Abstract
Background
Diabetic foot ulcers, although associated with macrovascular disease and neuropathy, have a microvascular disease causing ischemia not amenable to surgical intervention. Nitrite selectively releases nitric oxide in ischemic tissues, and diabetes subjects have low nitrite levels that do not increase with exercise. This study explores the safety and pharmacokinetics of a single dose of sodium nitrite in subjects with diabetic foot ulcers.
Subjects and Methods
Using a blinded, randomized crossover study design, 12 subjects with diabetes mellitus and active or healed foot ulcers received a single dose of sodium nitrite on two occasions 7–28 days apart, once with an immediate release (IR) formulation and once with an enteric-coated (EC) formulation for delayed release. Serum nitrite, nitrate, methemoglobin, sulfhemoglobin, blood pressure, pulse rate, complete blood count, chemistry panel, electrocardiogram, and adverse events were followed for up to 6 h after each dose. The IR and EC nitrite levels were analyzed by one-way analysis of variance and by pharmacokinetic modeling.
Results
The IR formulation elevated nitrite levels between 0.25 and 0.75 h (P<0.05). The EC formulation did not elevate nitrite levels significantly, but both formulations gave plasma nitrite levels previously suggested to be therapeutic (approximately 2–5 μM). The IR formulation gave an asymptomatic blood pressure drop of 10/6 mm Hg (P<0.003), and two subjects experienced mild flushing. There was no elevation of methemoglobin or other safety concerns. Pharmacokinetic modeling of plama nitrite levels gave r2 values of 0.81 and 0.97 for the fits for IR and EC formulations, respectively.
Conclusions
Oral sodium nitrite administration is well tolerated in diabetes patients.
Background
Diabetes represents an extremely large problem in today's society, affecting approximately 25 million people. Although the incidence of the disease increases with age, 0.2% of the population under the age of 20 years are afflicted, with the incidence rate rising. There are a large number of complications associated with diabetes, including increased heart disease and stroke caused by the narrowing of blood vessels due to fat deposits, leading to restricted blood flow. In addition, diabetes can often lead to diabetic foot ulcers, which, although associated with macrovascular disease and neuropathy, have a microvascular disease causing ischemia that is not amenable to surgical intervention. Thus, nonsurgical revascularization of the ischemic foot could represent an important means of treating diabetic foot ulcers. Modulation of ischemic tissue blood flow and survival is significantly influenced by nitric oxide (NO) bioavailability that can be governed by both NO synthases and nonenzymatic sources such as nitrite anion reduction.1–3 Moreover, sodium nitrite has been shown to selectively lead to revascularization of ischemic limbs,4 likely through its ability to act as a NO prodrug that is preferentially released in ischemic tissues.5 NO has been reported to be a beneficial adjuvant for both early and late phases of wound healing,6 with beneficial effects being manifold, including increasing extracellular matrix production, immune response modulation, and stimulation of cell proliferation (keratinocyte and endothelial cells).7,8
Although sodium nitrite is an inorganic salt that has been used as a preservative in meats, it can also be derived from dietary nitrate.9 Indeed, it has been suggested that nitrate and nitrite content of Mediterranean diets is involved in cardioprotection.10 Nitrates consumed in food are concentrated in the saliva and converted to nitrite by commensal bacteria in the mouth.11 Recent studies have shown that consumption of nitrate-rich foods, such as beetroot juice, can increase plasma nitrite levels through bioconversion involving the nitrate/nitrite enterosalivary conversion pathway12 that can lower blood pressure.13,14 Although this pathway can increase plasma nitrite from ingested nitrate, it is also subject to several variables such as the presence and type of oral flora as well as gastric pH, all of which can vary between individuals.11,15–17 Another important consideration is the fact that previous studies examining experimental therapeutic effects of nitrite and nitrate during pathophysiological conditions only found that sodium nitrite confers protection against ischemia-mediated tissue injury in either acute or chronic settings.4,18 Collectively, these facts indicate that sodium nitrite therapy may be quite efficacious in conferring tissue cytoprotection under ischemic conditions.
Although the above studies demonstrate nitrite's potential as a therapeutic, promoting tissue repair responses and angiogenesis, and suggest that it may be an effective treatment of diabetic foot ulcers, physiological data from human subjects also demonstrate the likely clinical efficacy of nitrite therapy. When risk factors associated with cardiovascular disease were correlated to circulating levels of nitrite in a patient's plasma, an inverse correlation was noted.19 Specifically, this study revealed a significantly lower level of circulating nitrite in patients with increased cardiovascular risk factors. In another study published earlier this year, circulating levels of nitrite were compared in normal volunteers, diabetes patients, patients with peripheral artery disease, and diabetes patients with peripheral artery disease.20 This study found that circulating nitrite levels in subjects with diabetes, peripheral artery disease, or both were lower than levels in normal subjects. An unexpected but potentially significant finding in this article was that, although nitrite levels increased in normal subjects following exercise, there was a significant decrease in nitrite plasma levels in the patients with peripheral artery disease and diabetes following exercise, which was below the already reduced levels found in these patient groups at baseline.
Reports in patients and animal models reveal that increases in plasma nitrite levels in the low micromolar range (1–10 μM) elicit various responses such as vasodilation and tissue cytoprotection during acute ischemia/reperfusion injury.18,21,22 Likewise, low plasma nitrite concentrations also stimulate angiogenic activity during chronic tissue ischemia.4 Given the deficits of circulating nitrite found in diabetes and cardiovascular patients,19,20 the ability of nitrite to stimulate vascular growth and tissue cytoprotection at low concentrations, the potential safety profile of sodium nitrite at the doses likely to be therapeutic (low micromolar from experimental studies), and the straightforward bioavailability of nitrite versus nitrate, sodium nitrite may represent a novel treatment of diabetes and its associated vascular and cardiovascular problems. This study examines the safety and single-dose pharmacokinetics of oral sodium nitrite in an immediate release (IR) form and an enteric-coated (EC) form designed to be released in the intestine as an initial step in the development of oral nitrite as a treatment for peripheral tissue ischemic conditions.
Subjects and Methods
Preparation of sodium nitrite capsules
The capsules were prepared by compounding (Prescription Compounds, Baton Rouge, LA) USP-grade sodium nitrite that was purchased from Spectrum Chemicals (Gardena, CA). In brief, a trace amount of blue food color was added, the nitrite was triturated, and microcrystalline cellulose was added using geometric dilutions and then mixed well. The mixture was sifted and encapsulated in #1 white capsules, such that each capsule contained 80 mg of sodium nitrite. For EC capsules, the capsules were coated with a 10% (wt/vol) solution consisting of cellacefate+triacetin+ethyl alcohol/acetone.
Potency/strength
Multiple small lots of capsules were compounded during the course of the study. All lots were tested for potency/strength prior to administration to study subjects and, in order to verify stability during the dosing interval, after all administration of a particular lot had been accomplished. Interim stability assessments were also done for several lots.
Testing for potency and capsule strength was done using the barbituric acid assay.
Dissolution and stability testing
Lots of non-EC capsules were tested for dissolution characteristics in a SOTAX (Horsham, PA) AT7 smart dissolution apparatus as in USP <711> (Dissolution) at various different time points. Five capsules were placed in sinkers and dropped at timed intervals into vessels each containing 1 L of 0.1 N HCl. Specimen samples were collected, replaced back in the 0.1 N HCl vessel at 10-, 30-, and 60-min intervals, and assayed at each sampling interval for the concentration of sodium nitrite. Because the potency/strength assay described above is linear over at least 2 logs, a modification of that assay was used for testing. Following a 3-min centrifugation at 3,500 g at room temperature, 1,100 μL of each collected sample was assayed. Standards for the standard curve were prepared from sodium nitrite in 0.1 N HCl. Three hundred microliters of a 16.67 mg/mL solution of barbituric acid in water (heated to 60–65°C to dissolve) was added to each standard or sample tube. Tubes were then vortex-mixed and incubated at room temperature for 30 min. Two hundred forty microliters of 2 N sodium acetate (pH 5.5) was then added to each tube. Tubes were vortex-mixed and incubated for 90 min at room temperature. Tube contents were then transferred to disposable plastic cuvettes, and the visible absorbance was read at 530 nm in a Beckman (Palo Alto, CA) DU800 spectrophotometer minus a baseline correction at 750 nm.
Bioburden
Production lots of non-EC capsules were tested for bioburden after compounding and at each stability point. Testing was conducted by Accugen Laboratories (Willowbrook, IL) and utilized USP <61> (Microbial Enumeration Tests) and USP <62> (Tests for Specified Microorganisms) for Escherichia coli. USP <61> allows quantitative enumeration of mesophilic bacteria and fungi that grow under aerobic conditions in nonsterile products. Because the lots were small (<100 capsules each), one capsule of each lot was added to 10 mL of phosphate buffer with Tween 80. Further dilutions were made as appropriate.
Measurement of plasma nitrite and nitrate
Blood was collected into either acid citrate dextrose- or K2EDTA-containing Vacutainer® tubes (BD, Fairlawn, NJ) and centrifuged (1,000 g, 5 min, 5°C). Two aliquots of plasma (300–500 μL) were obtained from the blood tube, with one immediately processed by methanol extraction (1:1 ratio) and another immediately snap-frozen in liquid nitrogen. Samples were transported overnight on dry ice to both the University of Alabama at Birmingham (methanol extraction specimen) and Emory University (Atlanta, GA) (frozen plasma) for separate independent analysis. After methanol processing, samples were centrifuged (10,000 g, 10 min, 5°C) to clarify, and the supernatant was removed and saved. Nitrite and nitrate were measured from 10 μL of the supernatant using the Griess reaction coupled to high-performance liquid chromatography separation with the ENO-20 analyzer (Eicom, Kyoto, Japan). Nitrite/nitrate levels were calculated by comparison with a standard curve of sodium nitrite (Sigma-Aldrich, St. Louis, MO). Randomly selected samples from each volunteer were also spiked with known amounts of nitrite and nitrate immediately upon sample collection to allow for extraction efficiency determination.
Clinical design
This open-label phase I single-dose study enrolled 12 patients with diabetes mellitus and active or healed foot ulcers (Table 1). All subjects gave written informed consent, and the study was approved by the Institutional Review Board of the Pennington Biomedical Research Center (IRB protocol number 10012). Subjects were given sodium nitrite (80 mg) on two occasions approximately 1 week apart, once in an IR form and on the other occasion in an EC form, followed by 14 blood draws over a 6-h period to define the pharmacokinetics.
Table 1.
Subject Demographics
| Screen | Visit 1 | Visit 2 | |
|---|---|---|---|
| Age (years) | 51.8±9.1 | ||
| Height (cm) | 179.1±5.9 | ||
| Body mass index (kg/m2) | 35.2±6.9 | ||
| Male/female | 9/3 | ||
| African American/ white | 4/8 | ||
| Weight (kg) | 112.7±19.2 | 112.4±19.2 | 112.5±20.0 |
| Blood pressure (mm Hg) | |||
| Systolic | 126.3±17.6 | 121.3±20.0 | 129.0±16.7 |
| Diastolic | 80.5±7.8 | 76.3±8.4 | 81.0±8.1 |
The 12 subjects participating in this study were between the ages of 18 and 85 years of age and had type 1 or type 2 diabetes mellitus with an active or healed foot ulcer. They agreed not to consume phosphodiesterase-5 inhibitor drugs during the study, and women with childbearing potential agreed to use a reliable form of contraception. Subjects who were pregnant, were nursing a baby, had a systolic blood pressure of <90 or >160 mm Hg, or had osteomyelitis, cardiovascular disease causing dyspnea at rest, anemia, a hemoglobin variant, glucose-6-phosphate dehydrogenase deficiency, or a history of substance abuse were excluded. Subjects taking medications for migraine like sumatriptan, allopurinol, tricyclic antidepressants, antihistamines, nitrates, meperidine, or related sedative medications were also excluded. Table 2 provides a list of medications taken by the subjects.
Table 2.
Medications Taken by the Subjects and the Number of Subjects Taking Them
| Medication | Number |
|---|---|
| Metformin | 9 |
| Insulin | 5 |
| Amlodipine | 3 |
| Exenatide | 3 |
| Hydrochlorothiazide | 3 |
| Aspirin | 2 |
| Fenofibrate | 2 |
| Glipizide | 2 |
| Quinipril | 2 |
| Rosuvastatin | 2 |
| Simvastatin | 2 |
| Atenolol | 1 |
| Bupropion | 1 |
| Carvedilol | 1 |
| Citalopram | 1 |
| Conjugated estrogen | 1 |
| Escitalopram | 1 |
| Fluoxetine | 1 |
| Furosemide | 1 |
| Gemfibrozil | 1 |
| Hydroxychloroquine | 1 |
| Ibuprofen | 1 |
| Latanoprost drops | 1 |
| Lisinopril | 1 |
| Lorazapam | 1 |
| Methotrexate | 1 |
| Olmesartin | 1 |
| ω-3-Acid ethyl esters | 1 |
| Omeprazole | 1 |
| Pravastatin | 1 |
| Sitagliptin | 1 |
| Testosterone | 1 |
| Thyroxine | 1 |
Subjects attended a screening visit, fasting for at least 10 h beforehand, where they signed an informed consent, had height, weight, and vital signs measured and blood was drawn for complete blood count (CBC) (hemoglobin, hematocrit, mean cell volume, platelet count, white blood cell count, granulocyte number, neutrophil number, eosinophil number, and basophil number), chemistry panel (glucose, blood urea nitrogen [BUN], creatinine, potassium, uric acid, albumin, calcium, magnesium, creatine phosphokinase, alanine-leucine transaminase, alkaline phosphatase, iron, cholesterol, triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol), urinalysis, hemoglobin electrophoresis, and glucose-6-phosphate dehydrogenase screen. Subjects returned for a second screening visit at which the lab work was reviewed, a medical history was taken, and a physical examination was performed. Subjects showing abnormalities in the various chemistries above were not eligible for participation in the study.
Subjects passing screening came to the first study visit at 7 a.m. after fasting from midnight the prior day, except for water. The subjects were given a 2,000 kcal/day diet consistent with American Diabetes Association guidelines with less than 8 ounces of meat and no nitrates. An electrocardiogram was obtained, weight and vital signs were measured, and an intravenous line was inserted into the arm for blood drawing and attached to normal saline. Women of childbearing potential had a pregnancy test, and results were returned prior to any test medication. Subjects were asked about any medications they were taking. Blood was drawn for a CBC, chemistry panel, nitrite, nitrate, methemoglobin, partial thromboplastin time, and prothrombin time. After the baseline labs, including a chemistry panel with BUN, creatinine, and CBC, were drawn, the subjects were give a capsule containing 80 mg of sodium nitrite in either IR or EC form, and blood was drawn for nitrite and nitrate measurement at 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, and 6 h. Blood was drawn for clinical methemoglobin and sulfhemoglobin measurement at baseline and 1, 2, 3, 4, 5, and 6 h after dosing, as methemoglobinemia is a potential adverse event associated with nitrite administration. Blood pressure and pulse rate were measured with each blood draw because nitrite is a vasodilator. Subjects were questioned about any adverse events with each blood draw, and an electrocardiogram was performed at 3 and 6 h. After the 6-h specimen was drawn, which included a chemistry panel with BUN, creatinine, and CBC, the subjects were released and given an appointment to return in 7–28 days to repeat the test day with the form of nitrite that they did not receive on the first test day.
The demographic data were presented using descriptive statistics and compared using a t test. The adverse events were compared by χ2 statistics, and the laboratory tests were compared using the general linear model and SAS software (SAS Institute Inc., Cary, NC). Nitrite, nitrate, methemoglobin, sulfhemoglobin, pulse rate, and blood pressure were analyzed using one-way analysis of variance with a correction for multiple comparisons. Pharmacokinetic modeling was also performed for the IR and EC formulations.
Results
Release and stability testing
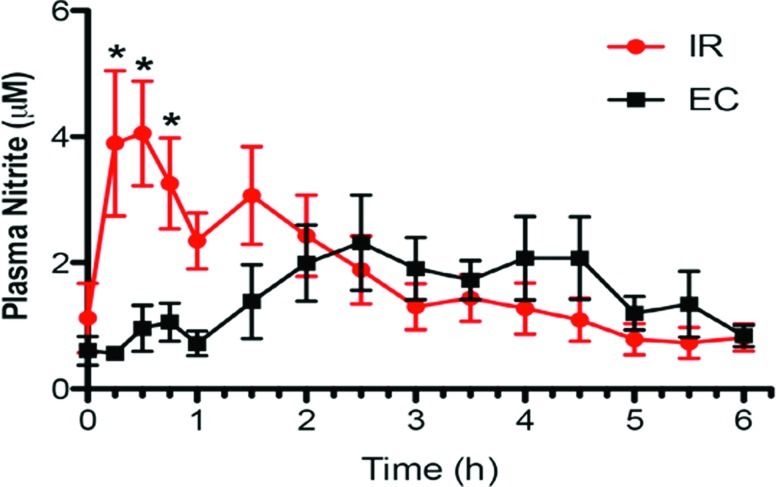
The barbituric acid assay for determination of sodium nitrite concentration was very sensitive and linear over at least 2 logs. It was therefore suitable for both the potency/strength assay and dissolution testing. The non-EC capsules (IR) were stable over a 8-month period at 25°C and 40% relative humidity (Fig. 1).
FIG. 1.
Plasma nitrite levels from different oral formulations of sodium nitrite. The immediate release (IR) formulation gave significant elevations of plasma nitrate compared with baseline at 0.25, 1.0, and 0.75 h, but there was no significant increase in nitrite above baseline with the enteric-coated (EC) formulation at any time point. Data are mean±SEM values. *P<0.05 versus baseline. Color graphics available online at www.liebertonline.com/dia
EC capsules were stored at room temperature. The content of the EC capsules was stable over at least 37 days. However, after that time the capsule coating began to yellow and show spots. EC capsules were therefore made at several time points during the study in order to maintain a more consistent appearance and contents.
Because the IR capsules were used for a longer time period, each lot was tested for bioburden and the presence of E. coli. The lots passed testing (total aerobic count, <10 colony-forming units/mL; yeast and mold, <10 colony-forming units/mL; absent E. coli).
Clinical
Table 1 reports study subject demographics. No statistical difference was observed for weight, blood pressure, or pulse rate from baseline compared with visits 1 and 2. Only one subject was taking a proton pump inhibitor (omeprazole, 40 mg once a day), which was taken in the evenings. Eight of the subjects took antihypertensive medications, and five of these took the medication on test days 2 h after dosing of nitrite. Two subjects took their antihypertensive medication 2 h after dosing on test day 1 but not test day 2 because they ran out of their medication and did not get the prescription filled, and their antihypertensive medication was not in stock at the study site. One subject routinely took the antihypertensive medication in the evening and did not take it during the test on either test day. Table 2 lists other medications taken by participants.
Nitrite
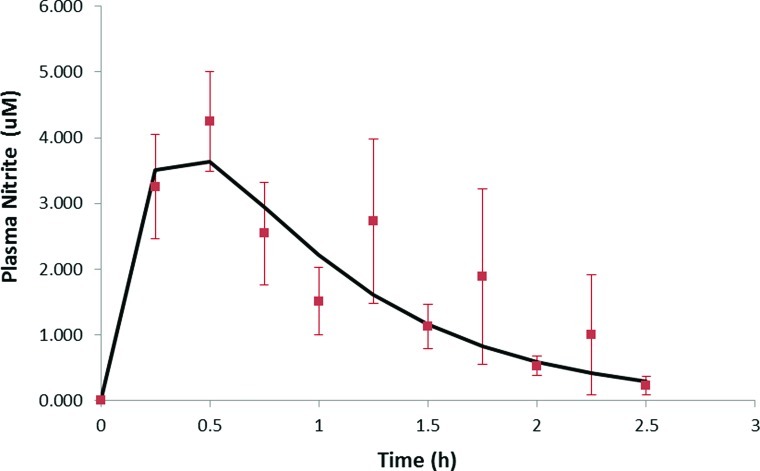
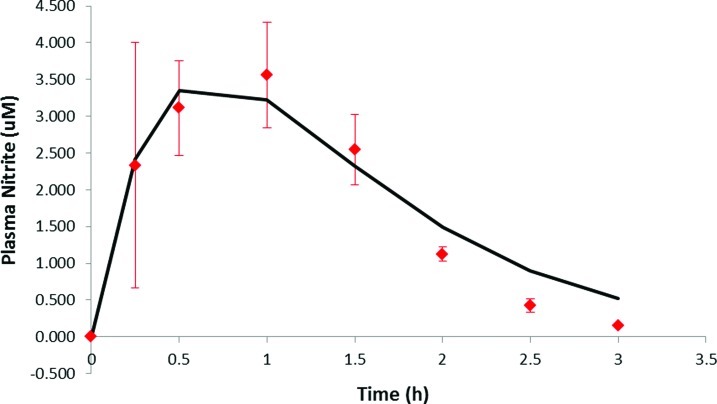
The IR formulation caused a significant elevation of average plasma nitrite levels at 15, 30, and 45 min (P<0.05) compared with baseline, but there was no significant increase in nitrite above baseline with the EC formulation at any time point. Results of the second assay done without methanol extraction confirmed these findings. Although the differences in nitrite levels in the two formulations were significant only with the IR formulation, there was a different shape to the two curves (Fig. 1). To further analyze the plasma profiles of nitrite after administration of the two formulations, corrections were applied to the plasma nitrite data for each subject. The first correction was for the initial nitrite plasma level that was determined from the initial baseline lab analysis for each subject. The initial nitrite level was subtracted from all later determined plasma nitrite levels for each subject. This plasma nitrite correction averaged 1.25 μM (range, 0.0–6.8 μM) for the IR formulation, whereas for the EC formulation the correction averaged 0.74 μM (range, 0.29–1.68 μM). The second correction was for the apparent delay in absorption (i.e., lag time) before plasma nitrite levels began to rise. For the IR formulation the average lag time was 0.17 h (range, 0–0.5 h), whereas the EC formulation had an average lag time of 2.1 h (range, 0.5–3.5 h). Additionally, some subjects exhibited second peaks in plasma nitrite levels well after sodium nitrite from either formulation could be still absorbed—these nitrite levels were not included in the pharmacokinetic analysis. After applying these corrections to each subject's data, the nitrite levels were averaged at each time, as was done for Figure 1, and the results are shown in Figures 2 and 3 for IR and EC formulations, respectively. The plasma profiles appear somewhat different for the IR formulation, showing a rapid rise to a peak plasma level at 0.5 h, whereas for the EC formulation the peak appeared at 1.0 h. These data were then fit by a one-compartment pharmacokinetic model with first-order absorption as shown below:
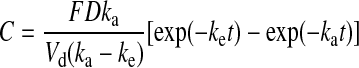 |
FIG. 2.
Corrected immediate release (IR) formulation plasma nitrite curve. Plasma nitrite levels at various time points after the IR formulation were ingested, averaged, and used to obtain a corrected pharmacokinetic profile analysis (smooth curve). Data are mean±SEM values. Color graphics available online at www.liebertonline.com/dia
FIG. 3.
Corrected enteric-coated (EC) formulation plasma nitrite curve. Plasma nitrite levels at various time points after the EC formulation were ingested, averaged, and used to obtain a corrected pharmacokinetic profile analysis (smooth curve). Data are mean±SEM values. Color graphics available online at www.liebertonline.com/dia
where ke is the elimination rate constant, ka is the absorption rate constant, Vd is the volume of distribution, D is the dose, F is the fraction of nitrite absorbed, and t is time.
Fitting the above equation to the average IR plasma data in Figure 2 gave a ke=1.37±0.09 h−1 and ka=4.5±2.0 h−1. The uncertainty in ka is large because the time to reach the peak plasma level is 0.5 h, which means there are only three plasma nitrite values that determine this constant. Thus, ka is viewed to be uncertain. From the data of Hunault et al.23 a value of ka=3 or 20 h−1 was obtained from the two oral doses of sodium nitrite given in solution to normal subjects. The ke ranged from 0.95 to 1.89 h−1, which agrees with the values in this study. The smooth curve in Figure 2 is the fit of the pharmacokinetic model to the IR data, with r2=0.81. For the EC formulation, the fit of the above equation to the plasma data gave a value of ke=1.38±0.01 h−1 and ka=1.55±0.01 h−1 with r2=0.97. The values for ke agree quite well between the two formulations, and the EC formulation has a lower ka because it may release nitrite more slowly in the intestine than the IR formulation. The smooth curve in Figure 3 is the fit of the pharmacokinetic model to the EC data.
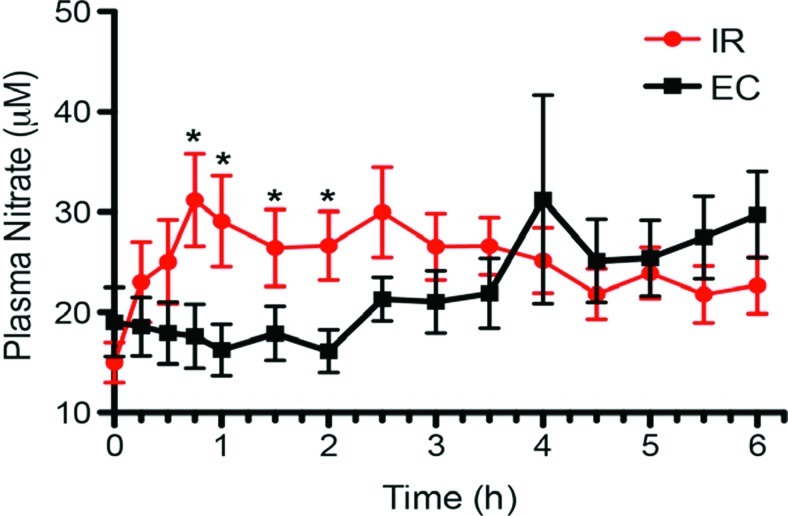
Nitrate
The IR formulation gave significant elevations of plasma nitrate compared with baseline at 0.25, 0.5, and 0.75 h (P<0.05), but there was no significant increase in nitrate above baseline with the EC formulation at any time point. Although the differences in nitrate levels in the two formulations were significant only with the IR formulation, there was again a different shape to the two curves (Fig. 4).
FIG. 4.
Plasma nitrate levels from different oral formulations of sodium nitrite. The immediate release (IR) formulation gave significant elevations of plasma nitrate compared with baseline at 0.75, 1.0, 1.5, and 2 h, but there was no significant increase in nitrate above baseline with the enteric-coated (EC) formulation at any time point. Data are mean±SEM values. *P<0.05 versus baseline. Color graphics available online at www.liebertonline.com/dia
Methemoglobin and sulfhemoglobin
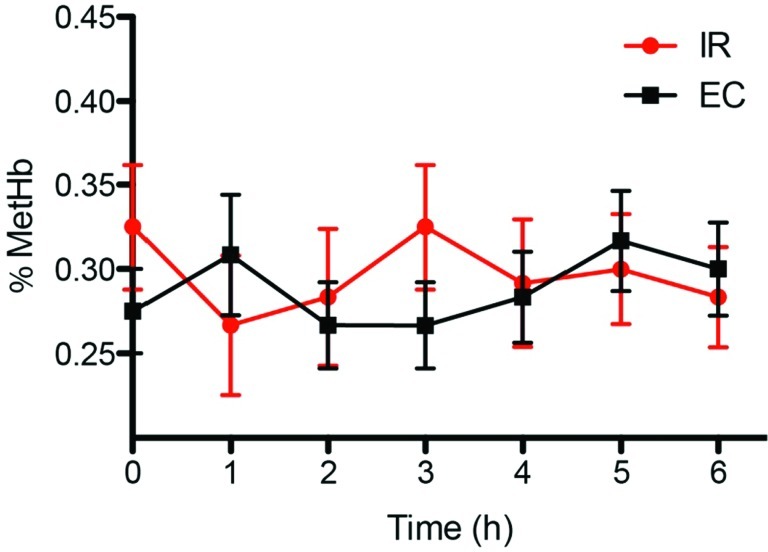
There was no significant increase in the methemoglobin levels with either the IR or the EC formulation (Fig. 5). Sulfhemoglobin was not detectable at any time point.
FIG. 5.
Methemoglobin (MetHb) levels with different sodium nitrite oral formulations. Blood was collected every hour during the study to measure changes in percentage MetHb. Neither the immediate release (IR) or enteric-coated (EC) sodium nitrite formulation significantly altered blood MetHb levels. Data are mean±SEM values. Color graphics available online at www.liebertonline.com/dia
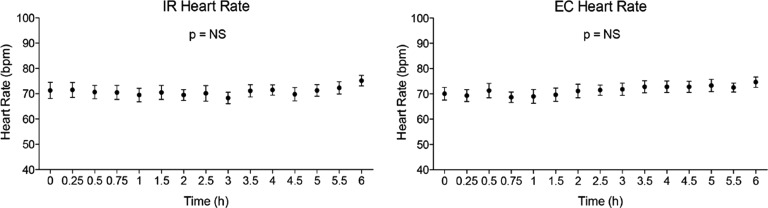
Heart rate
There was no significant change in heart rate with either the IR or EC formulations (Fig. 6).
FIG. 6.
Effects of different sodium nitrite formulations on heart rate. Subject heart rate was monitored over time after administration of either immediate release (IR) or enteric-coated (EC) sodium nitrite. No significant change in heart rate was observed with either formulation. Data are mean±SEM values. bpm, beats per minute; NS, not significant.
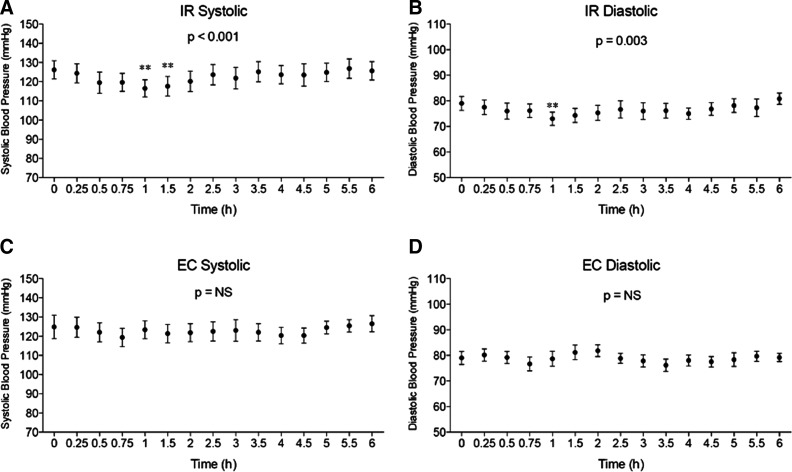
Blood pressure
There was a significant drop in systolic blood pressure in the IR formulation at 1.0 and 1.25 h (P<0.001) and in diastolic blood pressure at 1 h (P<0.003). There was no significant change in systolic or diastolic blood pressure at any time point with the EC formulation (Fig. 7). Although the drop in blood pressure was statistically significant in the IR group, the drop was from 125/78 to 115/72 mm Hg and was asymptomatic, and values returned to basal levels by 2 h.
FIG. 7.
Effects of different sodium nitrite formulations on blood pressure. Systolic and diastolic blood pressure was measured over time after administration of different sodium nitrite formulations. (A and B) Effect of immediate release (IR) sodium nitrite formulation on systolic and diastolic blood pressure, respectively. Systolic blood pressure significantly dropped with the IR formulation at 1.0 and 1.25 h (P<0.001), and diastolic blood pressure significantly dropped at 1.0 h (P<0.003). (C and D) Effect of enteric-coated (EC) sodium nitrite formulation on systolic and diastolic blood pressure, respectively. No significant change in systolic or diastolic blood pressure was observed at any time point for EC sodium nitrite formulation. Data are mean±SEM values. NS, not significant.
Adverse events
Adverse events are identified in Table 3. The only adverse event that seemed potentially related to the study intervention was headache and flushing in the IR group. None of the differences in adverse events, however, were statistically significant.
Table 3.
Sodium Nitrite Formulation Adverse Events
| |
Formulation |
|
|---|---|---|
| Event | Immediate release | Enteric-coated |
| Headache | 2 | 1 |
| Nausea | 0 | 1 |
| Hot flush | 2 | 0 |
| Knee pain | 1 | 1 |
| Ear infection | 1 | 0 |
Laboratory tests
The results of laboratory tests before and after test days with the IR and EC formulations were not statistically different. IR formulation pretreatment and post-treatment creatinine values were 0.85±0.33 and 0.83±0.27 mg/dL, respectively. Likewise, IR formulation pretreatment and post-treatment BUN values were 17.7±6.7 and 16.3±5.8 mg/dL, respectively. EC formulation pretreatment and post-treatment creatinine values were 0.88±0.37 and 0.79±0.29 mg/dL, respectively. EC formulation pretreatment and post-treatment BUN values were 17.9±5.2 and 16.1±4.4 mg/dL, respectively. There was no statistical change between pre- and post-treatment values, although the numbers were in the direction of improvement.
Physical examinations and electrocardiograms
The physical examinations were unchanged as were the interpretations of the electrocardiograms.
Discussion
Studies over the past decade have revealed that the nitrite anion may undergo selective reduction back to NO through a variety of mechanisms including, but not limited to, acidic disproportionation, deoxyhemoglobin and deoxymyoglobin, xanthine oxidoreductase, mitochondrial enzymes, and others.24 As such, use of sodium nitrite as an NO prodrug for the treatment of various pathophysiological conditions involving decreased NO bioavailability has become increasingly attractive.25 Oral sodium nitrite pharmacokinetics have been studied in healthy volunteers,23 but such similar studies have not been reported for diabetes patients.
Our study demonstrates that oral sodium nitrite therapy significantly increases plasma levels of nitrite in the IR formulation within 0.25–0.75 h after dosing. This correlated with a statistically significant rise in nitrate 0.25–0.75 h after dosing, along with a statistically significant fall in blood pressure 1.0–1.25 h after dosing. Although there was no statistically significant change in the levels of nitrite, nitrate, or blood pressure after dosing with the EC formulation, there was a difference in the shape of the curve suggesting that statistically significant changes in nitrite might be expected between 1.5 and 4.5 h at a larger dose. There were no significant changes in methemoglobin, sulfhemoglobin, pulse rate, laboratory tests, or other safety parameters with the possible exception of headache and a hot flush feeling after taking the IR formulation in two of the 12 subjects (17%).
A recent study by Kenjale et al.26 demonstrates the importance of developing sodium nitrite as a potential treatment for lower-extremity ischemia. Eight subjects with stable claudication on walking were given 500 mL of beet juice containing 9,090 μmol of nitrate and 36 nmol of nitrite or a 500 mL orange juice control in a crossover fashion 1–2 weeks apart. Subjects given beet juice walked a significant 18% longer than in the control condition (P<0.01) prior to the onset of claudication. During the beet juice condition, the nitrite level rose significantly between hours 2 and 3 with a nitrite peak of 0.943 μmol/L. The peak nitrite level in our study was 4 μmol/L at 0.25–0.5 h after subjects took the 80 mg of IR sodium nitrite and 2 μmol/L at 2.5 h after they took the 80 mg of EC sodium nitrite. Our findings are similar to that of oral sodium nitrite dosing in healthy subjects,23 with the difference that peak plasma levels achieved in oral administration to diabetes subjects are somewhat less than what would be anticipated through oral or intravenous administration. Nonetheless, the levels of nitrite achieved in our study would be expected to extend walking time in patients with peripheral vascular disease and claudication. Subjects in the study of Kenjale et al.26 had an average drop of 6/6 mm Hg in blood pressure and no change in pulse rate, whereas subjects in our study had an average drop of 10/6 mm Hg with our IR formulation and no change in pulse rate.
It is interesting that diabetes subjects given the IR formulation demonstrated a large increase in plasma nitrate levels above what would be expected from the approximately 4 μM plasma nitrite level. We do not currently know the reason for this sustained increase in plasma nitrate following oral administration of 80 mg of sodium nitrite. However, possible renal insufficiency or nitrate contamination are unlikely as patient BUN and creatinine levels do not indicate renal abnormalities and USP grade sodium nitrite was confirmed>97% pure. Clearly, additional studies will be needed to better understand this finding.
In summary, the IR formulation of sodium nitrite gave a statistically significant increase in serum nitrite and nitrate levels and an asymptomatic reduction in systolic and diastolic blood pressure. The only potential adverse event was mild flushing in 17% of those taking the IR formulation. Although there was no statistically significant elevation in blood levels of nitrite or nitrate with the EC formulation, the shape of the curve suggested a delayed release that would likely reach significance at a higher dose, and the levels with both release forms gave higher nitrite levels than a study giving beet juice,26 in which walking time was significantly extended in patients with peripheral vascular disease and stable claudication.
Acknowledgments
This study was supported by a grant from Theravasc, Inc. of Cleveland, OH. Pennington Biomedical Research Center received grant support from Theravasc, Inc., for which F.G. served as Principal Investigator and Medical Investigator in the study.
Author Disclosure Statement
F.L.G., C.G.K., and T.G. have a provisional patent (number 61/470,004) on the use of nitrite for dementia. D.J.L., R.K.P., and C.G.K. have a patent (number PCT/US2008/083830) for the therapeutic use of sodium nitrite. T.G. and C.G.K. have ownership in Theravasc, Inc. B.L.P., D.R.F., Y.Q., and A.B. have no conflicts of interest to disclose.
References
- 1.Zweier JL. Wang P. Samouilov A. Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 2.Gladwin MT. Shelhamer JH. Schechter AN. Pease-Fye ME. Waclawiw MA. Panza JA. Ognibene FP. Cannon RO., 3rd Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A. 2000;97:11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modin A. Björne H. Herulf M. Alving K. Weitzberg E. Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of 'acidic-metabolic' vasodilation. Acta Physiol Scand. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 4.Kumar D. Branch BG. Pattillo CB. Hood J. Thoma S. Simpson S. Illum S. Arora N. Chidlow JH., Jr Langston W. Teng X. Lefer DJ. Patel RP. Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Faassen EE. Bahrami S. Feelisch M. Hogg N. Kelm M. Kim-Shapiro DB. Kozlov AV. Li H. Lundberg JO. Mason R. Nohl H. Rassaf T. Samouilov A. Slama-Schwok A. Shiva S. Vanin AF. Weitzberg E. Zweier J. Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwentker A. Billiar TR. Nitric oxide and wound repair. Surg Clin North Am. 2003;83:521–530. doi: 10.1016/S0039-6109(02)00207-4. [DOI] [PubMed] [Google Scholar]
- 7.Luo JD. Chen AF. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin. 2005;26:259–264. doi: 10.1111/j.1745-7254.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 8.Schwentker A. Vodovotz Y. Weller R. Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide. 2002;7:1–10. doi: 10.1016/s1089-8603(02)00002-2. [DOI] [PubMed] [Google Scholar]
- 9.Lundberg JO. Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 10.Lundberg JO. Feelisch M. Bjorne H. Jansson EA. Weitzberg E. Cardioprotective effects of vegetables: is nitrate the answer? Nitric Oxide. 2006;15:359–362. doi: 10.1016/j.niox.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Duncan C. Dougall H. Johnston P. Green S. Brogan R. Leifert C. Smith L. Golden M. Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 12.Lundberg JO. Weitzberg E. Cole JA. Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 13.Larsen FJ. Ekblom B. Sahlin K. Lundberg JO. Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 14.Webb AJ. Patel N. Loukogeorgakis S. Okorie M. Aboud Z. Misra S. Rashid R. Miall P. Deanfield J. Benjamin N. MacAllister R. Hobbs AJ. Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doel JJ. Benjamin N. Hector MP. Rogers M. Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 2005;113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 16.Govoni M. Jansson EA. Weitzberg E. Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Petersson J. Carlström M. Schreiber O. Phillipson M. Christoffersson G. Jägare A. Roos S. Jansson EA. Persson AE. Lundberg JO. Holm L. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med. 2009;46:1068–1075. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Duranski MR. Greer JJ. Dejam A. Jaganmohan S. Hogg N. Langston W. Patel RP. Yet SF. Wang X. Kevil CG. Gladwin MT. Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleinbongard P. Dejam A. Lauer T. Jax T. Kerber S. Gharini P. Balzer J. Zotz RB. Scharf RE. Willers R. Schechter AN. Feelisch M. Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med. 2006;40:295–302. doi: 10.1016/j.freeradbiomed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Allen JD. Miller EM. Schwark E. Robbins JL. Duscha BD. Annex BH. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric Oxide. 2009;20:231–237. doi: 10.1016/j.niox.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosby K. Partovi KS. Crawford JH. Patel RP. Reiter CD. Martyr S. Yang BK. Waclawiw MA. Zalos G. Xu X. Huang KT. Shields H. Kim-Shapiro DB. Schechter AN. Cannon RO., 3rd Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 22.Dejam A. Hunter CJ. Tremonti C. Pluta RM. Hon YY. Grimes G. Partovi K. Pelletier MM. Oldfield EH. Cannon RO., 3rd Schechter AN. Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 23.Hunault CC. van Velzen AG. Sips AJ. Schothorst RC. Meulenbelt J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicol Lett. 2009;190:48–53. doi: 10.1016/j.toxlet.2009.06.865. [DOI] [PubMed] [Google Scholar]
- 24.Kevil CG. Kolluru GK. Pattillo CB. Giordano T. Inorganic nitrite therapy: historical perspective and future directions. Free Radic Biol Med. 2011;51:576–593. doi: 10.1016/j.freeradbiomed.2011.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lundberg JO. Weitzberg E. Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 26.Kenjale AA. Ham KL. Stabler T. Robbins JL. Johnson JL. Vanbruggen M. Privette G. Yim E. Kraus WE. Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J Appl Physiol. 2011;110:1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]