups.
Histology
At the end of each neural recording experiment, rats were given a bolus dose of pentobarbital (100 mg/kg, iv) and perfused through the heart with 10% formalin. The recording site was marked electrolytically (5 μA, 20 s). Recording and injections sites were confirmed from transverse sections (40 μm) and drawn onto a standard series of rat brainstem outlines (Takeshita et al., 2001).
Results
General and behavioral effects of FS conditioning
A total 75 rats were used for neural (n = 37) and EMG (n = 38) recording experiments and subjected to either FS or sham conditioning. Body weights were similar for FS and sham rats before conditioning (FS = 331.2 ± 11.5 g; sham = 323.9 ± 8.7 g). Three days of FS caused a significant reduction in body weight (-8.4 ± 2.3 %, F 3, 207 = 11.7, P < 0.01), while sham conditioning had no effect (0.1 ± 0.6 %). Immobility time (IT), as a percentage of the 10 min swim session, increased significantly after 2 days of FS compared to the initial session (94.6 ± 0.8% versus 81.8 ± 1.0%, F 2, 72 = 127, P < 0.001). Grip force (GF) was similar for FS and sham rats prior to conditioning (FS = 1084 ± 22 g; sham = 1074 ± 20 g). After 3 days of FS GF was reduced significantly compared to sham rats (-16.5 ± 3.1 % versus 2.6 ± 1.8%, F 3, 207 = 15.6, P < 0.01). These results indicated that the FS protocol used here was sufficient to cause significant changes in general behavior (decreased body weight), depression-like behavior (increased IT duration) and forelimb muscle weakness and/or hyperalgesia (decreased GF).
TMJ-responsive neurons: general properties
TMJ-responsive neurons were recorded in superficial laminae (147 ± 17 μm, n = 25) and deeper laminae (1148 ± 55 μm, n = 12) after penetration of the dorsal surface and within 1.5 mm rostral to the level of entrance of the C2 rootlets. However, since an acute angle of penetration was used, the exact vertical distance from the dorsal brainstem surface could not be determined. All units were spontaneously active. Units in superficial laminae from FS and sham rats displayed similar levels of background activity (FS = 0.9 ± 0.3 spikes/s, n = 12; sham = 1.2 ± 0.3 spikes/s, n = 13, F1,24 = 0.05, P > 0.1). By contrast, units in deep laminae displayed significantly higher background firing rate after FS than units from sham rats (FS = 9.6 ± 3.3, n = 6; sham = 0.7 ± 0.5 spikes/s, n = 6, F1,11 = 12.7, P < 0.01). Second, all TMJ-responsive units received convergent input from facial skin overlying the TMJ. TMJ units in superficial laminae were all classified as NS, while units in deep laminae lamina were classified as WDR. The cutaneous RF was positioned over the TMJ and extended anterior and ventral to the joint within the territories for the maxillary and mandibular branches of the trigeminal nerve (see Okamoto et al. 2003, Fig 4C). The high threshold RF areas of NS units in superficial laminae were similar for FS and sham rats; however, WDR units in deep laminae displayed enlarged RF areas (F3,31 = 3.65, P < 0.05) after FS compared to units in sham rats (Fig 1B). As seen in Fig 1, among NS units in superficial laminae FS also increased the Rmag to pinch stimulation of the cutaneous RF compared to the sham group (F1,18 = 6.87, P < 0.025, Fig 1C), while among WDR units in deep laminae, FS increased the Rmag to both press and pinch compared to the sham group (F2,18 = 4.35, P < 0.05, Fig. 1D). These results indicated that FS enhanced both the spontaneous activity and the response to convergent mechanical input from facial skin and that this enhancement occurred preferentially in TMJ units from deep laminae.
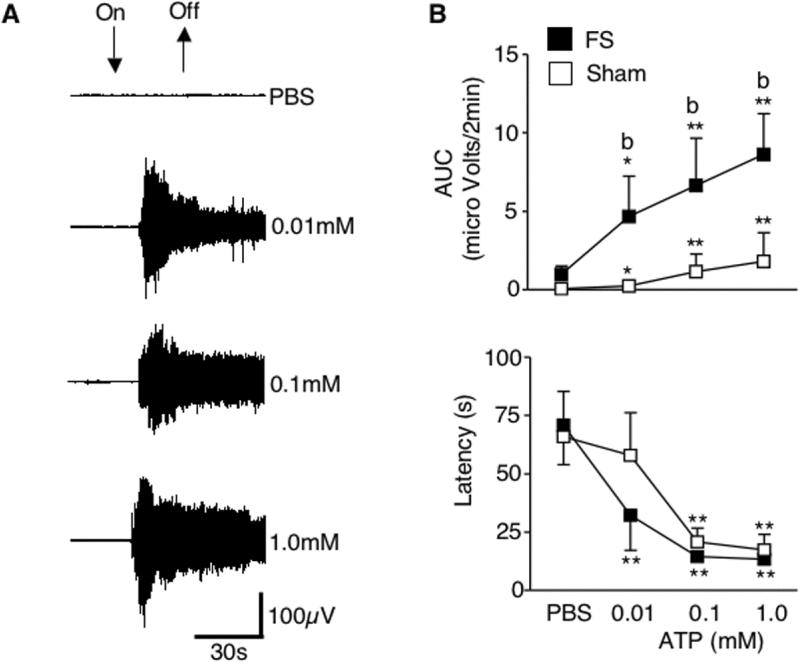
Figure 4.
Dose-response relationship for EMG activity in the masseter muscle evoked by ATP injection (0.01, 0.1 and 1 mM) into the TMJ. A. An example of EMG activity of the left masseter muscle evoked by the injection of ATP into the left TMJ region from FS rats. B. TMJ-evoked masseter muscle activity was significantly increased (upper panel) and response latency was decreased (lower panel) in a dose dependent manner in FS and sham rats. Compared with sham group, FS rats display greater EMG activity indicated by AUC. * P <0.05, ** P < 0.01 vs. response to PBS. b = P < 0.01 vs. sham group.
TMJ-responsive neurons and ATP-evoked responses
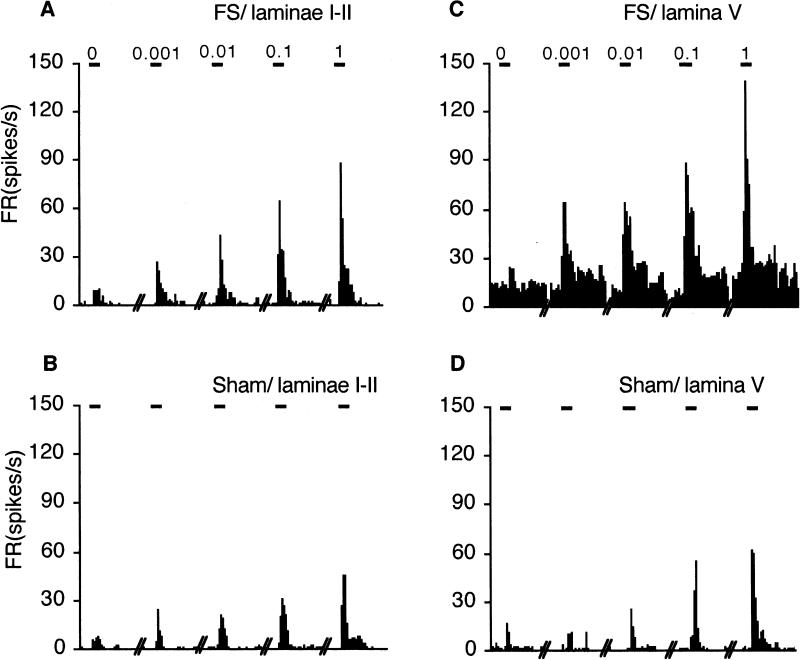
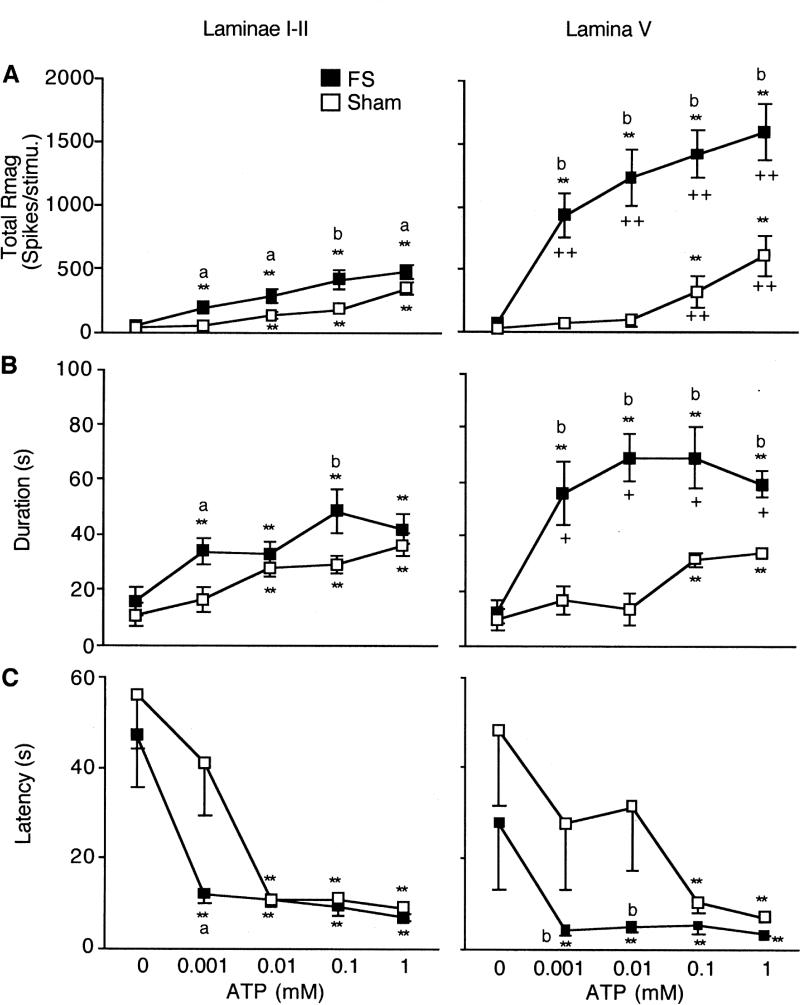
The examples in Fig 2 illustrate that intra-TMJ injections caused a significant ATP dose-related increase in total Rmag after FS and sham conditioning for units in both superficial (Fig 2A and 2B) and deep laminae (Fig 2C and 2D). In superficial laminae, FS increased the total Rmag at each ATP dose tested compared to units from sham rats (Fig 3A, left panel). At the lowest ATP dose tested (0.001 mM), 10 of 12 units in FS rats displayed an increase in Rmag of > 50% above that after PBS injection, while in sham rats only 4 of 13 units were activated. This difference was significant (Fisher's Exact Probability = 0.0011) and suggested that FS reduced the threshold dose of ATP necessary to activate TMJ-responsive units in superficial laminae. Among units in deep laminae, FS markedly increased the total Rmag to each dose of ATP tested compared to responses in sham rats (Fig 3A, right panel). Although the lowest dose of ATP was sufficient to activate 6 of 6 units in deep laminae from FS rats, 4 of 6 units from sham rats also were activated (Fisher's Exact Probability = 0.22) and suggested that FS did not affect the threshold dose of ATP necessary to activate TMJ units in deep laminae. As seen in Fig 3A, the ATP-evoked total Rmag for units in lamina V were significantly greater (F3,36 = 64.4, P < 0.001) than that seen for units in superficial laminae. In FS rats, deep units showed a significantly greater total Rmag at all ATP concentrations compared to superficial units (F1, 17 = 74.8, P < 0.001, Fig 3A), while in sham rats, deep units showed a greater response at the dose of 0.1 and 1.0 mM ATP (F1,18 = 4.51, P < 0.01, Fig 3A).
Figure 2.
Peristimulus time histograms of the response to ATP injected into the TMJ on superficial (A, B) and deep laminae (C, D) from FS and sham rats. Calibration bar above each histogram = 30 s and indicate stimulus periods for PBS, 0.001, 0.01, 0.1 and 1 mM ATP injections into the TMJ.
Figure 3.
Effect of ATP on total Rmag (A), response duration (B) and latency (C) for TMJ units recorded in superficial (left) and deep (right) laminae defined as ATP responsiveness from FS and sham rats. ** P < 0.01 vs. response to PBS (0 mM ATP). a = P < 0.05, b = P < 0.01 vs. sham group. ++ = P < 0.01 vs. superficial laminae units.
Response duration increased significantly after intra-TMJ injections of ATP for all groups in a dose-dependent manner (Fig 3B). In superficial laminae, FS caused a small, but significantly greater (F1, 24 = 9.0, P < 0.025) increase in response duration versus the sham group, whereas in deep laminae FS markedly increased response duration at each dose of ATP compared to sham rats (F1,11 = 36.9, P < 0.001). The effect of FS on ATP-evoked response duration was significantly greater for units in deep laminae (F1, 17 = 12.9, P < 0.005), whereas no laminar differences were seen for units from sham rats (F1, 18 = 0.5, P > 0.1). Response latency also was reduced following increasing doses of intra-TMJ injections of ATP in FS and sham groups (Fig 3C). FS significantly reduced the ATP-evoked response latency for units in superficial and deep laminae such that the lowest dose of ATP (0.001 mM) now resulted in the shortest latency (F4, 146 = 18.5, P < 0.01). The effect of FS on response latency was similar for units in superficial and deep laminae.
Electromyographic (EMG) activity ATP dose-effect
Baseline EMG activity was sampled for 2 min prior to intra-TMJ injections of test solutions and was similar for FS and sham rats (AUC: FS = 1.38 ± 0.23 μV/2 min, Sham = 1.24 ± 0.21 μV/2 min, n = 7 rats/group, F1, 49 = 0.8, P > 0.1). Intra-TMJ injections of ATP evoked an increase in EMG activity (AUC minus baseline AUC) in a dose-dependent manner for both groups (Fig 4A, B, F3, 42 = 5.9, p < 0.01), and group comparisons indicated a significantly greater response after FS (F1, 13 = 5.1 P < 0.05, Fig. 4B). By contrast, the lowest dose of ATP (0.01 mM) tested evoked a significant increase in the EMG response in a similar the number of rats after FS (5 of 7 rats) and sham conditioning (4 of 7 rats) and suggested little or no effect on threshold (Fisher's exact probability, P = 0.37). Increasing doses of ATP also evoked significant and similar decreases in EMG response latency for FS and sham groups (F3, 49 = 13.3, P < 0.01, Fig. 4B). The minimum latency following intra-TMJ injection of 1 mM ATP averaged 13.3 ± 2.1 s and 17.4 ± 6.5 s for FS and sham groups, respectively. These results indicated that ATP-evoked EMG responses, but not threshold doses of ATP, were significantly affected by FS, consistent with a central neural mechanism.
Effect of lidocaine on EMG responses
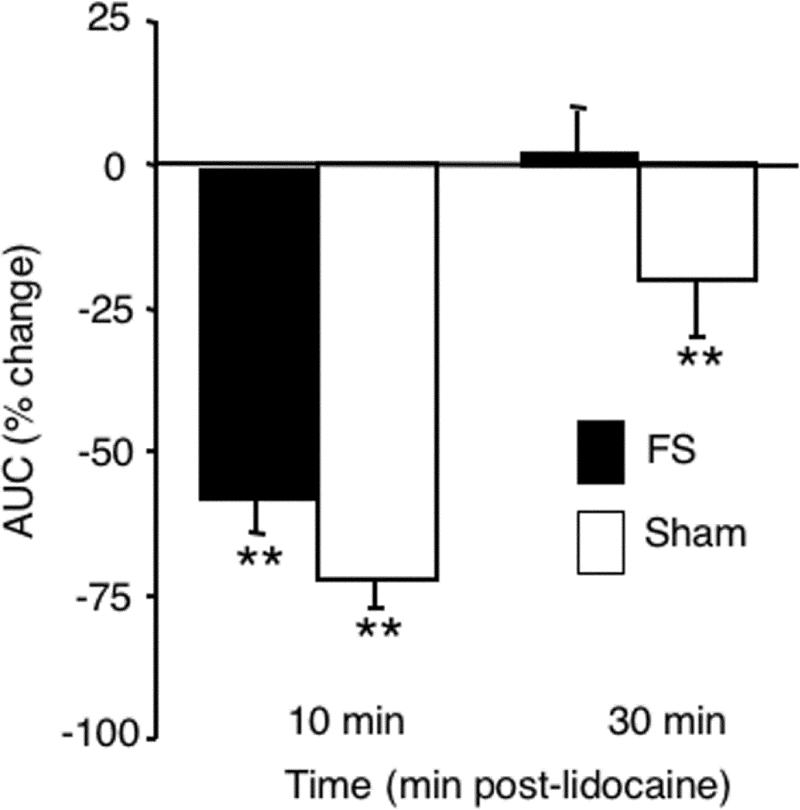
To determine if the spinomedullary junction plays a significant role in mediating the EMG response to TMJ stimulation, lidocaine (2%, 100 nl) was microinjected into the dorsal horn at the Vc/C1-2 region in FS and sham rats. Prior to lidocaine, the ATP-evoked EMG response was significantly greater (F1, 53 = 35.1, P < 0.001) in FS (2.79 ± 0.2 μV/2 min, n = 11) than sham rats (1.32 ± 0.22 μV/2 min, n = 13). As seen in Fig 5 lidocaine blockade of the spinomedullary junction caused a marked reduction in the ATP-evoked EMG response. By 10 min after lidocaine, ATP-evoked responses were reduced significantly (F2, 47 = 56.1, P < 0.001) and similarly in FS and sham rats; however, by 30 min, ATP-evoked EMG responses in FS rats returned to pre-lidocaine levels while responses in sham rats remained reduced (Fig 5, F3, 45 = 20.9, P < 0.01, FS versus sham). In control experiments, the average AUC evoked by the initial ATP injection in naïve animals (n = 11, AUC = 1.81) was similar to that in sham animals (n = 13, AUC = 1.32, F1, 23 = 1.1, P > 0.1) suggesting that sham conditioning did not alter TMJ-evoked EMG responses. The average AUC for the initial ATP injection and spontaneous activity prior to ATP injections were not significantly different from each of 3 subsequent injections (F3, 32 = 1.87, P > 0.1) in naïve rats. These results suggested TMJ-evoked EMG responses depended on a critical relay through the spinomedullary junction and that FS conditioning reduced but did not prevent dependency on this relay.
Figure 5.
Blockade of neural activity at the Vc/C1-2 junction reduces TMJ-evoked EMG activity in the masseter muscle. Microinjection of lidocaine (2% 100nl) into the Vc/C1-2 region decreases TMJ-evoked EMG activity in FS and sham rats. ** P < 0.01 vs. pre-lidocaine.
Discussion
The present study revealed that repeated psychophysical stress alone was sufficient to alter the encoding properties of TMJ-responsive neurons at spinomedullary (Vc/C1-2) junction. These results revealed three novel findings. Repeated FS enhanced the responses to sensory input of neurons in deep laminae significantly more than neurons in superficial laminae. FS-induced enhancement of neural responses was widespread and affected inputs from the TMJ region and facial skin. FS also enhanced TMJ-evoked masseter muscle EMG activity, an effect that depended on a relay through the spinomedullary junction.
TMJD is included among a group of comorbid pain conditions such as fibromyalgia, inflammatory bowel syndrome and chronic headache that share common features (Diatchenko et al. 2006; Yunus 2007). Psychological stress expressed either as anxiety and/or depression is a common feature of these idiopathic pain conditions and is recognized as a risk factor for TMJD (Huang et al., 2002; Gameiro et al. 2006; Slade et al., 2007; Kanehira et al., 2008) and orofacial pain (Korszun, 2002; Sipila et al., 2006; Benoliel et al., 2011). Although neuroimaging studies in TMJD patients have suggested that brain areas that process cognitive and emotional aspects of pain display structural abnormalities (Younger et al., 2010; Moayedi et al., 2011), the neural basis for the integrative relationship between psychological stress and pain expression remains poorly defined.
It has long been known that acute exposure to a noxious stimulus or psychophysical stress can lead to analgesia, whereas repeated presentation of these same stressors can induce behavioral hyperalgesia in animals (see Imbe et al., 2006). Several studies have suggested that repeated stress also increased orofacial pain-like behavior. For example, three days of non-noxious tooth movement increased aversive behavior (Yozgatian et al., 2008), and several days’ exposure to psychological stress decreased the threshold for head withdrawal to jaw muscle mechanical probing (Huang et al., 2011). Repeated restraint stress for several weeks increased jaw-related aversive behavior to intra-TMJ injection of formalin in male rats (Gameiro et al., 2005). However, little is known about the sites and/or mechanisms within the brain that mediate stress-induced facilitation of orofacial pain-like behavior. To address this issue in the context of TMJ nociceptive processing, we adapted the well established model for spinal pain of repeated FS developed by Quintero et al. (2000). Several lines of evidence suggest that this repeated FS protocol recruits central neural mechanisms to produce long lasting cutaneous and muscle hyperalgesia in rodents (Suarez-Roca et al., 2006a). First, repeated FS increased c-fos expression in spinal dorsal horn after formalin injection into the hind paw (Quintero et al., 2003) and increased c-fos and pCREB expression at the spinomedullary junction after mustard oil injection into the TMJ region (Duenes et al., 2010). Second, repeated FS enhanced nocifensive behavior to formalin injection into hindpaw and depended on descending pathways from the rostroventromendial medulla (RVM, Imbe et al. 2010) and monoaminergic transmitter availability (Suraez-Roca et al., 2006a). Third, neurochemical features consistent with mechanisms of central sensitization (Suzuki et al., 2004; Kuner, 2010) such as increased post-synaptic NMDA receptor activity (Quintero et al., 2011), reduced inward-rectifying potassium channel activity in dorsal horn (Ippolito et al., 2005) were involved in FS-induced enhancement of nocifensive behavior. Fourth, an initial reduced release of GABA followed by a delayed increased release of glutamate in spinal dorsal horn was consistent with initiation and maintenance of FS-induced enhanced nocifensive behavior (Quintero et al. 2011). These studies indicate that the repeated FS protocol used in the present study has widespread effects on somatosensory pathways that likely modify trigeminal as well as spinal nociceptive processing.
Repeated FS enhanced the TMJ-evoked responses of neurons in superficial and deep laminae over a wide physiological range of ATP concentration and was markedly greater for neurons in deep versus superficial laminae. FS also increased the responses to mechanical stimulation of facial skin and enlarged the convergent cutaneous RF area of individual TMJ neurons. Although it is well established that neurons in superficial and deep dorsal horn display significant differences in afferent inputs, efferent projection targets and neurochemical markers (see Hunt & Mantyh, 2001; Basbaum et al., 2009), their relative roles in nociceptive processing remain controversial (Craig 2003; Price et al. 2003). Both the superficial and deep laminae at the Vc/C1-2 junction region receive significant direct input from primary afferents that supply the TMJ and masticatory muscles (Jaquin 1983; Shigenaga et al., 1986; Takemura et al. 1987; Shigenaga et al. 1988: Dessem et al. 2007). Neural recording studies have confirmed that TMJ units in superficial and deep laminae at the Vc/C1-2 junction encoded the intensity of intra-TMJ stimuli (Takeshita et al., 2001; Okamoto et al., 2003: Tashiro et al., 2007).
The basis for laminae-preferred effects on dorsal horn neurons after FS is not known; however, stimulus modality and specific neurochemical pathways may be involved. In spinal cord mechanical and thermal-evoked central sensitization was greater for neurons in deep than superficial laminae and windup-like responses of deep dorsal horn neurons were more dependent on NMDA mechanisms (Seagrove et al., 2004). Mutant mice lacking the preprotachykinin gene, and thus deficient in substance P, displayed differential deficits in thermal and mechanical sensitization among neurons in superficial and deep dorsal horn (Mazario & Basbaum, 2007). Similarly, in TRPV1 mutant mice spinal dorsal horn neurons in superficial and deep laminae displayed differential responses to noxious heat and thermal sensitization after mustard oil (Eckert et al., 2006). However, it is not known if selective antagonists for TRPV1 or NK1 differentially alter the properties of TMJ-responsive neurons in superficial and deep laminae at the Vc/C1-2 region.
The present data cannot exclude that peripheral mechanisms also contributed to FS-induced enhancement of neural activity. Reduced response latency coupled with increased background neural activity often is ascribed to peripheral mechanisms of nociception (Treede et al., 1992). Indeed, response latencies of TMJ neurons in superficial and deep laminae to ATP were reduced after FS and a greater percentage of neurons in both laminae were activated by the lowest dose of ATP. We also cannot exclude that effects on non-neural peripheral tissues could have contributed to these results since repeated psychological stress caused structural and metabolic changes in masseter muscle of rats (Chen et al., 2011), while in humans, acute mental stress significantly affected hemodynamic variables and EMG activity in jaw muscles (Hidaka et al. 2004). An elevation in peripheral nerve activity has been proposed as a critical factor in chronic pain and after exposure to psychophysical stress (Reichling and Levine, 2009).
The nature of pain modulation by psychological stressors in idiopathic conditions such as TMJD suggests a role for endogenous pain control systems (Keay & Bandler, 2001; Bragdon et al., 2002; Gameiro et al., 2006; King et al., 2009) and several features of the repeated FS model in animals support this notion. FS-induced behavioral hyperalgesia was reduced by prior blockade of the RVM (Imbe et al., 2010) or by administration of monoamine reuptake inhibitors (Suarez-Roca et al., 2006b), while brain levels of 5HT were significantly altered after repeated FS (Kirby and Lucki, 1998; Baganz et al., 2010). The periaqueductal grey (PAG) – RVM system is critical for coordinating cognitive, emotional and sensory aspects of nociception (Bandler & Shipley, 1994; Keay et al., 2001) and may contribute to stress-induced hyperalgesia (Martenseon et al., 2009). Stimulation of the PAG had differential effects on A- versus C-fiber induced activation of nociceptive neurons in superficial and deep laminae in spinal dorsal horn (Koutsikou et al., 2007). Reversible spinalization increased both resting and articular-evoked neural activity with significantly greater effects on neurons located in deep than superficial dorsal horn (Cervero et al., 1991). Considerable evidence indicates that nociceptive neurons in superficial and deep laminae contribute differentially to nociception (Suzuki et al., 2002, 2004).
The present results demonstrated that intra-TMJ injections of ATP increased masseter muscle EMG activity in a dose-related manner that depended on a relay through the spinomedullary junction. The EMG response to intra-TMJ injections of algesic agents has been used extensively as a model for TMJ nociception (Broton and Sessle 1988; Yu et al., 1995; Cairns et al., 1998, 2001). The EMG response to intra-TMJ injections of ATP was enhanced after FS compared to sham rats at all doses of ATP tested The EMG response latency to intra-TMJ injections of ATP was significantly longer than the latency for TMJ unit activity at each ATP dose tested. This suggested that the relationship between EMG and TMJ-responsive neurons likely is complex and involves neurons at the Vc/C1-2 region. The finding that acute blockade of the spinomedullary region greatly reduced the evoked EMG response was consistent with previous studies indicating that chronic neurochemical lesion of Vc prevented the increase in masticatory muscle EMG activity evoked by mustard oil injection into the TMJ (Tsai et al., 1999). It remains to be determined if FS-induced modulation of EMG activity and TMJ-responsive spinomedullary neurons share common mechanisms and/or central pathways. This possibility was supported by evidence that stimulation of the PAG reduced the C-fiber, but not A-fiber evoked EMG activity (McMullan and Lumb, 2006a) similar to the preferential reduction of C-fiber input to deep dorsal horn neurons (McMullan and Lumb 2006b).
In conclusion, FS enhanced TMJ-evoked neural activity at the Vc/C1-2 junction and masseter muscle EMG activity. The facilitatory effects of FS on Vc/C1-2 units were notable in that neurons in deep laminae displayed markedly greater changes in response properties to TMJ and facial skin stimulation than neurons in superficial laminae. The Vc/C1-2 junction region also likely was critical for mediating the TMJ-evoked increases in EMG activity since lidocaine blockade of the spinomedullary region reversibly reduced by >60% the evoked EMG response in FS and sham groups. These findings indicated that neural signals evoked by psychological stress and TMJ stimulation were integrated by central mechanisms that included the Vc/C1-2 region and may underlie the induction and maintenance of TMJD pain in humans. This would suggest the need to treat the sensory and emotional components of TMJD in order to provide efficient pain relief for this complex condition. In addition, given the high prevalence of persistent TMJD in women compared to men (Maixner 2009; Bereiter and Okamoto 2011) and the marked effects of estrogen status on TMJ-responsive Vc/C1-2 neurons (Tashiro et el. 2007), it will be important to determine the relationship between sex hormones and FS in future experiments. These results supported the hypothesis that psychophysical stress alone was sufficient to significantly modify the encoding properties of TMJ-responsive neurons and that the spinomedullary junction was critical for the integration of reflexes relevant for TMJ nociception.
Acknowledgements
The authors have no financial or other relationships to report that might lead to a conflict interest. This study was supported by grants from the National Institute of Dental and Craniofacial Research: DE12758 (DAB) and the Office of Research on Women's Health.
Abbreviations
- ATP
adenosine triphosphate
- AUC
area under curve
- EMG
electromyography
- FS
forced swim conditioning
- GF
grip force
- IT
immobility time
- NS
nociceptive-specific
- RF
receptive field
- Rmag
response magnitude
- RVM
rostroventromendial medulla
- TMJ
temporomandibular joint
- TMJD
temporomandibular joint disorder
- Vc
trigeminal subnucleus caudalis
- Vc/C1-2
trigeminal subnucleus caudalis and upper cervical spinal cord junction
- WDR
wide dynamic range
References
- Aggarwal VR, Macfarlane GJ, Farragher TM, McBeth J. Risk factors for onset of chronic oro-facial pain--results of the North Cheshire oro-facial pain prospective population study. Pain. 2010;149:354–359. doi: 10.1016/j.pain.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz N, Horton R, Martin K, Holmes A, Daws LC. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun. J Neurosci. 2010;30:15185–15195. doi: 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends in neurosciences. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoliel R, Svensson P, Heir GM, Sirois D, Zakrzewska J, Oke-Nwosu J, Torres SR, Greenberg MS, Klasser GD, Katz J, Eliav E. Persistent orofacial muscle pain. Oral diseases. 2011;17(Suppl 1):23–41. doi: 10.1111/j.1601-0825.2011.01790.x. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Okamoto K. Neurobiology of estrogen status in deep craniofacial pain. Int Rev Neurobiol. 2011;97:251–284. doi: 10.1016/B978-0-12-385198-7.00010-2. [DOI] [PubMed] [Google Scholar]
- Bodere C, Tea SH, Giroux-Metges MA, Woda A. Activity of masticatory muscles in subjects with different orofacial pain conditions. Pain. 2005;116:33–41. doi: 10.1016/j.pain.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bragdon EE, Light KC, Costello NL, Sigurdsson A, Bunting S, Bhalang K, Maixner W. Group differences in pain modulation: pain-free women compared to pain-free men and to women with TMD. Pain. 2002;96:227–237. doi: 10.1016/S0304-3959(01)00451-1. [DOI] [PubMed] [Google Scholar]
- Broton JG, Sessle BJ. Reflex excitation of masticatory muscles induced by algesic chemicals applied to the temporomandibular joint of the cat. Arch. Oral Biol. 1988;33:741–747. doi: 10.1016/0003-9969(88)90008-8. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ, Hu JW. Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J. Neurosci. 1998;18:8056–8064. doi: 10.1523/JNEUROSCI.18-19-08056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BE, Sessle BJ, Hu JW. Temporomandibular-evoked jaw muscle reflex: role of brain stem NMDA and non-NMDA receptors. Neuroreport. 2001;12:1875–1878. doi: 10.1097/00001756-200107030-00022. [DOI] [PubMed] [Google Scholar]
- Cervero F, Schaible H-G, Schmidt RF. Tonic descending inhibition of spinal cord neurones driven by joint afferents in normal cats and in cats with an inflamed knee joint. Exp Brain Res. 1991;83:675–678. doi: 10.1007/BF00229846. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Huang F, Zhang M, Shang HY. Psychological stress alters ultrastructure and energy metabolism of masticatory muscle in rats. J Biomed Biotechnol. 2011;2010:302693. doi: 10.1155/2010/302693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1–30. doi: 10.1146/annurev.neuro.26.041002.131022. [DOI] [PubMed] [Google Scholar]
- Dessem D, Moritani M, Ambalavanar R. Nociceptive craniofacial muscle primary afferent neurons synapse in both the rostral and caudal brain stem. J Neurophysiol. 2007;98:214–223. doi: 10.1152/jn.00990.2006. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Nackley AG, Slade GD, Fillingim RB, Maixner W. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123:226–230. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Duenes SL, Thompson R, Chang Z, Okamoto K, Bereiter DA. Psychophysical stress increases the expression of phospho-CREB, Fos protein and neurokinin-1 receptors in superficial laminae of trigeminal subnucleus caudalis in female rats. Neurosci Lett. 2010;486:207–210. doi: 10.1016/j.neulet.2010.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert WA, 3rd, Julius D, Basbaum AI. Differential contribution of TRPV1 to thermal responses and tissue injury-induced sensitization of dorsal horn neurons in laminae I and V in the mouse. Pain. 2006;126:184–197. doi: 10.1016/j.pain.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Gameiro GH, Andrade Ada S, de Castro M, Pereira LF, Tambeli CH, Veiga MC. The effects of restraint stress on nociceptive responses induced by formalin injected in rat's TMJ. Pharmacol Biochem Behav. 2005;82:338–344. doi: 10.1016/j.pbb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Gameiro GH, da Silva Andrade A, Nouer DF, Ferraz de Arruda Veiga MC. How may stressful experiences contribute to the development of temporomandibular disorders? Clin Oral Investig. 2006;10:261–268. doi: 10.1007/s00784-006-0064-1. [DOI] [PubMed] [Google Scholar]
- Hathaway CB, Hu JW, Bereiter DA. Distribution of Fos-like immunoreactivity in the caudal brainstem of the rat following noxious chemical stimulation of the temporomandibular joint. J Comp Neurol. 1995;356:444–456. doi: 10.1002/cne.903560311. [DOI] [PubMed] [Google Scholar]
- Hidaka O, Yanagi M, Takada K. Changes in masseteric hemodynamics time-related to mental stress. J Dent Res. 2004;83:185–190. doi: 10.1177/154405910408300220. [DOI] [PubMed] [Google Scholar]
- Hirata H, Hu JW, Bereiter DA. Responses of medullary dorsal horn neurons to corneal stimulation by CO2 pulses in the rat. J Neurophysiol. 1999;82:2092–2107. doi: 10.1152/jn.1999.82.5.2092. [DOI] [PubMed] [Google Scholar]
- Huang F, Zhang M, Chen YJ, Li Q, Wu AZ. Psychological stress induces temporary masticatory muscle mechanical sensitivity in rats. J Biomed Biotechnol. 2011;2011:720603. doi: 10.1155/2011/720603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GJ, LeResche L, Critchlow CW, Martin MD, Drangsholt MT. Risk factors for diagnostic subgroups of painful temporomandibular disorders (TMD). J. Dent Res. 2002;8:284–288. doi: 10.1177/154405910208100412. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–2192. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- Imbe H, Okamoto K, Donishi T, Senba E, Kimura A. Involvement of descending facilitation from the rostral ventromedial medulla in the enhancement of formalin-evoked nocifensive behavior following repeated forced swim stress. Brain Res. 2010;1329:103–112. doi: 10.1016/j.brainres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Ippolito DL, Xu M, Bruchas MR, Wickman K, Chavkin C. Tyrosine phosphorylation of K(ir)3.1 in spinal cord is induced by acute inflammation, chronic neuropathic pain, and behavioral stress. J Biol Chem. 2005;280:41683–41693. doi: 10.1074/jbc.M507069200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin MF, Rhoades RW, Enfiejian HL, Egger MD. Organization and morphology of masticatory neurons in the rat: a retrograde HRP study. J Comp Neurol. 1983;218:239–256. doi: 10.1002/cne.902180302. [DOI] [PubMed] [Google Scholar]
- Kanehira H, Agariguchi A, Kato H, Yoshimine S, Inoue H. Association between stress and temporomandibular disorder. Nihon Hotetsu Shika Gakkai Zasshi. 2008;52:375–380. doi: 10.2186/jjps.52.375. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neuroscience and biobehavioral reviews. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Keay KA, Clement CI, Depaulis A, Bandler R. Different representations of inescapable noxious stimuli in the periaqueductal gray and upper cervical spinal cord of freely moving rats. Neurosci Lett. 2001;313:17–20. doi: 10.1016/s0304-3940(01)02226-1. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Somers TJ. Psychological approaches to understanding and treating arthritis pain. Nat Rev Rhematol. 2010;6:210–216. doi: 10.1038/nrrheum.2010.22. [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–343. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL., 3rd Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain. 2009;143:172–178. doi: 10.1016/j.pain.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Lucki I. The effect of repeated exposure to forced swimming on extracellular levels of 5-hydroxytryptamine in the rat. Stress. 1998;2:251–263. doi: 10.3109/10253899809167289. [DOI] [PubMed] [Google Scholar]
- Korszun A. Facial pain, depression and stress-connections and directions. J. Oral Pathol. Med. 2002;31:615–619. doi: 10.1034/j.1600-0714.2002.00091.x. [DOI] [PubMed] [Google Scholar]
- Koutsikou S, Parry DM, MacMillan FM, Lumb BM. Laminar organization of spinal dorsal horn neurones activated by C- vs. A-heat nociceptors and their descending control from the periaqueductal grey in the rat. Eur J Neurosci. 2007;26:943–952. doi: 10.1111/j.1460-9568.2007.05716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner R. Central mechanisms of pathological pain. Nature medicine. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- List T, Axelsson S. Management of TMD: evidence from systematic reviews and meta-analyses. Journal of oral rehabilitation. 2010;37:430–451. doi: 10.1111/j.1365-2842.2010.02089.x. [DOI] [PubMed] [Google Scholar]
- Maixner W. Temporomandibular joint disorders. In: Mayer E, Bushnell M, editors. Functional pain syndromes: presentation and pathology. IASP Press; Seattle: 2009. pp. 55–69. [Google Scholar]
- Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142:236–244. doi: 10.1016/j.pain.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazario J, Basbaum AI. Contribution of substance P and neurokinin A to the differential injury-induced thermal and mechanical responsiveness of lamina I and V neurons. J Neurosci. 2007;27:762–770. doi: 10.1523/JNEUROSCI.2992-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism: clinical and experimental. 2010;59(Suppl 1):S9–15. doi: 10.1016/j.metabol.2010.07.012. [DOI] [PubMed] [Google Scholar]
- McMullan S, Lumb BM. Midbrain control of spinal nociception discriminates between responses evoked by myelinated and unmyelinated heat nociceptors in the rat. Pain. 2006a;124:59–68. doi: 10.1016/j.pain.2006.03.015. [DOI] [PubMed] [Google Scholar]
- McMullan S, Lumb BM. Spinal dorsal horn neuronal responses to myelinated versus unmyelinated heat nociceptors and their modulation by activation of the periaqueductal grey in the rat. J Physiol. 2006b;576:547–556. doi: 10.1113/jphysiol.2006.117754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey H. Psychological medicine, pain and musculoskeletal disorders. Rheumatic Dis Clin North Am. 1996;22:623–637. doi: 10.1016/s0889-857x(05)70292-1. [DOI] [PubMed] [Google Scholar]
- Moayedi M, Weissman-Fogel I, Crawley AP, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Contribution of chronic pain and neuroticism to abnormal forebrain gray matter in patients with temporomandibular disorder. NeuroImage. 2011;55:277–286. doi: 10.1016/j.neuroimage.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Ohrbach R, Dworkin SF. Five-year outcomes in TMD: relationship of changes in pain to changes in physical and psychological variables. Pain. 1998;74:315–326. doi: 10.1016/s0304-3959(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Hirata H, Takeshita S, Bereiter DA. Response properties of TMJ neurons in superficial laminae at the spinomedullary junction of female rats vary over the estrous cycle. J. Neurophysiol. 2003;89:1467–1477. doi: 10.1152/jn.00795.2002. [DOI] [PubMed] [Google Scholar]
- Peck CC, Murray GM, Gerzina TM. How does pain affect jaw muscle activity? The Integrated Pain Adaptation Model. Aust Dent J. 2008;53:201–207. doi: 10.1111/j.1834-7819.2008.00050.x. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Price DD, Greenspan JD, Dubner R. Neurons involved in the exteroceptive function of pain. Pain. 2003;106:215–219. doi: 10.1016/j.pain.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Quintero L, Cardenas R, Suarez-Roca H. Stress-induced hyperalgesia is associated with a reduced and delayed GABA inhibitory control that enhances post-synaptic NMDA receptor activation in the spinal cord. Pain. 2011;152:1909–1922. doi: 10.1016/j.pain.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Quintero L, Cuesta MC, Silva JA, Arcaya JL, Pinerua-Suhaibar L, Maixner W, Suarez-Roca H. Repeated swim stress increases pain-induced expression of c-Fos in the rat lumbar cord. Brain Res. 2003;965:259–268. doi: 10.1016/s0006-8993(02)04224-5. [DOI] [PubMed] [Google Scholar]
- Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacol Biochem Behav. 2000;67:449–458. doi: 10.1016/s0091-3057(00)00374-9. [DOI] [PubMed] [Google Scholar]
- Rammelsberg P, LeResche L, Dworkin S, Mancl L. Longitudinal outcome of temporomandibular disorders: a 5-year epidemiologic study of muscle disorders defined by research diagnostic criteria for temporomandibular disorders. J Orofac Pain. 2003;17:9–20. [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends in neurosciences. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagrove LC, Suzuki R, Dickenson AH. Electrophysiological characterisations of rat lamina I dorsal horn neurones and the involvement of excitatory amino acid receptors. Pain. 2004;108:76–87. doi: 10.1016/j.pain.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Chen IC, Suemune S, Nishimori T, Nasution ID, Yoshida A, Sato H, Okamoto T, Sera M, Hosoi M. Oral and facial representation within the medullary and upper cervical dorsal horns in the cat. J Comp Neurol. 1986;243:388–408. doi: 10.1002/cne.902430309. [DOI] [PubMed] [Google Scholar]
- Shigenaga Y, Sera M, Nishimori T, Suemune S, Nishimura M, Yoshida A, Tsuru K. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J Comp Neurol. 1988;268:489–507. doi: 10.1002/cne.902680403. [DOI] [PubMed] [Google Scholar]
- Sipila K, Ylostalo PV, Joukamaa M, Knuuttila ML. Comorbidity between facial pain, widespread pain, and depressive symptoms in young adults. J Orofac Pain. 2006;20:24–30. [PubMed] [Google Scholar]
- Slade G, Diatchenko L, Bhalang K, Sigurdsson A, Fillingim R, Belfer I, Maixner W. Influence of Psychological Factors on Risk of Temporomandibular Disorders. J Dent Res. 2007;86:1120–1125. doi: 10.1177/154405910708601119. [DOI] [PubMed] [Google Scholar]
- Suarez-Roca H, Quintero L, Arcaya JL, Maixner W, Rao SG. Stress-induced muscle and cutaneous hyperalgesia: differential effect of milnacipran. Physiol Behav. 2006 a;88:82–87. doi: 10.1016/j.physbeh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Suarez-Roca H, Silva JA, Arcaya JL, Quintero L, Maixner W, Pinerua-Shuhaibar L. Role of mu-opioid and NMDA receptors in the development and maintenance of repeated swim stress-induced thermal hyperalgesia. Behav Brain Res. 2006b;167:205–211. doi: 10.1016/j.bbr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Takemura M, Sugimoto T, Sakai A. Topographic organization of central terminal region of different sensory branches of the rat mandibular nerve. Exp Neurol. 1987;96:540–557. doi: 10.1016/0014-4886(87)90217-2. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Hirata H, Bereiter DA. Intensity coding by TMJ-responsive neurons in superficial laminae of caudal medullary dorsal horn of the rat. J Neurophysiol. 2001;86:2393–2404. doi: 10.1152/jn.2001.86.5.2393. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Bereiter DA. Morphine modulation of temporomandibular joint-responsive units in superficial laminae at the spinomedullary junction in female rats depends on estrogen status. Eur J Neurosci. 2008;28:2065–2074. doi: 10.1111/j.1460-9568.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Okamoto K, Milam SB, Bereiter DA. Differential effects of estradiol on encoding properties of TMJ units in laminae I and V at the spinomedullary junction in females. J Neurophysiol. 2007;98:3242–3253. doi: 10.1152/jn.00677.2007. [DOI] [PubMed] [Google Scholar]
- Treede R-D, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Tsai CM, Chiang CY, Yu XM, Sessle BJ. Involvement of trigeminal subnucleus caudalis (medullary dorsal horn) in craniofacial nociceptive reflex activity. Pain. 1999;81:115–128. doi: 10.1016/s0304-3959(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Wang K, Arendt-Nielsen L, Jensen T, Svensson P. Reduction of clinical temporomandibular joint pain is associated with a reduction of the jaw-stretch reflex. J Orofac Pain. 2004;18:33–40. [PubMed] [Google Scholar]
- Younger JW, Shen YF, Goddard G, Mackey SC. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149:222–228. doi: 10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yozgatian JH, Zeredo JL, Hotokezaka H, Koga Y, Toda K, Yoshida N. Emotional stress- and pain-related behaviors evoked by experimental tooth movement. Angle Orthod. 2008;78:487–494. doi: 10.2319/040207-165.1. [DOI] [PubMed] [Google Scholar]
- Yu X-M, Sessle BJ, Vernon H, Hu JW. Effects of inflammatory irritant application to the rat temporomandibular joint on jaw and neck muscle activity. Pain. 1995;60:143–149. doi: 10.1016/0304-3959(94)00104-M. [DOI] [PubMed] [Google Scholar]
- Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]