Abstract
Heterodimeric hCG is one of the key hormones determining early pregnancy success. We have previously identified rare missense mutations in hCGβ genes with potential pathophysiological importance. The present study assessed the impact of these mutations on the structure and function of hCG by applying a combination of in silico (sequence and structure analysis, molecular dynamics) and in vitro (co-immunoprecipitation, immuno- and bioassays) approaches. The carrier status of each mutation was determined for 1086 North-Europeans [655 patients with recurrent miscarriage (RM)/431 healthy controls from Estonia, Finland and Denmark] using PCR-restriction fragment length polymorphism. The mutation CGB5 p.Val56Leu (rs72556325) was identified in a single heterozygous RM patient and caused a structural hindrance in the formation of the hCGα/β dimer. Although the amount of the mutant hCGβ assembled into secreted intact hCG was only 10% compared with the wild-type, a stronger signaling response was triggered upon binding to its receptor, thus compensating the effect of poor dimerization. The mutation CGB8 p.Pro73Arg (rs72556345) was found in five heterozygotes (three RM cases and two control individuals) and was inherited by two of seven studied live born children. The mutation caused ∼50% of secreted β-subunits to acquire an alternative conformation, but did not affect its biological activity. For the CGB8 p.Arg8Trp (rs72556341) substitution, the applied in vitro methods revealed no alterations in the assembly of intact hCG as also supported by an in silico analysis. In summary, the accumulated data indicate that only mutations with neutral or mild functional consequences might be tolerated in the major hCGβ genes CGB5 and CGB8.
Keywords: female reproduction, recurrent miscarriage, hCGβ, CGB5 and CGB8, missense mutations, structural analysis, functional assays
Introduction
Placental chorionic gonadotrophin (hCG) is a pleiotropic hormone that functions since early pregnancy in an endocrine/autocrine/paracrine way to support progesterone production by the corpus luteum, to promote angiogenesis (Zygmunt et al., 2002), trophoblast invasiveness (Guibourdenche et al., 2010) and decidualization of the endometrium (Zimmermann et al., 2009; Kajihara et al., 2010), to stimulate fetal testicular testosterone production (Huhtaniemi et al., 1977) and regulate maternal immunotolerance (Kayisli et al., 2003; Tsampalas et al., 2010). hCG is produced already by an 8-cell blastocyst prior to implantation and its concentration doubles every 2 days until peaking at gestational weeks 9–11 (Hay, 1988; Lopata and Hay, 1989). Large inter-individual variation in the levels of hCG during pregnancy has been documented and critically low amounts in maternal circulation may indicate an adverse pregnancy outcome (Korhonen et al., 1994; Rull and Laan, 2005).
Like all heterodimeric glycoprotein hormones (LH, FSH, TSH), hCG is formed by non-covalent association of the common α-subunit and the hormone-specific β-subunit (Morgan et al., 1975). The crystal structure of hCG has revealed a similar tertiary structure for both subunits, which are composed of three hairpin loops held together by three disulfide bonds that form a characteristic cystine knot structure (Lapthorn et al., 1994). The integrity of the cystine knot is essential for the folding, assembly and function of the hormone. The structural and functional properties of the subunits are determined by six cystine bonds and six oligosaccharide chains (two N-linked, four O-linked) in hCGβ and five cystine bonds and two N-linked carbohydrates in the α-subunit (Morgan et al., 1975; Lapthorn et al., 1994).
The chorionic gonadotrophin beta (CGB) genes encoding the hCG β-subunit (145 aa) have evolved in the primate lineage by serial duplications of the ancestral LH β-subunit coding gene (LHB) (Maston and Ruvolo, 2002; Hallast et al., 2008; Nagirnaja et al., 2010). In humans, the common gene cluster for LHB and six CGB genes is located at 19q13.32 (Policastro et al., 1986). Four genes (CGB, CGB5, CGB7 and CGB8) code for an almost identical hCGβ protein in the placenta, but have a highly variable transcriptional activity (Miller-Lindholm et al., 1997; Rull and Laan, 2005; Rull et al., 2008a). A normal uncomplicated pregnancy is characterized by a balanced biallelic expression of the maternal and paternal alleles of the hCGβ genes (Uusküla et al., 2010).
Addressing the genetic variation of CGB genes has been challenging due to a high DNA sequence similarity (up to 99%) between gene copies (Hallast et al., 2005). So far, only one naturally occurring variant of hCGβ (CGB5 p.Val79Met) has been functionally characterized leading to an inefficient hCG assembly in vitro (Miller-Lindholm et al., 1999). The p.Val79Met heterozygotes represented 14/334 (4.2%) of the studied random subjects from Omaha, NE, USA but no clinical information was available on the mutation carriers. Curiously, this substitution was totally absent in further screening of over 500 DNA samples from five European populations (Jiang et al., 2004).
We have recently identified heterozygous cases of novel missense mutations in the most actively transcribed hCGβ genes (CGB5, CGB8) in Estonian and Finnish samples with recurrent miscarriage (RM) (Rull et al., 2008b). RM affects up to 3% of fertile couples aiming to conceive a child and around half are defined as idiopathic (Bricker and Farquharson, 2002; Rai and Regan, 2006).The reported substantial familiar disposition to RM points to the contribution of genetic risk factors (Christiansen et al., 1990; Kolte et al., 2011). This current study addressed the prevalence of the identified hCGβ mutations in a larger Northern Europe sample set (total n = 1086 subjects; 655 RM cases and 431 fertile controls) and assessed their effect on the structure and function of the synthesized hCG hormone using in silico and in vitro approaches. The study showed that the CGB5 p.Val56Leu mutation substantially affects the assembly and functionality of intact hCG α/β heterodimers, the CGB8 p.Pro73Arg substitution alters the conformation of the hCGβ-subunit, while the CGB8 p.Arg8Trp is neutral in the structural and functional context.
Materials and Methods
Identification of subjects with CGB5 p.Val56Leu, CGB8 p.Arg8Trp and p.Pro73Arg mutations
This study was approved by the Ethics Review Committee on Human Research of the University of Tartu, Estonia, Ethics Committee of the Department of Obstetrics and Gynecology, Helsinki University Central Hospital outpatient clinic for women with RM and the Ethics Committee of the Fertility Clinics, Rigshospitalet, Copenhagen, Denmark. The study was conducted according to the Declaration of Helsinki principles. A written informed consent to participate in the study was obtained from each individual prior to recruitment.
Details of the initial identification of mutations in the hCGβ coding genes in Estonian and Finnish couples with RM have been published previously (Rull et al., 2008b). Briefly, subjects had been recruited at the Women's Clinic of Tartu University Hospital and Nova Vita Clinic, Tallinn, Estonia during 2003–2007; and at the Department of Gynecology and Obstetrics of the Helsinki University Hospital in Finland during 2001–2004. As maternally and paternally derived hCGβ gene variants contribute equally to the function of the fetal genome, the patient group included both the female and male partner of the couples experiencing idiopathic RM (≥3 consecutive miscarriages during the first trimester of pregnancy without any identified cause; age 18–40 years). Mutation screening was performed by resequencing the CGB5 and the CGB8 genes in 205 RM patients (82 couples, 41 single females) and 195 age-matched fertile women with no history of miscarriages, and either at least one (Finnish subjects) or three (Estonian subjects) successful pregnancies (Rull et al., 2008b). The screening led to the identification of CGB5 p.Val56Leu (rs72556325; g.1178G > C, position on the genomic sequence relative to mRNA start site), CGB8 p.Arg8Trp (rs72556341; g.806C > T) and p.Pro73Arg (rs72556345; g.1237C > G) mutations.
The current study addressed the presence of the three hCGβ gene mutations in an extended RM case–control sample from Denmark (n = 686). The Danish subjects have been recruited since 1986 at the Danish Recurrent Miscarriage Clinics, Copenhagen and Aalborg, Denmark: 450 RM patients (199 RM couples, 52 single patients; age 20–41 years) with ≥3 consecutive miscarriages before gestational week 20 (>95% during the first trimester) and 236 fertile controls (117 couples and 2 single females) with no history of miscarriages and at least two successful pregnancies. Mutational screening for the three hCGβ mutations (CGB5 p.Val56Leu, CGB8 p.Arg8Trp and p.Pro73Arg) was performed on genomic DNA using either PCR and allele-specific restriction fragment length polymorphism (RFLP) (CGB5), or a combination of long-range and nested PCR followed by RFLP (CGB8) (Rull et al., 2008b) (primers in Table I). Allele-specific restriction of amplified PCR products was performed with FastDigest®AlwNI (CaiI) (Thermo Fisher Scientific, Fermentas, Vilnius, Lithuania) for CGB5 p.Val56Leu and a combination of NcoI, PdiI and DraI (Fermentas) for mutual restriction analysis of CGB8 p.Arg8Trp and p.Pro73Arg (Supplementary data, Fig. S1). All mutation carriers identified by RFLP were confirmed by direct sequencing.
Table I.
Primer sequences used in the study.
| Primer name | Sequence 5′-3′ | Product length |
|---|---|---|
| I. PCR amplification for genotyping p.Arg8Trp, p.Val56Leu and p.Pro73Arg mutations by RFLP | ||
| Amplification of CGB5 gene | ||
| CGB5_F | CAGGAAAGCCTCAAGTAGAGGAG | 1757 bp |
| CGB5_R | CGCTCGACGATGTTTTCTATTTT | |
| Amplification of CGB8 gene | ||
| CGB8_F | CACGCCTGTAATTGTCGGAGGCTGT | 8384 bp |
| CGB8_R | GAAAAGAGAGTGAAGATGGGGGACGAC | |
| CGB8nested_F | CCCGGATAACTTTTCGTATTTTTA | 2544 bp |
| CGB8nested_R | TCCTCAGATCAACTCTCATGGAT | |
| II. PCR amplification and site-directed mutagenesis for hCGβ plasmid construction | ||
| Amplification of hCGβ coding region | ||
| CGB_coding_F | CACCAAGGATGGAGATGTTCC | 523 bp |
| CGB_coding_R | TGCGGATTGAGAAGCCTTTA | |
| Mutagenesisa | ||
| Mut_CGB5_V56L_F | GCCCTGCCTCAGGTGCTGTGCAACTACCGCG | |
| Mut_CGB5_V56L_R | CGCGGTAGTTGCACAGCACCTGAGGCAGGGC | |
| Mut_CGB8_R8W_F | GCCGCTTCGGCCATGGTGCCGCCCCATC | |
| Mut_CGB8_R8W_R | GATGGGGCGGCACCATGGCCGAAGCGGC | |
| Mut_CGB8_P73R_F | CTCCCTGGCTGCCGGCGCGGCGTGAAC | |
| Mut_CGB8_P73R_R | GTTCACGCCGCGCCGGCAGCCAGGGAG | |
| III. PCR amplification for the hCGα + β joint plasmid construction and production of ‘high yield’ hCGb | ||
| Amplification of hCGα coding region | ||
| CGA_coding_F | CGTACGAGCGCCATGGATTA (Pfl23II) | 369 bp |
| CGA_coding_R | ACCGGTTTAAGATTTGTGATAATA (BshTI) | |
| Amplification of FLAG-tagged hCGβ coding region | ||
| CGB_plasmid_F | TTCGAACACCAAGGATGGA (Bsp119I) | 551 bp |
| CGB_plasmid_R | ATTAATTTACTTATCATCATCATCT (VspI) | |
aMutagenesis site is underlined.
bRestriction sites are indicated in italics and respective restriction enzymes given in brackets.
In total, the screening of CGB5 p.Val56Leu, CGB8 p.Arg8Trp and p.Pro73Arg mutations among Northern Europeans was performed in 655 RM patients (281 couples, 93 single patients) and 431 fertile controls with no documented history of RM (117 couples, 197 single patients). In all analyzed RM cases, clinical risk factors known to increase the risk of RM had been excluded. All patients had a normal karyotype tested from peripheral blood lymphocyte cultures. Female patients had normal menstrual cycles and no uterine anomalies (by ultrasonography or hystero-sonogram) or antiphospholipid syndrome. Additionally, the female patients were screened for the presence of thrombophilic mutations [Factor V Leiden, p.Arg506Gln, rs6025 (Bertina et al., 1994); F2, prothrombin G20210A; rs1799963 (Poort et al., 1996)].
The DNA of live born children of mutation carriers was analyzed in order to assess the inheritance of studied mutations (Table II). Altogether nine children (two from a couple with the CGB8 p.R8W carrier and seven from couples with the CGB8 p.P73R carriers) were available for genotyping, performed as described above.
Table II.
Carriers of the hCGβ missense mutationsa and their pregnancy history.
| Mutation | Carrier nationality | Disease statusb | Mutation carrierb | No. of miscarriagesc | No. of childrenc | No. of genotyped children | Children with mutation |
|---|---|---|---|---|---|---|---|
| p.R8W (rs72556341) | Estoniand | RM | Male partner | 5 | 2 | 2 | 1 |
| p.V56L (rs72556325) | Finnishd | RM | Male partner | 3 | 1 | n.a. | n.a |
| p.P73R (rs72556345) | Estoniand | RM | Female partner | 4 + 2 (first, second partner) | 3 (second partner)e | 1 | 0 |
| Danish | RM | Female partner | 3 | 2 | 2 | 0 | |
| Danish | RM | Male partner | 9 (first partner) | 1 (second partner) | n.a. | n.a | |
| Danish | Fertile control | Male partner | 0 | 2 | 2 | 1 | |
| Danish | Fertile control | Male partner | 0 | 2 | 2 | 1 |
n.a., DNA not available.
aIn total 1086 individuals were screened, including 655 RM cases and 431 fertile controls from Estonia, Finland and Denmark.
bDetailed clinical information of mutation carriers is provided in ‘Materials and Methods’ section and Supplemental data, Text S2.
cNumber of miscarriages and live births in a couple with same partners or indicated if otherwise.
dDiscovery mutation carriers reported in Rull et al. (2008b).
ePreterm deliveries (2910 g, gestational week 36; 2488 g, gestational week 37; 2428 g, gestational week 35).
In silico protein sequence and structural analysis
To explore the evolutionary constraints on hCGβ amino acid positions Arg8, Val56 and Pro73, homologs of hCGβ were searched using BLAST (Altschul et al., 1997) with the hCGβ sequence against the National Center for Biotechnology Information (NCBI) non-redundant protein sequence database (ftp://ftp.ncbi.nih.gov/blast/db). Homologs with over 90% sequence identity to each other were excluded. The resulting 37 homologous protein sequences were aligned with ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/) to the protein sequence corresponding to the mature human hCGβ peptide (homology region 1–111 aa; Supplementary data, Table SI and Fig. S2). The sequence of the analyzed mature hCGβ protein does not include the signal peptide (20 aa; codons –1 up to –20) of the full precursor protein (165 aa). Positional variability was assessed by the analysis of sequence logos generated using WebLogo (Crooks et al., 2004).
Structural analysis of amino acid changes in the hCG β-subunit was based on the hCG crystal structure (1hcn) obtained from the Protein Data Bank (www.pdb.org). The fraction of solvent accessible surface (SAS) of a given amino acid in either the isolated hCGβ-subunit or the hCGα/β complex was calculated with Voroprot (Olechnovič et al., 2011) as the following ratio: (SAS of Cα and a side-chain in the context of the protein 3D structure)/(SAS of Cα and a side-chain of the isolated residue).
Molecular dynamics simulations
Molecular dynamics (MD) simulations of the hCG heterodimeric assemblies containing either the wild-type or one of the three mutant hCG β-subunits were performed using GROMACS package, versions 4.5.3 and 4.5.5 (Hess et al., 2008). GROMOS 43a1 united atom force field (van Gunsteren et al., 1996) was employed to model protein atoms. The protein was immersed into a cubic box extending at least by 0.9 nm from the protein. The rest of the box was filled with explicit simple point charge water molecules. Chloride ions were added to neutralize the charge of the protein and periodic boundary conditions were imposed for the simulations. Prior to running the simulation, the system was minimized for 1000 steepest descent iterations and then equilibrated for 20 ps with non-hydrogen protein atoms restrained.
During the simulations, a Berendsen pressure coupling with time constant τ = 0.5 ps, and a temperature coupling using velocity rescaling with stochastic term (τ = 0.1 ps) were applied. Long range electrostatic interactions were calculated using Fast Particle-mesh Ewald electrostatic summation (Darden et al., 1993) with a 0.9 nm cutoff between the short- and long-range interactions. A 1.4 nm cutoff was used for van der Waals interactions. An all-bond constraint was imposed during the simulation using the LINCS algorithm (Hess et al., 1997). The averaged MD structures were subjected to 400 steps of steepest descents optimization, followed by 3000 conjugated gradient minimization steps in vacuo. The secondary structures of α- and β-subunits were analyzed with the DSSP program (Kabsch and Sander, 1983).
Plasmid construction and site-directed mutagenesis for the analysis of hCG assembly
pM2-hCGα plasmid containing hCG α-subunit 2.4 kb minigene (Matzuk and Boime, 1988) was used for transient expression of the hCG α-subunit. For the construction of the reference wild-type hCG β-subunit plasmid, the full hCGβ coding region (identical for CGB5 and CGB8) was amplified from placental cDNA of a normal pregnancy (cDNA kindly provided by Dr Jaana Männik) (Supplementary data, Fig. S3). The amplified DNA fragment including natural hCGβ initiation and stop codons was ligated into a pcDNA3.1D/V5-His-TOPO plasmid (Invitrogen, Paisley, UK) and the construct was verified by sequencing.
The plasmid containing the entire coding region of wild-type CGB5/CGB8 genes was used as a template for the introduction of single base pair mutations g.806C > T, g.1178G > C and g.1237C > G leading to the amino acid substitutions p.Arg8Trp, p.Val56Leu and p.Pro73Arg, respectively (Supplementary data, Fig. S3). Primers for site-directed mutagenesis (Table I) were designed using the PrimerX software (http://www.bioinformatics.org/primerx/) and mutagenesis was performed employing a QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) as per the manufacturer's instructions. Mutations were verified by sequencing.
FLAG-tagged hCGβ isoforms were constructed by the insertion of a FLAG epitope in frame in front of the natural TAA STOP-codon of the CGB5/CGB8 coding region involving either a p.Arg8Trp, p.Val56Leu or p.Pro73Arg mutation. FLAG insertion was validated by sequencing. The C-terminal tagging of a gonadotrophin β-subunit has been demonstrated not to have an effect on the heterodimer assembly or the receptor binding (Sugahara et al., 1995; Wu et al., 1996; Garcia-Campayo and Boime, 2001; Kottler et al., 2010).
hCG expression for the analysis of hCG assembly
The Chinese hamster ovary (CHO) cells (American Type Culture Collection) were maintained in T75 flasks in Dulbecco's Modified Eagle Medium (DMEM) with an F12 nutrient mixture and supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma, Dorset, UK). Cells were transiently co-transfected with the pM2-hCGα and either wild-type or one of the mutated hCGβ plasmids using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's protocol. Four to six hours after transfection, the medium was replaced with the HyClone CHO Utility medium (Thermo Scientific, Waltham, MA, USA) without antibiotics. The media were collected 48 h after transfection and either stored at −80°C for immunoassays or immediately used for co-immunoprecipitation.
Co-immunoprecipitation of FLAG-tagged hCG for the analysis of hCG assembly
Prior to co-immunoprecipitation, the collected media were concentrated by centrifugation using Amicon Ultra-15 10-K filter devices (Millipore, Billerica, MA, USA) according to the manufacturer's instructions. To ensure an equal protein concentration input into the co-immunoprecipitation reaction, the total protein in the concentrated medium was determined using a protein assay kit (Bio-Rad, Hercules, CA, USA), and 250 µg of total protein of each sample was used for precipitation. Each FLAG-tagged hCGβ variant and associated hCGα was precipitated using anti-FLAG antibody conjugated beads and the FLAG-Tagged Protein Immunoprecipitation Kit (Sigma) as per the manufacturer's instructions. Binding was conducted overnight at 4°C and bound FLAG-tagged complexes were eluted under native conditions using the 3× FLAG peptide.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and western blotting of co-immunoprecipitated hCG complexes
Equal volumes (20 µl) of co-immunoprecipitated FLAG-tagged samples were resolved on 4–12% gradient NuPAGE Bis-Tris polyacrylamide gels (Invitrogen) under reducing or non-reducing conditions. Under reducing conditions 10% 2-mercaptoethanol was added to the lithium dodecyl sulfate sample buffer (Invitrogen) and samples were denatured at 95°C for 5 min. Under non-reducing conditions, samples were run in the absence of 2-mercaptoethanol and heat denaturation as this has previously been shown to cause dissociation of the hCG dimers (Ben-Menahem et al., 1999). For molecular weight estimation SeeBlue Plus2 Pre-Stained Standard (Invitrogen) was added on each gel. The separated proteins and protein complexes were transferred to nitrocellulose membranes (Invitrogen) for western blotting. The membranes were blocked with 5% non-fat dry milk for 1 h and then incubated overnight at 4°C either in anti-FLAG antibody (1:5000 dilution; Sigma) for the detection of the both heterodimeric and free FLAG-tagged hCGβ or in antiserum to human gonadotrophin α-subunit (1:500 dilution; NHPP-NIDDK, Torrance, CA, USA) for detection of co-immunoprecipitated hCG α-subunit, and therefore an intact hCG heterodimer only. The membrane was then incubated for 1.5 h at room temperature in appropriate horse-radish peroxidase conjugated secondary antibody (1:10 000 dilution; DAKO, Glostrup, Denmark). Chemiluminescence was probed using the ECL Plus or ECL Prime reagent (GE Healthcare, Buckinghamshire, UK) and detected using an X-ray film (GE Healthcare). Three independent transfections and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) experiments were performed for each mutant.
Immunoassays of heterodimeric hCG and hCG β-subunits
The concentration of the assembled intact hCG was measured with an hCG Human ELISA Kit (Abcam, Cambridge, MA) following the manufacturer's instructions (detection limit <50 pg/ml). The concentration of the total hCGβ was determined with an hCG + β kit on Roche Elecsys 1010 system (in Tartu University Hospital, Tartu, Estonia) with a detection limit of <0.1 mIU/ml and using the same aliquots of media as in the hCG ELISA assay. Two independent transfection experiments with duplicate reactions for each hCGβ variant were performed for hCG expression and analysis of hCG assembly.
Plasmid construction and production of ‘high yield’ hCG for estimating hCG bioactivity and glycosylation
To acquire a high yield of hCG for the bioassay and the enzymatic deglycosylation assay, hCGα and FLAG-tagged hCGβ coding regions were cloned into a mutual expression vector pQMCF-CMV-RSVLTR and expressed using the QMCF Technology (Silla et al., 2005) (Icosagen Cell Factory OÜ) (Supplementary data, Text S1). The full coding sequence of hCGα (CGA gene) was amplified from the placental cDNA of a normal pregnancy (cDNA kindly provided by Dr Jaana Männik) and hCGβ from the pcDNA3.1D/V5-His-TOPO plasmid containing either the wild-type or mutated (p.Arg8Trp, p.Val56Leu or p.Pro73Arg) FLAG-tagged CGB5/CGB8 coding region. PCR amplification was performed using primers listed in Table I and HOT FIREPol DNA polymerase (Solis BioDyne, Tartu, Estonia) according to the manufacturer's instructions. The cDNA variants of both hCG subunits were subsequently cloned into the pQMCF-CMV-RSVLTR vector (Icosagen Cell Factory OÜ) containing two expression cassettes. The construct was verified by sequencing. Additionally, wild-type hCGβ and hCGα were separately cloned into the pQMCF-CMV-RSVLTR expression vector for the production of respective monomers used as negative controls in the bioassay of hCG function.
For high-yield production of hCG variants a CHO-based cell line CHOEBNALT85 (Icosagen Cell Factory OÜ) maintained in chemically defined serum-free media (Invitrogen, Gibko) was used. Transfection was performed by electroporation (230 V and 975 µF) of 6 × 106 cells with 1 µg of expression plasmid containing the coding region of hCGα and either the wild-type or one of the hCGβ mutated variants, or one of the subunits alone. Forty-eight hours after the transfection, cell-conditioned media were collected and frozen at −80°C until being subjected to an ELISA immunoassay, bioassay of hCG function and co-immunoprecipitation of FLAG-tagged hCGβ for the deglycosylation assay.
Co-immunoprecipitation and enzymatic deglycosylation of hCGβ variants
‘High yield’ FLAG-tagged hCGβ variants were precipitated using 300 µl of CHOEBNALT85 cell-conditioned medium and applying a FLAG-Tagged Protein Immunoprecipitation Kit (Sigma) as described above. For the enzymatic deglycosylation assay, 2 µl of precipitated hCGβ samples were denatured and treated either with endoglycosidase H (Endo H) or N-glycosidase F (PNGase F) (New England BioLabs, Hitchin, UK) as per the manufacturer's instructions. The deglycosylation reactions were incubated at 37°C for 3 h and subsequently resolved on 4–12% gradient NuPAGE Bis-Tris polyacrylamide gels (Invitrogen) and detected using the anti-FLAG antibody as described above. For untreated control reactions, the samples were processed similarly but without the addition of the deglycosylation enzymes.
CRE-luciferase reporter gene bioassay of hCG function
The human embryonic kidney 293 (HEK293) (American Type Culture Collection) cell-line stably transfected with the human LH/CG receptor (HEK-hLHR) was maintained in T75 flasks in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin (Sigma) and 0.5 mg/ml geneticin (Invitrogen, Gibco).
Following the replacement of the medium with DMEM without antibiotics, serum or PhenolRed, the HEK-hLHR cells were transfected with a pADNeo2C6-BGL plasmid (kindly provided by Axel Themmen, Erasmus MC, The Netherlands) containing the cAMP-responsive (CRE) firefly luciferase reporter gene using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The following day the medium was removed and cells were stimulated with 100 µl of serially diluted cell-conditioned medium containing CHOEBNALT85-expressed ‘high yield’ wild-type or mutant hCG adjusted for concentration of hCG heterodimer measured with ELISA. Stimulation with a wild-type hCG β-subunit or α-subunit only was used as a negative control. After 5–6 h stimulation at 37°C, 100 µl of buffer from Steadylite™ plus Reporter Gene Assay System (PerkinElmer, MA) was added. Plates were shaken in the dark for 10 min and the CRE-luciferase activity determined using the plate-reading luminometer (Victor, PerkinElmer-Wallac). For calculation of the dosage response, CRE-luciferase activity values were normalized to values of unstimulated cells. All reactions were in triplicate and experiments were repeated five times. The EC50 values (±SD) (EC50 is defined as the concentration of the hormone required to produce 50% of the maximal response) were estimated using the GraphPad Prism 5 software and statistical significance was calculated using a paired t-test.
Results
Prevalence of hCGβ p.Arg8Trp, p.Val56Leu and p.Pro73Arg substitutions
Two of the studied substitutions CGB5 p.Val56Leu (rs72556325) and CGB8 p.Arg8Trp (rs72556341) were each identified in a single heterozygous RM patient among the full screened Northern-European sample set (Estonian, Finnish, Danish, n = 1086; RM cases/controls, n = 655/431) (Table II). The carrier of the CGB5 p.Val56Leu mutation was a Finnish male partner of a couple suffering from secondary RM (three consecutive miscarriages after a live birth) (Rull et al., 2008b). The carrier of the CGB8 p.Arg8Trp mutation was an Estonian male partner of a woman with a series of unexplained RMs (Supplementary data, Text S2). The mutation was inherited by one of two genotyped children of the mutation carrier (Table II).
The CGB8 p.Pro73Arg mutation (rs72556345) was identified with a similar carrier frequency (0.46%) among RM (3/655) and control (2/431) individuals. The five heterozygous mutation carriers represented one Estonian (female partner) and two Danish (one female and one male partner) RM patients and two Danish fertile male partners with no documented miscarriages (Table II; Supplementary data, Text S2). The CGB8 p.Pro73Arg mutation was inherited by two live born children (Danish couples with no miscarriages) out of seven available for genotyping (Table II).
The overall prevalence of the identified hCG beta missense mutations in RM patients (5/655, 0.76%) was higher compared with controls (2/431, 0.46%). The overall inheritance of the studied mutations was slightly lower than expected by chance (50%)-altogether three out of nine genotyped children (33%) were mutation carriers.
Positional context, evolutionary conservation and computational structural analysis of targeted hCGβ substitutions
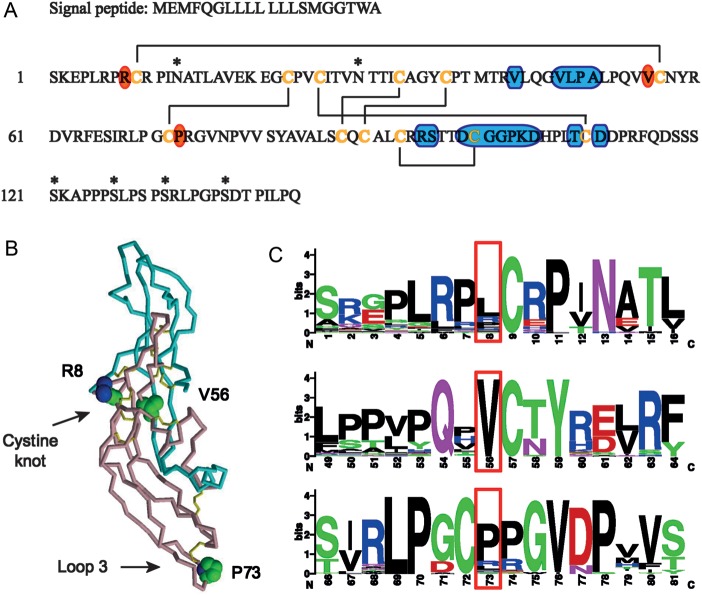
All three hCGβ missense mutations under study are located immediately next to disulfide bond-forming cysteins (Cys9, Cys57 and Cys72; Fig. 1A). The positions of point mutations in the hCGβ protein suggest that they are unlikely to affect hormone glycosylation sites or to directly alter the receptor-binding regions of the β-subunit. Instead, positions Arg8 and Val56 are involved in the central cystine knot structure essential for the hCGβ folding and the heterodimer assembly (Fig. 1B). Valine at position 56 is fully conserved among hCGβ homologs from mammals to fishes, whereas position 8 is less constrained with Leu being even more favored than Arg (Fig. 1C; Supplementary data, Table SI and Fig. S2). Position Pro73 is incorporated in a stable turn of the protein loop 3 that does not directly associate with the hCG α-subunit or the LH/CG receptor (Fig. 1A and B). Therefore, greater variation in amino acid usage is tolerated at position 73. Although Pro is the most frequent choice at this site, hCGβ protein homologs also frequently have Arg or Leu (Fig. 1C).
Figure 1.
Structural and evolutionary context of non-synonymous mutations in the hCGβ protein. (A) Amino acid sequence of a signal peptide (20 aa) and mature protein (145 aa) of identical hCGβ encoded by CGB5 and CGB8 genes (NCBI; NP_149032.1 for CGB5, NP_149439.1 for CGB8). Six disulfide bonds found in the crystal structure of the hCGβ protein (Lapthorn et al., 1994) are drawn with lines connecting respective disulfide bond-forming cysteine residues (orange letters). Glycosylation sites are marked by asterisks. Regions interacting with the LH/CG receptor have been identified by extrapolating the structural model for FSH receptor (Fan and Hendrickson, 2005) and are indicated on the blue background. The positions Arg8, Val56 and Pro73 in hCGβ, targeted in this study have been indicated with an orange background. (B) Three-dimensional (3D) structure of the assembled hCG molecule based on Protein Data Bank (PDB; http://www.pdb.org) entry 1hcn. The structure of the hCG α-subunit is depicted in blue, β-subunit in pink and disulfide bonds in yellow. The side-chains of amino acids Arg8, Val56 and Pro73 in hCGβ are shown in the space-filling representation. (C) Protein sequence logos surrounding hCGβ positions Arg8, Val56 and Pro73 (in red boxes). The letter size is proportional to the degree of conservation among hCGβ homologs (Supplemental Data, Table SI and Fig. S2).
In addition, a computational structural analysis was applied to estimate the SAS for the hCGβ positions 8, 56 and 73 using the published crystal structure of the hCG dimer (Protein Data Bank; 1hcn) (Lapthorn et al., 1994). Position Val56, located in the cystine knot structure, is largely buried within the hCGα/β heterodimer complex with only 3% exposed to the solvent compared with 46% in the case of the unassembled β-monomer (Fig. 2). Therefore, amino acid substitutions at this position might be expected to have a minor effect on the β-subunit alone but a more pronounced effect on the hCGα/β complex. In particular, the replacement of valine with leucine, which has a larger side-chain, could presumably hinder the formation/stability of the heterodimer.
Figure 2.
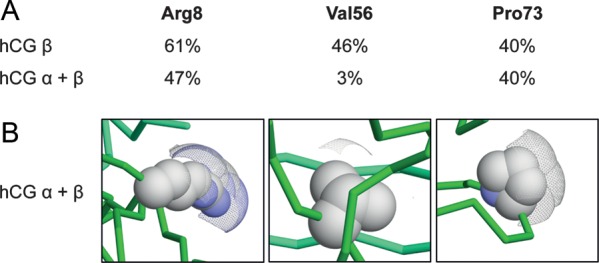
Solvent accessible surfaces of hCGβ Arg8, Val56 and Pro73 in hCGβ alone and in hCGα/β. (A) The percentage of solvent accessible surface areas in hCGβ alone and in the intact hCGα/β complex. (B) Visual representation of SAS areas of hCGβ Arg8, Val56 and Pro73 residues in the hCGα/β complex using Voroprot (Olechnovič et al., 2011). Solvent accessible surface areas are displayed as a mesh.
The hCGβ position Pro73 is localized within the solvent-exposed hCGβ loop and does not directly interact with the hCG α-subunit. Thus, the solvent accessibility of Pro73 remains unaffected after the formation of the hCGα/β complex (Fig. 2). Although the hCGβ position Arg8 is located in the structurally conserved cystine knot of hCGβ, the surface of position 8 also remains exposed to the solvent upon interaction with the α-subunit (47% in hCG versus 61% in hCGβ; Fig. 2). Therefore, neither of these two mutations would be expected to have a major effect on the α/β assembly.
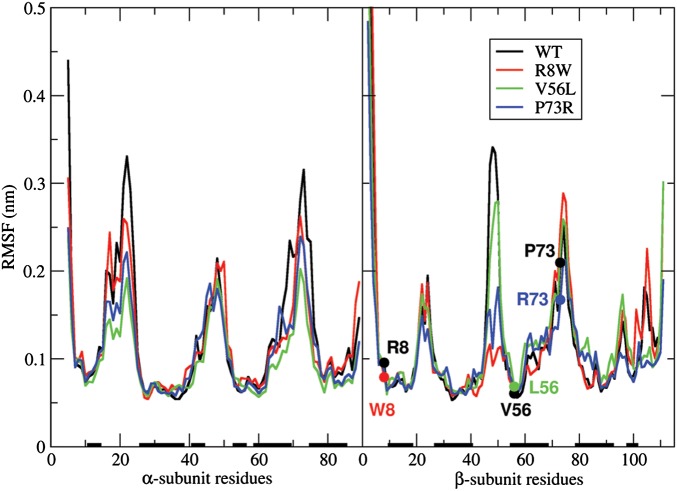
MD simulations
The impact of β-subunit mutations on the dynamics of the hCG heterodimer was explored by performing 30 ns MD simulations for the wild-type and mutant hCGs. MD simulations showed that the α/β heterodimer is very dynamic, partly because of the large amount of loops (∼50% of the residues). Interestingly, mutations of the β-subunit appeared to have an impact on the α-chain as well (Table III). To explore the position-dependent flexibility of the hCG heterodimer, root-mean-square fluctuations (RMSF) of Cα carbons with respect to the averaged and minimized structures (in the 10–30-ns interval) were computed for all four hCG variants (Fig. 3). In general, the observed changes did not reveal dramatic structural rearrangements. However, the data in Fig. 3, supported by the structural analysis of MD-generated structures (Supplementary data, Text S3 and Fig. S4) show that all three mutants exhibited less flexibility than the wild-type hCG. This can be explained by the formation of additional hydrogen bonds between the spatially neighboring loops (notably in p.P73R; Supplementary data, Text S3), and the increase of the amount of β-strands in the structure in the case of the p.V56L and p.R8W mutations. Overall, compared with the wild-type, the p.V56L assembly behaved most differently, followed by p.P73R, while the behavior of the p.R8W differed the least.
Table III.
Cα atom root-mean-square deviation (RMSD) (nm) between the X-ray structure and the 10–30 ns averaged/minimized structures resulting from the MD simulations.
| α | β | α/β | |
|---|---|---|---|
| WT | 0.253 | 0.287 | 0.303 |
| p.R8W | 0.179 | 0.304 | 0.278 |
| p.V56L | 0.266 | 0.326 | 0.346 |
| p.P73R | 0.304 | 0.288 | 0.320 |
The values (nm) are shown separately for the individual subunits and for the assembled hCG heterodimer.
Figure 3.
RMSF of the Cα carbons with respect to the average MD structures. Wild-type and mutated residues at positions under study are indicated with filled circles and labels. Secondary structure elements (α-helices or β-strands) in the WT X-ray structure are represented as black bars along the horizontal axis.
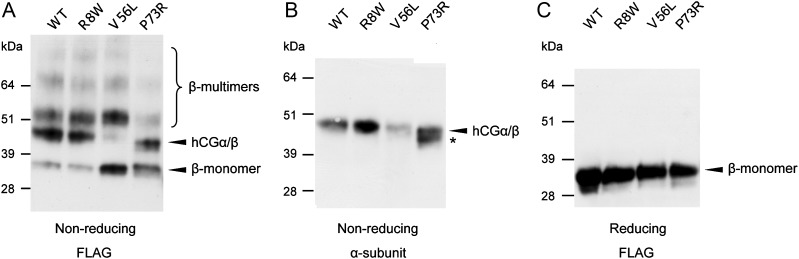
hCGβ p.Val56Leu and p.Pro73Arg substitutions affect assembly of the hCGα/β dimer in in vitro assays
Co-immunoprecipitation techniques were employed to assess the effect of hCGβ p.Arg8Trp, p.Val56Leu and p.Pro73Arg mutations on the assembly of the hCG dimer. FLAG-tagged wild-type and mutant hCG β-subunits were co-expressed with un-tagged α-subunits in CHO cell lines. Using the FLAG-tagged epitope, secreted-free hCGβ monomers and heterodimeric hCG were immunoprecipitated from the cell culture media and subjected to western blot testing for detection with either the anti-FLAG antibody, which probed for both hCGβ monomers and assembled α/β heterodimers (Fig. 4A and C), or via antiserum against the hCG α-subunit that specifically visualizes the assembled heterodimer hormone only (Fig. 4B). Under non-reducing conditions a ∼47 kDa hCG heterodimer was identified with both antiserum against hCGα and anti-FLAG antibody (Fig. 4A and B), whereas a ∼34 kDa hCGβ monomer was visualized via the anti-FLAG antibody in reducing and non-reducing conditions (Fig. 4A and C). In case of the p.Val56Leu substitution, the proportion of β-subunits incorporated into α/β heterodimers relative to the freely retained β-monomers was notably reduced compared with the wild-type (Fig. 4A), indicating a decreased capability of p.Val56Leu β-subunits to assemble into the heterodimer.
Figure 4.
Co-immunoprecipitation and western blot analysis of FLAG-tagged hCGβ variants co-expressed with hCGα in CHO cells. FLAG-tagged hCGβ monomers and associated complexes were immunoprecipitated from CHO cell culture media using anti-FLAG antibody-conjugated beads and separated by SDS–PAGE under non-reducing (A and B) or reducing (C) conditions. (A and C) Free and heterodimeric assembled FLAG-tagged hCGβ was detected using the anti-FLAG antibody. (B) A heterodimeric hCG was specifically visualized using antiserum to the hCG α-subunit. Bands corresponding to the heterodimeric hCG and unassembled hCGβ monomers are indicated by arrowheads; bands corresponding to β-subunit-specific multimeric complexes are indicated with a bracket. The data are drawn from the same experiment and they are representative of three independent co-immunoprecipitation experiments. An alternative α/β complex with the hCGβ conformational isoform caused by the p.Pro73Arg mutation is indicated by an asterisk.
The hCGβ p.Pro73Arg mutant gave rise to two alternative hCGβ isoforms, one corresponding to the molecular weight of the wild-type hCGβ (∼34 kDa) and an additional variant with ∼2 kDa lower molecular weight (Fig. 4A). Both isoforms were being assembled into the hCG dimer with approximately equal efficiency (Fig. 4A and B). In the case of hCGβ p.Arg8Trp substitution, the applied SDS–PAGE and western blot analysis did not reveal detectable differences in the fraction of free hCGβ or in the assembly of intact hCG compared with the wild-type variant (Fig. 4).
Several additional high-molecular-weight protein complexes were visualized by the anti-FLAG antibody under non-reducing experimental conditions (Fig. 4A). As these bands were neither visualized via antisera against the α-subunit incorporated into intact hCG (Fig. 4B) nor detected under reducing conditions disrupting all disulfide bonds (Fig. 4C), we speculate that they may represent covalent multimeric hCGβ-specific complexes shown to be secreted from the cells, especially in the presence of mutations that affect the β-subunit folding pathway (Bedows et al., 1994; Feng et al., 1995, 1996).
In the SDS–PAGE run under reducing conditions that causes dissociation of hCG dimers and disruption of disulfide bonds, all tested hCGβ variants collapsed into one major (∼34 kDa) and one minor (∼31 kDa) isoform (Fig. 4C), previously shown to contain either two or one N-linked oligosaccharide chain, respectively (Matzuk et al., 1987). No evidence of the effect of the studied substitutions on the glycosylation pattern of the hCGβ protein was seen in the enzymatic deglycosylation assay using either Endo H or PNGase F deglycosidases (Supplementary data, Fig. S5).
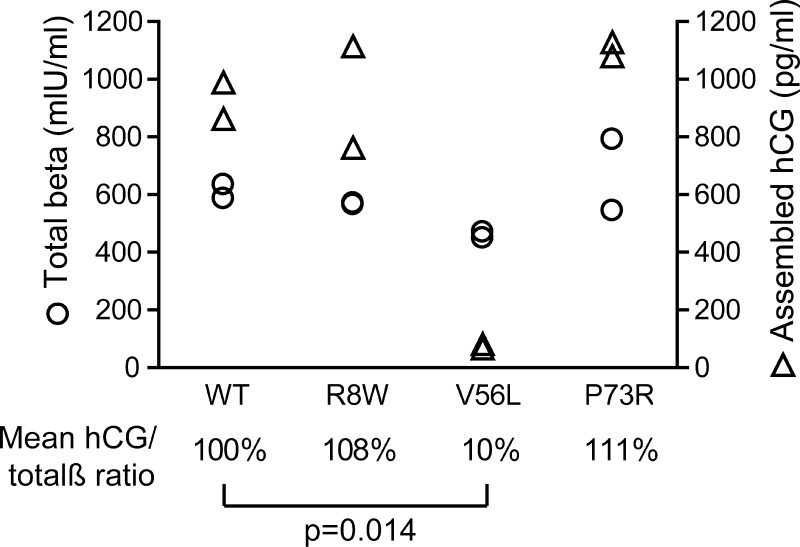
Quantitative immunoassays confirm the deficient assembly of intact hCG in the case of the hCGβ p.Val56Leu variant
The efficiency of hCG dimerization was estimated using the ratio of assembled intact hCG to the total amount of the secreted hCGβ subunit in the cell culture media of the transfected CHO cells. In case of the hCGβ p.Val56Leu mutation, on average only 10% of dimers were assembled compared with the wild-type ( = 100%) (Student's t-test, P = 0.014) (Fig. 5). The outcome of the immunoassay is consistent with the co-immunoprecipitation results on the p.Val56Leu mutation reported above. For hCGβ mutants p.Arg8Trp and p.Pro73Arg, the fraction of the secreted hCG β-subunit assembled into intact hormone did not significantly differ from the wild-type.
Figure 5.
Effect of hCGβ mutations on the formation of the hCG heterodimer as determined by immunoassays. The total β-subunit (free β-monomers + β-subunit fraction assembled into the hCGα/β heterodimer) in CHO cell culture media was quantified on the Elecsys 1010 system and the quantity of assembled α/β heterodimer only was determined with the intact hCG-specific ELISA assay. The data points represent the measurements of duplicate independent transfections. For each hCGβ mutation, the mean ratio of the assembled to the total β-subunits was calculated and compared with the wild-type (=100%). The P-value was calculated using Student's t-test. Circles indicate the concentration of the total β-subunit (mIU/ml) and triangles the concentration of assembled heterodimeric hCG (pg/ml) in the medium.
The hCGβ p.Val56Leu variant exhibits an increased bioactivity upon binding to the human LH/CG receptor in the in vitro bioassay
The QMCF technology was applied to produce ‘high yield’ intact hCG reaching a 10 times higher concentration in the medium compared with the expression of the hCG heterodimer with the conventional transient co-transfection of the α- and β-subunits. Serial dilutions of the CHOEBNALT85 cell-conditioned media adjusted for the concentration of heterodimeric hCG were used to stimulate HEK-hLHR cells, and the cAMP signaling response was measured. The cAMP response to stimulation with CGB5 p.Val56Leu was significantly more sensitive than to the wild-type hCG (Fig. 6), exhibiting a half-maximal response EC50 of 2.50 ± 0.81 pg/ml compared with the wild-type hCG EC50 of 11.41 ± 2.32 pg/ml (P < 0.0013). No significant differences were observed between the wild-type hCGβ and variants carrying either mutation CGB8 p.Arg8Trp or CGB8 p.Pro73Arg (EC50 of 13.51 ± 3.47 pg/ml, P = 0.131; 8.35 ± 3.54 pg/ml, P = 0.053, respectively) (Fig. 6). The results were confirmed by the same experiments performed on CHO cells transiently transfected with the human LH/CG receptor (data not shown).
Figure 6.
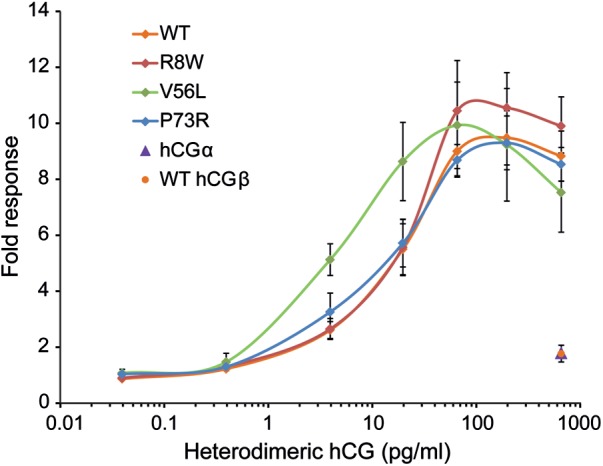
Bioactivity of hCGβ variants measured as an hLH/CG receptor-mediated cAMP signaling response to stimulation with the dosage gradient of heterodimeric wild-type and mutant hCG preparations. Stimulation with wild-type hCGβ or hCGα monomers was used as a negative control. The fold response is given as a ratio of the CRE luciferase activity to unstimulated cells. The data are the mean ± SD of five independent experiments.
Discussion
We have previously reported singleton heterozygous carriers of mutations CGB5 p.Val56Leu and CGB8 p.Arg8Trp, p.Pro73Arg among RM patients (Rull et al., 2008b). In the current study, we addressed the effect of the identified substitutions on hCG synthesis and function by combining in silico comparative genomics and computational structural analysis with in vitro experiments. For the mutation CGB5 p.Val56Leu in silico analyses predicted and cell-culture experiments consistently confirmed the effect of the substitution on the intact hCG α/β dimer assembly, but also on the bioactivity upon binding to the LH/CG receptor and stimulation of the cAMP response. The CGB8 p.Pro73Arg mutation was found to alter the hCGβ conformation and the heterodimer assembly. For the third mutation studied (CGB8 p.Arg8Trp) the chosen experimental design did not detect major structural or functional consequences, which was in agreement with the computational predictions.
Among the three studied substitutions, hCGβ p.Val56Leu had a substantial effect on the assembly of the bioactive hormone. The co-immunoprecipitation assay and quantitative immunoassays demonstrated that only 10% of the secreted hCGβ carrying the p.Val56Leu mutation was incorporated into the intact heterodimeric hCG compared with the wild-type. The effect of the p.Val56Leu substitution can be explained by its location in the core part of the hCGβ structure immediately next to Cys57, which forms one of the disulfide bonds (Cys9-Cys57) in the cystine knot (Fig. 1A) (Lapthorn et al., 1994). The cystine knot is a highly conserved structural feature not only in hCG α- and β-subunits, but also among growth factors such as transforming growth factor-β2, NGF and platelet-derived growth factor-BB (Murray-Rust et al., 1993; Lapthorn et al., 1994). Disruption of the Cys9-Cys57 bond has previously been shown to give rise to folding and assembly deficient hCGβ protein (Bedows et al., 1994; Mishra et al., 2003). Although not directly involved in the disulfide bond formation, Val56 becomes almost completely buried due to the interaction with the α-subunit when the intact hCG is formed (Figs 1B and 2). Therefore, it was expected that any substitution at this position would destabilize the assembled hormone. The high evolutionary conservation of Val56 among hCGβ homologs (Fig. 1C) (including human TSHβ and LHβ; except for Thr in FSHβ) further underlines its importance in the formation of a functional hormone (Pierce and Parsons, 1981).
Surprisingly, in addition to posing a hindrance on heterodimer formation, the p.Val56Leu mutation also modified the bioactivity of the assembled hormone (Fig. 6). The half-maximal response of the heterodimer carrying the p.Val56Leu mutation was increased 4.6-fold compared with the wild-type when binding to the LH/CG receptor and stimulating the cAMP response. It has been previously observed that in addition to its role in the interaction of the subunits the region involving the Cys9-Cys57 bond also exhibits the highest potency toward the LH/CG receptor (Mishra et al., 2001). The MD simulations with the hCGβ p.Val56Leu mutation predicted slight changes in the structural conformation of the hCGβ and altered dynamics for both hCG subunits and the heterodimer (Table III; Supplementary data, Text S3 and Fig. S4), providing a possible explanation for the increased potency of the assembled hormone. As a consequence, the shortage of the produced dimeric hormone (10% compared with the wild-type) may be partly or fully compensated for by its increased bioactivity.
The CGB8 p.Pro73Arg mutation is located near the top of the hCGβ loop 3 not interacting directly with the α-subunit or the LH/CG receptor (Fig. 1A and B). Interestingly, this substitution resulted in the formation of two alternative conformational variants of hCGβ (wild-type and ∼2 kDa smaller isoforms). These two β-monomers have a similar glycosylation pattern (Supplementary data, Fig. S5), were secreted in approximately equal amounts and were both assembly-competent (Fig. 4B). Pro73 is located next to Cys72, which forms one of the five disulfide bonds, Cys23–Cys72. Disruption of this bond has been demonstrated to affect the folding pathway of the β-subunit by destabilizing a conformational intermediate of the hCGβ protein. Consequently, an additional isoform lacking the Cys23–Cys72 bond and exhibiting a difference of 2 kDa in size on SDS–PAGE was secreted into the medium (Bedows et al., 1993, 1994). It has also been reported that the substitution of Pro73 with Gly slows down the final folding of the hCGβ subunit (Feng et al., 1996). Although changes in the quality of disulfide bonding and kinetics of the hCGβ folding may also be speculated in the case of the p.Pro73Arg mutation, it was not addressed directly in the current study. Importantly, the overall functional characteristics of the p.Pro73Arg heterodimeric isoforms remain comparable with the wild-type (Fig. 6), pointing to the functional neutrality of this mutation. Concurrently, the p.Pro73Arg mutation can be found with the same carrier frequency among RM patients and controls (0.46%) and it is inherited by two out of seven children of the p.Pro73Arg mutation carriers (Table II).
The position of the third mutation, hCGβ p.Arg8Trp is located in the conserved hCGβ cystine knot structure but it is largely exposed to the solvent and exhibits low evolutionary conservation (Figs 1B and C and 2). No major structural or functional effects were observed for this mutation in performed wetlab and in silico experiments.
It is noteworthy that, apart from apparently population-specific p.Val79Met (4.2% allele frequency in Omaha, USA) (Miller-Lindholm et al., 1999; Jiang et al., 2004), only a few other rare missense mutations have been identified in the CGB5 and CGB8 genes worldwide. In CGB8, p.Val29Ile was found in Estonians (9 heterozygotes/194 subjects) and Finns (1 heterozygote/185 individuals) (Rull et al., 2008b). In CGB5, p.Arg6Gln was identified in a single Han Chinese (among 25 screened subjects) and p.Asp117Ala in two African Mandekalu (n = 23) samples (Hallast et al., 2005), but no clinical information is available on these mutation carriers. The current study identified heterozygous carriers of rare hCGβ mutations among five North-European RM patients and two controls with proven fertility. Lack of common missense mutations in the most abundantly expressed genes CGB5 and CGB8, which contribute 62–82% to the pool of hCGβ mRNA (Miller-Lindholm et al., 1997; Rull and Laan, 2005), emphasizes the requirement of full transcription and production of functional protein from these genes for a successful pregnancy outcome. Interestingly, in the studied families, the wild-type CGB8 gene variants had a small preference to be inherited by the next generation (only three heterozygous mutation carriers out of nine children tested) (Table II). Furthermore, no individuals homozygous for any hCGβ mutations have been identified so far, which may indicate either an insufficient sample size in the conducted studies or that such genotypes result in a complete pregnancy failure. In summary, the accumulated data indicate that only mutations with neutral or mild functional consequences might be tolerated in the major hCG beta coding genes CGB5 and CGB8.
Supplementary data
Supplementary data are available at http://molehr.oxfordjournals.org/
Authors’ roles
M.L., I.T.H., L.N. and K.R.: conceived and designed the laboratory experiments . Č.V. and V.K. designed and performed the computational structural analysis. Recruited the study subjects: K.R., O.B.C. and R.S. Performed experiments: L.N., K.R. and G.K. Assisted in experimental performance: K.C.J. and H.P. Supervised the experimental conduct: I.T.H. and M.L. Analyzed and interpreted data: L.N., K.R., Č.V., V.K., K.C.J., H.P., I.T.H. and M.L. Contributed reagents/materials/analysis tools: I.T.H., M.L., Č.V. and O.B.C. Wrote the paper: L.N., M.L., Č.V., K.R. and V.K. The rest of the authors revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by the Wellcome Trust Programme grant (grant number 082101/Z/07/Z) to I.T.H., Howard Hughes Medical Institute (HHMI) International Scholarship Grant (55005617), Wellcome Trust International Senior Research Fellowship in Biomedical Science in Central Europe (070191/Z/03/A), Estonian Science Foundation (7471, 9030) and Estonian Ministry of Education and Science core grant (SF0180022s12) to M.L., HHMI International Scholarship grant (55005627) to Č.V., Estonian Women in Science Award from European Commission grant (205419) (ECOGENE) for Estonian Biocentre to K.R., research grant from the Research Council of the County of Nordjylland to O.B.C., financing from FP7-REGPOT-2009-1 project (245721) (MoBiLi) to V.K., stipends from Ernst Jaakson Memorial Fund, Kristjan Jaak Foundation and Estonian Students Foundation to L.N. and a PhD grant from The University Hospital Copenhagen, Rigshospitalet, Denmark to R.S. Funding to pay the Open Access publication charges for this article was provided by Wellcome Trust International Senior Research Fellowship in Biomedical Science in Central Europe 070191/Z/03/A to M.L.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
We are thankful to Frederic Jean-Alphonse and Aylin Hanyaloglu for advice on in vitro experiments, Mart Ustav and Andres Tover for the technical advice in the QMCF technology, and Urve Toots for her contribution in the production of ‘high yield’ hCG. We acknowledge all the participants of Estonian, Finnish and Danish RM studies.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedows E, Huth JR, Suganuma N, Bartels CF, Boime I, Ruddon RW. Disulfide bond mutations affect the folding of the human chorionic gonadotropin-beta subunit in transfected Chinese hamster ovary cells. J Biol Chem. 1993;268:11655–11662. [PubMed] [Google Scholar]
- Bedows E, Norton SE, Huth JR, Suganuma N, Boime I, Ruddon RW. Misfolded human chorionic gonadotropin beta subunits are secreted from transfected Chinese hamster ovary cells. J Biol Chem. 1994;269:10574–10580. [PubMed] [Google Scholar]
- Ben-Menahem D, Hyde R, Pixley M, Berger P, Boime I. Synthesis of multi-subunit domain gonadotropin complexes: a model for alpha/beta heterodimer formation. Biochemistry. 1999;38:15070–15077. doi: 10.1021/bi991510c. [DOI] [PubMed] [Google Scholar]
- Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, van der Velden PA, Reitsma PH. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- Bricker L, Farquharson RG. Types of pregnancy loss in recurrent miscarriage: implications for research and clinical practice. Hum Reprod. 2002;17:1345–1350. doi: 10.1093/humrep/17.5.1345. [DOI] [PubMed] [Google Scholar]
- Christiansen OB, Mathiesen O, Lauritsen JG, Grunnet N. Idiopathic recurrent spontaneous abortion. Evidence of a familial predisposition. Acta Obstet Gynecol Scand. 1990;69:597–601. doi: 10.3109/00016349009028702. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darden T, York D, Pedersen L. Particle mesh Ewald: an N log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Matzuk MM, Mountjoy K, Bedows E, Ruddon RW, Boime I. The asparagine-linked oligosaccharides of the human chorionic gonadotropin beta subunit facilitate correct disulfide bond pairing. J Biol Chem. 1995;270:11851–11859. doi: 10.1074/jbc.270.20.11851. [DOI] [PubMed] [Google Scholar]
- Feng W, Bedows E, Norton SE, Ruddon RW. Novel covalent chaperone complexes associated with human chorionic gonadotropin beta subunit folding intermediates. J Biol Chem. 1996;271:18543–18548. doi: 10.1074/jbc.271.31.18543. [DOI] [PubMed] [Google Scholar]
- Garcia-Campayo V, Boime I. Novel recombinant gonadotropins. Trends Endocrinol Metab. 2001;12:72–77. doi: 10.1016/s1043-2760(00)00338-6. [DOI] [PubMed] [Google Scholar]
- Guibourdenche J, Handschuh K, Tsatsaris V, Gerbaud P, Leguy MC, Muller F, Brion DE, Fournier T. Hyperglycosylated hCG is a marker of early human trophoblast invasion. J Clin Endocrinol Metab. 2010;95:E240–E244. doi: 10.1210/jc.2010-0138. [DOI] [PubMed] [Google Scholar]
- Hallast P, Nagirnaja L, Margus T, Laan M. Segmental duplications and gene conversion: human luteinizing hormone/chorionic gonadotropin beta gene cluster. Genome Res. 2005;15:1535–1546. doi: 10.1101/gr.4270505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallast P, Saarela J, Palotie A, Laan M. High divergence in primate-specific duplicated regions: human and chimpanzee chorionic gonadotropin beta genes. BMC Evol Biol. 2008;8:195. doi: 10.1186/1471-2148-8-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL. Placental histology and the production of human choriogonadotrophin and its subunits in pregnancy. Br J Obstet Gynaecol. 1988;95:1268–1275. doi: 10.1111/j.1471-0528.1988.tb06817.x. [DOI] [PubMed] [Google Scholar]
- Hess B, Bekker H, Berendsen HJC, Fraaije JGEM. LINCS: a linear constraint solver for molecular simulations. J Comput Chem. 1997;18:1463–1472. [Google Scholar]
- Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Korenbrot CC, Jaffe RB. HCG binding and stimulation of testosterone biosynthesis in the human fetal testis. J Clin Endocrinol Metab. 1977;44:963–967. doi: 10.1210/jcem-44-5-963. [DOI] [PubMed] [Google Scholar]
- Jiang M, Savontaus ML, Simonsen H, Williamson C, Mullenbach R, Gromoll J, Terwort N, Alevizaki M, Huhtaniemi I. Absence of the genetic variant Val79Met in human chorionic gonadotropin-beta gene 5 in five European populations. Mol Hum Reprod. 2004;10:763–766. doi: 10.1093/molehr/gah098. [DOI] [PubMed] [Google Scholar]
- Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kajihara T, Uchino S, Suzuki M, Itakura A, Brosens JJ, Ishihara O. Human chorionic gonadotropin confers resistance to oxidative stress-induced apoptosis in decidualizing human endometrial stromal cells. Fertil Steril. 2010;95:1302–1307. doi: 10.1016/j.fertnstert.2010.05.048. [DOI] [PubMed] [Google Scholar]
- Kayisli UA, Selam B, Guzeloglu-Kayisli O, Demir R, Arici A. Human chorionic gonadotropin contributes to maternal immunotolerance and endometrial apoptosis by regulating Fas-Fas ligand system. J Immunol. 2003;171:2305–2313. doi: 10.4049/jimmunol.171.5.2305. [DOI] [PubMed] [Google Scholar]
- Kolte AM, Nielsen HS, Moltke I, Degn B, Pedersen B, Sunde L, Nielsen FC, Christiansen OB. A genome-wide scan in affected sibling pairs with idiopathic recurrent miscarriage suggests genetic linkage. Mol Hum Reprod. 2011;17:379–385. doi: 10.1093/molehr/gar003. [DOI] [PubMed] [Google Scholar]
- Korhonen J, Stenman UH, Ylostalo P. Serum human chorionic gonadotropin dynamics during spontaneous resolution of ectopic pregnancy. Fertil Steril. 1994;61:632–636. doi: 10.1016/s0015-0282(16)56638-2. [DOI] [PubMed] [Google Scholar]
- Kottler ML, Chou YY, Chabre O, Richard N, Polge C, Brailly-Tabard S, Chanson P, Guiochon-Mantel A, Huhtaniemi I, Young J. A new FSHbeta mutation in a 29-year-old woman with primary amenorrhea and isolated FSH deficiency: functional characterization and ovarian response to human recombinant FSH. Eur J Endocrinol. 2010;162:633–641. doi: 10.1530/EJE-09-0648. [DOI] [PubMed] [Google Scholar]
- Lapthorn AJ, Harris DC, Littlejohn A, Lustbader JW, Canfield RE, Machin KJ, Morgan FJ, Isaacs NW. Crystal structure of human chorionic gonadotropin. Nature. 1994;369:455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- Lopata A, Hay DL. The potential of early human embryos to form blastocysts, hatch from their zona and secrete HCG in culture. Hum Reprod. 1989;4:87–94. doi: 10.1093/humrep/4.suppl_1.87. [DOI] [PubMed] [Google Scholar]
- Maston GA, Ruvolo M. Chorionic gonadotropin has a recent origin within primates and an evolutionary history of selection. Mol Biol Evol. 2002;19:320–335. doi: 10.1093/oxfordjournals.molbev.a004085. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Krieger M, Corless CL, Boime I. Effects of preventing O-glycosylation on the secretion of human chorionic gonadotropin in Chinese hamster ovary cells. Proc Natl Acad Sci USA. 1987;84:6354–6358. doi: 10.1073/pnas.84.18.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM, Boime I. The role of the asparagine-linked oligosaccharides of the alpha subunit in the secretion and assembly of human chorionic gonadotrophin. J Cell Biol. 1988;106:1049–1059. doi: 10.1083/jcb.106.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Lindholm AK, LaBenz CJ, Ramey J, Bedows E, Ruddon RW. Human chorionic gonadotropin-beta gene expression in first trimester placenta. Endocrinology. 1997;138:5459–5465. doi: 10.1210/endo.138.12.5618. [DOI] [PubMed] [Google Scholar]
- Miller-Lindholm AK, Bedows E, Bartels CF, Ramey J, Maclin V, Ruddon RW. A naturally occurring genetic variant in the human chorionic gonadotropin-beta gene 5 is assembly inefficient. Endocrinology. 1999;140:3496–3506. doi: 10.1210/endo.140.8.6915. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Mahale SD, Iyer KS. Mapping the receptor binding regions of human chorionic gonadotropin (hCG) using disulfide peptides of its beta-subunit: possible involvement of the disulfide bonds Cys(9)-Cys(57) and Cys(23)-Cys(72) in receptor binding of the hormone. J Pept Res. 2001;58:17–26. doi: 10.1034/j.1399-3011.2001.00866.x. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Mahale SD, Iyer KS. Disulfide bonds Cys(9)-Cys(57), Cys(34)-Cys(88) and Cys(38)-Cys(90) of the β-subunit of human chorionic gonadotropin are crucial for heterodimer formation with the α-subunit: experimental evidence for the conclusions from the crystal structure of hCG. Biochim Biophys Acta. 2003;1645:49–55.. doi: 10.1016/s1570-9639(02)00501-0. [DOI] [PubMed] [Google Scholar]
- Morgan FJ, Birken S, Canfield RE. The amino acid sequence of human chorionic gonadotropin. The alpha subunit and beta subunit. J Biol Chem. 1975;250:5247–5258. [PubMed] [Google Scholar]
- Murray-Rust J, McDonald NQ, Blundell TL, Hosang M, Oefner C, Winkler F, Bradshaw RA. Topological similarities in TGF-beta 2, PDGF-BB and NGF define a superfamily of polypeptide growth factors. Structure. 1993;1:153–159. doi: 10.1016/0969-2126(93)90029-g. [DOI] [PubMed] [Google Scholar]
- Nagirnaja L, Rull K, Uuskula L, Hallast P, Grigorova M, Laan M. Genomics and genetics of gonadotropin beta-subunit genes: unique FSHB and duplicated LHB/CGB loci. Mol Cell Endocrinol. 2010;329:4–16. doi: 10.1016/j.mce.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olechnovič K, Margelevicius M, Venclovas C. Voroprot: an interactive tool for the analysis and visualization of complex geometric features of protein structure. Bioinformatics. 2011;27:723–724. doi: 10.1093/bioinformatics/btq720. [DOI] [PubMed] [Google Scholar]
- Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Policastro PF, Daniels-McQueen S, Carle G, Boime I. A map of the hCG beta-LH beta gene cluster. J Biol Chem. 1986;261:5907–5916. [PubMed] [Google Scholar]
- Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698–3703. [PubMed] [Google Scholar]
- Rai R, Regan L. Recurrent miscarriage. Lancet. 2006;368:601–611. doi: 10.1016/S0140-6736(06)69204-0. [DOI] [PubMed] [Google Scholar]
- Rull K, Laan M. Expression of beta-subunit of HCG genes during normal and failed pregnancy. Hum Reprod. 2005;20:3360–3368. doi: 10.1093/humrep/dei261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rull K, Hallast P, Uuskula L, Jackson J, Punab M, Salumets A, Campbell RK, Laan M. Fine-scale quantification of HCG beta gene transcription in human trophoblastic and non-malignant non-trophoblastic tissues. Mol Hum Reprod. 2008a;14:23–31. doi: 10.1093/molehr/gam082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rull K, Nagirnaja L, Ulander VM, Kelgo P, Margus T, Kaare M, Aittomaki K, Laan M. Chorionic gonadotropin beta-gene variants are associated with recurrent miscarriage in two European populations. J Clin Endocrinol Metab. 2008b;93:4697–4706. doi: 10.1210/jc.2008-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silla T, Hääl I, Geimanen J, Janikson K, Abroi A, Ustav E, Ustav M. Episomal maintenance of plasmids with hybrid origins in mouse cells. J Virol. 2005;79:15277–15288. doi: 10.1128/JVI.79.24.15277-15288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara T, Pixley MR, Minami S, Perlas E, Ben-Menahem D, Hsueh AJ, Boime I. Biosynthesis of a biologically active single peptide chain containing the human common alpha and chorionic gonadotropin beta subunits in tandem. Proc Natl Acad Sci USA. 1995;92:2041–2045. doi: 10.1073/pnas.92.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsampalas M, Gridelet V, Berndt S, Foidart JM, Geenen V, Perrier d'Hauterive S. Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. J Reprod Immunol. 2010;85:93–98. doi: 10.1016/j.jri.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Uusküla L, Rull K, Nagirnaja L, Laan M. Methylation allelic polymorphism (MAP) in chorionic gonadotropin beta5 (CGB5) and its association with pregnancy success. J Clin Endocrinol Metab. 2010;96:E199–E207. doi: 10.1210/jc.2010-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gunsteren WF, Billeter SR, Eising AA, Hünenberger PH, Krüger P, Mark AE, Scott WRP, Tironi IG. Biomolecular Simulation: The GROMOS96 Manual and User Guide. Zürich, Germany: Vdf Hochschulverlag AG an der ETH Zürich; 1996. [Google Scholar]
- Wu C, Narayan P, Puett D. Protein engineering of a novel constitutively active hormone-receptor complex. J Biol Chem. 1996;271:31638–42. doi: 10.1074/jbc.271.49.31638. [DOI] [PubMed] [Google Scholar]
- Zimmermann G, Ackermann W, Alexander H. Epithelial human chorionic gonadotropin is expressed and produced in human secretory endometrium during the normal menstrual cycle. Biol Reprod. 2009;80:1053–1065. doi: 10.1095/biolreprod.108.069575. [DOI] [PubMed] [Google Scholar]
- Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Munstedt K, Rao CV, Lang U, Preissner KT. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87:5290–5296. doi: 10.1210/jc.2002-020642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.