Abstract
MRI pulse sequences designed to increase the speed and spatial resolution of fMRI have always been a hot topic. Here, we review and chronicle the history behind some of the pulse sequence ideas that have contributed not only to the enhancement of fMRI acquisition but also to diffusion imaging. (i) Partial Fourier EPI allows lengthening echo trains for higher spatial resolution while maintaining optimal TE and BOLD sensitivity. (ii) Inner-volume EPI renamed zoomed-EPI, achieves extremely high spatial resolution and has been applied to fMRI at 7 Tesla to resolve cortical layer activity and columnar level fMRI. (iii) An early non-BOLD approach while unsuccessful for fMRI created a diffusion sequence of bipolar pulses called ‘twice refocused spin echo’ now widely used for high resolution DTI and HARDI neuronal fiber track imaging. (iv) Multiplexed EPI shortens TR to a few hundred millisecond, increasing sampling rates and statistical power in fMRI.
Keywords: EPI, partial Fourier, zoomed EPI, inner volume, multiplexed EPI, multiband, simultaneous, SIR, SER, twice-refocused SE diffusion, bipolar gradient, layer specific, resting state, columnar level, BOLD, functional imaging, fMRI
Early Echo Planar Imaging in North America
Echo planar imaging (EPI), invented by Sir Peter Mansfield (Mansfield, 1977) at the University of Nottingham, England, is now the technique most frequently used for fMRI and diffusion imaging. Paradoxically, EPI required such fast gradient switching that it was not possible to perform on conventional MR scanners and therefore its use was confined to the Nottingham laboratory for many years. The first EPI research and imaging outside of Nottingham was in North America at the UCSF Radiological Imaging Laboratory (RIL) directed by Leon Kaufman. The experiments were conducted on a whole body scanner operating at 0.35 Tesla, the first to use a superconducting magnet (Oxford Magnets) in the USA, called “P-0” for prototype, that had relatively slow gradients so only a few echoes could be refocused in the echo train during the T2* signal decay, resulting in small image matrices and low SNR. The gradient rise time was 1.4 ms to a maximum of 4 mT/m. So, in an EPI acquisition, 2.8 ms was required to switch the read gradient (−peak to + peak), resulting in a 5.0 ms echo spacing with a 2.2 ms (ADC) echo readout. In comparison, a typical modern scanner’s gradients (Siemens, 3T Trio) with 40 mT/m maximum, can achieve an echo spacing of 0.5 ms. The P-0 scanner was about an order of magnitude slower than the Nottingham EPI scanner and it could only produce 24 echoes in a T2* decay period of 120 ms. The resulting image had very low spatial resolution, long TEs (~60ms) and considerable T2* dependent voxel blurring and geometric distortions.
Considerable innovation in pulse sequence design was directed to overcome the limitations of spatial resolution and low SNR in EPI due to the slow gradients on the P-0 scanner at UCSF RIL. This resulted in several (Fig. 1) novel EPI variants; partial Fourier EPI, zoomed-EPI and spin echo EPI, all now commonly employed in fMRI. When these EPI variants were later performed on fast gradient systems they were, as the EPI sequence itself, even more useful and are now common in both routine fMRI and for achieving very high spatial resolution fMRI at ultra-high field. What follows is a more detailed review of this early EPI development up to the more recent fMRI applications at columnar level resolutions, as well as early attempts to perform functional MRI that led to new diffusion sequences. Finally, we review a recent pulse sequence innovation, multiplexed-EPI (simultaneous slice and multiband EPI), which provides unprecedented increases in temporal resolution and higher statistical power for fMRI.
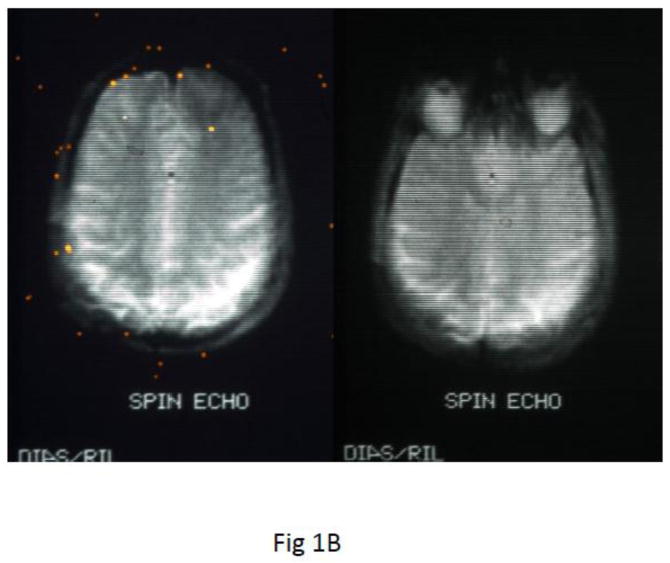
Fig. 1.
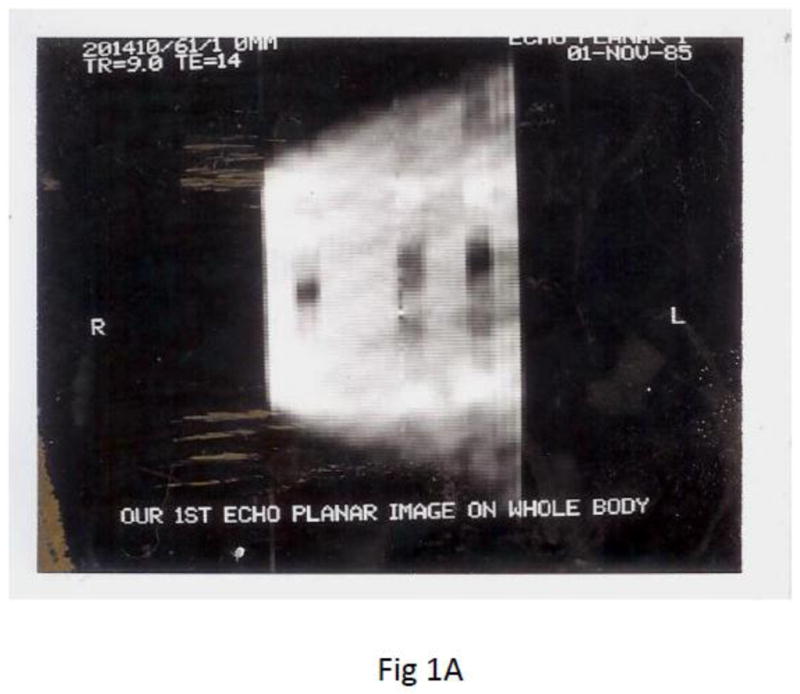
Earliest known EPI images in North American made on a 0.35 Tesla superconducting magnet P-0 scanner at UCSF RIL. A) EPI image of phantom made with slow gradient switching. To compensate for the only possible few echoes refocused, the EPI pulse sequence combined 3 novel techniques i) inner-volume (zoomed-EPI) to achieve 4 times higher resolution, ii) half Fourier for earlier TE to increase SNR and iii) spin echo CPMG refocusing for multiple gradient echoes and spin echoes to reduce field inhomogeneity errors. Spatial resolution 1.7mm × 1.7mm and 10mm thick (Feinberg and Hale, 1986). B) half Fourier spin echo EPI acquired on a faster gradient coil insert (Crooks et al., 1986) eliminated the first half of k-space allowing the center of k-space (Ko) to move to the beginning of the echo train, TE=14 ms for higher SNR.
Partial Fourier EPI
The realization that the requisite number of phase encoded signals in k-space can be nearly halved to reduce the scan time of MRI came from DF’s graduate research at Berkeley with electron diffraction in which only half of a digital diffraction pattern reconstructed by 2D FT was required to visualize the entire crystal lattice. The other half of the diffraction pattern was by Hermitian symmetry simply obtained from the transposed complex conjugate number, avoiding unnecessary printouts requiring additional computer paper. Two groups independently developed and published papers on the general technique of half Fourier imaging within months of each other, one effort was led by Paul Margosian at Siemens in Germany (Margosian, 1986) and the other effort led by DF at UCSF RIL (Feinberg et al., 1986) and in November 1985, DF combined Half Fourier with EPI to improve performance on the slow gradient system (Feinberg and Hale, 1986). In January of 1986 Lawrence Crooks completed the building of a faster switching gradient coil insert for head and pediatric imaging within the P-0 scanner at UCSF and the fast switching coil considerably improved the quality of Half Fourier EPI (Crooks et al., 1986). The full description of the pediatric echo planar scanner was published two years later (Crooks et al., 1988), by which time the fields attention was on the resonance coil technology of Richard Redzian and Ian Pykett (Pykett and Rzedzian, 1987) used to make EPI at MGH. The UCSF RIL gradient coil insert was used to acquire the first published diffusion weighted EPI images in 1988 with human brain diffusion imaging presented at the SMRM (Avram and Crooks, 1988).
Reduced Field of View EPI
Spatial resolution in EPI can be increased without additional signals provided the image field of view (FOV) can be reciprocally reduced. Reduced FOV imaging (Feinberg et al., 1985), originally called ‘inner-volume EPI’ (Feinberg and Hale, 1986) later called ‘zoomed EPI’ (Mansfield et al., 1988) uncoupled the otherwise direct dependence of spatial resolution (R) on the number of phase encoded echoes (N) in the echo train, limited by gradient performance and T2* decay, where in general terms: R = FOV/N. By restricting the signal volume and commensurately reducing FOV, the resolution is increased (smaller R) without increasing N and without signal aliasing. There are now three different means of restricting signal regions and reducing the FOV to achieve zoomed-EPI i) inner-volume imaging utilizing intersecting planes of excitation and refocusing creating a SE refocused region, ii) 2D selective excitation pulses (Pauly et al., 1989) with tailored RF pulses restricting excitation in two axes and iii) outer volume suppression (Heidemann et al., 2010; Le Roux et al., 1998; Pfeuffer et al., 2002) to reduce the extent of signal on phase encoded axes. The inner-volume variant is limited to single slice 2D or 3D imaging because one of the two perpendicular oriented RF regions has a secondary effect of saturating spins in locations of adjacent slices (T2* weighting, if desired, is still feasible using an asymmetric TE (Weisskoff and Kiihne, 1992)). Outer volume suppression methods can be used for GE EPI and for multi-slice acquisitions, but to varying degrees it has incomplete saturation/suppression which is a potential source of physiological noise in fMRI.
Spin Echo EPI
A major focus of the UCSF RIL lab throughout the 1980s was to explore and develop spin echo imaging. Many of the scientists at this lab came from UC Berkeley where Erwin Hahn who discovered spin echoes (Hahn, 1950), was a professor of physics. It was logical to develop spin echo refocused EPI to improve the image quality by controlling field inhomogeneity and susceptibility artifacts. On the slow gradient system of P-0, a CPMG spin echo sequence was combined with EPI so the signal decay was determined primarily by T2 instead of T2*, enabling a longer echo train for higher resolution and reduced distortions in the final images (Feinberg and Hale, 1986). The short EPI echo trains were between multiple 180° refocusing pulses to create both gradient echoes and spin echoes in one echo train. This EPI variant combining CPMG sequence was later more fully developed and given the acronym for gradient-and-spin echo (GRASE) (Oshio and Feinberg, 1991) and is now used at 7T for high resolution fMRI. Other variants of SE EPI; two images per echo train at different TE and inversion recovery EPI were also reported (Crooks et al., 1988).
Whereas BOLD T2* weighted EPI images are now mainly used for fMRI, it was not in the beginning unreasonable to explore other contrast mechanisms to make fMRI images, as certainly today it is known that changes in blood volume and blood flow also contribute to fMRI contrast mechanisms. One such early approach was explored by DF beginning in 1986. In an attempt to make functional images of activity in primary visual cortex, diffusion and T2 weighted SE imaging was used in block designed experiments (lights on versus lights off) first performed on a Diasonics 0.35 Tesla scanner identical to P-0 at Huntington Research Institute in Pasadena, California. Regrettably, several hours after using the scanner for this experiment the magnet quenched for no apparent reason; i.e. lost its electrical current and boiled off much of the liquid helium. Over the next six months the magnet quenched two more times, several hours after performing the fMRI experiment using large diffusion gradient pulses, so the experiment was discontinued until it could be performed on a more stable magnet. It was assumed, but never confirmed, that the long diffusion gradient pulses caused eddy currents and mechanical shaking of the magnet’s internal structures contributing to the instability in this second delivered Oxford superconducting magnet in the USA.
This diffusion based fMRI experiment continued a few years later in Boston in 1989, at Brigham and Women’s Hospital, Harvard Medical School, on a scanner built by Leo Neuringer’s group in an MIT-IBM collaboration, as IBM was considering entering the MRI business (the radar and radio manufacturing company, Marconi, was another early entrant in commercializing MRI scanners). This MIT-IBM scanner had for its time, extremely strong 20mT/m gradient strength, which would be very useful for diffusion encoding permitting much shorter pulses than was previously needed with the 5mT/m gradients. The gradients, however, had incorrectly calibrated shielding causing considerable eddy currents. Overcoming the eddy currents led to two distinct pulse sequence innovations for EPI-based imaging. A severe Nyquist ghost caused by eddy currents was entirely eliminated using an asymmetric read gradient waveform which eliminated echo time reversal, now known as the “fly-back” k-space trajectory (Feinberg et al., 1990).
Twice refocused spin echo diffusion sequence
The second pulse sequence innovation was a diffusion pulse sequence that drastically reduced problems of eddy currents by means of using two 180° refocusing pulses combined with four gradient pulses of alternating polarities (G+−+−) hence bipolar which cancelled eddy currents, and is now called the twice refocused spin echo (TRSE) diffusion sequence or simply the bipolar sequence (Feinberg and Jakab, 1990) (Fig. 2). Karlicek and Lowe earlier developed a very different alternating pulsed gradient sequence utilizing five 180° refocusing pulses and gradient polarities (G+−−+) to measure restricted diffusion independent of cross terms from field inhomogeneity (Karlicek and Lowe, 1980). In comparison, the Stejskal-Tanner diffusion sequence uses a pair of monopolar gradient pulses (G++) with a single 180° refocusing pulse between them. By exploring several different possible gradient polarities (Fig. 2) we discovered the TRSE diffusion sequence had much greater diffusion sensitivity, achieved a higher b-value in the same time than other polarity configurations, and had far less image artifact from eddy currents, which were largely cancelled by the opposite polarity (bipolar) gradients. Cautions on combining data from diffusion sequences with different eddy currents were discussed in our paper and eddy current improvements of the TRSE sequence were described at several conferences (Feinberg and Jakab, 1989), however, the sequence’s impact would come years later with the invention of diffusion tensor imaging (DTI) (Basser et al., 1994; Basser and Pierpaoli, 1996).
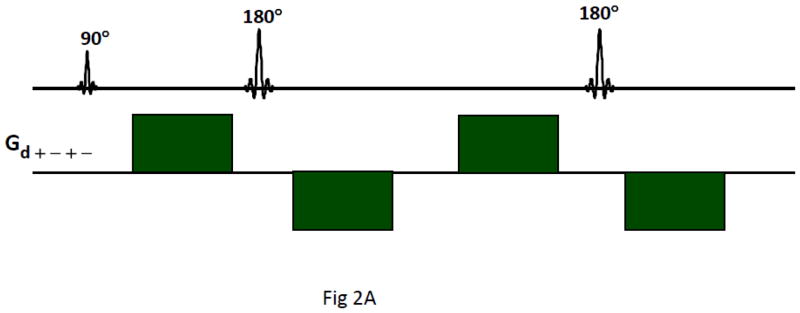
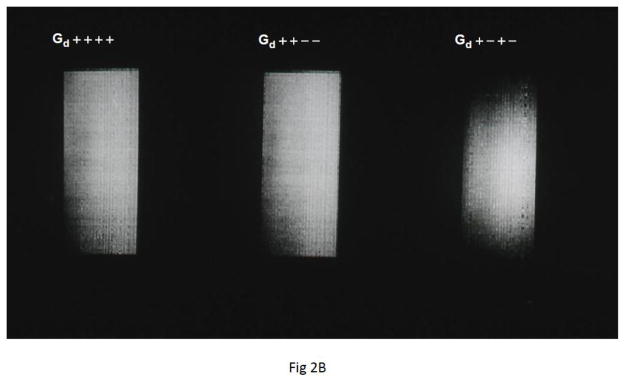
Fig. 2.
A) Twice refocused spin echo (TRSE) diffusion sequence with bipolar alternating polarity gradients was innovated for early fMRI experiments (Feinberg and Jakab, 1990). B) Initial comparison of diffusion encoding schemes revealed higher signal attenuation hence highest b-values in bipolar TRSE sequence (right) labeled Gd (+−+−) compared to the two monopolar gradient schemes (++++ and ++−−) in same time. One line from each image (horizontal axis) varying gradient amplitudes (vertical axis) incremented from maximum (−Gs to +Gs) in 128 TR repetitions, phantom 30mm diameter bottle of gadolinium doped water, acquired with inner-volume zoomed-EPI and fly-back k-space trajectory.
In 1990, Denis LeBihan and Robert Turner organized the ”SMRM Workshop on future directions in diffusion MRI” in Bethesda. At the workshop Dr. LeBihan politely introduced DF to Professor Edward Stejskal who said throughout the morning session he had been studying the diagrams in DF’s syllabus paper which compared the phase and moment squared diagrams of the Stejskal-Tanner and TRSE sequences. He opened his conference syllabus to the diagram pages held by his pencil and amazingly said that for the first time he saw where his equation’s −1/3 delta term originated from in the sequence timing. No less kind and encouraging, that evening Prof. Stejskal joined two junior scientists DF and James Pekar for dinner at an inexpensive restaurant where he described early experimental hardware used to make large diffusion gradients. Years later, in 1998, Timothy Reese and colleagues at MGH applied the TRSE diffusion sequence in DTI to reduce eddy current dependent misalignments and distortions in combined images (Reese et al., 1998) and subsequently, Oliver Heid at Siemens (Heid, 2000) modified the gradient pulse widths in TRSE as an additional means to spoil stimulated echoes and to achieve shorter TEs. The TRSE diffusion sequence is now in common use in neurosciences for high resolution neuronal fiber tracks, however, its invention was motivated by diffusion based functional MRI experiments.
Efforts towards higher spatial resolution fMRI in humans at the CMRR
At the Center for Magnetic Resonance (CMRR) at the University of Minnesota, under the support and direction of Kamil Ugurbil, the push to higher spatial resolution fMRI in humans was from the beginning a primary goal. Not long after fMRI images were produced in CMRR (Ogawa et al., 1992), the efforts to push the spatial resolution began in collaboration with Dr. Ravi Menon in the mid-90s, mapping ocular dominance columns in human subjects with fMRI (Menon et al., 1997). These early studies were temporally inefficient, taking on the order of 15 sec or more to make a single high-resolution FLASH image. It was also clear that the sensitivity and specificity of high-resolution GE-BOLD fMRI images were limiting, even at 4 Tesla. In 1998, with the installation of the world’s first 7 Tesla human magnet at the CMRR, the opportunity presented itself to increase both the specificity and sensitivity of BOLD images. Early studies established not only the feasibility of 7T fMRI in humans (Duong et al., 2003; Yacoub et al., 2003; Yacoub et al., 2001; Yacoub et al., 2005), but also the expected field-dependent increases in BOLD contrast and specificity to the microvasculature (Ugurbil et al., 2006a; Ugurbil et al., 2006b). SE-based BOLD contrast, as predicted (Ogawa et al., 1993; Ogawa et al., 1992), provided even more spatial specificity by reducing signals from large vessels whereby enhancing signals from small vessels in grey matter. The challenge at 7 T would be to generate BOLD images at high resolution with temporal efficiency and sufficient functional contrast-to-noise (fCNR). The strategy used was to reduce the imaging FOV sufficiently such that sub-millimeter resolution images could be generated quickly. Further, the RF coil (Adriany et al., 2001), built by Dr. Gregor Adriany, was optimized for studies of the human visual cortex. The coil had an open design for visual presentation and for use with a bite bar to restrict subject motion. It also provided the needed sensitivity in posterior parts of the visual cortex using 6-cm quadrature receiver loops and a separate large half volume transmitter for uniform B1+ fields. The RF coil profile maximized signal in visual areas, with a sharp drop off in sensitivity beyond that, permitting acquisition of high-resolution images with a smaller phase FOV. The echo train was then also reduced substantially, which was critical at 7T due to the short T2*s. Further, a high performance (Magnex) gradient coil insert, designed for head imaging (38 cm ID), had been installed at the 7T allowing gradients of 40 mT/m in 200 μsec further reducing the echo spacing. The use of multi-shot (2–4 segments) resulted in GE-EPI TRs of 6 s for 0.5 mm in-plane resolutions (Yacoub et al., 2007; Yacoub et al., 2005). Outer volume suppression was also explored to reduce the FOV enough so that single shot EPI might also be used (Pfeuffer et al., 2002), however, it did not achieve the necessary SNR nor the spatial coverage necessary for high resolution columnar imaging. Prior to parallel imaging techniques (Pruessmann et al., 1999; Sodickson et al., 1999), ‘zoomed’ EPI was useful for obtaining high resolution SE EPI. The needed refocusing pulse allowed selective excitation along a 2nd dimension, allowing FOV reduction to any arbitrary 2D volume. The acquisition of efficient high-resolution SE and GE BOLD images at 7T permitted robust columnar mapping, peaking with the first demonstrations of orientation columns in humans (Yacoub et al., 2008) and demonstrating the superiority of SE-based methods for high resolution mapping questions (Yacoub et al., 2007). Despite this success, it was clear that single slice (anisotropic) acquisitions for BOLD images would be problematic due to the locations and curvatures of cortical regions of interest and because of motion correction and anatomical registration in small volumes.
7 Tesla columnar level and cortical layer fMRI with Zoomed EPI and 3D GRASE
Some years later, in 2006 at a Gordon Conference on Cerebral blood flow and Metabolism held in Oxford, DF saw movies of ODC and orientation columns in humans (Yacoub et al., 2008), and was impressed by the spatial resolution but was unaware of the techniques used by Essa Yacoub (EY) and Noam Harrel (NH) at Minnesota. The 2D columnar images were acquired in individuals with a flat calcarine sulcus, positioning a thick slice with high in plane resolution over this flat region of gray matter. The limitation to a single slice plane was due to inner-volume 2D SE-EPI in which the orthogonal refocusing planes used to restrict the FOV prevented multi-slice acquisition. A collaboration was initiated by DF and NH at the 2007 ISMRM High Field Workshop when it was realized that making a single-shot inner-volume 3D GRASE sequence (Feinberg et al., 2008), would overcome several limitations. 3D-SE fMRI utilizing 3D GRASE increased the sensitivity of the high-resolution fMRI images by means of the longer echo trains possible using the multiple refocusings of GRASE rather than the single refocusing of SE-EPI. The GRASE sequence created stimulated echoes in addition to gradient and spin echoes, which came from magnetization stored on the longitudinal axis, hence they had slower T1 relaxation (~1500ms in grey matter) compared to T2 (~50ms) to maintain the signal amplitude in the echo train. This zoomed 3D GRASE image achieved SE type contrast in a single-shot, with resolutions as high as 0.6 mm isotropic. Recently, 3D GRASE was used to map axis of motion organization in human MT (Zimmermann et al., 2011). The SE contrast mechanism has been repeatedly demonstrated to be important at high fields because it minimizes signals from large vessels, which can preclude accurate high-resolution mapping. This was especially the case in human studies were limited imaging FOVs are needed, limiting the flexibility of avoiding areas with contaminating vessels. Approaches for zoomed GE-EPI using outer volume suppression with parallel imaging for fMRI at 7 Tesla have been demonstrated by Bob Turner’s group at Max Planck Institute in Leipzig (Heidemann et al., 2010). Future directions will aim to optimize zoomed imaging to extend the volume coverage with even more slices, likely involving parallel imaging, multi-slab, and multiplexed-EPI approaches. These advances can also be applied to increase spatial and temporal resolution in whole brain coverage using EPI at 7 Tesla (Fig. 3).
Fig. 3.
Recent 7 Tesla GE-EPI images acquired with high spatial resolution 0.75 mm isotropic resolution. Shown are two of 128 slices acquired in TR =5s. Image parameters: matrix 256×256 partial Fourier=5/8, parallel imaging IPAT=4, TE=20 msec, 48 msec readout per image.
High Speed fMRI
An inherent demand of high-resolution imaging is the need for thinner slices. Many more slices are required to cover the entire brain than in a lower resolution study. This severely limits the temporal efficiency of high-resolution applications, possibly lengthening the TR from 2–3 sec to 4–8 sec, giving fewer time points in fMRI time series and precluding certain event-related paradigms. For multi-slice SE EPI at 7T this is further complicated because the refocusing pulses result in the SAR limits being reached rather quickly, preventing acquisitions of many slices in reasonable TRs for fMRI. A sequence developed for simultaneous image readout (SIR) EPI (Feinberg et al., 2002) appeared particularly useful at 7T due to the sharing of refocusing pulses between adjacent EPI slices simultaneously acquired. The fewer refocusing pulses resulted in a reduced SAR. The drawback of the longer echo train time in SIR was countered by using high performance gradient systems and parallel imaging to shorten the echo train. The images looked promising on the fast 7T gradient insert (80 mT/m maximum, 333 T/m/s slew rate). When SIR SE EPI was implemented on 7T we noticed a further SAR advantage as the inherent chemical shifted lipid ghost was eliminated without fat sat pulses leading to a further reduction of SAR. This was achieved without lengthening RF refocusing pulses (Feinberg et al., 2010) as required in related methods (Ivanov et al., 2010) and without slice gradient reversal (Nagy and Weiskopf, 2008).
While the initial collaboration involved extending high resolution images to larger 3D volumes, it was then extended to explore multiplexed EPI sequences to improve the temporal efficiency of multi-slice EPI acquisitions for fMRI. The CMRR at the University of Minnesota had been developing and published the first demonstration of multi-banded (MB) 2D-EPI slice excitation for fMRI at 7T, resulting in several fold reductions in volume TRs (Moeller et al., 2008; Moeller et al., 2010). While the achievable acceleration using the SIR technique was limited by longer echo trains, the MB technique was limited by coil geometry and the higher RF power of the MB pulses. What was obvious was that even larger slice accelerations could be achieved if the two techniques were combined. In collaboration between CMRR in Minnesota and UC-Berkeley, a multiplexed imaging approach was implementing resulting in: M = SIR × MB, where M is the number of slices recorded instead of a single image in each EPI echo train. We now had a multiplexed-EPI sequence, modified for higher resolution and higher acquisition speed, utilizing partial Fourier, SIR, MB, parallel imaging, and high fields to increase SNR. This work coincided with the onset of the NIH Human Connectome Project (HCP) and the WashU-Minn Consortium. Our HCP collaborator at Oxford, Steve Smith performed ICA analysis of the highly accelerated resting state fMRI time series once the data file sizes were reduced to several gigabytes facilitating downloading at Oxford. It was not clear to us and certainly not to Steve, whether there would be any significant advantages (either from hemodynamic information or fMRI sensitivity) to the much faster TRs due to the source images’ SNR losses (i.e. from faster TRs/reduced flip angles) and because of the slow hemodynamic response. After modifying his tools to handle the unprecedented data sizes and after days of computational time per data set, it was found that the RSN Z-scores, after high dimensional ICA analysis, improved by 60% in the fast TR acquisitions (Fig. 4), as we reported in 2010 in a PLoS ONE paper (Feinberg et al., 2010).
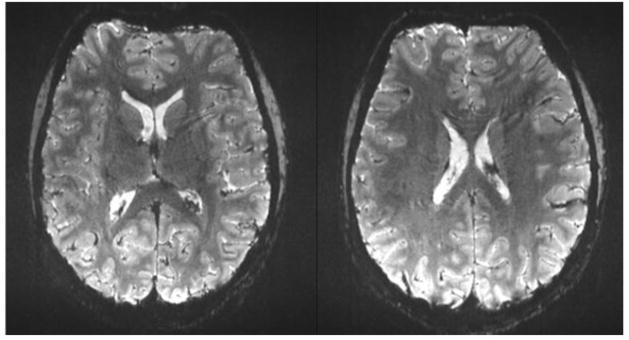
Fig. 4.
Comparison of resting state fMRI performed with whole brain imaging using conventional GE-EPI at TR= 2.5 sec and multiplexed-EPI at TR = 0.4 sec with noticeably improved definition of resting state networks (Feinberg et al., 2010).
Controlled aliasing techniques by RF phase cycling in multi-banded simultaneous slice excitations (Breuer et al., 2005) were modified for EPI using linearly blipped gradients (Nunes et al., 2006) but with limitations from voxel tilting. This was recently modified to use a balanced blipped gradient approach which resulted in minimal voxel tilting in controlled aliasing EPI (Setsompop et al., 2011) and significantly reduced g-factors. With this refinement and continued improvements in signal refocusing and image reconstructions (Feinberg et al., 2011) we are able to image the entire brain in under 100 ms at 2.5 mm isotropic resolution using higher SIR-3 × MB-12 while also using in-plane parallel imaging (Grappa factor-2 (Griswold et al., 2002)) (a 72 fold acceleration at 3T) and more conservatively with 300–500 ms TRs using MB factors of up to 8. We anticipate more advanced RF receiver arrays and further optimizations of MB and SIR with closer echo spacing will together give routine performance of fast fMRI sequences regardless of field strength. However, because of the sluggish and variable hemodynamic response function, it is unclear whether higher sampling rates alone will prove fruitful in further disentangling neuronal processes from the BOLD response. This topic just recently generated much debate at the 2011 OHBM meeting in Quebec City. While the debate focused on modeling and theories, what brought life to one side of the debate was the unprecedented opportunity, made possible by multiplexed EPI, of achieving whole brain TRs within hundreds of milliseconds. The faster scan times may also have advantages in suppressing motion, allowing exclusion of data with motion artifacts while still having sufficient sampling rates to observe BOLD changes in an fMRI time series. It is clear that the greater power in the data, because of the many more time points, will permit a better understanding of functional processes and/or allow for significant reduction in scan times for fMRI or diffusion imaging of fiber tracks.
Future directions and new dimensions in fMRI
What is also promising is if the significantly larger number of data points yields not only more powerful analyses in functional connectivity studies, but also whether more robust analyses in the temporal as opposed to the spatial domain can be performed. Similarly, in task based fMRI faster sampling rates may be advantageous in measuring BOLD responses with greater statistical power. Potential advances in neurosciences achieved by exploring higher bandwidths and spatial resolutions will likely change fMRI from that of time averaged stationary maps to new spatial and temporal views of brain activity made possible only with faster MR imaging.
Acknowledgments
Work supported in part by National Institutes of Health (grants, R01 EB000331, P41 RR08079, RO1EB002009, 1R44NS073417 and 5R44NS063537).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriany G, Pfeuffer J, Yacoub E, Van de Moortele P-F, Shmuel A, Andersen P, Hu X, Vaughan JT, Ugurbil K. A half-volume transmit/receive coil combination for 7 Tesla applications. ISMRM; Glasgow, U.K: 2001. p. 1097. [Google Scholar]
- Avram H, Crooks LE. Effect of self-diffusion on echo planar imaging. Proc. of 7th Annual Soc Mag Res Med; San Francisco. 1988. [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Breuer FA, Blaimer M, Heidemann RM, Mueller MF, Griswold MA, Jakob PM. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi-slice imaging. Magn Reson Med. 2005;53:684–691. doi: 10.1002/mrm.20401. [DOI] [PubMed] [Google Scholar]
- Crooks LE, Arakawa M, Hale JD, Hoenninger JC, Watts JC, Kaufman L, Feinberg DA. Echo planar imaging at 0.35T with one half of a data set. Proc. of Soc. Mag. Res. Med; Montreal. 1986. pp. 946–947. [Google Scholar]
- Crooks LE, Arakawa M, Hylton NM, Avram H, Hoenninger JC, Watts JC, Hale JD, Kaufman L. Echo-planar pediatric imager. Radiology. 1988;166:157–163. doi: 10.1148/radiology.166.1.3336674. [DOI] [PubMed] [Google Scholar]
- Duong TQ, Yacoub E, Adriany G, Hu X, Ugurbil K, Kim SG. Microvascular BOLD contribution at 4 and 7 T in the human brain: gradient-echo and spin-echo fMRI with suppression of blood effects. Magn Reson Med. 2003;49:1019–1027. doi: 10.1002/mrm.10472. [DOI] [PubMed] [Google Scholar]
- Feinberg D, Hoenninger J, Crooks L, Kaufman L, Watts J, Arakawa M. Inner volume MR imaging: technical concepts and their application. Radiology. 1985;156:743–747. doi: 10.1148/radiology.156.3.4023236. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Hale JD, Watts JC, Kaufman L, Mark A. Halving MR imaging time by conjugation: demonstration at 3.5 kG. Radiology. 1986;161:527–531. doi: 10.1148/radiology.161.2.3763926. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Hale JS. Echo Planar-Inner Volume Imaging at 0.35T. Proc. of Soc Magn. Res. Med; Montreal. 1986. p. 950. [Google Scholar]
- Feinberg DA, Harel N, Ramanna S, Ugurbil K, Yacoub E. Sub-millimeter single-shot 3D GRASE with inner volume selection for T2-weighted fMRI applications at 7 Tesla. 16th Annual Meeting of the International Society for Magnetic Resonance in Medicine; Toronto. 2008. [Google Scholar]
- Feinberg DA, Jakab PD. Perfusion imaging by 3D FT Echo Planar utilizing separate pulse sequences for low velocity and diffusion encoding SMRM; Montreal. 1989. p. 140. [Google Scholar]
- Feinberg DA, Jakab PD. Tissue perfusion in humans studied by Fourier velocity distribution, line scan, and echo-planar imaging. Magn Reson Med. 1990;16:280–293. doi: 10.1002/mrm.1910160209. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith S, Auerbach E, Ugurbil K, Yacoub E. Multiplexed Echo Planar Imaging with Sub-Second Whole Brain FMRI and Fast Diffusion Imaging. ISMRM; Montreal: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Glasser MF, Miller KL, Ugurbil K, Yacoub E. Multiplexed Echo Planar Imaging for Sub-Second Whole Brain FMRI and Fast Diffusion Imaging. PLoS One. 2010;5:e15710. doi: 10.1371/journal.pone.0015710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg DA, Reese TG, Wedeen VJ. Simultaneous echo refocusing in EPI. Magn Reson Med. 2002;48:1–5. doi: 10.1002/mrm.10227. [DOI] [PubMed] [Google Scholar]
- Feinberg DA, Turner R, Jakab PD, von Kienlin M. Echo-planar imaging with asymmetric gradient modulation and inner-volume excitation. Magn Reson Med. 1990;13:162–169. doi: 10.1002/mrm.1910130116. [DOI] [PubMed] [Google Scholar]
- Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47:1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- Hahn EL. Spin Echos. Phys Rev. 1950;80:580–594. [Google Scholar]
- Heidemann RM, Ivanov D, Trampel R, Fasano F, Pfeuffer J, Turner R. Zoomed GRAPPA (ZOOPPA) for Functional MRI. ISMRM; Stockholm: 2010. p. 2889. [Google Scholar]
- Ivanov D, Schafer A, Streicher MN, Heidemann RM, Trampel R, Turner R. A simple low-SAR technique for chemical-shift selection with high-field spin-echo imaging. Magn Reson Med. 2010;64:319–326. doi: 10.1002/mrm.22518. [DOI] [PubMed] [Google Scholar]
- Karlicek R, Lowe JA. A modified pulsed gradient technique for measuring diffusion in the presence of large background gradients. J Magn Reson. 1980:37. [Google Scholar]
- Le Roux P, Gilles RJ, McKinnon GC, Carlier PG. Optimized outer volume suppression for single-shot fast spin-echo cardiac imaging. J Magn Reson. 1998;8:1022–1032. doi: 10.1002/jmri.1880080505. [DOI] [PubMed] [Google Scholar]
- Mansfield P. Multi-planar image formation using NMR spin echoes. Jphys C: Solid State Phys. 1977;10:L55–L58. [Google Scholar]
- Mansfield P, Ordidge RJ, Coxon R. Zonally magnified EPI in real time by NMR. J Phys E: Sci Instrum. 1988;21:275–280. [Google Scholar]
- Margosian P. Faster MR Imaging-Imaging with Half the Data. Soc. of Magn. Reson. Med; London. 1986. pp. 1024–1025. [Google Scholar]
- Menon R, Ogawa S, Strupp JP, Ugurbil K. Ocular dominance in human V1 demonstrated by functional magnetic resonance imaging. J Neurophysiol. 1997;77:2780–2787. doi: 10.1152/jn.1997.77.5.2780. [DOI] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Auerbach E, van de Moortele PF, Adriany G, Ugurbil K. fMRI with 16 fold reduction using multibanded multislice sampling. Proc Int Soc for Mag Reson Med; Toronto. 2008. [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med. 2010;63:1144–1153. doi: 10.1002/mrm.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Weiskopf N. Efficient fat suppression by slice-selection gradient reversal in twice-refocused diffusion encoding. Magn Reson Med. 2008;60:1256–1260. doi: 10.1002/mrm.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes R, Hajnal J, Golay X, Larkman D. Simultaneous slice excitation and reconstruction for single shot EPI. ISMRM 14th Annual Meeting; Seattle, Washington. 2006. p. 293. [Google Scholar]
- Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. Biophys J. 1993;64:800–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshio K, Feinberg DA. GRASE (Gradient- and spin-echo) imaging: a novel fast MRI technique. Magn Reson Med. 1991;20:344–349. doi: 10.1002/mrm.1910200219. [DOI] [PubMed] [Google Scholar]
- Pauly J, Nishimura D, Macovski A. A k-Space Analysis of Small-Tip-Angle Excitation. J Magn Reson. 1989;81:43–56. doi: 10.1016/j.jmr.2011.09.023. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Van De Moortele PF, Yacoub E, Adriany G, Andersen P, Merkle H, Garwood M, Ugurbil K, Hu X. Zoomed functional imaging in the human brain at 7 Tesla with simultaneously high spatial and temporal resolution. Neuroimage. 2002;17:272–286. doi: 10.1006/nimg.2002.1103. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42:952–962. [PubMed] [Google Scholar]
- Pykett IL, Rzedzian RR. Instant images of the body by magnetic resonance. Magn Reson Med. 1987;5:563–571. doi: 10.1002/mrm.1910050607. [DOI] [PubMed] [Google Scholar]
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planer imaging with reduced g-factor penalty. Magn Reson Med. 2011 Aug 19;2011 doi: 10.1002/mrm.23097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson DK, Griswold MA, Jakob PM. SMASH imaging. Magn Reson Imaging Clin N Am. 1999;7:237–254. vii–viii. [PubMed] [Google Scholar]
- Ugurbil K, Adriany G, Akgün C, Andersen P, Chen W, Garwood M, Gruetter R, Henry P-G, Marjanska M, Moeller S, Van de Moortele P-F, Prüssmann K, Tkac I, Vaughan JT, Wiesinger F, Yacoub E, Zhu X-H. High Magnetic Fields for Imaging Cerebral Morphology, Function and Biochemistry. In: Robitaille PMLaB, LJ, editors. Biological Magnetic Resonance: Ultra High Field Magnetic Resonance Imaging. Springer; New York: 2006a. pp. 285–342. [Google Scholar]
- Ugurbil K, Chen W, Harel N, Van de Moortele P-F, Yacoub E, Zhu XH, Uludag K. Brain Function, Magnetic Resonance Imaging of. In: Akay M, editor. Wiley Encyclopedia of Biomedical Engineering. Hoboken: John Wiley & Sons, Inc, Hoboken; 2006b. pp. 647–668. [Google Scholar]
- Weisskoff RM, Kiihne S. MRI susceptometry: image-based measurement of absolute susceptibility of MR contrast agents and human blood. Magn Reson Med. 1992;24:375–383. doi: 10.1002/mrm.1910240219. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Duong TQ, Van De Moortele PF, Lindquist M, Adriany G, Kim SG, Ugurbil K, Hu X. Spin-echo fMRI in humans using high spatial resolutions and high magnetic fields. Magn Reson Med. 2003;49:655–664. doi: 10.1002/mrm.10433. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Harel N, Ugurbil K. High-field fMRI unveils orientation columns in humans. Proc Natl Acad Sci U S A. 2008;105:10607–10612. doi: 10.1073/pnas.0804110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Logothetis N, Ugurbil K. Robust detection of ocular dominance columns in humans using Hahn Spin Echo BOLD functional MRI at 7 Tesla. Neuroimage. 2007;37:1161–1177. doi: 10.1016/j.neuroimage.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub E, Shmuel A, Pfeuffer J, Van De Moortele PF, Adriany G, Andersen P, Vaughan JT, Merkle H, Ugurbil K, Hu X. Imaging brain function in humans at 7 Tesla. Magn Reson Med. 2001;45:588–594. doi: 10.1002/mrm.1080. [DOI] [PubMed] [Google Scholar]
- Yacoub E, Van De Moortele PF, Shmuel A, Ugurbil K. Signal and noise characteristics of Hahn SE and GE BOLD fMRI at 7 T in humans. Neuroimage. 2005;24:738–750. doi: 10.1016/j.neuroimage.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Zimmermann J, Goebel R, De Martino F, Van de Moortele PF, Feinberg DA, Adriany G, Chaimow D, Shmuel A, Ugurbil KEY. Mapping the organization of axis of motion selective features in human area MT using high-field fMRI. PLoS One. 2011 doi: 10.1371/journal.pone.0028716. [DOI] [PMC free article] [PubMed] [Google Scholar]