Abstract
Objective
To analyse associations between symptoms and bacteriuria in uncomplicated lower urinary tract infection in women (LUTIW) and to evaluate outcome of therapy with three different regimens of pivmecillinam or placebo.
Design
Prospective, multicentre, randomized, double-blind, and placebo-controlled therapy study. Symptoms registered at inclusion, during therapy and at follow-up visits after 8–10 and 35–49 days. Significant bacteriuria defined according to current European guidelines.
Setting
A total of 18 primary healthcare centres in northern Sweden.
Subjects
Women aged 18 years and above with symptoms of urgency, dysuria, supra pubic or loin pain.
Main outcome measures
Symptoms and bacteriuria at inclusion and course of symptoms, bacteriuria, and their combinations during and post-therapy.
Results
At inclusion, no associations or significant differences were found between symptom scores and bacteriuria, bacterial counts, or species. The 884 patients (77%) with significant bacteriuria were followed up. All pivmecillinam therapies were superior to placebo (p < 0.001). From day six until first follow-up, the mean values of all symptoms were higher and the bacteriological cure was lower at first follow-up in the three days (84%) compared with the seven days regimens (93–94%, p < 0.001). At final follow-up clinical cure was similar in all pivmecillinam regimens (65–72%) as was bacteriological cure (83–89%). Pivmecillinam had few low to mild adverse reactions, comparable to placebo.
Conclusions
Symptoms are not conclusive for diagnosis of LUTIW. Pivmecillinam therapies are superior to placebo and seven days regimens are more efficient than three days. Pivmecillinam 200 mg×2×7 days is recommended as a first-line therapy for LUTIW.
Keywords: Bacteriuria, family practice, pivmecillinam, placebo, symptoms, therapy study, urinary tract infection
Literature on lower urinary tract infection in women (LUTIW) is usually focused on bacteriology and bacteriological outcome of antimicrobial therapy, while symptoms and clinical outcome and the natural course by placebo therapy are sparsely documented.
Symptoms are not conclusive for the diagnosis of LUTIW.
Outcomes of pivmecillinam therapies are superior to placebo therapy.
Seven days of pivmecillinam therapies are more efficient than three days and pivmecillinam 200 mg×2×7 days is recommended as a first-line therapy for LUTIW.
About half of all women suffer at least once in their lifetime from urinary tract infection (UTI) [1]. In Sweden approximately one million patients annually visit mainly primary healthcare because of lower UTI in women (LUTIW) [2], and 14% of all antibiotics prescribed to outpatients are due to UTI [3]. Common symptoms of LUTIW are urgency/frequency, dysuria, suprapubic pain and loin pain without signs of pyelonephritis [4], [5]. The definition of uropathogens and cut-off limits for significant bacteriuria have been debated [6], and different guidelines have been used, which vary between 103 and 105 colony forming units/ mL depending on uropathogens, sex, and type of urine sample [7–9].
Among UTI therapies pivmecillinam has shown good efficacy, few ecological side effects as adverse reactions [10], [11], and low induction of antibiotic resistance [12]. The increasing resistance to many antibiotics commonly prescribed is often associated with high consumption of antibiotics [13], [14] and may cause therapeutic problems. Optimal doses and length of pivmecillinam therapy have not been established [10], [15], though empirical treatment of uncomplicated cystitis for three days has been recommended for most common antibiotics including pivmecillinam [16].
The aims of the present study were to analyse the associations between symptoms and bacteriuria and to evaluate the effect on symptoms, bacteriuria, and their combinations by three different regimens of pivmecillinam compared with placebo during and 5–7 weeks post-therapy in patients with significant bacteriuria at inclusion.
Material and methods
Study design
A prospective, multicentre, randomized, double-blind, and placebo-controlled therapy study was performed at 18 primary healthcare centres in the county of Västerbotten in northern Sweden with inclusion period from April 1995 to December 1997. Women aged 18 years and above with symptoms suggestive of lower UTI – urgency, dysuria, suprapubic pain, or loin pain – were eligible to participate. The severity of each symptom was graded as none, light, moderate, or severe (score 0–3), and a total symptom score of ≥2 was required for inclusion. Patients were randomized to three different regimens of pivmecillinam (Selexid®): 200 mg×3×7 days, 200 mg×2×7 days or 400 mg×2×3 days or placebo (i.e. all patients were given 2 + 1+2 identical tablets each day for 7 days). Prior to inclusion patients gave informed written consent to participate. Patients with significant bacteriuria were followed up at two visits, after 8–10 days and 35–49 days, respectively.
Exclusion criteria
Antibiotic therapy for UTI within the last month, participation in other studies within last three months, known/suspected penicillin allergy, genital infection, complicating factors (diabetes or abnormality of the urinary tract), one or more signs of pyelonephritis (pyrexia ≥ 38.5°C, CRP ≥ 25 mg/L, kidney tenderness by palpation), urine incontinence requiring catheter/pads, pregnancy/planned pregnancy, or previous participation in the study form the exclusion criteria.
Controls
Patients fulfilling the inclusion criteria but who rejected participation in the study were voluntarily registered as controls (reject log) at consultation, when compared with the included patients.
Bacteriological methods
After spreading of the labia, a mid-stream urine sample was collected and transported at <6° C within 24 h to the Laboratory of Clinical Bacteriology, University Hospital of Umeå [17]. The uropathogens were quantified in colony-forming units/mL, and significant bacteriuria was defined according to current European guidelines in patients with symptoms as ≥103 /mL for primary pathogens, ≥104 for secondary pathogens, and ≥105 for doubtful pathogens, and as ≥105 for all species in patients without symptoms [9] (see Table II). Samples without bacteriuria or with nonsignificant bacterial counts were classified as negative cultures as were samples with mixed flora without one dominating species. In cultures with mixed flora and one species dominating (i.e. at least 10 times higher than any other species and in significant bacterial counts), this species was denoted as a uropathogen. Bacterial species in significant counts were registered at inclusion and at the follow-up visits.
Table II.
Relation between bacteriuria according to current European guidelines and mean values of all symptom scores at inclusion.
| Bacteria CFU/ mL |
|||||||||||
| Negative 1 |
103 |
104 |
≥105 |
Total |
|||||||
| Species | Symptom score 2 | n | Symptom score 2 | n | Symptom score 2 | n | Symptom score 2 | n | Symptom score 2 | n | % |
| E. coli 3 | 5.7 | 38 | 5.5 | 70 | 5.3 | 602 | 5.3 | 710 | 62.1 | ||
| CNS 5, 6 | 5.8 | 43 | 5.8 | 43 | 3.8 | ||||||
| S. saprophyticus 3 | 5.0 | 4 | 5.9 | 26 | 5.8 | 30 | 2.6 | ||||
| Klebsiella species 4 | 4.0 | 1 | 4.8 | 28 | 4.8 | 29 | 2.5 | ||||
| Enterococcus species 4 | 6.2 | 6 | 6.0 | 16 | 6.1 | 22 | 1.9 | ||||
| Citrobacter species 4 | 5.6 | 14 | 5.6 | 14 | 1.2 | ||||||
| Enterobacter species 4 | 6.1 | 13 | 6.1 | 13 | 1.1 | ||||||
| S. aureus 4 | 5.0 | 2 | 4.8 | 5 | 4.9 | 7 | 0.6 | ||||
| Group B streptococci 5 | 3.8 | 6 | 3.8 | 6 | 0.5 | ||||||
| Proteus species 4 | 5.0 | 2 | 6.0 | 4 | 5.7 | 6 | 0.5 | ||||
| Pseudomonas species 5 | 6.0 | 2 | 6.0 | 2 | 0.2 | ||||||
| Gram negative rods 5 | 4.0 | 2 | 4.0 | 2 | 0.2 | ||||||
| Gram positive rods 5 | 5.0 | 1 | 5.0 | 1 | 0.1 | ||||||
| Negative culture 1 | 5.1 | 258 | 5.1 | 258 | 22.7 | ||||||
| Total | 5.1 | 258 | 5.7 | 38 | 5.5 | 85 | 5.4 | 762 | 5.3 | 1143 | |
| % of total | 23 | 3 | 7 | 67 | 100 | ||||||
1Non-significant bacteriuria, see Material and methods; 2mean value of symptom scores, see Material and methods; 3primary pathogen; 4secondary pathogen; 5doubtful pathogen; 6coagulase-negative staphylococci other than S. saprophyticus.
Adverse events
At both follow-up visits suspected adverse events were registered.
Outcome of therapy
Symptom scores for each symptom were registered at inclusion, at medication three times daily in diaries during therapy (days 1–7) when the mean value for all symptom scores was calculated daily, and at the follow-up visits. The clinical effect of therapy was illustrated by mean values for all symptom scores or proportion of symptom-free patients daily during therapy and at the follow-up visits. Clinical cure was defined as no persisting symptoms during and post-therapy. Bacteriological cure was defined as eradication of initial bacteriuria at the follow-up visits.
Sample size calculation
Assuming a clinical cure rate of 80% at last follow-up in the 200 mg×3×7 days regimen, a total of 250 patients per treatment group were required, in order to ensure with 80% certainty that the upper 95% (one-alpha) confidence limit for difference in cure rates between the 200 mg×3×7 days and the other pivmecillinam regimens was less than 10%.
Statistics
Comparison of proportions was done using the chi-squared test. P-values < 0.05 were considered significant. Analysis of variance with post hoc tests and Bonferroni correction was used when mean values were compared [18]. The software used for statistical calculation was SPSS 11.0 (SPSS Inc., Chicago, IL).
Results
Patients included and controls
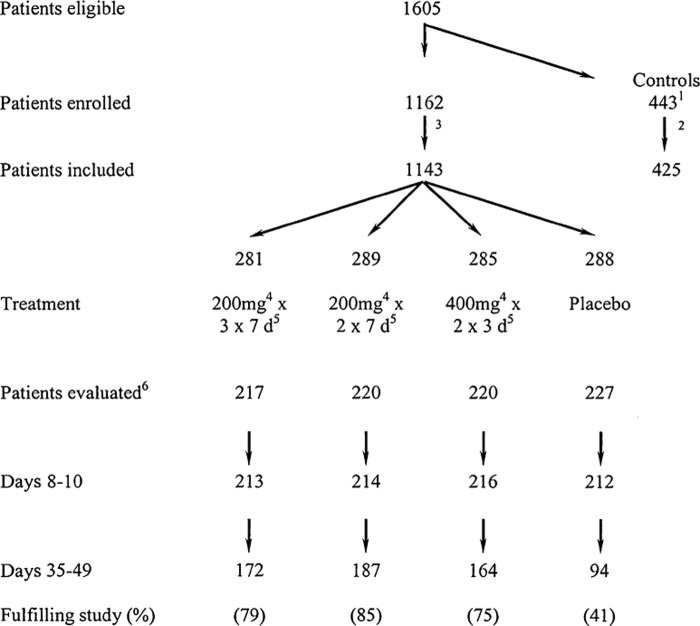
A total of 1605 women were eligible for recruitment of which 443 rejected participation but accepted to serve as controls (Figure 1). Of the 1162 patients, 19 were excluded due to total symptom scores < 2 (n = 8) or missing cultures (n = 11), as were 18 controls, resulting in 1143 included patients and 425 controls. The patients had a mean age of 43 years, mean symptom duration of 10 days, mean value for all symptom scores of 5.3 points (Table I), urgency in 96%, dysuria in 88%, suprapubic pain in 60%, and loin pain in 40%. E. coli was the most common bacterial species (62.1% of cultures, Table II), followed by coagulase negative staphylococci other than S. saprophyticus (3.8%) and S. saprophyticus (2.6%). Among cultures, 67% had ≥105 colony forming units/mL, 7% had 104, 3% had 103, and 23% were negative. The controls had similar characteristics except that they were older (mean value 47 years, p < 0.001) and had shorter symptom duration (mean value 7 days, p < 0.001). No associations or significant differences were found between mean values for all symptom scores, bacteriuria, bacterial counts, or species (Table II).
Figure 1.
Flow chart of patients receiving pivmecillinam or placebo treatment and controls. 1443 patients fulfilled the inclusion criteria but rejected participation (reject log); 218 patients excluded at consultation (missing cultures) resulting in 425 controls; 319 patients excluded at consultation (11 missing cultures, 8 with symptom score < 2); 4pivmecillinam; 5days of treatment; 6patients with significant bacteriuria according to current European guidelines.
Table I.
Characteristics at inclusion of different patient groups and controls (reject log).
| Placebo group | Pivmecillinam 200mg×3×7d1 | Pivmecillinam 200mg×2×7d1 | Pivmecillinam 400mg×2×3d1 | Controls | |
| Number of patients | 288 | 281 | 289 | 285 | 425 |
| Age mean value (years) | 42 (±17) | 44 (±18) | 43 (±18) | 44 (±18) | 47 (±19) |
| Age median and range (years) | 41 (18–82) | 44 (18–85) | 42 (18–88) | 40 (18–83) | 46 (18–89) |
| Age distribution (%) ≤24 years | 23 | 22 | 20 | 21 | 17 |
| 25–54 years | 52 | 47 | 51 | 50 | 48 |
| ≥ 55 years | 25 | 31 | 29 | 29 | 35 |
| Symptom duration (days) | |||||
| Mean value (SD) | 9 (±16) | 11 (±22) | 9 (±21.0) | 10 (±18) | 7 (±12) |
| Median | 4 | 4 | 4 | 5 | 3 |
| Mean value of symptom scores (SD) | 5.3 (±2.0) | 5.5 (±2.0) | 5.2 (±2.0) | 5.3 (±2.0) | 5.5 (±.2.1) |
| Mean bladder incubation time (hours) | 4 (±2) | 4 (±3) | 4 (±2) | 4 (±3) | 4 (±3) |
| Significant bacteriuria (%) | |||||
| E. coli | 59 | 67 | 61 | 62 | 62 |
| Other species | 20 | 11 | 15 | 15 | 15 |
| Negative culture | 22 | 23 | 24 | 23 | 23 |
1Days of treatment.
Patients with significant bacteriuria were studied further
Of the 1143 included patients, 884 had significant bacteriuria (77%, see Table II) and were studied further. At the first follow-up, 97% of these participated (see Figure 1) but patients with unbearable symptoms received antibiotics other than pivmecillinam and left the study. Significantly fewer placebo-treated patients fulfilled the study (41%) compared with patients receiving pivmecillinam (75–85%, p < 0.001), among whom fewer patients fulfilled the 3 days (75%) compared with the 200 mg×2×7 days regimen (85%, p = 0.006).
Clinical outcome
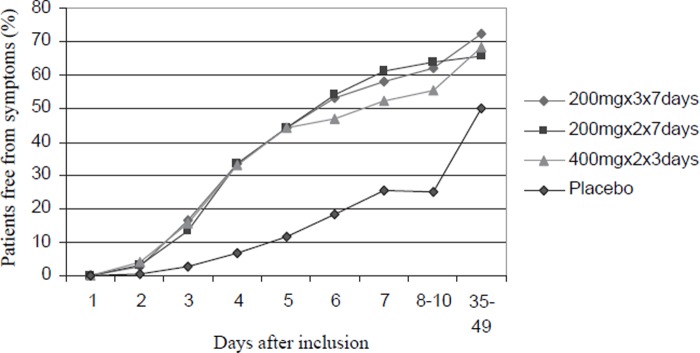
The symptoms disappeared gradually and faster among all pivmecillinam regimens compared with placebo (Figure 2, p < 0.001), with higher clinical cure rates post-therapy (Table III, p < 0.001). The proportion of pivmecillinam treated patients free from symptoms was 44%, 55–65%, and 65–72% at day five, first, and final follow-up, respectively (Figure 2). Comparable figures for placebo-treated patients were 12%, 26%, and 51%. From day six until first follow-up, the proportion of symptom-free patients tended to be lower (Figure 2), and mean values for all symptom scores were higher in the 3-day compared with the 200 mg×3×7 days and 200 mg×2×7 days regimens (day 6: 1.32, 1.00 and 0.93, respectively, p = 0.045, and first follow-up: 1.33, 1.03 and 0.85, p = 0.024). At final follow-up, these differences in clinical outcome between the pivmecillinam regimens had disappeared. Clinical cure at both follow-up visits was similar in 59–62% in the pivmecillinam regimens compared with 37% after placebo therapy (Table III, p < 0.001). Clinical recurrence of symptoms at last follow-up was similar in 12–13% of all pivmecillinam- as placebo-treated patients.
Figure 2.
Eradication of symptoms during and post-therapy among pivmecillinam- and placebo-treated patients.
Table III.
Clinical and bacteriological cure of treatment in different pivmecillinam and placebo regimens (%).
| Pivmecillinam |
Placebo |
p-values comparison |
||||
| 200mg×3×7d1 | 200mg×2×7d1 | 400mg×2×3d1 | ||||
| (A) | (B) | (C) | (P) | |||
| Outcome of therapy | n = 217 | n = 220 | n = 220 | n = 227 | A vs. B vs. C vs. P | A vs. B vs. C |
| Clinical cure | ||||||
| Days 8–10 | 62 | 64 | 55 | 25 | <0.001 | 0.16 |
| Days 35–49 | 72 | 65 | 68 | 51 | 0.006 | 0.37 |
| Days 8–10 and 35–49 | 62 | 59 | 60 | 37 | <0.001 | 0.80 |
| Bacteriological cure | ||||||
| Days 8–10 | 93 | 94 | 84 | 34 | <0.001 | <0.0012 |
| Days 35–49 | 89 | 83 | 86 | 70 | <0.001 | 0.21 |
| Days 8–10 and 35–49 | 86 | 80 | 80 | 44 | <0.001 | 0.25 |
| Clinical and bacteriological cure at days 8–10 and 35–49 | 58 | 57 | 56 | 21 | <0.001 | 0.96 |
1Days of treatment; 2p-values comparing A vs. B: p = 0.18; A vs. C: p = 0.01; B vs. C: p = 0.003.
Bacteriological outcome
Bacteriological cure was more efficient for all pivmecillinam regimens compared with placebo (see Table III, p < 0.001) and more successful at first follow-up in the 7 days regimens (93–94%) compared with 3 days regimens (84%, p < 0.001). However, at final follow-up, the bacteriological cure rates were similar in all pivmecillinam regimens (83–89%).
Clinical and bacteriological outcome
Clinical and bacteriological cure at both follow-up visits was 56–58% in the pivmecillinam regimens (see Table III) compared with 21% after placebo therapy (p < 0.001). At first follow-up after pivmecillinam therapy, 96–97% of patients free from symptoms were also free from bacteriuria (Table IV). Among patients with symptoms, 10–30% also had persisting bacteriuria. At final follow-up after pivmecillinam therapy, 93–98% of patients free from symptoms were also bacteriologically cured, and 25–48% of the patients with symptoms also had persisting bacteriuria (Table IV).
Table IV.
Bacteriological outcome in relation to clinical outcome in different regimens of pivmecillinam and placebo therapy.
| Pivmecillinam |
Placebo |
|||
| 200mg×3×7d1 | 200mg×2×7d1 | 400mg×2×3d1 | ||
| (A) | (B) | (C) | (P) | |
| Outcome of therapy: Bacteriological response in relation to clinical response | n = 217 | n = 220 | n = 220 | n = 227 |
| Days 8–10 | n (%) | n (%) | n (%) | n (%) |
| Clinically cured | 132 (62) | 136 (64) | 119 (55) | 54 (25) |
| Bacteriologically cured among clinically cured | 128 (97) | 132 (97) | 114 (96) | 30 (56) |
| Clinical failure | 81 (38) | 78 (36) | 97 (45) | 158 (76) |
| Bacteriological failure among clinical failure | 10 (12) 2 | 8 (10) 2 | 29 (30) 2 | 117 (74) |
| Days 35–49 | ||||
| Clinically cured | 124 (72) | 122 (65) | 111 (68) | 48 (51) |
| Bacteriologically cured among clinical cured | 115 (93) | 119 (98) | 105 (95) | 40 (83) |
| Clinical failure | 48 (28) | 65 (35) | 53 (32) | 46 (49) |
| Bacteriological failure among clinical failure (%) | 12 (25) 3 | 31 (48) 3 | 20 (38) 3 | 24 (52) |
1Days of treatment; 2p-values comparing A vs. B: p = 1.0; A vs. C: p = 0.015; B vs. C: p = 0.005; 3p-values comparing A vs. B: p = 0.042; A vs. C: p = 0.510; B vs. C: p = 0.840.
Adverse drug reactions
The most common adverse drug reactions were gastrointestinal (5–8% of pivmecillinam- and 4% of placebo-treated patients) and only seven patients (<1%) given pivmecillinam withdrew from treatment due to adverse reactions. The majority of adverse reactions were mild to moderate and similar in the pivmecillinam regimens as in placebo (17% in 200 mg×3×7 days, 12% in 200 mg×2×7 days, 14% in 3 days, and 12% in placebo, respectively, p = 0.17). The risk of complications was low and two patients developed pyelonephritis, one receiving placebo and one pivmecillinam for seven days.
Discussion
The LUTIW project is to our knowledge the largest prospective, multicentre, randomized, double-blind, and placebo-controlled therapy study of UTI. The patients included were representative of uncomplicated LUTIW consulting primary healthcare with similar symptoms and bacteriology to the controls. The majority of clinical trials of UTI focus on bacteriological cure [19], but in the present study we also highlight symptoms and clinical outcome. This study is unique as current international ethical recommendations do not support placebo therapy [20].
In accordance with other studies [9], E coli was the dominating bacterial species. The low incidence of S. saprophyticus (2.6%) may have been due to a high concentration of novobiocin in agar plates used in the multipoint inoculation tests for identification. Hence a number of S. saprophyticus (novobiocin resistant) may have been misidentified as other coagulase negative staphylococci (sensitive) [17]. However, the present distribution of staphylococci had no significant influence on outcome of therapy, as both staphylococcal groups had similar spontaneous clinical and bacterial cure [17] and similar outcome of pivmecillinam therapy to E. coli, despite in vitro resistance to pivmecillinam for staphylococci (data not shown). These results can partly be explained by the low predictive values of antibiotic resistance for outcome of LUTIW therapy [21], [22].
No associations or significant differences between mean symptom scores and bacteriuria, bacterial counts, or species were found at inclusion, and 23% of the patients did not have significant bacteriuria, indicating that suggestive symptoms only are not conclusive for diagnosis of LUTIW. In order to prevent unnecessary consumption of antibiotics we consider that antibiotics should be prescribed for symptomatic bacteriuria and not for symptoms only, and therefore we focus outcome of therapy on patients with significant bacteriuria. This is supported by Belgian, Dutch, and German guidelines [23], which advise both symptoms and urinalysis for diagnosis and therapy of UTI, but is opposed by Norwegian guidelines [24], which recommend empirical therapy for symptoms only. This also contradicts the increasing tendency in clinical practice to prescribe antibiotics for symptoms only, which has been reported to be cost-effective [25] and has been proposed in the US [26]. Moreover, equal relief of symptoms was found in women with bacterial cystitis or urethral syndrome given empirical antibiotic therapy [27], and trimethoprim was reported as more efficient than placebo in relieving urgency in women with urethral syndrome [28]. However, these studies had few patients and short follow-up times and are not conclusive for prescribing antibiotics in the case of symptoms only.
In a recent meta-analysis of 32 studies of LUTIW the efficacy of 3 days therapies was compared with 5 days and longer therapies [29]. No significant differences in clinical cure rates were found at short- and long-term follow-up, but 3 days therapies were less effective in bacteriological outcome at both short- and long-term follow-up. However, comparison and assessment of outcome between different studies are difficult due to differences in: definitions of significant bacteriuria, evaluation of symptoms and clinical outcome, and times for follow-up. For instance, the bacteriological failure rate for norfloxacin could vary between 1% and 43% due to different cut-off limits used for significant bacteriuria [30].
In the present study, we used four-graded symptom scores and patient diaries, which probably contributed to lower clinical cure rates and illustrated the clinical course of LUTIW better, since mild symptoms were also considered. Recently, the benefit of using graded symptom scores was reported concluding that moderate or severe symptoms had higher predictive values of diagnosing UTI than slight symptoms [31]. We found that symptoms disappeared gradually and similarly during the first five days in most patients given pivmecillinam. At days 6–10, the clinical outcome in the 3 days regimen was less efficient with higher symptom scores compared with the 7 days regimen, which probably contributed to a higher dropout frequency in the 3 days regimen. At final follow-up, no differences in clinical efficacy were seen among all pivmecillinam regimens. The clinical cure after placebo therapy was 51% at final follow-up, but this result was highly biased due to many dropouts. If dropouts were considered as clinical failures, the clinical cure was 21%.
Traditionally, outcome of therapy focus on bacteriological cure, of which the 7 days pivmecillinam regimens were more effective than the 3 days at first but not at final follow-up. However, as asymptomatic bacteriuria is harmless unless during pregnancy [32], only symptomatic bacteriuria needs to be diagnosed and treated with antibiotics. In the present study almost all clinically cured patients were also bacteriologically cured (96–97% at first and 93–98% at final follow-up, respectively), which also supports that outcome of therapy should focus more on clinical cure.
We found that the 7 days regimens were more effective than the 3 days, especially between days 6–10 in respect of relief of symptoms and bacteriological cure. The 200 mg×2×7 days dosage had similar efficacy to the 200 mg×3×7 days, which could be explained by the high concentrations and long half-life of mecillinam in urine (T½ = 17 h) [33]. Hence, the 200 mg×2×7 days regimen is preferred with 1/3 reduction of antibiotic consumption compared with the traditional Swedish 200 mg×3×7 days dosage.
From the present project we have published a study of the natural course of LUTIW illustrated by placebo therapy [17], which had previously been only sparsely documented [34–36]. Also, at a symposium a preliminary summary of the outcomes of therapies has been reported [10]. The results in that preliminary report differed from this study due to bacteriuria defined according to modified American guidelines [7] and a less strict definition of “free from symptoms” (score ≤ 1). However, we consider that the present evaluation of the study is more comprehensive and clinically relevant.
In summary, symptoms are not conclusive for diagnosis of LUTIW. Pivmecillinam therapies are superior to placebo and 7 days regimens are more efficient than 3 days regimens. We recommend pivmecillinam 200 mg×2×7 days as a first-line therapy for LUTIW.
Acknowledgements
The authors are grateful to all general practitioners, staffs, and patients at the 18 primary healthcare centres: Anderstorp, Byske, Ersboda, Hörnefors, Lövånger, Malå, Mariehem, Lycksele (Medicinkonsulten), Moröbacke, Stenbergska, Teg, Tärna, Umeå East, Umeå West, Vilhelmina, Vindeln, Åsele, and the Student Health (Studenthälsan) of Umeå University. Thanks are also offered to Carina Karlsson and Catharina Lundgren, Department of Clinical Bacteriology, University Hospital of Umeå, for their valuable technical assistance.
Contributors
Sven A. Ferry initiated, coordinated, and supervised the study and together with Stig E. Holm, Rolf Lundholm, and Tor J. Monsen he planned the study, analysis, interpretation of results, and writing of the paper. Hans Stenlund designed and performed the data analysis, and participated in the interpretation of the results and writing of the paper. Ulf Diehl, LEO Pharma Sweden, was data reviewer and monitor together with Gunilla Ljunghagen, LEO Pharma Sweden.
Funding
The study was financed by LEO Pharma, Denmark, and was also sponsored by grants from the County Council of Västerbotten and Umeå University, Sweden.
Competing interests
Sven A. Ferry and Hans Stenlund have received consulting fees from LEO Pharma.
Ethical approval
The study was conducted in accordance with the Swedish Medical Product Agency guidelines and was approved by the Agency 1995 03 01 (Dnr 151: 01783/94) as well as by the Ethics Committee of Umeå University 1995 03 07 (Dnr 93-178).
References
- 1.Hummers-Pradier E, Kochen MM. Urinary tract infections in adult general practice patients. British J Gen Pract. 2002;52:752–61. [PMC free article] [PubMed] [Google Scholar]
- 2.Strandberg K, Beerman B, Lönnerholm G, editors. UppsalaSweden: Medical Products Agency; 1990. Workshop: Treatment of urinary tract infections; pp. 7–194. 2. [Google Scholar]
- 3.Stalsby Lundborg C. Olsson E. Molstad S and the Swedish Study Group on Antibiotic Use. Antibiotic prescribing in outpatients: A 1-week diagnosis-prescribing study in 5 counties in Sweden. Scand J Infect Dis. 2002;34:442–8. doi: 10.1080/00365540110080647. [DOI] [PubMed] [Google Scholar]
- 4.Ferry S, Burman LG, Mattsson B. Urinary tract infection in primary health care in northern Sweden, II: Clinical presentation. Scand J Prim Health Care. 1987;5:176–80. doi: 10.3109/02813438709014000. [DOI] [PubMed] [Google Scholar]
- 5.Hooton TM, Stamm W E. Diagnosis and treatment of uncomplicated urinary tract infection. Inf Dis Clin North Am. 1997;11:551–80. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- 6.Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307:463–8. doi: 10.1056/NEJM198208193070802. [DOI] [PubMed] [Google Scholar]
- 7.Rubin RH, Shapiro ED, Andriole VT, Davis RJ, Stamm WE. Evaluation of new antiinfective drugs for the treatment of urinary tract infection. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis. 1992;15:216–27. doi: 10.1093/clind/15.supplement_1.s216. [DOI] [PubMed] [Google Scholar]
- 8.Aspevall O, editor; Hallander H, editor. 1993. Referensmetodik för laboratoriediagnostik vid kliniska bakteriologiska laboratorier. I. Infektionsdiagnostik. I 5. Urinvägs-infektioner/bakteriuri. Svenska Läkarsällskapets sektion för medicinsk mikrobiologi, referensgruppen för klinisk bakteriologi i samverkan med Statens Bakteriologiska Laboratorium [Reference methodology for laboratory diagnostics at clinical bacteriological laboratories, I: Infectious diagnostics. I 5: Urinary tract infections/bacteriuria. Swedish Medical Association, Section for Microbiology in cooperation with the Swedish Institute for Infectious Disease Control]. ISSN 0283-328X. [Google Scholar]
- 9.Kouri T, Fogazzi G, Gant V, Hallander H, Hofmann W, Guder WG. European urinalysis guidelines. Scand J Clin Lab Invest. 2000;60:1–96. doi: 10.1016/s0009-8981(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 10.Nicolle LE. Pivmecillinam in the treatment of urinary tract infections. J Antimicrob Chemother. 2000;46:35–9. [PubMed] [Google Scholar]
- 11.Edlund C, Nord CE. Effect on the human normal microflora of oral antibiotics for treatment of urinary tract infections. J Antimicrob Chemother. 2000;46:41–8. [PubMed] [Google Scholar]
- 12.Graninger W. Pivmecillinam: Therapy of choice for lower urinary tract infection. Int J Antimicrob Agents. 2003;((Suppl 2)):73–8. doi: 10.1016/s0924-8579(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 13.Olafsson M, Kristinsson K G, Sigurdsson JA. Urinary tract infections, antibiotic resistance and sales of antimicrobial drugs: An observational study of uncomplicated urinary tract infections in Icelandic women. Scand J Prim Health Care. 2000;18:35–8. doi: 10.1080/02813430050202532. [DOI] [PubMed] [Google Scholar]
- 14.Kahlmeter G. Prevalence and antimicrobial susceptibility of pathogens in uncomplicated cystitis in Europe. The ECO.SENS study. Int J Antimicrob Agents. 2003;((Suppl 2)):49–52. doi: 10.1016/s0924-8579(03)00229-2. [DOI] [PubMed] [Google Scholar]
- 15.Norrby SR. Short-term treatment of uncomplicated lower urinary tract infections in women. Rev Infect Dis. 1990;12:458–67. doi: 10.1093/clinids/12.3.458. [DOI] [PubMed] [Google Scholar]
- 16.Baerheim A. Empirical treatment of uncomplicated cystitis: Keep it simple. BMJ. 2001;323:1197–8. doi: 10.1136/bmj.323.7323.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand J Infect Dis. 2004;6:296–301. doi: 10.1080/00365540410019642. [DOI] [PubMed] [Google Scholar]
- 18.Howell David C. 5th ed. 2002. Statistics of Psychology. Duxbury, 384. [Google Scholar]
- 19.Bent S, Nallamothu BK, Simel DL, Fihn SD, Saint S. Does this woman have an acute uncomplicated urinary tract infection? JAMA. 2002;287:2701–10. doi: 10.1001/jama.287.20.2701. [DOI] [PubMed] [Google Scholar]
- 20.WMA. Ferney-VoltaireFrance: World Medical Association; 2002. Ethical principles for medical research involving human subjects. [Google Scholar]
- 21.Ferry S, Burman LG, Holm SE. Clinical and bacteriological effects of therapy of urinary tract infection in primary health care: Relation to in vitro sensitivity testing. Scand J Infect Dis. 1988;20:535–44. doi: 10.3109/00365548809032503. [DOI] [PubMed] [Google Scholar]
- 22.Burman LG, Ahlmén J, Calmenius C, Ferry S, Johnson B. Urinvägsinfektion av S. Saprophyticus. Självläker sällan men pivmecillinam har god effect [Urinary tract infections caused by S. Saprophyticus rarely cure spontaneously but pivmecillinam has good efficacy] Läkartidningen. 1993;90:481–4. [PubMed] [Google Scholar]
- 23.Christiaens T, De Backer D, Burgers J, Baerheim A. Guidelines, evidence, and cultural factors: Comparison of four European guidelines on uncomplicated cystitis. Scand J Prim Health Care. 2004;22:141–5. doi: 10.1080/02813430410006521. [DOI] [PubMed] [Google Scholar]
- 24.Flottorp S, Oxman AD, Cooper JG, Hjortdahl P, Sandberg S, Vorland LH. Retningslinjer for diagnostikk og behandling av akutte vannlatingsplager hos kvinner [Guidelines for diagnosis and treatment of acute urinary tract problems in women] Tidsskr Nor Laegeforen. 2000;120:1748–53. [PubMed] [Google Scholar]
- 25.Fenwick EAL, Briggs AH, Hawke CI. Management of urinary tract infection in general practice: A cost-effectiveness analysis. Br J Gen Pract. 2000;50:635–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Hooton TM. Practice guidelines for urinary tract infection in the era of managed care. Int J Antimicrob Agents. 1999;11:241–5. doi: 10.1016/s0924-8579(99)00023-0. [DOI] [PubMed] [Google Scholar]
- 27.Baerheim A, Digranes A, Hunskaar S. Equal symptomatic outcome after antibacterial treatment of acute urethral syndrome in adult women. Scand J Prim Health Care. 1999;17:170–3. doi: 10.1080/028134399750002593. [DOI] [PubMed] [Google Scholar]
- 28.Richards D, Toop L, Chambers S, Fletcher L. Response to antibiotics of women with symptoms of urinary tract infection but negative dipstick urine test results: Double blind randomised controlled trial. BMJ. 2005;331:143–6. doi: 10.1136/bmj.38496.452581.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katcman E A, Milo G, Paul M, Christiaens T, Baerheim A, Leibovici L. Three-days vs. longer duration of antibiotic treatment for cystitis in women: Systemic review and meta-analysis. Am J Med. 2005;118:1196–1207. doi: 10.1016/j.amjmed.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 30.The Swedish Urinary Tract Infection Study Group Interpretation of the bacteriologic outcome of antibiotic treatment for uncomplicated cystitis: Impact of the definition of significant bacteriuria in a comparison of ritipenem acoxil with norfloxacin. Clin Infect Dis. 1995;20:507–13. [PubMed] [Google Scholar]
- 31.Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al. Developing clinical rules to predict urinary tract infection in primary care settings: Sensitivity and specificity of near patient tests (dipsticks) and clinical scores. Br J Gen Pract. 2006;56:606–12. [PMC free article] [PubMed] [Google Scholar]
- 32.Ferry S. Workshop on Treatment of Urinary Tract Infections. Diagnosis and Treatment of Urinary Tract Infections. Asymptomatic bacteriuria in primary health care – when to seek, treat or control? Swedish Medical Products Agency. 1990;2:149–55. [Google Scholar]
- 33.Anderson JD, Adams MA. Urinary excretion of Mecillinam by volunteers receiving film-coated tablets of Pivmecillinam Hydrochloride. Chemotherapy. 1979;25:1–4. doi: 10.1159/000237814. [DOI] [PubMed] [Google Scholar]
- 34.Mabeck CE. Treatment of uncomplicated urinary tract infection in non-pregnant women. Postgrad Med J. 1972;48:69–75. [PMC free article] [PubMed] [Google Scholar]
- 35.Asbach HW. Single dose oral administration of cefixime 400 mg in the treatment of acute uncomplicated cystitis and gonorrhoea. Drugs. 1991;42((Suppl 4)):10–13. doi: 10.2165/00003495-199100424-00005. [DOI] [PubMed] [Google Scholar]
- 36.Christiaens TC, De Meyere M, Verschraegen G, Peersman W, Heytens S, De Maeseneer JM. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br J Gen Pract. 2002;52:729–34. [PMC free article] [PubMed] [Google Scholar]