Abstract
Macroautophagy (autophagy) is a lysosomal degradation pathway that is conserved from yeast to humans that plays an important role in recycling cellular constituents in all cells. A number of protein complexes and signaling pathways impinge on the regulation of autophagy, with the mammalian target of rapamycin (mTOR) as the central player in the canonical pathway. Cytoplasmic Ca2+ signaling also regulates autophagy, with both activating and inhibitory effects, mediated by the canonical as well as non-canonical pathways. Here we review this regulation, with a focus on the role of an mTOR-independent pathway that involves the inositol trisphosphate receptor (InsP3R) Ca2+ release channel and Ca2+ signaling to mitochondria. Constitutive InsP3R Ca2+ transfer to mitochondria is required for autophagy suppression in cells in nutrient-replete media. In its absence, cells become metabolically compromised due to insufficient production of reducing equivalents to support oxidative phosphorylation. Absence of this Ca2+ transfer to mitochondria results in activation of AMPK, which activates mTOR-independent pro-survival autophagy. Constitutive InsP3R Ca2+ release to mitochondria is an essential cellular process that is required for efficient mitochondrial respiration, maintenance of normal cell bioenergetics and suppression of autophagy.
1. Introduction
Autophagy, a lysosomal degradation pathway that is conserved from yeast to humans, plays an important role in degrading and recycling cellular constituents, including damaged organelles. It operates as a bulk degradation system in all cells as a complementary system to the ubiquitin-proteasome degradation pathway [1]. At least three types of autophagy have been described according to their lysosomal delivery mechanisms: microautophagy, chaperone-mediated autophagy and macroautophagy [2]. Among these, macroautophagy is the only one that has been observed to date to be regulated by Ca2+ [3] and will therefore be the focus of this review. Macroautophagy involves the formation of a double membrane cistern, possibly derived from several sources including endoplasmic reticulum [4] and mitochondria [5], that enlarges and fuses with itself, engulfing cytoplasmic constituents within an autophagosome in a process involving an evolutionary set of over 20 conserved proteins (known as Atg proteins) essential for the execution of autophagy [1, 6]. Autophagosomes fuse with late endosomes and lysosomes, promoting the delivery of organelles, aggregated proteins and cytoplasm to the luminal acidic degradative milieu that enables their breakdown into constituent molecular building blocks that can be recycled by the cell [1].
Macroautophagy is a bulk cytoplasmic degradation pathway, but under some situations it appears to operate in an organelle-selective way, for example towards mitochondria, referred to as mitophagy, and the endoplasmic reticulum, referred to as reticulophagy [7]. Macroautophagy, hereafter referred to as autophagy, plays different cellular roles depending on physiological context. In unstressed cells, low rates of autophagy perform a housekeeping function, termed quality control autophagy, that is essential for maintenance of normal cellular homeostasis [8]. Autophagy also has important roles in cellular responses to certain invading pathogens including bacteria and viruses [2], and it also functions in developmental cell death, tumor suppression, and aging, and it has been implicated in neurodegeneration, cardiovascular disease and cancer [1, 9]. Under conditions of stress, most famously starvation, autophagy is strongly activated as a pro-survival mechanism by promoting the recycling of fatty acids and amino acids to meet cellular metabolic demands, either through synthesis of new macromolecules or by their oxidation in mitochondria to maintain cellular ATP and viability until nutrient supplies are restored [10]. Autophagy has also been implicated in cell death, referred to as programmed cell death type II [11]. However, because there is little direct evidence for autophagy as the primary driver of cell death under (patho)physiological conditions, it has been referred to as cell death “with autophagic features” [12].
2. mTOR dependent autophagy and cytoplasmic calcium
A number of protein complexes and signaling pathways are involved in the initiation of autophagy, the maturation of autophagosomes, and their delivery to and fusion with lysosomes [1, 13]. The central player in the regulation of autophagy, representing the canonical pathway of autophagy activation, is the mammalian target of rapamycin (mTOR), specifically the complex 1 (mTORC1) [14, 15]. mTORC1 is a serine-threonine kinase that plays important roles in regulating cell growth, cell cycle progression, nutrient import and protein synthesis [16–18]. The mTORC1 complex is positively regulated by the active, GTP-bound form of the small GTPase Rheb, which can be inactivated by the GTPase activating protein (GAP) formed by the heterodimer TSC1/TSC2 (tuberous sclerosis complex 1/2) [16]. The active mTORC1 complex promotes cell growth and inhibits ULK1/2 kinases, important regulators of autophagosome formation [15]. Autophagy is activated as a survival mechanism in response to metabolic stress associated with insufficient growth factor stimulation or nutrient availability [19]. Although several signal transduction pathways converge to regulate mTORC1 activity, one important mediator of this response is AMP-activated protein kinase (AMPK), a critical metabolic sensor [20]. AMPK phosphorylates and activates the TSC1/TSC2 complex [21]. Activation of TSC1/TSC2 suppresses both Rheb and mTORC1 activity, inducing autophagy [22–24]. In addition, AMPK can also phosphorylate and activate ULK1/2 kinases to promote autophagy [15, 25, 26].
AMPK can be phosphorylated and activated by upstream kinases LKB1 and Ca2+-calmodulin dependent protein kinase kinase beta (CaMKKβ, also referred to as CaMKK2) [27]. The latter provides a link between Ca2+ signaling, mTOR and autophagy. Elevations of cytoplasmic free Ca2+ concentration ([Ca2+]i) by various mechanisms, including ligand stimulation of plasma membrane receptors coupled to the release of Ca2+ from intracellular stores, can activate autophagy [3, 28–34]. Before discussing this literature, it is important to note that the physiological relevance of many of these observations is unclear. In some studies, [Ca2+]I was experimentally elevated by non-physiological means (Ca2+ ionophores; thapsigargin-induced depletion of ER Ca2+ stores) and for prolonged periods (24–72 hours) [28, 29, 32], whereas physiological [Ca2+]I signals are generally of much shorter duration with smaller amplitudes with different kinetic features. In other studies, cytoplasmic Ca2+ as a regulator of autophagy was only inferred, based on the effects of strongly buffering [Ca2+]I with high concentrations of BAPTA-AM [31, 33, 34]. With these caveats in mind, Hoyer-Hansen et al. originally described that prolonged elevation of [Ca2+]i can activate autophagy by a mechanism that requires CaMKKβ and AMPK and involves the inhibition of mTORC1 signaling [28]. Similarly, amyloid beta induces autophagy by a mechanism that requires CaMKKβ and AMPK signaling [29]. Over-expression of leucine-rich repeat kinase-2 (LRRK2), mutations in which cause late-onset Parkinson’s disease, activates a Ca2+-dependent CaMKKβ – AMPK pathway that causes a persistent increase in autophagosome formation [35]. Recently, nutrient deprivation was observed to trigger inositol 1,4,5-trisphosphate receptor (InsP3R)-mediated Ca2+ release from the endoplasmic reticulum (ER) that caused a rise of [Ca2+]I that was found to be required for starvation-induced autophagy [36]. Starvation induced autophagy is generally mTORC1 dependent, but whether autophagy observed in this study involves the CaMKKβ-AMPK-mTORC1 pathway was not determined. An obligate role for InsP3R-mediated Ca2+ signaling for nutrient deprivation-induced autophagy is somewhat unexpected since cells with all InsP3R genes knocked out can mount a normal mTOR-dependent starvation-induced pro-survival autophagic response [37]. In addition to a “canonical” CaMKKβ-AMPK-mTORC1 pathway for Ca2+ activation of autophagy, it has been reported that thapsigargin-induced, BAPTA-AM-sensitive autophagy can be AMPK independent [33], although the mechanisms are unknown. Prolonged thapsigargin treatment can have pleiotropic effects unrelated to Ca2+ signaling per se. Nevertheless, some evidence suggests that Ca2+ may directly regulate mTORC1 activity. However, in these cases, Ca2+ was found to have an opposite, inhibitory effect on autophagy. Thus, Khan reported that lack of InsP3R-mediated Ca2+ release from the ER had no effects on AMPK activity, but autophagy was activated nevertheless, correlated with inhibition of mTORC1 activity [38]. In addition, amino acid restoration to starving cells was reported to induce a rise in [Ca2+]I that activates mTORC1 by Ca2+-calmodulin binding to the Vps34 class III phosphatidylinositol 3-kinase that is required for mTORC1 activation [39] (although see [40]).
In summary, evidence suggests that elevations of [Ca2+]I can either activate or inhibit autophagy with the bulk of the data suggesting that Ca2+ can induce autophagy by a signal transduction pathway involving Ca2+ activation of CaMKKβ, its phosphorylation and activation of AMPK, and AMPK inhibition of mTOR (Figure 1). Nevertheless, as noted, in many of these studies the [Ca2+]I signals were not physiological nor directly measured. Required are more studies that explore the roles of physiologically-relevant [Ca2+]I signals in both normal as well as stressed cells.
Fig. 1.
A mechanism for activation of autophagy by elevated cytoplasmic [Ca2+] in the canonical mTOR pathway. Various Ca2+ mobilizing agents have been shown to induce autophagy. Activation of calmodulin (CaM) by Ca2+ activates CaMKKβ, which phosphorylates and activates AMPK. Activated AMPK phosphorylates the TSC1/TSC1 complex, enhancing its GTPase activity to maintain Rheb in its GDP-bound inhibited state. Absence of Rheb-GTP activity inactivates mTORC1, releasing its break on autophagy. Inhibition of elevated [Ca2+]i by buffering it with BAPTA prevents activation of CaMKKβ and AMPK, enabling Rheb to maintain mTORC1 activity, suppressing autophagy.
3. mTOR independent autophagy and InsP3R Ca2+ signaling
Ca2+ signaling has also been linked to non-canonical, mTOR-independent autophagy. In a seminal study, Sarkar et al. observed that lithium (Li+) stimulates autophagy in an mTOR-independent manner by inhibiting inositol monophosphatase (IMPase), the enzyme responsible for maintaining cellular levels of free inositol required for phosphatidylinositol signaling [41]. Li+ activation of autophagy could be reversed by manipulations that raised cytoplasmic InsP3 levels, implicating phosphatidylinositol signaling in the regulation of autophagy. Importantly, Li+ was without effect on mTORC1 activity, suggesting that phosphatidylinositol regulation of autophagy was mediated by a non-canonical pathway that operated additively and independently of mTOR.
Subsequently, two independent screens identified compounds that induced mTORC1-independent autophagy [42, 43]. Of note, some compounds discovered in each screen are known Ca2+ channel inhibitors, again suggesting that constitutive Ca2+ signals may play a role in inhibiting autophagy. In an extensive study of one of these compounds, Xia et al. found that agonist-induced InsP3-mediated Ca2+ release was blocked in all of many cell types examined [44]. They suggested that reducing intracellular [Ca2+] prevents the calpain-mediated cleavage of ATG5, which in turn increases the levels of full-length ATG5 and ATG12-ATG5 conjugate, activating autophagy. In contrast, the Rubinsztein group found that inhibition of InsP3 production was without effect on ATG5 cleavage [43], suggesting that InsP3 and Ca2+ inhibited autophagy by other mechanisms. Criollo et al. also observed that IMPase inhibition and Li+ activated autophagy [45]. Importantly, they demonstrated that RNAi knockdown of the InsP3R Ca2+ release channel or pharmacological inhibition of InsP3R activity with xestospongin B (XeB) rapidly (<2 hr) induced autophagy. Although the role of mTORC was not examined in this study, the similar results obtained by Sarkar et al. [41] regarding the effects of Li+ and IMPase inhibition suggest that the activation of autophagy by inhibition of InsP3R proceeds by a mechanism independent of the canonical mTOR pathway, as subsequently confirmed [37]. Neither ER Ca2+ levels or [Ca2+]I were affected during autophagy induction by InsP3R inhibition [45]. Thus, the link between InsP3R activity and autophagy was not established. It was suggested that Ca2+ release by the InsP3R may not be an intrinsic part of the mechanism, whereas protein interactions with the channel may play a dominant role. Thus, it was proposed that the major effect of XeB or InsP3R knockdown was to disrupt an interaction of the channel with beclin-1 [46].
Beclin-1 (yeast atg6) interacts with a Vps34-associated protein complex involved in early autophagosome biogenesis [47, 48]. It was proposed that the InsP3R buffers the levels of beclin-1 available to interact with this complex, inhibiting autophagy [46]. However, other studies suggest that model cannot sufficiently account for the role of the InsP3R in regulating autophagy. Glucocorticoids were shown to induce autophagy in lymphocytes by reducing InsP3R-mediated [Ca2+]i signaling, although here the induction of autophagy appeared to be mediated by the canonical mTOR pathway [49]. Cardenas et al. observed that DT40 cells lacking all InsP3R (DT40-KO), or wild-type DT40 cells exposed to XeB or inhibitors of phospholipase C or IMPase, displayed constitutive autophagy due to enhanced autophagic flux, in agreement with these previous studies, that was mTOR-independent and functioned as a cell survival mechanism [37]. Stable expression of recombinant rat InsP3R-3 rescued elevated autophagy in the DT40-KO cells. However, DT40-KO cells expressing a mutant InsP3R-3 that lacked ion channel activity as a result of a defective channel gate did not suppress constitutive autophagy. Similarly, DT40-KO cells expressing a mutant InsP3R-3 that gated normally but had no Ca2+ permeability also failed to suppress constitutive autophagy [37, 38]. Of note, the mutant and WT InsP3R-3 bound similarly to beclin-1 [37] and no differences in beclin-1-Vps34 complexes in wild-type and DT40-KO cells were observed [38], indicating that disruption of beclin-1 binding [46] likely does not account for autophagy induction by InsP3R inhibition. Rather, these results suggest that the Ca2+ release activity of the InsP3R is necessary for autophagy suppression.
A clue regarding a mechanism that could link Ca2+ release activity of the InsP3R and mTOR-independent suppression of autophagy was the observation that AMPK was constitutively activated in DT40-KO cells [37]. Although this was not observed in DT40-KO cells in another study [38], pharmacological inhibition or genetic knockdown of InsP3R caused a rapid and constitutive activation of AMPK in all of several cell types examined [37]. As noted, enhanced AMPK activity can induce autophagy by inhibition of mTOR [50]. Nevertheless, phosphorylation of mTOR as well mTOR substrates were similar in DT40-KO and WT cells, and unchanged in WT cells exposed to XeB. Thus, autophagy induced by lack of InsP3R activity correlates with enhanced AMPK activity, but the mTOR pathway does not appear to be involved. Enhanced constitutive AMPK activity by disruption of InsP3R Ca2+ signaling was shown to be necessary for activation of autophagy, since pharmacological or genetic inhibition of AMPK activity blocked autophagy activation induced by InsP3R inhibition [37].
4. Calcium signals and mitochondria
AMPK is a highly sensitive indicator of cellular energy status, whose activity increases under conditions of metabolic stress that elevate the cytoplasmic AMP:ATP ratio [20]. Treatment of hepatocytes with XeB increased AMP levels and the AMP:ATP ratio [37]. The presence of elevated [AMP] and the requirement for AMPK activation to induce pro-survival autophagy in response to loss of InsP3R Ca2+ signaling suggested that cells lacking this pathway have compromised bioenergetics. In agreement, basal O2 consumption rate (OCR) was lower by 60% in InsP3R-KO cells compared with WT cells and in several cell types with InsP3R activity inhibited by XeB [37]. Reduced oxidative phosphorylation observed in the DT40-KO cells was not associated with altered numbers of mitochondria, suggesting that oxidative phosphorylation is constitutively compromised in the absence of InsP3R activity [37].
Regulation of mitochondrial enzyme activity by Ca2+ has been known for many years. The activities of three key metabolic enzymes, the pyruvate-, α-ketoglutarate-, and isocitrate-dehydrogenases, are enhanced by Ca2+, resulting in increased reduction of nicotinamide adenine dinucleotide (NAD) to its reduced form, NADH, which fuels the mitochondrial respiratory chain to enhance ATP synthesis [51, 52]. In addition, aralar1 and citrin, aspartate/glutamate carriers, are regulated by Ca2+, enhancing aerobic metabolism during stimulation [53]. Ca2+ uptake into mitochondria is relatively unimpeded at the outer membrane due the presence of the voltage-dependent anion channel (VDAC). Ca2+ permeation into mitochondria is rate-limited at the inner membrane, where its uptake is mediated by MCU [54, 55], the pore-forming subunit of a Ca2+ selective ion channel (MiCa [56]) formerly referred to as the Ca2+ uniporter. Mitochondrial Ca2+ uptake by MCU is driven by the highly negative transmembrane voltage generated by electron transport in normally respiring mitochondria [57]. Studies in mitochondrial suspensions and mitoplasts (mitochondria without the outer mitochondrial membrane (OMM) have suggested that mitochondrial Ca2+ uptake takes place over a range of 1–100 µM Ca2+ [56–59]. Nevertheless, InsP3-linked agonists that generate sub-micromolar global Ca2+ elevations trigger larges increases in mitochondrial [Ca2+] in a wide variety of cell types [60–62]. The efficient transmission of InsP3-induced Ca2+ release into mitochondria is achieved by close appositions between the mitochondria and the ER, supported by physical linkages that bring the organelles to with 19 to 30 nm of each other [60, 63], that exposes MiCa to high [Ca2+]. The functional consequence of ER-released mitochondrial Ca2+ uptake include regulation of mitochondria-mediated cell death [64, 65] and, conversely, enhanced ATP synthesis [66–68].
5. Essential regulation of cell bioenergetics by constitutive InsP3R Ca2+ transfer to mitochondria
Elevated [AMP] and the requirement for AMPK activation to induce pro-survival autophagy in response to loss of InsP3R Ca2+ signaling suggests that cells lacking this pathway have compromised bioenergetics. In a simple model, constitutive low-level InsP3R-mediated Ca2+ transfer to mitochondria promotes oxidative phosphorylation, and that cells lacking this pathway have diminished bioenergetics that are sensed by AMPK that activates autophagy. In agreement, incubating cells with methyl-pyruvate that is oxidized to provide reducing equivalents (NADH) to drive oxidative phosphorylation and ATP production [19] blocked autophagy and reduced elevated AMPK activity in cells with InsP3R activity inhibited [37]. Furthermore, inhibition of mitochondrial Ca2+ uptake by the specific Ca2+ uniporter blocker Ru360 [37] or RNAi knockdown of MCU (our unpublished observations) lowers OCR, activates AMPK and induces mTOR-independent autophagy in cells expressing InsP3R, effects that are strikingly similar to those of XeB or genetic knockdown of InsP3R, suggesting that their targets are in the same biochemical pathway. In agreement, the effects of InsP3R and MCU inhibition on AMPK activity and autophagy were not additive [37].
A model in which InsP3R Ca2+ transfer to mitochondria inhibits autophagy by providing ongoing support for optimal mitochondrial function provides testable predictions. First, the activities of Ca2+ dependent mitochondrial enzymes should be relatively acutely-dependent on InsP3R mediated Ca2+ release. Mitochondrial matrix Ca2+ can regulate oxidative phosphorylation at several sites, including the F1F0-ATPase and several dehydrogenases [69, 70]. The activity of pyruvate dehydrogenase (PDH) is activated by Ca2+-dependent pyruvate phosphatase-mediated dephosphorylation [71]. PDH was found to be hyper-phosphorylated in cells with InsP3R inhibited. Furthermore, pharmacological inhibition of PDH kinases in cells with InsP3R inhibited reduced PDH phosphorylation, restored OCR and reduced autophagy to control levels [37]. These results suggest that mitochondrial respiration is compromised in the absence of InsP3R function, and implicate insufficient PDH activity as an important component.
A second prediction of the model is that InsP3R-mediated Ca2+ release should be constitutively ongoing. The InsP3/Ca2+ signaling system has been widely considered within the context of agonist stimulation. Nevertheless, cells in their normal milieu in vivo are continuously bathed in a variety of factors that impinge on the PLC/InsP3 system. Spontaneous Ca2+ signals in the absence of external stimulation due to the presence of growth factors and low levels of other soluble factors in serum have been observed [72–74] and shown to be involved in cell differentiation and other processes and to be InsP3R dependent [75, 76]. Rubinsztein et al., who originally described InsP3 dependent mTOR-independent autophagy [41], proposed a cyclical scheme in which InsP3R released-Ca2+ activates calpain, which in turn cleaves the heterotrimeric protein Gsα, activating adenylyl cyclase resulting in an increase in the cAMP levels and activation of Epac. Epac activates a small G-protein Rap2B, which activates PLC-ε, resulting in the production of InsP3, activation of InsP3R and further Ca2+ release [77]. Through this and other mechanisms, it is likely that low [InsP3]i exist in all cells in vivo and can be expected to drive low-level InsP3R Ca2+ release activity. Spontaneous InsP3R-mediated Ca2+ release events are rare in saline buffer in vitro because [InsP3]i falls to subphysiological basal levels in the absence of these factors. Transient and highly localized release events observed with total internal reflection fluorescence microscopy, consistent with stochastic release from one to a few InsP3R throughout the cytoplasm [78, 79], are greatly enhanced in cells bathed in medium containing nutrient and growth factors that more closely mimics in vivo conditions [37]. It is notable that optimal mitochondrial NADH production is achieved by repetitive Ca2+ transients [59, 61, 68, 80]. It seems likely that most ongoing release events under these conditions are not observed because of the optical limits of imaging and the highly localized and transient nature of each event. The fact that global levels of ATP and cellular OCR are rapidly reduced upon inhibition of InsP3R mediated Ca2+ signaling [37] suggests that most mitochondria in the cell experience these Ca2+ release events, implying that the majority of Ca2+ release events have remained beyond the limits of optical detection.
Several studies have demonstrated that expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL suppresses autophagy [81]. In many cases this has been attributed to a direct molecular interaction of the anti-apoptotic proteins with a BH3 domain in beclin-1, that effectively sequesters beclin-1 from the autophagy machinery [81]. In addition, it has been proposed that a tri-molecular interaction exists between Bcl-2, the InsP3R and beclin-1 [46]. In this model, the InsP3R acts as a scaffold, independent of its channel function, to facilitate the interaction between Bcl-2 and beclin-1, keeping autophagy suppressed. It is notable that both the pro-autophagy functions of beclin-1 as well as the anti-autophagy properties of Bcl-2 require their targeting to the ER (reviewed in [81]). It was suggested that the major mechanism whereby the InsP3R impinged on the regulation of autophagy was by sequestration of beclin-1 [46]. However, no differences in the interaction between Bcl-2 and beclin-1 were observed between WT and DT40-KO cells [38], nor could we observe altered interactions of beclin-1 and InsP3R in response to XeB [37]. Alternately, it was proposed that Bcl-2 inhibits autophagy by reducing of the Ca2+ content of the ER [28, 34]. However, because of the dependence of the magnitude of the Ca2+ flux through the InsP3R on the concentration of Ca2+ in the ER lumen [82], it might be expected that, if anything, reduced ER luminal [Ca2+] would diminish Ca2+ transfer to mitochondria and consequently promote autophagy. Indeed, given the critical housekeeping role of low-level autophagy, this could be an adaptive mechanism, although this has not been explored. We propose here a different mechanism. It was previously demonstrated that Bcl-xL directly interacts with all isoforms of the InsP3R and sensitizes them to low [InsP3]i that exist in resting cells [83–85]. This sensitization promotes low-level Ca2+ release activity and accounts for the sometimes-observed reduction of ER [Ca2+] associated with Bcl-2 or Bcl-xL over-expression (see references in [83]). Importantly, enhanced InsP3R-mediated Ca2+ signaling by anti-apoptotic Bcl-2 family members provides enhanced apoptosis resistance [83–85]. It was suggested that apoptosis resistance was linked to increased bioenergetic fitness of cells with enhanced low level InsP3R-mediated Ca2+ signaling [83], the same mechanism described above that provides optimal bioenergetics that suppresses autophagy [37]. It is possible, therefore, that a mechanism by which Bcl-2 and Bcl-xL inhibit autophagy involves their ability to enhance InsP3R-mediated low level Ca2+ transfer to mitochondria. Future studies however are required to test this hypothesis, and to evaluate the role of beclin-1 in such a mechanism.
6. Summary
In summary, an essential cellular process that is required for efficient mitochondrial respiration and maintenance of normal cell bioenergetics involves constitutive Ca2+ transfer from the ER to mitochondria mediated by the InsP3R (Figure 2). In the absence of this ongoing uptake of Ca2+ by mitochondria, oxidative phosphorylation is reduced, lowering cellular levels of ATP. This bioenergetic deficit is sensed by AMPK, which in turn activates autophagy as a survival mechanism. Activation of autophagy as a response to reduced cellular ATP is not unexpected, as this is a response that cells employ when cellular energy stores become depleted during nutrient deprivation. But why do cells respond in this way to the reduction of InsP3R signals? The cells remain in nutrient replete media with normal or even enhanced nutrient uptake. It seems surprising that cells do not compensate for reduced oxidative phosphorylation in the absence of ongoing InsP3R-mediated Ca2+ transfer to mitochondria by using other mechanisms, for example enhanced nutrient uptake or proliferation of mitochondria or enhanced expression of mitochondrial enzymes. DT40-KO cells have survived for years in culture, employing autophagy as a cell survival mechanism rather than using other compensatory mechanisms to ensure bioenergetic homeostasis. Cells that lack InsP3R Ca2+ signaling behave as if they truly are starving, activating a seemingly inappropriate, since nutrients are abundant, autophagic response that must involve a major re-wiring of their metabolic pathways. In the future, mapping the metabolic programs that are activated by reductions of constitutive InsP3R-mediated Ca2+ signaling may shed light on the rationale for this strategy.
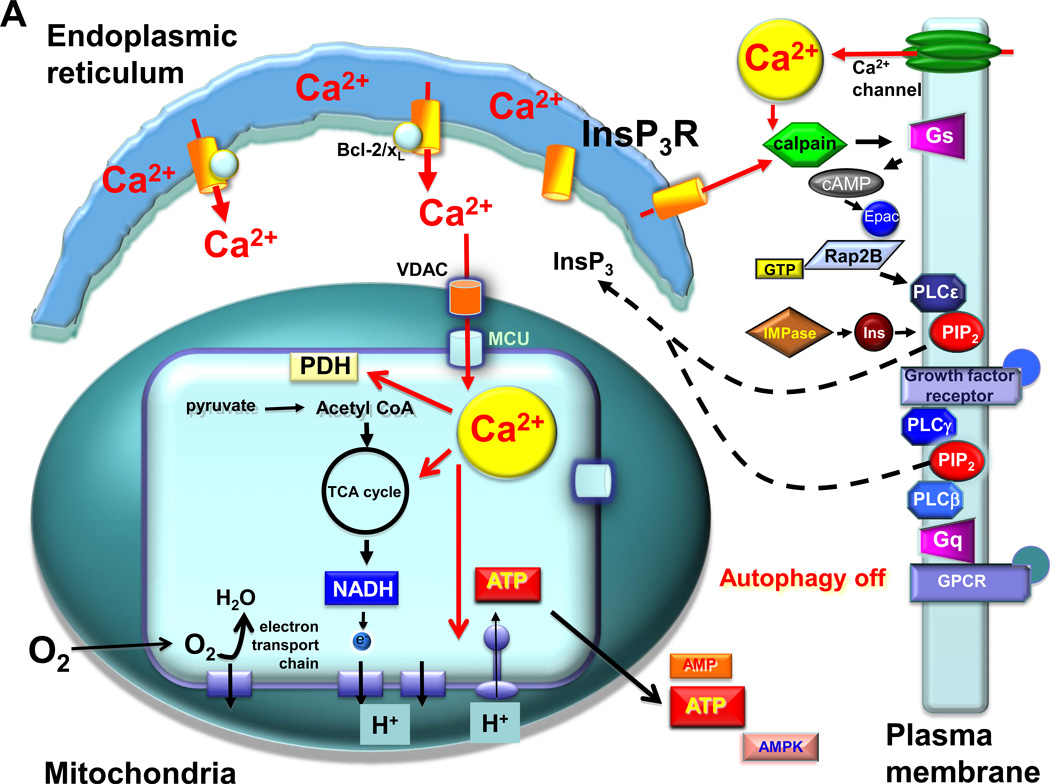
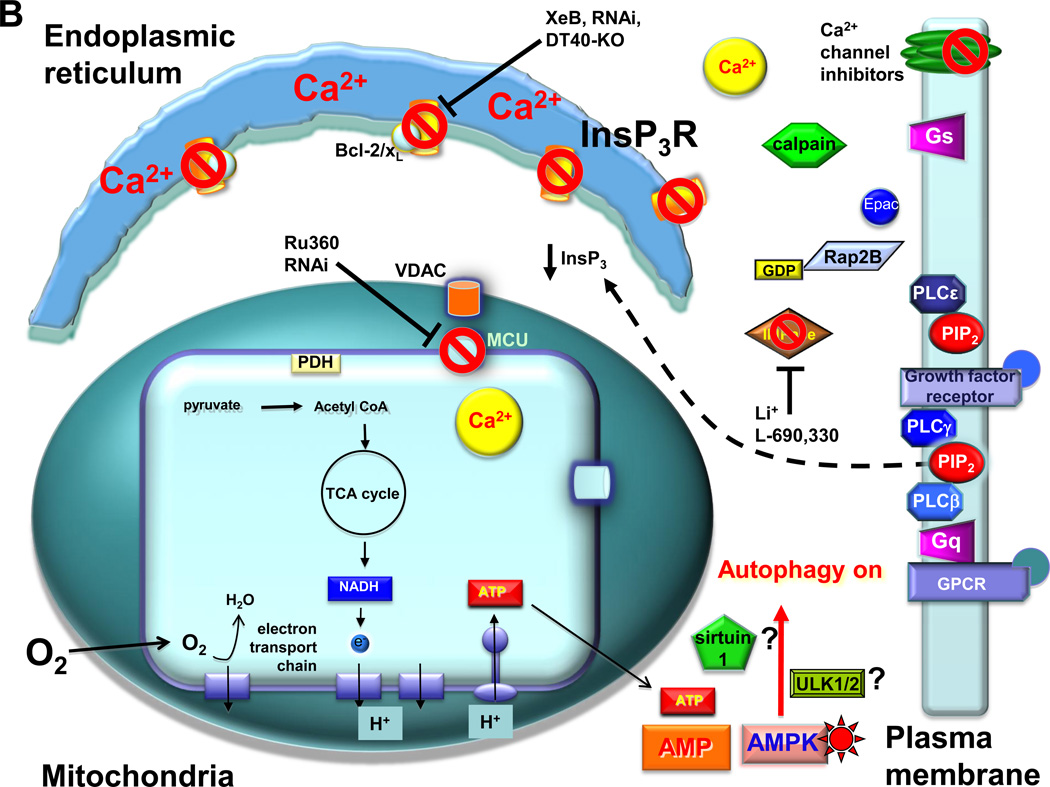
Fig. 2.
Suppression of InsP3R Ca2+ transfer to mitochondria induces mTOR-independent autophagy. A. In resting cells, low-level InsP3 production is maintained by circulating ligands for receptors that couple to phospholipases C (PLC) beta and gamma. In addition, InsP3R-mediated Ca2+ release and/or extracellular influx the Ca2+ can activate calpain, which cleaves and activates the heterotrimeric G-protein alpha subunit Gs, increasing the levels of cAMP, activating Epac to stimulate the small G protein Rap2B that activates PLCε. Phosphatidylinositol 4–5-biphosphate (PIP2) levels are maintained by a constant supply of inositol (Ins) by the inositol monophosphatase (IMPase). Low-level stochastic Ca2+ release from the endoplasmic reticulum by InsP3R, enhanced by Bcl-2 and Bcl-xL bound to the channel, provides Ca2+ to mitochondria in close proximity, mediated by the outer membrane VDAC and MCU at the inner mitochondrial membrane. Matrix Ca2+ activates pathways, including dehydrogenases and the F1-F0-ATPase, that fuel the electron transport chain and O2 consumption by providing reducing equivalents in the form of NADH. ATP production maintains a low cytoplasmic AMP:ATP ratio. This mechanism provides the cell with bioenergetic fitness that suppresses autophagy. B. The absence of constitutive delivery of Ca2+ from ER to mitochondria, due either to inhibition of InsP3R-mediated Ca2+ release (by genetic deletion of InsP3R (DT40-KO), inhibition of InsP3R activity (by xestospongin B (XeB), molecular ablation of the InsP3R (by RNAi), inhibition of InsP3 production by blocking the IMPase (by lithium (Li+) or L-690,330), blocking PLC (not shown; [37]), or inhibiting the calpain cascade that impinges on PLCε)); or by inhibition of mitochondrial Ca2+ uptake (Ru360 or MCU RNAi), results in diminished pyruvate dehydrogenase (PDH) activity (as well as the activities possibly other Ca2+-sensitive dehydrogenases and the ATP-synthase) that results in insufficient production of NADH, limiting the activity of the electron transport chain, diminishing O2 consumption and reducing ATP production. The consequent rise of the AMP:ATP ratio activates AMPK, which induces pro-survival, mTOR-independent autophagy, possibly involving ULK1/2 and/or sirtuin1.
The signal transduction pathways that couples AMPK activation by reduced InsP3R-mediated Ca2+ transfer to mitochondria to activation of autophagy are unknown. mTORC1 remains activated when this transfer is blocked, so the canonical pathway of autophagy activation is not involved. Ongoing mTORC1 activity could explain why DT40-KO cells are able to proliferate and survive indefinitely in culture in the absence of InsP3R Ca2+ signaling and ongoing autophagy. Of note, moderate ATP depletion induced by low concentrations of 2-deoxyglucose activates AMPK without affecting mTORC1 activity [86], whereas acute depletion of cellular ATP induced by starvation and glucose deprivation may require the cessation of all catabolic process controlled by mTOR [23, 24]. Thus, inhibition of InsP3R mediated Ca2+ transfer to mitochondria may simulate mild nutrient starvation and ATP depletion that activates AMPK and autophagy without affecting mTORC1 activity. Nevertheless, activation of autophagy in the absence of InsP3R-mediated Ca2+ transfer to mitochondria has an absolute requirement for AMPK activity [37]. How does enhanced AMPK activity impinge on autophagy in the absence of mTORC1 involvement? Many proteins are essential for the execution of autophagy [1, 6], and it is possible that some of them may be AMPK substrates. The ULK1/2 kinases that play critical roles in the initiation of autophagy have been shown to be activated by AMPK-mediated phosphorylation [15, 87]. Another possibility is sirtuin1, a NAD+ dependent deacetylase involved in cellular bioenergetics sensing, which regulates and is regulated by AMPK [88]. Sirtuin1 can stimulate autophagy by deacetylation of essential components of the autophagic machinery [89], by-passing the activity of mTORC1, similar to that observed after InsP3R inhibition. Further experiments are necessary to clarify the molecules involve in the mTORC1-independent autophagy induced by absence of InsP3R-mediated Ca2+ transfer to mitochondria.
Acknowledgements
The authors acknowledge financial support from the National Institutes of Health (GM/DK56328 to JKF). CC was supported by an award from the American Heart Association.
List of abbreviations
- mTOR
mammalian target of rapamycin
- InsP3R
inositol 1,4,5-trisphosphate receptor
- AMPK
adenosine monophosphate activated protein kinase
- mTORC1
mammalian target of rapamycin complex 1
- GAP
GTPase activating protein
- TSC1/TSC2
tuberous sclerosis complex 1/2
- ULK
Unc-51-like kinase
- LKB1
liver kinase B1
- CaMKKβ
Ca2+-calmodulin dependent protein kinase kinase beta
- [Ca2+]i
cytoplasmic free Ca2+ concentration
- ER
endoplasmic reticulum
- LRRK2
leucine-rich repeat kinase-2
- BAPTA-AM
1,2-bis(o-aminophenoxy) ethane-N,N,N',N'-tetraacetic acid acetoxy-methy ester
- IMPase
inositol monophosphatase
- XeB
xestospongin B
- DT40-KO
DT40 chicken B lymphoblasts lacking at three InsP3R isoforms
- AMP
adenosine monophosphate
- OCR
oxygen consumption rate
- NAD
nicotinamide adenine dinucleotide
- NADH
reduced nicotinamide adenine dinucleotide
- VDAC
voltage-dependent anion channel
- MCU
mitochondrial calcium uptake uniporter pore
- MiCa
mitochondrial inner membrane calcium channel
- OMM
mitochondrial outer membrane
- PDH
pyruvate dehydrogenase
- PLC
phospholipase C
- Gsα
type S alpha subunit of heterotrimeric guanine nucleotide binding protein
- BH3
Bax homology domain 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 2.Wirawan E, Vanden Berghe T, Lippens S, Agostinis P, Vandenabeele P. Autophagy: for better or for worse. Cell Res. 2012;22:43–61. doi: 10.1038/cr.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decuypere JP, Bultynck G, Parys JB. A dual role for Ca2+ in autophagy regulation. Cell Calcium. 2011;50:242–250. doi: 10.1016/j.ceca.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol. 2008;182:685–701. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell. 2010;141:656–667. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizushima N, Klionsky DJ. Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 7.Klionsky DJ, Cuervo AM, Dunn WA, Jr, Levine B, van der Klei I, Seglen PO. How shall I eat thee? Autophagy. 2007;3:413–416. doi: 10.4161/auto.4377. [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Yao TP. Quality control autophagy: A joint effort of ubiquitin, protein deacetylase and actin cytoskeleton. Autophagy. 2010;6:555–557. doi: 10.4161/auto.6.4.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marino G, Lopez-Otin C. Autophagy: molecular mechanisms, physiological functions and relevance in human pathology. Cell Mol Life Sci. 2004;61:1439–1454. doi: 10.1007/s00018-004-4012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scarlatti F, Granata R, Meijer AJ, Codogno P. Does autophagy have a license to kill mammalian cells? Cell Death Differ. 2009;16:12–20. doi: 10.1038/cdd.2008.101. [DOI] [PubMed] [Google Scholar]
- 12.Shen S, Kepp O, Kroemer G. The end of autophagic cell death? Autophagy. 2012;8:1–3. doi: 10.4161/auto.8.1.16618. [DOI] [PubMed] [Google Scholar]
- 13.Yang YP, Liang ZQ, Gu ZL, Qin ZH. Molecular mechanism and regulation of autophagy. Acta Pharmacol Sin. 2005;26:1421–1434. doi: 10.1111/j.1745-7254.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 14.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the Regulation of Autophagy: Cross Talk, Shortcuts, and Feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arsham AM, Neufeld TP. Thinking globally and acting locally with TOR. Curr Opin Cell Biol. 2006;18:589–597. doi: 10.1016/j.ceb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 20.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 21.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Wilson WA, Fujino MA, Roach PJ. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol Cell Biol. 2001;21:5742–5752. doi: 10.1128/MCB.21.17.5742-5752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281:34870–34879. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 24.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 25.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witters LA, Kemp BE, Means AR. Chutes and Ladders: the search for protein kinases that act on AMPK. Trends Biochem Sci. 2006;31:13–16. doi: 10.1016/j.tibs.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Son SM, Jung ES, Shin HJ, Byun J, Mook-Jung I. Abeta-induced formation of autophagosomes is mediated by RAGE-CaMKKbeta-AMPK signaling. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.09.039. (Available online 1 November 2011). [DOI] [PubMed] [Google Scholar]
- 30.Knoferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, Tonges L, Stadelmann C, Bruck W, Bahr M, Lingor P. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci USA. 2010;107:6064–6069. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Weiss LM, Orlofsky A. Host cell autophagy is induced by Toxoplasma gondii and contributes to parasite growth. J Biol Chem. 2009;284:1694–1701. doi: 10.1074/jbc.M807890200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, Jaattela M. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grotemeier A, Alers S, Pfisterer SG, Paasch F, Daubrawa M, Dieterle A, Viollet B, Wesselborg S, Proikas-Cezanne T, Stork B. AMPK-independent induction of autophagy by cytosolic Ca2+ increase. Cell Signal. 2010;22:914–925. doi: 10.1016/j.cellsig.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Brady NR, Hamacher-Brady A, Yuan H, Gottlieb RA. The autophagic response to nutrient deprivation in the hl-1 cardiac myocyte is modulated by Bcl-2 and sarco/endoplasmic reticulum calcium stores. Febs J. 2007;274:3184–3197. doi: 10.1111/j.1742-4658.2007.05849.x. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Suaga P, Luzon-Toro B, Churamani D, Zhang L, Bloor-Young D, Patel S, Woodman PG, Churchill GC, Hilfiker S. Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP. Hum Mol Genet. 2012;21:511–525. doi: 10.1093/hmg/ddr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decuypere JP, Welkenhuyzen K, Luyten T, Ponsaerts R, Dewaele M, Molgo J, Agostinis P, Missiaen L, De Smedt H, Parys JB, Bultynck G. Ins(1,4,5) P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy. 2011;7:1472–1489. doi: 10.4161/auto.7.12.17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan MT, Joseph SK. Role of inositol trisphosphate receptors in autophagy in DT40 cells. J Biol Chem. 2010;285:16912–16920. doi: 10.1074/jbc.M110.114207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan Y, Flinn RJ, Wu H, Schnur RS, Backer JM. hVps15, but not Ca2+/CaM, is required for the activity and regulation of hVps34 in mammalian cells. Biochem J. 2009;417:747–755. doi: 10.1042/BJ20081865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Yu J, Pan H, Hu P, Hao Y, Cai W, Zhu H, Yu AD, Xie X, Ma D, Yuan J. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc Natl Acad Sci U S A. 2007;104:19023–19028. doi: 10.1073/pnas.0709695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, O'Kane CJ, Floto RA, Rubinsztein DC. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia HG, Zhang L, Chen G, Zhang T, Liu J, Jin M, Ma X, Ma D, Yuan J. Control of basal autophagy by calpain1 mediated cleavage of ATG5. Autophagy. 2010;6:61–66. doi: 10.4161/auto.6.1.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D, Molgo J, Diaz J, Lavandero S, Harper F, Pierron G, di Stefano D, Rizzuto R, Szabadkai G, Kroemer G. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 46.Vicencio JM, Ortiz C, Criollo A, Jones AW, Kepp O, Galluzzi L, Joza N, Vitale I, Morselli E, Tailler M, Castedo M, Maiuri MC, Molgo J, Szabadkai G, Lavandero S, Kroemer G. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 2009;16:1006–1017. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- 47.Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 49.Harr MW, McColl KS, Zhong F, Molitoris JK, Distelhorst CW. Glucocorticoids downregulate Fyn and inhibit IP3-mediated calcium signaling to promote autophagy in T lymphocytes. Autophagy. 2010;6:912–921. doi: 10.4161/auto.6.7.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimball SR. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc. 2006;38:1958–1964. doi: 10.1249/01.mss.0000233796.16411.13. [DOI] [PubMed] [Google Scholar]
- 51.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 52.Hansford RG. Physiological role of mitochondrial Ca2+ transport. J Bioenerg Biomembr. 1994;26:495–508. doi: 10.1007/BF00762734. [DOI] [PubMed] [Google Scholar]
- 53.Lasorsa FM, Pinton P, Palmieri L, Fiermonte G, Rizzuto R, Palmieri F. Recombinant expression of the Ca2+-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chinese hamster ovary cells. J Biol Chem. 2003;278:38686–38692. doi: 10.1074/jbc.M304988200. [DOI] [PubMed] [Google Scholar]
- 54.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 57.Spat A, Szanda G, Csordas G, Hajnoczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium. 2008;44:51–63. doi: 10.1016/j.ceca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 59.Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 60.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 61.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 62.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. Embo J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duchen MR. Ca2+-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem J. 1992;283:41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pralong WF, Hunyady L, Varnai P, Wollheim CB, Spat A. Pyridine nucleotide redox state parallels production of aldosterone in potassium-stimulated adrenal glomerulosa cells. Proc Natl Acad Sci U S A. 1992;89:132–136. doi: 10.1073/pnas.89.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci U S A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balaban RS. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim Biophys Acta. 2009;1787:1334–1341. doi: 10.1016/j.bbabio.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Territo PR, Mootha VK, French SA, Balaban RS. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol Cell Physiol. 2000;278:C423–C435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 71.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34:217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- 72.Ciccolini F, Collins TJ, Sudhoelter J, Lipp P, Berridge MJ, Bootman MD. Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. J Neurosci. 2003;23:103–111. doi: 10.1523/JNEUROSCI.23-01-00103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng B, McMahon DG, Mattson MP. Modulation of calcium current, intracellular calcium levels and cell survival by glucose deprivation and growth factors in hippocampal neurons. Brain Res. 1993;607:275–285. doi: 10.1016/0006-8993(93)91517-v. [DOI] [PubMed] [Google Scholar]
- 74.Ferrari MB, Spitzer NC. Calcium signaling in the developing Xenopus myotome. Dev Biol. 1999;213:269–282. doi: 10.1006/dbio.1999.9387. [DOI] [PubMed] [Google Scholar]
- 75.Carey MB, Matsumoto SG. Neurons differentiating from murine neural crest in culture exhibit sensory or sympathetic-like calcium currents. J Neurobiol. 1999;39:501–514. doi: 10.1002/(sici)1097-4695(19990615)39:4<501::aid-neu4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 76.Kanemaru K, Okubo Y, Hirose K, Iino M. Regulation of neurite growth by spontaneous Ca2+ oscillations in astrocytes. J Neurosci. 2007;27:8957–8966. doi: 10.1523/JNEUROSCI.2276-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009;16:46–56. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- 78.Smith IF, Parker I. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc Natl Acad Sci USA. 2009;106:6404–6409. doi: 10.1073/pnas.0810799106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith IF, Wiltgen SM, Parker I. Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium. 2009;45:65–76. doi: 10.1016/j.ceca.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robb-Gaspers LD, Burnett P, Rutter GA, Denton RM, Rizzuto R, Thomas AP. Integrating cytosolic calcium signals into mitochondrial metabolic responses. Embo J. 1998;17:4987–5000. doi: 10.1093/emboj/17.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vais H, Foskett JK, Daniel Mak DO. Unitary Ca2+ current through recombinant type 3 InsP3 receptor channels under physiological ionic conditions. J Gen Physiol. 2010;136:687–700. doi: 10.1085/jgp.201010513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem. 2010;285:13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-xL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci U S A. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horman S, Browne G, Krause U, Patel J, Vertommen D, Bertrand L, Lavoinne A, Hue L, Proud C, Rider M. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol. 2002;12:1419–1423. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 87.Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011;7:643–644. doi: 10.4161/auto.7.6.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salminen A, Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009;15:217–224. doi: 10.1016/j.molmed.2009.03.004. [DOI] [PubMed] [Google Scholar]