Abstract
Administration of the proinflammatory molecule lipopolysaccharide (LPS) alters transport rates for many peptides across the blood-brain barrier (BBB). We and others have previously shown that effects of LPS on BBB transport are highly dependent on the injection paradigm used, and timing of the study. Cytokine expression in both brain and serum compartments influence the BBB response to an inflammatory stimulus, and mediate changes in BBB transport. Here, we used multianalyte technology to simultaneously determine the responses of 13 cytokines and chemokines (G-CSF, GM-CSF, IL- 1α, IL-1β, IL-6, IL-10, IL-13, IP-10, KC, MCP-1, MIP-lα, RANTES, and TNF- α) in brain and blood to single and repeated injections of LPS and path analysis to determine the major relations among these analytes. Major findings are: 1) in comparison to measurements taken from a time course after a single injection of LPS, the three injection regimen of LPS produced significantly higher levels in brain for G-CSF, IL-1 alpha, IL-6, MCP-1, MIP-1 alpha, and TNF and in serum for G-CSF, IL-6, and GM-CSF and 2) path analysis distinguished direct from indirect correlations between analyte pairs, with MCP-1, IL-6, G-CSF, and KC mediating relations among these cytokines both within and between serum and brain compartments. These results suggest that potentiation of cytokine levels in brain and serum compartments could play important roles in the regulation of BBB transport, and that our novel application of an established statistical method can be used to assess direct correlations within multiplexed datasets.
Keywords: cytokines, chemokines, multiplex, brain, serum, LPS, path analysis
Introduction
The blood-brain barrier (BBB) is an important part of the central nervous system whose primary functions include regulation of the permeation of molecules between the brain and circulation. The BBB accomplishes this by both restricting passage of potentially harmful circulating molecules through expression of intercellular tight junctions and lowered rates of macropinocytosis while promoting passage of nutrients and regulatory peptides through expression of specific transporters (Abbott et al., 2010). Alterations in BBB transport have been implicated in a number of diseases affecting the CNS, including obesity, diabetes, Alzheimer’s disease, and neuro-AIDS (Banks, 2008; Banks et al., 2006; Castaneda et al., 2010; Horani and Mooradian, 2003; Zlokovic et al., 2010). Many of these transporters also show altered functions under inflammatory conditions, suggesting that inflammation could influence the course of disease through its actions at the BBB. Peripheral injection of lipopolysaccharide (LPS) is one model of systemic inflammation which has routinely been used to study alterations in BBB transport. We and others have shown that a peripheral injection of LPS significantly alters BBB transport systems for insulin, leptin, gp120, amyloid beta peptide, PACAP, P-glycoprotein substrates and TNF-a (Banks et al., 1999; Jaeger et al., 2009; Jin et al., 2011; Nonaka et al., 2004; Nonaka et al., 2005; Osburg et al., 2002; Xaio et al., 2001). Notably, temporal patterns of altered transporter function differed depending on the peptide studied (Nonaka et al., 2004; Xaio et al., 2001). It was also found in some of these studies that repeated injection of LPS was more effective at altering BBB transport of these molecules than a single injection (Banks et al., 1999; Nonaka et al., 2005; Xaio et al., 2001). The mechanisms behind both of these effects remain to be understood, but likely involve changes in inflammatory mediators such as cytokines and chemokines.
Addressing the question of how cytokines and chemokines could affect BBB transport in vivo is complex for a number of reasons. First, cytokines and chemokines produced either in the blood or brain compartments, as well as by brain endothelial cells themselves could influence BBB function (Verma et al., 2006). This is further complicated by the ability of the BBB itself to transport cytokines from one compartment to the other (Banks and Erickson, 2010). This spatial component necessitates measuring cytokines and chemokines at the protein level in both brain and blood. Second, cytokine signaling is highly dependent on the context of their receptor environment as well as other pro-inflammatory and anti-inflammatory signals that are present (Opal and DePalo, 2000). Therefore, measuring panels of cytokines together may provide a better overall picture of their influence on a physiological process. Third, cytokines and chemokines show distinct temporal profiles following an inflammatory insult (Zetterstrom et al., 1998), so timing of the study must also be taken into consideration.
In this study, we used multianalyte technology to measure simultaneously in mouse brain and serum levels of granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukins-lα (IL-lα), -1β (IL-1β), -6 (IL-6), -10 (IL-10), and -13 (IL-13), interferon-inducible protein-10 (IP-10/CXCL10), keratinocyte chemoattractant (KC/ CXCL1), monocyte chemoattractant protein-1 (MCP-1/CCL2), macrophage inflammatory protein-lα (MIP-1α/CCL3), regulated upon activation, normal T-cell expressed, and secreted (RANTES/ CCL5), and tumor necrosis factor-α (TNF- α) at multiple time points after receiving a single injection or at a single time point after receiving repeated injections of LPS. These 13 analytes were chosen based on previous findings that levels were altered in serum after 3 injections of LPS within a 24 hour period (Jaeger et al., 2009), or based on established roles in the neuroinflammatory process (Roth and De Souza, 2001; Zetterstrom et al., 1998). This study was designed to address the following questions: 1) Do changes in temporal patterns of cytokine expression in brain or serum parallel changes observed for BBB transport, 2) Do cytokine profiles change in brain or serum when comparing single to repeated injections, and 3) How do cytokine networks change within and between serum and brain compartments when comparing single vs. repeated LPS injections. The first two questions were addressed by generating time curves for cytokine expression and ANOVA analysis. The third question was addressed by applying path analysis (Baron and Kenny, 1986) to produce a diagram of direct correlations between measured analytes in brain and serum compartments. Our results identified cytokines and chemokines that are temporally matched to changes in BBB transport, as well as those whose expression significantly changes with one vs. three LPS injections. Path analysis identified cytokines and chemokines with the most direct connections to other analytes, and how these connections change when comparing single to repeated LPS injections. This demonstrates a novel application to an established, relatively simple statistical method that can be used to better understand cytokine biology in health and disease.
Methods
Experimental design
A total of 42 CD-1 mice were separated into six groups of seven, treated with LPS, and their brains and serum harvested following time points specified in this section. An illustration of the injection paradigms is shown in Scheme 1. The first group remained untreated, and was used as the zero time point. The second through fifth groups received a single LPS injection, and were sacrificed 4, 8, 16, or 28 hours post-injection. The sixth group received three injections of LPS, with the second and third injections administered 6 and 24 hours after the first. This group was sacrificed 28 hours after the first injection. For statistical comparisons between single vs. repeated LPS injections, the three injection group was compared to time zero as well as single injection times 4 and 28 hours because the endpoint of the three injection paradigm was 28 hours after the first injection and 4 hours after the last injection. For the method of path analysis (described in greater detail under “statistical analysis”), XY pairing of individual cytokine measurements for correlation analysis was done for all possible analyte combinations within and between brain and serum compartments for measurements taken from a single animal. This method of analysis was repeated for three separate data sets. The first included all six groups of injection regimens and time points studied. The second included time zero and all single injection time points, but excluded the repeated injection regimen and the third included only the time zero and repeated injection group.
Animal use and tissue collection
All animal studies were performed under protocols approved by the VA animal care and use committee, and in accordance with IACUC guidelines. LPS from Salmonella typhimurium (Sigma, St. Louis, MO) was dissolved in normal saline and administered to 6-8 week-old male CD-1 mice by giving one or three intraperitoneal injections at a dose of 3 mg/kg per injection. Single or repeated injections of LPS at this dose produce outward signs of sickness behavior (Kelley et al., 2003), including significant weight loss (Banks et al., 1999). All mice receiving a single or repeated LPS injection at this dose survived in the present study, although mortality occurs occasionally for the three injection regimen at a rate under 5% (unpublished results). Brains and serum were harvested at specified times following LPS injection as detailed in the experimental design section. Brains collected were promptly removed of meninges, cut in half, weighed, and snap-frozen in isopentane. Serum was collected after centrifuging the blood samples at 1000g for 10 minutes. Both hemibrains and serum were stored at -80°C until further use.
Protein extraction from brain tissue
For total protein extraction we used a modified protocol adapted from methods previously described for analysis of cytokine/chemokine panels in brain (Fox et al., 2005). A single hemibrain was weighed and homogenized with a polytron bench top homogenizer (Kinematica, Switzerland) in a 5 X volume of extraction buffer (20mM Tris HCl, 0.15 M NaCl, 2mM EDTA, 1mM EGTA, and Protease Inhibitor Cocktail; Sigma, St. Louis, MO). Samples were centrifuged (1,000 x g) for 10 min at 4°C, then the supernatant removed and centrifuged a second time (20,000 x g for 40 min at 4 °C) to remove any remaining debris. For all samples, protein levels were quantified with a BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL).
Measurement of cytokines and chemokines in serum and brain supernatants
All cytokine and chemokine measurements were performed by Millipore, Inc. (St. Charles, MO), and the procedure for measuring cytokines in brain tissue has been described elsewhere (Fox et al., 2005). Concentrations of G-CSF, GM-CSF, IL- 1α, IL-1β, IL-6, IL-10, IL-13, IP-10 (CXCL 10), KC (CXCL 1), MCP-1 (CCL 2), MIP-1α (CCL 3), RANTES (CCL 5), and TNF- α were simultaneously determined in brain and serum samples using a LINCOplexTM mouse cytokine kit. Fluorescent signal was measured with a Luminex200 reader (Luminex Corp., Austin, TX). Data were calculated by generating a calibration curve obtained using recombinant cytokines specified above, diluted in kit matrix for serum samples and extraction buffer for brain samples. Concentrations of cytokines were calculated using StatLIAs software (Brendan Scientific Corp., Calrsbad, CA) with a five- parameter logistic curve-fitting method. Cytokine and chemokine concentrations in brain samples were then normalized to the total protein concentration determined for each sample. Samples reading out of range for any given cytokine or chemokine (>10,000 pg/ml) were diluted and re-assayed. Cytokines that were not detected were assigned a value of zero in all analyses. Information on which cytokines/chemokines were re-assayed and which were not detected for each time point and injection regimen is provided in supplementary tables 1-4.
Correction of Brain Vascular Space with Radioactive Albumin
Bovine Serum Albumin (BSA, Sigma-Aldrich, St. Louis, MO) was radioactively labeled with 125I using the chloramine-T method, and purified on a Sephadex G-10 column. Mice were then injected with 1 × 106 CPM 125I-BSA in the left jugular, and following 5 minutes of circulation, serum and hemibrains were collected and processed as described above. Radioactivity was quantified in aliquots of both hemibrain extracts and serum suspensions using a gamma counter. Total protein concentration in the hemibrain extract was quantified using a BCA protein assay kit (Thermo Scientific, Rockford, IL). The percent contribution of serum CPM to final hemibrain extract CPM was then calculated by determining the tissue-serum ratio in units of milliliters per mg total protein. All brain values were then corrected for individual serum measurements using the following equation:
Statistical Analysis
Concentrations of analyte from brain and blood samples at each time point following single injections were compared to time zero for time-course analysis and the single injection time points specified in the experiemental design section were compared to each other and the group receiving three LPS injections. Significant differences between groups were found using one-way ANOVA and Dunnett’s or Neuman-Keuls multiple comparisons tests for time course and repeated vs. single injection comparisons respectively. A normal distribution of data was assumed for the above analysis. Correlation analyses were performed on cytokine pairs measured in an individual animal using three datasets as described under experimental design. Spearman correlation coefficients were used so that results from analyte pairings were not heavily dependent on linear relationships or outliers. The level of statistical significance based on the Bonferroni correction for 300 comparisons was adjusted to a p = 0.0002 (r = 0.545). Prism 5 software (GraphPad Inc, San Diego, CA) was used for all statistical analyses described above.
Path analysis based on methods as outlined by Baron and Kenney (Baron and Kenny, 1986) was used to further explore relations among cytokines. All analyses described using this method were done in Excel and by hand. First, the statistically significant correlation coefficients (r) derived from the simple correlations of all cytokine/chemokine pairings in each data set (Table 3) were ranked in order from high to low. Starting with the pairs with the highest correlation coefficients, pairs in which the correlation coefficient was statistically significant were graphed (see Figure 4) unless the cytokines were already connected indirectly through other pairings. Violation of this rule would create a chain passing through a cytokine more than once and was most easily noted visually in figure 4 in that it would produce a closed loop. The cytokines producing this indirect pathway are termed mediators. To determine the extent to which the mediators accounted for the correlation between the pair producing the violation (i.e., closing the loop), the correlation coefficients of all the mediators were multiplied and subtracted from the correlation coefficient of the violating pair. If the residual (direct) correlation coefficient remained statistically significant, then it was deemed that the pair was directly connected and not just related through the mediating cytokines.
Table 3.
Spearman r- values of significant correlations used for path analysis in figure 4a following Bonferroni correction (p = 0.0002, r = 0.545) between cytokines measured in serum only A), brain only B), or serum and brain C). Letters preceding cytokine/chemokine abbreviations denote brain or serum origin (B- and S- respectively).
| A) Serum-serum | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-G-CSF | S-GM-CSF | S-IL-1a | S-IL-1b | S-IL-6 | S-IL-10 | S-IL-13 | S-IP-10 | S-KC | S-MCP-1 | S-MIP-1a | S-RANTES | S-TNF-a | |
| S-G-CSF | 1 | NS | NS | NS | 0.5783 | NS | NS | NS | 0.7770 | NS | NS | NS | 0.5714 |
| S-GM-CSF | 1 | NS | NS | 0.6280 | NS | NS | NS | NS | 0.5955 | 0.7032 | NS | 0.6099 | |
| S-IL-1a | 1 | NS | 0.8234 | NS | NS | 0.7102 | 0.6751 | 0.8210 | 0.6568 | 0.6567 | 0.7662 | ||
| S-IL-1b | 1 | NS | NS | NS | NS | NS | NS | NS | NS | NS | |||
| S-IL-6 | 1 | NS | NS | 0.7859 | 0.7821 | 0.9323 | 0.7939 | 0.7308 | 0.9299 | ||||
| S-IL-10 | 1 | NS | NS | NS | NS | NS | NS | NS | |||||
| S-IL-13 | 1 | -0.5489 | NS | NS | NS | -0.5750 | NS | ||||||
| S-IP-10 | 1 | 0.6488 | 0.7977 | 0.7044 | 0.8559 | 0.7810 | |||||||
| S-KC | 1 | 0.7512 | 0.5768 | 0.6777 | 0.7533 | ||||||||
| S-MCP-1 | 1 | 0.8424 | 0.7446 | 0.9212 | |||||||||
| S-MIP-1a | 1 | 0.6943 | 0.8147 | ||||||||||
| S-RANTES | 1 | 0.7083 | |||||||||||
| S-TNF-a | 1 | ||||||||||||
| B) Brain-brain | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B-G-CSF | B-IL-1a | B-IL-1b | B-IL-6 | B-IL-10 | B-IL-13 | B-IP-10 | B-KC | B-MCP-1 | B-MIP-1a | B-RANTES | B-TNF-a | ||
| B-G-CSF | 1 | 0.7201 | NS | 0.7636 | NS | NS | NS | 0.7234 | 0.8208 | 0.7110 | 0.8528 | 0.5456 | |
| B-IL-1a | 1 | NS | 0.6278 | NS | NS | NS | 0.7126 | 0.6524 | 0.7687 | 0.6703 | NS | ||
| B-IL-1b | 1 | NS | NS | 0.6196 | NS | NS | NS | NS | NS | NS | |||
| B-IL-6 | 1 | NS | NS | 0.5760 | 0.6624 | 0.9109 | 0.6258 | 0.6674 | 0.7020 | ||||
| B-IL-10 | 1 | NS | NS | NS | NS | NS | NS | NS | |||||
| B-IL-13 | 1 | NS | NS | NS | NS | NS | NS | ||||||
| B-IP-10 | 1 | 0.7545 | 0.7112 | NS | NS | NS | |||||||
| B-KC | 1 | 0.7524 | 0.5678 | 0.6928 | NS | ||||||||
| B-MCP-1 | 1 | 0.6587 | 0.7337 | 0.6070 | |||||||||
| B-MIP-1a | 1 | 0.8188 | 0.5875 | ||||||||||
| B-RANTES | 1 | NS | |||||||||||
| B-TNF-a | 1 | ||||||||||||
| C) Serum-brain | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-G-CSF | S-GM-CSF | S-IL-1a | S-IL-1b | S-IL-6 | S-IL-10 | S-IL-13 | S-IP-10 | S-KC | S-MCP-1 | S-MIP-1a | S-RANTES | S-TNF-a | |
| B-G-CSF | 0.913 | NS | NS | NS | NS | NS | NS | NS | 0.695 | NS | NS | NS | NS |
| B-IL-1a | 0.773 | NS | NS | NS | NS | NS | NS | NS | 0.585 | NS | NS | NS | NS |
| B-IL-1b | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| B-IL-6 | 0.744 | NS | NS | NS | 0.545 | NS | NS | NS | 0.632 | 0.560 | NS | NS | 0.561 |
| B-IL-10 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| B-IL-13 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| B-IP-10 | NS | NS | NS | NS | NS | NS | NS | 0.594 | NS | 0.546 | NS | NS | NS |
| B-KC | 0.809 | NS | 0.693 | NS | 0.760 | NS | -0.575 | 0.607 | 0.785 | 0.762 | 0.555 | NS | 0.735 |
| B-MCP-1 | 0.794 | NS | NS | NS | 0.582 | NS | NS | NS | 0.672 | 0.616 | NS | NS | 0.571 |
| B-MIP-1a | 0.664 | NS | NS | NS | NS | 0.567 | NS | NS | NS | NS | NS | NS | NS |
| B-RANTES | 0.832 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| B-TNF-a | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
Figure 4.
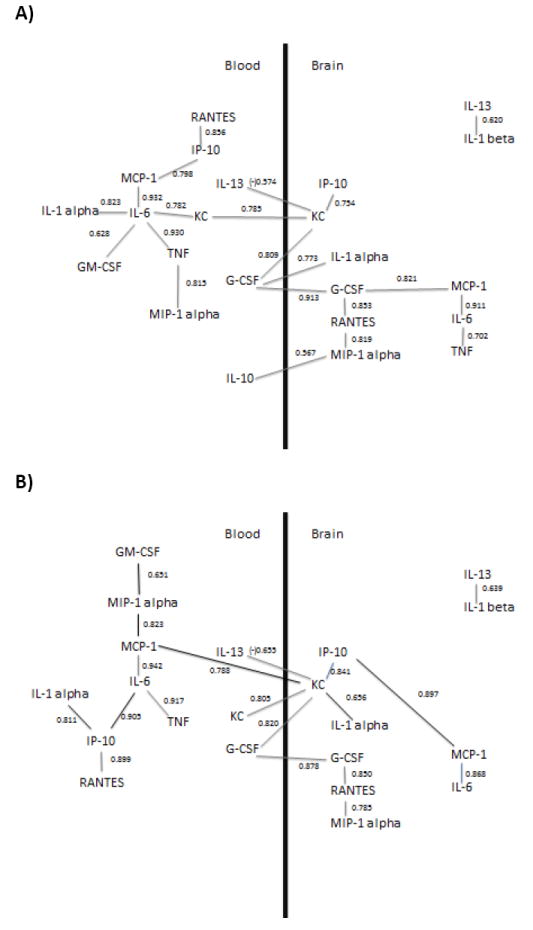
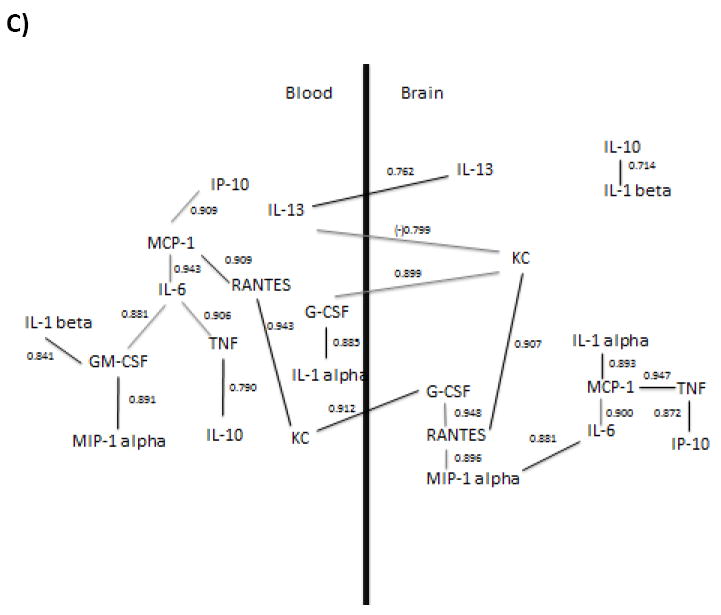
Determination of relations among brain and blood cytokine and chemokine correlations by path analysis. Brain levels were corrected for vascular space contamination. Numerical values near lines are r values and statistical significance set at p = 0.0002 after Bonferroni correction. The inverse correlation between serum IL-13 and brain KC is indicated by the negative r value. Path analysis was applied to a) correlations of all cytokines from all groups b) correlations of only time zero and single injections, and c) correlations of only time zero and repeated injections. Black lines in b) and c) indicate a shift in analyte relationships as compared to the data set in a).
Results
Temporal expression patterns following a single LPS injection
Following initial measurement of cytokines and chemokines in brain and serum, many analytes either read above the standard curve range or were not detected (supplementary table 1). To determine the potential contribution of serum retained in the brain vasculature to the levels of cytokines and chemokines in brain, bovine serum albumin (BSA), a commonly used marker of vascular space, was labeled with 125I and injected intravenously. After 5 minutes of circulation, serum and brains were collected and processed identically to samples used for cytokine analysis. The radioactivity in brain homogenate was corrected for protein concentration, and the volume of serum per milligram of protein in brain extract was calculated to be 0.20 ± 0.06 μl (n=4, SD). Therefore, 0.02% of the analyte value for serum is predicted to contribute to the value for brain in Figure 1. For example, a serum value of 2 (106) pg/ml for G-CSF would account for 400 pg/mg of the G-CSF found in brain and 60,000 pg/ml of IL-6 in serum would account for about 12 pg/mg of IL-6 in brain. To eliminate the possibility of serum values influencing the results for brain analytes in this study, we subtracted the calculated serum contamination from brain values for each individual mouse used in this study prior to analysis.
Figure 1.
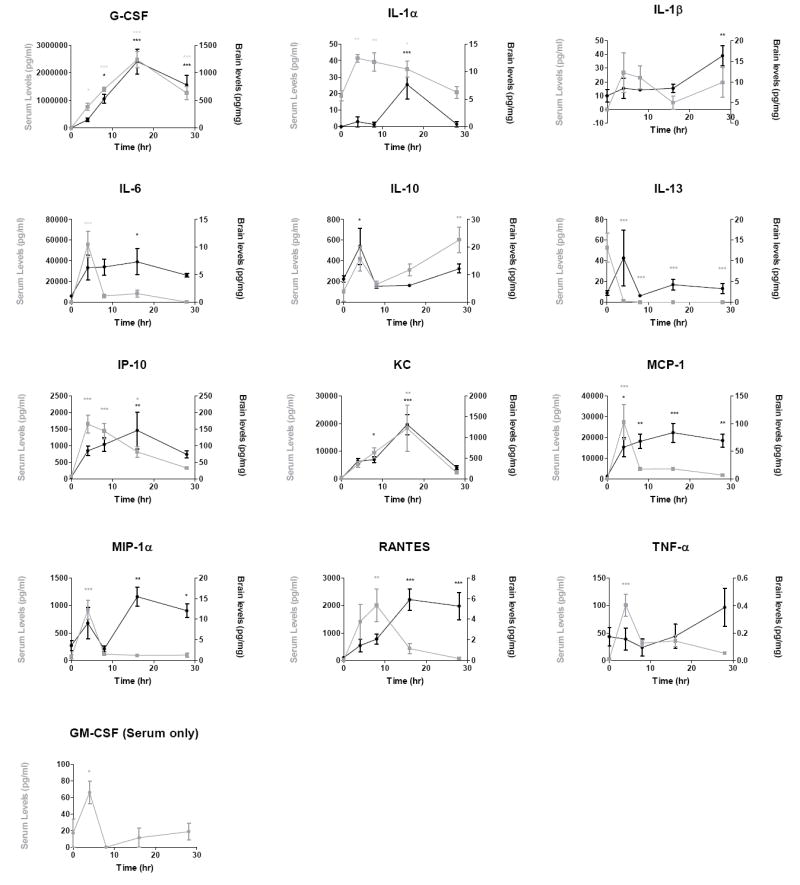
Time curves of 13 cytokines and chemokines quantified in serum and brain 0, 4, 8, 16, and 28 hours following a single injection of LPS. Data were analyzed by one-way ANOVA followed by Dunnett’s multiple comparisons test. Individual points were plotted as mean ± SEM, n = 7 per time point, * p≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 compared to t=0.
Cytokine and chemokine levels were measured in serum and brain at 0 (no LPS treatment), 4, 8, 16, and 28 hours following a single LPS injection of 3mg/kg. Expression patterns of all cytokines and chemokines measured after this single injection are shown in Figure 1. Brain and blood showed similar temporal patterns for G-CSF, IL-6, IL-10, and KC, whereas brain patterns were distinct from those of serum for the remainder of the cytokines and chemokines measured. Levels in brain and serum tended to move in different directions for IL-13 and TNF. Peaks in serum preceded peaks in brain for IL-1α, IL-1β, MCP-1, RANTES, IP-10, and TNF-α. MIP-lα showed an early peak in brain that corresponded temporally to a peak in serum but subsequently showed a peak in brain with no corresponding peak in serum. GM-CSF was not detected in brain for any of the time points or injection paradigms used in these studies, and therefore only serum values are shown. Cytokines which remained significantly elevated in brain at 28 h were IL-1β, MCP-1, MIP-1a, and RANTES.
Single vs. repeated injections of LPS
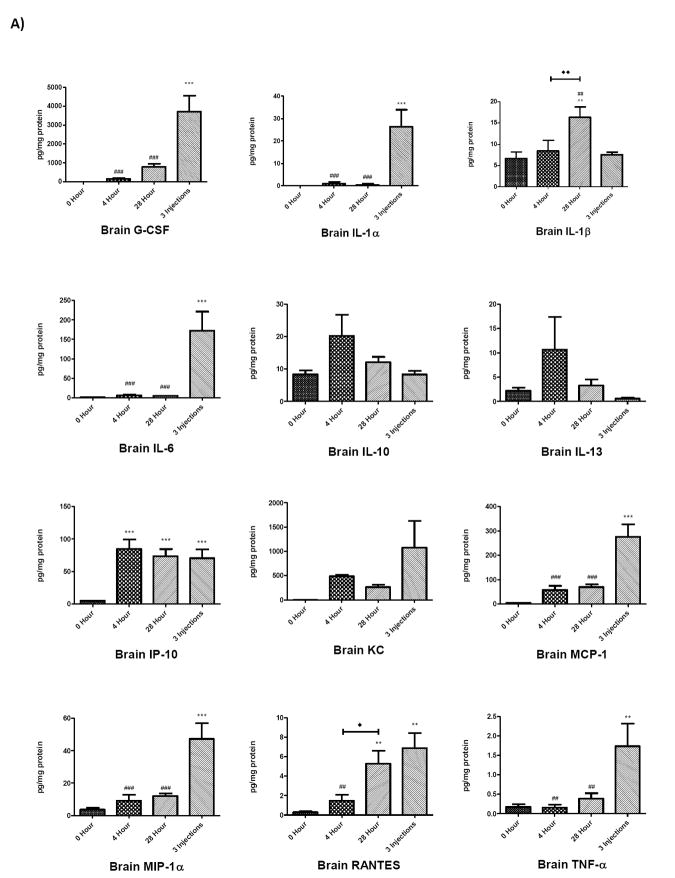
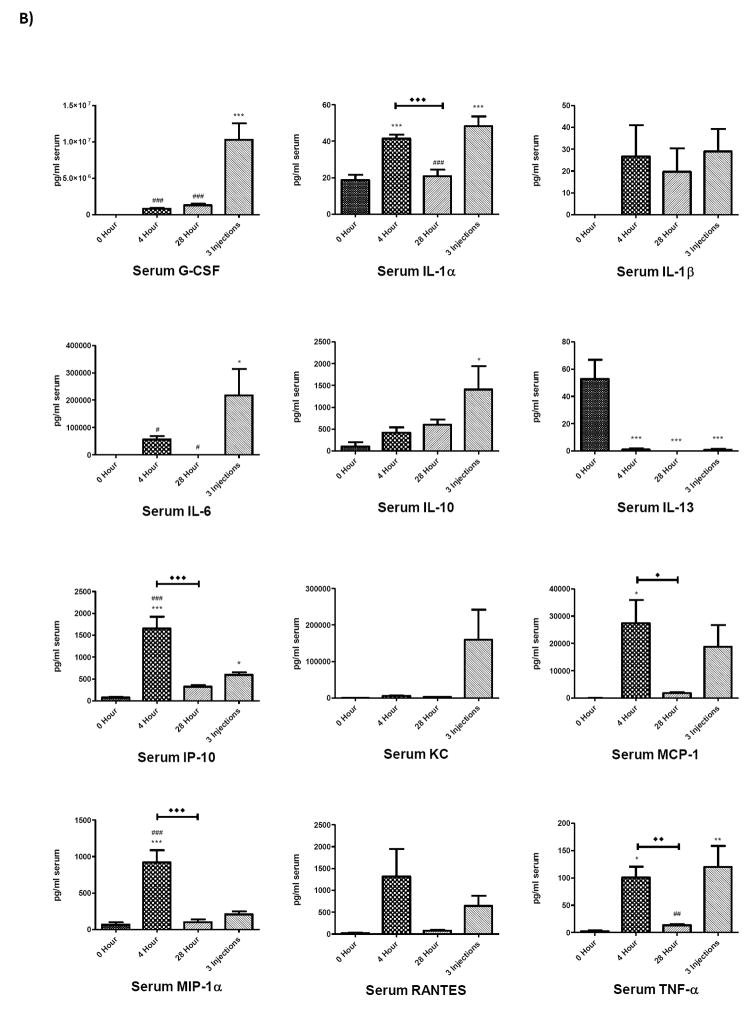
To determine whether brain and serum cytokine and chemokine profiles differed in mice receiving a single LPS injection compared to multiple LPS injections, cytokine and chemokine levels in brain and serum at 0, 4, and 28 hours following a single injection of LPS were compared to levels in brain and serum from mice treated with three injections of LPS over a 24 hour period (Figure 2). The 4 and 28 hour time points in the single injection group were chosen for comparison because mice receiving repeated LPS injections were sacrificed 28 h after receiving their first injection, a time point that was also 4 hours after receiving their third, final injection (Scheme 1). Analysis of these groups showed that cytokine or chemokine levels after repeated injections of LPS demonstrated distinct patterns compared to single injection time points that were dependent on the analyte measured as well as the tissue source. These patterns were categorized into four groups, shown in Table 1: those in which the 3 injection value i) did not differ from the 4 or 28 h single injection values nor from the 0 time (control) value, ii) differed from the 0 (control) value, iii) differed from the 4 h single injection value, iv) differed from the 28 h single injection value; the latter 3 categories are not mutually exclusive. In serum, G-CSF, GM-CSF and IL-6 were significantly elevated compared to the two single-injection time points and to the control value. In brain, 3 injections of LPS elevated G-CSF, IL-lα, IL-6, MCP-1, MIP-lα, and TNF-α compared to controls and the values for a single LPS injection. Notably, cytokines and chemokines from the three-injection group showing significant increases compared to 0, 4, and 28 hours also showed significant increases compared to 8 and 16 hour single injection time points (data not shown). The injection time point/paradigm showing the greatest magnitude of change compared to time 0 for each cytokine/chemokine in each compartment is shown in Table 2.
Figure 2.
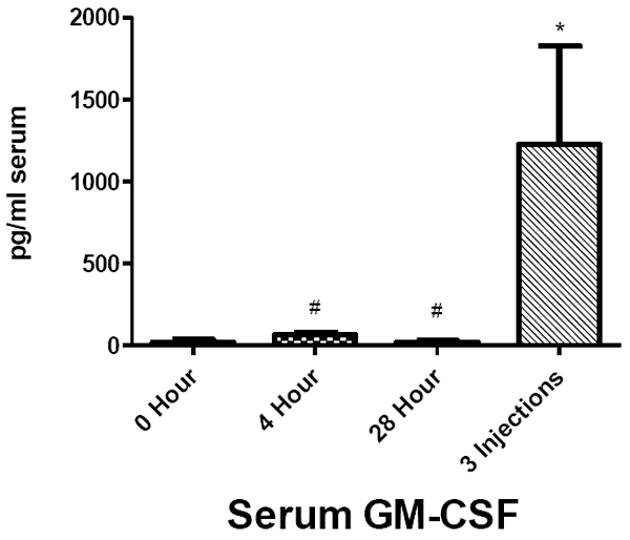
Comparison of cytokines and chemokine levels in brain A) and serum B) 4 and 28 h after a single and after repeated LPS injections. The value for repeated injections (given at 0, 6, and 24 h) was taken 4 h after the last injection which also corresponds to 28 h after the first injection (see scheme 1). Data were analyzed by one-way ANOVA followed by Newman-Keuls multiple comparisons test. Graphs plotted as mean ± SEM. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 compared to 0 Hour; # p ≤ 0.05, ## p ≤ 0.01, ### p ≤ 0.001 compared to 3 Injections; ◆ p ≤ 0.05, ◆◆ p ≤ 0.01, ◆◆◆ p ≤ 0.001 between groups indicated, n= 7 per group.
Scheme 1.
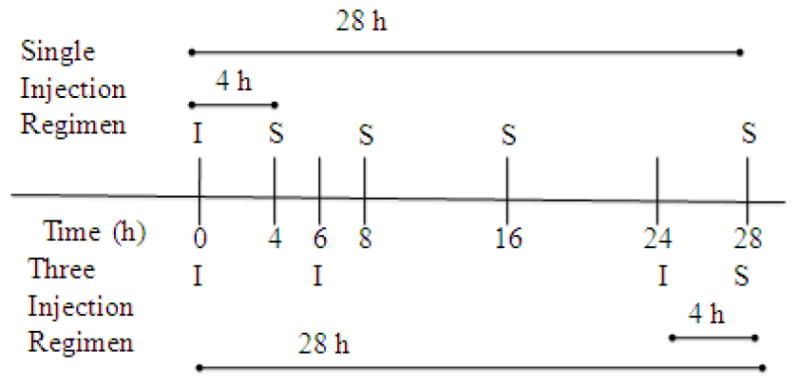
Injection and Sampling Regimens. Single injection regimen: Blood and brain were sampled (S) 4, 8, 16, and 28 h after a single injection (I) of LPS at 0 h. Three injection regimen: LPS injections were given at 0, 6, and 24 h. Brain and blood were sampled 28 h after the 1st injection (4 h after the last injection).
Table 1.
Summary of results comparing cytokine values taken after 3 LPS injections to controls (0 h) and to the values taken at 4 and 28 h after a single injection of LPS in serum a) and brain b). Asterisk (*) indicates the categorie(s) under which each cytokine or chemokine is grouped. All differences are relative to single injection time points. Cytokines analyzed were: G-CSF, GM-CSF, IL- 1α, IL-1β, IL-6, IL- 10, IL-13, IP-10, KC, MCP-1, MIP-1α, RANTES, and TNF- α.
| A) Serum | ||||
|---|---|---|---|---|
| No Difference | Different from 0 Hour | Different from 4 Hour | Different from 28 Hour | |
| IL-1β | * | |||
| KC | * | |||
| RANTES | * | |||
| MCP-1 | * | |||
| IL-10 | * | |||
| IL-13 | * | |||
| MIP-1α | * | |||
| IP-10 | * | * | ||
| IL-1α | * | * | ||
| TNF-α | * | * | ||
| IL-6 | * | * | * | |
| G-CSF | * | * | * | |
| GM-CSF | * | * | * | |
| B) Brain | ||||
| No Difference | Different from 0 Hour | Different from 4 Hour | Different from 28 Hour | |
| IL-10 | * | |||
| IL-13 | * | |||
| KC | * | |||
| IP-10 | * | |||
| IL-1β | * | |||
| RANTES | * | * | ||
| G-CSF | * | * | * | |
| IL-1α | * | * | * | |
| IL-6 | * | * | * | |
| MCP-1 | * | * | * | |
| MIP-1α | * | * | * | |
| TNF-α | * | * | * | |
Table 2.
Cytokine and chemokine levels in brain and serum compartments at all time points following single injections, as well as from the three injection model were compared to time zero. Time points showing the greatest magnitude of change compared to time zero are indicated, and magnitude changes +/- standard error of the mean are shown above. Data were ranked from greatest to least magnitude of change. Letters preceding cytokine/chemokine abbreviations denote brain or serum origin (B- and S- respectively).
| Cytokine | Time point | Mag. Change | SEM |
|---|---|---|---|
| S-G-CSF | 3 inj. | 10268984.0 | 2278000.0 |
| S-IL-6 | 3 inj. | 217445.0 | 96881.0 |
| S-KC | 3 inj. | 159138.9 | 82761.2 |
| S-MCP-1 | 4 hrs. | 27361.0 | 8493.0 |
| B-G-CSF | 3 inj. | 3694.6 | 854.1 |
| S-RANTES | 8hrs. | 1980.8 | 584.1 |
| S-IP-10 | 4hrs. | 1582.0 | 270.7 |
| S-IL-10 | 3 inj. | 1306.6 | 543.0 |
| B-KC | 16 hrs. | 1306.0 | 245.3 |
| S-GM-CSF | 3 inj. | 1207.1 | 600.2 |
| S-MIP-1a | 4 hrs. | 854.4 | 171.6 |
| B-MCP-1 | 3 inj. | 271.6 | 51.0 |
| B-IL-6 | 3 inj. | 170.8 | 49.5 |
| B-IP-10 | 16 hrs. | 141.0 | 55.1 |
| S-TNF-a | 3 inj. | 117.5 | 38.6 |
| S-IL-13 | 8, 16, 28 hrs. | -52.7 | 14.1 |
| B-MIP-1a | 3 inj. | 43.6 | 9.8 |
| S-IL-1a | 3 inj. | 29.6 | 6.1 |
| S-IL-1b | 3 inj. | 29.0 | 10.3 |
| B-IL-1a | 3 inj. | 26.5 | 7.5 |
| B-IL-10 | 4 hrs. | 11.8 | 6.6 |
| B-IL-1b | 28 hrs. | 9.7 | 2.9 |
| B-IL-13 | 4 hrs. | 8.4 | 6.7 |
| B-RANTES | 3 inj. | 6.6 | 1.6 |
| B-TNF-a | 3 inj. | 1.6 | 0.6 |
Correlations among cytokine expression and path analysis
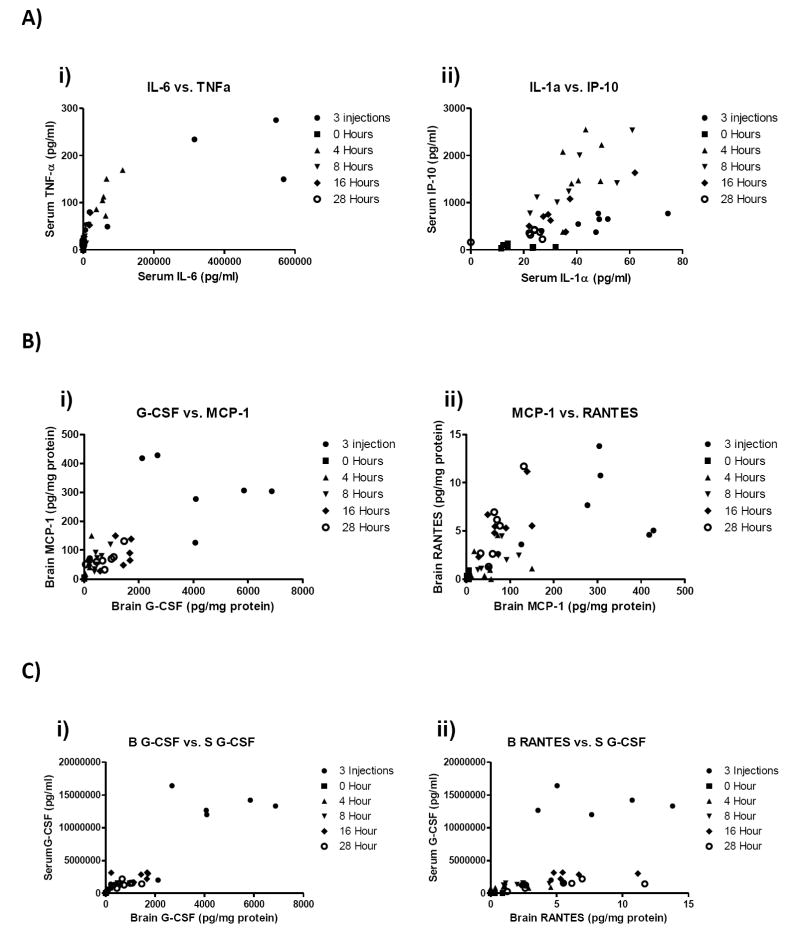
To determine correlative relationships within cytokine/chemokine networks and how these change according to injection paradigm, three data sets were used. The first included all injection regimens and time points, the second included only time points after single injections or time zero, and the third only time points after repeated injections or time zero. Analytes within each data set were then paired to all other analytes within the same data set that were measured in the same animal at the same time point. This included pairings within and between brain and serum compartments. Analyses were carried out using Spearman correlations and significant r values for all correlations after Bonferroni correction are presented in table 3. Of 300 pairings, 93 were statistically significant. Correlation analysis showed that all cytokines and chemokines were found to be correlated with at least one other substance with the exception of brain GM-CSF for which levels were not detectable in brain and brain IL-10. Representative XY pairings from the first data set are shown in figure 3.
Figure 3.
Individual correlation graphs of selected cytokine pairs in serum (A), brain (B) or between serum and brain (C) compartments. These data were taken from correlation analysis of all injection paradigms. Specific pairings were chosen to show both direct (i) and indirect (ii) relationships, as determined by path analysis in Figure 4a. The number of pairings per correlation graph was 42.
Graphical models of analyte correlations within and between brain and serum compartments for each data set were then constructed by hand based on the method of path analysis, as described in methods. Figure 4 shows the graphical results of our models for each data set. Path analysis found no residual correlation to be statistically significant after Bonferroni correction. Two groupings were found in all datasets: brain IL-13 correlating with brain IL-1 beta formed one group in figures 4 a and b and brain IL-10 correlating with brain IL-1 beta in figure 4c; all other correlated cytokines were connected in the second group for all three figures. The serum IL-13 correlation with brain KC in all datasets was the only inverse relation. In the data set including all treatments, 21 connections were found within and between brain and serum compartments. Six pairs connected brain and serum compartments: brain KC correlated with serum KC, serum IL-13, and serum G-CSF. Serum G-CSF also correlated with brain IL-1 alpha and brain GCSF, and serum IL-10 correlated brain MIP-1 alpha. Eight pairs connected analytes within serum compartments and seven connected analytes within brain. The cytokines involved in the greatest correlative complexity were serum IL-6, and brain KC, which were connected to 5 and 4 other cytokines respectively. The greatest complexity relating brain and serum compartments was for serum G-CSF and for brain KC, each of which connected to 3 other cytokines in the opposing compartment. Changes in cytokine relationships in path analysis from single injections or repeated injections were then compared to the dataset using all injection groups. For single injections (Figure 4b), 18 connections were formed, with 5 between brain and serum compartments, 7 in serum, and 7 in brain This was accounted for by a loss in connects between serum IL-10 and brain MIP-1a, and IL-6 and TNF-a in brain, as well as new connections formed which are indicated in black. For repeated injections (Figure 4c), 23 connections were formed, with 4 between brain and serum compartments, 10 between serum compartments, and 9 between brain compartments. This was accounted for by a loss of connects between serum IL-10 and brain MIP-1a, as well as new connections formed which are indicated in black.
Discussion
From comparisons of cytokine and chemokine expression in serum and brain at different times following a single LPS injection, we first found that cytokines in the blood compartment can achieve such high levels that they significantly contribute to measurements in the brain compartment. We therefore corrected all of our measurements in brain to account for serum artifact. This emphasizes the importance of considering concentrations of analytes in the blood when measuring them in brain. Second, we found that cytokines and chemokines could be grouped based on their expression in brain relative to serum. Profiles of G-CSF and KC in brain were well-matched to those in serum, which suggests that they are expressed at the same time in brain and serum compartments, and/or that these analytes in serum can cross the BBB to significantly contribute to brain levels. Many saturable transport systems at the BBB for cytokines have been identified (Banks, 2005), and BBB transport has been previously described for G-CSF (Zhao et al., 2007). We found no published results, however, addressing whether KC has a saturable transport system at the BBB. Analytes which peaked after serum were IL-1a, IP-10, IL-6, IL-1b, MCP-1, RANTES, and MIP-1a. These results support findings that the immune response in the brain is latent compared to that in the periphery, and that expression of these cytokines in the brain likely require initiation of signaling pathways and transcriptional events within the CNS (Tonelli and Postolache, 2005). In addition to finding cytokines and chemokines in brain which peaked after serum, it was also found that levels of IL-1b, MCP-1, MIP-1a and RANTES were significantly elevated above baseline levels 28 hours after LPS injection. Only IL-10 in serum remained significantly elevated above baseline at 28 hours post-injection, which supports its anti-inflammatory role in the response to a systemic insult (Conti et al., 2004). These data also show unique temporal patterns within critical time points found for changes in BBB peptide transport. For example, following a single LPS injection, transport of insulin into the brain was found to significantly increase, with a peak between 16 and 24 hours. In contrast, transport of leptin into the brain was found to be maximally suppressed between 6 and 12 hours following a single LPS injection (Nonaka et al., 2004; Xaio et al., 2001). Differences in transport of these two peptides could be explained by differences found in cytokine profiles in either time window, although future studies would be required to substantiate this claim. However, cytokines or chemokines whose peak changes are temporally matched to changes observed in BBB transport would serve as ideal targets to study inflammatory regulation of these transporters. Together, our data from this section first support the important concept that the inflammatory response in the brain initiates later and resolves more slowly than peripheral inflammatory responses, as has been shown by other groups (Qin et al., 2007; Weberpals et al., 2009) and may be mediated in part by differences in TLR4 signaling in the CNS vs. periphery (Chakravarty and Herkenham, 2005; Steiner et al., 2006), as well as restricted access of LPS to the CVOs of the brain due to minimal penetration of LPS across the BBB (Banks and Robinson, 2009; Banks and Robinson, 2010) Although we did not detect sustained changes in brain for all cytokines previously reported such as TNF-a, this may be due to enhanced sensitivity of detecting mRNA expression over protein signal. Furthermore, protein signal is likely a more accurate indicator of cytokine activity than message, especially since cytokines such as TNF-a require post-transcriptional and translational events for their activity (Moro et al., 2003; Stamou and Kontoyiannis, 2010). Second, we have outlined cytokine targets which might mediate LPS-induced alterations in BBB transport.
After assessing expression of cytokines at various time points after a single LPS injection, we determined whether a three LPS injection paradigm showed significant differences when compared to a single injection. Our findings were that IL-6, G-CSF, and GM-CSF in serum and RANTES, G-CSF, IL-1a, IL-6, MCP-1, MIP-1a, and TNF-a in brain were significantly increased compared to a single LPS injection. Given the increased levels of cytokines in serum and brain, we first concluded that our three injection model was distinct from models of endotoxin tolerance, where depressed peripheral proinflammatory cytokine responses are typically observed (Cavaillon et al., 2003) (Baykal et al., 1999; Erroi et al., 1993). In addition to our findings, others have also measured cytokine profiles in serum and brain using repeated LPS dosages (Chen et al., 2005; del Rey et al., 2009; Erroi et al., 1993). It is important, however, to distinguish that these studies used different doses of LPS, and different paradigms for repeated LPS injections. Therefore, cytokine profiles evoked in response to repeated inflammatory insults such as LPS injections are highly dependent on the dose, timing of administration, and duration of the study. This further emphasizes the necessity of our study in understanding cytokine regulation of blood-brain barrier transport. Due to our findings that BBB transport of peptides such as insulin and gp120 is potentiated using repeated vs. single LPS injections, we predict that cytokines found in our study to change significantly with three injections could mediate these effects.
Third, we determined which cytokines correlated both within and between the brain and serum compartments. Following Bonferroni correction, correlations that remained significant were used to produce a structural model of the relations among cytokines by path analysis. This model is based on the strength of statistical associations and not on preconceived relations, nor did we make a priori assumptions about directionality or cause and effect. Path analysis using our three data sets comparing cytokine measurements for all groups, time zero and single injections only, or time zero and repeated injections illustrated that shifts occur in cytokine correlation networks depending on the data set analyzed. Direct relationships which were preserved in all three models were MCP-1 and IL-6 and IL-6 and TNF-a in serum, brain KC and serum G-CSF, serum IL-13 and brain KC, and G-CSF and RANTES, RANTES and MIP-1a, and MCP-1 and IL-6 in brain. This suggests that these cytokines influence each other regardless of LPS injection paradigm used. Likewise, changes which were observed in cytokine connections between models suggest that altered cytokine pairings are driven by the injection paradigm used.
One advantage of this analysis is that it eliminates many pairs of cytokine correlations that can be explained by indirect correlations between other cytokines. For example, in our data set using all paradigms of LPS injections, serum TNF and serum MCP-1 were highly correlated with an r of 0.921 (Table 3). However, serum IL-6 had a higher r with both serum TNF (r = 0.930) and serum MCP-1 (r = 0.932) that were, according to this analysis, primary correlations. Therefore, TNF would be expected to be indirectly correlated with MCP-1 through IL-6. The strength of the indirect correlation can be calculated by multiplying the r of its components: 0.932 × 0.930 = 0.867. Subtracting this indirect r from the r correlating TNF and MCP-1 (0.921 − 0.867) left a residual or direct r for the MCP-1 vs. TNF correlation of 0.054; as this r value is not statistically significant, the model would suggest that MCP-1 and TNF are correlated indirectly through the mediation of IL-6 rather than directly to one another. Such modeling can indicate the relative strength of cytokine relations and which of those relations may be direct and which indirect. In this case, modeling eliminated most cytokine correlations as indirect. One limitation of the application of our model in the present study is that it may miss important cytokine relationships that are temporally mismatched. Cross-time correlations were not possible in this study or any study where only endpoints may be measured. This is because criteria for matching are restricted to analytes measured in a single animal. Cross-time correlation analysis over a time-course following an inflammatory stimulus would be possible, however, if serial collection of tissue such as serum was incorporated into the design. Inferences on directionality can be made in our data set using a different approach. In figure 4b, the same data set used to generate time curves was used for path analysis. Although many correlations from this data set may be explained by time-matched peaks in expression, others which correlate show a mismatch in peaks. For example, brain KC shows a peak at 16 hours whereas serum MCP-1 peaks at 4 hours. Therefore, the inference can be made that this relationship is largely driven by serum MCP-1. Another limitation of our study is that our analysis cannot rule out the real possibility that there may be unmeasured intermediates between two cytokines. Introduction of additional cytokines could substantially shift the relationships of the model. Likewise, it is possible that treatments other than LPS could produce other models of relationships. Main uses of the model are to provide a simpler schema explaining relations among cytokines and a hypothetical, working basis for testing relations among cytokines. For example, it could be conjectured based on the model shown in figure 4a that suppression of serum IL-6 levels would disrupt the relationships among serum IL-1 alpha, serum TNF, serum KC, and serum MCP-1 much more drastically than the relations among serum MCP-1, serum IL-1 beta, serum IP-10, and serum MIP-1 alpha.
One interesting suggestion of the models shown in figure 4 is a relative importance of brain-serum interactions. Brain KC and serum G-CSF as well as brain KC and serum IL-13 are present in all three models generated by path analysis, suggesting they may be especially important brain-peripheral tissue communication. There are many known routes by which a cytokine or chemokine in one of these compartments can influence another (Kelley et al., 2003; Stefani and Liguri, 2009). For example, cytokines in the peritoneal cavity can stimulate vagal afferent fibers to induce the release of cytokines in brain(Goehler et al., 1999), circulating cytokines can induce endothelial or tanycytic barrier tissues to release cytokines or cytokine-releasing substances into brain (Blatteis, 1992; Komaki et al., 1992), and isolated BBB endothelial cells can themselves respond to LPS directly by secreting cytokines into either the vascular or CNS compartment (Verma et al., 2006). Microglia and tanycytes in circumventricular organs such as the area postrema (Komaki et al., 1992; Wuchert et al., 2008) likely participate in a relay of signals between the circulation and the regions of the brain behind the BBB. Some cytokines such as IL-6, IL-1 alpha and beta, and TNF can cross the BBB (Banks et al., 1994; Banks et al., 1991; Gutierrez et al., 1993). Furthermore, transport of cytokines across the BBB may be regulated by binding proteins and/or receptor antagonists present in the circulation (Banks, 2005), as well as by the function of BBB transporters, which have been shown to be altered with LPS (Pan et al., 2008) and under conditions related to inflammation, such as opiate use and spinal cord injury (Lynch and Banks, 2008; Pan et al., 1997). The current analysis found brain-serum correlations for G-CSF and for KC; such relations could be caused by their BBB transport (Zhao et al., 2007) or through one of the other mechanisms discussed above. For example, serum and brain IL-6 were highly correlated with an r = 0.545, but the model suggests this correlation is better explained by mediation involving brain and serum KC and G-CSF and brain MCP-1.
In conclusion, cytokines and chemokines play important roles in the brain during normal function as well as in disease states. Alterations in their expression centrally and/or peripherally have been shown in a number of neurodegenerative diseases including Alzheimer’s disease, neuro-aids as well as diseases that show CNS complications such as diabetes (Craft, 2009; Marcondes et al., 2007; Mrak and Griffin, 2001; O’Connor et al., 2008; Reale et al., 2009)with evidence suggesting that peripheral inflammatory events can promote disease progression as well as exacerbate disease pathology (Combrinck et al., 2002; Jaeger et al., 2009). In many of these disease conditions, alterations in BBB functions are also observed, suggesting that the BBB/immune interaction is important in a spectrum of CNS diseases. Although much work has been done to elucidate the roles of individual cytokines and chemokines in the CNS, the growing use of multianalyte technology allows for higher throughput assessment of how panels of cytokines and chemokines change at the protein level under various treatments and disease states, and is even capable of measuring analytes in discrete brain regions (Abazyan et al., 2010; Brown et al., 2010; Datta and Opp, 2008). A combined strategy of analysis of multiple cytokines combined with powerful techniques for analyzing the relationships among those cytokines will be useful in determing the roles that cytokines play in health and disease.
Supplementary Material
Footnotes
Conflict of interest statement: All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, et al. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol Psychiatry. 2010;68:1172–81. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 2005;11:973–84. doi: 10.2174/1381612053381684. [DOI] [PubMed] [Google Scholar]
- Banks WA. The blood-brain barrier as a cause of obesity. Curr Pharm Des. 2008;14:1606–14. doi: 10.2174/138161208784705496. [DOI] [PubMed] [Google Scholar]
- Banks WA, Ercal N, Price TO. The blood-brain barrier in neuroAIDS. Curr HIV Res. 2006;4:259–66. doi: 10.2174/157016206777709447. [DOI] [PubMed] [Google Scholar]
- Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Brennan JM, Vallance KL. Adsorptive endocytosis of HIV-1gp120 by blood-brain barrier is enhanced by lipopolysaccharide. Exp Neurol. 1999;156:165–71. doi: 10.1006/exnr.1998.7011. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179:53–6. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharmacol ExpTher. 1991;259:988–96. [PubMed] [Google Scholar]
- Banks WA, Robinson SM. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Robinson SM. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav Immun. 2010;24:102–9. doi: 10.1016/j.bbi.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Baykal A, Kaynaroglu V, Hascelik G, Sayek I, Sanac Y. Epinephrine and endotoxin tolerance differentially modulate serum cytokine levels to high-dose lipopolysaccharide challenge in a murine model. Surgery. 1999;125:403–10. [PubMed] [Google Scholar]
- Blatteis CM. Role of the OVLT in the febrile response to circulating pyrogens. Prog Brain Res. 1992;91:409–12. doi: 10.1016/s0079-6123(08)62360-2. [DOI] [PubMed] [Google Scholar]
- Brown CM, Mulcahey TA, Filipek NC, Wise PM. Production of proinflammatory cytokines and chemokines during neuroinflammation: novel roles for estrogen receptors alpha and beta. Endocrinology. 2010;151:4916–25. doi: 10.1210/en.2010-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda TR, Tong J, Datta R, Culler M, Tschop MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Endotoxin tolerance: is there a clinical relevance? J Endotoxin Res. 2003;9:101–7. doi: 10.1179/096805103125001487. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–96. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhou H, Beltran J, Malellari L, Chang SL. Differential expression of cytokines in the brain and serum during endotoxin tolerance. J Neuroimmunol. 2005;163:53–72. doi: 10.1016/j.jneuroim.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–49. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–5. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SC, Opp MR. Lipopolysaccharide-induced increases in cytokines in discrete mouse brain regions are detectable using Luminex xMAP technology. J Neurosci Methods. 2008;175:119–24. doi: 10.1016/j.jneumeth.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey A, Randolf A, Wildmann J, Besedovsky HO, Jessop DS. Re-exposure to endotoxin induces differential cytokine gene expression in the rat hypothalamus and spleen. Brain Behav Immun. 2009;23:776–83. doi: 10.1016/j.bbi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erroi A, Fantuzzi G, Mengozzi M, Sironi M, Orencole SF, Clark BD, et al. Differential regulation of cytokine production in lipopolysaccharide tolerance in mice. Infect Immun. 1993;61:4356–9. doi: 10.1128/iai.61.10.4356-4359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargo KN, Iwema CL, Clark-Phelps MC, Sengelaub DR. Exogenous testosterone reverses age-related atrophy in a spinal neuromuscular system. Hormones and Behavior. 2007;51:20–30. doi: 10.1016/j.yhbeh.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, et al. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25:1138–49. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehler LE, Gaykema RP, Nguyen KT, Lee JE, Tilders FJ, Maier SF, et al. Interleukin-1beta in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–76. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Horani MH, Mooradian AD. Effect of diabetes on the blood brain barrier. Curr Pharm Des. 2003;9:833–40. doi: 10.2174/1381612033455314. [DOI] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, et al. Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav Immun. 2009;23:507–17. doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, et al. Lipopolysaccharide Alters the Blood-brain Barrier Transport of Amyloid Beta Protein: A Mechanism for Inflammation in the Progression of Alzheimer’s Disease. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Li J, Nation RL, Nicolazzo JA. Impact of p-glycoprotein inhibition and lipopolysaccharide administration on blood-brain barrier transport of colistin in mice. Antimicrob Agents Chemother. 2011;55:502–7. doi: 10.1128/AAC.01273-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, et al. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17(Suppl 1):S112–8. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Komaki G, Arimura A, Koves K. Effect of intravenous injection of IL-1 beta on PGE2 levels in several brain areas as determined by microdialysis. Am J Physiol. 1992;262:E246–51. doi: 10.1152/ajpendo.1992.262.2.E246. [DOI] [PubMed] [Google Scholar]
- Lynch JL, Banks WA. Opiate modulation of IL-1alpha, IL-2, and TNF-alpha transport across the blood-brain barrier. Brain Behav Immun. 2008;22:1096–102. doi: 10.1016/j.bbi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Burdo TH, Sopper S, Huitron-Resendiz S, Lanigan C, Watry D, et al. Enrichment and persistence of virus-specific CTL in the brain of simian immunodeficiency virus-infected monkeys is associated with a unique cytokine environment. J Immunol. 2007;178:5812–9. doi: 10.4049/jimmunol.178.9.5812. [DOI] [PubMed] [Google Scholar]
- Moro MA, Hurtado O, Cardenas A, Romera C, Madrigal JL, Fernandez-Tome P, et al. Expression and function of tumour necrosis factor-alpha-converting enzyme in the central nervous system. Neurosignals. 2003;12:53–8. doi: 10.1159/000071814. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Interleukin-1, neuroinflammation, and Alzheimer’s disease. Neurobiol Aging. 2001;22:903–8. doi: 10.1016/s0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Nonaka N, Hileman SM, Shioda S, Vo TQ, Banks WA. Effects of lipopolysaccharide on leptin transport across the blood-brain barrier. Brain Res. 2004;1016:58–65. doi: 10.1016/j.brainres.2004.04.066. [DOI] [PubMed] [Google Scholar]
- Nonaka N, Shioda S, Banks WA. Effect of lipopolysaccharide on the transport of pituitary adenylate cyclase activating polypeptide across the blood-brain barrier. Exp Neurol. 2005;191:137–44. doi: 10.1016/j.expneurol.2004.09.013. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol. 2008;252:91–110. doi: 10.1016/j.cellimm.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–72. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- Osburg B, Peiser C, Domling D, Schomburg L, Ko YT, Voigt K, et al. Effect of endotoxin on expression of TNF receptors and transport of TNF-alpha at the blood-brain barrier of the rat. Am J Physiol Endocrinol Metab. 2002;283:E899–908. doi: 10.1152/ajpendo.00436.2001. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Kastin AJ. Blood-brain barrier permeability to ebiratide and TNF in acute spinal cord injury. Exp Neurol. 1997;146:367–73. doi: 10.1006/exnr.1997.6533. [DOI] [PubMed] [Google Scholar]
- Pan W, Yu C, Hsuchou H, Zhang Y, Kastin AJ. Neuroinflammation facilitates LIF entry into brain: role of TNF. Am J Physiol Cell Physiol. 2008;294:C1436–42. doi: 10.1152/ajpcell.00489.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, Greig NH, Kamal MA. Peripheral chemo-cytokine profiles in Alzheimer’s and Parkinson’s diseases. Mini Rev Med Chem. 2009;9:1229–41. doi: 10.2174/138955709789055199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J, De Souza GE. Fever induction pathways: evidence from responses to systemic or local cytokine formation. BrazJ Med Biol Res. 2001;34:301–14. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- Stamou P, Kontoyiannis DL. Posttranscriptional regulation of TNF mRNA: a paradigm of signal-dependent mRNA utilization and its relevance to pathology. Curr Dir Autoimmun 2010. 11:61–79. doi: 10.1159/000289197. [DOI] [PubMed] [Google Scholar]
- Stefani M, Liguri G. Cholesterol in Alzheimer’s disease: unresolved questions. Curr Alzheimer Res. 2009;6:15–29. doi: 10.2174/156720509787313899. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Chakravarty S, Rudaya AY, Herkenham M, Romanovsky AA. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood. 2006;107:4000–2. doi: 10.1182/blood-2005-11-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonelli LH, Postolache TT. Tumor necrosis factor alpha, interleukin-1 beta, interleukin-6 and major histocompatibility complex molecules in the normal brain and after peripheral immune challenge. Neurol Res. 2005;27:679–84. doi: 10.1179/016164105X49463. [DOI] [PubMed] [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: A polarized response to lipopolysaccharide. Brain Behav Immun. 2006;20:449–55. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Weberpals M, Hermes M, Hermann S, Kummer MP, Terwel D, Semmler A, et al. NOS2 gene deficiency protects from sepsis-induced long-term cognitive deficits. J Neurosci. 2009;29:14177–84. doi: 10.1523/JNEUROSCI.3238-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuchert F, Ott D, Murgott J, Rafalzik S, Hitzel N, Roth J, et al. Rat area postrema microglial cells act as sensors for the toll-like receptor-4 agonist lipopolysaccharide. J Neuroimmunol. 2008;204:66–74. doi: 10.1016/j.jneuroim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Xaio H, Banks WA, Niehoff ML, Morley JE. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res. 2001;896:36–42. doi: 10.1016/s0006-8993(00)03247-9. [DOI] [PubMed] [Google Scholar]
- Zetterstrom M, Sundgren-Andersson AK, Ostlund P, Bartfai T. Delineation of the proinflammatory cytokine cascade in fever induction. Ann N Y Acad Sci. 1998;856:48–52. doi: 10.1111/j.1749-6632.1998.tb08311.x. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Navalitloha Y, Singhal S, Mehta J, Piao CS, Guo WP, et al. Hematopoietic growth factors pass through the blood-brain barrier in intact rats. Exp Neurol. 2007;204:569–73. doi: 10.1016/j.expneurol.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J Neurochem. 2010;115:1077–89. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.