Abstract
Background
Brain dysfunction in prefrontal cortex (PFC) and dorsal striatum (DS) contributes to habitual drug use. These regions are constituents of brain networks thought to be involved in drug addiction. To investigate whether networks containing these regions differ between nicotine dependent female smokers and age-matched female non-smokers, we employed functional MRI (fMRI) at rest.
Methods
Data were processed with independent component analysis (ICA) to identify resting state networks (RSNs). We identified a subcortical limbic network and three discrete PFC networks: a medial prefrontal cortex (mPFC) network and right and left lateralized fronto-parietal networks common to all subjects. We then compared these RSNs between smokers and non-smokers using a dual regression approach.
Results
Smokers had greater coupling versus non-smokers between left fronto-parietal and mPFC networks. Smokers with the greatest mPFC-left fronto-parietal coupling had the most DS smoking cue reactivity as measured during an fMRI smoking cue reactivity paradigm. This may be important because the DS plays a critical role in maintaining drug-cue associations. Furthermore, subcortical limbic network amplitude was greater in smokers.
Conclusions
Our results suggest that prefrontal brain networks are more strongly coupled in smokers, which could facilitate drug-cue responding. Our data also are the first to document greater reward-related network fMRI amplitude in smokers. Our findings suggest that resting state PFC network interactions and limbic network amplitude can differentiate nicotine-dependent smokers from controls, and may serve as biomarkers for nicotine dependence severity and treatment efficacy.
Keywords: Resting State, Functional Connectivity, Smoking, Limbic, Prefrontal Cortex
1. Introduction
Drug addiction is a complex, difficult to treat brain-based disorder. A number of studies have identified key brain regions thought to participate in addiction disorders, such as the nucleus accumbens (NAc), which has been a main focus of addiction research due to its role in processing initial drug reward (Di Chiara and Imperato, 1988). Other brain regions such as the dorsal striatum (DS) and prefrontal cortex (PFC) also are thought to play critical roles in addiction disorders because they help maintain compulsive drug use (Everitt and Robbins, 2005; Goldstein and Volkow, 2002; 2011). However, increasing evidence suggests that groups of brain regions, e.g., brain networks, act in a coordinated manner to moderate addiction-related behaviors. For example, cortical-striatal interactions have been implicated as playing a role in habitual drug use (Graybiel, 2008), suggesting that network-level abnormalities may contribute to addiction.
Brain functional networks can be readily observed using functional magnetic resonance imaging (fMRI). Traditional fMRI research typically measures brain function during task performance. However, brain networks can be observed at rest as groups of regions with blood-oxygen level dependent (BOLD) signals that spontaneously fluctuate in a highly correlated manner. Networks demonstrating this behavior, also termed resting state networks (RSNs), are associated with many known brain systems including visual, sensorimotor, and auditory systems, and include areas involved in reward and possibly in addiction (e.g., Beckmann et al., 2005; Biswal et al., 1995; Fox et al., 2007; Hampson et al., 2002; Lowe et al., 1998; Smith et al., 2009). RSNs can be observed during sleep and anesthesia and have been interpreted to reflect a fundamental, intrinsic property of functional brain organization (Greicius, 2008; Horovitz et al., 2008; Vincent et al,, 2007; 2008). Thus, evaluating RSNs can provide information regarding inherent brain function that may help identify networks key to addiction-related behaviors, which may be diagnostically or therapeutically useful.
RSNs have been assessed in studies of smoking addiction. However, past research focused on nicotine-induced changes in the default mode network (DMN) and separately evaluated non-smokers (Tanabe et al., 2011) and abstinent smokers (Cole et al., 2010). Previous comparisons of functional connectivity between smokers and non-smokers did not evaluate RSNs, but focused on connectivity patterns of circumscribed brain regions (Hong et al., 2009). Our work differs by identifying differences between smokers and non-smokers within brain networks thought to be important in addiction disorders. We focused on four addiction-related networks that overlapped with RSNs reported elsewhere (Laird et al., 2011; Smith et al., 2009): a subcortical network that included the dorsal and ventral striatum, and three prefrontal networks containing regions thought to be abnormal in drug users (Goldstein and Volkow, 2002; 2011) a mPFC network that included the ACC, OFC, and mPFC, and individual right and left lateralized fronto-parietal networks that included lateral frontal and posterior parietal regions. We hypothesized that network interactions would differ between smokers and controls, and that such differences would correlate with smoking-related measures such as nicotine dependence, recent smoking, and dorsal striatal fMRI cue reactivity to smoking versus neutral images (Janes et al., 2010). We focused on DS fMRI reactivity to smoking cues, because the dorsal striatum appears to be involved in maintaining reactivity to drug associated cues (Porrino et al., 2004; See et al., 2007; Volkow et al., 2006), and DS cue reactivity has been described as possibly representing “the neural basis for the habitual link between conditioned cues and smoking behavior “ (Yalachkov et al., 2009, pg. 4926). In addition, DS cue reactivity is potentiated during smoking abstinence (McClernon et al., 2009) and does not seem to be blunted by smoking cessation treatment with nicotine replacement therapy (Janes et al., 2009). Thus, determining relationships between DS cue reactivity and addiction-related RSN functional connectivity may help clarify whether resting state intrinsic brain function is related to habitual responding to drug cues.
2. Methods
2.1 Subjects
Twenty-nine women, 13 nicotine-dependent smokers and 16 age- and sex-matched healthy controls, underwent neuroimaging at McLean Hospital. Smokers were screened and referred from a smoking cessation clinical trial at Massachusetts General Hospital (MGH, NCT00218465). Smokers reported consuming ≥10 cigarettes/day in the last 6 months, met DSM-IV criteria for current nicotine dependence, and had expired air carbon monoxide (CO)>10 ppm (Bedfont Micro IV Smokerlyzer, Bedfont Scientific, Kent, England) at screening. Nicotine dependence also was assessed with the Fagerstrom Test for Nicotine Dependence (FTND) Fagerström, (Fagerström, 1978). Smokers were not abstinent at the time of fMRI scanning and were allowed to smoke until shortly before the study visit. Healthy nonsmoking controls were recruited at McLean Hospital and were required to have an expired CO ≤ 5ppm. Thirteen of the 16 non-smokers never smoked a cigarette, two smoked < 2 packs of cigarettes in their lifetime approximately 30 years previously, and one smoked < 10 packs of cigarettes 14 years previously. Subjects were excluded if they had any of the following conditions as assessed by the Structured Clinical Interview for DSM (SCID): alcohol use disorder and/or major depressive episode in the past 6 months, lifetime DSM-IV diagnosis of organic mental disorder, bipolar disorder or any schizophrenia spectrum disorder or current unstable medical illness. Subjects also were excluded for pregnancy, current psychotropic drug use, or recent illicit drug or excessive alcohol use (QuickTox 11 Panel Drug Test Card, Branan Medical Corporation, Irvine California; Alco-Sensor IV, Intoximeters Inc., St. Louis, MO). The smoking cessation clinical trial from which smokers were referred involved an investigational medication not approved for use in men; thus only women were enrolled. McLean Hospital and MGH institutional Review Boards approved this study.
2.2 Functional Neuroimaging
Scans were acquired on a Siemens Trio 3 Tesla scanner (Erlangen, Germany) with a circularly polarized (CP) head coil. Multiplanar rapidly acquired gradient-echo (MPRAGE) structural images (TR=2.1 sec, TE=2.7 msec, slices=128, matrix=256×256, flip angle=12, resolution= 1.0×1.0×1.33 mm) and gradient echo echo-planar fMRI images (EPI; TR=2 sec, TE=30 msec, matrix=64×64, field of view=224, flip angle=75, slices=30, resolution=3.5mm isotropic with 0mm gap) were acquired. Resting state and cue-reactivity task activation data were acquired during separate runs in a single scan session. During the 10-minute resting state fMRI scan subjects were asked to remain still with their eyes open, and to remain awake. The same fMRI acquisition parameters were used for the smoking cue-reactivity study to assess DS fMRI reactivity to smoking-related and neutral images (Janes et al., 2010).
2.3 fMRI Resting-state Independent Components Analysis
We used independent component analysis (ICA), a data-driven approach, for delineating RSNs that were comprised of brain regions implicated in drug abuse. Independent component analysis was conducted using tools from the Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl). Functional data pre-processing included: motion correction with MCFLIRT (Jenkinson et al., 2002), brain extraction using BET (Smith, 2002), spatial smoothing with a Gaussian kernel of full-width half-maximum 6mm and high-pass temporal filter with Gaussian-weighted least-squares straight-line fitting with σ = 100s. Registration of the functional EPI volumes to each individual subject’s high-resolution MPRAGE image and registration MPRAGE to the MNI152 2mm3 standard space template (Montreal Neurological Institute, Montreal, QC, Canada) were both done using FLIRT. All subjects’ four-dimensional time-series data were transformed into standard space at 2×2×2 mm resolution using the registration transformation matrices. To identify the set of resting state networks common to all subjects, the resampled functional data from the16 controls and 13 smokers were temporally concatenated and a multivariate group probabilistic ICA (PICA) was conducted using FSL MELODIC (Beckmann et al., 2005; Beckmann and Smith, 2004). To investigate large-scale RSNs, the dimensionality was fixed to 35, to prevent RSN splitting into finer parcellations of large-scale RSNs. However, this dimensionality is lower than the optimal dimensionality that would be estimated by the PICA algorithm. Thus, to ensure stable convergence of the ICA, the ICA was run 8 times followed by a meta-level ICA fed by all of the spatial maps from the 8 decompositions (Smith et al., 2009). This meta ICA was conducted to identify the set of independent components, including both resting state networks and artifact-related components, that were common to all subjects. Through comparison with brain networks reported previously (Laird et al., 2011; Smith et al., 2009) the resulting independent component maps were visually inspected to identify a subcortical network, a mPFC network, and left and right fronto-parietal networks (see Fig. 1). These RSNs then were compared between smokers and non-smokers. We assessed 1) between-network measures, which evaluate the functional connectivity between the 4 defined RSNs and 2) within-network measures, which assesses both the functional connectivity between each individual RSN and all voxels within the whole brain and fMRI signal amplitude within each of the 4 defined RSNs.
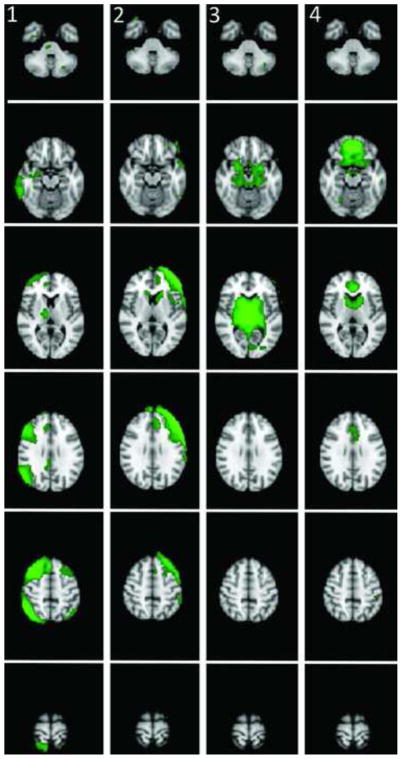
Figure 1. Addiction-Related RSNs defined by the Group ICA.
1) Right fronto-parietal network, 2) Left fronto-parietal network 3) The subcortical limbic network 4) The mPFC network.
2.4 Dual Regression
To investigate RSN behavior, subject-specific fMRI time courses and spatial maps corresponding to each group independent component (IC) must be estimated as they reflect variability in RSNs that may distinguish smokers from non-smokers. To calculate these time courses and spatial maps, we used a dual regression approach (Beckmann et al., 2009; Cole et al., 2010; Filippini et al., 2009). In the first stage of dual regression, the full set of group ICs, which include all RSNs and artifact components, are used in a multiple spatial regression against each individual subject’s dataset to estimate the average time course of voxels in each RSN. By including all of the ICs in the multiple regression, any voxel with contributions from multiple signal sources (for example, from coupling with an RSN and from motion effects) will have these effects “partialled out” into their separate contributions by the multiple regression prior to the averaging. The subject-specific time courses were normalized to unit variance and then used in a second multiple regression against the individual subject’s dataset, to identify voxels correlated with each of the RSN time courses, thus identifying the spatial map unique to the subject. These maps of partial regression coefficients reflect both the strength of an individual voxel’s time course relative to the IC time course (e.g., amplitude), as well as the correlation between the individual voxel’s time course and the IC time course, or the degree of integration of that voxel into the network. Conversion of these maps into semi-partial correlations will result in spatial maps that reflect a pure measure of coherence, which provides a second metric of within-network functional connectivity.
2.5 Network Interaction Comparisons Between Smokers and Non-Smokers
Correlation coefficients (Pearson’s r) were computed between the individual subject time courses for each of the four RSNs of interest that were generated by the first stage of the dual regression procedure. These correlation coefficients were then Fisher z-transformed and unpaired t-tests were conducted to compare between-network connectivity in smokers and non-smokers. P-values less than 0.01 were considered significant. As smokers, relative to non-smokers, exhibited greater coupling only between the mPFC and left fronto-parietal networks, we assessed whether this greater coupling was correlated with CO level, nicotine dependence severity, and DS fMRI reactivity to smoking cues. One-way correlation analyses (Pearson’s r) were performed to directly assess relationships between tobacco smoking variables and mPFC- left fronto-parietal network interactions.
2.6 Within Network Functional Connectivity Differences: Smokers Versus Non-Smokers
To assess within-network functional connectivity differences between smokers and non-smokers, subject-specific spatial maps from stage two of the dual regression were probed in a voxel-wise manner using a linear model contrast in a multiple regression general linear model (GLM) framework. We assessed group functional connectivity differences by comparing both RSN within-network coherence and amplitude using the partial regression coefficient and partial correlation coefficient spatial maps that were output from stage 2 of the dual regression. Partial correlation maps were z-transformed prior to GLM analyses using the Fisher r to z transformation. For both metrics, the subject-specific spatial maps corresponding to each of the four networks of interest were collected across subjects into four separate 4-dimensional files (one per original component map, with the fourth dimension being subject). These maps were assessed for group differences in within-network functional connectivity using non-parametric permutation testing (FSL Randomise; Nichols and Holmes, 2002; Smith et al., 2004) with cluster-based thresholding corrected for multiple comparisons by using the null distribution of the maximum cluster size (across the image). The cluster-forming threshold was z=2.3, and a family-wise error corrected threshold of p<0.01 was used to define clusters showing significant differences in connectivity (Hayasaka and Nichols, 2003). Similar to the between-network analysis conducted in smokers, we also evaluated associations between smoking relevant measures (CO, nicotine dependence severity, and DS fMRI reactivity to smoking vs. neural images) and within-network RSN functional connectivity. To assess whether between-network associations differed between smokers and non-smokers we conducted a repeated measures analysis of variance (ANOVA) with six within factors corresponding to correlations between each of the 4 RSNs. These analyses were conducted using the same strategy as above using separate models that individually included CO, FTND, or cue-reactivity as covariates (cue-reactivity analysis described below). These associations were family-wise error corrected to p<0.01.
2.7 Cue-reactivity
During the scan day, smoking-related DS cue reactivity was collected just prior to resting state measures. Smoking cue reactivity was then analyzed using BrainVoyager QX 1.10.4 as described in our previous work (Janes et al., 2010). Images were slice-time corrected, motion corrected, spatially smoothed (6-mm Gaussian kernel), resampled to 3mm isotropic voxels, and spatially normalized into Talairach space (Talairach and Tournoux 1988). Beta-weights for the smoking > neutral image contrast were extracted from right and left anterior caudate nuclei that overlapped with DS areas known to exhibit drug cue-induced dopamine increases (Volkow et al., 2006). The anterior caudate portion of the DS also receives prefrontal input and in electrophysiological studies has been shown to respond to environmental cues prior to behavior initiation (Rolls et al,1983). The anterior caudate DS ROIs were comprised of two cubes centered around Talairach coordinates (Talairach and Tournoux 1988): x= ± 10, y = 15, z = 9, and x = ± 11, y = 5, z =14, equaling a total volume of 427mm3 (shown in Supplemental Figure 11). The general linear model used to assess smoking > neutral cue-reactivity included motion confound regressors and task-related regressors (smoking, neutral, animal target images) convolved with the two-gamma hemodynamic response function. Beta-weights were averaged across all voxels within each ROI and these averaged values were correlated with within and between RSN measures to assess relationships between RSNs with DS smoking cue-reactivity.
3. Results
3.1 Subject Demographics
Controls were 42.4 (+/− 10.8; s.d) years old and smokers 39.9 (+/− 10.1) years old, and there was no age difference between groups (t27 = 0.71, p =0.47). The average FTND score in smokers was 5.5 +/− 2.3. Expired CO in smokers was 18.6 +/− 8.1 ppm at the time of fMRI scanning, and in non-smokers was 1.1 +/− 1.3 ppm.
3.2 Addiction-related RSNs
We identified four addiction-related networks that were common to smokers and non-smokers: 1) the right fronto-parietal network, which includes the anterior cingulate cortex (ACC), medial frontal cortex, right dorsolateral prefrontal (DLPFC) cortex, bilateral insula extending into the putamen, right temporal cortex, and portions of parietal cortex 2) the left fronto-parietal network, which includes the bliateral caudate nucleus with a larger extent on the left, left DLPFC, bilateral insula with a larger extent on the left, left inferior frontal gyrus, left dorsal ACC extending to the medial frontal gyrus, and portions of left temporal and parietal cortex. 3) The subcortical limbic network, which includes the entire striatum and extends into the thalamus, brainstem, hippocampus, and amygdala. 4) The mPFC network, which includes the bilateral ACC, medial PFC/OFC and extends into rostral portions of the striatum. These networks have been reported previously in healthy individuals (Smith et al., 2009) and are shown in Figure 1.
3.3 Network differences between Smokers and Non-Smokers
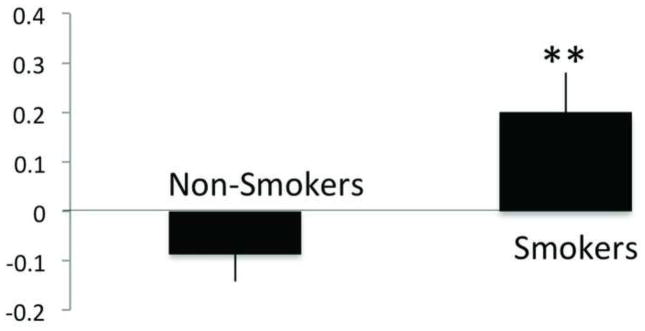
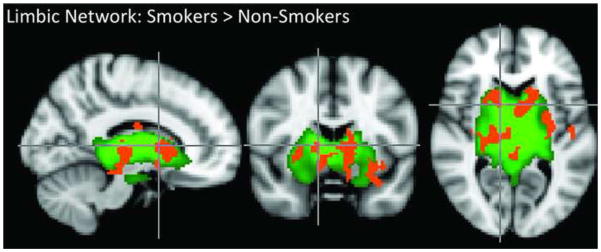
We found a significant main effect of between-network correlation (F5, 135 = 10.7, p < 0.001) and a significant interaction effect between between-network correlation and subject group (F5, 135 = 3.7, p = 0.003). A post-hoc t-test revealed that relative to non-smokers, smokers exhibited a greater correlation between the mPFC and left fronto-parietal networks (t=2.8, p = 0.009; Figure 2). Smokers also exhibited greater subcortical limbic network amplitude within the network. In comparison to non-smokers, the subcortical limbic network in smokers had greater amplitude in bilateral regions including the ventral striatum (including the nucleus accumbens (NAc), the caudate nucleus, putamen, thalamus, globus pallidus, amygdala, and hippocampus (pcorrected =0.01, Figure 3).
Figure 2. Left fronto-parietal and mPFC Network Correlation Differences between Smokers and Non-Smokers.
Relative to non-smokers, smokers had greater coupling between the Left fronto-parietal and mPFC networks at rest, *p < 0.01.
Figure 3. Limbic Network Activation Differences Between Smokers and Non-Smokers.
Green voxel highlighting indicates the subcortical limbic network RSN defined by the group ICA and shown in Figure 1. The orange overlay shows voxels in smokers exhibiting greater subcortical limbic network amplitude relative to non-smokers.
3.4 Network correlations in smokers
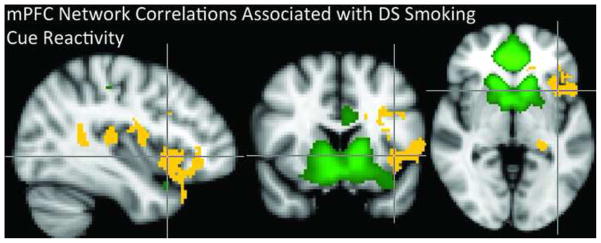
In smokers, increased coupling between the mPFC network and the left fronto-parietal network was correlated with left DS reactivity to smoking vs. neutral images (r > 0.55, p < 0.03, Figure 4) and FTND scores (at a trend level, r > 0.46, p < 0.057). When assessing within-network functional connectivity, we found that greater left caudate cue reactivity was positively correlated with greater functional connectivity of the mPFC network with several left-lateralized brain regions including: the insular cortex, inferior frontal gyrus (IFG), orbital frontal cortex (OFC), caudate, putamen, hippocampus, posterior cingulate cortex (PCC), precuneus, and the right thalamus (p < 0.01, Figure 5).
Figure 4. Dorsal Striatal Cue Reactivity is associated with mPFC-left fronto-parietal network coupling.
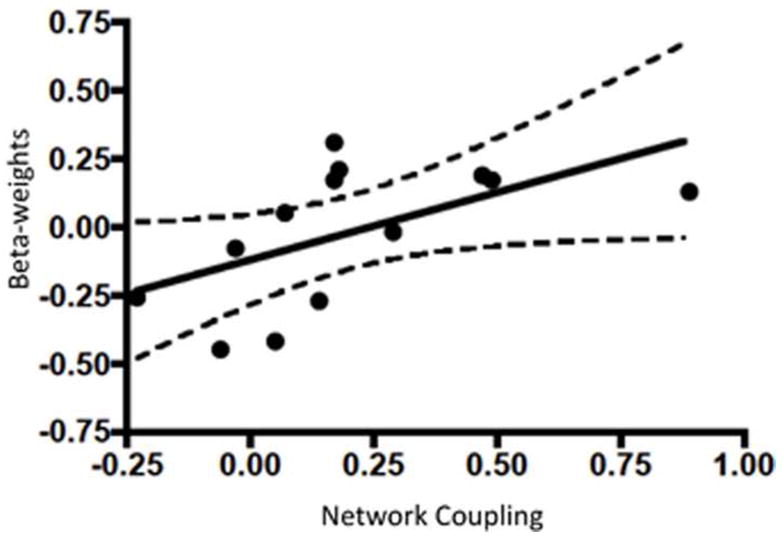
Within smokers, mPFC and left fronto-parietal network coupling was positively correlated with left dorsal striatal (caudate nucleus) reactivity to smoking vs. neutral images. The x-axis represents mPFC-left fronto-parietal network correlations while the y-axis shows beta-weights for dorsal striatum smoking vs. neutral image reactivity. Dotted lines represent 95% confidence intervals. Dorsal striatal region of interest is shown in supplemental figure 1.
Figure 5. mPFC Network Correlations Associated with Dorsal Striatal Cue Reactivity.
The green voxel highlighting indicates the mPFC network RSN defined by the group ICA and shown in Figure 1. mPFC network coupling with the brain regions marked with yellow voxels is correlated with dorsal striatal cue reactivity.
4. Discussion
Our results indicate that intrinsic resting state brain networks comprised of frontal and striatal regions differ between smokers and non-smokers on measures of network coupling and within-network amplitude. This finding is consistent with prior work showing that frontal and striatal regions play key roles in compulsive drug use (for review see: Everitt and Robbins, 2005; Goldstein and Volkow, 2002; 2011).
Relative to non-smokers, smokers exhibited greater coupling between the mPFC and left fronto-parietal networks. In smokers, this coupling was positively correlated with left DS reactivity to smoking cues, and (at a trend level) with nicotine dependence severity. We did not find any association between network coupling and CO levels, so it seems unlikely that smokers’ enhanced mPFC-left fronto-parietal coupling was due to physiological effects of CO or recent smoking.
In smokers, heightened DS cue reactivity was associated with greater mPFC-left fronto-parietal network coupling. Since RSNs were evaluated at rest following exposure to smoking-related stimuli, one interpretation of this finding is that enhanced mPFC-left fronto-parietal network coupling in smokers (versus non-smokers) may be related to the role brain regions in these networks play in cue reactivity, reward prediction, and cue-induced action planning. In this regard, medial prefrontal regions (part of our mPFC network) encode information both of the value of reward-predicting cues (Kahnt et al., 2010; 2011) and the perceived probability of obtaining a reward (Knutson et al.,2005). The DLPFC (which is part of our left fronto-parietal network) has been linked with value-based choices (Kahnt et al., 2010) and medial and lateral PFC regions code both for actual and predicted rewards (Kahnt et al., 2010). The left fronto-parietal network also includes brain regions involved in action planning such as the inferior frontal gyrus (IFG), (Johnson-Frey et al., 2005). Enhanced mPFC-left fronto-parietal coupling in smokers suggests that cue exposure may increase communication between cue-evaluative and action planning brain regions. This could facilitate responses to reward predictive cues.
Both the left fronto-parietal and mPFC networks included anterior caudate regions, which overlap with the DS ROI we used to evaluate cue reactivity. The anterior caudate involvement in both frontal networks is consistent with prior studies showing that the head of the caudate nucleus receives prefrontal input (Lehéricy et al., 2004; Rolls et al., 1983). Further, cortical-striatal interactions have been implicated in habitual behavior (for review see: Graybiel, 1995; Packard and Knowlton, 2002) including habitual drug use (Gerdeman et al., 2003; Graybiel, 2008). Like several of the regions in our frontal brain networks discussed previously, the DS is involved in reward prediction (Kahnt et al., 2011). The DS also is involved in selection of appropriate actions following conditioned learning (Atallah et al., 2007), suggesting that prefrontal-DS interactions play a critical role in responding to reward-predictive cues such as those associated with smoking.
While the between-network analysis described above identified group differences in mPFC-left fronto-parietal network coupling, we did not find group differences in mPFC coupling with other regions of the brain. However, we did find that DS cue-reactivity in smokers was strongly associated with mPFC coupling with several brain regions that overlapped with the left fronto-parietal network. These brain regions included the IFG and DS (discussed above) as well as the left insular cortex. The insula is of interest since it is highly reactive to smoking stimuli (Versace et al., 2011) and it is thought to play a role in the maintenance of smoking (Naqvi et al., 2007) and smoking relapse vulnerability (Janes et al., 2010). Furthermore, the insular cortex has been described as an area linking the limbic and motor control systems (Ackermann and Riecker, 2004). Thus, in combination with other cue-evaluative and action planning brain regions in the mPFC network, the insula could contribute to behavioral responses to smoking cues, especially in highly cue reactive smokers.
Relative to non-smokers, smokers also exhibited greater amplitude within the subcortical limbic network, which contains brain regions involved in primary reward processing. This suggests that while the subcortical limbic network is anatomically similar in smokers and non-smokers, amplitude within the subcortical limbic network is enhanced in smokers. Resting state amplitude is thought to reflect neuronal activity, whereby an increase in amplitude may indicate greater local neuronal activity (Yang et al., 2007). The relationship between resting state amplitude and neuronal activity suggests that, relative to non-smokers, smokers may have greater intrinsic neuronal activity in the subcortical limbic network at rest. This enhancement is consistent with preclinical reports showing that nicotine induces a persistent increase in the baseline sensitivity of brain reward systems (Kenny and Markou, 2006; Kenny et al., 2008). Additionally, behavioral clinical studies indicate that a single dose of nicotine increases sensitivity to non-drug rewards (Barr et al., 2008). Thus, the greater subcortical limbic network amplitude we found in smokers relative to non-smokers may represent a chronic nicotine-induced increase in baseline reward-system sensitivity. Subcortical limbic network amplitude was not correlated with any smoking-related covariate (CO, nicotine dependence, or DS cue reactivity). However, future work is needed to determine whether subcortical limbic network amplitude is associated with more direct measures of reward sensitivity.
4.1 Limitations
A limitation of this study is that resting state measures were conducted only a few minutes after smoking-cue exposure. Since cognitive task performance can impact subsequent measures of RSN function (Albert et al., 2009), it is possible that group RSN differences may have been a consequence of group differences in reactivity to smoking-related cues. An important next step will be to determine whether drug cue exposures influence subsequent RSN measures and whether any cue-induced changes in RSNs relate to clinical measures. Another limitation of this study is that only women smokers were included, so we cannot determine whether our findings generalize to men. While we plan to include men in future studies, smoking cue reactivity studies in women are important because women tend to be more reactive to smoking-related cues (Field and Duka, 2004; Perkins et al., 2001).
4.2 Conclusions
Our results suggest that mPFC-left fronto-parietal network coupling differs in smokers and non-smokers, and in smokers, network characteristics appear to be associated with clinical (FTND) and neurobiological (smoking cue fMRI reactivity) measures of addiction. Our findings also suggest that inter-PFC network coupling could be a biomarker for enhanced cue reactivity and nicotine dependence severity. We also showed greater amplitude of resting state activity in smokers at rest. To our knowledge, this is the first report of a smoking associated with greater reward-related network activity. Additional research is needed to determine whether this greater limbic network amplitude reflects a trait or a state (e.g., a function of recent nicotine use as suggested by preclinical research, (Kenny and Markou, 2006). We plan future studies to evaluate RSN changes following smoking cessation treatment to determine whether RSN measures reflect treatment efficacy.
Supplementary Material
Acknowledgments
Role of Funding Source
Funding for this study was provided by NIH grants K01DA029645, T32DA015036 and U01DA019378. The NIH had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
We would like to thank Drs. Eden Evins and Maurizio Fava for their role in screening and referring nicotine dependent subjects to this imaging study.
Footnotes
The supplemental figure can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors
Drs. Janes, Nickerson, and Kaufman conceptualized the study. Drs. Janes, Nickerson, and Frederick oversaw imaging assessments and data analyses. Drs. Janes, Nickerson, and Kaufman drafted the manuscript. Dr. Janes consolidated edits from coauthors. All authors approved the final manuscript.
Conflicts of Interest
No Disclosures: ACJ, LN, BBF
MJK: Research Support: GSK, Varian Inc., the Michael J. Fox Foundation for Parkinson’s Research, PhotoThera, Inc., and Air Products and Chemicals, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann H, Riecker A. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89:320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Cur Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah HE, Lopez-Paniagua D, Rudy JW, O’Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat Neurosci. 2007;10:126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biol Psychiatry. 2008;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Mackay CE, Filippini N, Smith SM. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage. 2009;47:S148. [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Mag Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T. Cue reactivity in smokers: the effects of perceived cigarette availability and gender. Pharmacol Biochem Behav. 2004;78:647–652. doi: 10.1016/j.pbb.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;5:733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurobiol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. NeuroImage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Hong LE, Hong G, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horovitz SG, Fukunaga M, de Zwart JA, van Gelderen P, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008;29:671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Frederick BdeB, Richardt S, Burbridge C, Merlo-Pich E, Renshaw PF, Evins AE, Fava M, Kaufman MJ. Brain fMRI reactivity to smoking-related images before and during extended smoking abstinence. Exp Clin Psychopharmacol. 2009;17:365–373. doi: 10.1037/a0017797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Frederick Bde B, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67:722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton ST. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Heinzle J, Park SQ, Haynes JD. The neural code of reward anticipation in human orbitofrontal cortex. Proc Natl Acad Sci USA. 2010;107:6010–6015. doi: 10.1073/pnas.0912838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahnt T, Heinzle J, Park SQ, Haynes JD. Decoding the formation of reward predictions across learning. J Neurosci. 2011;31:14624–14630. doi: 10.1523/JNEUROSCI.3412-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. J Neurosci. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology. 2008;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J Cog Neurosci. 2011;23:4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. NeuroImage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology. 2009;204:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res. 2001;3:141–150. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Thorpe SJ, Maddison SP. Responses of striatal neurons in the behaving monkey. 1 head of the caudate nucleus. Behav Brain Res. 1983;7:179–210. doi: 10.1016/0166-4328(83)90191-2. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology. 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Dobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady MM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme Medical Publishers; NY: 1988. [Google Scholar]
- Tanabe J, Nyberg E, Martin LF, Martin J, Cordes D, Kronberg E, Tregellas JR. Nicotine effects on default mode network during resting state. Psychopharmacology. 2011;216:287–295. doi: 10.1007/s00213-011-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Jackson EF, Costa VD, Robinson JD, Lam CY, Minnix JA, Brown VL, Wetter DW, Cinciripini PM. Do brain responses to emotional images and cigarette cues differ? An fMRI study in smokers. Eur J Neurosci. 2011;34:2054–2063. doi: 10.1111/j.1460-9568.2011.07915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Brain regions related to tool use and action knowledge reflect nicotine dependence. J Neurosci. 2009;29:4922–4929. doi: 10.1523/JNEUROSCI.4891-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. NeuroImage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.