Abstract
Humans and mice deficient in the adaptor protein SAP (Sh2d1a) have a major defect in humoral immunity, due to lack of T cell help for B cells. The role of SAP in this process is incompletely understood. We found that deletion of receptor Ly108 (Slamf6) in CD4+ T cells reversed the Sh2d1a−/− phenotype, eliminating the SAP requirement for germinal centers. This potent negative signaling by Ly108 required immunotyrosine switch motifs (ITSMs) and SHP-1 recruitment, resulting in high amounts of SHP-1 at the T:B synapse, limiting T:B adhesion. Ly108 negative signaling was not only important in CD4+ T cells, as we found that NKT cell differentiation was substantially restored in Slamf6−/−Sh2d1a−/− mice. The ability of SAP to regulate both positive and negative signals in T cells can explain the severity of SAP-deficiency and highlights the importance of SAP and SHP-1 competition for Ly108 ITSM binding as a rheostat for the magnitude of T cell help to B cells.
INTRODUCTION
The formation of germinal centers is critical for the development of most humoral immunity. This includes affinity maturation, long lived plasma cell generation, and an overall effective neutralizing antibody response (Allen et al., 2007; Tarlinton, 2008). The development of germinal centers is controlled by follicular helper CD4+ T cells (Tfh), which are the specialized T cells for B cell help (Crotty, 2011). Therefore, understanding Tfh cells and germinal centers is important for rational approaches to vaccine design, and new therapeutic approaches for autoimmune diseases involving B cells. Tfh cell differentiation is dependent on the transcription factor Bcl6 (Johnston et al., 2009; Nurieva et al., 2009; Yu et al., 2009), and Tfh cells express proteins that facilitate co-localization with B cells (high CXCR5 and CXCR4, concomitant with low CCR7 and S1P receptor expression or function) and molecules important for T cell help to B cells, such as IL-21, IL-4, and CD40L (Crotty, 2011). Tfh cells within germinal centers, GC Tfh, have notably high expression of signaling lymphocyte activation molecule (SLAM)-associated protein (SAP) (Ma et al., 2009; Yusuf et al., 2010).
Sh2d1a, the gene encoding SAP, was originally cloned as the causal locus of the frequently lethal human immunodeficiency X-linked lymphoproliferative disease (XLP), characterized by difficulty controlling a variety of pathogens (Cannons et al., 2011). It was then determined that SAP plays a central role in the development of B cell immunity, as SAP is required in CD4+ T cells for germinal center B cell development and development of long term humoral immunity (Crotty et al., 2003). In the absence of SAP, virtually no memory B cells, long-lived plasma cells, or sustained antibody responses are present after acute viral infections (Crotty et al., 2003; Kamperschroer et al., 2006; McCausland et al., 2007; Moyron-Quiroz et al., 2009), chronic viral infections (Chen et al., 2005; Crotty et al., 2006; Harker et al., 2011), parasite exposure (Cannons et al., 2006), immunizations with a variety of simple or complex protein antigens (Cannons et al., 2011; Cannons et al., 2006; Veillette et al., 2008), or when ablated in autoantibody prone mice (Jennings et al., 2008; Linterman et al., 2009). XLP patients were later confirmed to have severe loss of germinal centers and memory B cells (Ma et al., 2005; Ma et al., 2006; Malbran et al., 2004). SAP has also been shown to be critically important for the development of NKT cells (Chung et al., 2005; Griewank et al., 2007; Nichols et al., 2005; Pasquier et al., 2005).
These findings have spurred extensive interest in delineating the molecular functions of SAP and the SLAM family receptors. Structurally, SAP consists primarily of an SH2 domain (Poy et al., 1999). SAP is expressed in the cytoplasm and binds tyrosines found in the intracellular domains of SLAM family receptors such as SLAM (CD150, Slamf1), CD84 (Cd84), Ly9 (SLAMF3, Slamf3), 2B4 (CD244, SLAMF4, Cd244), and Ly108 (Slamf6, NTB-A in humans). Multiple SLAM family receptors are expressed on both T and B cells and have been shown to bind SAP through an uncommon tyrosine motif, termed an immunotyrosine switch motif (ITSMs) (Sayos et al., 1998; Shlapatska et al., 2001). All SAP-binding SLAM family members contain two or more ITSMs in their cytoplasmic tail. SLAM family receptors all possess a similar ectodomain structure, and most exhibit homotypic adhesion (e.g., SLAM-SLAM, Ly108-Ly108) (Cao et al., 2006; Mavaddat et al., 2000; Yan et al., 2007). The receptor tails serve as docking sites for multiple signaling molecules upon homotypic receptor binding (Cannons et al., 2011; Latour et al., 2001; Schwartzberg et al., 2009; Veillette et al., 2009). SLAM family receptors are known to mediate IL-4 production by CD4+ T cells (Davidson et al., 2004; Wang et al., 2004; Yusuf et al., 2010), cytotoxicity by NK cells (Nakajima et al., 2000; Pasquier et al., 2005; Tangye et al., 2000), thymic selection of NKT cells (Griewank et al., 2007) and bactericidal activity of neutrophils (Howie et al., 2005) and macrophages (Berger et al., 2010), among other functions. While findings on inhibitory aspects of SLAM family receptor signaling are present in the literature (Cannons et al., 2011; Veillette et al., 2009), some of this was controversial (Latour et al., 2001; Nichols et al., 2001; Sayos et al., 1998), and most recent analysis has focused on the positive SAP-dependent signaling activities of SLAM-family receptors (Cannons et al., 2010b; Chan et al., 2003; Latour et al., 2003; Yusuf et al., 2010; Zhong and Veillette, 2008). Most notably, SAP plays a central role in CD4+ T cell adhesion to B cells (Qi et al., 2008). Sh2d1a−/− CD4+ T cells were unable to form long term conjugates with cognate B cells in vivo and in vitro (Cannons et al., 2010a; Qi et al., 2008). SLAM family members CD84 and Ly108 were shown to collaborate in this process in vitro in a SAP dependent manner, and CD84 was shown to be important for germinal center development after some protein immunizations, though it did not fully phenocopy SAP-deficiency (Cannons et al., 2010a). Here we show that single gene ablation of Slamf6 or Cd84 does not result in significant germinal center or antibody defects after an acute viral infection, unlike the severe germinal center defect seen in Sh2d1a−/− mice. This left an inability to explain the severity of the humoral immunity defects globally observed in the absence of SAP. To better understand the role of Ly108 signaling in T cell help to B cells, we developed a Sh2d1a−/−Slamf6−/− double deficient mouse. Surprisingly, the absence of Ly108 eliminated the requirement for SAP in CD4+ T cells for B cell help. This observation led us to identify a potent Ly108 negative signaling pathway, active in both CD4+ T cells and NKT cells, which may act as a general regulator of lymphocyte:lymphocyte adhesion.
RESULTS
Ly108 Expression and Function in an Acute Viral Infection
We have been examining the role of individual SAP-binding SLAM family receptors in the process of T cell help to B cells in the germinal center. With the exception of 2B4 (CD244), which binds to CD48, and is not expressed by CD4+ T cells, the remaining SLAM family members are homophilic receptors. Murine CD319 (CRACC, Slamf7) does not bind SAP (Bouchon et al., 2001). Mice deficient in individual SLAM family members have provided evidence that Ly9 (Slamf3) is not required for germinal centers (Graham et al., 2006). SLAM (Slamf1) is not required for Tfh cell differentiation or adhesion (Cannons et al., 2006; McCausland et al., 2007), but is required for GC Tfh cell IL-4 production (Cannons et al., 2010b; Yusuf et al., 2010). Interestingly, Ly108 (NTB-A in humans, Slamf6) is known to bind SAP (Bottino et al., 2001; Zhong and Veillette, 2008) and Ly108 isoforms are linked to the development of autoantibody mediated autoimmune diseases (Keszei et al., 2011; Wandstrat et al., 2004). Thus, we evaluated whether Ly108 expression is modulated on Tfh cells and B cells in the context of an acute viral infection, lymphocytic choriomeningitis virus (LCMV). Activated CD4+ T cells express elevated levels of Ly108 compared to naïve CD4+ T cells (Figure 1A). Tfh cells express more Ly108 than other virus-specific CD4+ T cells (predominately Th1 cells), consistent with previous observations (Cannons et al., 2010b). The Tfh cells present in germinal centers (GC Tfh cells) are known to have elevated levels of SAP and BCL6 (Kroenke et al., 2012; Ma et al., 2009; Yusuf et al., 2010) but had equivalent Ly108 to other Tfh cells (Figure 1A). Given that Ly108 is a homophilic receptor, we measured Ly108 expression on activated B cells during an LCMV infection. We observed that both germinal center B cells and plasma cells have elevated Ly108 expression compared to naïve B cells (Figure 1B). CD4+ T cells and B cells from Slamf6−/− mice are shown for comparison, demonstrating constitutive expression of Ly108 by both CD4+ T cells and B cells (Figure 1C-D).
Figure 1. Ly108 expression and function in an acute viral infection.
Ly108 expression on splenic (a) effector (Th1) (CXCR5lo), Tfh (CXCR5hi), and GC Tfh (CXCR5hiPD1hi) CD44hi CD4+ T cells eight days post LCMV infection, and (b) Naïve B cells, plasma cells (CD19+CD138hiIgDlo), and germinal center B cells (CD19+GL7hiFashi) 8 days after LCMV infection. (c-d) CD4+ T cells (c) and B cells (d) from uninfected wild type (WT) and Slamf6−/− mice. (e) Slamf6+/+ and Slamf6−/− mice or (f) Slamf6−/− and SAP−/− mice were infected with LCMV and splenocytes were analyzed for GC B cells 8 days later. (g) WT, Slamf6+/-, and Slamf6−/− mice were infected with LCMV and virus specific serum IgG was measured day 30 post infection. (h) Slamf6−/− and SAP−/− mice were infected with LCMV and virus specific serum IgG was measured day 8 post infection. (a-h) Data are representative of 2 or more independent experiments. N = 4 or more per group. *** P < 0001. Error bars are SEM.
To investigate the effects of loss of Ly108, we scrutinized antiviral B cell and T cell responses by wild type (WT), Slamf6+/-, and Slamf6−/− mice. Eight days after an acute LCMV infection, germinal center B cell numbers were unaffected by absence of Ly108 (Figure 1E). Additionally, antibody responses 30 days after infection were normal in Slamf6−/− mice (Figure 1G). In contrast, Sh2d1a−/− mice display a striking germinal center defect 8 days after LCMV infection, and have severely reduced anti-LCMV antibody titers 30 days post infection (Figure 1F and 1H).
Previous work demonstrated that CD84 has a partial role in germinal center development after protein immunizations (Cannons et al., 2010a), but we observed no defect in Cd84−/− mice in the context of an acute LCMV infection (Figure S1). We therefore examined a second infection model, vaccinia virus (VACV), and again no defect in germinal centers or Tfh cells was observed in Cd84−/− mice (Figure S1 and data not shown). These observations implicated a robust Tfh cell functional redundancy between SLAM family receptors, which has also been observed for SLAM family receptor participation in NKT cell development (Griewank et al., 2007).
Ly108 function in the absence of SAP
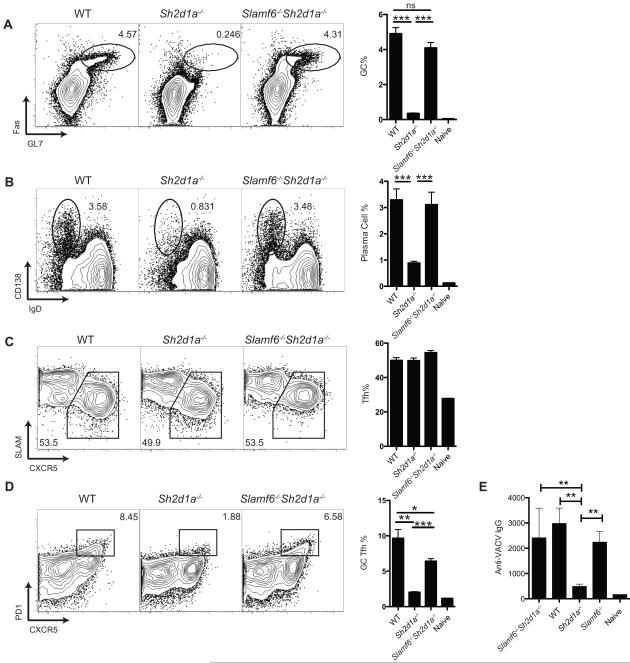
To strategically delineate the requirements of Ly108 in the SAP-deficient phenotype, we generated Sh2d1a−/−Slamf6−/− double deficient mice. Surprisingly, the removal of Ly108 expression from SAP-deficient mice eliminated the SAP requirement for germinal center formation in response to an LCMV infection (P < 0.001, Figure 2A). Plasma cell development was also recovered in Slamf6−/−Sh2d1a−/− mice, in stark contrast to Sh2d1a−/− mice (P < 0.001, Figure 2B). Tfh cell percentages were unaffected (Figure 2C). Sh2d1a−/− mice have a severe defect in GC Tfh cell formation (Yusuf et al., 2010). In contrast to the absence of GC Tfh cells in Sh2d1a−/− mice, GC Tfh cell numbers in the combined absence of SAP and Ly108 (Slamf6−/− Sh2d1a−/−) were 80% of WT GC numbers (P < 0.001, Figure 2D). Antibody responses to an acute viral infection were recovered in Slamf6−/−Sh2d1a−/− mice, in contrast to Sh2d1a−/− mice (P < 0.01, Figure 2E). In contrast, neither Slamf1−/−Sh2d1a−/− mice (Yusuf et al., 2010) nor Cd84−/−Sh2d1a−/− mice (Figure S2) showed evidence of amelioration of the negative signaling occurring in the absence of SAP, with germinal center defects equal in severity to Sh2d1a−/− mice. These data suggested that Ly108 transmits a potent negative signal in the absence of SAP, potentially explaining the gross defect of humoral immunity in SAP deficient mice and humans.
Figure 2. Loss of Ly108 eliminates the SAP expression requirement for germinal center development.
(a-d) Wild type, Sh2d1a−/− and Slamf6−/−Sh2d1a−/− mice were infected with LCMV. Splenocytes were analyzed day 8 after infection. (a) Germinal center B cells (FashiGL7hi) and (b) plasma cells (IgDloCD138hi) are shown as a % of total B cells (CD19+CD4−). (c) Tfh cells (CXCR5+SLAMlo) and (d) GC Tfh cells (CXCR5+PD1hi) are shown as a % of activated CD4+ T cells (CD44hiCD4+). Data are representative of 3 (a-c) or 2 (d) independent experiments. N = 5 per group. (e) Wild type, Sh2d1a−/−, Slamf6−/−, and Slamf6−/−Sh2d1a−/− mice were infected with vaccinia virus. Serum antibody titers were analyzed day 8 after infection. * P < 0.05, ** P < 0.005, *** P < 0001. n.s., no statistically significant difference (P > 0.05). Error bars are SEM.
CD4+ T cell intrinsic effects of Ly108 signaling
SAP has been shown to be required in CD4+ T cells for humoral immunity and, with the exception of one study, not required in B cells or APCs for the development of germinal centers (Cannons et al., 2011; Veillette et al., 2008). To determine if Ly108 inhibitory signaling in the absence of SAP was CD4+ T cell intrinsic, we transferred naïve WT, Sh2d1a−/−, or Slamf6−/− Sh2d1a−/− purified SMARTA TCR transgenic CD4+ T cells (‘SM’, LCMV gp66-77 I-Ab specific) into Sh2d1a−/− recipients. Given that Sh2d1a−/− mice are unable to mount an endogenous germinal center response to LCMV, any germinal center B cells that develop are a direct result of the transferred virus-specific SM CD4+ T cells. Eight days post-LCMV, mice receiving Sh2d1a−/− SM CD4+ T cells failed to mount a germinal center response, while Slamf6−/−Sh2d1a−/− SM CD4+ T cells rescued germinal center formation (58% of WT. Sh2d1a−/− vs. Slamf6−/−Sh2d1a−/− P < 0.001, Figure 3A). Slamf6−/−Sh2d1a−/− SM cells also supported robust plasma cell development in response to the acute infection (90% of WT), in contrast to Sh2d1a−/− SM cells (P < 0.001, Figure 3B). While Sh2d1a−/− SM cells had a defect in Tfh cell frequency (P < 0.05, Figure 3C) (Yusuf et al., 2010), this defect was reversed in the absence of Ly108 (Slamf6−/−Sh2d1a−/− vs. Sh2d1a−/− SM, P < 0.01. Figure 3C). Notably, Slamf6−/−Sh2d1a−/− SM CD4+ T cells were able to differentiate into GC Tfh cells (P < 0.001, Figure 3D), consistent with the strong B cell response observed in the recipient mice. These data show that the germinal center phenotype seen in Slamf6−/−Sh2d1a−/− mice is due to a CD4+ T cell intrinsic function, revealing a potent Ly108 inhibitory pathway that requires counteraction by SAP.
Figure 3. Loss of Ly108 reverses the SAP requirement in CD4+ T cells for T cell help to B cells.
(a-d) WT, Sh2d1a−/−, or Slamf6−/−Sh2d1a−/− CD45.1+ SM TCR transgenic CD4+ T cells were transferred into Sh2d1a−/− recipient mice subsequently infected with LCMV. Splenocytes were analyzed day 8 after infection. (a) Germinal center B cells (FashiGL7hi) and (b) plasma cells (CD138+IgDlo) shown as % of total B cells (CD19+CD4−). (c) Tfh cells (CXCR5+) and (d) GC Tfh cells (CXCR5+PD1hi) shown as a % of SM (CD45.1+CD4+B220−). (e-g) Slamf6−/−Sh2d1a−/− SM CD4+ T cells (CD45.1+) were transduced with Ly108-1, Ly108-2, SAP or GFP vector in vitro, transferred into Sh2d1a−/− recipient mice, and mice were infected with LCMV. (e) Germinal center B cells (FashiGL7hi) are shown 8 days after infection, as a % of total B cells. (f) Plasma cell (CD19+ IgDloCD138+) frequencies, as a % of total B cells. (g) MFI of Ly108 expression on Slamf6−/−Sh2d1a−/− SM CD4+ T cells transduced with empty vector (GFP) or Ly108 vector (Ly108-2), in comparison to endogenous Ly108 expression on WT SM cells. Data are representative of 4 (a-c,e) or 2 (d,f,g) independent experiments. N = 4 or more mice per group. * P < 0.05, ** P < 0.005, *** P < 0001. Error bars are SEM.
One challenge to studying SLAM family receptors is that the genes are clustered together on chromosome 1. This impacts the interpretation of certain experiments because of SLAM family receptor allelic differences between mouse strains (Keszei et al., 2011; Veillette et al., 2006; Wandstrat et al., 2004). Therefore, we designed an experiment to confirm that Slamf6−/−Sh2d1a−/− reversal of the severe Sh2d1a−/− humoral immunity defect phenotype was specifically due to the absence of Ly108. Ly108 was reintroduced by retroviral vector (RV) transduction of Slamf6−/− Sh2d1a−/− SM CD4+ T cells (Figure 3E). The two widely expressed Ly108 isoforms, Ly108-1 and Ly108-2, both of which contain two canonical ITSMs, were investigated for activity. Introduction of either isoform of Ly108 into Slamf6−/− Sh2d1a−/− SM cells “reversed” the phenotype of these cells, and suppressed germinal center development (P < 0.05 and P < 0.01, Figure 3E). Plasma cell responses to the viral infection were also inhibited by Ly108 expression (P < 0.012 and P < 0.04, Figure 3F). Inhibition of germinal center and plasma cell development by Ly108 expression in the absence of SAP was incomplete, probably due to moderate expression of Ly108 by the RV expression vector (Figure 3G). This experimental setting allowed for the demonstration of negative signaling involving Ly108, but it also allowed for complementary experiments demonstrating that overexpression of SAP in Slamf6−/− Sh2d1a−/− CD4+ T cells led to enhanced germinal center formation (P < 0.05, Figure 3E). This is consistent with SAP both blocking a negative signal through Ly108 and providing a positive signal for CD4+ T cell help to B cells. Taken together, these results provide strong evidence for a CD4+ T cell intrinsic effect of Ly108 inhibition of humoral immunity which is modulated by SAP expression.
The role of Ly108 in formation of T:B conjugates
To evaluate the how Ly108 functions in the absence of SAP, we asked what aspect of T cell help to B cells is inhibited by Ly108. Previous studies have shown that SAP plays an important role in CD4+ T cell: B cell conjugate contact time in germinal centers. SAP deficiency leads to decreased contact time. This defect can be recapitulated in vitro using a flow cytometry based T:B conjugation assay (Cannons et al., 2010a; Qi et al., 2008) wherein absence of SAP expression in CD4+ T cells results in a severe defect in adhesion to B cells in the presence of cognate peptide (Figure 4A-B). We therefore examined if T:B conjugates were impacted by the absence of Ly108 in Sh2d1a−/− CD4+ T cells. Combined loss of SAP and Ly108 again reversed the Sh2d1a−/− CD4+ T cell phenotype, back to T:B conjugate percentages seen for WT CD4+ T cells (Figure 4 A-B). These data demonstrate that Ly108 signaling can actively inhibit T:B adhesion and indicates this is the likely cause of the in vivo Ly108-dependent block to germinal center development.
Figure 4. Slamf6−/−Sh2d1a−/− CD4+ T cells form stable conjugates with B cells, and ITSM phosphotyrosine motifs are required for inhibitory signals transmitted by Ly108 in vivo.
(a-b) Conjugation efficiency of WT, Sh2d1a−/−, and Slamf6−/− Sh2d1a−/− SM CD4+ T cells with B cells pulsed with cognate peptide (LCMV gp66-77). (a) Representative flow cytometry plots, gated on CD4+ T cells. (b) Mean frequency of CD4+CD19+ conjugates in total CD4+ events. N = 2. (c-g) Roles of Ly108 tyrosines in vivo. (c-e) Slamf6−/−Sh2d1a−/− SM CD4+ T cells were transduced with GFP, Ly108-2 (“Ly108”), Ly108-Y3 mutant, or Ly108-AllF mutant RV and transferred into Sh2d1a−/− recipient mice. An additional group received Sh2d1a−/− SM cells transduced with RV-GFP. Mice were infected with LCMV and B cell responses in spleen were analyzed 8 days following infection. (c) Representative germinal center B cell FACS plots are shown, gated on total B cells (CD19+CD4−). (d) Quantitation of GC B cells as gated in (c). (e) Quantitation of the plasma cell response. (f-g) Slamf6−/−Sh2d1a−/− SM -Ly108-Y1 mutant or - Ly108-Y2 mutant CD4+ T cells were transduced with RV-GFP, -Ly108-2 (“Ly108”), and transferred into Sh2d1a−/− recipient mice subsequently infected with LCMV. (f) Representative germinal center B cell FACS plots are shown, gated on total B cells (CD19+CD4−), analyzed at day 8 after infection. (g) Quantitation of GC B cells as gated in (f). Data are representative of 2 independent experiments. N = 4 or more per group. * P < 0.05, ** P < 0.005 Error bars are SEM.
ITSM phosphotyrosine motifs are required for Ly108 inhibitory signals
Based on the previous experiments, we hypothesized that Ly108 transmits a potent negative signal to CD4+ T cells in conditions of low or absent SAP protein. SAP protein levels are low in naïve CD4+ T cells and many activated CD4+ T cells, but SAP protein expression is substantially upregulated in Tfh cells, both in mice (Yusuf et al., 2010) and humans (Kroenke et al., 2012; Ma et al., 2009). We therefore addressed how Ly108 transmits this negative signal. Ly108 isoforms contain multiple tyrosines, which may be potential docking sites for inhibitory signaling molecules. In addition to two ITSMs, Ly108-1 contains one additional unique tyrosine, while Ly108-2 contains two additional unique tyrosines (Cannons et al., 2011). Because Ly108-1 and Ly108-2 both are able to inhibit germinal center formation in the absence of SAP (Figure 3E-G), we focused on Ly108-2 and generated a Ly108-2 (‘Ly108’) mutant expression construct in which all cytoplasmic tyrosines were mutated to phenylalanines (Ly108-AllF) (Figure S3). We also evaluated a construct that contained only the single conserved non-canonical ITSM (Ly108-Y3) to test the importance of the ITSMs for Ly108 negative signaling. Slamf6−/−Sh2d1a−/− SM CD4+ T cells were transduced with Ly108, Ly108-AllF, Ly108-Y3, or a control construct (RV-GFP) and transferred into Sh2d1a−/− recipients. RV-GFP transduced Sh2d1a−/− SM CD4+ T cells transferred into Sh2d1a−/− recipients were used as a negative control. Following cell transfer, mice were infected with LCMV and germinal center formation evaluated at day 8. As anticipated, Slamf6−/−Sh2d1a−/− SM + RV-GFP drove significantly more germinal centers than Sh2d1a−/−SM + RV-GFP (P < 0.05, Figure 4C-D). Additionally, reintroduction of Ly108 in Slamf6−/− Sh2d1a−/− SM cells inhibited germinal center formation (P < 0.05, Figure 4C-D). However Ly108-Y3 (P < 0.01) or Ly108-AllF (P < 0.05) expressing Slamf6−/−Sh2d1a−/− SM cells were unable to inhibit germinal center formation in comparison to Slamf6−/−Sh2d1a−/− SM cells expressing intact Ly108 (Figure 4C-D). In follow up experiments with Ly108-AllF, the inhibition of plasma cell development by Ly108 in the absence of SAP was lost when CD4+ T cells expressed the Ly108-AllF mutant (P < 0.013, Figure 4E). Thus, ITSM phosphotyrosines are important for the transduction of inhibitory signals through Ly108 that restrict humoral immune responses.
To examine the roles of individual ITSMs in Ly108 negative signaling we created two additional Ly108 mutants containing only the first or second ITSM tyrosine (Ly108-Y1 and Ly108-Y2). Slamf6−/−Sh2d1a−/− SM CD4+ T cells were transduced with RV-Ly108, Ly108-Y1, or Ly108-Y2 and transferred into Sh2d1a−/− recipients that were then infected with LCMV. Ly108 with only a single ITSM, either the first ITSM (P < 0.05) or second ITSM (P < 0.05), provided strong inhibitory signaling in the absence of SAP protein (Figure 4F-G). Since the ITSMs are the SAP binding motifs, these results indicate that the ITSM phosphotyrosines can serve as an on-off switch for CD4+ T cell help to B cells via competition for phosphotyrosine binding between SAP (positive signaling) and an unknown negative signaling protein.
Increased SHP-1 association with Ly108 in the absence of SAP and disruption of the T:B synapse
Previous literature suggested that in addition to binding SAP, different SLAM family members could recruit negative signaling molecules including the protein phosphatases SHP-2, SHP-1, Cbl, or Csk (Chen et al., 2006; Eissmann et al., 2005; Kim et al., 2010; Sayos et al., 1998; Snow et al., 2009; Zhong and Veillette, 2008), as well as the lipid phosphatase SHIP-1 (Latour et al., 2001; Shlapatska et al., 2001). Limited evidence was also available for recruitment of negative signaling molecules to Ly108 (or NTB-A) (Bottino et al., 2001; Snow et al., 2009; Valdez et al., 2004; Zhong and Veillette, 2008). To understand the mechanisms by which Ly108 transduces an inhibitory signal, we immunoprecipitated Ly108 from WT and Sh2d1a−/− CD4+ T cells after stimulation with peptide-pulsed B cells. Stimulation with B cells led to a specific increase in SHP-1 association with Ly108 in the Sh2d1a−/− CD4+ T cells (Figure 5A), with less SHP-1 association in WT CD4+ T cells. Thus, in the absence of SAP, CD4+ T cells show increased association of the phosphatase SHP-1 with Ly108.
Figure 5. Ly108 recruits SHP-1 to the T:B immunological synapse.
(a) Activated WT and Sh2d1a−/− AND TCR transgenic (PCC-specific) CD4+ T cells were lysed without stimulation or post-incubation with LPS-activated B cells that were untreated or pulsed with PCC peptide. Lysates were immunoprecipated for Ly108 and blotted for SHP-1 and Ly108. Total cell lysates (TCL) were examined for pERK activation and total ERK protein levels. NP = no peptide. (b-d) WT, Sh2d1a−/−, and Slamf6−/− Sh2d1a−/− SM CD4+ T cell conjugates with LPS-activated B cells pulsed with cognate peptide (LCMV gp66-77) were stained with Hoechst (blue), and antibodies to CD4 (white) and SHP-1 (green). (b) Cells were examined from the side as a confocal projection in the x-y plane (top row), at 45° (middle row), and 90° (en face, bottom row) rotations in the y-z plane. (c) Representative immunofluorescence images of SHP-1 localization at the immune synapse. (d) Quantification of SHP-1 localization at the immune synapse. Data represent two independent experiments with over 40 conjugates scored / genotype for each experiment. (e) Activated Slamf6−/− and Slamf6−/−Sh2d1a−/− SM CD4+ T cells expressing Ly108-GFP or Ly108-AllF-GFP (‘Ly108-AllF’) constructs were incubated with activated B cells pulsed with cognate peptide (LCMV gp66-77). Lysates were immunoprecipated for Ly108 and blotted for SHP-1 and Ly108. (f-h) Slamf6−/− and Slamf6−/−Sh2d1a−/− SM CD4+ T cells were transfected with either Ly108-GFP (‘WTLy108’) or Ly108-AllF-GFP, and conjugated to WT B cell targets pulsed with cognate peptide (LCMV gp66-77). Cells were stained with Hoechst (blue) and antibodies against SHP-1 (red). Green is Ly108-GFP fluorescence. (f) Quantitation of SHP-1 localization at the synapse. (g) Representative immunofluorescence images of cells expressing Ly108-GFP. (h) Representative immunofluorescence images of cells expressing Ly108-AllF-GFP. Further examples are shown in Figure S4. Data for each experiment depicted is representative of two or more experiments.
We subsequently used confocal immunofluorescence microscopy to examine SHP-1 localization in T:B conjugates. SHP-1 accumulated at the synapse of WT, Sh2d1a−/−, and Sh2d1a−/−Slamf6−/− CD4+ T cell conjugates with WT B cells pulsed with cognate peptide (Figure 5B, top row). Rotation of the images and a more detailed en face evaluation of the T:B synapse revealed marked differences in SHP-1 localization (Figure 5B, bottom row). WT CD4+ T cells exhibited a clearance of SHP-1 from the center of the B cell contact site, resulting in an O shaped SHP-1 pattern around the perimeter of the synapse (Figure 5B-D). In contrast, Sh2d1a−/− CD4+ T cells failed to restrict SHP-1 localization and SHP-1 was diffusely spread throughout the entire T:B synapse (Figure 5B-D). Strikingly, SHP-1 was cleared from the central synapse in a large fraction of Sh2d1a−/−Slamf6−/− T:B conjugates (Figure 5B-D). Together, these data suggest that the impaired clearance of SHP-1 from the immune synapse has a pronounced negative impact on the adhesion of Sh2d1a−/− CD4+ T cells with antigen presenting B cells.
To validate the requirements of the Ly108 tyrosine residues and SHP-1 recruitment, Slamf6−/− and Slamf6−/−Sh2d1a−/− CD4+ SM T cells were retrovirally reconstituted with either WT Ly108 or Ly108-AllF. Following stimulation with peptide pulsed B cells, SHP-1 selectively associated with WT Ly108 in the Slamf6−/−Sh2d1a−/− CD4+ T cells (Figure 5E), consistent with the increased SHP-1 associated with Ly108 observed in Sh2d1a−/− cells (Figure 5A). Additionally, to visualize Ly108 and SHP-1 localization, Ly108 constructs were designed as fusion proteins with GFP and introduced into Slamf6−/− and Slamf6−/−Sh2d1a−/− CD4+ SM T cells. Ly108 is present both in central and peripheral regions of the synapse, as seen in Slamf6−/− cells reconstituted with Ly108-GFP or Ly108-AllF-GFP (Figure 5G-H, and Figure S4). SHP-1 is predominantly restricted to an outer ring at the synapse in Slamf6−/− + Ly108-GFP cells (Figure 5F-G), comparable to WT and Slamf6−/−Sh2d1a−/− CD4+ T cells (Figure 5B-D). Slamf6−/−Sh2d1a−/− CD4+ T cells reconstituted with Ly108 show increased SHP-1 recruitment in the center of the synapse (Figure 5F-G), consistent with enhanced SHP-1 recruitment by Ly108 in the absence of SAP. However, Slamf6−/−Sh2d1a−/− CD4+ T cells reconstituted with the Ly108-AllF mutant show SHP-1 localization in an outer ring, comparable to WT CD4+ T cells (Figure 5F, H). These experiments support a model whereby Ly108 recruits SHP-1 to the central area of the synapse in the absence of SAP and confirms the requirements for the tyrosine residues for such recruitment. Thus, both in vitro and in vivo experiments suggest that in the absence of SAP, Ly108 mediates a potent negative signal primarily via SHP-1 phosphatase recruitment to ITSM motifs.
Sh2d1a− T:B cell adhesion can be restored by inhibition of SHP-1 or reduced Ly108 expression
If the negative signaling through Ly108 depends on SHP-1 recruitment, disruption of SHP-1 recruitment or function is predicted to prevent Ly108 negative signaling. Furthermore, disruption of SHP-1 recruitment or function would be predicted to reverse the severe cell:cell adhesion defect observed for SAP-deficient CD4+ T cells. To test this hypothesis, we pre-incubated SM CD4+ T cells with sodium stibogluconate (SSG), a specific SHP-1 inhibitor (Iype et al., 2010), and evaluated T:B conjugate formation. Sh2d1a−/− CD4+ T cell adhesion to B cells in the presence of cognate peptide is severely defective (Figure 6A-B). Strikingly, treatment of Sh2d1a−/− CD4+ T cells with SSG restores T:B adhesion to the same level achieved by wildtype CD4+ T cells (Figure 6A-C). Thus, excessive Ly108-mediated SHP-1 recruitment is the primary functional cause of the profound Sh2d1a−/− CD4+ T cell adhesion to B cell defect.
Figure 6. Inhibition of SHP-1 reverses the adhesion defect of SAP-deficient CD4+ T cells.
(a-c) Activated WT, Sh2d1a−/−, Slamf6−/−, and Slamf6−/−Sh2d1a−/− SM CD4+ T cells were incubated with activated WT B cells pulsed with cognate peptide (LCMV gp66-77. 0, 0.01, or 1.0 μg/ml), in the presence or absence of SSG, followed by flow cytometry. (a) Representative flow cytometry plots, gated on CD4+ T cells. (b-c) Mean frequency of T:B conjugates in total CD4+ events, in the (b) absence or (c) presence of SSG. (d) CD4+ T cells incubated with activated Slamf6−/− B cells pulsed with cognate peptide (LCMV gp66-77. 0, 0.01, or 1.0 μg/ml). Data are shown from one of two experiments with equivalent results.
Ly108 is a self-ligand. This implicates a requirement for Ly108 on both the CD4+ T cell and B cell for Ly108 and SHP-1 dependent inhibitory signaling to occur in CD4+ T cells. Inhibition of T:B adhesion does require Ly108 expression on both the CD4+ T cells and the B cells, as Sh2d1a−/− CD4+ T cells exhibit adhesion to Slamf6−/− B cells that is comparable to WT CD4+ T cell adhesion to WT B cells in the presence of high dose cognate peptide (Figure 6D). In addition, at 100-fold lower peptide concentrations, Sh2d1a−/− CD4+ T cells exhibit reduced adhesion to Slamf6−/− B cells compared to WT CD4+ T cell adhesion to Slamf6−/− B cells (Figure 6B, D). We infer from these results that positive SAP-dependent signaling through SLAM family receptors is most important under limiting concentrations of antigen (Figure 6D), while negative signaling through Ly108 can be potent at all antigen concentrations (Figure 6B). In summary, ligation of Ly108 on CD4+ T cells by Ly108 on B cells triggers SHP-1 recruitment in the absence of SAP, resulting in truncated synapse formation and abortive T cell help (Figure S5). This potent inhibitory mechanism downstream of Ly108 can be reversed by selective inhibition of SHP-1.
NKT cell development is rescued in Sh2d1a−/− mice by the elimination of Ly108
In addition to the dramatic humoral immunity defect, a severe defect in NKT cell development is a second prominent phenotype in both SAP-deficient mice and humans (Griewank et al., 2007; Ma et al., 2005; Nichols et al., 2005; Pasquier et al., 2005). SLAM family receptors Ly108 and SLAM have been shown to contribute to the development of NKT cells in the thymus (Griewank et al., 2007). We therefore examined if the absence of NKT cells in Sh2d1a−/− animals was related to negative signaling by Ly108. WT, Sh2d1a−/−, Slamf6−/− and Slamf6−/−Sh2d1a−/− mice were analyzed for the presence of splenic NKT cells. Consistent with previous studies, NKT cells were absent in Sh2d1a−/− mice (99.9% loss, P < 0.0001), while Slamf6−/− animals had 61% reduced frequencies compared to WT (P < 0.0001, Figure 7A, C). Quantifying absolute cell numbers gave the same results (P < 0.001, P < 0.002. Figure 7B). Surprisingly, NKT cell frequencies were substantially rescued by deletion of Slamf6 from Sh2d1a−/− mice (252-fold NKT cell increase in Slamf6−/−Sh2d1a−/− vs. Sh2d1a−/− mice. P < 0.0001. Figure 7A, C). The Slamf6−/−Sh2d1a−/− mice did, however, have fewer splenic NKT cells than WT (P < 0.0001). Notably, the Slamf6−/−Sh2d1a−/− mice also had few splenic NKT cells than Slamf6−/− mice (P < 0.0034, Figure 7A, C).
Figure 7. Ly108 provides both positive and negative signals for NKT cell development.
WT, Slamf6−/−, Sh2d1a−/− and Slamf6−/−Sh2d1a−/− (‘DKO’) mice were analyzed for NKT cells. (a) Frequencies of splenic NKT cells, gated as shown in (c). (b) Absolute numbers of splenic NKT cells. (c) Representative flow cytometry plots of splenic NKT cells, gated on total CD4+ cells. Data are representative of 2 independent experiments. (d) Representative flow cytometry plots of thymic NKT cells, gated on total live CD8− cells. (e) Quantitation of (d), combining two independent experiments. (f) Developmental profiles of thymic NKT cells, gated as described in (d), from 1 of 2 representative experiments. (g) Representative flow cytometry plots of liver NKT cells, gated on total live CD19− mononuclear cells. (h) Quantitation of (g), combining two independent experiments. (i) Developmental profiles of liver NKT cells, gated as described in (g), from 1 of 2 representative experiments. ** P < 0.005, *** P < 0.0005. Error bars are SEM.
NKT cell development depends on thymocyte-thymocyte interactions, where CD1d on one thymocyte is engaged by the TCR of another thymocyte (Bendelac et al., 2007). NKT cell development in Sh2d1a−/− mice is blocked at an extremely early stage, referred to as Stage 0. NKT cell thymic development is rescued in Slamf6−/−Sh2d1a−/− mice (Sh2d1a−/− vs. Slamf6−/− Sh2d1a−/−, 0.008% and 2.62% respectively. Figure 7D, E). However, the rescue does not completely restore developing thymic NKT cell numbers to wildtype numbers (Figure 7D, E). Liver is a major site of NKT cells in the periphery. While Sh2d1a−/− mice have a complete absence of liver NKT cells, Slamf6−/−Sh2d1a−/− mice have substantial liver NKT cells (0.007% vs. 3.1%, Figure 7G, H). Nevertheless, the liver NKT cell frequency in Slamf6−/−Sh2d1a−/− mice is still reduced in comparison to wildtype mice (Figure 7G,H), comparable to the NKT cells in spleen. Slamf6−/−Sh2d1a−/− NKT cells are fully differentiated, with normal proportions of Stage 2 (NK1.1−) and Stage 3 (NK1.1+) NKT cells (Figure 7F,I). Overall, Slamf6−/−Sh2d1a−/− mice have a significant loss of NKT cells, in the rank order: wildtype > Slamf6−/− > Slamf6−/−Sh2d1a−/−. This shows the positive signaling role of Ly108 in NKT cell development also. In conclusion, inhibitory signaling by Ly108 is potent in both CD4+ T cell function and NKT cell development.
DISCUSSION
A central role for SAP has been shown in Tfh cell differentiation and function and the generation of B cell immunity, both in mice and humans; however, how SAP facilitates these feats has been incompletely understood. We have found that Ly108 can transmit positive and negative signals to CD4+ T cells and NKT cells, such that absence of SAP results in both the loss of a positive signal and the exacerbation of a negative signal. Our findings help resolve the conundrum of why SAP-deficiency results in such a severe humoral immunity defect encompassing virtually all T-dependent immune responses. Equally importantly, these findings reveal a mechanism whereby Ly108 appears to serve as a rheostat for T:B interactions and other lymphocyte:lymphocyte interactions (e.g., NKT cell thymic selection), via modulation of the ratio of positive and negative signals transmitted by SAP and SHP-1 competing for occupancy of the same ITSMs of Ly108. The broader role of Ly108 is confirmed by similar findings in CD8 T cells (Zhao et al., accompanying paper).
Negative signaling by SLAM family receptors was previously observed, but this was primarily in the context of 2B4 in SAP-deficient human NK cells (Moretta et al., 2001; Nakajima et al., 2000; Parolini et al., 2000), and it was generally considered to be an unusual feature of NK cell receptor inhibitory signaling biology (Moretta et al., 2001; Raulet et al., 2001; Veillette et al., 2009) that was extended to include both 2B4 and NTB-A (Slamf6) (Bottino et al., 2001). Our CD4+ T cell and NKT cell work presented here, and new studies on CD8 T cells (Zhao et al., co-submission)(Palendira et al., 2011), indicate that a potent negative signaling role of Ly108 is broadly active in lymphocytes. This is not a generalized property of SLAM family receptors. Slamf1−/−Sh2d1a−/− mice phenocopy the severe germinal center defect of Sh2d1a−/− mice, indicating that SLAM only has positive signaling functions (Yusuf et al., 2010). Disruption of Cd84 also failed to rescue the negative signaling observed in Sh2d1a−/− mice. Therefore, the capacity for both negative and positive signaling appears to be restricted to Ly108 and 2B4 SLAM family receptors, of which only Ly108 is expressed on CD4+ T cells and developing NKT cells.
The potent negative signaling by Ly108 is primarily mediated by SHP-1. Ptpn6−/− (motheaten, SHP-1 deficient) mice have a phenotype consistent with the importance of SHP-1 in Ly108 negative signaling and balancing signaling through Ly108, as Ptpn6−/− mice have rapid hyperglobulinemia (Green and Shultz, 1975; Shultz and Green, 1976). SHP-1 is a key protein phosphatase, functioning in a variety of signaling pathways, and the importance of SHP-1 is reinforced by the severe phenotype of Ptpn6−/− mice, which become severely ill and die at approximately 3 weeks of age due to autoimmune pneumonitis and other autoimmune sequelae. Further examination of the kinetics of SHP-1 regulation of T:B interactions and other Ly108-dependent functions of lymphocytes is an important area for future investigation, which will require refined experimental approaches.
Positive signaling mediated by SAP has been shown previously for multiple SLAM family receptors, including SLAM, CD84, and Ly108. Positive signaling through SLAM has been studied in the most molecular detail, and has served as a template for understanding SLAM family receptor signaling (Veillette, 2006). SAP binds phosphotyrosines of the SLAM ITSM motifs and recruits Fyn kinase and PKCθ, and together this signaling complex mediates induction of IL-4 expression (Cannons et al., 2011; Crotty, 2011). The biological role of this pathway in CD4+ T cells has recently become clearer, as GC Tfh cells produce IL-4 (Crotty, 2011; Harada et al., 2012; Vijayanand et al., 2012), and SLAM receptor engagement induction of SAP signaling is required for expression of IL-4 by GC Tfh cells (Yusuf et al., 2010). A positive signaling role for SAP in CD4+ T cells is also seen for CD84 adhesion and Ly108 adhesion (Cannons et al., 2010a). A positive signaling role for Ly108 is clearest from the study of NKT cell development. A consistent ~50% reduction in NKT cell numbers was observed in Slamf6−/− mice (Griewank et al., 2007) and confirmed here. Notably, Slamf6−/−Sh2d1a−/− mice have fewer NKT cells than Slamf6−/− mice. This indicates that not only does Ly108 provide a negative signal in the absence of SAP and a positive signal in the presence of SAP, additional SLAM family receptors also provide positive SAP-dependent signals that facilitate NKT cell development. The role of SLAM itself in NKT cell development was primarily revealed only in the combined absence of Ly108 and SLAM, highlighting the redundancy between SLAM family receptors for positive signaling via SAP.
The positive signaling contribution of SAP in CD4+ T cells is best revealed in the studies of Slamf6−/−Sh2d1a−/− CD4+ T cell intrinsic defects. Both germinal center B cell and GC Tfh cell frequencies were ~50% lower compared to WT CD4+ T cell recipients. This shows a genetic requirement for positive signals through SAP and/or Ly108, paralleling the NKT cell biology. Furthermore, SAP overexpression in Slamf6−/−Sh2d1a−/− CD4+ T cells enhanced germinal center B cell numbers, again showing a positive role for SAP signaling. A positive signaling role for Ly108 in CD8 T cells is seen in reduced signaling and killing by Slamf6−/− CD8 T cells (Zhou et al., co-submitted manuscript).
The observation that Ly108 can transmit both positive and negative signals led us to examine the molecular mechanism of this process. We found that the negative signal is ITSM dependent, and requires SHP-1 recruitment to Ly108 and the immunological synapse. This intimate linking of both positive and negative signaling to a single Ly108 binding site, the ITSM, forces a direct competition between SAP and SHP-1 for occupancy. Therefore the simplest interpretation of these data is that the magnitude of negative or positive signaling transmitted by engaged Ly108 during T:B interaction is determined by the ratio of available SAP vs. SHP-1 in the local subcellular microenvironment. This suggests that the ratio of SAP and SHP-1 occupancy of Ly108 acts as a rheostat for the magnitude of T cell help to B cells. Ly108 expression is dynamically regulated on many hematopoietic cells. SAP expression is dynamically regulated in T cells. SHP-1 recruitment to membrane is dynamically regulated. We suggest that this Ly108 rheostat concept may apply to a variety of cell:cell interactions.
SAP’s impact on the duration of cell:cell interaction is particularly important for Tfh cell function. Tfh cells are specialized for B cell help, which is primarily provided via cell:cell interactions (Crotty, 2011). In addition, Tfh cell differentiation itself is strongly dependent on T:B interactions. Tfh cell differentiation is a multi-stage process (Crotty, 2011), such that in the absence of SAP there is still sufficient T:B interaction for early Tfh cell differentiation (Bcl6+CXCR5+), but not full polarization to GC Tfh cells (Bcl6hiPD1hi)(Choi et al., 2011; Crotty, 2011; Deenick et al., 2010; Qi et al., 2008; Yusuf et al., 2010). This is also important for the cognate B cells, because in the absence of extended T:B interactions, germinal center B cells fail to develop (Qi et al., 2008). Furthermore, in the absence of sufficient survival signals from CD4+ T cells, germinal center B cells apoptose within hours (Liu et al., 1989). GC Tfh cells regulate maintenance of germinal center B cells (Eto et al., 2011; Linterman et al., 2010; Victora et al., 2010), and altering the duration of T:B contact controls the quantity of information transfer between the two cells, thereby controlling germinal center B cell survival and further differentiation (Crotty, 2012). Altering the duration of T:B contact also likely alters the quality of the information transferred, as some information transfer likely takes the form of an initial contact-dependent B→T signal (i.e., MHCII-TCR engagement), and then “help” from the T cell back to the B cell after a lag phase of additional signal integration and protein translation. The duration of the contact is critical for such information transfer. As such, Ly108 modulation of the overall time of adhesion appears to serve as a powerful rheostat for T→B help, indirectly influencing a range of receptor:ligand interactions. This is consistent with the observation that GC Tfh cells have the highest SAP protein expression among CD4+ T cells. NKT cell development is also consistent with this, given that thymocyte interactions are potentially of insufficient duration to facilitate early NKT cell development in the thymus in the absence of SAP. In support of this model, recently it has been shown that thymic NKT cell development depends on strong sustained TCR signaling (Moran et al., 2011). Further understanding the stages of Ly108 and SLAM family mediated cell:cell communications is important for unraveling germinal center biology.
The characterization of the ITAM and ITIM motifs has greatly informed our understanding of how lymphocytes interpret interactions with other cells. The ITSM motif has proven to be challenging to understand. The data herein highlights the bimodal positive-negative signaling that can occur through this motif. The fact that SAP or SHP-1 need only one pY for binding, while Ly108 and other SLAM family receptors have two ITSMs, adds complexity to the signaling competition possibilities. The presence of non-ITSM tyrosine motifs adds a further level of complexity yet to be examined. A third isoform of Ly108 with only a single ITSM was recently identified, and this isoform ameliorates autoimmunity (Keszei et al., 2011). It is also worth noting that PD-1, a potent inhibitory receptor expressed on T cells with great interest as an immunotherapeutic target for treating chronic viral infections and tumors (Barber et al., 2006), is unusual in that it possesses a single ITSM (Sidorenko and Clark, 2003) and has not been reported to bind SAP. In conclusion, these surprising results illuminate several interesting aspects of lymphocyte biology centered on the elucidation that the severe humoral immunity and NKT cell development defects observed in SAP-deficiency stem from the duality of Ly108 functions.
METHODS
Mice
C57BL/6J (B6) mice were purchased from the Jackson Laboratory. Sh2d1a−, Slamf6−/−, Slamf6−/− Sh2d1a−, SMARTA TCR transgenic (SM, LCMV gp66-77 I-Ab specific) CD45.1+, Sh2d1a− SM, Slamf6−/− SM, and Slamf6−/−Sh2d1a− SM mice were all on a fully B6 background and bred at LIAI. AND TCR transgenic mice were purchased from Jackson. Cd84−/− mice were generated as previously described (Cannons et al., 2010a). Slamf6−/− mice were generated by Lexicon Genetics on the 129 background via homologous recombination targeting exon 1 of Slamf6. The neomycin resistance gene cassette remains. Ly108 protein expression is completely absent (Dutta and Schwartzberg, 2012). Slamf6−/− mice have no gross B cell, CD4+ T cell, or CD8 T cell defects. Mice were obtained through the NIH KOMP program, and then backcrossed 10 generations to the B6 background at LIAI. Whole genome microsatellite analysis through the University of California, Los Angeles Southern California Genotyping Consortium verified that the Slamf6−/− mice were 99% B6. The remaining 1% was of the Sv129 background around the SLAM locus, incorporating the region between the SNP markers mCv22849619 and rs13476259. Expression of SLAM and CD84 on Slamf6−/− lymphocytes is normal (data not shown). Sh2d1a− mice were greater than 99% B6 by SNP analysis, with a small region of the X chromosome remaining Sv129. All animal experiments were conducted in accordance with approved animal protocols.
Adoptive Transfers, Retroviral Transductions, and Transfections
Sh2d1a (SAP) expressing retroviral vector (pMIG-SAP) was reported previously (McCausland et al., 2007). Ly108-1 and Ly108-2 sequences were cloned into the pMIG vector. Site directed mutagenesis of Ly108-2 was done to create single tyrosine to phenylalanine mutants. Viral particles containing expression constructs of interest (RV) were produced from the Plat-E cell line as previously described (Johnston et al., 2009). SM CD4+ T cells were purified from spleen by negative selection using magnetic beads (Miltenyi). Cell transfers were done with either 5×103 for naïve cells, or 2.5 × 104 for retrovirally-transduced cells by intravenous injection via the retro-orbital sinus. For biochemical analysis, retrovirally transduced cells were sorted based on GFP expression and restimulated with peptide pulsed B cells. Ly108 and Ly108-AllF were cloned into a GFP fusion expression construct (Zhao F, submitted). Constructs were introduced into cells via Amaxa nucleofactor as previously described (Qi, 2008 #1136).
Viruses
LCMV Armstrong stocks were prepared and quantified as previously described (McCausland et al., 2007). All infections were done by bilateral intraperitoneal injection of 2 × 105 plaque-forming units of LCMV Armstrong per mouse. Vaccinia virus Western Reserve strain (VACV-WR) stocks (mature virion) were prepared and quantified as previously described (Benhnia et al., 2009). Mice were infected with VACV by bilateral intraperitoneal injection of 2 × 105 plaque-forming units per mouse.
Flow Cytometry
Single-cell suspensions of spleen were prepared by standard gentle mechanical disruption. Monoclonal antibodies against surface markers were used with FACS buffer (PBS + 0.5% BSA): GL7, Fas, and CD138-biotin (281-2) came from BD Pharmingen; CD4 (RM4-5 and GK1.5), CD8a (Ly-2), B220 (RA3-6B2), PD1 (J43), CD45.1 (A20), CD45.2 (104), CD62L (MEL-14), Ly108 (13G3-19D), and CD44 (IM7) came from eBioscience; SLAM (TC15-12F12.2) came from Biolegend. FITC-labeled peanut agglutinin (PNA) was from Vector Laboratories. CXCR5 staining was done using purified anti-CXCR5 (2G8, BD Pharmingen), followed by biotinylated goat anti-rat IgG (Jackson Immunoresearch), and then PE or APC-labeled streptavidin (Caltag Laboratories) with each staining step done in PBS + 0.5% BSA + 2% FCS + 2% Normal Mouse Serum on ice; samples were acquired without fixation. CD1d tetramers were provided by M. Kronenberg (Sidobre and Kronenberg, 2002). All FACS samples were washed twice with FACS buffer, acquired with an LSRII or Canto (BD Biosciences) and then analyzed with FlowJo (Tree Star).
ELISA
Serum from mice 30 days after LCMV infection was used. Anti-LCMV IgG was quantified by ELISA using LCMV infected cell lysate as the capture antigen. 96 well Polysorp microtiter plates (Nunc) were coated overnight with LCMV infected cell lysate in PBS. Following incubation of sample serum, HRPO-conjugated goat anti-mouse IgGγ (Invitrogen) was used for detection. VACV ELISAs used a similar procedure, with VACV antigen (Moyron-Quiroz et al., 2009).
Immunoprecipitation and Immunoblotting
LPS-activated B cells were untreated or pulsed with PCC peptide for 1 hr at 37°C, washed and mixed with either WT or Sh2d1a−/− antigen-specific CD4+ T cells for 20 mins at 37°C. Note that pervanadate treatment of T cells did not result in Ly108 SHP-1 binding, indicating that physiological Ly108 ligation is required (data not shown). Stimulated cells were lysed with cold HNGT buffer (pH 7.4) containing 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 1 mM MgCl2, 10% glycerol, 1% Triton-X 100, and protease inhibitors. Lysates were incubated overnight with 2.5μg/ml anti-mouse Ly108 (eBio13G3-191) at 4°C, followed by 2 hr incubation with protein-A (Santa Cruz). Immunocomplexes were washed and boiled in non-reducing SDS sample buffer for 5 mins at 95°C. Proteins were separated by SDS-PAGE and transferred to nitrocellulose. Membranes were blocked with TBS containing 5% BSA, 0.1% Tween-20. For western blotting, the following reagents were used: rabbit anti-SHP-1, rabbit anti-pERK, rabbit anti-ERK (Cell Signaling Technologies), mouse anti-Ly108 (eBioscience) and rabbit anti-Ly108 (Dutta and Schwartzberg, 2012). HRP-conjugated secondary reagents were from Jackson ImmunoResearch Laboratories.
Conjugate Adhesion Assay
SMARTA CD4+ T cells (5 × 105/well) were incubated for 30 min (37°C) in 96-well U-bottom plates with LPS-activated B cells (2 × 106/well) pulsed with gp66 peptide (LCMV gp66-77, or gp61-80 in some assays). Conjugate frequencies were enumerated by flow cytometry after the cell mixture was stained at 4°C for CD4 and CD19 as previously described (Qi et al., 2008). Sodium stibogluconate (SSG, Calbiochem) was resuspended in H2O at 100 mg/ml.
Immunofluorescence Microscopy
LPS-activated B cells pulsed with gp66 LCMV peptide for 1 hr at 37°C were washed and mixed with either activated, WT SMARTA, Sh2d1a−/− SMARTA, Slamf6−/− SMARTA or Sh2d1a−/− Slamf6−/− SMARTA CD4+ T cells at a 1:1 ratio in serum free media for 5 mins at 37°C to allow conjugates to form. Conjugates were plated on glass multi-well slides for 15 mins at 37°C. Samples were fixed and permeablized with −20°C methanol, washed several times in PBS and blocked with PBS containing 0.1% BSA for 20 mins at room temp. Samples were incubated with primary antibody in PBS containing 0.1% BSA for 1 hr at room temp, washed 5 times with PBS followed by staining with secondary reagents for 40 mins at room temp. For immunofluorescence the following reagents were used: rabbit anti-SHP-1 (AbCam), rat anti-CD4 (BD Pharmingen). Secondary reagents conjugated with Alexa Fluor dyes (excited at 488, 568, 633) were purchased from Invitrogen. Hoechst staining was completed in PBS for 5 min at room temp. Conjugates were examined by immunofluorescence using a Zeiss LSM 510 confocal microscope with a 63X oil immersion objective. Three dimensional reconstruction of z-stacks were made using the Imaris Scientific 3D/4D image processing and analysis software (Bitplane Scientific Software). 40 conjugates were examined per genotype per experiment.
Statistical Analysis
Statistical tests were performed using Prism 5.0 (GraphPad). P-values were calculated using two-tailed unpaired Student’s t tests with a 95% confidence interval. Error bars depict the standard error of the mean (SEM).
Supplementary Material
Acknowledgements
We thank I. Engel and M. Kronenberg for technical advice. Slamf6 mice were obtained through the NIH KOMP Repository program. This work was supported by NIH grants and LIAI institutional funds (SC), and the NIH NHGRI intramural program (PLS, JLC, FZ). FZ is a Scholar in the NIH-Oxford-Cambridge Scholars in Biomedical Research program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen CDC, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annual review of immunology. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Benhnia MR-E-I, McCausland MM, Laudenslager J, Granger SW, Rickert S, Koriazova L, Tahara T, Kubo RT, Kato S, Crotty S. Heavily isotype-dependent protective activities of human antibodies against vaccinia virus extracellular virion antigen B5. J Virol. 2009;83:12355–12367. doi: 10.1128/JVI.01593-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SB, Romero X, Ma C, Wang G, Faubion WA, Liao G, Compeer E, Keszei M, Rameh L, Wang N, et al. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat Immunol. 2010;11:920–927. doi: 10.1038/ni.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino C, Falco M, Parolini S, Marcenaro E, Augugliaro R, Sivori S, Landi E, Biassoni R, Notarangelo LD, Moretta L, Moretta A. NTB-A, a novel SH2D1A-associated surface molecule contributing to the inability of natural killer cells to kill Epstein-Barr virus-infected B cells in X-linked lymphoproliferative disease. The Journal of experimental medicine. 2001;194:235–246. doi: 10.1084/jem.194.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchon A, Cella M, Grierson HL, Cohen JI, Colonna M. Activation of NK cell-mediated cytotoxicity by a SAP-independent receptor of the CD2 family. J Immunol. 2001;167:5517–5521. doi: 10.4049/jimmunol.167.10.5517. [DOI] [PubMed] [Google Scholar]
- Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010a;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- Cannons JL, Wu JZ, Gomez-Rodriguez J, Zhang J, Dong B, Liu Y, Shaw S, Siminovitch KA, Schwartzberg PL. Biochemical and genetic evidence for a SAP-PKC-theta interaction contributing to IL-4 regulation. J Immunol. 2010b;185:2819–2827. doi: 10.4049/jimmunol.0902182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Ramagopal UA, Fedorov A, Fedorov E, Yan Q, Lary JW, Cole JL, Nathenson SG, Almo SC. NTB-A receptor crystal structure: insights into homophilic interactions in the signaling lymphocytic activation molecule receptor family. Immunity. 2006;25:559–570. doi: 10.1016/j.immuni.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol. 2003;5:155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- Chen G, Tai AK, Lin M, Chang F, Terhorst C, Huber BT. Signaling lymphocyte activation molecule-associated protein is a negative regulator of the CD8 T cell response in mice. J Immunol. 2005;175:2212–2218. doi: 10.4049/jimmunol.175.4.2212. [DOI] [PubMed] [Google Scholar]
- Chen R, Latour S, Shi X, Veillette A. Association between SAP and FynT: Inducible SH3 domain-mediated interaction controlled by engagement of the SLAM receptor. Mol Cell Biol. 2006;26:5559–5568. doi: 10.1128/MCB.00357-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Crotty S. The 1-1-1 fallacy. Immunological reviews. 2012;247:133–142. doi: 10.1111/j.1600-065X.2012.01117.x. [DOI] [PubMed] [Google Scholar]
- Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- Crotty S, McCausland MM, Aubert RD, Wherry EJ, Ahmed R. Hypogammaglobulinemia and exacerbated CD8 T-cell-mediated immunopathology in SAP-deficient mice with chronic LCMV infection mimics human XLP disease. Blood. 2006;108:3085–3093. doi: 10.1182/blood-2006-04-018929. [DOI] [PubMed] [Google Scholar]
- Davidson D, Shi X, Zhang S, Wang H, Nemer M, Ono N, Ohno S, Yanagi Y, Veillette A. Genetic evidence linking SAP, the X-linked lymphoproliferative gene product, to Src-related kinase FynT in T(H)2 cytokine regulation. Immunity. 2004;21:707–717. doi: 10.1016/j.immuni.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, Tangye SG. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010;33:241–253. doi: 10.1016/j.immuni.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta M, Schwartzberg PL. Characterization of ly108 in the thymus: evidence for distinct properties of a novel form of ly108. Journal of immunology (Baltimore, Md: 1950) 2012;188:3031–3041. doi: 10.4049/jimmunol.1103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C. Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244) Blood. 2005;105:4722–4729. doi: 10.1182/blood-2004-09-3796. [DOI] [PubMed] [Google Scholar]
- Eto D, Lao C, Ditoro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. IL-21 and IL-6 Are Critical for Different Aspects of B Cell Immunity and Redundantly Induce Optimal Follicular Helper CD4 T Cell (Tfh) Differentiation. PLoS ONE. 2011;6:e17739. doi: 10.1371/journal.pone.0017739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DB, Bell MP, McCausland MM, Huntoon CJ, van Deursen J, Faubion WA, Crotty S, McKean DJ. Ly9 (CD229)-deficient mice exhibit T cell defects yet do not share several phenotypic characteristics associated with SLAM- and SAP-deficient mice. J Immunol. 2006;176:291–300. doi: 10.4049/jimmunol.176.1.291. [DOI] [PubMed] [Google Scholar]
- Green MC, Shultz LD. Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J Hered. 1975;66:250–258. doi: 10.1093/oxfordjournals.jhered.a108625. [DOI] [PubMed] [Google Scholar]
- Griewank K, Borowski C, Rietdijk S, Wang N, Julien A, Wei DG, Mamchak AA, Terhorst C, Bendelac A. Homotypic interactions mediated by Slamf1 and Slamf6 receptors control NKT cell lineage development. Immunity. 2007;27:751–762. doi: 10.1016/j.immuni.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y, Tanaka S, Motomura Y, Ohno S, Yanagi Y, Inoue H, Kubo M. The 3′ enhancer CNS2 is a critical regulator of interleukin-4-mediated humoral immunity in follicular helper T cells. Immunity. 2012;36:188–200. doi: 10.1016/j.immuni.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Harker JA, Lewis GM, Mack L, Zuniga EI. Late Interleukin-6 Escalates T Follicular Helper Cell Responses and Controls a Chronic Viral Infection. Science. 2011 doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie D, Laroux FS, Morra M, Satoskar AR, Rosas LE, Faubion WA, Julien A, Rietdijk S, Coyle AJ, Fraser C, Terhorst C. Cutting edge: the SLAM family receptor Ly108 controls T cell and neutrophil functions. J Immunol. 2005;174:5931–5935. doi: 10.4049/jimmunol.174.10.5931. [DOI] [PubMed] [Google Scholar]
- Iype T, Sankarshanan M, Mauldin IS, Mullins DW, Lorenz U. The protein tyrosine phosphatase SHP-1 modulates the suppressive activity of regulatory T cells. Journal of immunology (Baltimore, Md: 1950) 2010;185:6115–6127. doi: 10.4049/jimmunol.1000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings P, Chan A, Schwartzberg P, Wakeland EK, Yuan D. Antigen-specific responses and ANA production in B6.Sle1b mice: a role for SAP. J Autoimmun. 2008;31:345–353. doi: 10.1016/j.jaut.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, Ditoro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamperschroer C, Dibble JP, Meents DL, Schwartzberg PL, Swain SL. SAP is required for Th cell function and for immunity to influenza. J Immunol. 2006;177:5317–5327. doi: 10.4049/jimmunol.177.8.5317. [DOI] [PubMed] [Google Scholar]
- Keszei M, Detre C, Rietdijk ST, Muñoz P, Romero X, Berger SB, Calpe S, Liao G, Castro W, Julien A, et al. A novel isoform of the Ly108 gene ameliorates murine lupus. J Exp Med. 2011;208:811–822. doi: 10.1084/jem.20101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Das A, Gross CC, Bryceson YT, Long EO. Synergistic signals for natural cytotoxicity are required to overcome inhibition by c-Cbl ubiquitin ligase. Immunity. 2010;32:175–186. doi: 10.1016/j.immuni.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. Journal of immunology. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour S, Gish G, Helgason CD, Humphries RK, Pawson T, Veillette A. Regulation of SLAM-mediated signal transduction by SAP, the X-linked lymphoproliferative gene product. Nat Immunol. 2001;2:681–690. doi: 10.1038/90615. [DOI] [PubMed] [Google Scholar]
- Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, Davidson D, Veillette A. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat Cell Biol. 2003;5:149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- Ma CS, Hare NJ, Nichols KE, Dupré L, Andolfi G, Roncarolo M-G, Adelstein S, Hodgkin PD, Tangye SG. Impaired humoral immunity in X-linked lymphoproliferative disease is associated with defective IL-10 production by CD4+ T cells. J Clin Invest. 2005;115:1049–1059. doi: 10.1172/JCI23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Pittaluga S, Avery DT, Hare NJ, Maric I, Klion AD, Nichols KE, Tangye SG. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J Clin Invest. 2006;116:322–333. doi: 10.1172/JCI25720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naïve human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunology and cell biology. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- Malbran A, Belmonte L, Ruibal-Ares B, Baré P, Massud I, Parodi C, Felippo M, Hodinka R, Haines K, Nichols KE, de Bracco MM. Loss of circulating CD27+ memory B cells and CCR4+ T cells occurring in association with elevated EBV loads in XLP patients surviving primary EBV infection. Blood. 2004;103:1625–1631. doi: 10.1182/blood-2003-07-2525. [DOI] [PubMed] [Google Scholar]
- Mavaddat N, Mason DW, Atkinson PD, Evans EJ, Gilbert RJ, Stuart DI, Fennelly JA, Barclay AN, Davis SJ, Brown MH. Signaling lymphocytic activation molecule (CDw150) is homophilic but self-associates with very low affinity. J Biol Chem. 2000;275:28100–28109. doi: 10.1074/jbc.M004117200. [DOI] [PubMed] [Google Scholar]
- McCausland MM, Yusuf I, Tran H, Ono N, Yanagi Y, Crotty S. SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase. J Immunol. 2007;178:817–828. doi: 10.4049/jimmunol.178.2.817. [DOI] [PubMed] [Google Scholar]
- Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. The Journal of experimental medicine. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz JE, McCausland MM, Kageyama R, Sette A, Crotty S. The smallpox vaccine induces an early neutralizing IgM response. Vaccine. 2009;28:140–147. doi: 10.1016/j.vaccine.2009.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Cella M, Bouchon A, Grierson HL, Lewis J, Duckett CS, Cohen JI, Colonna M. Patients with X-linked lymphoproliferative disease have a defect in 2B4 receptor-mediated NK cell cytotoxicity. European Journal of Immunology. 2000;30:3309–3318. doi: 10.1002/1521-4141(200011)30:11<3309::AID-IMMU3309>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Nichols KE, Hom J, Gong S-Y, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols KE, Koretzky GA, June CH. SAP: natural inhibitor or grand SLAM of T cell activation? Nat Immunol. 2001;2:665–666. doi: 10.1038/90595. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang Y-H, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palendira U, Low C, Chan A, Hislop AD, Ho E, Phan TG, Deenick E, Cook MC, Riminton DS, Choo S, et al. Molecular pathogenesis of EBV susceptibility in XLP as revealed by analysis of female carriers with heterozygous expression of SAP. PLoS Biol. 2011;9:e1001187. doi: 10.1371/journal.pbio.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini S, Bottino C, Falco M, Augugliaro R, Giliani S, Franceschini R, Ochs HD, Wolf H, Bonnefoy JY, Biassoni R, et al. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein-Barr virus-infected cells. J Exp Med. 2000;192:337–346. doi: 10.1084/jem.192.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquier B, Yin L, Fondanèche M-C, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poy F, Yaffe MB, Sayos J, Saxena K, Morra M, Sumegi J, Cantley LC, Terhorst C, Eck MJ. Crystal structures of the XLP protein SAP reveal a class of SH2 domains with extended, phosphotyrosine-independent sequence recognition. Mol Cell. 1999;4:555–561. doi: 10.1016/s1097-2765(00)80206-3. [DOI] [PubMed] [Google Scholar]
- Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol. 2001;19:291–330. doi: 10.1146/annurev.immunol.19.1.291. [DOI] [PubMed] [Google Scholar]
- Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- Schwartzberg PL, Mueller KL, Qi H, Cannons JL. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat Rev Immunol. 2009;9:39–46. doi: 10.1038/nri2456. [DOI] [PubMed] [Google Scholar]
- Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, Clark EA, Sidorenko SP. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001;166:5480–5487. doi: 10.4049/jimmunol.166.9.5480. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Green MC. Motheaten, an immunodeficient mutant of the mouse. II. Depressed immune competence and elevated serum immunoglobulins. Journal of immunology. 1976;116:936–943. [PubMed] [Google Scholar]
- Sidobre S, Kronenberg M. CD1 tetramers: a powerful tool for the analysis of glycolipid-reactive T cells. J Immunol Methods. 2002;268:107–121. doi: 10.1016/s0022-1759(02)00204-1. [DOI] [PubMed] [Google Scholar]
- Sidorenko SP, Clark EA. The dual-function CD150 receptor subfamily: the viral attraction. Nat Immunol. 2003;4:19–24. doi: 10.1038/ni0103-19. [DOI] [PubMed] [Google Scholar]
- Snow AL, Marsh RA, Krummey SM, Roehrs P, Young LR, Zhang K, van Hoff J, Dhar D, Nichols KE, Filipovich AH, et al. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J Clin Invest. 2009;119:2976–2989. doi: 10.1172/JCI39518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangye SG, Phillips JH, Lanier LL, Nichols KE. Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J Immunol. 2000;165:2932–2936. doi: 10.4049/jimmunol.165.6.2932. [DOI] [PubMed] [Google Scholar]
- Tarlinton DM. Evolution in miniature: selection, survival and distribution of antigen reactive cells in the germinal centre. Immunology and cell biology. 2008;86:133–138. doi: 10.1038/sj.icb.7100148. [DOI] [PubMed] [Google Scholar]
- Valdez PA, Wang H, Seshasayee D, van Lookeren Campagne M, Gurney A, Lee WP, Grewal IS. NTB-A, a new activating receptor in T cells that regulates autoimmune disease. J Biol Chem. 2004;279:18662–18669. doi: 10.1074/jbc.M312313200. [DOI] [PubMed] [Google Scholar]
- Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nat Rev Immunol. 2006;6:56–66. doi: 10.1038/nri1761. [DOI] [PubMed] [Google Scholar]
- Veillette A, Cruz-Munoz M-E, Zhong M-C. SLAM family receptors and SAP-related adaptors: matters arising. Trends in immunology. 2006;27:228–234. doi: 10.1016/j.it.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Veillette A, Dong Z, Pérez-Quintero L-A, Zhong M-C, Cruz-Munoz M-E. Importance and mechanism of ‘switch’ function of SAP family adapters. Immunological Reviews. 2009;232:229–239. doi: 10.1111/j.1600-065X.2009.00824.x. [DOI] [PubMed] [Google Scholar]
- Veillette A, Zhang S, Shi X, Dong Z, Davidson D, Zhong M-C. SAP expression in T cells, not in B cells, is required for humoral immunity. Proc Natl Acad Sci USA. 2008;105:1273–1278. doi: 10.1073/pnas.0710698105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M, Huang X, Interlandi J, Djuretic IM, Brown DR, et al. Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity. 2012;36:175–187. doi: 10.1016/j.immuni.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian X-H, Yim Y-S, Pertsemlidis A, Garner HR, Morel L, Wakeland EK. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Wang N, Satoskar A, Faubion W, Howie D, Okamoto S, Feske S, Gullo C, Clarke K, Sosa MR, Sharpe AH, Terhorst C. The cell surface receptor SLAM controls T cell and macrophage functions. J Exp Med. 2004;199:1255–1264. doi: 10.1084/jem.20031835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Malashkevich VN, Fedorov A, Fedorov E, Cao E, Lary JW, Cole JL, Nathenson SG, Almo SC. Structure of CD84 provides insight into SLAM family function. Proc Natl Acad Sci USA. 2007;104:10583–10588. doi: 10.1073/pnas.0703893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Rao S, Tsai L, Lee S, He Y, Sutcliffe E, Srivastava M, Linterman M, Zheng L, Simpson N, et al. The Transcriptional Repressor Bcl-6 Directs T Follicular Helper Cell Lineage Commitment. Immunity. 2009 doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M-C, Veillette A. Control of T lymphocyte signaling by Ly108, a signaling lymphocytic activation molecule family receptor implicated in autoimmunity. J Biol Chem. 2008;283:19255–19264. doi: 10.1074/jbc.M800209200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.