Abstract
Objectives:
Chronic inflammation and oxidative stress are associated with health and the disease status. The objective of the present study was to investigate the association among white blood cell (WBC) counts, neutrophil counts as a WBC subpopulation, and diacron reactive oxygen metabolites (d-ROMs) levels in an asymptomatic population.
Methods:
The clinical data, including general cardiovascular risk variables and high-sensitivity C-reactive protein (hs-CRP), were collected from 100 female subjects (mean age, 62 years) in outpatient clinics. The correlation of the d-ROMs with hs-CRP, WBC, and neutrophil counts was examined.
Results:
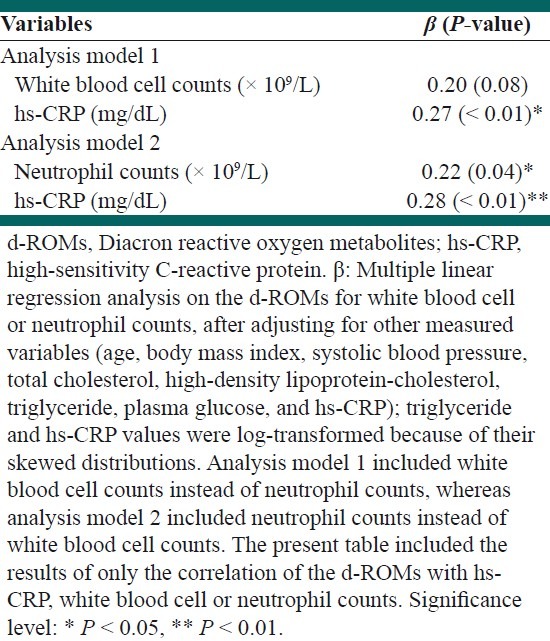
The mean/median levels were WBC counts 5.9 × 109/L, neutrophil counts 3.6 × 109/L, hs-CRP 0.06 mg/dL, and d-ROMs 359 CURR U. A simple correlation analysis showed a significant positive correlation of the d-ROMs with the WBC counts, neutrophil counts, or hs-CRP levels. The correlation between d-ROMs and neutrophil counts (β = 0.22, P < 0.05), as well as that between d-ROMs and hs-CRP (β = 0.28, P < 0.01), remained significant and independent in a multiple linear regression analysis adjusted for other variables. A multiple linear regression analysis showed that WBC counts had only a positive correlation tendency to the d-ROMs.
Conclusions:
Neutrophils may be slightly but more involved in the oxidative stress status, as assessed by d-ROMs, in comparison to the overall WBC. Further studies are needed to clarify the biologic mechanism(s) of the observed relationship.
Keywords: C-reactive protein, inflammation, leukocyte, neutrophil, oxidative stress
INTRODUCTION
Subclinical chronic inflammation and oxidative stress are concomitantly involved in the pathogenesis of degenerative disorders, such as atherosclerotic disease.[1,2] Inflammation induces an oxidative burden, and oxidative stress also enhances inflammation.[3] Accordingly, markers that evaluate inflammatory and oxidative conditions have been explored.[2,4] The white blood cell (WBC) counts and high-sensitivity C-reactive protein (hs-CRP) in blood serve as inflammatory markers,[2,5,6] although these markers have nonspecific features.[7] Most studies on the relationship of WBC with the risk of cardiovascular disease (CVD) have used total WBC counts,[5,8] although neutrophils (a differential WBC subtype) can play crucial roles in oxidation of lipoproteins and endothelial dysfunction.[9,10] Some studies have reported a significant positive association between the WBC subpopulations (neutrophil counts, in particular) and CVD outcomes (eg, the SOLVD study and the EPIC study).[11,12] The relationship of WBC and its subpopulations with oxidative stress-related markers has not been thoroughly examined; for instance, a significant positive relationship between sputum 8-isoprostane and neutrophil counts,[13] as well as between blood 3-chlorotyrosine and WBC counts,[14] has been reported.
The limited information may be due to the limited number of oxidative stress-related markers, which can be used to easily assess the oxidative stress status in clinical settings.[4] The diacron reactive oxygen metabolites (d-ROMs) test was recently introduced as an easy clinical marker to evaluate the total oxidative burden.[3] Although there are prior studies showing a significant positive correlation between the hs-CRP and d-ROMs levels,[3] the association of WBC and its subpopulations with d-ROMs has not been shown. The aim of the present study was thus to investigate the correlation between WBC, neutrophil counts, and d-ROMs levels in an asymptomatic population.
METHODS
The study included 100 female subjects (mean age, 62 ± 9 years) recruited from outpatient clinics. The inclusion criteria were asymptomatic, nonsmokers, and participants not taking any medications. Subjects with blood hemoglobin values between 11.5 and 17.5 g/dL and WBC counts between 3.0 and 10 × 109/L were included (these levels were based on reference intervals[15] and clinical experience). The exclusion criteria were acute infectious diseases, such as the common cold, or a history of cardio/cerebrovascular, endocrine, hematologic, collagen, severe kidney, or liver diseases. This study was approved by the Institutional Ethics Committee, and all subjects gave their informed consent.
The clinical data from each subject were obtained following an overnight fast. The body mass index (BMI) was calculated based on the weight and height measured while subjects wore light clothing without shoes. The systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in the subject's right arm with a mercury sphygmomanometer while the subject was in the seated position. Blood hemoglobin, total WBC and neutrophil counts (plus lymphocyte counts) were measured with an autoanalyzer (COULTER LH750 model, BECKMAN COULTER Co. Ltd., Tokyo, Japan). The serum total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), triglyceride (TG), and plasma glucose levels were measured by enzymatic methods. The serum hs-CRP levels were measured by an enzyme-linked immunosorbent assay (Assaypro Co. Ltd., St. Charles, MO, USA). The d-ROMs levels were determined by a photometric assay that measures the total oxidant capacity (mainly dependent on the total amount of hydroperoxides, a class of reactive oxygen metabolites) of a sample against N,N-diethyl paraphenylenediamine, used as chromogenic substrate. Serum samples were mixed with an acidic buffered solution and incubated at 37°C with the chromogenic substrate for 3 min, and after that they underwent photometric reading at 505 nm, according to the kinetic mode, in 2 min, at 37°C, by using a dedicated photometer (F.R.E.E. system, Diacron International s.r.l., Grosseto, Italy). The d-ROMs test showed acceptable analytic performance based on the low intra- and interassay coefficients of variation (2.1% and 3.1%, respectively).[3]
The data are expressed as the means ± standard deviation (SD) or the medians (interquartile range). A simple correlation test (Pearson's test) and a multiple linear regression analysis were used to observe the correlation between the d-ROMs and other measured variables, including hs-CRP, WBC, and neutrophil counts. The multiple linear regression analysis to evaluate the correlation of the d-ROMs (as an independent variable) with WBC counts or neutrophil counts was adjusted by the variables: age, BMI, SBP, TC, HDL-C, TG, plasma glucose, and hs-CRP. The SBP only was entered into these models, because of a collinearity of DBP. The TG and hs-CRP values were log-transformed in these analyses, because of their skewed distributions. Statistical significance was defined as a P value < 0.05.
RESULTS
The mean (±SD)/median (interquartile range) levels of the measured variables were BMI, 24.0 ± 3.3 kg/m2; hemoglobin, 13.4 ± 1.1 g/dL; WBC counts, 5.9 ± 1.7 × 109/L; neutrophil counts, 3.6 ± 1.4 × 109/L; lymphocyte counts, 1.8 ± 0.6 × 109/L; SBP, 142 ± 22 mmHg; DBP, 80 ± 11 mmHg; TC, 250 ± 30 mg/dL; HDL-C, 68 ± 17 mg/dL; TG, 143 (96–224) mg/dL; plasma glucose, 126 ± 44 mg/dL; hs-CRP, 0.06 (0.04–0.12) mg/dL; and d-ROMs, 359 ± 88 CARR U.
A simple correlation analysis showed that there was a significant positive correlation between the d-ROMs and BMI, plasma glucose, WBC counts, neutrophil counts, or hs-CRP levels [Table 1]. The other variables did not show any relative significance. In addition, there was a significant positive correlation between the WBC and neutrophil counts (r = 0.91, P < 0.01). The WBC counts were significantly and positively correlated with hs-CRP levels (r = 0.25, P = 0.01), whereas the neutrophil counts showed a positive correlation tendency to hs-CRP levels (r = 0.17, P = 0.09).
Table 1.
A simple correlation of the d-ROMs with other measured variables
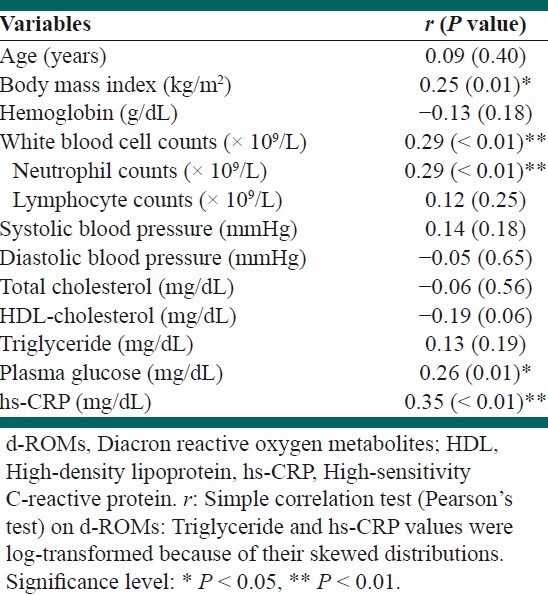
A subsequent multiple linear regression analysis revealed that there remained a significant positive correlation between the d-ROMs and neutrophil counts, independent of other variables [Table 2]. A multiple linear regression analysis revealed that the WBC counts had only a positive correlation tendency to the d-ROMs. There remained an independent, significant, and positive correlation between the d-ROMs and hs-CRP levels in the multiple linear regression analysis.
Table 2.
A multiple regression analysis of the d-ROMs with hs-CRP, white blood cell or neutrophil counts
DISCUSSION
The present study found that there was an independent, significant, and positive correlation between neutrophil counts and d-ROMs levels in asymptomatic female subjects. An independent, significant, and positive correlation between hs-CRP and d-ROMs levels is consistent with a prior study.[3] The correlation of the d-ROMs with neutrophil counts was significant relative to the total WBC counts (although the difference in the correlation of the d-ROMs with the neutrophil or WBC counts was not very large), and this suggests that neutrophils may be similarly or slightly but more involved in the oxidative stress status, as assessed by this test, in comparison to the overall WBCs. This is noteworthy because of the specific roles of neutorophils on the oxidative milieu in bloodstream and various tissues, including the vasculature.[9,10] This is also useful as the basic information in considering the link between inflammation and oxidative stress in various pathophysiologies using the d-ROMs test, a recently established marker to easily assess the oxidative burden in clinical settings.[3]
Although the biologic mechanism(s) underlying the correlation between neutrophils and d-ROMs remain unclear, there are possible explanations. Neutrophils can directly reflect the relevant pathophysiology as a WBC subpopulation in comparison to the total WBCs.[9,10] Neutrophils secrete various types of inflammatory chemokines and cytokines (eg, interluekin-6),[16] and the inflammation triggered by these molecules can both induce an oxidative stress response and further inflammation via cell dysfunction in systemic organs, including the adipose tissue, liver, and vasculature.[3] Neutrophils-released mediators, such as leukotriene B4, can cause neutrophils to move to inflammatory sites and generate oxidative stress.[17] Oxidative stress also activates the inflammatory pathway.[18] Therefore, this cycle of oxidative stress and inflammation in relation to neutrophils may partly explain the correlation between neutrophils and d-ROMs levels.
There were some limitations to the present study. The sample size was relatively small, and the study had a cross-sectional design nature that cannot completely define the cause-and-effect relationship of the results. The study population was restricted to female participants with asymptomatic health states. Future studies with a prospective design and with larger and more varied populations are needed.
In summary, the present study showed that there was an independent, significant, and positive correlation between neutrophil counts and d-ROMs levels in asymptomatic female subjects. The data suggest that neutrophils may be slightly but more involved in the oxidative stress status, as assessed by the d-ROMs test, relative to the overall WBCs. Further studies are necessary to clarify the biologic mechanism(s) underlying the observed relationship.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: An overview. Free Radic Biol Med. 2000;28:1815–26. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 2.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 3.Kotani K, Taniguchi N. Correlation between high-sensitivity C-reactive protein and reactive oxygen metabolites during a one-year period among asymptomatic subjects. J Clin Med Res. 2012;4:52–5. doi: 10.4021/jocmr755w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202:321–9. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman M, Blum A, Baruch R, Kaplan E, Benjamin M. Leukocytes and coronary heart disease. Atherosclerosis. 2004;172:1–6. doi: 10.1016/s0021-9150(03)00164-3. [DOI] [PubMed] [Google Scholar]
- 6.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: A systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:483–95. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- 7.Stuart J, George AJ, Davies AJ, Aukland A, Hurlow RA. Haematological stress syndrome in atherosclerosis. J Clin Pathol. 1981;34:464–7. doi: 10.1136/jcp.34.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helfand M, Buckley DI, Freeman M, Fu R, Rogers K, Fleming C, et al. Emerging risk factors for coronary heart disease: A summary of systematic reviews conducted for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:496–507. doi: 10.7326/0003-4819-151-7-200910060-00010. [DOI] [PubMed] [Google Scholar]
- 9.Harlan JM, Killen PD, Harker LA, Striker GE, Wright DG. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981;68:1394–403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Görög P. Neutrophil-oxidized low density lipoprotein: Generation in and clearance from the plasma. Int J Exp Pathol. 1992;73:485–90. [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper HA, Exner DV, Waclawiw MA, Domanski MJ. White blood cell count and mortality in patients with ischemic and nonischemic left ventricular systolic dysfunction (an analysis of the Studies Of Left Ventricular Dysfunction [SOLVD]) Am J Cardiol. 1999;84:252–7. doi: 10.1016/s0002-9149(99)00272-6. [DOI] [PubMed] [Google Scholar]
- 12.Gurm HS, Bhatt DL, Lincoff AM, Tcheng JE, Kereiakes DJ, Kleiman NS, et al. Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary intervention: Insights from the EPIC, EPILOG, and EPISTENT trials. Heart. 2003;89:1200–4. doi: 10.1136/heart.89.10.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinnula VL, Ilumets H, Myllärniemi M, Sovijärvi A, Rytilä P. 8-Isoprostane as a marker of oxidative stress in nonsymptomatic cigarette smokers and COPD. Eur Respir J. 2007;29:51–5. doi: 10.1183/09031936.00023606. [DOI] [PubMed] [Google Scholar]
- 14.Cheng ML, Chen CM, Gu PW, Ho HY, Chiu DT. Elevated levels of myeloperoxidase, white blood cell count and 3-chlorotyrosine in Taiwanese patients with acute myocardial infarction. Clin Biochem. 2008;41:554–60. doi: 10.1016/j.clinbiochem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Flegar-Mestrić Z, Nazor A, Jagarinec N. Haematological profile in healthy urban population (8 to 70 years of age) Coll Antropol. 2000;24:185–96. [PubMed] [Google Scholar]
- 16.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: Potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–9. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 17.Jala VR, Haribabu B. Leukotrienes and atherosclerosis: New roles for old mediators. Trends Immunol. 2004;25:315–22. doi: 10.1016/j.it.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Kokura S, Yoshida N, Yoshikawa T. Anoxia/reoxygenation-induced leukocyte-endothelial cell interactions. Free Radic Biol Med. 2002;33:427–32. doi: 10.1016/s0891-5849(02)00852-3. [DOI] [PubMed] [Google Scholar]


